Abstract
Objective
Neuromuscular electrical stimulation that incorporates wide pulse widths (1 ms) and high frequencies (100 Hz; wide pulse-NMES (WP-NMES)) augments contractions through an increased reflexive recruitment of motoneurons in individuals without neurological impairments and those with spinal cord injury. The current study was designed to investigate whether WP-NMES also augments contractions after stroke. We hypothesised that WP-NMES would generate larger contractions in the paretic arm compared to the non-paretic arm due to increased reflex excitability for paretic muscles after stroke.
Methods
The biceps brachii muscles were stimulated bilaterally in 10 individuals with chronic hemiparetic stroke. Four stimulation patterns were delivered to explore the effects of pulse width and frequency on contraction amplitude: 20-100-20 Hz (4 s each phase, 1 ms pulse width); 20-100-20 Hz (4 s each phase, 0.1 ms); 20 Hz for 12 s (1 ms); and 100 Hz for 12 s (1 ms). Elbow flexion torque and electromyography were recorded.
Results
Stimulation that incorporated 1 ms pulses evoked more torque in the paretic arm than the non-paretic arm. When 0.1 ms pulses were used there was no difference in torque between arms. For both arms, torque declined significantly during the constant frequency 100 Hz stimulation and did not change during the constant frequency 20 Hz stimulation.
Conclusions
The larger contractions generated by WP-NMES are likely due to increased reflexive recruitment of motoneurons, resulting from increased reflex excitability on the paretic side.
Significance
NMES that elicits larger contractions may allow for development of more effective stroke rehabilitation paradigms and functional neural prostheses.
Keywords: Reflex transmission, motoneuron excitability, sensori-motor integration, biceps brachii
Introduction
Individuals who have experienced a stroke often have difficulty generating sufficient and appropriate joint torques required to produce functional movements in the paretic upper limb. Neuromuscular electrical stimulation (NMES) has been applied to muscles affected by stroke to assist with muscle strengthening and activities of daily living (Chae, 2003; de Kroon et al., 2005; Popovic et al., 2009; Stein et al., 2006). Traditionally, parameters used to stimulate muscles affected by stroke include pulse widths of 200–300 μs and frequencies of 20–50 Hz (de Kroon et al., 2005). During NMES the size of the evoked contraction is often limited by the individual’s discomfort, as discomfort increases with increasing stimulation currents. For individuals who have experienced a stroke, relatively high currents are sometimes required to generate muscle contractions sufficient to produce functional movements especially when the need to overcome co-contraction and abnormal joint torque couplings is considered (Keller et al., 2005). Fatigability of NMES induced contractions also limits contraction amplitude due to the non-physiological order in which motor axons are activated during NMES (Feiereisen et al., 1997; Gregory and Bickel, 2005; Jubeau et al., 2007). Thus, there is a need for the continued development of NMES techniques that generate large muscle contractions while minimizing discomfort and muscular fatigue.
NMES that incorporates higher frequencies (up to 100 Hz) and wider pulse widths (1 ms) (wide pulse NMES; WP-NMES) than those traditionally used for electrical stimulation can enhance NMES-evoked contractions in individuals with no neurological impairments (Collins et al., 2001; 2002; Collins, 2007) and in those with a spinal cord injury (Clair et al., 2006; Nickolls et al., 2004; Thompson et al., 2011) through an increase in the “central contribution”. This central effect is thought to develop due to the recruitment of spinal motoneurons by the electrically-evoked afferent volley travelling along reflex pathways through the spinal cord (see Collins, 2007). Mechanistically, the high frequencies and wide pulse widths are thought to send a relatively larger afferent volley to the spinal cord than traditional NMES, augmenting contraction amplitude by increasing H-reflex amplitudes (Bergquist et al. 2011; Klakowicz et al., 2006) and potentially increasing the activation of persistent inward currents in spinal neurons (Collins et al., 2001; 2002; Collins, 2007). A potential advantage of using WP-NMES for rehabilitation, compared to more traditional NMES, is that lower stimulus currents may be sufficient to generate functional muscle contractions. Furthermore, synaptic activation of motoneurons follows the size principle (Henneman et al., 1965) thereby recruiting fatigue-resistant motor units first, which may help generate contractions that are more fatigue-resistant (Lagerquist et al., 2009).
The extent to which the central nervous system (CNS) contributes to contractions evoked by WP-NMES in individuals who have experienced a stroke has not been tested. Several changes occur in the CNS after stroke that may enhance the central contribution to contractions evoked by WP-NMES in the paretic limb. Decreases in the efficacy of pre-synaptic inhibitory mechanisms that regulate the transmission of afferent input to motoneurons would allow a larger afferent volley to reach the motoneurons (Aymard et al., 2000; Lamy et al., 2009). Putative post-synaptic increases in the excitability of the motoneurons would amplify the motoneuron’s response to afferent input (Dewald et al., 1995; Mazavet et al., 2003; McPherson et al., 2008; Kline et al., 2007). Together these changes increase reflex excitability, thus a given afferent volley would lead to a larger motor output.
The current study was therefore designed to investigate whether WP-NMES augments muscle contractions after stroke. These experiments were prompted in part by the finding that tonic vibration reflexes were larger in the paretic arm compared to the non-paretic arm in individuals with chronic stroke (McPherson et al., 2008). The tonic vibration reflex and WP-NMES both generate contractions through the reflexive recruitment of motoneurons, thus we expected that WP-NMES would also generate larger contractions in the paretic arm compared to the non-paretic arm. Specifically, we hypothesized that stimulation incorporating a 1 ms pulse width would generate larger contractions in the paretic arm versus the non-paretic arm, but contractions generated using 0.1 ms pulses would not be different between arms. We also hypothesized that stimulation delivered using 1 ms pulses at 100 Hz would evoke contractions with a larger central contribution compared to stimulation using 1 ms pulses at 20 Hz. The results of the current study provide further evidence of an increase in reflex excitability in the paretic arm after stroke and have implications for the use of NMES in the neurorehabilitation of stroke.
Methods
Participants
NMES was applied via surface electrodes over the right and left biceps brachii muscles of 14 individuals who had experienced a hemiparetic stroke resulting in upper limb paresis. Out of 14 participants in the current study, 4 participants withdrew due to discomfort experienced during the stimulation. The ten participants that were included in he current study responded to the experimenter by saying that the stimulation was comfortable. Additionally, the experimenter observed the participant throughout the experiment for any signs of discomfort (i.e. changes in skin coloring, sweating, tensing up). The ten participants that were included in the current study did not show any of these signs during the experiment. Thus, we do not believe that discomfort was a factor that affected the results of the remaining 10 participants (7 males and 3 females; age range: 53–83 yrs). Each participant provided informed, written consent. The experiments were conducted in accordance with the standards set by the Declaration of Helsinki for research involving human participants and were approved by the Institutional Review Board of Northwestern University.
