Abstract
Background
Stimulating an immune response against cancer with the use of vaccines remains a challenge. We hypothesized that combining a melanoma vaccine with interleukin-2, an immune activating agent, could improve outcomes. In a previous phase 2 study, patients with metastatic melanoma receiving high-dose interleukin-2 plus the gp100:209–217(210M) peptide vaccine had a higher rate of response than the rate that is expected among patients who are treated with interleukin-2 alone.
Methods
We conducted a randomized, phase 3 trial involving 185 patients at 21 centers. Eligibility criteria included stage IV or locally advanced stage III cutaneous melanoma, expression of HLA⋆A0201, an absence of brain metastases, and suitability for high-dose interleukin-2 therapy. Patients were randomly assigned to receive interleukin-2 alone (720,000 IU per kilogram of body weight per dose) or gp100:209–217(210M) plus incomplete Freund’s adjuvant (Montanide ISA-51) once per cycle, followed by interleukin-2. The primary end point was clinical response. Secondary end points included toxic effects and progression-free survival.
Results
The treatment groups were well balanced with respect to baseline characteristics and received a similar amount of interleukin-2 per cycle. The toxic effects were consistent with those expected with interleukin-2 therapy. The vaccine–interleukin-2 group, as compared with the interleukin-2–only group, had a significant improvement in centrally verified overall clinical response (16% vs. 6%, P = 0.03), as well as longer progression-free survival (2.2 months; 95% confidence interval [CI], 1.7 to 3.9 vs. 1.6 months; 95% CI, 1.5 to 1.8; P = 0.008). The median overall survival was also longer in the vaccine–interleukin-2 group than in the interleukin-2–only group (17.8 months; 95% CI, 11.9 to 25.8 vs. 11.1 months; 95% CI, 8.7 to 16.3; P = 0.06).
Conclusions
In patients with advanced melanoma, the response rate was higher and progression-free survival longer with vaccine and interleukin-2 than with interleukin-2 alone. (Funded by the National Cancer Institute and others; ClinicalTrials.gov number, NCT00019682.)
Although it is clear that vaccines are important in the prevention of infectious diseases, their benefits with respect to metastatic cancer have been less clear. One of the first studies to show improved survival with vaccines among patients with metastatic cancer was reported recently in a study involving men who received sipuleucel-T vaccine for the treatment of metastatic castration-resistant prostate cancer.1 We hypothesized that the effectiveness of cancer vaccines could be improved by the simultaneous administration of specific antigens and cytokines to drive the immune response.2
Melanoma, a tumor that may be innately immunogenic in humans, is an important model for the study of tumor immunity. Although early stages of melanoma can be cured by means of surgery, the prognosis for patients with metastatic melanoma is grim, with a 5-year survival rate of less than 10%. To date, only three agents have been approved for the treatment of metastatic disease, but these agents have low response rates. Interleukin-2, a cytokine that induces T-cell activation and proliferation, is associated with an overall response rate of 13 to 16%, and up to 6% of patients have a complete response that can be quite durable.3,4 A variety of agents have been combined with interleukin-2 in an effort to improve its efficacy, including chemotherapy and other cytokines.
Vaccination with the gp100:209–217(210M) peptide has resulted in very high levels of circulating T cells that were capable of recognizing and killing melanoma cancer cells in vitro, leading to the hypothesis that activation of these T cells with cytokines such as interleukin-2 could be synergistic. In a previous single-group, phase 2 study, patients with metastatic melanoma were immunized with the gp100:209–217(210M) peptide vaccine in Montanide ISA-51 (incomplete Freund’s adjuvant), followed by high-dose interleukin-2, leading to objective clinical responses in 13 of 31 patients (42%).5 The fact that the response rate was apparently higher than that previously reported with interleukin-2 alone provided the impetus for the current randomized trial comparing vaccine plus interleukin-2 with interleukin-2 alone.
