Molecular logic gates made of DNA have attracted significant attention because of biocompatibility, simple design and their ability to analyze and control biological systems.[1] To fuel further development of the field, applications of DNA-based gates to solve significant biological problems are required. Recently we characterized a set of DNA logic gates (YES, NOT, AND, and OR) and demonstrated their connectivity by designing ANDNOT and XOR operations.[1g,h] The gates used hybridization of DNA strands with a molecular beacon (MB) probe to produce a fluorescent output. Here we demonstrate how DNA logic gates can be applied to solve an important biomedical task of analysis of multiple DNA sequences containing a complex set of mutations.
Mycobacterium tuberculosis (Mtb) infects approximately 2 billion people all over the world and is responsible for about 2 million deaths each year.[2] Approximately 10% of all patients are infected by strains of Mtb that are drug-resistant; these strains are primarily resistant to antibiotics rifampin (Rif) and isoniazid.[3] Currently, there is an urgent need for cost-effective diagnostic tools that can detect Mtb in clinical samples and differentiate between drug-susceptible and drug-resistant Mtb strains.[2-4] Several assays exist to detect mutations responsible for antibiotic resistance.[5] One of the most advanced commercial assays, Cepheid's Expert MTB/RIF,[5i-n] takes advantage of real-time PCR (rtPCR) and MB probes.[6] MB probes, first introduced by Tyagi and Kramer,[6a] are stem-loop folded oligonucleotides with fluorophore and quencher dyes attached at opposite ends (Fig. 1). Hybridization of an MB probe to a complementary DNA or RNA switches the probe to an elongated form, thus separating the fluorophore from the quencher. The resultant fluorescence increase can be quantitatively measured, which is the basis for the widespread application of MB probes in real-time detection of nucleic acids.[6] In the Expert MTB/RIF assay, five MB probes were designed to span the highly variable 81-nt core of the bacterial amplicon where about 96% of all Rif-resistant mutations are located (Fig.1A). The probes were complementary to the drug-susceptible wildtype (wt) sequence.[5g] A signal from all five MB probes indicated the presence of the wt (no mutation in the core region). Absence of a signal from all five probes indicated that a sample was Mtb negative. Failure of at least one MB probe to produce a signal indicated the presence of a Rif-resistant Mtb (Fig. 1A, right).
Figure 1.
Two molecular beacon (MB) probe-based strategies for the analysis of Rif-resistant Mtb. A) Currently used Expert MTB/RIF five MB probe approach.[5i-n] Absence of a signal from at least one MB probe ( e.g. MB5) indicates the presence of Rif-resistant Mtb DNA. B) Combination of YES and OR logic gates for the detection of Mtb and its resistance to Rif. The three DNA strands (C, D, and E) form a complex with UMB1 and the analyte to report the presence of Mtb DNA. A pair of strands M and F of the OR gate forms fluorescent complex with UMB2 and the analyte only if a mutation responsible for Rif-resistance is present in the sequence.
Formal logic suggests that the Expert MTB/RIF scheme is redundant: it uses five outputs to answer ‘yes’ or ‘no’ to the two following questions: (i) Is there Mtb DNA present in a sample? (ii) Does the Mtb DNA contain a mutation that confers rifampin resistance? Additionally, five expensive hard-to-optimize MB probes along with a five-channel fluorescent reader are needed for the assay. Formally, the task can be executed by using only two outputs. Here we propose to use a DNA-based YES logic gate to answer the first question and an OR logic gate to answer the second question. Each gate uses a separate fluorescent output, thus the detection scheme requires only two MB probes.
