Abstract
The polyether ionophoric antibiotics including monensin, salinomycin, and narasin, are widely used in veterinary medicine and as food additives and growth promoters in animal husbandry including poultry farming. Their effects on human health, however, are not fully understood. Recent studies showed that salinomycin is a cancer stem cell inhibitor. Since poultry consumption has risen sharply in the last three decades, we asked whether the consumption of meat tainted with growth promoting antibiotics might have effects on adipose cells. We showed in this report that the ionophoric antibiotics inhibit the differentiation of preadipocytes into adipocytes. The block of differentiation is not due to the induction of apoptosis nor the inhibition of cell proliferation. In addition, salinomycin also suppresses the transcriptional activity of the CCAAT/enhancer binding proteins and the peroxisome proliferator-activated receptor γ. These results suggest that the ionophoric antibiotics can be exploited as novel anti-obesity therapeutics and as pharmacological probes for the study of adipose biology. Further, the pharmacological effects of salinomycin could be a harbinger of its toxicity on the adipose tissue and other susceptible target cells in cancer therapy.
Keywords: Adipogenesis, salinomycin, polyether antibiotic, ionophoric antibiotic, obesity
Introduction
Antimicrobials are used extensively in livestock farming as growth promoter and to improve health and welfare of animals by controlling coccidiosis and decreasing the shedding of zoonotic pathogens [1]. The ionophoric antibiotics including monensin, narasin, and salinomycin, which are not used in human therapy and thus not considered medically important, are therefore, widely used as growth promoters in animal husbandry. The widespread use of these antibiotics at subtherapeutic levels in feed and water has led to the emergence of antibiotic-resistant pathogenic strains of bacteria, which is a major public health concern. The potential human health impacts associated with the use of these antimicrobials in animal feeds have not been fully examined. It is also interesting that salinomycin has recently been shown to be a specific inhibitor of cancer stem cells [2], and is undergoing clinical development for cancer therapy [3].
We recently analyzed the Food Availability data from the USDA Economic Research Service in relation to the rising obesity trends and observed that while the consumption of red meat (beef, veal, lamb, and pork) has declined within the same period, poultry consumption in the United States has close to doubled [4]. In light of the use of ionophoric antibiotics in poultry and other animals farming, this raises the question about the potential impact of consuming meat tainted with growth-promoting antibiotics on human health, thus prompting us to ask whether these antibiotics may have biological effects on the adipose tissue.
Adipose tissue is considered as a major endocrine organ that produces a diverse cadre of adipokines including leptin and adiponectin, which are involved in the modulation of energy metabolism, inflammatory response and the immune system [5]. Therefore, disruption of the homeostasis or adipogenesis of the adipose tissue is expected to have significant consequences on the function of this endocrine system. Obesity is characterized by an increase in adipose mass and associated with increased energy intake [6] that ultimately triggers inflammation in the adipose tissue [7].
In this report, using a cultured preadipocyte adipogenesis assay, we examined the effects of the ionophoric antibiotics on the differentiation of the 3T3-L1 (L1) [8] and the OP9 mouse stromal cells [9]. Our results showed that the ionophoric polyether antibiotics are inhibitors of preadipocyte differentiation into adipocyte, with salinomycin as the most potent inhibitor. The block of adipogenesis is not due to the induction of apoptosis nor the inhibition of cell proliferation. Further analysis showed that salinomycin also inhibited the transactivation potential of the CCAAT/enhancer binding proteins (C/EBPs) and the peroxisome proliferator-activated receptor γ (PPARγ), which are master transcriptional regulators of adipogenesis [10]. Our findings, therefore, suggest the potential development of the ionophoric antibiotics or small molecule derivatives possessing similar anti-adipogenic activity as novel therapeutics for combating obesity. In addition, the biological effect of salinomycin on adipogenesis further suggests potential toxicity associated with its use in cancer therapy.
Materials and methods
Cell culture, chemicals, adipogenesis, apoptosis, and reporter assays
The bone marrow-derived mouse stromal OP9 cells [9] (a gift of Dr. Perry Bickel, University of Texas Health Science Centre, Houston) were cultured at 37°C with 10% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen) supplemented with 10% (v/v) fetal calf serum (Invitrogen), 1 mM sodium pyruvate, 0.1 mM non-essential amino acids, 2 mM L-glutamine, 100 μg ml−1 streptomycin sulfate, and 100 U ml−1 penicillin. 3T3-L1 (L1) cells were obtained from the American Type Culture Collection, and cultured as described [8]. Ionophoric antibiotics were obtained from Sigma and dissolved according to manufacturer’s recommendation; briefly, narasin and salinomycin were solubilized in DMSO and monensin in methanol.
