Abstract
Background
Accurate estimation of favorable neurological survival after in-hospital cardiac arrest could provide critical information for physicians, patients, and families.
Methods
Within the Get With The Guidelines-Resuscitation registry, we identified 42,957 patients from 551 hospitals admitted between January 2000 and October 2009 who were successfully resuscitated from an in-hospital cardiac arrest. A simple prediction tool for favorable neurological survival in patients successfully resuscitated from an in-hospital cardiac arrest was developed using multivariable logistic regression, with two-thirds of the sample randomly selected as the derivation cohort and one-third as the validation cohort. Favorable neurological status was defined as the absence of severe neurological deficits (Cerebral Performance Category score of ≤2).
Results
Rates of favorable neurological survival were similar in the derivation (n=7052; 24.6%) and validation cohorts (n=3510; 24.5%). Eleven variables were associated with favorable neurological survival: younger age, initial cardiac arrest rhythm of ventricular fibrillation or pulseless ventricular tachycardia with a defibrillation time of ≤2 minutes, baseline neurological status without disability, arrest location in a monitored unit, shorter duration of resuscitation, and absence of mechanical ventilation, renal insufficiency, hepatic insufficiency, sepsis, malignancy, and hypotension prior to the arrest. The model had excellent discrimination (C statistic of 0.80 for both the derivation and validation cohorts) and calibration. The prediction tool demonstrated the ability to identify patients across a wide range of rates of favorable neurological survival: patients in the top decile had a 70.7% probability of this outcome while patients in the bottom decile had a 2.8% probability.
Conclusion
Among successfully resuscitated patients with an in-hospital cardiac arrest, a simple, bedside prediction tool provides robust estimates of the probability of favorable neurological survival. This tool permits accurate prognostication after cardiac arrest for physicians, patients, and families.
Keywords: Prediction, Risk Score, Cardiac Arrest, Survival, Neurological Disability
In-hospital cardiac arrests are common and associated with poor survival, despite immense efforts to treat them. To improve prognostication, several risk models have been developed over the last 2 decades,1–3 but for several reasons these have had limited clinical application First, prior models were developed in all patients with in-hospital cardiac arrest, including those who never survive the initial event. As cardiac arrests are medical emergencies in which rapid decision-making is required, these models are impractical to use in the midst of an acute resuscitation. After the resuscitation, they are unlikely to be valid, as survival rates and patient characteristics among those who are successfully resuscitated differ significantly from the initial sample of cardiac arrest patients in whom these models were developed. Another concern is that earlier models examined survival to discharge and did not routinely consider neurological status, which is very important to patients and families. Finally, most studies have not translated their models into meaningful and valid tools that can assist with clinical prognostication at the bedside.
Accordingly, a prediction tool developed specifically for patients successfully resuscitated from an in-hospital cardiac arrest which also considers neurological status would be of great value, but has not yet been developed. To optimize prognostication, such a tool would incorporate key patient characteristics before the cardiac arrest and clinically important factors associated with the cardiac arrest (e.g., duration of resuscitation). From a clinical perspective, providing this type of evidence-based estimate of the likelihood of survival with favorable neurological recovery would offer important advantages to current approaches. After a cardiac arrest, clinicians often provide broad comments about prognosis while families and other care-givers are eager for more precise information about the likelihood of survival and neurological outcome. More accurate prognostication in this setting would help confirm that decisions about the intensity of life support are aligned with patients’ preferences and advanced directives.
To address this gap in care, we sought to develop a valid and clinically useful risk prediction tool—the Cardiac Arrest Survival Post-Resuscitation In-hospital (CASPRI) score—among successfully resuscitated patients with an in-hospital cardiac arrest in the large, multicenter Get With the Guidelines (GWTG)-Resuscitation registry. To maximize its utility, our score focused on survival to hospital discharge without severe neurological deficits using standardized cerebral performance category (CPC) scores, as this is an easily available and clinically relevant outcome for most patients and their families after cardiac arrest.