The sequences of all strands of the proposed oligonucleotide sensor as well as analytes are given in Table S1. Analyte WT represents wt Mtb sequence with no resistance-causing mutations. All five mutant sequences (named here M1-M5; see Table S1) were from the highly variable 81-nt core, each with a separate known mutation which confers Rif resistance. They were previously used for characterization of Rif resistance Mtb strains by conventional methods.[5]
The YES logic gate consisted of the three DNA stands (C, D, and E in Fig. 1B) and a universal MB probe (UMB1). In the presence of any Mtb DNA (either WT or Rif-resistant M1-M5) the three strands formed a complex with the target DNA and UMB1. The fluorescent complex that contained UMB1 had an arrangement of DNA strands similar to DX tile,[7] which we recently investigated and characterized.[8] Importantly, the DX complex was tolerant to mutations since it formed an extended 36 base pair hybrid with the analyte (Fig. S1). The OR gate was designed to report the presence of any of the 5 different drug resistance-conferring mutations across all 3 separate regions within the hyper-variable 81nt region of the Mtb genome. The OR gate consisted of strands F and M. Each strand M was designed to recognize a specific mutant strain of Mtb that is known to cause Rif resistance. Together, the five M and the three F strands formed five fluorescent complexes with UMB2 and the five Mtb mutants, M1, M2, M3, M4 and M5 as shown in details in Figs S2 and S3. Each fluorescent complex was formed only in the presence of the cognate mutant analyte, but not WT. Therefore, overall performance of the complete set of all M and F strands corresponded to OR logic, which produced a high fluorescent output in the presence of any antibiotic resistant Mtb DNA, but low in the presence of WT. Pairs of strands M and F were optimized to produce high signals only in the presence of cognate mutant sequence (Fig. S3). Two different F strands, M1_M4-F and M2_M3-F were utlilized as uniform components for recognition of M1, M4 or M2, M3, respectively. Importantly, no signal above the background was produced by the wt Rif-susceptible sequence (Fig. S2 and S3). This was achieved by designing relatively short analyte-binding arms of all M strands (7 nucleotides), which increased sensitivity for a mutation.
To create a functional assay, all DNA strands of both the YES and OR gates were combined with UMB1 and UMB2 in one reaction mixture and the outputs of the UMBs were measured at two different wavelengths (517 nm and 580 nm). It was found that both OR and YES gates functioned according to the design: the YES gate produced high output at 580 nm in the presence of both WT and the mutant sequences (Fig. 2, grey bars); the OR gate generated high signal at 517 nm in the presence of any of the mutants but a low signal for the WT analyte (Fig. 2, black bars). Therefore, the combination of the YES and OR gates produced a high signal at 580 nm if any Mtb sequence is present; a signal at 517 nm was observed only if a Rif-resistant mutant sequence was present. This signal pattern can be unambiguously used for diagnostic purposes to answer the questions of the presence of an Mtb sequence and its Rif susceptibility.
Figure 2.
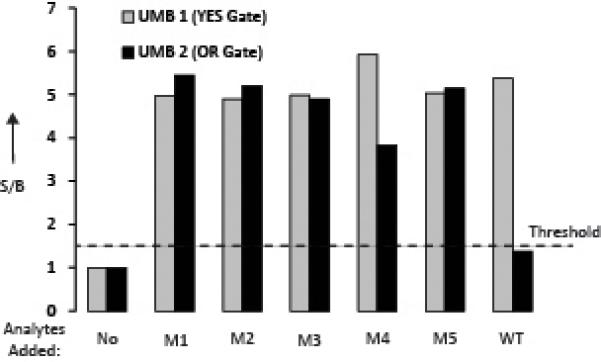
Combination of YES and OR gates for the detection and mutation analysis of Mtb DNA. All OR gate, YES gate, UMB1, and UMB2 oligonucleotides were mixed together in the absence or presence of each separate mutant or wildtype analytes. Fluorescence at 580 nm for UMB1 (grey) or 517 nm for UMB2 (black) was recorded after a 15 minute incubation at room temperature. The data is an average of three independent experiments, presented as the ratio of signal to background (S/B). S/B was calculated as a ration of fluorescence in the presence of analyzed sequence to that in the absence.
PCR-based Mtb analysis is considered to be a new method to replace time consuming culturing or error-prone sputum smear microscopy techniques.[5] The Expert MTB/RIF assay can detect Mtb resistance in 2 hours with high specificity.[5i-n] However, the approach is limited to interrogation of the 81nt hypervariable fragment of the Mtb genome. Detection of other mutations (e.g. mutations responsible for isoniazid resistance) would require introduction of additional MB probes, while current rtPCR instruments register fluorescence from only a few (5-6) channels. Importantly, if a sample is contaminated with the wt sequence, the assay would produce a false negative for antibiotic resistance, since all five MB probes would preferably bind the wt analyte, producing high fluorescent signal.