Cytotoxicity of the polyether antibiotics in OP9 and L1 cells was measured by the conversion of tetrazolium MTT salt into a formazan product [11], and cell growth was measured by daily counting using the Beckman Coulter Counter Z1 as previously described [12]. Apoptosis was monitored as described [13] by the Guava Nexin assay on the Guava cytometer according to manufacturer’s specification (EMD Millipore, Billerica, MA). The assay measures membrane changes associated with apoptosis using Annexin V-PE and 7-aminoactinomycin D to identify dead cells. OP9 cells were induced to differentiate for two days either with or without salinomycin and then replenished in propagation media either with or without salinomycin for two additional days. Cells were then harvested and diluted to approximately 500,000 cells/ml and the Guava Nexin reagent was added and incubated on ice for 20 min prior to analysis on the Guava PCA system. Results were quantified as the percentage of gated cells positive for Annexin V staining.
Transfection was performed using the LTX transfection reagent (Invitrogen) with 25 ng of reporter plasmids mixed with 10 ng of expression constructs as indicated in the experiment. Equal amounts of DNA were used for all transfection by adding the appropriate amount of salmon sperm DNA and the Renilla luciferase reporter as internal control. Relative luciferase activities were determined 24 h following transfection and normalized to the Renilla luciferase activity. Briefly, OP9 cells were grown to confluence and then further cultured for two additional days, and cells were then induced to differentiate in a serum replacement medium composed of MEM-α with 15% KnockOut SR (Invitrogen, Carlsbad, CA) supplemented either with or without salinomycin for 2 days and then replenished in the propagation medium, also supplemented either with or without the drug for two additional days followed by Nile red lipid staining and visualization by fluorescence microscopy. L1 cells differentiation was performed as described [8], and cells were further cultured with or without the polyether antibiotics for an additional 6–7 days followed by Nile red staining and visualization as described above.
Reporter constructs
C/EBP and PPARγ response-elements reporter plasmids were constructed by ligating oligonucleotides containing either three C/EBP- or PPARγ-response elements in tandem into the pGL3-Luc reporter expression vector and sequence-verified. The mouse PPARγ2 gene promoter was amplified as previously described [14] using primers (5′-CTAGCTAGCGCTCCCACGTTAGCAGTTTGGCAC-3′ and 5′-CCCAAGCTTCTTGCAGCAACATCAGGAAGGAC-3′) with Nhe I and Hind III sites and then subcloned into the pGL3-basic vector (Promega) and sequence verified. Activity of MITF on a 280 bp fragment of the tyrosinase gene promoter luciferase reporter, encompassing 200 bp upstream of the transcription start site and 80 bp downstream [15], was used as control to assess the specificity of salinomycin.
Statistical analyses
Commercially available software package (PRISM, GraphPad Software, Inc., La Jolla, CA) or the data analysis add-in for Microsoft Excel was used for performing statistical analysis. Either single factor ANOVA or Student’s t-tests were used for all analysis. P values of less than or equal to 0.05 were considered statistically significant.