Inclusion criteria were the following: 1) A cortical or sub-cortical stroke at least one year prior to the study (range: 26–255 months post-stroke; mean: 102 ± 23 months). 2) An upper limb Fugl-Meyer Assessment (FMA) score for the paretic limb of less than 50 (out of 66). In this study scores ranged from 10–46 (mean: 25 ± 4), which indicates moderate to severe impairment (Fugl-Meyer et al., 1975). 3) An FMA score for the non-paretic limb of 66, which indicates no impairment. 4) Passive range of motion in both limbs of at least 90° for shoulder flexion, shoulder abduction, and elbow flexion. 5) No inflammatory conditions at the shoulder, elbow, wrist, or fingers as screened for by applying overpressure at the end of the range of motion. 6) No recent changes in medications used to manage hypertension. 7) Not taking medications to treat spasticity.
Experimental Protocol
The position of the participant and the equipment used in the current study closely follow that used by McPherson and colleagues (2008). Participants were seated in the chair of a Biodex dynamometer system (Biodex Medical Systems, Shirley, NY, USA) with seatbelts placed over the shoulders and across the waist to help maintain a consistent upright posture for the duration of the experiment. Both feet were supported by a foot rest. Participants wore a custom-made fibreglass cast over the forearm, wrist, and hand of the arm receiving stimulation in order to minimize movement during the experiment. The cast also allowed for tight coupling between the arm and the 6 degrees-of-freedom load cell used to measure elbow torques (JR3, Model 45E15A, Woodland, CA, USA). The load cell was attached to the cast at the wrist with a Delrin ring attachment piece and orthogonal forces and moments generated in the x, y, and z planes were recorded and converted into elbow torques. The arm was positioned with 75° of shoulder abduction, 40° horizontal shoulder flexion, and 90° of elbow flexion (see figure 1). The weight of the arm was supported throughout the experiment to help participants remain fully relaxed.
Figure 1.
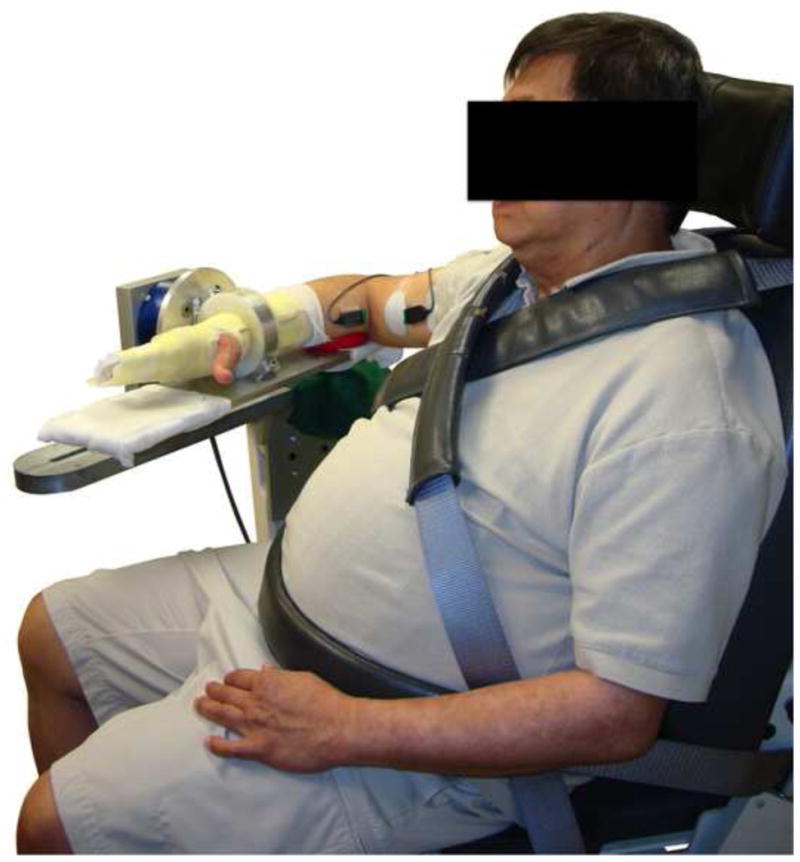
Image of the experimental setup with the paretic arm in the test configuration. The metal forearm interface plate and ring are mounted directly to a 6-DOF load cell. A delrin interface ring connects the wrist of the test subject with a Fiberglass cast to the metal ring. EMG recording and stimulating electrodes are placed over the biceps brachii muscle.
To monitor whether participants remained relaxed throughout the experiment, surface electromyography (EMG) was recorded via electrodes placed over the muscle belly with a 1 cm inter-electrode distance (Delsys, 16-channel Bagnoli EMG System, Boston, MA, USA). In the arm receiving stimulation, the muscles recorded from were: brachioradialis, biceps brachii, lateral head of the triceps brachii, long head of the triceps brachii, anterior deltoid, intermediate deltoid, posterior deltoid, and pectoralis major.
The paretic and non-paretic arms were tested on separate days. For a given participant, the sessions were conducted at the same time of day, between 1 and 5 days apart. The non-paretic arm was tested on the first day because stimulation intensities for both arms were matched to a percentage of the maximum voluntary elbow flexion torque in that arm. Participants started each experiment by performing maximal voluntary isometric elbow flexion contractions, using visual feedback of their maximal voluntary torque (MVT) provided on a computer monitor. Verbal encouragement to perform maximally was provided by the experimenters. The participants completed as many trials as necessary to record three maximal contractions within 10% of each other (typically 3–5). For all other trials participants were instructed to relax to minimize muscle activity in the arm being tested and to stay as still as possible.
NMES was delivered through bipolar surface electrodes (oval 3.81 x 6.35 cm, Uni-patch Superior Silver Electrodes, Wabasha, MN, USA) over the biceps brachii muscle. The electrodes were positioned over the proximal and distal ends of the muscle belly to allow for the biceps brachii EMG electrodes to be placed between the stimulating electrodes. A Compex Motion stimulator (Keller et al., 2002) was used to control the parameters of the stimulation. Stimulation intensity was set based on the peak torque generated during a 250 ms stimulus train (25 pulses at 100 Hz; 1 or 0.1 ms pulse width). These short trains were chosen because they provided an indication of primarily peripheral motor axon activation in each arm (Baldwin et al., 2006; Collins et al., 2001; 2002). This procedure for matching stimulus intensity was used because we could not measure EMG during the stimulation due to the presence of large stimulation artifacts, and therefore could not use M-wave amplitude as an indication of stimulation intensity. Intensity was adjusted to generate peak torque during the short train in both arms that was approximately 6% of the non-paretic MVT. This stimulation intensity was chosen because it was the highest that was comfortable for participants in pilot experiments. In 7 out of 10 subjects this was achieved by using the same stimulation current for both arms. For the other 3 subjects to generate the same torque in both arms the current had to be increased by 2, 6, and 12 mA for the paretic arm.