Methods
Study Design
The study was designed by the authors, in consultation with the study sponsor, the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI). The primary end point of the trial was the rate of clinical response. Secondary end points included progression-free survival, toxic effects, immunologic response, and quality of life. After providing written informed consent, patients were randomly assigned, in a 1:1 ratio, to receive a high-dose bolus of interleukin-2 alone every 8 hours (Proleukin, Prometheus; provided through the patients’ health plans), or gp100:209–217(210M) plus Montanide ISA-51 (provided by the CTEP), given once per cycle, and the same high-dose interleukin-2 regimen beginning the second day of the cycle. Patients were stratified according to the site of disease (cutaneous or subcutaneous only vs. any site). Stratified randomization was performed with the use of random block sizes to ensure balance with respect to a potentially important prognostic feature. The full protocol, including the statistical analysis plan, is available with the full text of this article at NEJM.org.
The data were collected and the study was monitored by EMMES, a contract research organization. An independent data and safety monitoring board met annually and performed a planned interim analysis after 93 patients were enrolled. The data were analyzed by statisticians at EMMES and by the corresponding author. The first draft of the manuscript was prepared by the corresponding author. All the authors had input into the subsequent and final drafts and made the decision to submit the manuscript for publication. All the authors vouch for the accuracy of the data and for the fidelity of the study to the protocol. No pharmaceutical company had any role in the design of the study, the accrual or analysis of the data, or the preparation of the manuscript.
Patients
Patients were eligible for inclusion in the study if they had metastatic cutaneous melanoma, either stage IV or locally advanced stage III, and were HLA⋆A0201-positive (to allow presentation of the peptide vaccine to T cells). All genotyping for HLA status was performed at the National Institutes of Health HLA laboratory. All pathology slides were reviewed by pathologists at the NCI. All patients met the criteria for receiving high-dose interleukin-2 treatment and had no major cardiac, pulmonary, or renal diseases. Details regarding the inclusion criteria are provided in the Supplementary Appendix, available at NEJM.org.
Treatment
All patients received interleukin-2 at a dose of 720,000 IU per kilogram of body weight every 8 hours as an intravenous bolus. The vaccine group also received the HLA⋆A0201-restricted peptide gp100:209–217(210 M), amino acid sequence IMDQVPFSV (National Service Center [NSC] no. 683472). It was obtained from the CTEP under investigational-new-drug application BB6123 and was supplied in vials containing 1 mg of the peptide per milliliter of sterile water. Before administration in the subcutaneous tissues of the thigh, the peptide was mixed (with the use of a vortexer) with Montanide ISA-51 adjuvant (NSC no. 675756) provided by the CTEP under investigational-new-drug application BB5924.
Each patient was treated with interleukin-2, as tolerated, up to a maximum of 12 doses per cycle. Each cycle of treatment was repeated every 3 weeks, with 1 extra week added after every two cycles to allow for evaluation of the response. Patients with stable disease continued treatment for an additional two cycles. Patients with progressive disease or new sites of disease discontinued therapy.
Assessments
Tumor response was assessed every 6 weeks according to modified World Health Organization criteria (see the Supplementary Appendix). In vitro studies, in which peptide-specific T cells and CD4+foxp3+ T regulatory cells in the circulation were measured, were performed before the start of treatment and after the completion of four cycles of therapy (see the Supplementary Appendix). Toxic effects and adverse events were assessed according to the NCI Common Toxicity Criteria, version 2.0 (http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf).
Statistical Analysis
We estimated that with 83 patients in each group, the study would have 80% power to detect a difference between the two groups, assuming a clinical response rate of 15% in the interleukin-2–only group3 and 35% in the vaccine–interleukin-2 group,5 at an overall alpha level of 0.05 (two-tailed). In order to account for patients who could not be evaluated, we aimed to enroll a total of 185 patients.
All the analyses presented were specified before the data were unblinded. A Wilcoxon rank-sum test was used for the between-group comparison of skewed continuous variables, a chi-square or Fisher’s exact test for the comparison of categorical variables, and a log-rank test for the comparison of progression-free survival and overall survival. All reported P values are two-tailed. Bonferroni adjustment was applied in the analyses of toxic effects.