Results
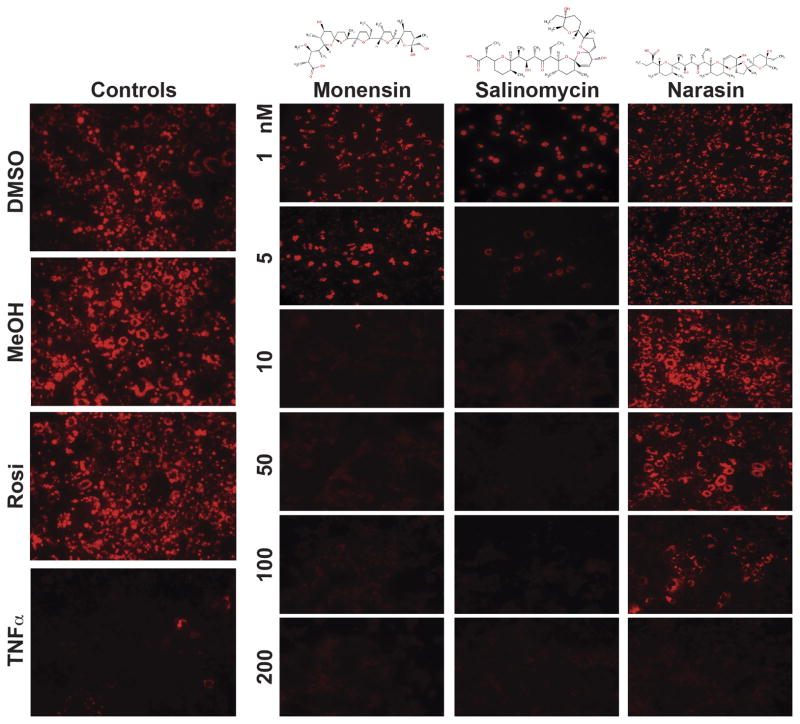
The coccidiostatic ionophoric antibiotics are a group of structurally similar polyether alkali monovalent cationic transporters with monensin exhibiting selectivity for Na+, and salinomycin and narasin (4-methylsalinomycin) showing preference for K+ over Na+ (Fig. 1) [16]. Since they are used as growth promoter in animal husbandry [1], we assessed the biological activity of these antibiotics on adipogenesis using the OP9 stromal cells and the L1 preadipocytes, which can be induced to differentiate into adipocytes in defined culture media [8,9]. OP9 stromal cells and L1 preadipocytes were differentiated into adipocytes either in the absence or the presence of the antibiotics, monitored by Nile Red staining of the differentiated cells [13,17]. Our results showed that salinomycin, monensin, and narasin inhibited the differentiation of OP9 cells in a dose-dependent manner, with salinomycin showing the higher potency than monensin and narasin (Fig. 1). TNFα, which inhibits the differentiation of preadipocytes into mature adipocytes, [18] served as control (Fig. 1). Greater than 90% inhibition of OP9 cells differentiation was observed with 10 nM of either salinomycin or monensin (Fig. 1). In contrast, narasin, a derivative of salinomycin that has an additional methyl group (Fig. 1), showed markedly reduced potency in inhibiting OP9 cells differentiation, with observable inhibition of adipogenesis at 100 nM of the drug (Fig. 1). A similar pharmacological profile of inhibition was observed in L1 preadipocyte differentiation, with salinomycin as the most potent inhibitor compared to monensin and narasin (Supplementary Fig. 1).
Fig. 1.
Polyether antibiotics inhibit adipogenesis. Dose-dependent (1, 5, 10, 50, 100, and 200 nM) inhibition of OP9 stromal cells differentiation into adipocytes by monensin, salinomycin and narasin, compared to TNFα, a cytokine and potent inhibitor of adipogenesis, as well as rosiglitazone (Rosi) an inducer of preadipocyte differentiation into adipocyte. Differentiated adipocytes were stained with Nile Red. Vehicle treated OP9 cells including dimethylsulfoxide (DMSO) and methanol (MeOH) served as controls. Representative micrographs of one of the experiments, which were performed for at least three or more times, are shown.
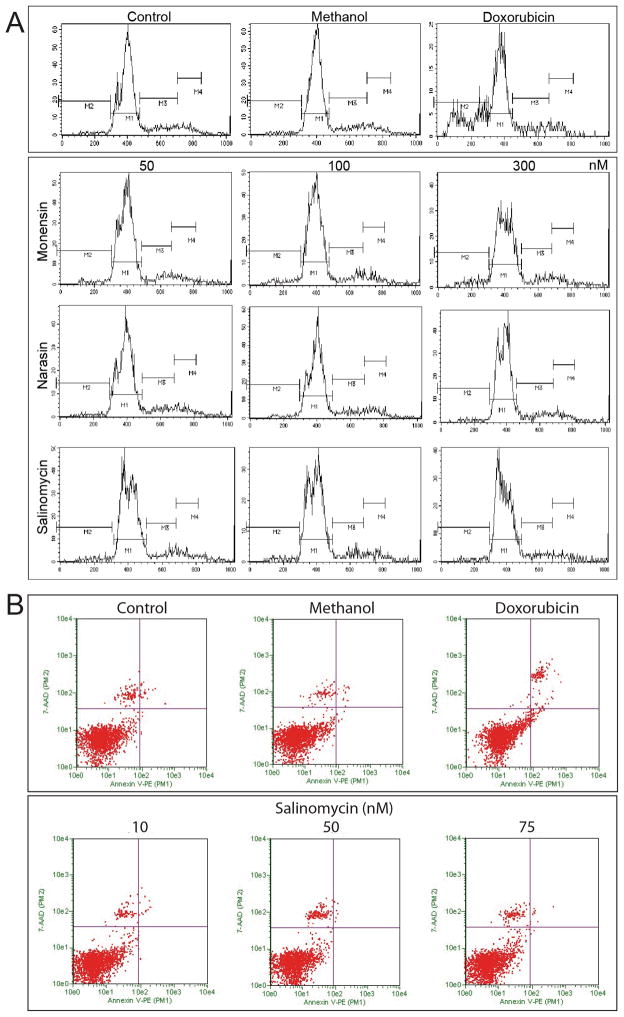
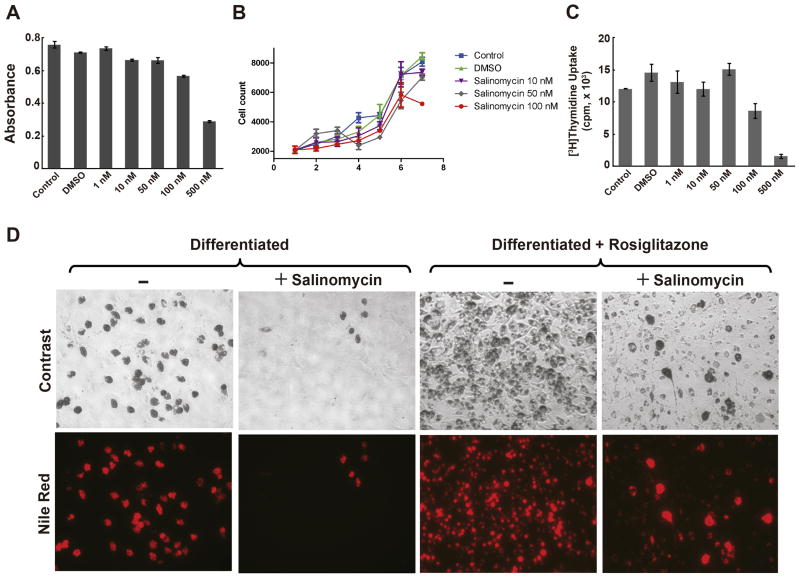
