Abstract
We report nine new individuals from six families who have homozygous mutations of HOXA1 with either the Bosley-Salih-Alorainy Syndrome (BSAS) or the Athabascan Brainstem Dysgenesis Syndrome (ABDS). Congenital heart disease was present in four BSAS patients, two of whom had neither deafness nor horizontal gaze restriction. Two ABDS probands had relatively mild mental retardation. These individuals blur the clinical distinctions between the BSAS and ABDS HOXA1 variants and broaden the phenotype and genotype of the homozygous HOXA1 mutation clinical spectrum.
Introduction
We recently reported a Mendelian syndrome associated with truncating mutations in HOXA11, a homeodomain transcription factor critical for the proper development of hindbrain rhombomeres2, 3. Homozygous 175-176insG guanine base-pair insertions were found in several families from Saudi Arabia, while a homozygous 84C>G nonsense mutation resulted in substitution of a stop codon for a tyrosine residue in a Turkish individual. These two mutations cause a phenotype referred to as the Bosley-Salih-Alorainy Syndrome (BSAS; OMIM #601536) characterized by bilateral Duane retraction syndrome (DRS) type 3, deafness, malformations of the cerebral vasculature, and autism in some patients1, 4. This syndrome differed from another homozygous HOXA1 variant, the Athabascan Brainstem Dysgenesis Syndrome (ABDS) reported in Native Americans, which is marked by horizontal gaze restriction, deafness, mental retardation, facial and bulbar weakness, central hypoventilation, frequent conotruncal cardiac malformations5, and cerebral vascular malformations1.
We now report nine additional affected individuals from three consanguineous Saudi families and three Native American families. These patients extend the phenotype and genotype of the homozygous HOXA1 clinical spectrum.
Materials and Methods
The three probands from consanguineous Saudi families were examined in the Neuro-ophthalmology and Pediatric Neurology clinics of the King Faisal Specialist Hospital and Research Centre in Riyadh, Saudi Arabia. All probands had neurologic and ophthalmologic examinations, and other affected family members had neurologic and cardiologic evaluations appropriate for clinical circumstances. The three Native American probands were examined at the Children’s Rehabilitation Services in Flagstaff, AZ. All families signed informed consent at the appropriate institution. The HOXA1 gene was sequenced as described previously1 in Saudi individuals, while a restriction enzyme-based assay (using Bpu 10I) identified the Native American variant 76C>T.
Results
Table 1 details the genetic and clinical status of the nine patients reported here. Patient A1 had a novel homozygous HOXA1 mutation causing the typical BSAS clinical syndrome. The other Saudi individuals (Patients B1, C1, C2, C3, and C4) had the previously reported Saudi HOXA1 mutation1. Patient A1 had two unaffected siblings, and Patient B1 had two unaffected siblings and six unaffected half siblings. Patients C1-C4 came from an inbred extended family in which two brothers had married two sisters who were first cousins. The three Native American probands (Patients D1, E1, and F1) had the somatic ABDS phenotype5 and genotype1. They were each from singleton families which were not consanguineous, and each had unaffected siblings.
Table 1.
General information
| ID | Sex | YOB |
HOXA1 mutation |
Hearing | Cardiac | Cognition | Comments |
|---|---|---|---|---|---|---|---|
| A1 | M | 1987 | 185delG | Deaf | Normal | Normal | |
| B1 | F | 1998 | 175- 176insG |
Deaf | Dual outlet right ventricle; subpulmonic VSD; interrupted aortic arch; PDA |
Autistic features; developmental delay |
Stroke during second cardiac surgery; seizures |
| C1 | M | 2003 | 175- 176insG |
Deaf | Tetrology of Fallot | Moderate developmental delay; seizures |
Low set ears; hypertrichosis; club foot |
| C2 | F | 2006 | 175- 176insG |
Deaf | VSD | Reportedly delayed | Club foot |
| C3 | F | 2003 | 175- 176insG |
Normal | Infantile CHF due to multiple VSD, closing spontaneously |
Developmentally normal |
|
| C4 | M | 2006 | 175- 176insG |
Normal | VSD | Developmentally normal |
|
| D1 | M | 1985 | 76C>T | Deaf | VSD closing spontaneously |
Severely delayed | Asymmetric face with mouth and nose deviated to left |
| E1 | F | 2003 | 76C>T | Deaf | Total anomalous pulmonary venous return |
Mildly delayed | |
| F1 | F | 1992 | 76C>T | Deaf | Normal | Mildly delayed |
Reported group includes individuals from six families (A, B, C, D, E, and F); 1 = proband in each family; additional numbers in family C indicate sibling (C2) and cousins (C3 and C4); YOB = year of birth; PDA = patent ductusarteriosus; VSD = ventriculoseptal defect. HOXA1 mutations were homozygous in all listed individuals.