Four stimulation patterns were delivered: 20-100-20 Hz (4 s each phase, 1 ms pulse width); 20-100-20 Hz (4 s each phase, 0.1 ms pulse width); 20 Hz for 12 s (1 ms pulse width); and 100 Hz for 12 s (1 ms pulse width). The second period of 20 Hz stimulation in the 20-100-20 Hz pattern was used to determine whether the increases in torque that occur during the 100 Hz period were sustained once the frequency has gone back to 20 Hz. If the torque remains elevated during the second period of 20 Hz, we have shown previously in individuals with no neurological impairments that it was due to enhanced activation in the CNS (Collins et al., 2001; 2001). If there was no additional contribution from the CNS during the second period of 20 Hz stimulation, we would expect the torque during the second 20 Hz period to return to, or close to, the level it was at prior to the 100 Hz burst. The 20-100-20 Hz stimulation pattern was also delivered using a narrower pulse width (20-100-20 Hz, 0.1 ms) to assess the effect of pulse width on the evoked contraction. The torque evoked by each of these stimulation patterns was compared between arms. The 20 and 100 Hz constant frequency patterns were included to assess the effect of stimulation frequency on the evoked contraction. The central contribution has been quantified in other studies by measuring the difference in the torque between the beginning and end of the stimulation train (Baldwin et al., 2006; Dean et al., 2007; Klakowicz et al., 2006); an increase in torque throughout the stimulation train was evidence of an increasing central contribution. In the present study, this difference in torque was compared between the 20 and 100 Hz stimulation patterns within each arm and for each stimulation pattern between arms. In a single trial, one stimulation pattern was delivered 5 times with 2 minutes of rest between each stimulation train. The order of delivery of the stimulation patterns was randomized across subjects.
The experimenter visually inspected the torque and all EMG channels after each trial to determine if that trial was acceptable. For each trial, if the participant had a pre-activation level of greater than 5% MVC, that trial was re-collected and the trial with the lowest pre-activation level was included. Pre-activation levels ranged from ~ 1–15% non-paretic MVC. The size of this range is partly due to 3 of the 10 participants who had particular difficulty relaxing their paretic arm prior to each trial. We chose to include these 3 participants in the main study group for the following two reasons. Firstly, it is common for individuals with chronic stroke to have involuntary activation of some muscles and by including this type of natural variance in our study group, we felt our results would be more generalizable to the general stroke population. Secondly, the torque responses evoked by the trains of WP-NMES in these 3 participants were qualitatively not distinguishable from the rest of the participants in the study. A trial was also rejected, and subsequently re-collected, if the participant moved before the trial finished (i.e. coughed, moved their head) or fell asleep.
Data Collection and Analyses
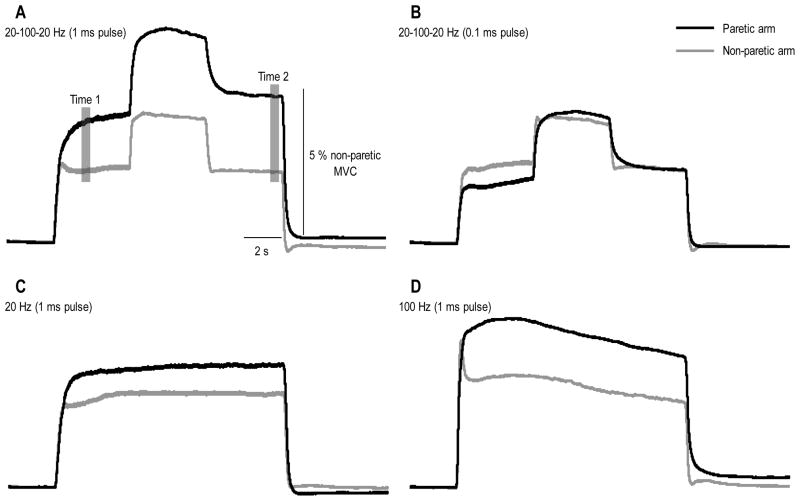
Data were sampled at 1000 Hz. EMG data were amplified 1000 times, high pass filtered (cut-off frequency, 20 Hz) (16-channel Bagnoli EMG System), and then low pass filtered using an eighth-order low-pass Butterworth filter (cut off frequency, 500 Hz) (Model 9016; Frequency Devices, Havelhill, MA). Custom written Matlab software (The Mathworks Inc., Natick, MA, USA) was used to analyze the torque and biceps brachii EMG data. A Jacobian-based algorithm was used to convert the load cell forces and moments measured at the wrist into elbow flexion torque. The torque data were filtered using a digital 8th order low pass Butterworth filter (cut-off frequency, 50 Hz). All torque data were then normalized to the MVT of the non-paretic arm. This normalization was performed for two reasons: 1) the MVT of the paretic arm can be unreliable and may not truly represent the force generating capacity of the muscle due to reduced central drive, the possibility of increased co-contraction between elbow flexors and extensors, and changes in muscle properties (Dietz and Sinkjaer, 2007; Sinkjaer and Magnussen, 1994); and 2) it was not possible to measure the electrically-stimulated maximum torque in the paretic arm due to pain tolerance of the participants. For statistical analyses, torque data were averaged over two 500 ms windows centred around: 1.5 s (Time 1) and 11.5 s (Time 2) into the stimulus train (see shaded regions Figure 2A).
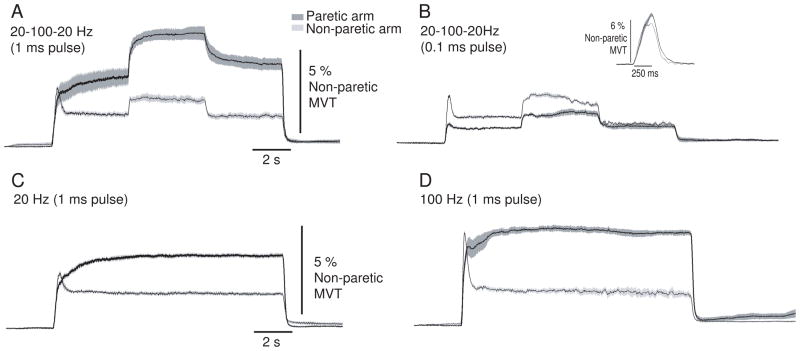
Figure 2.
Torque generated by each stimulation pattern in a single participant. Torque in the paretic arm is shown by the dark trace and in the non-paretic arm by the light trace. Each panel shows the torque generated by a specific stimulation pattern: A) 20-100-20 Hz (1 ms pulse width); B) 20-100-20 Hz (0.1 ms pulse width); C) 20 Hz constant frequency (1 ms pulse width); and D) 100 Hz constant frequency (1 ms pulse width). The inset in panel B shows the torque evoked in each arm by the short stimulus train (25 pulses at 100 Hz; 1 ms pulse width) used to set stimulus intensity. Each trace is an average of 5 repetitions of each stimulation pattern. The shaded bands represent ± 2 SE. The shaded regions in Panel A represent the time periods (Time 1 and Time 2) over which data were quantified for statistical analyses (see Figures 4 and 5).