Salinomycin has been shown to induce apoptosis in cancer stem cells [2,3]. It is noteworthy that inhibition of OP9 cell differentiation by either monensin, narasin, or salinomycin, at 10, 50, and 75 nM of drug, was not a result of drug induced apoptosis as indicated by the relative lack of sub-G0/G1 degraded DNA on the histograms of flow cytometric analysis. Compared to the positive control, OP9 cells treated with doxorubicin, a cytotoxic chemotherapeutic agent that causes cell death by apoptosis (Fig. 2B, Supplementary Fig. 2), showed significant accumulation of sub-G0/G1 particles in the histogram (Fig. 2A). The lack of apoptosis induced by salinomycin was confirmed by the lack of annexin V binding to phosphatidylserine, membrane changes that is associated with cell death [19]. These results were further supported by the negligible growth inhibition of OP9 cells assessed by the MTT (Fig. 3A) and the cell count assays (Fig. 3B), as well as the lack of inhibition of [3H]thymidine uptake by salinomycin at doses equal to or below 50 nM (Fig. 3C). Although at concentrations higher than 100 nM, salinomycin was growth inhibitory and cytotoxic, however, we did not apply these concentrations in the adipogenesis assay. A similar lack of cytotoxic effect was also found in L1 cells treated with salinomycin (Supplementary Fig. 3). In contrast, doxorubicin caused a dose-dependent cytotoxic killing in L1 cells (Supplementary Fig. 4), thus suggesting that salinomycin inhibits adipogenesis without the induction of apoptosis in these cells. Since salinomycin is the most potent inhibitor, it is used as the prototype for all subsequent studies.
Fig. 2.
Inhibition of adipogenesis by polyether antibiotics is not associated with apoptosis. A, Flow cytometric analysis by propidium iodide staining of OP9 cells undergoing differentiation into adipocytes in the presence of the polyether antibiotics, at 10, 50, and 75 nM each, compared to untreated differentiated adipocytes. Doxorubicin, a cytotoxic anticancer drug that induces apoptosis, indicated by the presence of sub-G0/G1 particles, was included as control. In contrast to doxorubicin, no significant apoptosis was observed following treatment with monensin, salinomycin, or narasin, compared to differentiated and vehicle-treated controls. B, Lack of apoptosis induced by salinomycin in OP9 cells was confirmed by the Guava Nexin assay. Doxorubicin induced-apoptosis was measured by annexin V binding to cell surface phosphatidylserine, in contrast to the lack of apoptosis following salinomycin treatment.