Methods
Study Design
The Get With the Guidelines (GWTG)-Resuscitation registry, formerly known as the National Registry of Cardiopulmonary Resuscitation (NRCPR), is a large, prospective, quality improvement registry of in-hospital cardiac arrests, and its design has been previously described in detail.4 Briefly, all patients with cardiac arrest (defined as the absence of a palpable central pulse, apnea, and unresponsiveness) and without Do-Not-Resuscitation (DNR) orders are identified and enrolled by specially-trained quality improvement personnel. Cases are identified by multiple methods, including centralized collection of cardiac arrest flow sheets, reviews of hospital paging system logs, and routine checks of code carts, pharmacy tracer drug records, and hospital billing charges for use of resuscitation medications.4
The GWTG-Resuscitation registry uses standardized Utstein-style definitions for patient variables and outcomes to facilitate uniform reporting of care delivered during cardiac arrests across hospitals.5–6 Data accuracy is ensured by certification of hospital staff and use of standardized software with data checks for data completeness and accuracy.7
Study Population
Our cohort was derived from 551 acute-care hospitals that submitted clinical data to GWTG-Resuscitation between January 1, 2000 and October 20, 2009. Within these hospitals, we identified 122,490 patients 18 years of age or older with clinical information on an index pulseless in-hospital cardiac arrest. We excluded 28,975 patients with cardiac arrest occurring in the emergency room, operating suites, procedure areas (cardiac catheterization, electrophysiology, and angiography suites), and post-procedural areas, because resuscitation response times, treatment, and causes of cardiac arrest differ markedly in these environments, as compared with inpatient ward and intensive care units—the focus of this study. As we were interested in examining only those patients who were successfully resuscitated after cardiac arrest, we excluded 48,061 patients who died during the initial resuscitation. Also excluded were patients with missing information on survival at discharge (n=148) or discharge neurological status among survivors (n=2349), both of which precluded an assessment of the primary outcome. The latter were similar to patients with information on discharge neurological status with regards to demographics, pre-arrest neurological status, and clinical factors (results not shown). Our final study cohort therefore included 42,957 patients from 551 hospitals who were successfully resuscitated from an initial in-hospital cardiac arrest on an inpatient ward or intensive care unit.
Study Outcome and Variables
The primary study outcome was favorable neurological survival to hospital discharge, which was defined as survival without severe neurological disability. Neurological status was evaluated using previously-developed CPC scores and determined from clinical assessments by medical staff.8 A CPC score of 1 is used to describe patients with mild to no neurological disability, 2 for moderate disability, 3 for severe disability, 4 for coma or vegetative state, and 5 for brain death. Based on prior work, we classified favorable neurological survival as those patients surviving to hospital discharge with a CPC score of ≤2.9
A total of 37 baseline characteristics were screened as candidate predictors for the study outcome. These included demographics (age [categorized in 10 year intervals], sex), location of arrest (intensive care, monitored, non-monitored unit), initial cardiac arrest rhythm (ventricular fibrillation, pulseless ventricular tachycardia, asystole, pulseless electrical activity), calendar year of event, time (work hours: 7am to 10:59pm vs. after hours: 11pm to 6:59am) and day (weekday vs. weekend) of cardiac arrest,7 neurological status before cardiac arrest (‘pre-arrest CPC score’, which was determined from family members and/or medical record notes), co-morbidities or medical conditions present prior to cardiac arrest (heart failure, myocardial infarction, or diabetes mellitus; renal, hepatic, or respiratory insufficiency; baseline evidence of motor, cognitive, or functional deficits [CNS depression]; acute stroke; acute non-stroke neurologic disorder; pneumonia; hypotension; sepsis; major trauma; metabolic or electrolyte abnormality; metastatic or hematologic malignancy), and critical-care interventions already in place at the time of cardiac arrest (mechanical ventilation, intravenous vasopressor medications, pulmonary artery catheter, intra-aortic balloon pump, dialysis). Definitions of select variables are provided in the supplementary appendix (eTable 1). Moreover, we collected information on key cardiac resuscitation variables that would be known at the time of calculating the score after successful resuscitation. These included: 1) duration of resuscitation to achieve return of spontaneous circulation, categorized as <2, 2–4, 5–9, 10–14, 15–19, 20–24, 25–29, and ≥30 minutes, and 2) time to defibrillation, categorized as ≤2, 3, 4–5, and >5 minutes.9 Race was not considered for model inclusion, as prior studies have found that racial differences in survival after in-hospital cardiac arrest are largely mediated by differences in comorbidity burden and hospital care quality for blacks and whites.10
Model Development
Baseline differences between patients who survived with favorable neurological status (CPC score ≤2) and those who did not (death or CPC score >2 at discharge) were evaluated using X2 tests for categorical variables and Student’s t-tests for continuous variables.
We randomly selected two-thirds of the study population for the derivation cohort and one-third for the validation cohort. Within the derivation sample, multivariable logistic regression analyses were used to identify significant predictors of favorable neurological survival to discharge. Generalized estimating equation (GEE) models with an exchangeable correlation matrix were employed to account for clustering of patients within hospitals. Multicollinearity was excluded for each variable prior to model inclusion.11 As defibrillation time pertained only to cardiac arrests due to ventricular fibrillation or pulseless ventricular tachycardia, we first confirmed that it was a significant predictor of favorable neurological survival in patients with these rhythms and that our outcome was similar for cardiac arrests due to ventricular fibrillation and pulseless ventricular tachycardia (results not shown). We then re-categorized initial cardiac arrest rhythm as asystole, pulseless electrical activity, and ventricular fibrillation or pulseless ventricular tachycardia with defibrillation times of ≤2, 3, 4–5, or >5 minutes.
To ensure parsimony and inclusion of only those variables that provided incremental prognostic value, we employed the approximation of full model methodology for model reduction.12 The contribution of each significant model predictor was ranked, and variables with the smallest contribution to the model were sequentially eliminated. This was an iterative process until further variable elimination led to a greater than 5% loss in model prediction as compared with the initial full model.