RESULTS
PATIENTS
Between June 6, 2000, and November 6, 2007, a total of 479 patients underwent HLA testing, and 185 patients at 21 institutions throughout the United States were randomly assigned to one of two groups. A total of 4 patients withdrew (1 in the interleukin-2–only group and 3 in the vaccine–interleukin-2 group), and 2 were ineligible (both in the vaccine–interleukin-2 group) and received no treatment. These 6 patients could not be evaluated for a response or for toxic effects. One patient received therapy off-protocol after receiving vaccine–interleukin-2 therapy and could not be evaluated for a response. All the patients were included in the analysis of progression-free survival and overall survival. In the interleukin-2–only group, 94 patients were enrolled, and 93 received treatment and could be evaluated for a response; in the vaccine–interleukin-2 group, 91 patients were enrolled, 86 received treatment, and 85 could be evaluated for a response.
The baseline characteristics of the patients in the two groups were well balanced (Table 1), except that patients in the vaccine–interleukin-2 group were slightly younger (P = 0.04, with no adjustment for multiple comparisons). Baseline weight, white-cell count, platelet count, and levels of creatinine, bilirubin, and aminotransferases were similar in the two groups (data not shown). The number of doses of interleukin-2 that patients received within each cycle of treatment was similar in the two groups. Patients in the interleukin-2–only group received an average of 8.9, 7.9, 7.7, and 8.0 doses in cycles 1, 2, 3, and 4, respectively; patients in the vaccine–interleukin-2 group received an average of 9.3, 7.9, 7.7, and 6.5 doses, respectively, suggesting that the two groups were treated with similar intensity. Each patient received 1 to 10 cycles of treatment depending on his or her clinical response and the side effects, for a total of 244 cycles in the interleukin-2–only group and 286 cycles in the vaccine–interleukin-2 group. The greater number of total cycles of interleukin-2 in the vaccine–interleukin-2 group can be potentially attributed to the protocol design, which called for the continuation of treatment in patients with a clinical response. Consequently, patients in the interleukin-2–only group received fewer total doses of interleukin-2 than did patients in the vaccine–interleukin-2 group (mean number of doses, 21.5 vs. 25.7; P = 0.04). Weight gain, thrombocytopenia, and peak creatinine and peak bilirubin levels during treatment were similar in the two groups (data not shown).
Table 1.
Baseline Characteristics of the Patients.*
| Characteristic | Interleukin-2 Alone (N = 94) |
Vaccine–Interleukin-2 (N = 91) |
P Value |
|---|---|---|---|
| Sex — no. (%) | 0.53 | ||
| Male | 63 (67) | 57 (63) | |
| Female | 31 (33) | 34 (37) | |
| Mean age — yr | 50.3 | 46.9 | 0.04 |
| Race or ethnic group — no. (%)† | 1.00 | ||
| White | 91 (97) | 90 (99) | |
| Hispanic | 2 (2) | 1 (1) | |
| Other | 1 (1) | 0 | |
| ECOG performance status — no. (%)‡ | 0.92 | ||
| 0 | 78 (83) | 76 (84) | |
| 1 | 16 (17) | 15 (16) | |
| Site of disease — no. (%) | |||
| Cutaneous or subcutaneous only | 8 (9) | 8 (9) | 0.95 |
| Any other site | 86 (91) | 83 (91) | |
| Disease stage — no. (%)§ | 0.33 | ||
| Locally advanced III | 3 (3) | 5 (5) | |
| IV | 91 (97) | 83 (91) | |
| M1a | 25 (27) | 22 (24) | |
| M1b | 29 (31) | 32 (35) | |
| M1c | 37 (39) | 29 (32) | |
| Data missing | 0 | 3 (3) | |
| Previous treatment — no. (%) | |||
| Surgery | 86 (91) | 86 (95) | 0.42 |
| Interferon-alfa | 39 (41) | 50 (55) | 0.07 |
| Chemotherapy | 11 (12) | 11 (12) | 0.94 |
| Radiation | 14 (15) | 14 (15) | 0.93 |
| Low-dose interleukin-2 | 7 (7) | 11 (12) | 0.29 |
Interleukin-2 was administered in patients in both groups at a dose of 720,000 IU per kilogram of body weight every 8 hr. The vaccine administered in the vaccine–interleukin-2 group was a gp100:209–217(210 M) peptide vaccine (1 mg) plus incomplete Freund’s adjuvant (Montanide ISA-51). P values were calculated with the use of a Wilcoxon rank-sum test for skewed continuous variables and a chi-square test or Fisher’s exact test for categorical variables.