Fig. 3.
A, Cytotoxicity of salinomycin. OP9 cells were treated with various concentrations (1, 10, 50, 100, 500 nM) of salinomycin and the percentage of cells surviving salinomycin treatment was determined by the ability of live cells to convert the tetrazolium MTT salt into a formazan product measured spectrophotometrically. B, Effects of salinomycin on cell growth. Proliferation of OP9 cells in the presence of 10, 50 or 100 nM salinomycin was measured by daily counting. C, OP9 cells were differentiated for 12 hrs followed by [3H]thymidine uptake to determine whether postconfluent mitosis and clonal expansion were inhibited by salinomycin. Experiments shown are representatives repeated in triplicate for at least 3 times. Statistics were conducted as t-test; asterisk, P<0.05 versus untreated and vehicle-treated controls. Error bars indicate s.e.m. (n=3). D, Salinomycin attenuates rosiglitazone induced OP9 cells differentiation into adipocytes. Rosiglitazone-induced OP9 cells differentiation either in the absence or the presence of salinomycin was compared to normally differentiated controls either in the absence or the presence of salinomycin. All experiments were performed for at least three or more times and representative micrographs from one of the experiments are shown.
Rosiglitazone, a member of the thiazolidinedione class of anti-diabetic drugs, is an activator of PPARγ and induces adipogenesis [20]. Consistent with the above results, salinomycin inhibited OP9 cells differentiation into adipocytes compared to control (Fig. 3D). Salinomycin, however, also markedly attenuated rosiglitazone-induced adipogenesis. These results suggest that salinomycin blocks the transcriptional regulation of adipogenesis mediated by rosiglitazone-induced PPARγ activity.
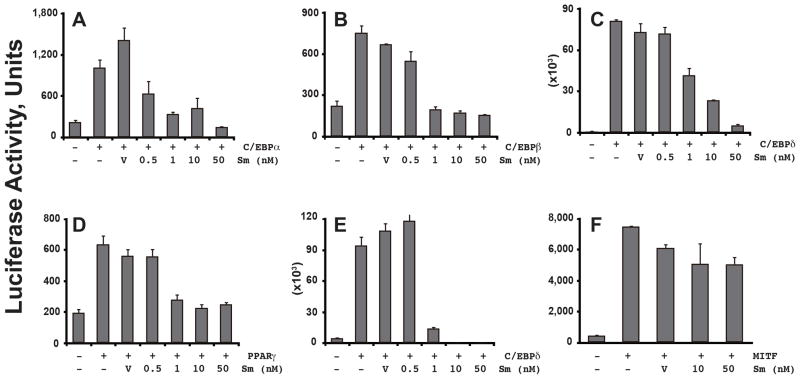
The differentiation of preadipocytes into adipocytes involves the activation of a cascade of transcription factors that trigger the downstream expression of a large number of adipogenic genes responsible for maintaining the adipocytic phenotype [10]. The transcription factors C/EBPα, β, and δ, and PPARγ are considered the master regulators of adipogenesis [10]. Hence, we asked whether salinomycin might pharmacologically repress their transactivation potential using promoter reporter assays driven by the PPARγ- and the C/EBP-response elements, respectively. Our results showed that salinomycin inhibited C/EBPα- (Fig. 4A), β- (Fig. 4B), and δ- (Fig. 4C), and PPARγ- (Fig. 4D) mediated transactivation from their respective response element driven reporters. In addition, salinomycin also inhibited C/EBPδ-induced transactivation from the PPARγ2 native gene promoter (Fig. 4E). In contrast, salinomycin failed to inhibit the melanogenesis master regulator microphthalmia-associated transcription factor (MITF)-activated tyrosinase gene promoter reporter (Fig. 4F), thus suggesting a specific inhibition of the adipogenesis pathway by salinomycin.
Fig. 4.
Salinomycin inhibits transcriptional regulation of adipogenesis. Promoter reporter assay by transfection was conducted in OP9 cells to determine the effects of salinomycin on the transactivation potential of C/EBPα (A), β (B), and δ(C), PPARγ (D), and MITF (F). Effect of salinomycin on C/EBPδ was further determined using the native PPARγ2 gene promoter (E), which contained a C/EBP response element. Statistics were conducted as t-test; asterisk, P<0.05 versus untreated and vehicle-treated controls that were either transfected with C/EBPs or PPARγ. Error bars indicate s.e.m. (n=3).