Biceps brachii EMG data were full-wave rectified and then smoothed using a causal one-sided moving average filter (window duration, 250 ms). This was followed by baseline correction which involved subtracting the average biceps brachii EMG over the first 250–500 ms in a trial, during which the subject was at rest and there was no stimulation, from all other EMG data within that trial. The EMG data were then normalized to the maximum EMG recorded during maximum voluntary contractions in the non-paretic arm. The EMG was quantified prior to the stimulation to verify if the muscle was fully relaxed. Although analysis of the EMG recorded during the stimulation was not possible due to interference from the stimulation artifacts, a comparison of EMG activity before and after stimulation was made. Data were averaged over two 500 ms windows centred around 1 s prior to and 1 s after the stimulation. However, it must be noted that in individuals with no neurological impairments we do not always see residual EMG activity after the stimulation when the torque has been augmented during the stimulation train (i.e. after the 100 Hz burst; Lagerquist and Collins, 2010). Therefore, in the current study we were not expecting to see a direct relationship between torque during the stimulation and residual EMG after the stimulation. The EMG data from muscles other than the biceps brachii (i.e. brachioradialis, lateral head of the triceps brachii, long head of the triceps brachii, anterior deltoid, intermediate deltoid, posterior deltoid, and pectoralis major) were only used to determine whether participants were relaxed prior to the start of each trial. One participant’s EMG data were excluded due to poor signal quality.
Statistical Analysis
A two-way repeated measures analysis of variance test (rm-ANOVA) was used to assess differences in torque evoked by the 250 ms train used to set stimulus intensity based on the following two factors: arm (2 levels: paretic and non-paretic) and pulse width (2 levels: 1 and 0.1 ms). A three-way rm-ANOVA was used to assess differences in torque during the 12 s stimulation trains based on the following three factors: stimulation pattern (4 levels: 20-100-20 Hz (1 ms pulses), 20-100-20 Hz (0.1 ms pulses), 20 Hz (1 ms pulses), 100 Hz (1 ms pulses)), arm (2 levels: paretic and non-paretic), and time (2 levels: Time 1 and Time 2). A similar three-way rm-ANOVA was used to assess changes in biceps brachii EMG activity with the following three factors: stimulation pattern (4 levels: 20-100-20 Hz (1 ms pulses), 20-100-20 Hz (0.1 ms pulses), 20 Hz (1 ms pulses), 100 Hz (1 ms pulses)), arm (2 levels: paretic and non-paretic), and time (2 levels: pre-stim and post-stim). The Huynh-Feldt correction was applied if the data violated the assumption of sphericity for rm-ANOVA. Tests of simple effects, followed by simplecomparisons, if necessary, were used post-hoc to assess significant 3-way interactions identified in the rm-ANOVA results. Tukey’s HSD tests were performed on significant 2-way interactions or main effects when appropriate. Descriptive statistics are reported as the mean ± 1 SE. All statistical tests were conducted with an alpha level of 0.05.
Results
Stimulation intensity
For all trials stimulus intensity was adjusted such that a 250 ms stimulus train (25 pulses at 100 Hz) evoked torque of approximately 6% of the non-paretic MVT. Examples of the torque recorded during these short trains in the paretic and non-paretic arms of a single participant are shown in Figure 2B (inset). For the group of participants, the torque evoked by the 250 ms train was not different between the paretic and non-paretic arms for either pulse width [F(1,9) = 4.186, p = 0.071]. For trials that used a 1 ms pulse width, the evoked contractions were 6.4 ± 0.5 and 5.9 ± 0.1 % of the non-paretic MVT in the paretic and non-paretic arms, respectively. For trials that used a 0.1 ms pulse width, the evoked contractions were 5.7 ± 0.4 and 6.1 ± 0.1 % of the non-paretic MVT in the paretic and non-paretic arms, respectively.
Single participant torque data
Elbow flexion torque evoked for all stimulation patterns in the paretic and non-paretic arms of one participant is shown Figure 2(A–D). This participant had a stroke 12.5 years prior to being involved in the study and a Fugl-Meyer score of 34 for the impaired upper limb. A clear effect of pulse width on torque generation in the paretic arm was evident for this participant. For all stimulation patterns that incorporated the wide (1 ms) pulse width there was more torque evoked in the paretic arm than the non-paretic arm (dark vs. light trace, respectively, Fig. 2A, 2C, and 2D). In contrast, when the stimulation was delivered with a 0.1 ms pulse width, torque generated in the paretic and non-paretic arms was not different (Fig. 1B). For this participant, there was no influence of stimulation frequency on the amplitude of the central contribution because the 100 Hz constant frequency stimulation pattern (1 ms pulses) (Fig. 1D) did not show a greater increase in the amplitude of the central contribution compared to the 20 Hz constant frequency stimulation pattern (1 ms pulses) (Fig. 1C) in either arm.
Group torque data: Effect of pulse width
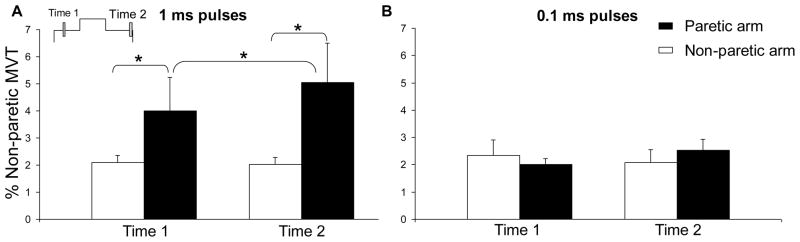
The torque evoked by each stimulation pattern, averaged over the 10 participants, is shown in Figure 3. The shaded areas in Figure 3A represent the time windows over which data were quantified for statistical analyses. There was a significant three-way interaction between stimulation pattern, arm, and time for the torque generated by NMES [F(1,12) = 4.43, p < 0.05]. To assess the effect of pulse width on torque, we used post-hoc analyses of this interaction to compare the two-way interaction of arm by time for the two stimulation patterns that used the same stimulation frequencies, but different pulse widths: 20-100-20 Hz (1 ms pulse width) and 20-100-20 Hz (0.1 ms pulse width). The arm by time interaction for the 20-100-20 Hz (1 ms pulse width) pattern was significant [F(1,12) = 5.79, p < 0.05]. Simple comparisons analyses revealed that for this stimulation pattern more torque was evoked in the paretic arm than the non-paretic arm at Time 1 [F(1,12) = 33.9, p < 0.05] and Time 2 [F(1,12) = 85.1, p < 0.05]. These statistically significant differences can be seen by comparing the dark and light traces in Figure 3A and each pair of black and white columns in Figure 4A. For the 20-100-20 Hz stimulation pattern that used a 0.1 ms pulse width, significant interactions or main effects were not found which indicates that the torque was not different between arms at either Time 1 or Time 2 (Fig. 3B; each pair of black and white columns in Fig. 4B).
