Summary
Mycobacterium tuberculosis persists within macrophages in an arrested phagosome and depends upon necrosis to elude immunity and disseminate. Although apoptosis of M. tuberculosis-infected macrophages is associated with reduced bacterial growth, the bacteria are relatively resistant to death mechanisms, leaving the mechanisms underlying this observation unresolved. We find that following apoptosis, M. tuberculosis-infected macrophages are rapidly taken up by uninfected macrophages through efferocytosis, a dedicated apoptotic cell engulfment process. Efferocytosis of M. tuberculosis sequestered within an apoptotic macrophage further compartmentalizes the bacterium and delivers it along with the apoptotic cell debris to the lysosomal compartment. M. tuberculosis is killed only after efferocytosis, indicating that apoptosis itself is not intrinsically bactericidal but requires subsequent phagocytic uptake and lysosomal fusion of the apoptotic body harboring the bacterium. While efferocytosis is recognized as a constitutive housekeeping function of macrophages, these data indicate that it can also function as an antimicrobial effector mechanism.
Cell death frequently occurs following intracellular infection and many pathogens target cell death pathways as a virulence strategy (Faherty and Maurelli, 2008). Following infection of macrophages by Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis, the bacilli persist within arrested, immature phagosomes. A strategy for Mtb virulence is the induction of necrosis, which enables the bacterium to evade host defenses, disperse and infect surrounding macrophages (Keane et al., 2000). In contrast, attenuated Mtb strains and mutants lacking key virulence factors induce far less necrosis and instead stimulate apoptosis (Chen et al., 2006; Miller et al., 2010). Apoptosis of Mtb-infected macrophages is associated with reduced bacterial growth (Molloy et al., 1994; Oddo et al., 1998). While it is assumed that apoptosis is intrinsically bactericidal, there is little evidence to support this assertion. In fact, as Mtb emerges unscathed following necrotic cell death it seems unlikely that apoptosis could harm the bacterium.
As cells undergo apoptosis they emit ‘find me’ signals such as ATP to attract circulating phagocytes (Elliott et al., 2009; Hume, 2008; Kono and Rock, 2008). Additionally, the dying cells expose ‘eat me’ signals on their surface, such as phosphatidylserine (PS). Responding phagocytes, predominantly macrophages, employ a variety of surface receptors to bind these ‘eat me’ signals, and mediate the recognition, tethering, and engulfment of apoptotic cells (Erwig and Henson, 2008; Zhou and Yu, 2008). This process of apoptotic cell engulfment, called efferocytosis, is distinct from phagocytosis and is crucial for organogenesis and the resolution of inflammation (Kinchen and Ravichandran, 2010). If apoptotic cells are not cleared by efferocytosis, they undergo secondary necrosis in which inflammatory cellular contents leak into the extracelluar space, contributing to autoimmunity and persistent inflammation (Fernandez-Boyanapalli et al., 2010; Peng and Elkon, 2011). Despite the substantive cell death that accompanies infection, the role of efferocytosis during infection, particularly with respect to efferocytosis of infected cells, has not been thoroughly investigated. Based on the known association between macrophage apoptosis and host resistance to Mtb, we hypothesized that the antibacterial role of apoptosis is mediated through efferocytosis.
We report here that efferocytosis of Mtb-infected macrophages occurs in vitro and in vivo. Apoptosis itself is not intrinsically bactericidal; instead, efferocytosis is required to mediate control of the bacterium following apoptosis. We find that the efferocytic phagosome containing Mtb readily fuses with lysosomes and this leads to bacterial killing both in vitro and in vivo. We speculate that engulfed Mtb confined within an apoptotic cell further compartmentalizes the bacterium and prevents its virulence factors from interfering with phagosome maturation. Thus, efferocytosed Mtb is destroyed along with the apoptotic cell debris. Efferocytosis has long been known to be a crucial macrophage function, but we report here that efferocytosis is also an innate immune effector function.
Results
Mtb-infected macrophages undergo apoptosis and are engulfed by uninfected macrophages
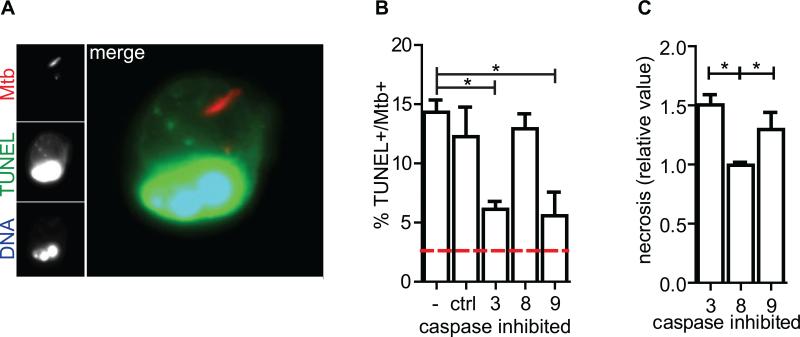
While virulent Mtb predominantly induces necrosis of both human and murine macrophages in vitro, particularly after several days or at high multiplicity of infection (MOI), careful analysis revealed that a spectrum of cell death modalities are observed, including apoptosis (Danelishvili et al., 2003; Divangahi et al., 2009; Duan et al., 2002). Thioglycollate-elicited peritoneal macrophages were infected with mCherry-expressing H37Rv, resulting in the majority of macrophages harboring 1-5 bacteria. Two days after infection 15% of infected macrophages were apoptotic as determined by TdT-mediated d-UTP nick end labeling (TUNEL) (Figures 1a, b). Under these conditions we found that apoptosis is triggered via the intrinsic apoptosis pathway, depending predominantly on caspases 9 and 3 as inhibition of these caspases reduced the incidence of TUNEL+ nuclei and subsequently drove more cells towards necrosis (Figure 1c). There was not significant bystander apoptosis of uninfected macrophages within the infected macrophage conditions (data not shown). Thus, a significant population of H37Rv-infected macrophages undergoes apoptosis.
Figure 1. Mtb-infected macrophages undergo apoptosis.
(A) Epifluorescence microscopy image of apoptotic Mtb-infected CD11b+ thioglycollate-elicited peritoneal macrophage (pMφ). Mtb (red) TUNEL (green), DNA (UV/pseudocolor blue) and merge. (B) TUNEL+ Mtb-infected pMφs as a percent of total infected pMφs two days post-infection in the presence of 10μM caspase inhibitors or control peptide, ctrl. Red dashed line is the mean percent TUNEL+ of uninfected Mφs. (C) Cell Death ELISA assay for necrosis 2 days post Mtb-infection of pMφs in the presence of 10μM caspase inhibitors relative to infected control-treated Mφs. Data is representative of ≥3 experiments. One way ANOVA with Dunnet's post-test *, p <0.05. Error bars, mean ± SEM.