Model discrimination was assessed with the C-statistic, and the C-statistic of the parsimonious model was compared with the full model to ensure no meaningful loss in prediction. Model calibration was evaluated by the Hosmer-Lemeshow goodness-of-fit statistic and observed vs. predicted plots. A scoring algorithm was developed by rounding coefficients from the multivariable regression model, and the risk score was validated within the validation sample. To make the risk score useful for clinicians in routine practice, we developed a reference plot nomogram for a range of risk scores to show the corresponding likelihood of favorable neurological survival to discharge.
At least 1 study variable was missing in 14.6% (6279/42,957) of patients, and the average number of variables with missing data fields per patient was 0.19. The data element that was missing most often was pre-arrest CPC score (n=4814), for which we created a separate indicator variable (“unknown”). The remaining missing data were assumed to be missing at random and were imputed using multiple imputation with IVEWARE software.13 Results with and without imputation were not meaningfully different, so only the former are presented.
Finally, as sensitivity analyses, we constructed models using the approach described above among only those patients with 1) a pre-arrest CPC score of ≤2, or 2) duration of resuscitation >2 minutes, to assess the robustness of our findings. All study analyses were performed with SAS 9.2 (SAS Institute, Cary, NC), IVEWARE (University of Michigan, MI),13 and R version 2.10.0.14
RESULTS
Baseline characteristics of the 28,629 patients in the derivation cohort and the 14,328 patients in the validation cohort were similar (Table 1). The mean patient age was 66 years, 56% were male, and 19% were black. Eighteen percent of patients had a CPC score of >2 prior to the arrest. Over 40% of patients had respiratory insufficiency, one-third had renal insufficiency or diabetes mellitus, 1 in 5 patients were admitted with acute heart failure or myocardial infarction, and 18% had coexisting sepsis syndrome.
Table 1.
Patient Characteristics for the Derivation and Validation Cohorts
| Derivation Cohort (n= 28,629) | Validation Cohort (n= 14,328) | |
|---|---|---|
| DEMOGRAPHICS | ||
| Age | ||
| Mean (SD) | 66.0 (15.2) | 66.2 (15.4) |
| Median (IQR) | 68.0 (56.0, 78.0) | 68.0 (56.0, 78.0) |
| Male Sex, % | 16,046 (56.0) | 8081 (56.4) |
| Black Race, % | 5531 (19.3) | 2813 (19.6) |
| PRE-EXISTING CONDITIONS, % | ||
| CPC Score Prior to Arrest | ||
| 1 | 13404 (52.8) | 6745 (52.9) |
| 2 | 7346 (28.9) | 3704 (29.0) |
| 3 | 3136 (12.4) | 1585 (12.4) |
| 4 or 5 | 1498 (5.9) | 725 (5.7) |
| Missing | 3245 (11.3) | 1569 (11.0) |
| Respiratory Insufficiency | 12739 (44.5) | 6380 (44.5) |
| Renal Insufficiency | 10625 (37.1) | 5319 (37.1) |
| Arrhythmia | 10601 (37.0) | 5348 (37.3) |
| Diabetes Mellitus | 9606 (33.6) | 4892 (34.1) |
| Hypotension | 8172 (28.5) | 4033 (28.1) |
| Heart failure prior to admission | 6792 (23.7) | 3387 (23.6) |
| Heart failure this admission | 6039 (21.1) | 2983 (20.8) |
| Myocardial Infarction prior to admission | 5250 (18.3) | 2712 (18.9) |
| Myocardial Infarction this admission | 5399 (18.9) | 2668 (18.6) |
| Metabolic or Electrolyte Abnormality | 5351 (18.7) | 2708 (18.9) |
| Sepsis | 5087 (17.8) | 2552 (17.8) |
| Pneumonia | 4408 (15.4) | 2110 (14.7) |
| Metastatic or Hematologic Malignancy | 3444 (12.0) | 1721 (12.0) |
| Baseline Depression in CNS Function | 3810 (13.3) | 1867 (13.0) |
| Hepatic Insufficiency | 2489 (8.7) | 1221 (8.5) |
| Acute CNS non-Stroke event | 2388 (8.3) | 1172 (8.2) |
| Acute Stroke | 1212 (4.2) | 622 (4.3) |
| Major Trauma | 1002 (3.5%) | 486 (3.4%) |
Abbreviations: CNS, central nervous system; CPC, Cerebral Performance Score; IQR, inter-quartile range; SD, standard deviation.
Cardiac arrest event characteristics for the derivation and validation cohorts are shown in Table 2. Approximately 74% of all successfully resuscitated patients had an initial cardiac arrest rhythm of asystole or pulseless electrical activity, while 26% had ventricular fibrillation or pulseless ventricular tachycardia. The median time to achieve return of spontaneous circulation was 11 minutes (inter-quartile range: 6–21). About one-third of arrests occurred at night and on the weekend. The majority of successfully resuscitated patients had a cardiac arrest in the intensive care unit, with 31% of patients on mechanical ventilation and 29% on intravenous vasopressor therapy prior to the arrest.