Figure 3.
Torque generated throughout each stimulation pattern averaged across the group. Torque in the paretic arm is shown by the dark trace and in the non-paretic arm by the light trace. Each panel shows the torque generated by a specific stimulation pattern: A) 20-100-20 Hz (1 ms pulse width); B) 20-100-20 Hz (0.1 ms pulse width); C) 20 Hz constant frequency (1 ms pulse width); and D) 100 Hz constant frequency (1 mspulse width). Error bars have been omitted for the sake of clarity. The shaded regions in Panel A represent the time periods (Time 1 and Time 2) over which data were quantified for statistical analyses (see Figures 4 and 5).
Figure 4.
The group mean effect of pulse width (1 vs. 0.1 ms) on torque generation for the 20-100-20 Hz stimulation pattern. Torque evoked in the paretic arm is represented by the black columns; in the non-paretic arm by the white columns. Panel A shows torque evoked using 1 ms pulses at Time 1 (1.5 s into the stimulation train) and Time 2 (11.5 s into the stimulation train). Panel B shows the torque evoked at Time 1 and Time 2 by stimulation using 0.1 ms pulses. 1 SE is shown. Data columns connected by brackets are significantly different from each other (p < 0.05).
Effect of stimulation frequency
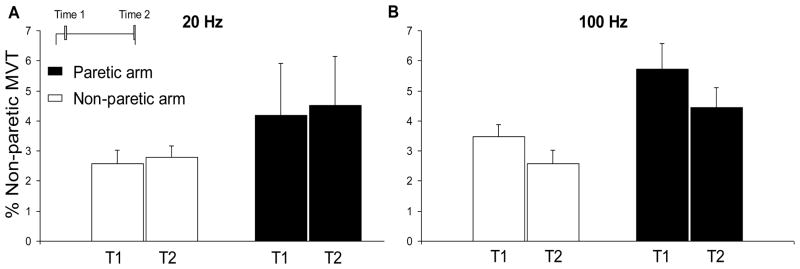
To assess the effect of stimulation frequency on torque, we used post-hoc analyses of the significant 3-way interaction mentioned above, to compare the two-way interaction of arm by time for the two stimulation patterns that used the same pulse width, but different stimulation frequencies: 20 Hz (1 ms pulse width) and 100 Hz (1 ms pulse width). For the 20 Hz stimulation pattern no significant interactions or main effects were found which indicates that the torque was not different between arms at Time 1 or Time 2 (Fig. 2C; Fig. 4A). For the 100 Hz stimulation pattern the main effects of arm [F(1,12) = 5.66, p < 0.05] and time [F(1,12) = 14.36, p < 0.05] were significant. The main effect of arm showed that the torque was larger in the paretic arm compared to the non-paretic arm throughout the stimulation train. The main effect of time highlighted the significant decline in torque from the beginning (Time 1) to the end (Time 2) of the stimulation train in both arms (Fig. 3D; each pair of columns in Fig. 5B). This decrease was not significantly different between arms. These main effects are not shown in Figure 5 for clarity.
Figure 5.
The group mean effect of stimulation frequency (20 vs 100 Hz) on torque generation for the group. Torque evoked in the paretic arm is represented by the black columns; in the non-paretic arm by the white columns. Panel A shows the torque evoked by 20 Hz stimulation (1 ms pulse width) for each arm at the beginning (Time 1; T1) and end (Time 2; T2) of the stimulation train. Panel B shows the torque evoked by 100 Hz stimulation (1 ms pulse width) for each arm at Time 1 and Time 2. 1 SE is shown. For the 100 Hz stimulation pattern, there were significant main effects of arm and time which are not shown here for clarity (see text).
To investigate whether the 4 s period of 100 Hz stimulation during the 20-100-20 Hz (1 ms pulse width) pattern resulted in a prolonged increase in torque, post-hoc analyses of the significant 3-way interaction mentioned above was used to compare the two-way interaction of arm by time during the 20-100-20 Hz (1 ms pulse width) stimulation pattern. The arm by time interaction was significant [F(1,12) = 5.79, p < 0.05] and simple comparisons analyses revealed that the torque increased significantly from Time 1 to Time 2 in the paretic arm only [F(1,12) = 10.04, p < 0.05] (dark trace, Figure 3A; black columns in Fig. 4A). When a 0.1 ms pulse width was used, an increase in torque after the 4 s period of 100 Hz stimulation was not observed in either arm.
Group EMG data
All participants had some difficulty completely relaxing the muscles in their paretic arm during some trials. Therefore the average group pre-stimulus biceps brachii EMG recorded from the paretic arm, across all trials, was 11 ± 3 % of the EMG recorded from the non-paretic arm during the maximum voluntary contraction. For the non-paretic arm the average group pre-stimulus EMG was 2 ± 0.4 % of the non-paretic maximal EMG. To assess whether the stimulation caused a sustained increase in EMG amplitude once the stimulation had been turned off, we measured EMG over two half second windows centred around: 1 s before and 1 s after stimulation. There was a significant stimulation pattern by time interaction [F(3,24) = 3.51, p = 0.031] and no main effect of arm. With the data from both arms grouped together, there was significantly more biceps brachii EMG 1 s after the 100 Hz stimulation pattern as compared to 1 s before the stimulation. There was also more activity 1 s after the 100 Hz stimulation as compared to 1 s after the 20 Hz stimulation. There were no differences in the EMG amplitudes before and after the 20 Hz constant frequency stimulation using a 1 ms pulse width or the 20-100-20 Hz stimulation using a 1 or 0.1 ms pulse width.
Discussion
In the current study a novel stimulation paradigm (WP-NMES) was delivered to the biceps brachii muscles of the paretic and non-paretic arms in individuals with chronic hemiparetic stroke. Consistent with our first hypothesis, more torque was generated in the paretic arm compared to the non-paretic arm when a 1 ms pulse width was used. There was no difference in torque between arms when a 0.1 ms pulse width was used. Our second hypothesis, which stated that higher stimulus frequencies would increase the central contribution more than lower frequencies, was not supported. As predicted, there was no difference in torque from the beginning to the end of the stimulation for each arm during constant frequency stimulation at 20 Hz using a 1 ms pulse width. However, for both arms during the 100 Hz constant frequency stimulation (1 ms pulses), there was a decline in torque from the beginning to the end of the stimulation train. Interestingly, when only 4 s of 100 Hz stimulation was incorporated into the 12 s stimulation train (1 ms pulses), torque was larger in the paretic arm compared to the non-paretic arm after the 100 Hz burst.