Phagocytes rapidly clear dying cells in vivo; however, whether Mtb-infected apoptotic cells are engulfed by macrophages has not been systematically addressed. We observed TUNEL+ nuclei contained within healthy, intact macrophages, indicating that Mtb-infected apoptotic cells could be engulfed by other macrophages (data not shown). To formally study efferocytosis of Mtb-infected macrophages we developed a confocal microscopy assay to monitor the fate of infected macrophages after they die and are engulfed by neighboring macrophages (Figure 2a). Macrophages from C57Bl/6 mice, which express the CD45.2 allele (hereafter referred to as CD45.2 macrophages) were labeled with DiO and infected with mCherry-H37Rv before adding uninfected congenic CD45.1 macrophages. Under these conditions there was no discernible transfer of dye between the macrophage membranes and bacterial membranes (data not shown). There exist three predicted scenarios after overnight co-culture. First, Mtb can remain within the first cell it infects – a primary infected cell (Figure 2ai). Second, necrosis of an infected macrophage allows Mtb to disperse into the culture supernatant and to infect other macrophages (Figure 2aii). These are secondarily infected macrophages. Third, an adjacent CD45.1 macrophage could engulf an Mtb-infected apoptotic macrophage (Figure 2aiii). This is efferocytosis. One day after co-culture of infected and uninfected macrophages, all three scenarios were observed (Figure 2b). The majority of Mtb were within primary infected macrophages. However, 21.7±1.7% of Mtb were within efferocytic cells and 9.8±1.2% spread into secondarily infected cells (mean ± SEM; n=12 experiments). Secondarily infected cells and efferocytic cells are distinguished based on whether or not Mtb co-localizes within discrete DiO+ vesicles within a CD45.1 macrophage. This analysis may underestimate the frequency of efferocytosis if the DiO label is degraded. The DiO signal did not appear to decay within the first 24 hours of coculture, the time point chosen for these experiments, although loss of signal was observed after 48 hours. Additionally, secondary infection might be overestimated, as not all extracellular Mtb can be removed prior to the addition of uninfected macrophages, even by extensive washing.). Efferocytosis was observed using thioglycollate-elicited peritoneal macrophages, bone-marrow derived macrophages, human peripheral blood monocyte-derived macrophages and resident peritoneal macrophages (Figures 2c,d and Figure S1a). We also considered whether Mtb infection could inhibit efferocytosis. Uninfected and infected macrophages similarly engulfed apoptotic thymocytes, indicating that Mtb does not inhibit efferocytosis (Figure S1b). Thus, by using a microscopy based co-culture assay, we detected significant efferocytosis of Mtb infected macrophages in vitro.
Figure 2. Efferocytosis of Mtb-infected apoptotic macrophages in vitro and in vivo.
(A) Assay for detecting efferocytosis of Mtb-infected apoptotic cells in vitro by confocal microscopy and its three predicted outcomes. (B) Confocal microscopy images of CD45.2+ pMφs dyed with DiO, infected with mCherry-H37Rv and co-cultured with uninfected undyed CD45.1+ pMφs for 16 hours before fixation and visualization. Depicted are the three predicted outcomes of the efferocytosis assay; primary infection (i), secondary infection (ii) and efferocytosis (iii). (C) Efferocytosis seen using the above assay performed with bone marrow-derived macrophages (BMDMφs). Images are representative of >20 experiments. (D) Efferocytosis seen following mCherry-H37Rv infection of DiO-dyed monocyte-derived human macrophages (hMDMφs) and co-culture of uninfected Dextran-649 labeled hMDMφs. (E) Representative images of recovered cells following bronchial alveolar lavage (BAL) 16 hours post transfer of infected pMφs. Images are representative of two experiments. See also Figure S1
Efferocytosis of Mtb-infected macrophages occurs in the lung
To determine whether efferocytosis of Mtb-infected cells occurs in vivo, mCherry-H37Rv infected DiO-labeled CD45.2 macrophages were transferred intratracheally into the lungs of CD45.1 congenic mice. Sixteen hours later the airways of recipient mice were lavaged, stained with antibodies directed against CD45.1 and inspected by confocal microscopy (Figure S1c). All three scenarios observed in vitro (Figure 2a). Recipient alveolar cells contained Mtb together with the remnants of the primary infected macrophages (Figure 2e). Similar results were seen following the intraperitoneal transfer of DiO-dyed CD45.2 mCherry-infected macrophages into CD45.1 recipient mice (data not shown). These results indicate that efferocytosis of infected cells is a general phenomenon that can be mediated by macrophages in different anatomical compartments. Importantly, alveolar macrophages are capable of engulfing Mtb-infected apoptotic macrophages and efferocytosis of Mtb-infected macrophages occurs in the lung.
Conditions that favor efferocytosis restrict Mtb growth
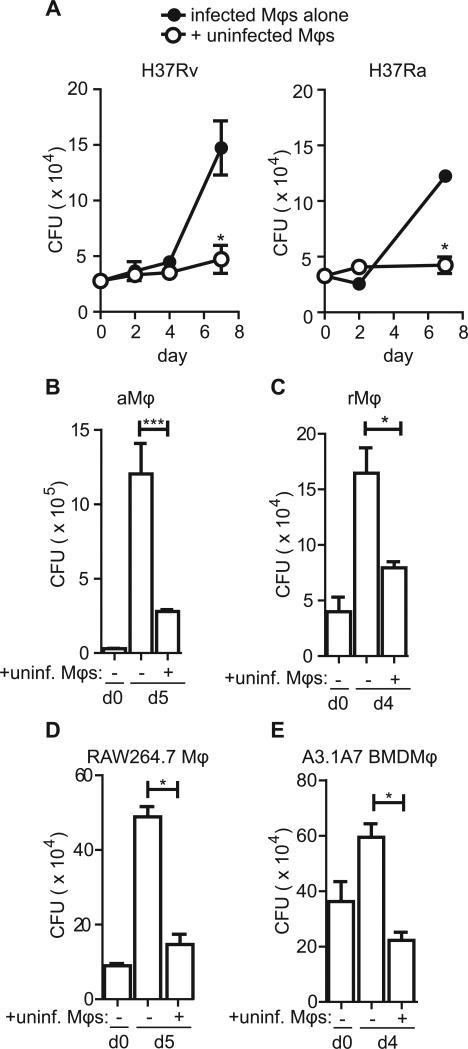
A substantial number of primary infected macrophages underwent apoptosis and the bacteria in these dying cells were engulfed along with cell debris by other macrophages in the assays described above. To determine whether the conditions in which we observed efferocytosis affects bacterial viability, uninfected peritoneal macrophages were co-cultured with Mtb-infected peritoneal macrophages and CFU were subsequently measured. The co-culture of uninfected macrophages with virulent H37Rv- or avirulent H37Ra-infected macrophages significantly suppressed bacterial growth (Figure 3a). This antibacterial activity was a feature of all types of macrophages tested including thioglycollate-elicited peritoneal macrophages, alveolar macrophages (Figure 3b), resident peritoneal macrophages (Figure 3c), RAW264 cells (Figure 3d) and the A3.1A7 bone marrow derived macrophage cell line (Figure 3e). Thus, under conditions in which we detect efferocytosis by microscopy bacterial growth is restricted.
Figure 3. Uninfected macrophage co-culture limits bacterial growth.
(A,B) pMφs were infected with a 10:1 MOI of H37Rv (A) or H37Ra (B) for four hours before uninternalized bacteria were washed away and uninfected pMφs were added at a ratio of 2:1. Bacterial burden was assessed by CFU enumeration by plating serial dilutions of each condition in quadruplicate at the indicated timepoints. Data is representative of >20 experiments (A, H37Rv) and 3 experiments (B, H37Ra). (C) Alveolar Mφs (aMφs) were infected with an MOI of 10:1 H37Rv for 4 hours before the addition of uninfected aMφs (+unif. Mφs). CFU was enumerated as described on days 0 (d0) and day 5 (d5). Data is representative of 2 experiments. (D) Resident peritoneal Mφs (rMφs) were infected as described with H37Rv before the addition of uninfected rMφs and CFU enumeration on indicated days. Data is representative of 3 experiments. (E) RAW264.7 Mφs were infected with H37Rv as previously described prior to the addition of uninfected Mφs of the same type. CFU was determined. Data is representative of 2 experiments. (F) RAW264.7 Mφs were infected with H37Rv as previously described prior to the addition of uninfected Mφs of the same type. Data is representative of 2 experiments. Error bars ± SEM, p ≤ 0.05, Student's T-test.