Table 2.
Arrest Characteristics for the Derivation and Validation Cohorts.
| Derivation Cohort (n= 28,629) | Validation Cohort (n= 14,328) | |
|---|---|---|
| CHARACTERISTICS OF ARREST, % | ||
| Initial Cardiac Arrest Rhythm | ||
| Pulseless Electrical Activity | 11,853 (41.4) | 6001 (41.9) |
| Asystole | 9256 (32.3) | 4705 (32.8) |
| Ventricular Fibrillation | 4497 (15.7) | 2130 (14.9) |
| Pulseless Ventricular Tachycardia | 3023 (10.6) | 1492 (10.4) |
| Duration of Resuscitation, min | ||
| Mean (SD) | 16.1 ± 17.7 | 16.1 ± 18.5 |
| Median (IQR) | 11.0 (6.0, 21.0) | 11.0 (6.0, 21.0) |
| Defibrillation Time*, min | ||
| Mean (SD) | 1.9 (3.2) | 1.8 (3.0) |
| Median (IQR) | 1.0 (0.0, 2.0) | 1.0 (0.0, 2.0) |
| Hospital Location | ||
| Intensive Care Unit | 17,240 (60.2) | 8617 (60.1) |
| Monitored Unit | 7042 (24.6) | 3530 (24.6) |
| Non-monitored Unit | 4347 (15.2) | 2181 (15.2) |
| Time and Day of Arrest | ||
| Night | 9376 (32.8) | 4603 (32.1) |
| Weekend | 8940 (31.2) | 4337 (30.3) |
| Hospital-wide Response Activated | 24,361 (85.1) | 12,192 (85.1) |
| Use of Automated External Defibrillator | 3478 (12.1) | 1743 (12.2) |
| INTERVENTIONS IN PLACE PRIOR TO ARREST, % | ||
| Mechanical ventilation | 8955 (31.3) | 4492 (31.4) |
| Intravenous Vasopressors | 8227 (28.7) | 4116 (28.7) |
| Intravenous Antiarrhythmics | 2242 (7.8) | 1111 (7.8) |
| Pulmonary Artery Catheter | 1381 (4.8) | 749 (5.2) |
| Dialysis | 1207 (4.2) | 644 (4.5) |
| Intra-Aortic Balloon Pump | 556 (1.9) | 330 (2.3) |
| Implantable Cardioverter Defibrillator | 492 (1.7) | 235 (1.6) |
Abbreviations: IQR, inter-quartile range; SD, standard deviation.
For arrests due to ventricular fibrillation or pulseless ventricular tachycardia
The rate of favorable neurological survival to discharge was similar in the derivation (n=7052; 24.6%) and validation cohorts (n=3510; 24.5%), and the main reason for not achieving this outcome was death (Table 3). Overall rates of survival and discharge CPC score by pre-arrest CPC score are presented in eTable 2. The rate of favorable neurological survival was 34.6%, 24.2%, 7.3%, and 8.2% for pre-arrest CPC scores of 1, 2, 3, and 4, respectively.
Table 3. Summary of Clinical Outcomes in the Derivation and Validation Cohorts.
Approximately one-quarter of patients survived to hospital discharge with a favorable neurological outcome. Death was the primary reason for not achieving favorable neurological survival, and survival with severe neurological disability accounted for a small proportion of both cohorts.
| Data Set | Favorable Neurological Survival* | Died | Survived, Severe Neurological Deficits** |
|---|---|---|---|
| Derivation (n = 28,629) | 7052 (24.6%) | 19,966 (69.7%) | 1611 (5.6%) |
| Validation (n = 14,328) | 3510 (24.5%) | 10,009 (69.9%) | 809 (5.7%) |
Cerebral Performance Category score of 1 or 2
Cerebral performance Category score of >2
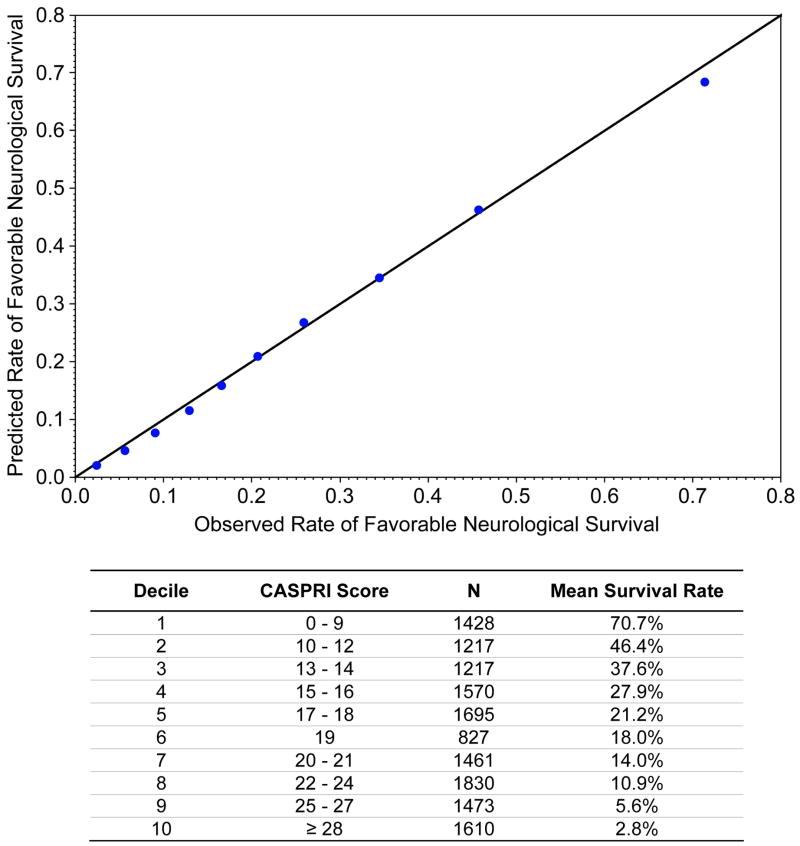