Here we propose the combination of YES and OR DNA logic gates to achieve accurate detection of both wt and Rif-resistant DNA. The approach minimizes the number of MB probes required for both the detection of Mtb and Rif resistance. Therefore, a qPCR instrument with five detection channels can be replaced with a more affordable two-channel thermal cycler. Alternatively, additional OR gates can be employed to detect other antibiotic-resistant Mtb strains if a multichannel qPCR instrument is available. Thus, the study presented here can serve as the framework for the development of a multiplex assay to determine Mtb presence as well as drug resistance for all major first line antibiotics. A multiplex assay of this nature would be a valuable tool for health care providers. In either case, the presented approach is cost-efficient since the logic gates use unmodified DNA as adaptor strands, which are inexpensive synthetic oligonucleotides.
Earlier we reported the design of DNA logic gates that take advantage of MB probes as fluorescent reporters.[9] However, the constructs used expensive non-nucleotide modifications, which added to the cost of custom-made oligonucleotides. The combination of adaptor strands M and F reported here is a new cost-efficient OR gate that demonstrates robust performance. Impressively, the 13 oligonucleotides working together in solution clearly demonstrated predictable digital response with no detectable cross-talk. We anticipate that the addition of more strands for detection of other mutations, including those responsible for resistance to isoniazid and other antibiotics, will not compromise the performance of the assay. Indeed, it has been recently shown by DNA nanotechnology that complex pre-designed DNA associates of hundreds of self-assembling DNA strands can be produced at a yield of >90%.[10]
Among numerous DNA logic gates reported so far there are few applications to solve challenging practical problems. Here we demonstrated that the combination of DNA logic gates can be used to perform a complex diagnostic task efficiently, affordably, and reliably. This work is a step towards employing logic gates as versatile tools for diagnosis of tuberculosis and other infectious diseases, which opens a new venue in application of DNA nanotechnology and DNA computation in biology and medicine.
Experimental Section
All oligonucleotides were custom-made by Integrated DNA Technologies, Inc (Coralville, IA). For the fluorescence assay with both YES and OR logic functions combined, all five M strands (50 nM), three F strands (200 nM), strands C, D (200nM each), and E (100 nM), UMB1 (25 nM), and UMB2 (25 nM) were mixed in a buffer containing 50 nM MgCl2, 50 mM Tris-HCl, pH 7.4, in the presence or absence of 250 nM wildtype (WT) or mutant analyte (M1-M5). Final sample volumes were 120 μL. Fluorescence spectra were recorded on a Perkin-Elmer (San Jose, CA) LS-55 Luminescence Spectrometer with a Hamamatsu xenon lamp (excitation at 550 nm; emission at 580 for UMB1 and excitation at 485 nm; emission 517 nm for UMB2) after 15 min incubation at room temperature (22°C). The data was analyzed using Microsoft Excel. The means of three independent experiments were calculated and converted to signal-to-background Figure 2. The background was considered the sample with all oligonucleotides except analyte. All signals above a threshold set at S/B = 1.5 were considered true outputs for either the YES or the OR logic gates.
Supplementary Material
Footnotes
The authors are grateful to Yulia V. Gerasimova for helpful discussion and corrections and to A. Trevor Longino for careful reading of the manuscript. Support from NIHGRI (R21 HG004060) and NSF CCF (1117205) is greatly appreciated.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the authors.