Efferocytosis of Mtb-infected apoptotic macrophages controls Mtb growth
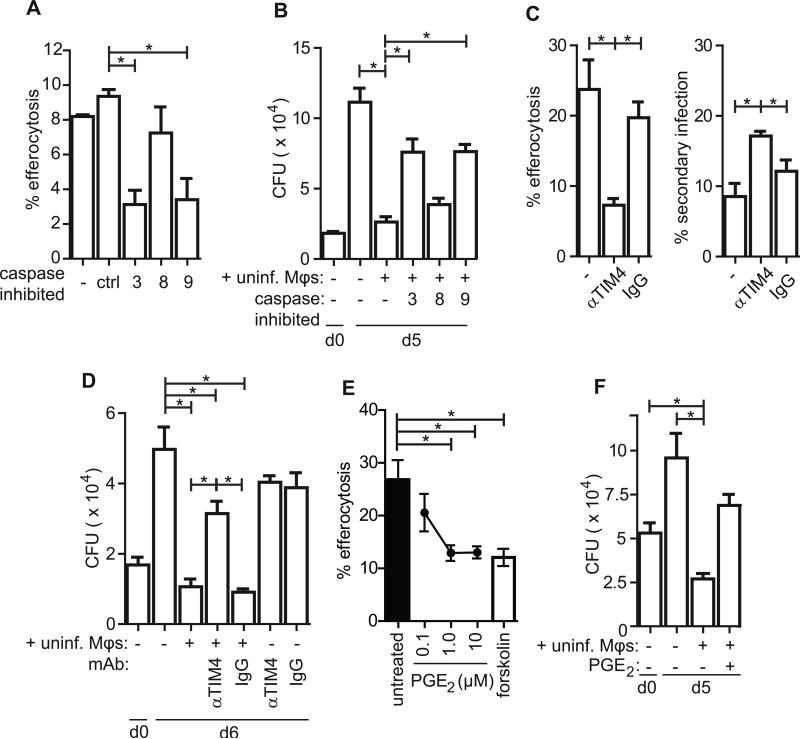
To prove that efferocytosis is responsible for the bacterial control observed and not other contact-dependent or -independent mechanisms, we sought to block efferocytosis itself. As efferocytosis requires the specific recognition and engulfment of apoptotic cells, we predicted that inhibition of apoptosis by caspase inhibitors would prevent efferocytosis and abrogate the CFU reduction mediated by uninfected macrophages. Conversely, if bacterial control by uninfected macrophage co-culture were independent of apoptosis, caspase inhibition would have no effect on Mtb growth. As predicted, inhibition of the intrinsic apoptosis pathway (caspase 9-dependent), but not the extrinsic pathway (caspase-8 dependent), reduced efferocytosis of Mtb-infected macrophages by uninfected macrophages as determined by confocal microscopy (Figure 4a). Similarly, caspase 3 and 9 inhibition abrogated CFU control observed following co-culture of infected and uninfected macrophages (Figure 4b). Caspase inhibition of infected macrophages, in the absence of uninfected macrophages had no effect on bacterial growth. Thus, apoptosis is a prerequisite for restricting Mtb replication in our macrophage co-culture model.
Figure 4. Efferocytosis controls Mtb growth.
(A) pMφs were infected as described in the confocal microscopy efferocytosis assay in the presence of 10μM caspase inhibitors. Percent efferocytosis is defined as the number of Mφs containing efferocytosed Mtb as a percent of total infected Mφs. Data is representative of 3 experiments. (B) pMφs were infected as described in Fig.4 (see legend), in the presence of 10μM caspase inhibitors. CFU were determined by plating conditions in quadruplicate on days 0 and 5. Data is representative of 3 experiments. (C) rMφs were infected as per the confocal microscopy efferocytosis assay in the presence of 10μg/mL TIM4 blocking antibody 5G3 or control rat IgG1 and efferocytosis and secondary infection was enumerated as above. Data is representative of 3 experiments. (D) rMφs were infected as in Fig.4 in the presence of αTIM4 or control IgG as indicated and CFU were plated in quadruplicate on days 0 and 6 post infection. Data is representative of 4 experiments. (E) Confocal microscopy efferocytosis assay with infected pMφs as previously described. Uninfected pMφs were pre-treated with PGE2 (at indicated concentrations) or forskolin (100μM) for 4 hours prior to washing and addition to the infected pMφ culture. Data is representative of 2 experiments. (F) aMφs were infected according to the efferocytosis assay prior to the addition of uninfected aMφs or PGE2-pretreated aMφs. CFU was determined by plating in quadruplicate on days 0 and 5 post infection. Data is representative of 2 experiments. Error bars ± SEM, p ≤ 0.05, One-way ANOVA with Dunnet's post-test. See also Figure S2.
To prove that efferocytosis was required for bacterial growth restriction, we sought to inhibit the engulfment process itself. Most macrophages express a variety of redundant receptors that mediate the recognition and uptake of apoptotic cells (Elliott and Ravichandran, 2010) and inhibition of any individual receptor yielded little or no inhibition of apoptotic thymocyte uptake by thioglycolate-elicited peritoneal macrophages in a non-infectious model of efferocytosis (data not shown). An early event during apoptosis is PS flipping to the outer leaflet of the plasma membrane. PS is a ligand for many efferocytosis receptors and bridging molecules that aid in apoptotic cell recognition and engulfment (Fadok et al., 1992; Hoffmann et al., 2001). Resident peritoneal macrophages rely exclusively on the PS receptor TIM4 to recognize apoptotic cells and to mediate efferocytosis; however, TIM4 is not expressed by most other macrophage subsets (Miyanishi et al., 2007; Rodriguez-Manzanet et al., 2010). Blocking TIM4 in resident peritoneal macrophages significantly reduced efferocytosis of Mtb-infected apoptotic cells (Figure 4c). TIM4 blockade also significantly increased the incidence of secondarily infected cells. This occurs presumably because the failure to clear apoptotic cells leads to more secondary necrosis and dispersal of intracellular contents including Mtb. Importantly, blocking TIM4 reduced the ability of uninfected macrophages to restrict bacterial growth (Figure 4d). These data conclusively demonstrate that efferocytosis is an innate antibacterial mechanism as engulfment is specifically required for control.
Pro-inflammatory cytokines and immune mediators such as TNF and PGE2 have been shown to reduce efferocytosis both in vitro and in vivo. PGE2 has been previously reported to inhibit efferocytosis in alveolar macrophages (Aronoff et al., 2004). We confirmed this finding and found PGE2 also inhibits efferocytosis in peritoneal macrophages as well, without significantly impacting phagocytosis (Figure S2a). To target the effects of PGE2 on efferocytic macrophages (and not infected macrophages) uninfected macrophages were pre-treated with high doses of PGE2 prior to their addition to infected macrophages. PGE2 inhibited those macrophages’ ability to engulf Mtb-infected apoptotic cells as determined by confocal microscopy (Figure 4e). The inhibitory effect of PGE2 is thought to be mediated by increasing intracellular cAMP, a crucial regulator of engulfment by phagocytes (Rossi et al., 1998). Thus, we showed that forskolin treatment also inhibited efferocytosis (Figure 4e). Importantly, pre-treatment of uninfected peritoneal macrophages with PGE2 prior to their co-culture with infected macrophages limited their ability to control Mtb growth (Figure S2b). Next, we determined whether PGE2 could inhibit efferocytosis-mediated control in alveolar macrophages. PGE2 pre-treatment of uninfected alveolar macrophages prevented their ability to restrict bacterial growth in vitro. In several experiments co-culture of uninfected and infected macrophages significantly reduced the number of recovered bacteria to below the level of the initial inoculums, which is indicative of bacterial killing, a phenomenon observed in other studies (Lee et al., 2006) (Figure 4f). These data demonstrate that efferocytosis is an important innate immune mechanism of bacterial control and is one that can be used by alveolar macrophages to control Mtb growth or even mediate bacterial killing.