WP-NMES enhances the central contribution to contractions after stroke
The present experiments were prompted by two previous findings: 1) In individuals with no neurological impairments and those with a spinal cord injury, WP-NMES enhances electrically-evoked contractions, compared to more traditional NMES that uses narrower pulse widths and lower stimulus frequencies, due to an increased central or reflexive activation of motoneurons (Collins et al., 2002; Klakowicz et al., 2006); and 2) After a stroke, tonic vibration reflexes were larger in the paretic arm than the non-paretic arm (McPherson et al., 2008). Larger contractions evoked by WP-NMES in the present study may share a similar central mechanism with the tonic vibration reflexes and the sensory volleys evoked during the stimulation (vibration or electrical) may have resulted in greater reflexive recruitment of motoneurons in the paretic arm than the non-paretic arm. In the present study, contractions were larger in the paretic arm when 1 ms pulses were used, but not when 0.1 ms pulses were used. This effect of pulse width occurred despite adjusting the stimulation intensity so that a brief 100 Hz stimulus train delivered using both pulse widths evoked contractions of equal amplitude in both arms (i.e. a similar peripheral contribution via direct motor axon depolarization). The reflexive contribution to contractions during the brief 100 Hz stimulus trains should have been minimal because in individuals with no neurological impairments a reflexive contribution develops slowly (over seconds) when stimulation is applied over the muscle (Baldwin et al., 2006), as in the present study. There is some evidence that after stroke motor axons change and there is a reduction in the efficacy of inwardly rectifying channels (IH) in the paretic arm versus the non-paretic arm (Jankelowitz et al., 2007). A reduced IH current on motor axons in the paretic arm would mean the axons show less accommodation to hyperpolarizing currents and would be more difficult to activate repetitively. Since the stimulation intensity was adjusted to recruit a similar proportion of motor axons with the different pulse widths in both arms, changes to motor axons after stroke would, if anything, reduce contraction amplitude during NMES. Thus, we propose that the enhanced contractions in the paretic limb in the present study resulted from an increased recruitment of motoneurons centrally and were not due to a purely peripheral mechanism such as the non-linear torque generating capacity of muscle (Binder-Macleod and Clamann, 1989; Frigon et al., 2011)
If the effect of pulse width in the paretic limb is not due to a difference in activating motor axons, it may be due to a differential ability of the two pulse widths to depolarize sensory axons in individuals with chronic stroke. However, unlike motor axons, there is no evidence for differences in properties of sensory axons between the paretic and non-paretic arms (Jankelowitz et al., 2007). Accordingly, changing the pulse width should have a similar effect on the afferent volley for the paretic and non-paretic arms and cannot explain how increasing the pulse width enhanced contractions in the paretic arm only. Thus, the enhanced elbow flexion torque responses in the paretic arm are more likely to be of central origin, due to pre- and post-synaptic changes that occur in the spinal cord.
Pre-synaptic changes may enhance contractions evoked by WP-NMES after stroke
WP-NMES will activate large diameter afferents from muscle and cutaneous receptors and both of these may contribute to the central recruitment of motoneurons during the stimulation. After a stroke, changes occur in the spinal cord that are pre-synaptic to motoneurons and influence the regulation of afferent input to motoneurons. For example, post-activation depression (Crone and Nielsen, 1989; Hultborn et al., 1996) in reflex pathways controlling the paretic limb is reduced compared to the non-paretic limb (Aymard et al., 2000; Lamy et al., 2009; Masakado et al., 2005). There is also a decrease in presynaptic inhibition on Ia afferent terminals in humans after stroke (Artieda et al., 1991; Aymard et al., 2000; Kagamihara and Masakado, 2005; Lamy et al., 2009; Nakashima et al., 1989). Similar to reductions in post-activation depression, reduced presynaptic inhibition would also increase neurotransmitter release each time an action potential reaches the afferent terminal (Rudomin and Schmidt, 1999). The net effect of these changes is that more afferent input will reach motoneurons of the paretic arm versus the non-paretic arm for a given input to the spinal cord. Thus, a WP-NMES protocol designed to maximize afferent activation may contribute to the larger muscle contractions evoked in the paretic arm in the current study.
Post-synaptic changes may enhance contractions evoked by WP-NMES after stroke
In addition to changes that occur pre-synaptic to the motoneuron after a stroke, there may be post-synaptic changes that affect motoneuron excitability. After a stroke, the disruption of corticospinal input may lead to an increased influence on motoneuron excitability of bulbospinal projections that provide monoaminergic input to the spinal cord (Dewald et al., 1995; Dewald and Beer, 2001; Ellis et al., 2007; Kline et al., 2007; Zaaimi et al., 2009). Increased monoaminergic drive could increase subthreshold depolarization, reduce the action potential threshold, reduce the spike afterhyperpolarization, or augment PIC amplitude (Fedirchuk and Dai, 2004; Heckman et al., 2005; 2008; Heckman, 2003; Powers and Binder, 2001). Mottram and colleagues (2009) showed no difference in an indirect measure of PIC amplitude in paretic muscle and suggested that increases in the number of spontaneously active motor units often seen in the paretic limb may be due to a low-level tonic depolarizing synaptic drive either of cortical or segmental origin. If monoaminergic drive increases after a stroke, synaptic inputs may more readily activate motoneurons innervating paretic muscles; this would be consistent with the finding that tonic vibration reflexes in the biceps muscle were larger in the paretic arm compared to the non-paretic arm (McPherson et al., 2008). Regardless, both pre- and post-synaptic changes could contribute to the enhanced flexion torques in the paretic arm and the present study was not designed to distinguish between the two.
Clinical Significance
This work represents the first time WP-NMES has been used to generate contractions for individuals who have experienced a stroke and is a first step towards the potential application of WP-NMES for rehabilitation in this population. By using WP-NMES it may be possible to generate larger muscle contractions in the paretic arm for a given stimulus current. Electrically-evoked muscle contractions that involve a large central contribution from the recruitment of motoneurons in the spinal cord may also be more fatigue-resistant due to the physiological motor unit recruitment order followed with synaptic activation, in which small fatigue-resistant motor units are activated first. This type of recruitment is preferable to that which occurs during contractions that primarily involve the direct depolarization of motor axons underneath the stimulating electrodes and employs a random motor unit recruitment order. In the present study, since torque declined during the 12 s train of 100 Hz constant frequency stimulation, but was augmented after a shorter (4 s) burst of 100 Hz stimulation, including brief periods of high frequency stimulation may be the most effective way to augment muscle contractions in the paretic limb while limiting peripheral fatigue.