All six probands had severe restriction of horizontal gaze and deafness bilaterally. Five BSAS patients had conotruncal or septal heart defects not previously reported in BSAS1, 4. Patient B1 had a double outlet right ventricle and Patient C1 had Tetrology of Fallot. Patients C1 and C2 had two first cousins (C3 and C4) with ventricular septal defects (VSDs) but no horizontal ocular motility abnormality or deafness. Two of the Native American probands also had cardiac defects, one a VSD that closed spontaneously (Patient D1) and one a total anomalous pulmonary venous return (TAPVR; Patient E1). Some children with congenital heart disease had developmental delay, sitting (Patient B1) or walking (Patients C1 and C2) at approximately three years of age; however, the patient with TAPVR was only minimally delayed. One had autistic features (Patient B1), and another had low set ears, hypertrichosis, and a club foot (Patient C1). Patients A1 and B1 had facial twitching, while Patient D1 had unilateral facial weakness noted previously in ABDS5.
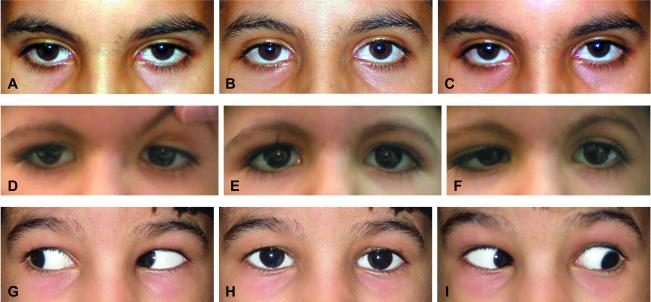
Table 2 details their ophthalmologic evaluation. Two BSAS probands (Patients A1 and B1) had no horizontal gaze and no globe retraction noted OU (Figure 1 A,B,C). Patient C1 had obvious DRS OU including globe retraction and narrowing of the palpebral fissure (Figure 1 D,E,F). Patient C3 had normal ocular motility except for restricted up gaze and ptosis on the left, and Patient C4 had no ocular motility abnormality (Figure 1 G,H,I). Native American patients all had complete HGP. Interestingly, the two patients with the mildest ocular motility abnormalities both had amblyopia, Patient C3 because of an accommodative esotropia untreated during early childhood and Patient C4 because of anisometropic amblyopia.
Table 2.
Ocular motility and alignment
| Patient | Abduction | Adduction | Retraction | Vertical | Alignment | Convergence | Comments |
|---|---|---|---|---|---|---|---|
| A1 | 0% OU | 0% OU | None observed |
Full | Orthotropic at distance; exotropic at near |
None observed | No nystagmus; facial twitching; mild scoliosis |
| B1 | 0% OU | 0% OU | None observed |
Grossly full | Grossly ortho | None observed |
No nystagmus; facial twitching; mild scoliosis; poorly cooperative |
| C1 | 0% OU | 25% OU | Mild OU | Full | Ortho | Good | Intermittent rapid horizontal pendular nystagmus |
| C2 | 0% OU | 0% OU | None observed |
Grossly full | Grossly ortho | None observed |
No nystagmus; poorly cooperative infant |
| C3 | 100% OU | 100% OU | None | Modest elevation restriction OS |
Incomitant left hypotropia; orthotropic in RG; ET without correction |
Intact | No nystagmus; accommodative ET controlled with glasses; strabismic amblyopia OS; left ptosis |
| C4 | 100% OU | 100% OU | None | Full | Ortho | Excellent | No nystagmus; dense anisometropic amblyopia OS |
| D1 | 0% OU | 0% OU | None observed |
Grossly full | Grossly ortho | None observed |
No nystagmus |
| E1 | 0% OU | 0% OU | None observed |
Grossly full | Grossly ortho | Excellent | No nystagmus |
| F1 | 0% OU | 0% OU | Mild OU | Full | Orthophoric | Excellent | No nystagmus |
Adduction and Abduction = % of normal excursion; OU = both eyes; OS = left eye; Ortho = orthophoric; RG = right gaze; ET = esotropia
Figure 1. Ocular motility.