Mtb and cell debris are found within vacuous phagosomes following efferocytosis
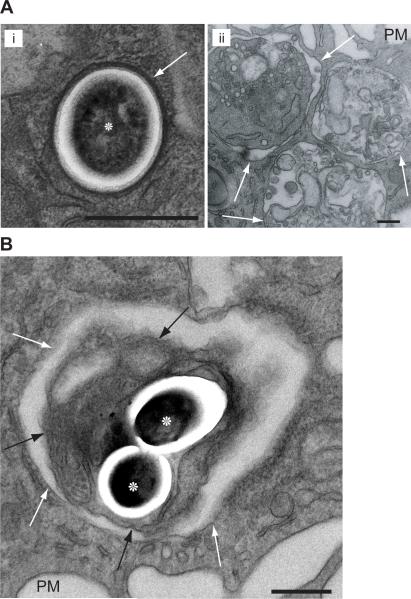
Given that efferocytosis of an Mtb-infected apoptotic cell exerts significant control over Mtb growth, we sought to understand the unique features of efferocytosis that restrict bacterial growth. We asked whether the intracellular compartments containing Mtb differed following internalization by phagocytosis versus efferocytosis. Using transmission electron microscopy (TEM) we confirmed that one day post-infection, Mtb was contained within a tight-fitting phagosome membrane closely juxtaposed to the bacterial surface, as has been previously seen (van der Wel et al., 2007) (Figure 5ai). Mtb were also intact and showed no sign of degradation. Following efferocytosis, apoptotic thymocytes within uninfected Mφs occupied very different phagosomes (Figure 5aii). Characteristically, the efferocytic phagosome is vacuous, with fluid filling the space between apoptotic cell membrane and phagosome membrane (Vandivier et al., 2006). Thymocytes, as well as apoptotic macrophages were found in various states of digestion and destruction of both organelle and plasma membranes (Figure 5aii & Figure S3). After co-culture of Mtb-infected and uninfected macrophages, Mtb was found within efferocytosed apoptotic blebs encased within a spacious phagosome (Figure 5b), as well as within large phagosomes containing apoptotic cell remnants (Figure S3). Membranes and intact cellular components were discerned within some of the apoptotic blebs, indicating an early stage of digestion (Figure 5b). Thus, the efferocytic Mtb phagosome is structurally distinct from a bacterium within a primary infected macrophage. Damaged Mtb with characteristic ‘onion’ morphology were also observed within vacuous phagosomes along with cellular debris, possibly indicating killed bacteria following efferocytosis (Armstrong and Hart, 1971) (Figure S3). This raised the possibility that bacteria efferocytosed within or among apoptotic cell debris are processed differently than phagocytosed bacteria.
Figure 5. Mtb are found within vacuous efferocytic phagosomes along with cell debris following uninfected macrophage co-culture.
(A) Transmission electron micrograph of (i) Mtb (asterisks) within a pMφ stained with lead citrate. Apoptotic thymocytes co-cultured with pMφs for 1 hour (ii) are engulfed and retained in phagsomes. Note the three thymocyte apoptotic blebs in increasing stages of digestion and disintegration. (B) Mtb within vacuous efferocytic phagosomes inside apoptotic cell blebs after the co-culture of uninfected pMφs with infected pMφs. PM, plasma membrane; scale bar, 1μM; white arrows, phagosome membrane; black arrow, apoptotic bleb membrane. Images are representative from 2 experiments with >1000 Mφs examined on multiple non-serial 60nM sections. See also Figure S3.
The Mtb-efferocytic phagosome matures and acquires lysosomal characteristics
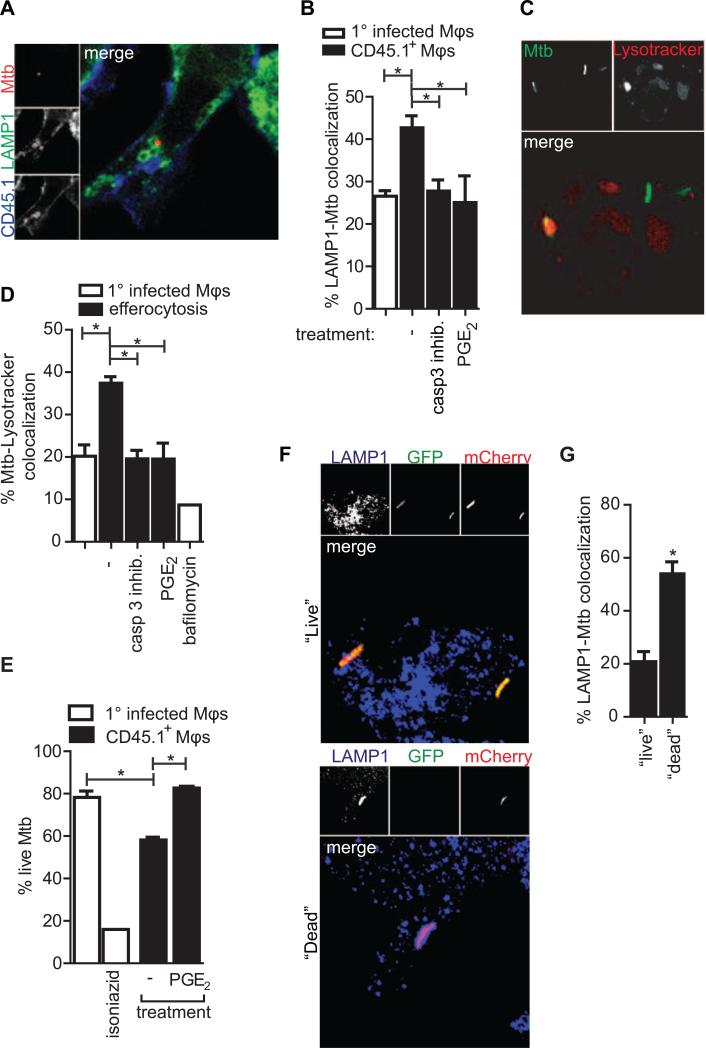
Mtb possesses virulence factors that subvert phagosome maturation and allow it to exploit the intracellular environment of the macrophage (Fratti et al., 2003). We reasoned that the uptake of Mtb contained within an apoptotic cell further compartmentalizes Mtb and limits its ability to interfere with phagosome maturation. To this end we measured the colocalization of Mtb with markers of mature lysosomes following efferocytosis (Figure 6a). More Mtb within efferocytic bone marrow-derived macrophages co-localized with LAMP1 than in primary infected macrophages (Figure 6b). Inhibiting apoptosis (with a caspase 3 inhibitor) or efferocytosis (with PGE2) both reduced the Mtb-LAMP1 co-localization back to levels characteristic of primary infection. The vacuolar ATPase is responsible for maintaining the acidic pH of the lysosomal compartment is excluded from Mtb-containing phagosomes (Sturgill-Koszycki et al., 1994). Following efferocytosis, there was greater Mtb-vATPase co-localization in efferocytic macrophages than in primary infected Mφs (Figure S4a). Ultimately, low pH marks a functional, mature lysosome. While the Mtb containing phagosome limits acidification, more Mtb were detected within acidic compartments following efferocytosis (Figures 6c, d). Many bacteria were within large acidified vesicles, reminiscent of the vacuous efferocytic phagosomes observed by confocal and electron microscopy. Blocking efferocytosis reduced the bacteria's co-localization with acidic compartments to levels seen in primary infection. Components of the autophagic machinery have been implicated in facilitating phagosome maturation, efferocytic phagosome maturation and Mtb control (Gutierrez et al., 2004; Martinez et al., 2011; Sanjuan et al., 2007). However, no specific enrichment of LC3, an early marker of autophagosomes, was found on Mtb-containing phagosomes following efferocytosis (Figure S4b). The fusion of lysosomes with the efferocytic phagosome delivers catabolic enzymes capable of destroying the bacterium. These data demonstrate that Mtb is unable to inhibit maturation of the efferocytic phagosome, exposing the bacterium to the caustic environment of degradative lysosomes.
Figure 6. Efferocytosis delivers Mtb to the lysosome.