Future Directions
These experiments have shown that WP-NMES enhances contractions in the paretic arm after stroke, ostensibly due to an enhanced reflexive recruitment of motoneurons. Studies in which reflex responses and motor units are recorded from the paretic limb during WP-NMES may help verify the central contribution to the evoked contractions and may provide insights into the pre- and post-synaptic changes that occur in the spinal cord after stroke. An investigation to determine the optimal combination of stimulation pulse width, frequency and intensity for maximizing the central contribution to electrically-evoked contractions after stroke would help identify the best stimulation parameters for rehabilitation. It would also be interesting to characterize responses to WP-NMES in a variety of muscles, given that some muscles have stronger reflexive input (Eccles et al., 1957; Jusiet al., 1995; Palmieri, 2002; Pierrot-Deseilligny and Burke, 2005) and thus may generate contractions with a larger central contribution than others. Gaining a better understanding of the afferent origin of the central contribution (i.e. muscle vs. cutaneous afferents) may lead to improved methods for enhancing the evoked contractions. Experiments designed to test muscle fatigue and the recruitment characteristics of single motor units during WP-NMES in the paretic limb are needed to determine the extent to which WP-NMES reduces muscle fatigue after stroke. Ultimately, a study comparing training programs that use WP-NMES to programs that incorporate more traditional NMES, in conjunction with functional evaluations before and after the training programs, would answer the question of whether it would be beneficial to use WP-NMES for rehabilitation for people who have had a stroke.
Highlights.
Wide pulse width (1 ms) neuromuscular electrical stimulation at high frequencies (100 Hz) generates larger contractions in the paretic limb as compared to the non-.paretic limb in individuals with chronic hemiparetic stroke.
The larger contractions evoked during WP-NMES in the paretic limb were likely due to increased reflexive recruitment of motoneurons, as a result of increased reflex excitability on the paretic side.
NMES that elicits larger contractions in the paretic limb may allow for the development of more effective stroke rehabilitation paradigms and functional neural prostheses.
Acknowledgments
We would like to thank Carolina Carmona and Dan Krainak for their assistance with participant recruitment and data collection. We would also like to acknowledge Laura Miller for the advice she provided regarding statistical analyses, Jacob McPherson for his editorial input to the manuscript, and Alejandro Ley for his assistance with data analyses. This work was supported by the Natural Sciences and Engineering Research Council of Canada and a University of Alberta Research Abroad Grant awarded to David Collins and Joanna Clair, as well, by a National Institute of Health grant (R01HD39343) and a National Institute for Disability and Rehabilitation Research grant (H133G070089) awarded to Jules Dewald.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- Artieda J, Quesada P, Obeso JA. Reciprocal inhibition between forearm muscles in pastic hemiplegia. Neurology. 1991;41:286–89. doi: 10.1212/wnl.41.2_part_1.286. [DOI] [PubMed] [Google Scholar]
- Aymard C, Katz R, Lafitte C, Lo E, Penicaud A, Pradat-Diehl P, et al. Presynaptic inhibition and homosynaptic depression: a comparison between lower and upper limbs in normal human subjects and patients with hemiplegia. Brain. 2000;123:1688–1702. doi: 10.1093/brain/123.8.1688. [DOI] [PubMed] [Google Scholar]
- Baldwin ER, Klakowicz PM, Collins DF. Wide-pulse-width, high-frequency neuromuscular stimulation: implications for functional electrical stimulation. J Appl Physiol. 2006;101:228–40. doi: 10.1152/japplphysiol.00871.2005. [DOI] [PubMed] [Google Scholar]
- Bergquist AJ, Clair JM, Collins DF. Motor unit recruitment when neuromuscular electrical stimulation is applied over a nerve trunk compared with a muscle belly: triceps surae. J Appl Physiol. 2011;110:627–37. doi: 10.1152/japplphysiol.01103.2010. [DOI] [PubMed] [Google Scholar]
- Binder-Macleod SA, Clamann HP. Force output of cat motor units stimulated with trains of linearly varying frequency. J Neurophysiol. 1989;61:208–217. doi: 10.1152/jn.1989.61.1.208. [DOI] [PubMed] [Google Scholar]
- Chae J. Neuromuscular electrical stimulation for motor relearning in hemiparesis. Phys Med Rehabil Clin N Am. 2003;14:93–109. doi: 10.1016/s1047-9651(02)00051-7. [DOI] [PubMed] [Google Scholar]
- Clair JM, Lagerquist O, Collins DF. Changes in H-reflex amplitude during tetanic neuromuscular stimulation in human spinal cord injury. Society for Neuroscience Annual Meeting Abstract; 2006. [Google Scholar]
- Collins DF. Central contributions to contractions evoked by tetanic neuromuscular electrical stimulation. Exerc Sport Sci Rev. 2007;35:102–09. doi: 10.1097/jes.0b013e3180a0321b. [DOI] [PubMed] [Google Scholar]
- Collins DF, Burke D, Gandevia SC. Large involuntary forces consistent with plateau-like behavior of human motoneurons. J Neurosci. 2001;21:4059–65. doi: 10.1523/JNEUROSCI.21-11-04059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Burke D, Gandevia SC. Sustained contractions produced by plateau-like behaviour in human motoneurones. J Physiol. 2002;538:289–301. doi: 10.1113/jphysiol.2001.012825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Methodological implications of the post activation depression of the soleus H-reflex in man. Exp Brain Res. 1989;78:28–32. doi: 10.1007/BF00230683. [DOI] [PubMed] [Google Scholar]
- de Kroon JR, Ijzerman MJ, Chae J, Lankhorst GJ, Zilvold G. Relation between stimulation characteristics and clinical outcome in studies using electrical stimulation to improve motor control of the upper extremity in stroke. J Rehabil Med. 2005;37:65–74. doi: 10.1080/16501970410024190. [DOI] [PubMed] [Google Scholar]
- Dean JC, Yates LM, Collins DF. Turning on the central contribution to contractions evoked by neuromuscular electrical stimulation. J Appl Physiol. 2007;103:170–76. doi: 10.1152/japplphysiol.01361.2006. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjectswith hemiparesis. Muscle Nerve. 2001;24:273–83. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118:495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- Dietz V, Sinkjaer T. Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol. 2007;6:725–33. doi: 10.1016/S1474-4422(07)70193-X. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957;137:22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis MD, Acosta AM, Yao J, Dewald JP. Position-dependent torque coupling and associated muscle activation in the hemiparetic upper extremity. Exp Brain Res. 2007;176:594–602. doi: 10.1007/s00221-006-0637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedirchuk B, Dai Y. Monoamines increase the excitability of spinal neurones in the neonatal rat by hyperpolarizing the threshold for action potential production. J Physiol. 2004;557:355–61. doi: 10.1113/jphysiol.2004.064022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiereisen P, Duchateau J, Hainaut K. Motor unit recruitment order during voluntary and electrically induced contractions in the tibialis anterior. Exp Brain Res. 1997;114:117–23. doi: 10.1007/pl00005610. [DOI] [PubMed] [Google Scholar]