References
- 1.a Elbaz J, Lioubashevski O, Wang F, Remacle F, Levine RD, Willner I. Nat. Nanotechnol. 2010;5:417–422. doi: 10.1038/nnano.2010.88. [DOI] [PubMed] [Google Scholar]; b Qian L, Winfree E. Science. 2011;332:1196–1201. doi: 10.1126/science.1200520. [DOI] [PubMed] [Google Scholar]; c Qian L, Winfree E, Bruck J. Nature. 2011;475:368–372. doi: 10.1038/nature10262. [DOI] [PubMed] [Google Scholar]; d Zhang DY, Seelig G. Nat. Chem. 2011;3:103–113. doi: 10.1038/nchem.957. [DOI] [PubMed] [Google Scholar]; e Macdonald J, Stefanovic D, Stojanovic MN. Sci. Am. 2008;299:84–91. doi: 10.1038/scientificamerican1108-84. [DOI] [PubMed] [Google Scholar]; f Stojanovic MN. Prog. Nucleic Acid Res. Mol. Biol. 2008;82:199–217. doi: 10.1016/S0079-6603(08)00006-8. [DOI] [PubMed] [Google Scholar]; g Lake A, Shang S, Kolpashchikov DM. Angew. Chem. Int. Ed. Engl. 2010;49:4459–4462. doi: 10.1002/anie.200907135. [DOI] [PubMed] [Google Scholar]; h Gerasimova YV, Kolpashchikov DM. Chem. Asian J. 2012;7:534–540. doi: 10.1002/asia.201100664. [DOI] [PubMed] [Google Scholar]
- 2.a Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]; b Murray CJ, Salomon JA. Proc. Natl. Acad. Sci. USA. 1998;95:13881–13886. doi: 10.1073/pnas.95.23.13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a WHO Anti-Tuberculosis Drug Resistance in the World. 2008. ; b Multidrug and Extensively Drug-Resistant TB (M/XDR-TB): 2010 Global Report on Surveillance and Response. 2010.
- 4.a Abu-Raddad LJ, Sabatelli L, Achterberg JT, Sugimoto JD, Longini IM, Jr., et al. Proc. Natl. Acad. Sci. U S A. 2009;106:13980–13985. doi: 10.1073/pnas.0901720106. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Jordan TS, Davies PD. Int. J. Tuberc. Lung Dis. 2010;14:683–688. [PubMed] [Google Scholar]; c Loddenkemper R, Sagebiel D, Brendel A. Eur. Respir. J. Suppl. 2002;36:66s–77s. doi: 10.1183/09031936.02.00401302. [DOI] [PubMed] [Google Scholar]; d Ellner JJ. Clin. Transl. Sci. 2009;2:80–84. doi: 10.1111/j.1752-8062.2008.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Jindani A, Griffin GE. Tuberculosis (Edinb) 2010;90:168–170. doi: 10.1016/j.tube.2010.03.006. [DOI] [PubMed] [Google Scholar]; f Sharma S, Yoder MA. Am. J. Ther. 2011;18:e101–112. doi: 10.1097/MJT.0b013e3181c3509c. [DOI] [PubMed] [Google Scholar]
- 5.a Telenti A, Imboden P, Marchesi F, Lowrie D, Cole S, Colston MJ, Matter L, Schopfer K, Bodmer T. Lancet. 1993;341:647–650. doi: 10.1016/0140-6736(93)90417-f. [DOI] [PubMed] [Google Scholar]; b Donnabella V, Martiniuk F, Kinney D, Bacerdo M, Bonk S, Hanna B, Rom WN. Am. J. Respir. Cell Mol. Biol. 1994;11:639–643. doi: 10.1165/ajrcmb.11.6.7946393. [DOI] [PubMed] [Google Scholar]; c Morris S, Bai GH, Suffys P, Portillo-Gomez L, Fairchok M, Rouse D. J. Infect. Dis. 1995;171:954–960. doi: 10.1093/infdis/171.4.954. [DOI] [PubMed] [Google Scholar]; d Helb D, et al. J. Clin. Microbiol. 2010;48:229–237. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]; e El-Hajj HH, Marras SA, Tyagi S, Kramer FR, Alland D. J. Clin. Microbiol. 2001;39:4131–4137. doi: 10.1128/JCM.39.11.4131-4137.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Cooksey RC, Morlock GP, Glickman S, Crawford JT. J. Clin. Microbiol. 1997;35:1281–1283. doi: 10.1128/jcm.35.5.1281-1283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Troesch A, Nguyen H, Miyada CG. J. Clin. Microbiol. 