(A) Mtb within a LAMP1+ vesicle inside an efferocytic BMDMφ. (B) Quantification of (A), percent of Mtb co-localizing with LAMP1 of total Mtb in primary infected BMDMφs (clear bar) or CD45.1+ BMDMφs (black bars). Uninfected CD45.1+ BMDMφs were untreated, pretreated with PGE2 or added to infected BMDMφs in the presence of caspase 3 inhibitor. Data is representative of 4 experiments. (C) Mtb within acidic vesicles pMφs. (D) Quantification of (C) showing percent Mtb-Lysotracker Red colocalization of total GFP-Mtb in primary infected pMφs or following efferocytosis and the depicted treatments. Bafilomycin was added at 1μM for 2 hours before and during Lysotracker staining. Data is representative of 2 experiments. (E) Percent “Live” Mtb of total Mtb counted in primary infected pMφs (clear bar) or CD45.1+ pMφs following co-culture with infected macrophages. Isoniazid was added for 24 hours. Data is representative of two experiments. Error bars ± SEM, p ≤ 0.05, One-way ANOVA with Dunnet's post-test. (F) “Live” (top) Mtb do not co-localize with LAMP1 as frequently as “Dead” (bottom) Mtb in bone marrow-derived macrophages (BMDMφs). (G) Quantification of (F). Percent LAMP1 colocalization with “Live” or “Dead” Mtb of total “Live” or “Dead” Mtb in BMDMφs. Data is representative of two experiments. Error bars ± SEM, p ≤ 0.05. Student's T-test. See also Figure S4.
Bacteria in LAMP1+ vesicles are more likely to be dead
To determine the effect of efferocytosis on bacterial viability at the single cell level, we infected macrophages with H37Rv that constitutively express mCherry (red) and inducibly express GFP (green) using a TetON promoter. After tetracycline induction ‘live’ bacteria expressed both red and green fluorescent proteins while bacteria that are dead only fluoresce red. We confirmed that changes in viability observed using the Live/Dead reporter Mtb correlate with changes in CFU (Figure S4c). Using these Live/Dead reporter Mtb, we find that fewer live or transcriptionally active Mtb were present within efferocytic macrophages (Figure 6e). We also found that the majority of the dead bacteria were found within LAMP1+ compartments (Figures 6f, g). Collectively, these data indicate that following efferocytosis, the efferocytic phagosome containing the infected apoptotic cell fuses with lysosomes, which are capable of killing Mtb.
Efferocytosis controls Mtb in vivo
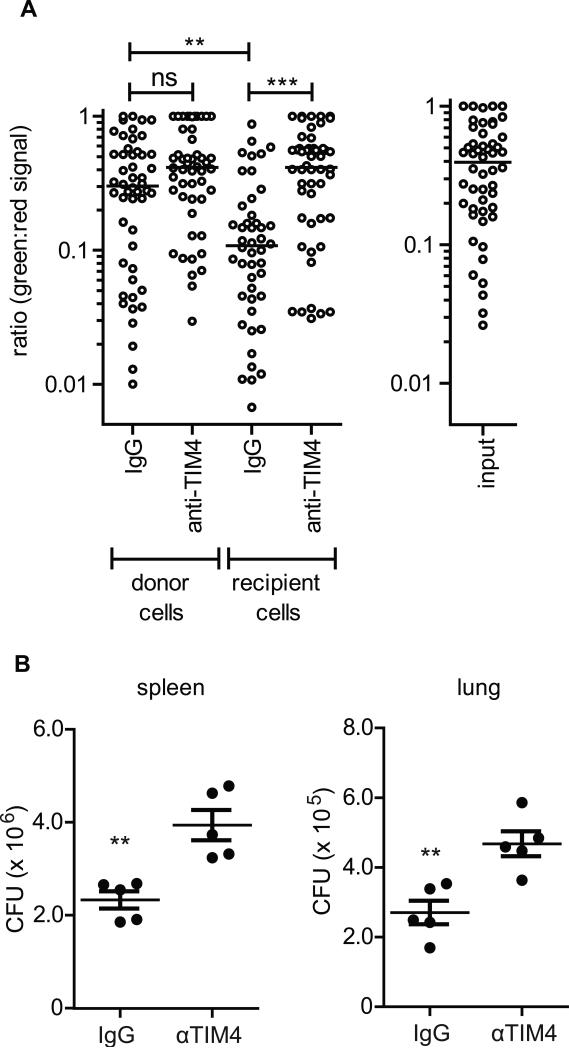
Having shown that efferocytosis of Mtb-infected apoptotic macrophages occurs in vivo (Figure 2), we were next interested in whether this process contributes to bacterial control in vivo. We used two approaches to answer this question. First, utilizing the Live/Dead reporter Mtb, we asked if efferocytosis kills Mtb in vivo. 5-lipoxygenase deficient macrophages (5LO-/-, CD45.2) that undergo more apoptosis after infection (Divangahi et al., 2009) and consequently more efferocytosis (Figure S5), were infected with Live/Dead-H37Rv and injected intraperitoneally into CD45.1 mice along with αTIM4 or IgG control antibodies. 16 hours later the peritoneal cavity was lavaged and the retrieved cells were plated with tetracycline to induce GFP expression in the live bacteria. The next day, cells were fixed and analyzed by microscopy. The viability of Mtb detected in the transferred (e.g., CD45.2) macrophages was not affected by the antibody treatment and was similar to Mtb in “input” macrophages that were exclusively cultured in vitro (Figure 7a). In contrast, the recipient CD45.1 macrophages from mice treated with IgG contained significantly fewer ‘live’ Mtb (as indicated by a low GFP:mCherry signal) compared with recipient CD45.1 macrophages from mice treated with TIM4 blocking antibody (Figure 7a). The former condition (“IgG”) is enriched for recipient macrophages that had engulfed Mtb infected macrophages. In the latter condition (“TIM4”), αTIM4 prevents the efferocytosis of apoptotic CD45.2 macrophages. Consequently, these macrophages die by secondary necrosis, and the released bacteria secondarily infect peritoneal macrophages. The bacteria in the secondarily infected macrophages have the same viability as Mtb in the input cells. Thus efferocytosis can kill Mtb in vivo and secondary necrosis is a mechanism of Mtb spread.
Figure 7. Efferocytosis controls Mtb in vivo.
(A) Mtb is less viable following efferocytosis in vivo. Live/Dead-H37Rv 5LO-/- infected pMφs were intraperitoneally transferred into CD45.1 mice with IgG or aTIM4 antibody. Sixteen hours later cells were removed and treated with 200ng/mL anhydrous tetracycline to induce GFP expression. Mtb viability was ascertained by measuring the ratio of GFP:mCherry expression for each bacterium. Input is expression in untransferred infected pMφs. Data is representative of 2 experiments. Median, p ≤ 0.001. Mann-Whitney test. (B) Bacterial burden in spleen and lung of Rag-/- mice two weeks post intravenous transfer of H37Rv-infected 5LO-/- pMφs and intraperitoneal administration of IgG or aTIM4. Data is representative of one experiment. Error bars ± SEM, p ≤ 0.001. Student's T-test. See also Figure S5.
Next we asked if efferocytosis could control bacterial burden in vivo. Efferocytosis is highly redundant and thus targeting a single efferocytic receptor for blockade in vivo presents many challenges. TIM4 is an efferocytic receptor responsible for apoptotic cell uptake on only a minority of macrophages, but we confirmed previous reports that intraperitoneal administration of TIM4 blocking antibody reduces efferocytosis of CFSE-labeled apoptotic macrophages in the spleen 2-3 hours after intravenous transfer (Figure S5) (Albacker et al., 2010). Rag-/- recipient mice were administered αTIM4 or IgG by the intraperitoneal route and 5LO-/- H37Rv infected macrophages were injected intravenously. Two weeks later we found a significantly higher Mtb burden in both the spleen and lungs of αTIM4-treated mice (Figure 7b). Efferocytosis is therefore a protective innate antibacterial effector mechanism that contributes to control of Mycobacterium tuberculosis.