Images A, D, and G are right gaze; images B, E, and H are primary gaze; and images C, F, and I are left gaze. Figures A, B, and C show Patient A1 with total horizontal gaze palsy, while Figures D, E, and F show Patient C1 with bilateral DRS type 3, and Figures G, H, and I are Patient C4 with entirely normal ocular motility. All of these patients had full vertical gaze OU. This montage illustrates the spectrum of horizontal gaze with homozygous HOXA1 mutations, from complete horizontal gaze restriction (top row) to DRS type 3 OU (middle row) to full motility (bottom row).
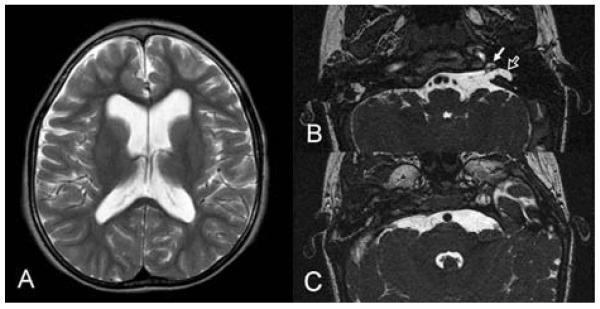
Table 3 details neuroimaging results, including evidence of cerebrovascular malformations in all three appropriately studied patients (A1, B1, and C1). Patient B1 had a severe developmental setback after her second cardiac surgery at age six months, and subsequent neuroimaging revealed diffuse cerebral hemispheric atrophy and white matter thinning compatible with global cerebral ischemia (Figure 2A). Patient C1 was deaf but had partial development of left inner ear structures (Figure 2B) with no obvious abducens nerve on either side (Figure 2C).
Table 3.
Neuroimaging
| Patient | Exams | Inner Ear | Vasculature | Comments |
|---|---|---|---|---|
| A1 | CT brain and petrous bone |
Bilateral CCD with narrow right internal auditory canal |
Bilateral hypoplastic ICA; enlarged vertebrobasilar system and Pcoms |
|
| B1 | MRI brain |
Right: Aplasia of inner ear structures (cochlea, vestibule, and semicircular canals); absent internal auditory canal Left: CCD with narrow internal auditory canal |
Hypoplastic left ICA; enlarged basilar artery |
White matter volume reduction and thin corpus callosum due to periventricular leukomalacia secondary to hypoxic-ischemic insult |
| C1 | MRI brain |
Right: Aplasia of inner ear structures (cochlea, vestibule, and semicircular canals); absent internal auditory canal and vestibulocochlear nerve; facial nerve runs in an anomalous canal in the petrous bone Left : Hypoplastic cochlea with basal turn and part of the second turn present; hypoplastic vestibule and semicircular canals; normal vestibulocochlear nerve with wide internal auditory canal |
Hypoplastic left ICA Enlarged left Pcom and basilar artery; absent 6th CNs bilaterally |
Abducens cranial nerves not seen; oculomotor nerves visible |
| F1 | MRI brain | Reported normal, but images not reviewed |
CCD = common cavity deformity; ICA = internal carotid artery; Pcom = posterior communicating artery; CNs = cranial nerves
Figure 2. Neuroimaging.
(A) Axial T2-weighted image of Patient B1 at the level of the lateral ventricles showing reduced amount of the periventricular white matter adjacent to the frontal horns and atria of the lateral ventricles secondary to hypoxic-ischemic injury.
(B) Axial steady state free precession (SSFP) images of Patient C1 at the level of the temporal bone showing hypoplastic cochlea (solid arrow) and hypoplasia of the vestibule and semicircular canals (open arrow).
(C) Axial SSFP images of Patient C1 at the level of the pons where the abducens cranial nerves should be visible. Both 6th cranial nerves are absent.
Discussion
The probands in this study were identified because of deafness and severe horizontal gaze restriction, cardinal features of both BSAS and ABDS. Each came from a consanguineous Saudi family or the Athabascan population, which experienced a genetic “bottleneck” in the 1800s6, and each had homozygous HOXA1 mutations. Three BSAS individuals (Patients B1, C1, and C2) had horizontal gaze palsy (HGP), deafness, and congenital heart disease, a constellation similar to ABDS. Meanwhile, two ABDS patients (Patients E1 and F1) had only mild cognitive change more similar to BSAS. These patients blur the distinction between homozygous HOXA1 BSAS and ABDS variants.
Seven patients had horizontal gaze restriction, one of whom (patient C1) had obvious globe retraction and narrowing of the palpebral fissure diagnostic of DRS type 3, confirming that either of these two ocular motility phenotypes can result from the same genetic mutation4. The abducens nerves are probably congenitally absent in both BSAS and ABDS4 with variable dysinnervation of the lateral rectus7. Variable facial, bulbar, and respiratory abnormalities in ABDS5 and autism in BSAS1 probably also imply brainstem developmental abnormalities, while inner ear malformations and deafness in HOXA1-/- patients are likely the sequela of abnormal inductive signals from hindbrain neuroectoderm4. Variable genetic loss of function implies partial ability of other genes related to the HOX cascade to assume the actions of the mutated HOXA1 gene1, 8, 9.