Race or ethnic group was determined by the study coordinators.
The Eastern Cooperative Oncology Group (ECOG) performance status ranges from 0 to 5, with higher scores indicating greater impairment (5 indicates death). ECOG 0 indicates that the patient is fully active, and ECOG 1 that a patient is restricted in the performance of physically strenuous activity but is ambulatory and able to carry out work of a light or sedentary nature.
The stage was determined according to the criteria of the American Joint Committee on Cancer, 6th edition, which are based on the sites of disease. (No measurements of lactate dehydrogenase levels were performed.)
CLINICAL RESPONSE
The primary objective of the study was to determine whether the addition of a peptide vaccine to high-dose interleukin-2 would result in a higher rate of clinical response than that with interleukin-2 alone. The response rate as assessed by the investigators was 10% (complete response, 2%; partial response, 8%) among the 93 patients receiving interleukin-2 alone and 20% (complete response, 11%; partial response, 9%) among the 85 patients receiving vaccine and interleukin-2 who could be evaluated for a response (P = 0.05) (Table 2). After blinded central radiologic review, the response rate was 6% (complete response, 1%; partial response, 5%) among the patients receiving interleukin-2 alone and 16% (complete response, 9%; partial response, 7%) among the patients receiving vaccine and interleukin-2 (P = 0.03) (Table 2). The difference between the two groups was greatest among patients with M1b metastatic disease (classified according to the tumor–node–metastasis [TNM] categorization for melanoma of the American Joint Committee on Cancer), because of lung involvement (0% vs. 25%, P = 0.005); however, since in this study, stratification was not performed according to M1 disease status, the analysis of response in this small subgroup of patients has limited power and must be interpreted with caution.
Table 2.
Response to Treatment, as Assessed by Investigators and by Central Review.
| Response | Assessment by Investigators | Assessment by Central Review | ||
|---|---|---|---|---|
| Interleukin-2 Alone (N = 93) |
Vaccine–Interleukin-2 (N = 85) |
Interleukin-2 Alone (N = 93) |
Vaccine–Interleukin-2 (N = 85) |
|
| number (percent) | ||||
| Complete* | 2 (2) | 9 (11) | 1 (1) | 8 (9) |
| Partial | 7 (8) | 8 (9) | 5 (5) | 6 (7) |
| Complete or partial† | 9 (10) | 17 (20) | 6 (6) | 14 (16) |
| Stable disease | 25 (27) | 21 (25) | 25 (27) | 20 (24) |
| Progressive disease | 59 (63) | 47 (55) | 62 (67) | 51 (60) |
P = 0.02 for complete response as assessed by investigators, and P = 0.01 for complete response as assessed by central review.
P = 0.05 for response as assessed by investigators, and P=0.03 for response as assessed by central review.