Discussion
Apoptosis is thought to be an innate antimycobacterial mechanism. Wild-type (virulent) Mtb are believed to actively inhibit apoptosis and instead induce necrosis. This idea is based on the finding that attenuated mutants of Mtb induce greater macrophage apoptosis (Velmurugan et al., 2007). This evidence, together with studies on host factors such as eicosanoids that modulate the death modality of Mtb infected macrophages, has led to the paradigm that proapoptotic conditions restrict bacterial replication while pro-necrotic conditions favor bacterial dispersal, infection of new cells and bacterial growth (Chen et al., 2008; Divangahi et al., 2009). However, there is little evidence that apoptosis has direct antibacterial activity and a specific mechanism for apoptosis-induced Mtb death has never been named. Thus, we hypothesized that cellular events following apoptosis might be crucial for bacterial control. Efferocytosis is a crucial function of macrophages, but its role during infection has not been systematically addressed. We sought to test the hypothesis that efferocytosis of Mtb infected apoptotic cells is a cellular mechanism that limits intracellular bacterial replication.
We found that Mtb-infected apoptotic macrophages are engulfed by surrounding uninfected macrophages both in vitro and in vivo. Blocking the engulfment of Mtb-infected apoptotic macrophages, whether by increasing cAMP levels or specifically blocking the PS receptor TIM4; prevented Mtb control. We do not believe that these effects are limited to TIM4-mediated recognition and uptake of apoptotic infected macrophages. Since the majority of resident peritoneal macrophages rely exclusively on TIM4 as the dominant PS receptor, the use of resident peritoneal macrophages provided a model that could be used to study the consequences of inhibiting efferocytosis (Ghosn et al., 2010). We previously reported that PGE2 protects Mtb-infected macrophages from necrotic cell death in a cAMP-dependent fashion (Chen et al., 2008). Clearly PGE2-cAMP signaling has many effects. We have shown here that efferocytosis plays a major role in suppressing bacterial growth following apoptosis. Indeed, the phenomenon of uninfected macrophages contributing to the control of intracellular mycobacteria following apoptosis was observed over a decade ago (Fratazzi et al., 1997). At that time, it was speculated that engulfment of apoptotic infected macrophages could be responsible for bacterial control. Our current study provides a mechanistic basis for this observation.
Presumably, there is a basal level of efferocytosis occurring all the time during culture of macrophages. As efferocytosis is a constitutive process, many of the phenomenon previously attributed solely to apoptosis, may in fact require efferocytosis. The assay system developed here will be an important tool to study how efferocytosis is regulated (or subverted) in the context of infection. Our data does not exclude the possibility of other cell contact and non-contact mechanisms of bacterial control exerted by uninfected macrophages seen in other high-dose Mtb-induced cytolysis models (Hartman and Kornfeld, 2011). In fact, in the recent study by Hartman and Kornfeld, efferocytosis appeared to be inhibited and, in the context of our results, may indicate that high-MOI non-apoptotic cell death inhibits efferocytosis or prevents the expression of relevant ‘eat me’ signals on the dying cell.
Mtb exerts tremendous control over the host macrophage from the relative safety of an arrested phagosome. The bacteria's ability to impair phagolysosome maturation is a major part of its virulence strategy; however, how the bacteria accomplishes this is still largely unknown. In contrast, it appears that Mtb is no longer able to inhibit phagolysosome maturation when contained in an efferocytic phagosome and is shuttled to lysosomes. We envision that this is an issue of compartmentalization: Mtb encased within an apoptotic cell, within an efferocytic phagosome has at least one additional layer of membrane distancing itself from the host cytosol. We were unable to determine if the phagosome membrane from the primary infected macrophage still surrounds Mtb within the efferocytic phagosome. There is evidence that Mtb translocation to the cytosol induces cell death, thus perhaps Mtb is only behind one and not two additional layers of membrane within the efferocytic phagosome (van der Wel et al., 2007). Regardless, Mtb packaged behind this additional membrane layer cannot inhibit phagosome maturation and is thus killed. Several studies suggest that Mtb must be in contact with the phagosome membrane in order to inhibit lysosome fusion. Mtb might require close proximity to the phagosome membrane to efficiently direct its effector molecules to host cell targets to halt phagolysosome fusion or to induce cell death (de Chastellier et al., 2009). Or simply, the bioactive lipids displayed on Mtb's cell wall can't find their targets behind an additional membrane (Chua et al., 2004). Additional study is required to ascertain what property of efferocytosis allows for phagosome maturation.
This report has focused on the role of macrophage apoptosis as an innate response following Mtb infection. However, the killing of infected cells is an important arm of the adaptive immune response to tuberculosis (Woodworth et al., 2008). T cells can induce apoptotic death in target cells via the release of cytotoxic granules containing granzymes or through the engagement of surface receptors containing death domains such as CD95/95L (Lavrik and Krammer, 2012; Trapani, 2012). We envision the capacity of CTL to kill infected cells restricts bacterial growth via apoptosis induction followed by their engulfment by uninfected macrophages recruited to inflammatory foci.
The relative efficiency of efferocytosis will depend on the microenvironment. Some inflammatory signals, such as abundant TNF or PGE2, can impair efferocytosis (Aronoff et al., 2004; Michlewska et al., 2009). This could lead to greater secondary necrosis. If efferocytosis is inhibited during active Mtb infection, pharmacologically releasing this inhibition could benefit the infected individual in two ways. First, tuberculosis is a disease of rampant cell death, inflammation and tissue destruction. Efferocytosis would be key in maintaining proper lung tissue architecture during disease and minimizing the development of immunopathology. Second, enhancing efferocytosis would restrict bacterial replication and could prevent recrudescence of infection. Additionally, the role of efferocytosis during the maintenance of latency is completely unknown and might play an important role during this stage of disease.
Some pathogens may to subvert efferocytosis to evade immunity. For example, Leishmania employ neutrophil apoptosis and subsequent efferocytosis as a mechanism of establishing infection within macrophages (Peters et al., 2008; van Zandbergen et al., 2004). Indeed, some pathogens might rely on uptake of infected dying cells to spread the infection, as is the case in M. marinum infection of zebrafish embryos (Davis and Ramakrishnan, 2009). Efferocytosis may control or exacerbate many infectious diseases and it will be important to explore how macrophages contribute to microbial immunity by executing this vital ‘housekeeping’ function.
Experimental Procedures
Mice and macrophage isolation
C57Bl/6 (CD45.2), B6.SJL-Ptprca Pepcb/BoyJ (CD45.1) and B6.129S7-Rag1tm1Mom/J (Rag-/-) mice were purchased from Jackson Laboratories (Bar Harbor USA), and were kept and bread using standard humane animal husbandry protocols. Mtb-infected mice were housed under BSL3 conditions. Thioglycollate-elicited peritoneal Mφs (pMφs) were lavaged from peritoneal cavities 4-5 days following 3% IP thioglycollate injection. Mφs were isolated using CD11b microbeads and magnetic columns (Miltenyi Biotec) and cultured in 24 or 96 well dishes overnight prior to infection. Resident peritoneal Mφs (rMφs) were lavaged from peritoneal cavities and selected based on CD11b+CD19-. Bone marrow Mφs (BMMφs) were differentiated from bone marrow cells 7-10 days in RPMI supplemented with 20% L929 cell supernatant. Alveolar Mφs (aMφs) were lavaged from the lungs of mice with PBS. Primary human peripheral blood (Research Blood Components, Boston, MA) monocyte-derived macrophages (MDMφs) were generated from adherent bead-selected CD14+ selected cells following 7 day culture with human serum.