The cardiac and cerebrovascular abnormalities now identified in both ABDS and BSAS patients could reflect an action of the HOXA1 gene outside the brainstem1, 4, 5 that has not been described in Hoxa1-/- mice2, 3. This may be a primary effect of HOXA1 on the aortic sac and paired dorsal aortae and aortic arches10, 11. The cardiovascular abnormalities in the HOXA1 spectrum converge with those in DiGeorge syndrome12, 13 resulting from loss of TBX1 function14, 15. Hoxa1 in mouse shares similar spatial and temporal expression patterns to TBX1 in embryonic mesoderm14, 16, 17, and HOXA1 may function similarly to TBX1 in human cardiovascular and cerebrovascular development by regulating the formation of the aortic sac, paired dorsal aortae and aortic arches10, 11. Whatever the mechanism, it is noteworthy that two children with VSDs (Patients C3 and C4) had no horizontal ocular motility abnormality or deafness, which had been considered sine qua non of homozygous HOXA1 mutations in humans4. These children raise the possibility that congenital heart disease might be a clinically isolated, or relatively isolated, manifestation of homozygous HOXA1 mutations.
Cognitive limitations in ABDS may be secondary to global brain hypoxia precipitated by the combination of central hypoventilation, cerebrovascular malformations, and the relatively high altitude at which the Athabascan population lives5. This speculation is supported by the two mildly affected Native Americans included in this report (E1 and F1), who were raised at an altitude of 1000 m, while the more severely affected individual (D1) was raised above 1500 m. Autism, on the other hand, seems to be a primary but infrequent sequela of HOXA1 mutations1, 4.
A severity gradient of involvement has been noted in BSAS4, and some patients described here (e.g., Patients C1 and D1) were much more affected than others (e.g., Patients C3 and C4). The homozygous HOXA1 clinical spectrum appears to be bounded at the more severe end by ABDS and at the other end by milder versions of BSAS including isolated bilateral DRS1, 4 and isolated, mild congenital heart disease (Patient C4). Thus far, facial and bulbar weakness and symptomatic central hypoventilation have only been present among the Athabascan population, while somatic abnormalities have only been observed in BSAS (see Table 4).
Table 4.
Frequency of clinical characteristics in the homozygous HOXA1 clinical spectrum
| BSAS | ABDS |
HOXA1 Spectrum |
|
|---|---|---|---|
| DRS/HGP | 14/16 | 13/13 | 27/29 |
| Deafness | 13/16 | 13/13 | 26/29 |
| Delayed motor development | 10/16 | 11/13 | 21/29 |
| Cognitive abnormality | 3/16 | 13/13 | 16/29 |
| Cerebrovascular anomalies * | 9/13 | 1/3 | 10/16 |
| Congenital heart disease | 4/16 | 9/13 | 13/29 |
| Central hypoventilation | 0/16 | 11/13 | 11/29 |
| Facial twitching or paresis | 2/16 | 7/13 | 9/29 |
| Somatic abnormalities | 8/16 | 0/13 | 8/29 |
| Seizure disorder | 1/16 | 4/13 | 5/29 |
| Bulbar paresis | 0/16 | 2/13 | 2/29 |
Cognitive abnormalities = autism or mental retardation; “Somatic abnormalities” include low set ears, flattened ear helix, bony facial asymmetry, hypertrichosis, polydactyly; brachydactyly, club foot, and duplex ureteral system;
= in patients appropriately studied
Patients presented here broaden the clinical spectrum of homozygous HOXA1 mutations and begin to merge the BSAS and ABDS HOXA1 variants. Table 4 lists the main characteristics of the homozygous HOXA1 spectrum with their respective frequencies in patients genotyped thus far4, 5. DRS/HGP and deafness due to inner ear maldevelopment are cardinal features, although congenital heart disease, cerebrovascular abnormalities, and cognitive disturbances figure prominently as well. Making a clinical diagnosis of homozygous HOXA1 mutations is now complicated by the observation that not all patients have horizontal gaze restriction and not all patients are deaf. Isolated bilateral DRS or isolated congenital heart disease can be the sole manifestation of homozygous HOXA1 mutations, and isolated deafness, autism, or cerebrovascular abnormalities might potentially also occur. In fact, homozygous HOXA1 mutations might not be universally penetrant or an affected individual may be asymptomatic, such as with an asymptomatic VSD (e.g., Patient C4) or cerebrovascular abnormality. These are important issues because patients with homozygous HOXA1 mutations have the potential for cerebrovascular maldevelopment that could constitute a risk in certain environmental settings or at the time of surgery.