Initially, 20 independent predictors were identified in the derivation cohort with the multivariable model, resulting in a model C-statistic of 0.808 (eTable 3). After elimination of 9 predictors which did not improve model discrimination, the final model included 11 variables and had a similar C-statistic of 0.802 (Table 4). Notably, the 3 variables with the largest predictive ability (defined by total t-statistic) were initial cardiac arrest rhythm, duration of resuscitation to achieve return of spontaneous circulation (i.e., code duration), and pre-arrest neurological status. When the model was tested in the independent validation cohort, model discrimination was similar (see Table 4). Calibration was confirmed with observed vs. predicted plots (Figure 1) and the Hosmer-Lemeshow goodness-of-fit test (p=0.29). When we restricted the cohort to only those patients with pre-arrest CPC scores of 1 or 2 (eTable 4) or duration of resuscitation >2 minutes (eTable 5), our findings were essentially unchanged.
Table 4. Multivariate Predictors for Favorable Neurological Survival to Discharge.
Adjusted estimates for model predictors of favorable neurological survival to discharge in the derivation and validation cohorts are shown. The total t-statistic provides the contribution of each predictor to the overall model for the derivation cohort.
| Predictors | Total t-statistic | Derivation Cohort (n= 28,629) | Validation Cohort (n= 14,328) |
|---|---|---|---|
| Age Group, yrs | 24.0 | ||
| < 50 | 1.22 (1.11–1.35) | 1.16 (1.01–1.33) | |
| 50 to 59 | 1.11 (1.01–1.23) | 1.13 (0.98–1.30) | |
| 60 to 69 | Reference | Reference | |
| 70 to 79 | 0.76 (0.70–0.82) | 0.73 (0.64–0.83) | |
| ≥ 80 | 0.57 (0.52–0.63) | 0.60 (0.52–0.69) | |
| Initial Arrest Rhythm | 57.1 | ||
| Ventricular Fibrillation or Pulseless VT | |||
| Time to defibrillation ≤ 2 minutes | Reference | Reference | |
| Time to defibrillation 3 minutes | 1.19 (0.97–1.45) | 0.95 (0.71, 1.30) | |
| Time to defibrillation 4 to 5 minutes | 0.81 (0.66–0.98) | 0.80 (0.60–1.06) | |
| Time to defibrillation > 5 minutes | 0.64 (0.53–0.78) | 0.80 (0.60–1.06) | |
| Asystole | 0.31 (0.28–0.35) | 0.31 (0.27–0.35) | |
| Pulseless Electrical Activity | 0.32 (0.30–0.35) | 0.31 (0.28–0.35) | |
| Pre-Arrest CPC Score | 51.2 | ||
| 1 | Reference | Reference | |
| 2 | 0.70 (0.65–0.75) | 0.72 (0.64–0.81) | |
| 3 | 0.17 (0.14–0.20) | 0.18 (0.14–0.22) | |
| 4 or 5 | 0.15 (0.12–0.21) | 0.20 (0.15–0.26) | |
| Hospital Location | 15.2 | ||
| Non-monitored unit | Reference | ||
| Telemetry unit | 1.70 (1.53–1.89) | 1.76 (1.53–2.02) | |
| Intensive Care Unit | 1.37 (1.23–1.54) | 1.47 (1.27–1.71) | |
| Duration of Resuscitation, min | 67.6 | ||
| < 2 | Reference | Reference | |
| 2 to 4 | 0.84 (0.70–1.01) | 0.92 (0.75–1.12) | |
| 5 to 9 | 0.52 (0.44–0.63) | 0.63 (0.52–0.77) | |
| 10 to 14 | 0.37 (0.31–0.45) | 0.48 (0.39–0.59) | |
| 15 to 19 | 0.28 (0.23–0.34) | 0.34 (0.27–0.43) | |
| 20 to 24 | 0.33 (0.27–0.40) | 0.40 (0.31–0.52) | |
| 25 to 29 | 0.31 (0.25–0.38) | 0.32 (0.24–0.42) | |
| ≥ 30 | 0.22 (0.18–0.27) | 0.25 (0.20–0.32) | |
| Mechanical Ventilation | 15.7 | 0.50 (0.46–0.55) | 0.46 (0.40–0.52) |
| Renal Insufficiency | 13.1 | 0.65 (0.61–0.69) | 0.63 (0.57–0.70) |
| Hepatic Insufficiency | 9.0 | 0.48 (0.41–0.56) | 0.36 (0.28–0.45) |
| Sepsis | 12.7 | 0.50 (0.45–0.56) | 0.60 (0.53–0.69) |
| Malignancy | 13.1 | 0.46 (0.41–0.52) | 0.44 (0.37–0.52) |
| Hypotension | 10.7 | 0.60 (0.54–0.66) | 0.66 (0.59–0.75) |
|
| |||
| Model C-Statistic | 0.80 | 0.80 | |
Abbreviations: CPC, cerebral performance score; VT, ventricular tachycardia.