Discussion
The widespread use of ionophoric antibiotics in animal husbandry and the concerns for such practice on the rising obesity trends prompted us to investigate the biological effects of these antimicrobials on adipogenesis. Our results in this study showed unexpectedly that the ionophoric polyether antibiotics including salinomycin and monensin are potent inhibitors of adipogenesis (Fig. 1). The block of differentiation is not due to the induction of apoptosis nor the inhibition of cell proliferation, as salinomycin is neither cytotoxic nor growth inhibitory at the concentrations applied in the adipogenesis assay (Figs. 2 and 3). Salinomycin, which was shown recently to be a selective and potent cytotoxic agent against mammary and other cancer stem cells [2,3], also inhibits the transactivation potential of C/EBPs and PPARγ, master transcriptional regulators of adipogenesis. Our findings here are novel, showing that these agents are potent inhibitors of preadipocytes differentiation into adipocytes via their repression of the transcriptional activity of C/EBPα, β, and δ, and PPARγ. These observations suggest the potential development of polyether compounds devoid of antimicrobial activity as inhibitors of the transcriptional activation of adipogenesis for the treatment of obesity. In addition, the current development of salinomycin as a novel anticancer stem cell therapeutic should take into consideration the potential toxicity arising from the effects of salinomycin on preadipocyte and other potential target cells.
The notion of targeting adipose tissues for obesity treatment has been increasingly investigated [21]. The adipose tissue is an important endocrine organ with indispensible metabolic regulatory function [22]. It has been found that adipocyte number is higher in obese individuals during adipose tissue expansion compared to lean individuals [23]. Moreover, fat mass in obese subjects is determined by both fat cell number and fat cell size (or volume). Therefore, targeting the emergence of new fat cells in adipogenesis during the normal process of adipocyte turnover could potentially lead to a normalization or restoration of the adipocyte number in obese individuals to levels comparable to those in lean individuals. Whether this is a prudent strategy remains to be determined.
The development of salinomycin or its congeners for cancer treatment is an exciting possibility. However, in light of our findings, the potential toxicity of these agents on the adipose tissue needs to be further explored. Although polyether ionophores have been used in animal husbandry as coccidiostat for about three decades, their effects on human health resulting from the consumption of meat containing residual levels of these compounds and the chronic low-level exposure to these drugs are unknown. It has been reported that accidental ingestion of these polyether antibiotics [24,25,26] and also occupational exposure to high doses of these drugs (>1 g/kg) [27] caused death and severe physiological toxicities in human. Residual levels of salinomycin at approximately 20 μg/kg have been found in some poultry eggs [28] and approximately 2 to 200 μg/kg in various edible tissues in chicken [29]. To date, no overt toxicity has been reported in human from the consumption of poultry meat and products containing residual levels of these polyether antibiotics. Clearly, the residual antibiotic levels found in poultry meat and eggs, which are either close to or higher than the concentrations used in our studies, inhibit adipogenesis. Therefore, the long-term effects of these antibiotics on human health and other biological processes need to be rigorously examined.
The mechanisms of action of salinomycin are complex. Salinomycin induces necrosis and apoptosis in tumor in mice [2]. In cultured cancer cells, salinomycin also induces apoptosis when applied at concentrations up to 100-folds higher than those used in our study [30]. We posit that inhibition of adipogenesis by salinomycin is not likely a result of its cytotoxicity, which is supported by the lack of annexin V positive cells in salinomycin-treated OP9 and L1 cells, as well as negligible cytotoxicity and growth inhibition in these cells following exposure to the lower concentrations of salinomycin (Fig. 2).
Salinomycin has been shown to inhibit mitochondrial oxidative phosphorylation, causing the release of K+ from mitochondria [16]. It is conceivable that either Na+ or K+ may be required for the action of these ionophoric antibiotics. We consider this unlikely because both monensin and salinomycin inhibit preadipocyte differentiation into adipocyte, thus suggesting that the mechanism of inhibition of adipogenesis is probably independent of the presence of a specific alkali cation. The Na, K-ATPase α2 subunit gene (Atp1a2) is responsible for the maintenance of Na+ and K+ gradient across the cell membrane [31]. Homologous recombination knockout of Atp1a2 showed no difference in the capacity of adipocyte differentiation among the wild-type, heterozygous, and homozygous embryonic fibroblasts [32], thus suggesting that Atp1a2 is not required for the inhibition of adipogenesis by these antimicrobials.