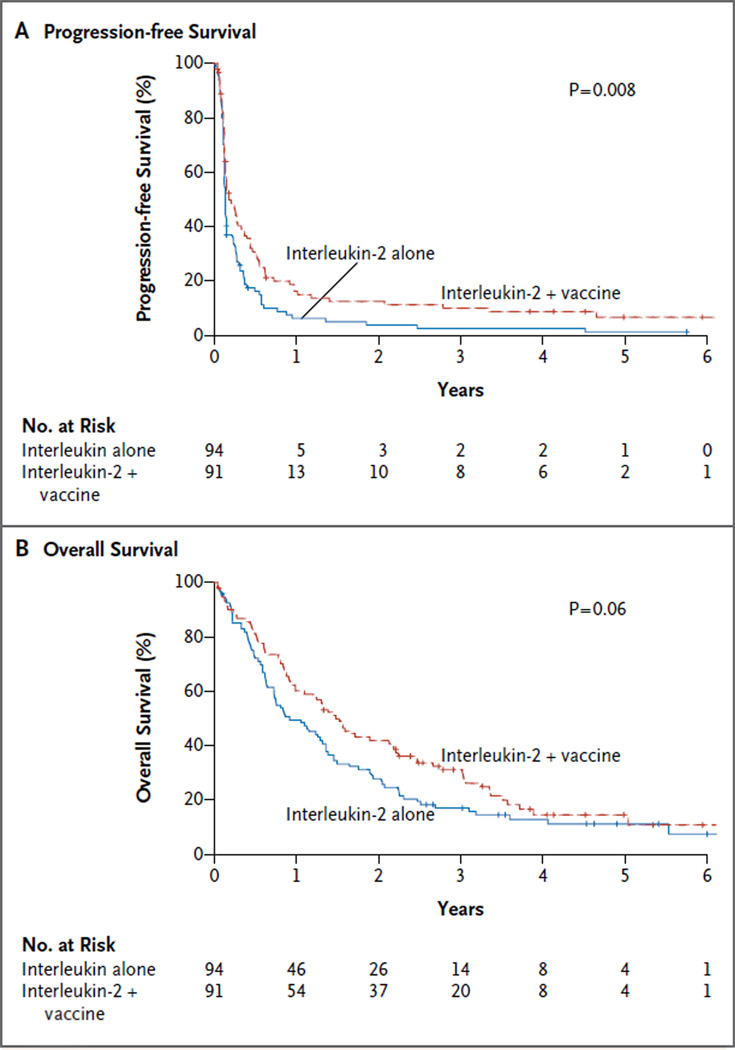
A secondary objective of the study was to compare the two groups with respect to progression-free survival. The time to progression was defined as the time from randomization until documented progression or death from any cause. The median progression-free survival was 1.6 months (95% confidence interval [CI], 1.5 to 1.8) among patients receiving interleukin-2 alone and 2.2 months (95% CI, 1.7 to 3.9) among patients receiving vaccine and interleukin-2 (P = 0.008) (Fig. 1A). The study was not powered to detect a difference in overall survival, but a trend in favor of the vaccine was observed. The median overall survival was 11.1 months (95% CI, 8.7 to 16.3) among patients receiving interleukin-2 alone and 17.8 months (95% CI, 11.9 to 25.8) among patients receiving vaccine and interleukin-2 (P = 0.06) (Fig. 1B). The median follow-up time among surviving patients is 41.5 months.
Figure 1. Progression-free and Overall Survival.
Progression-free survival (Panel A) was longer among patients receiving vaccine and interleukin-2 than among those receiving interleukin-2 alone. The median progression-free survival among patients who received the vaccine was 2.2 months (95% confidence interval [CI], 1.7 to 3.9), as compared with 1.6 months (95% CI, 1.5 to 1.8) among patients who did not receive the vaccine. There was a trend toward longer overall survival (Panel B) among patients receiving vaccine and interleukin-2 than among those receiving interleukin-2 alone. The median survival among patients who received the vaccine was 17.8 months (95% CI, 11.9 to 25.8), as compared with 11.1 months (95% CI, 8.7 to 16.3) among patients who did not receive the vaccine.