Figure 1. Comparison of Predicted vs. Observed Outcome Rate for the Validation Cohort.
Each data point represents a decile of risk for the outcome of favorable neurological survival to discharge.
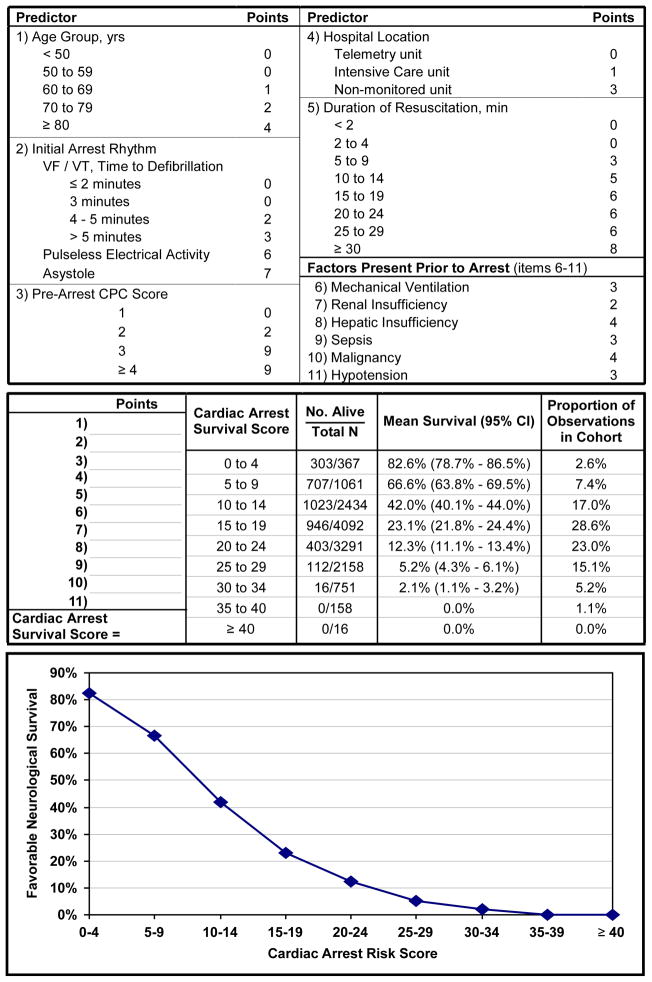
Based on the coefficients of the model predictors, we developed the Cardiac Arrest Survival Post-Resuscitation In-hospital (CASPRI) score (Figure 2). Individual scores for each predictor can be summed, with a higher summary score denoting a decreased likelihood of favorable neurological survival. Patients in the top decile (CASPRI score <10) had a 70.7% mean probability of favorable neurological survival, while patients in the bottom decile (CASPRI score ≥28) had a 2.8% mean probability of favorable neurological survival (see Figure 1). For clinical use, the mean probabilities for favorable neurological survival for each 5-point increase in the CASPRI score are described in Figure 2.
Figure 2. The Cardiac Arrest Survival Post-Resuscitation In-hospital (CASPRI) Score Card and Nomogram for Favorable Neurological Survival.
For this in-hospital cardiac arrest risk score, points for each variable are determined and a summary score is obtained. The corresponding likelihood of surviving to hospital discharge without severe neurological disability is determined from the risk table or plot.
COMMENT
By leveraging the size and scope of the GWTG-Resuscitation registry, we have developed and validated a simple bedside prediction tool—the Cardiac Arrest Survival Post-Resuscitation Inhospital (CASPRI) score—that can be used to estimate the likelihood of survival to discharge with favorable neurological status for patients initially resuscitated from an in-hospital cardiac arrest. To our knowledge, this is the first risk score developed among successfully resuscitated patients who develop cardiac arrest in the hospital setting. Because this prediction tool was developed in over 40,000 patients from 551 hospitals and used clinical factors that can be readily assessed, we believe that it offers the potential to provide physicians reliable and valuable prognostic information for discussions with patients and their families after successful resuscitation.