The Wnt/β-catenin signaling pathway plays a role in maintaining preadipocytes in an undifferentiated state through its inhibition of the transcriptional activity of C/EBPα and PPARγ [33]. Salinomycin was recently shown to inhibit the Wnt/β-catenin signaling pathway in cancer cells [34]. This finding excludes Wnt/β-catenin as a molecular target for the action of salinomycin on adipogenesis because inhibition of Wnt/β-catenin signaling promotes adipogenesis, which is counterintuitive to our observations.
We also found that narasin is less potent than monensin and salinomycin in inhibiting OP9 cells and L1 preadipocytes differentiation. Narasin (4-methylsalinomycin) is a derivative of salinomycin with an additional methyl group on the terminal tetrahydropyran moiety. We speculate that the tetrahydropyran moiety tethering to the monocarboxylic acid may be a pharmacophore that is essential for the anti-adipogenic activity of these antibiotics. Whether the anti-adipogenic potential and the anti-cancer stem cell biological activity are overlapping remains to be determined.
In summary, our results demonstrated the unexpected anti-adipogenic activity of the ionophoric antibiotics on the differentiation of adipocytes. These compounds also inhibit the transcriptional activity of the master regulators of adipogenesis, thus suggesting that they may be potentially developed as novel anti-obesity therapeutics and also as pharmacological tools for probing the biology of adipose tissue. Paradoxically, these pharmacological effects of salinomycin could be a harbinger of its toxicity profile in cancer therapy.
Supplementary Material
Salinomycin inhibits preadipocyte differentiation into adipocytes
Salinomycin inhibits transcriptional regulation of adipogenesis
Pharmacological effects of salinomycin suggest toxicity in cancer therapy
Acknowledgments
This research is supported in part by a High Impact Research Grant UM.C/625/1/HIR/003/2 from the University of Malaya.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sapkota AR, Lefferts LY, McKenzie S, Walker P. What do we feed to food-production animals? A review of animal feed ingredients and their potential impacts on human health. Environ Health Perspect. 2007;115:663–670. doi: 10.1289/ehp.9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huczynski A. Salinomycin: a new cancer drug candidate. Chem Biol Drug Des. 2012;79:235–238. doi: 10.1111/j.1747-0285.2011.01287.x. [DOI] [PubMed] [Google Scholar]
- 4.Shao Q, Chin KV. Survey of American food trends and the growing obesity epidemic. Nutr Res Pract. 2011;5:253–259. doi: 10.4162/nrp.2011.5.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2009 doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr. 2009;90:1453–1456. doi: 10.3945/ajcn.2009.28595. [DOI] [PubMed] [Google Scholar]
- 7.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 314:1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- 9.Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Park C, Choi K, Bickel PE. OP9 mouse stromal cells rapidly differentiate into adipocytes: characterization of a useful new model of adipogenesis. J Lipid Res. 2006;47:450–460. doi: 10.1194/jlr.D500037-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Lefterova MI, Lazar MA. New developments in adipogenesis. Trends Endocrinol Metab. 2009;20:107–114. doi: 10.1016/j.tem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 12.Cvijic ME, Chin KV. Characterization of a cAMP-dependent protein kinase mutant resistant to cisplatin. Int J Cancer. 1997;72:345–350. doi: 10.1002/(sici)1097-0215(19970717)72:2<345::aid-ijc24>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 13.Kablan A, Saunders RA, Szkudlarek-Mikho M, Chin AJ, Bosio RM, Fujii K, Shapiro J, Chin KV. Prieurianin Causes Weight Loss in Diet-Induced Obese Mice and Inhibits Adipogenesis in Cultured Preadipocytes. J Diabetes Metab. 2010;1 doi: 10.4172/2155-6156.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y, Qi C, Korenberg JR, Chen XN, Noya D, Rao MS, Reddy JK. Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPAR gamma) gene: alternative promoter use and different splicing yield two mPPAR gamma isoforms. Proc Natl Acad Sci U S A. 1995;92:7921–7925. doi: 10.1073/pnas.92.17.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schepsky A, Bruser K, Gunnarsson GJ, Goodall J, Hallsson JH, Goding CR, Steingrimsson E, Hecht A. The microphthalmia-associated transcription factor Mitf interacts with beta-catenin to determine target gene expression. Mol Cell Biol. 2006;26:8914–8927. doi: 10.1128/MCB.02299-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riddell FG. Structure, conformation, and mechanism in the membrane transport of alkali metal ions by ionophoric antibiotics. Chirality. 2002;14:121–125. doi: 10.1002/chir.10052. [DOI] [PubMed] [Google Scholar]