1999;37:49–55. doi: 10.1128/jcm.37.1.49-55.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Gingeras TR, et al. Genome Res. 1998;8:435–448. doi: 10.1101/gr.8.5.435. [DOI] [PubMed] [Google Scholar]; i Blakemore R, Story E, Helb D, Kop J, Banada P, Owens MR, Chakravorty S, Jones M, Alland D. Clin Microbiol. 2010;48:2495–2501. doi: 10.1128/JCM.00128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Miotto P, Bigoni S, Migliori GB, Matteelli A, Cirillo DM. Eur. Respir. J. 2012;39:1269–1271. doi: 10.1183/09031936.00124711. [DOI] [PubMed] [Google Scholar]; k Armand S, Vanhuls P, Delcroix G, Courcol R, Lemaître N. J. Clin. Microbiol. 2011;49:1772–1776. doi: 10.1128/JCM.02157-10. [DOI] [PMC free article] [PubMed] [Google Scholar]; l Malbruny B, Le Marrec G, Courageux K, Leclercq R, Cattoir V. Int. J. Tuberc. Lung Dis. 2011;15:553–555. doi: 10.5588/ijtld.10.0497. [DOI] [PubMed] [Google Scholar]; m Vadwai V, Boehme C, Nabeta P, Shetty A, Alland D, Rodrigues C. J. Clin. Microbiol. 2011;49:2540–2545. doi: 10.1128/JCM.02319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]; n Gous N, Scott LE, Wong E, Omar T, Venter WD, Stevens W. Clin. Microbiol. 2012;50:2100–2103. doi: 10.1128/JCM.00252-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a Tyagi S, Kramer FR. Nat. Biotechnol. 1996;14:303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]; b Marras SA, Tyagi S, Kramer FR. Clin. Chim. Acta. 2006;363:48–60. doi: 10.1016/j.cccn.2005.04.037. [DOI] [PubMed] [Google Scholar]; c Chakravorty S, Aladegbami B, Burday M, Levi M, Marras SA, Shah D, El-Hajj HH, Kramer FR, Alland DJ. Clin. Microbiol. 2010;48:258–267. doi: 10.1128/JCM.01725-09. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Wang F, Niu G, Chen X, Cao F. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:1572–1579. doi: 10.1007/s00259-011-1786-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Song P, Xiang Y, Xing H, Zhou Z, Tong A, Lu Y. Anal Chem. 2012 doi: 10.1021/ac203488p. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Meng HM, Fu T, Zhang XB, Wang NN, Tan W, Shen GL, Yu RQ. Anal. Chem. 2012;84:2124–2128. doi: 10.1021/ac300005f. [DOI] [PubMed] [Google Scholar]; g Shire ZJ, Loppnow GR. Anal. Bioanal. Chem. 2012;403:179–184. doi: 10.1007/s00216-012-5790-4. [DOI] [PubMed] [Google Scholar]
- 7.Fu TJ, Seeman NC. Biochemistry. 1993;32:3211–3220. doi: 10.1021/bi00064a003. [DOI] [PubMed] [Google Scholar]
- 8.Kolpashchikov DM, Gerasimova YV, Khan MS. Chembiochem. 2011;12:2564–2567. doi: 10.1002/cbic.201100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a Kolpashchikov DM. J. Am. Chem. Soc. 2006;128:10625–10628. doi: 10.1021/ja0628093. [DOI] [PubMed] [Google Scholar]; b Gerasimova YV, Hayson A, Ballantyne J, Kolpashchikov DM. Chembiochem. 2010;11:1762–1768. doi: 10.1002/cbic.201000287. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Grimes, Gerasimova YV, Kolpashchikov DM. Angew. Chem. Int. Ed. Engl. 2010;49:8950–8953. doi: 10.1002/anie.201004475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a Rothemund PW. Nature. 2006;440:297–302. doi: 10.1038/nature04586. [DOI] [PubMed] [Google Scholar]; b Barish RD, Schulman R, Rothemund PW, Winfree E. Proc. Natl. Acad. Sci. U S A. 2009;106:6054–6059. doi: 10.1073/pnas.0808736106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