Previous risk models of survival among patients with an in-hospital cardiac arrest have had limited utility in routine practice.1–3 These models focused upon estimating the likelihood of survival for all patients with a cardiac arrest. While this approach could be useful in risk-adjusted feedback reports on performance for quality improvement activities, they have less clinical utility, as they are impractical to use during an acute resuscitation. During resuscitation, the focus is typically on rapidly delivering acute cardiac life support therapies, which are frequently driven by established expert consensus and protocols. Moreover, most studies have not incorporated information on neurological status in their assessment of outcomes and few have translated their models into meaningful risk scores for routine practice.
Unlike models constructed from study populations that included all patients who suffered a cardiac arrest, the CASPRI score was developed exclusively among successfully resuscitated patients—a population in which prognostication is particularly useful. A prediction tool in this population is not subject to the acute time constraints of an emergent resuscitation. Moreover, it allows for inclusion of patient factors from both before and during the cardiac arrest, including important time-related variables that strongly correlate with outcomes. In this regard, it is noteworthy that the strongest predictors of a meaningful survival outcome among successfully resuscitated patients—duration of resuscitation, initial cardiac arrest rhythm and defibrillation time (for arrests due to ventricular fibrillation and pulseless ventricular tachycardia)—were cardiac arrest variables, and many patient factors, such as a cardiac etiology for hospitalization (e.g., myocardial infarction, heart failure), were not retained in the final model.
Quantifying the likelihood of meaningful survival after an in-hospital cardiac arrest is important but can be difficult. In an era of increasing emphasis on personalized medicine, providing concrete probabilities for favorable neurological survival after cardiac arrest is an important discussion that clinicians have with patients and their families to manage expectations. By converting our prediction model into a risk score, we have sought to create an infrastructure with which clinicians can identify 10% of patients successfully resuscitated from an in-hospital cardiac arrest who have a >70% probability of favorable neurological survival to discharge, as compared with another 10% who have less than a 3% chance of this outcome. Unlike other prediction tools in clinical care in which deciles of risk are often clustered around a narrow range of probabilities, the CASPRI prediction tool is unusual in its ability to predict a wide range of rates for a survival outcome. To facilitate its adoption, we have developed a reference plot nomogram (Figure 2) to access this risk score.
Our study should be interpreted with the following limitations. Our model was developed from data submitted to GWTG-Resuscitation and outcomes may differ at other facilities. Second, we did not evaluate the use of therapeutic hypothermia in our model. This variable is not routinely collected within the registry, and its efficacy has not been established for in-hospital cardiac arrest.15–16 Third, we did not have comprehensive data on other resuscitation process factors, such as quality of chest compressions and extent of interruptions of cardiopulmonary resuscitation. Many of these process factors are difficult to document accurately; therefore, their inclusion would make a risk score difficult to apply in routine practice. Additionally, our prediction tool is not generalizable to hospitalized patients with out-of-hospital cardiac arrest. Fifth, we did not re-evaluate neurological status after hospital discharge or throughout the hospitalization; therefore, our risk score was only able to prognosticate in-hospital outcomes at the time of return of spontaneous circulation. Sixth, although the CASPRI risk score underwent internal validation within GWTG-Resuscitation, its external validity in a separate data set has not been established but is desirable. Finally, the CASPRI tool is not intended to recommend subsequent treatment, as thresholds for treatment and medical futility vary by patient and family. Rather, the tool generates an individualized, evidence-based probability of favorable neurological survival, thereby allowing patients and their families to make informed treatment decisions that are most aligned with the patient’s goals and values.
In conclusion, we have developed a valid and robust tool for predicting survival with a favorable neurological outcome in successfully resuscitated patients after an in-hospital cardiac arrest. We believe that this tool is simple to use, addresses a critical unmet need for better prognostication after cardiac arrest, and has the potential to enhance communication with patients and families.
Supplementary Material
Acknowledgments
Funding
Dr. Chan was supported by Grant Number K23HL102224 from the National Heart, Lung, And Blood Institute. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health.
Dr. Krumholz is supported by Grant U01 HL105270-02 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute.
The American Heart Association provided funding for the collection and management of data in the NRCPR and reviewed and approved the final manuscript. However, it had no role in the design and conduct of this study, the analysis or interpretation of the data, or the preparation of this manuscript.