TOXIC EFFECTS OF TREATMENT
The grades 3 to 5 toxic effects that were observed over the course of all the cycles among the patients in both groups who received treatment were consistent with the expected side effects in patients receiving high-dose interleukin-2 (Table 3). With respect to most categories of toxicity, patients in the two groups had similar side effects, which were predominantly grade 3. There were more arrhythmias, more abnormalities of laboratory-test results, and more neurologic events among patients in the vaccine–interleukin-2 group than among patients in the interleukin-2–only group. (The P values have not been adjusted for multiple comparisons; therefore, at P = 0.05, one result would be expected to be significant purely by chance.) Since these differences may have been related to the increased number of interleukin-2 treatment cycles received by patients in the vaccine–interleukin-2 group as compared with patients in the interleukin-2–only group (286 vs. 244), we also evaluated the toxic effects that were observed only in the first two cycles of therapy. When only the first two cycles of treatment were analyzed and adjusted for multiple comparisons, only the rate of arrhythmias remained significantly higher in the vaccine–interleukin-2 group than in the interleukin-2–only group (15% vs. 2%), owing predominantly to events of sinus tachycardia (5 vs. 1 grade 3 events) and supraventricular arrhythmia (5 vs. 1 grade 3 events). These toxic effects were transient and reversible in all the affected patients. Three treatment-related deaths occurred — one in the interleukin-2–only group and two in the vaccine–interleukin-2 group.
Table 3.
Grades 3 to 5 Toxic Effects of Treatment over the Course of All Cycles.*
| Toxic Effect | Interleukin-2 Alone (N = 93) |
Vaccine–Interleukin-2 (N = 85)† |
P Value |
|---|---|---|---|
| no. of patients (%) | |||
| Hearing | 0 | 1 (1) | 0.48 |
| Blood or bone marrow | 33 (35) | 41 (48) | 0.08 |
| Cardiovascular | |||
| Arrhythmia | 4 (4) | 16 (19) | 0.002‡ |
| General | 25 (27) | 31 (36) | 0.17 |
| Coagulation | 2 (2) | 3 (4) | 0.67 |
| Constitutional symptoms | 15 (16) | 24 (28) | 0.06 |
| Skin | 6 (6) | 6 (7) | 0.87 |
| Gastrointestinal | 17 (18) | 18 (21) | 0.63 |
| Hemorrhage | 1 (1) | 2 (2) | 0.61 |
| Hepatic | 36 (39) | 34 (40) | 0.86 |
| Infection or febrile neutropenia | 6 (6) | 7 (8) | 0.65 |
| Lymphatic system | 0 | 1 (1) | 0.48 |
| Metabolic or laboratory-testing results | 19 (21)§ | 36 (42) | 0.002‡ |
| Musculoskeletal | 3 (3) | 6 (7) | 0.31 |
| Neurologic | 11 (12) | 22 (26) | 0.02 |
| Ocular or visual | 0 | 1 (1) | 0.48 |
| Pulmonary | 19 (21)§ | 19 (22) | 0.81 |
| Pain | 10 (11) | 11 (13) | 0.65 |
| Renal or genitourinary | 14 (15) | 16 (19) | 0.50 |
| Sexual or reproductive function | 1 (1) | 0 | 1.00 |
| Syndromes¶ | 1 (1) | 2 (2) | 0.61 |
| Maximum reported grade 3–5 | 74 (80) | 73 (86) | 0.27 |
Toxic effects were assessed according to the National Cancer Institute Common Toxicity Criteria, version 2.0. P values were calculated with the use of the chi-square test or Fisher’s exact test.
A total of 86 patients in this group were treated, but 1 was not assessed for toxic effects.
After Bonferroni adjustment, P values of 0.002 or less were considered to be significant in order to maintain the 0.05 error rate.
Data for two patients were missing.
Included are tumor flare, the tumor lysis syndrome, and other syndromes.
RESULTS OF IN VITRO STUDIES
Immunologic analyses were performed before and after four cycles of treatment. Anti-peptide reactivity developed in none of the 12 patients in the interleukin-2–only group from whom samples could be evaluated, as compared with 7 of the 37 patients in the vaccine–interleukin-2 group from whom samples could be evaluated. There was no relationship between the development of anti-peptide reactivity and the objective clinical response.