- Frigon A, Thompson CK, Johnson MD, Manuel M, Hornby TG, Heckman CJ. Extra forces evoked during electrical stimulation of the muscle or its nerve are generated and modulated by a length-dependent intrinsic property of muscle in humans and cats. J Neurosci. 2011;31:5579–88. doi: 10.1523/JNEUROSCI.6641-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance Scand. J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Gregory CM, Bickel CS. Recruitment patterns in human skeletal muscle during electrical stimulation. Phys Ther. 2005;85:358–64. [PubMed] [Google Scholar]
- Heckman CJ. Active conductances in motoneuron dendrites enhance movement capabilities. Exerc Sport Sci Rev. 2003;31:96–101. doi: 10.1097/00003677-200304000-00008. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistant inward currents in motoneuron dendrites: Implications for motor output. Muscle Nerve. 2005;31:135–56. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Johnson M, Mottram C, Schuster J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist. 2008;14:264–75. doi: 10.1177/1073858408314986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Excitability and inhibitibility of motoneurons of different sizes. J Neurophysiol. 1965;28:599–620. doi: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res. 1996;108:450–62. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- Jankelowitz SK, Howells J, Burke D. Plasticity of inwardly rectifying conductances following a corticospinal lesion in human subjects. J Physiol. 2007;581:927–40. doi: 10.1113/jphysiol.2006.123661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubeau M, Gondin J, Martin A, Sartorio A, Maffiuletti NA. Random motor unit activation by electrostimulation. Int J Sports Med. 2007;28:901–4. doi: 10.1055/s-2007-965075. [DOI] [PubMed] [Google Scholar]
- Jusić A, Baraba R, Bogunovi A. H-reflex and F-wave potentials in leg and arm muscles. Electromyogr Clin Neurophysiol. 1995;35:471–8. [PubMed] [Google Scholar]
- Kagamihara Y, Masakado Y. Excitability of spinal inhibitory circuits in patients with spasticity. J Clin Neurophysiol. 2005;22:136–47. doi: 10.1097/01.wnp.0000158948.00901.4e. [DOI] [PubMed] [Google Scholar]
- Keller T, Ellis MD, Dewald JP. Overcoming abnormal joint torque patterns in paretic upper extremities using triceps stimulation. Artif Organs. 2005;29:229–32. doi: 10.1111/j.1525-1594.2005.29041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T, Popovic MR, Pappas IP, Muller PY. Transcutaneous functional electrical stimulator “Compex Motion”. Artif Organs. 2002;26:219–23. doi: 10.1046/j.1525-1594.2002.06934.x. [DOI] [PubMed] [Google Scholar]
- Klakowicz PM, Baldwin ER, Collins DF. Contribution of m-waves and h-reflexes to contractions evoked by tetanic nerve stimulation in humans. J Neurophysiol. 2006;96:1293–1302. doi: 10.1152/jn.00765.2005. [DOI] [PubMed] [Google Scholar]
- Kline TL, Schmit BD, Kamper DG. Exaggerated interlimb neural coupling following stroke. Brain. 2007;130:159–69. doi: 10.1093/brain/awl278. [DOI] [PubMed] [Google Scholar]
- Lagerquist O, Collins DF. Influence of stimulus pulse width on M-waves, H-reflexes, and torque during tetanic low-intensity neuromuscular stimulation. Muscle Nerve. 2010;42:886–93. doi: 10.1002/mus.21762. [DOI] [PubMed] [Google Scholar]
- Lagerquist O, Walsh LD, Blouin JS, Collins DF, Gandevia SC. Effect of a peripheral nerve block on torque produced by repetitive electrical stimulation. J Appl Physiol. 2009;107:161–7. doi: 10.1152/japplphysiol.91635.2008. [DOI] [PubMed] [Google Scholar]
- Lamy JC, Wargon I, Mazevet D, Ghanim Z, Pradat-Diehl P, Katz R. Impaired efficacy of spinal presynaptic mechanisms in spastic stroke patients. Brain. 2009;132:734–48. doi: 10.1093/brain/awn310. [DOI] [PubMed] [Google Scholar]
- Masakado Y, Kagamihara Y, Takahashi O, Akaboshi K, Muraoka Y, Ushiba J. Post- activation depression of the soleus H-reflex in stroke patients. Electromyogr Clin Neurophysiol. 2005;45:115–22. [PubMed] [Google Scholar]
- McPherson JG, Ellis MD, Heckman C, Dewald JP. Evidence for increased activation of persistent inward currents in individuals with chronic hemiparetic stroke. J Neurophysiol. 2008;100:3236–42. doi: 10.1152/jn.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram CJ, Suresh NL, Heckman CJ, Gorassini MA, Rymer WZ. Origins of abnormal excitability in biceps brachii motoneurons of spastic-paretic stroke survivors. J Neurophysiol. 2009;102:2026–38. doi: 10.1152/jn.00151.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Rothwell JC, Day BL, Thompson PD, Shannon K, Marsden CD. Reciprocal inhibition between forearm muscles in patients with writer’s cramp and other occupational cramps, symptomatic hemidystonia and hemiparesis due to stroke. Brain. 1989;112:681–97. doi: 10.1093/brain/112.3.681. [DOI] [PubMed] [Google Scholar]
- Nickolls P, Collins DF, Gorman RB, Burke D, Gandevia SC. Forces consistent with plateau-like behaviour of spinal neurons evoked in patients with spinal cord injuries. Brain. 2004;127:660–70. doi: 10.1093/brain/awh073. [DOI] [PubMed] [Google Scholar]
- Palmieri RM. Intersession reliability for H-reflex measurements arising from the soleus, peroneal, and tibialis anterior musculature. Int J Neurosci. 2002;112:841–50. doi: 10.1080/00207450290025851. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. The circuitry of the human spinal cord: its role in motor control and movement disorders. Cambridge: Cambride University Press; 2005. [Google Scholar]
- Popovic DB, Sinkjaer T, Popovic MB. Electrical stimulation as a means for achieving recovery of function in stroke patients. NeuroRehabilitation. 2009;25:45–58. doi: 10.3233/NRE-2009-0498. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol. 2001;143:137–263. doi: 10.1007/BFb0115594. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Sinkjaer T, Magnussen I. Passive, intrinsic and reflex-mediated stiffness in the ankle extensors of hemiparetic patients. Brain. 1994;117:355–63. doi: 10.1093/brain/117.2.355. [DOI] [PubMed] [Google Scholar]
- Stein RB, Chong S, Everaert DG, Rolf R, Thompson AK, Whittaker M, et al. A multicenter trial of a footdrop stimulator controlled by a tilt sensor. Neurorehabil Neural Repair. 2006;20:371–9. doi: 10.1177/1545968306289292. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Lewek MD, Jayaraman A, Hornby TG. Central excitability contributes to supramaximal volitional contractions in human incomplete spinal cord injury. J Physiol. 2011;589:3739–52. doi: 10.1113/jphysiol.2011.212233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaaimi B, Edgley SA, Baker SN. Reticulospinal and ipsilateral corticospinal tract contributions to functional recovery after unilateral corticospinal lesion. Society for Neuroscience Annual Meeting Abstract; 2009. [Google Scholar]