Beside the authors Paul S. Chan, Robert A. Berg, and Comilla Sasson, members of Get with the Guidelines-Resuscitation include:
Emilie Allen, MSN, RN, Parkland Health & Hospital System; Michael W. Donnino, MD, Beth Israel Deaconess Medical Center; Dana P. Edelson, MD, MS, University of Chicago Medical Center; Kathy Duncan, Institute for Healthcare Improvement; Brian Eigel, PhD and Lana Gent, PhD, American Heart Association; Robert T. Faillace, MD, St. Joseph’s Regional Medical Center; Romergryko G. Geocadin, MD, Johns Hopkins School of Medicine; Elizabeth A. Hunt, MD, MPH, PhD, Johns Hopkins Medicine Simulation Center; Lynda Knight, RN, Lucile Packard Children’s Hospital at Stanford; Kenneth LaBresh, MD, RTI International; Mary E. Mancini, RN, PhD, University of Texas at Arlington; Vinay M. Nadkarni, MD, University of Pennsylvania School of Medicine; Graham Nichol, MD, MPH and Samuel A. Warren, MD, University of Washington; Mary Ann Peberdy, MD and Joseph P. Ornato, MD, Virginia Commonwealth University Health System.
Footnotes
Disclosures: Dr. Krumholz is a recipient of a research grant from Medtronic, Inc. through Yale University. The other authors report no potential conflicts of interest or disclosures.
Authorship: Dr. Chan had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study Concept and design: Chan, Nallamothu
Acquisition of Data: Chan
Statistical Analysis: Li, Sasson
Analysis and interpretation of data: Chan, Spertus, Krumholz, Berg, Li, Sasson, Nallamothu
Drafting of the manuscript: Chan, Li, Sasson, Nallamothu
Critical revision of the manuscript for important intellectual content: Chan, Spertus, Krumholz, Berg, Nallamothu
Funding/Administrative Support: Spertus
Study Supervision: Chan, Spertus, Berg, Nallamothu
References
- 1.George AL, Jr, Folk BP, 3rd, Crecelius PL, Campbell WB. Pre-arrest morbidity and other correlates of survival after in-hospital cardiopulmonary arrest. Am J Med. 1989 Jul;87(1):28–34. doi: 10.1016/s0002-9343(89)80479-6. [DOI] [PubMed] [Google Scholar]
- 2.Cohn EB, Lefevre F, Yarnold PR, Arron MJ, Martin GJ. Predicting survival from inhospital CPR: meta-analysis and validation of a prediction model. J Gen Intern Med. 1993 Jul;8(7):347–353. doi: 10.1007/BF02600069. [DOI] [PubMed] [Google Scholar]
- 3.Cooper S, Janghorbani M, Cooper G. A decade of in-hospital resuscitation: outcomes and prediction of survival? Resuscitation. 2006 Feb;68(2):231–237. doi: 10.1016/j.resuscitation.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003 Sep;58(3):297–308. doi: 10.1016/s0300-9572(03)00215-6. [DOI] [PubMed] [Google Scholar]
- 5.Cummins RO, Chamberlain D, Hazinski MF, et al. Recommended guidelines for reviewing, reporting, and conducting research on in-hospital resuscitation: the inhospital ‘Utstein style’. American Heart Association. Circulation. 1997 Apr 15;95(8):2213–2239. doi: 10.1161/01.cir.95.8.2213. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs I, Nadkarni V, Bahr J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa) Circulation. 2004 Nov 23;110(21):3385–3397. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 7.Peberdy MA, Ornato JP, Larkin GL, et al. Survival from in-hospital cardiac arrest during nights and weekends. JAMA. 2008 Feb 20;299(7):785–792. doi: 10.1001/jama.299.7.785. [DOI] [PubMed] [Google Scholar]
- 8.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975 Mar 1;1(7905):480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 9.Chan PS, Krumholz HM, Nichol G, Nallamothu BK. Delayed time to defibrillation after in-hospital cardiac arrest. N Engl J Med. 2008 Jan 3;358(1):9–17. doi: 10.1056/NEJMoa0706467. [DOI] [PubMed] [Google Scholar]
- 10.Chan PS, Nichol G, Krumholz HM, et al. Racial differences in survival after in-hospital cardiac arrest. JAMA. 2009 Sep 16;302(11):1195–1201. doi: 10.1001/jama.2009.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belsley DAKE, Welsch RE. Regression diagnostics : Identifying influential data and sources of collinearity. New York: 1980. [Google Scholar]
- 12.Harrell FE. Regression Modeling Strategies with Applications to Linear Models, Logistic Regression and Survival Analysis. New York: Springer-Verlag; 2001. [Google Scholar]
- 13.Raghunathan TESP, Van Hoeyk J. IVEware: Imputation and Variance Estimation Software - User Guide. Michigan: Survey Research Center, Institute for Social Research University of Michigan; 2002. [Google Scholar]
- 14.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing V; Austria: 2008. URL http://www.R-project.org. [Google Scholar]
- 15.Dumas F, Grimaldi D, Zuber B, et al. Is hypothermia after cardiac arrest effective in both shockable and nonshockable patients?: insights from a large registry. Circulation. 2011 Mar 1;123(8):877–886. doi: 10.1161/CIRCULATIONAHA.110.987347. [DOI] [PubMed] [Google Scholar]
- 16.Kim F, Olsufka M, Longstreth WT, Jr, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation. 2007 Jun 19;115(24):3064–3070. doi: 10.1161/CIRCULATIONAHA.106.655480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.