Post-treatment levels of CD4+foxp3+ cells were higher in patients who had a clinical response to treatment than in those who did not have a response (16.84±2.22% vs. 11.08±1.01%, P=0.01). There was no effect of the vaccine on this difference. Additional results are provided in the Supplementary Appendix.
DISCUSSION
This randomized study showed the clinical benefit of a vaccine in the treatment of patients with measurable metastatic melanoma. Patients receiving the HLA⋆A0201-restricted peptide gp100:209–217(210 M) with interleukin-2 were more than twice as likely to have a clinical response as those receiving interleukin-2 alone (Table 2). Though the response rate among the patients receiving interleukin-2 alone was lower than the rate of 16% reported initially from a limited number of experienced programs,3 our study was conducted at a large number of institutions, reflecting an outcome that is more likely to be achieved when experimental therapies are applied more widely. In addition, the response rates seen in this study are similar to those reported recently in a large, nonrandomized, single-institution study of interleukin-2 with a vaccine as compared with interleukin-2 without a vaccine (22% vs. 13%).4
After the initial report on the gp100 peptide vaccine5 that prompted this randomized trial, the Cytokine Working Group initiated a series of three phase 2 studies to confirm the efficacy of the vaccine.6 These three studies (involving 39 to 42 patients in each study) used the same peptide vaccine administered every 3 weeks but combined it with a lower dose of interleukin-2 than that used in our study, administered on variable schedules. The response rates ranged from 13% to 24%. These phase 2 studies did not include an interleukin-2–only control group, and consequently, conclusions about the efficacy of the vaccine itself could not be drawn.
The peptide vaccine added some toxic effects to those seen with interleukin-2 therapy. Aside from the skin reactions of redness, swelling, and pain at the local injection site, the vaccine was associated with higher incidences of transient, reversible sinus tachycardia and supraventricular arrhythmia than those seen with interleukin-2 alone. These side effects are within the expected range of side effects of high-dose interleukin-2; the reason for their increased frequency in the vaccine–interleukin-2 group is not known. The vaccine or the adjuvant may have induced additional circulating cytokines that could cause greater cardiac toxic effects.
A number of immune-suppressing molecules within the tumor microenvironment, as well as immune regulatory checkpoints, have been described that may inhibit antitumor T-cell responses in vivo.7,8 Overcoming these factors may be important for improving the efficacy of cancer vaccines. For example, the cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) molecule is capable of suppressing effector immune responses on T cells, and blockade of this molecule with the specific antibody ipilimumab has led to clinical responses in patients with melanoma.9 In a recent randomized trial, treatment with ipilimumab with or without the gp100 vaccine resulted in a survival advantage over treatment with the vaccine alone.10 In that study, the combination of ipilimumab with the vaccine was not superior to ipilimumab alone. It is not clear why we observed an improved response when we combined the gp100 vaccine with interleukin-2, whereas this was not seen when a similar vaccine was combined with ipilimumab. This disparity highlights the need for further validation of the conclusions from both of these studies but also points to potential differences in the mechanisms of action of interleukin-2 and ipilimumab. Among the numerous potential explanations for the difference is the possibility that immune responses in patients after anti–CTLA-4 therapy are largely dependent on CD4+ T cells; in contrast, the gp100 peptide vaccine was designed to stimulate CD8+ T cells. Anti–CTLA-4 antibody can augment CD8+ T-cell responses, and it can also independently augment CD4+ antitumor immune responses in murine models.11 Indeed, CD4+/ICOS+ T cells were shown to be increased in both the peripheral blood and tumor sites after anti–CTLA-4 antibody treatment in patients with bladder cancer.12 Additional studies are needed to understand these complex mechanisms of action.