- 17.Fowler SD, Greenspan P. Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections: comparison with oil red O. J Histochem Cytochem. 1985;33:833–836. doi: 10.1177/33.8.4020099. [DOI] [PubMed] [Google Scholar]
- 18.Simons PJ, van den Pangaart PS, van Roomen CP, Aerts JM, Boon L. Cytokine-mediated modulation of leptin and adiponectin secretion during in vitro adipogenesis: evidence that tumor necrosis factor-alpha- and interleukin-1beta-treated human preadipocytes are potent leptin producers. Cytokine. 2005;32:94–103. doi: 10.1016/j.cyto.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 20.Sharma AM, Staels B. Review: Peroxisome proliferator-activated receptor gamma and adipose tissue--understanding obesity-related changes in regulation of lipid and glucose metabolism. J Clin Endocrinol Metab. 2007;92:386–395. doi: 10.1210/jc.2006-1268. [DOI] [PubMed] [Google Scholar]
- 21.Marcelin G, Chua S., Jr Contributions of adipocyte lipid metabolism to body fat content and implications for the treatment of obesity. Curr Opin Pharmacol. 2010;10:588–593. doi: 10.1016/j.coph.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J, Arner P. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 24.Caldeira C, Neves WS, Cury PM, Serrano P, Baptista MA, Burdmann EA. Rhabdomyolysis, acute renal failure, and death after monensin ingestion. Am J Kidney Dis. 2001;38:1108–1112. doi: 10.1053/ajkd.2001.28618. [DOI] [PubMed] [Google Scholar]
- 25.Kouyoumdjian JA, Morita MP, Sato AK, Pissolatti AF. Fatal rhabdomyolysis after acute sodium monensin (Rumensin) toxicity: case report. Arq Neuropsiquiatr. 2001;59:596–598. doi: 10.1590/s0004-282x2001000400022. [DOI] [PubMed] [Google Scholar]
- 26.Sharma N, Bhalla A, Varma S, Jain S, Singh S. Toxicity of maduramicin. Emerg Med J. 2005;22:880–882. doi: 10.1136/emj.2004.020883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Story P, Doube A. A case of human poisoning by salinomycin, an agricultural antibiotic. N Z Med J. 2004;117:U799. [PubMed] [Google Scholar]
- 28.Mortier L, Huet AC, Charlier C, Daeseleire E, Delahaut P, Van Peteghem C. Incidence of residues of nine anticoccidials in eggs. Food Addit Contam. 2005;22:1120–1125. doi: 10.1080/02652030500199355. [DOI] [PubMed] [Google Scholar]
- 29.Henri J, Maurice R, Postollec G, Dubreil-Cheneau E, Roudaut B, Laurentie M, Sanders P. Comparison of the oral bioavailability and tissue disposition of monensin and salinomycin in chickens and turkeys. J Vet Pharmacol Ther. 2012;35:73–81. doi: 10.1111/j.1365-2885.2011.01285.x. [DOI] [PubMed] [Google Scholar]
- 30.Fuchs D, Heinold A, Opelz G, Daniel V, Naujokat C. Salinomycin induces apoptosis and overcomes apoptosis resistance in human cancer cells. Biochem Biophys Res Commun. 2009;390:743–749. doi: 10.1016/j.bbrc.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 31.Jaitovich AA, Bertorello AM. Na+, K+-ATPase: an indispensable ion pumping-signaling mechanism across mammalian cell membranes. Semin Nephrol. 2006;26:386–392. doi: 10.1016/j.semnephrol.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Kawakami K, Onaka T, Iwase M, Homma I, Ikeda K. Hyperphagia and obesity in Na, K-ATPase alpha2 subunit-defective mice. Obes Res. 2005;13:1661–1671. doi: 10.1038/oby.2005.204. [DOI] [PubMed] [Google Scholar]
- 33.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 34.Lu D, Choi MY, Yu J, Castro JE, Kipps TJ, Carson DA. Salinomycin inhibits Wnt signaling and selectively induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl Acad Sci U S A. 2011;108:13253–13257. doi: 10.1073/pnas.1110431108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.