Acknowledgements
The authors would like to thank Dr. Rido Cha for his assistance.
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Tischfield MA, Bosley TM, Salih MA, et al. Homozygous HOXA1 mutations disrupt human brainstem, inner ear, cardiovascular and cognitive development. Nat Genet. 2005;37:1035–1037. doi: 10.1038/ng1636. [DOI] [PubMed] [Google Scholar]
- 2.Chisaka O, Musci TS, Capecchi MR. Developmental defects of the ear, cranial nerves and hindbrain resulting from targeted disruption of the mouse homeobox gene Hox-1.6. Nature. 1992;355:516–520. doi: 10.1038/355516a0. [DOI] [PubMed] [Google Scholar]
- 3.Lufkin T, Dierich A, LeMeur M, et al. Disruption of the Hox-1.6 homeobox gene results in defects in a region corresponding to its rostral domain of expression. Cell. 1991;66:1105–1119. doi: 10.1016/0092-8674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- 4.Bosley TM, Salih MA, Alorainy IA, et al. Clinical charactization of the HOXA1 Syndrome BSAS variant. Neurology. 2007 doi: 10.1212/01.wnl.0000276947.59704.cf. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holve S, Friedman B, Hoyme HE, et al. Athabascan brainstem dysgenesis syndrome. Am J Med Genet A. 2003;120:169–173. doi: 10.1002/ajmg.a.20087. [DOI] [PubMed] [Google Scholar]
- 6.Erickson RP. Southwestern Athabaskan (Navajo and Apache) genetic diseases. Genet Med. 1999;1:151–157. doi: 10.1097/00125817-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Gutowski NJ, Bosley TM, Engle EC. 110th ENMC International Workshop: the congenital cranial dysinnervation disorders (CCDDs). Naarden, The Netherlands, 25-27 October, 2002. Neuromuscul Disord. 2003;13:573–578. doi: 10.1016/s0960-8966(03)00043-9. [DOI] [PubMed] [Google Scholar]
- 8.Gavalas A, Studer M, Lumsden A, et al. Hoxa1 and Hoxb1 synergize in patterning the hindbrain, cranial nerves and second pharyngeal arch. Development (Cambridge, England) 1998;125:1123–1136. doi: 10.1242/dev.125.6.1123. [DOI] [PubMed] [Google Scholar]
- 9.Gavalas A, Trainor P, Ariza-McNaughton L, Krumlauf R. Synergy between Hoxa1 and Hoxb1: the relationship between arch patterning and the generation of cranial neural crest. Development (Cambridge, England) 2001;128:3017–3027. doi: 10.1242/dev.128.15.3017. [DOI] [PubMed] [Google Scholar]
- 10.Larsen WJ. Human Embryology. 3rd ed 2001. [Google Scholar]
- 11.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 12.Baldini A. DiGeorge syndrome: an update. Current opinion in cardiology. 2004;19:201–204. doi: 10.1097/00001573-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Momma K, Matsuoka R, Takao A. Aortic arch anomalies associated with chromosome 22q11 deletion (CATCH 22) Pediatric cardiology. 1999;20:97–102. doi: 10.1007/s002469900414. [DOI] [PubMed] [Google Scholar]
- 14.Xu H, Morishima M, Wylie JN, et al. Tbx1 has a dual role in the morphogenesis of the cardiac outflow tract. Development (Cambridge, England) 2004;131:3217–3227. doi: 10.1242/dev.01174. [DOI] [PubMed] [Google Scholar]
- 15.Yagi H, Furutani Y, Hamada H, et al. Role of TBX1 in human del22q11.2 syndrome. Lancet. 2003;362:1366–1373. doi: 10.1016/s0140-6736(03)14632-6. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Huynh T, Baldini A. Mesodermal expression of Tbx1 is necessary and sufficient for pharyngeal arch and cardiac outflow tract development. Development (Cambridge, England) 2006;133:3587–3595. doi: 10.1242/dev.02539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Cerrato F, Xu H, et al. Tbx1 expression in pharyngeal epithelia is necessary for pharyngeal arch artery development. Development (Cambridge, England) 2005;132:5307–5315. doi: 10.1242/dev.02086. [DOI] [PubMed] [Google Scholar]