In vitro studies of T-cell reactivity showed that some patients in the vaccine group had an increase in circulating gp100 reactive T cells after vaccination (see the Supplementary Appendix). However, the number of patients in whom gp100 reactive T cells developed was small, which is consistent with previous studies that noted that interleukin-2 decreased the number of antigen-specific T cells in the peripheral blood after vaccination,5,13,14 Perhaps this finding was the result of increased migration of antigen-specific T cells into the tumor after activation by interleukin-2. As with previous studies, circulating levels of antigen-specific T cells did not correlate with a clinical response, suggesting that future studies should incorporate additional assays and analysis of other areas, such as the tumor site. However, we noted an increase in T regulatory cells (CD4+foxp3+) in patients in both treatment groups who had a response to treatment (see the Supplementary Appendix). We postulate that this effect is related to interleukin-2. Although T regulatory cells are generally thought to be suppressive, they have been associated with improved survival in patients with colon cancer.15 We hypothesize that the increased levels of T regulatory cells in patients who had a response to treatment represent a counterregulatory response after a strong antitumor immune reaction; however, the increased levels may also be related to the timing of collection of the sample.
Our study showed that overall survival appeared to be somewhat better in the vaccine–interleukin-2 group in the period from 6 months through 3 years of follow-up (Fig. 1B). Additional data are needed to ascertain whether the finding in our study was due to a direct effect of the vaccine or to the possibility that vaccinated patients were more responsive to salvage regimens or that the nature of progression differed between the two groups or that other factors were involved. Our study showed that a vaccine can enhance cytokine therapy in patients with melanoma and highlights the potential of using rational combinations of immune agents in treating patients with metastatic cancer.
Supplementary Material
Acknowledgments
Supported by the National Cancer Institute, Indiana University Health Goshen, Goshen Hospital and Health Care Foundation, Chiron, and Novartis.
We thank Juliann Wu, M.S., of EMMES, Rockville, MD, for data management and statistics; Paul Duray, M.D., for review of pathology slides; Lien Ngo, M.S., Surgery Branch, National Cancer Institute (NCI), Bethesda, MD, for performing in vitro studies; personnel at the NCI Cancer Therapy Evaluation Program and Division of Cancer Treatment and Diagnosis for guidance and support for this project; and Jana Risemas for assistance with the preparation of the manuscript.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 2.Overwijk WW, Theoret MR, Restifo NP. The future of interleukin-2: enhancing therapeutic anticancer vaccines. Cancer J Sci Am. 2000;(Suppl 1):S76–S80. [PMC free article] [PubMed] [Google Scholar]
- 3.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 4.Smith FO, Downey SG, Klapper JA, et al. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res. 2008;14:5610–5618. doi: 10.1158/1078-0432.CCR-08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sosman JA, Carrillo C, Urba WJ, et al. Three phase II cytokine working group trials of gp100 (210M) peptide plus high-dose interleukin-2 in patients with HLA-A2-positive advanced melanoma. J Clin Oncol. 2008;26:2292–2298. doi: 10.1200/JCO.2007.13.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lizée G, Cantu MA, Hwu P. Less yin, more yang: confronting the barriers to cancer immunotherapy. Clin Cancer Res. 2007;13:5250–5255. doi: 10.1158/1078-0432.CCR-07-1722. [DOI] [PubMed] [Google Scholar]
- 9.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [Erratum, N Engl J Med 2010;363:1290.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quezada SA, Simpson TR, Peggs KS, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liakou CI, Kamat A, Tang DN, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008;105:14987–14992. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slingluff CL, Jr, Petroni GR, Yamshchikov GV, et al. Immunologic and clinical outcomes of vaccination with a multiepitope melanoma peptide vaccine plus low-dose interleukin-2 administered either concurrently or on a delayed schedule. J Clin Oncol. 2004;22:4474–4485. doi: 10.1200/JCO.2004.10.212. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Impact of cytokine administration on the generation of antitumor reactivity in patients with metastatic melanoma receiving a peptide vaccine. J Immunol. 1999;163:1690–1695. [PMC free article] [PubMed] [Google Scholar]
- 15.Salama P, Phillips M, Grieu F, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.