Mtb culture and infection
mCherry-H37Rv and Live/Dead-H37Rv were cultured with 0.5ug/mL Hygromycin B. Mφs were grown in 96-well plates and infected at a multiplicity of infection (MOI) of 10:1 for 4 hours. Cultures were then washed 3-5 times. Efferocytic Mφs were added at 2x the number of infected Mφs. As indicated efferocytic Mφs were pretreated with 1-10μM PGE2 (Cayman Chemical) for 4 hours at 37°C before washing and addition to infected Mφs. Caspase inhibitors (10μM, Calbiochem), αTIM4 (10μg/mL- kind gift of Vijay Kuchroo, Harvard Medical School) or rat IgG1 (10μg/mL) were added following infection. CFU were determined immediately following infection and at indicated timepoints per condition in quadruplicate by removing culture supernatant, lysing Mφs with 1% TritonX/PBS solution, plating serial dilutions on 7H11 plates and incubating at 37°C/5% CO2 for 3 weeks.
Assays for cell death
Apoptosis was assessed by TUNEL (Roche) according to manufacturer's specifications on Mφs grown on coverglass and fixed with 3.7% paraformaldehyde. 100 infected cells were counted per condition in triplicate by conventional epifluorescence microscopy using a Nikon TE2000-U inverted microscopy fitted with a SPOT-RT CCD camera. Apoptosis was scored as cells possessing a TUNEL+ pyknotic nucleus. Cell Death ELISAPLUS (Roche) was performed as per manufacturer's recommended protocol on H37Rv-infected macrophages to measure necrosis as was described previously (Divangahi et al., 2009).
Confocal microscopy efferocytosis assay
Mφs from CD45.2 mice were cultured in 24 well dishes in triplicate per condition on coverglass and dyed with Vybrant DiO (Invitrogen) as per manufacturer's specifications as needed prior to infection with mCherry-H37Rv or GFP-H37Rv for 4 hours. Bacteria were washed away and uninfected CD45.1 Mφs were added at 2x the number of infected Mφs. Treatment conditions were added as appropriate. 16-24 hours later cells were washed and stained with mouse monoclonal CD45.1 (Abcam) before affixing to microscopy slides with Prolong Gold plus DAPI (Invitrogen). LAMP1 (Abcam) and LC3 (MBL) staining was done 16-24 hours post infection following fixation and TritonX permeabalization. vATPase (Abcam) staining was done 16-24 hours post infection following fixation and saponin permeabalization. Lysotracker Red (Invitrogen) was added 16-24 hours post infection for 1 hour at 37°C at 1uM prior to washing and fixation. Tetracycline was added 16 hours post infection/addition of uninfected Mφs at 200ng/mL and induced GFP expression for 24 hours in Live/Dead H37Rv-infected Mφs. Conditions were analyzed in triplicate and for each replicate in each condition 100-300 Mtb were counted. Images were taken using a Nikon TE2000-U inverted microscopy equipped with C1 Plus confocal laser scanner.
In vivo efferocytosis assays
Microscopy: Adoptive transfer of Mtb-infected Mφs was as described previously (Divangahi et al., 2009). DiO-dyed CD45.2 pMφs were infected in standing culture with mCherry-H37Rv for one hour. 100uL of infected macrophage solution was transferred into the lungs of uninfected CD45.1 mice via the intratracheal route. 16-20 hours later airway cells were retrieved by bronchoalveolar lavage, fixed with PFA, stained for CD45.1 and cytospun onto microscopy slides for confocal microscopy analysis. Live/Dead: 5 million 5LO-/- macrophages infected with Live/Dead-H37Rv were injected intraperitoneally into CD45.1 mice along with 400μg α TIM4 or IgG. 16 hours later peritoneal cavities were lavaged and cells plated onto coverglass in the presence of 200ng/mL tetracycline. Untransferred macrophages were similarly plated and tetracycline treated to ascertain baseline ‘input’ Mtb viability. The next day cells were antibody stained for CD45.1, paraformaldehyde fixed and examined by confocal microscopy. A ratio of green (GFP) to red (mCherry) signal was measured for each bacterium. CFU: H37Rv-infected C57Bl/6 macrophages were IV injected into Rag-/- mice intraperitoneally treated with 400μg α TIM4 or IgG. Approximately 5 million macrophages were transferred harboring 100,000 bacteria. Two weeks later bacterial burden was assessed in spleen and lung by serial dilution.
Transmission electron microscopy
Macrophages were grown in 6 well dishes and infected and treated as described above. 24 hours post infection cells were fixed with glutaraldehyde/paraformaldehyde/picric acid in sodium cacodylate buffer for 1 hour. Cells were postfixed for 30 minutes in Osmium tetroxide/Potassium ferrocyanide, washed in water and incubated in uranyl acetate for 30 minutes. Cells were washed and dehydraded in alcohol, removed from culture dish in propuleneoxide, pelleted and infiltrated for 2 hours in propuleneoxide and TAAB Epon (Marivac Canada). Samples were embedded in TAAB Epon and polymerized at 60°C for 48 hours. 60nM sections were cut on a Reichert Ultracut-S microtome, picked up onto copper grids and stained with lead citrate and examined using a TecnaiG2 Spirit BioTWIN transmission electron microscopy and recorded with an AMT 2k CCD camera.
Image Analysis
Microscopy images were analyzed using Image J and co-localization analysis macros or user-generated pipelines in Cell Profiler (Carpenter et al., 2006). Investigators were blinded during colocalization analysis.
Statistical Analysis
Statistical analysis and graphical output was done with GraphPad Prism. One-way ANOVA with Dunnet's post-test. * = p≤0.05
Supplementary Material
Highlights.
Apoptosis occurs following virulent Mycobacterium tuberculosis (Mtb) infection
Mtb-infected dead cells are engulfed by uninfected macrophages via efferocytosis
Efficient maturation of the bacteria-containing efferocytic phagosome kills Mtb
Efferocytosis is an antibacterial effector mechanism both in vitro and in vivo
Acknowledgements
We are most grateful to members of the Behar, Fortune and Remold labs for reagents, helpful discussion and insights. TIM4 blocking antibodies were a generous gift of Vijay Kuchroo. Members of the Harvard Electron Microscopy Core Facility helped in the preparation, staining and operation of the electron microscope. The Small Animal Biocontainment (ABC) Suite is supported by CFAR 5P30AI060354. T.R.R and S.M.F were supported by CFAR 5P30AI060354, DP2-0d001378 and T32-AI07387. C.N.A. is the recipient of a fellowship from FCT. S.M.B and H.G.R. were supported by R56AI084161 and R01AI072143.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albacker LA, Karisola P, Chang YJ, Umetsu SE, Zhou M, Akbari O, Kobayashi N, Baumgarth N, Freeman GJ, Umetsu DT, et al. TIM-4, a receptor for phosphatidylserine, controls adaptive immunity by regulating the removal of antigen-specific T cells. J Immunol. 2010;185:6839–6849. doi: 10.4049/jimmunol.1001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JA, Hart PD. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. The Journal of experimental medicine. 1971;134:713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol. 2004;173:559–565. doi: 10.4049/jimmunol.173.1.559. [DOI] [PubMed] [Google Scholar]
- Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Divangahi M, Gan H, Shin DS, Hong S, Lee DM, Serhan CN, Behar SM, Remold HG. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. The Journal of experimental medicine. 2008;205:2791–2801. doi: 10.1084/jem.20080767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Gan H, Remold HG. A mechanism of virulence: virulent Mycobacterium tuberculosis strain H37Rv, but not attenuated H37Ra, causes significant mitochondrial inner membrane disruption in macrophages leading to necrosis. J Immunol. 2006;176:3707–3716. doi: 10.4049/jimmunol.176.6.3707. [DOI] [PubMed] [Google Scholar]
- Chua J, Vergne I, Master S, Deretic V. A tale of two lipids: Mycobacterium tuberculosis phagosome maturation arrest. Current Opinions Microbiology. 2004;7:71–77. doi: 10.1016/j.mib.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Danelishvili L, McGarvey J, Li YJ, Bermudez LE. Mycobacterium tuberculosis infection causes different levels of apoptosis and necrosis in human macrophages and alveolar epithelial cells. Cellular microbiology. 2003;5:649–660. doi: 10.1046/j.1462-5822.2003.00312.x. [DOI] [PubMed] [Google Scholar]
- Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chastellier C, Forquet F, Gordon A, Thilo L. Mycobacterium requires an all-around closely apposing phagosome membrane to maintain the maturation block and this apposition is re-established when it rescues itself from phagolysosomes. Cellular microbiology. 2009;11:1190–1207. doi: 10.1111/j.1462-5822.2009.01324.x. [DOI] [PubMed] [Google Scholar]
- Divangahi M, Chen M, Gan H, Desjardins D, Hickman TT, Lee DM, Fortune S, Behar SM, Remold HG. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nature immunology. 2009 doi: 10.1038/ni.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L, Gan H, Golan DE, Remold HG. Critical role of mitochondrial damage in determining outcome of macrophage infection with Mycobacterium tuberculosis. J Immunol. 2002;169:5181–5187. doi: 10.4049/jimmunol.169.9.5181. [DOI] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. The Journal of cell biology. 2010;189:1059–1070. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwig LP, Henson PM. Clearance of apoptotic cells by phagocytes. Cell death and differentiation. 2008;15:243–250. doi: 10.1038/sj.cdd.4402184. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- Faherty CS, Maurelli AT. Staying alive: bacterial inhibition of apoptosis during infection. Trends Microbiol. 2008;16:173–180. doi: 10.1016/j.tim.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Boyanapalli R, McPhillips KA, Frasch SC, Janssen WJ, Dinauer MC, Riches DW, Henson PM, Byrne A, Bratton DL. Impaired phagocytosis of apoptotic cells by macrophages in chronic granulomatous disease is reversed by IFN-gamma in a nitric oxide-dependent manner. J Immunol. 2010;185:4030–4041. doi: 10.4049/jimmunol.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratazzi C, Arbeit RD, Carini C, Remold HG. Programmed cell death of Mycobacterium avium serovar 4-infected human macrophages prevents the mycobacteria from spreading and induces mycobacterial growth inhibition by freshly added, uninfected macrophages. J Immunol. 1997;158:4320–4327. [PubMed] [Google Scholar]
- Fratti RA, Chua J, Vergne I, Deretic V. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5437–5442. doi: 10.1073/pnas.0737613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosn EE, Cassado AA, Govoni GR, Fukuhara T, Yang Y, Monack DM, Bortoluci KR, Almeida SR, Herzenberg LA, Herzenberg LA. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2568–2573. doi: 10.1073/pnas.0915000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Hartman ML, Kornfeld H. Interactions between Naive and Infected Macrophages Reduce Mycobacterium tuberculosis Viability. PLoS ONE. 2011;6:e27972. doi: 10.1371/journal.pone.0027972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann PR, deCathelineau AM, Ogden CA, Leverrier Y, Bratton DL, Daleke DL, Ridley AJ, Fadok VA, Henson PM. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. The Journal of cell biology. 2001;155:649–659. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume DA. Bring out your dead. Nature immunology. 2008;9:12–14. doi: 10.1038/ni0108-12. [DOI] [PubMed] [Google Scholar]
- Keane J, Remold HG, Kornfeld H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J Immunol. 2000;164:2016–2020. doi: 10.4049/jimmunol.164.4.2016. [DOI] [PubMed] [Google Scholar]
- Kinchen JM, Ravichandran KS. Identification of two evolutionarily conserved genes regulating processing of engulfed apoptotic cells. Nature. 2010 doi: 10.1038/nature08853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrik IN, Krammer PH. Regulation of CD95/Fas signaling at the DISC. Cell death and differentiation. 2012;19:36–41. doi: 10.1038/cdd.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Remold HG, Ieong MH, Kornfeld H. Macrophage apoptosis in response to high intracellular burden of Mycobacterium tuberculosis is mediated by a novel caspase-independent pathway. J Immunol. 2006;176:4267–4274. doi: 10.4049/jimmunol.176.7.4267. [DOI] [PubMed] [Google Scholar]
- Martinez J, Almendinger J, Oberst A, Ness R, Dillon CP, Fitzgerald P, Hengartner MO, Green DR. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:17396–17401. doi: 10.1073/pnas.1113421108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michlewska S, Dransfield I, Megson IL, Rossi AG. Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: key role for TNF-alpha. Faseb J. 2009;23:844–854. doi: 10.1096/fj.08-121228. [DOI] [PubMed] [Google Scholar]
- Miller JL, Velmurugan K, Cowan MJ, Briken V. The type I NADH dehydrogenase of Mycobacterium tuberculosis counters phagosomal NOX2 activity to inhibit TNF-alpha-mediated host cell apoptosis. PLoS pathogens. 2010;6:e1000864. doi: 10.1371/journal.ppat.1000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. The Journal of experimental medicine. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo M, Renno T, Attinger A, Bakker T, MacDonald HR, Meylan PR. Fas ligand-induced apoptosis of infected human macrophages reduces the viability of intracellular Mycobacterium tuberculosis. J Immunol. 1998;160:5448–5454. [PubMed] [Google Scholar]
- Peng Y, Elkon KB. Autoimmunity in MFG-E8-deficient mice is associated with altered trafficking and enhanced cross-presentation of apoptotic cell antigens. The Journal of clinical investigation. 2011;121:2221–2241. doi: 10.1172/JCI43254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, Lawyer P, Fay MP, Germain RN, Sacks D. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science (New York, NY. 2008;321:970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Manzanet R, Sanjuan MA, Wu HY, Quintana FJ, Xiao S, Anderson AC, Weiner HL, Green DR, Kuchroo VK. T and B cell hyperactivity and autoimmunity associated with niche-specific defects in apoptotic body clearance in TIM-4-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8706–8711. doi: 10.1073/pnas.0910359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AG, McCutcheon JC, Roy N, Chilvers ER, Haslett C, Dransfield I. Regulation of macrophage phagocytosis of apoptotic cells by cAMP. J Immunol. 1998;160:3562–3568. [PubMed] [Google Scholar]
- Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, Fok AK, Allen RD, Gluck SL, Heuser J, Russell DG. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science (New York, NY. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- Trapani JA. Granzymes, cytotoxic granules and cell death: the early work of Dr. Jurg Tschopp. Cell death and differentiation. 2012;19:21–27. doi: 10.1038/cdd.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, Pierson J, Brenner M, Peters PJ. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129:1287–1298. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- van Zandbergen G, Klinger M, Mueller A, Dannenberg S, Gebert A, Solbach W, Laskay T. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J Immunol. 2004;173:6521–6525. doi: 10.4049/jimmunol.173.11.6521. [DOI] [PubMed] [Google Scholar]
- Vandivier RW, Henson PM, Douglas IS. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 2006;129:1673–1682. doi: 10.1378/chest.129.6.1673. [DOI] [PubMed] [Google Scholar]
- Velmurugan K, Chen B, Miller JL, Azogue S, Gurses S, Hsu T, Glickman M, Jacobs WR, Jr., Porcelli SA, Briken V. Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cells. PLoS pathogens. 2007;3:e110. doi: 10.1371/journal.ppat.0030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth JS, Wu Y, Behar SM. Mycobacterium tuberculosis-specific CD8+ T cells require perforin to kill target cells and provide protection in vivo. J Immunol. 2008;181:8595–8603. doi: 10.4049/jimmunol.181.12.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Yu X. Phagosome maturation during the removal of apoptotic cells: receptors lead the way. Trends Cell Biol. 2008;18:474–485. doi: 10.1016/j.tcb.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.