Summary
Plant-based oral vaccines are a promising emergent technology that could help alleviate disease burden worldwide by providing a low-cost, heat stable, oral alternative to parenterally administered commercial vaccines. Here we describe high-level accumulation of the hepatitis B surface antigen (HBsAg) at a mean concentration of 0.51%TSP in maize T1 seeds using an improved version of the globulin1 promoter. This concentration is more than four-fold higher than any previously reported lines. HBsAg expressed in maize seeds was extremely heat stable, tolerating temperatures up to 55°C for one month without degradation. Optimal heat stability was achieved after oil extraction of ground maize material, either by supercritical fluid extraction or hexane treatment. The contributions of this material towards the development of a practical oral vaccine delivery system are discussed.
Keywords: Hepatitis B surface antigen, mucosal vaccine, plant vaccine, bioencapsulation, immunogenicity, supercritical fluid extraction
Introduction
Over 350 million people are chronically infected with the hepatitis B virus (HBV) worldwide, with 15–25% dying prematurely of cirrhosis of the liver or hepatocellular carcinoma (CDC, 2006; Shepard et al., 2006). The disease burden is primarily felt in developing countries where the lack of refrigeration, paucity of trained health professionals, and low income levels conspire to inflate infection rates despite the existence of an effective commercial vaccine. In developed countries there are still large segments of the population that do not have access to, or do not respond well to, the commercial, injected vaccine. Hemodialysis patients, the elderly, Celiac’s disease patients, the morbidly obese, individuals with inflammatory bowel disease, and immunodeficient individuals are all poor responders (Ahishali et al., 2008; Chaves et al., 2011; Leonardi et al., 2009; Perez et al., 2009; Roome et al., 1993; Tohme et al., 2011; van den Berg et al., 2009).
A heat stable, low-cost oral vaccine could alleviate the disease burden significantly by eliminating the need for a cold-chain, by circumventing reliance on healthcare professionals, and by mitigating the requirement for large capital investment by poor countries. Furthermore, mucosal delivery could conceivably improve seroconversion rates in poor responders.
Edible plant expression systems offer a promising platform for producing oral vaccines which could provide a low-cost alternative to parenteral vaccines. These systems bioencapsulate the antigen and can be ingested after minimal processing, circumventing the need for protein extraction, purification, and formulation, thus reducing cost substantially (Daniell et al., 2009). They also have demonstrated efficacy in inducing immune responses (Lamphear et al., 2004; Tacket et al., 2004; Thanavala et al., 2005) and can provide better protection against pathogenic insult than parenteral vaccines (Lamphear et al., 2002). However, they have not gained traction as oral delivery systems due to relatively poor accumulation of antigens (Daniell et al., 2009; Rybicki, 2009). In particular, the hepatitis B surface antigen (HBsAg) used in the parenteral vaccine has been recalcitrant to accumulation in several different edible plant systems (Gao et al., 2003; Kapusta et al., 1999; Kumar et al., 2005; Qian et al., 2008; Richter et al., 2000). HBsAg is a membrane-bound protein, a class of proteins that are typically difficult to express in heterologous systems (Grisshammer, 2006). This has greatly hampered the production of an efficacious oral vaccine.
Recently, accumulation of HBsAg in maize grain reached the highest levels in an edible system reported to date, enabling a strong immune response when orally fed to mice (Hayden et al, 2012). Here we describe additional constructs engineered to improve HBsAg accumulation such that small amounts of material can be fed to animals while maintaining high dose rates of HBsAg, leading to improved immunogenicity. These DNA constructs demonstrate improved accumulation of HBsAg over previously reported material and deliver maize grain suitable for oral vaccination that is cost-effective, heat stable, and highly concentrated. This material should enable vaccine doses to be administered in small amounts of easily consumed material and to be stored long-term at ambient temperatures.
Results and Discussion
HBsAg accumulation in maize
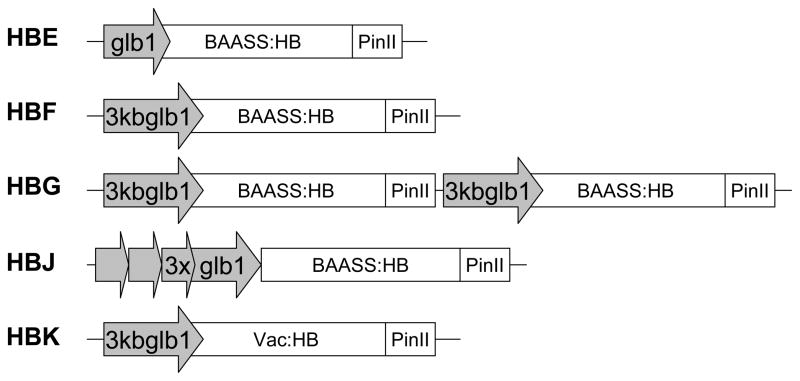
The construct accumulating the highest level of HBsAg reported to date, (Hayden et al, 2012) was used as a standard against which all new constructs were compared. New constructs (Figure 1) incorporated the 3kb extended globulin1 promoter (HBF), a promoter which has been shown to increase accumulation of GUS and trypsin relative to the shorter 1.4kb globulin1 promoter (Streatfield et al., 2010). In an attempt to further boost HBsAg levels, a double transcription cassette was formed by tandem duplication of the HBF construct (HBG), a conformation that previously increased concentrations of three independent recombinant proteins (data not shown) and therefore was a good candidate for increased protein accumulation. A synthetic promoter in which the 5′ region of the extended globulin1 promoter was repeated three times (HBJ) was also evaluated. Finally, a vacuolar targeting sequence (HBK) replaced the barley alpha amylase signal sequence (BAASS; HBF construct). The BAASS and vacuolar signal sequence have been shown to differentially affect levels of various recombinant proteins (Hood et al., 2003; Hood et al., 2007; Streatfield et al., 2003), therefore they were both included in an attempt to increase HBsAg accumulation.
Figure 1.
Construct design for the production of HBsAg in Zea mays. glb1, 1.4kb globulin1 promoter; 3kbglb1, extended globulin1 promoter; 3xglb1, tandemly repeated extended globulin1 promoter; BAASS, barley alpha amylase signal sequence; Vac, Vacuolar targeting sequence; HB, hepatitis B surface antigen; PinII, potato proteinase inhibitor II termination sequence. All constructs also contained an herbicide resistance gene following the PinII termination sequence.
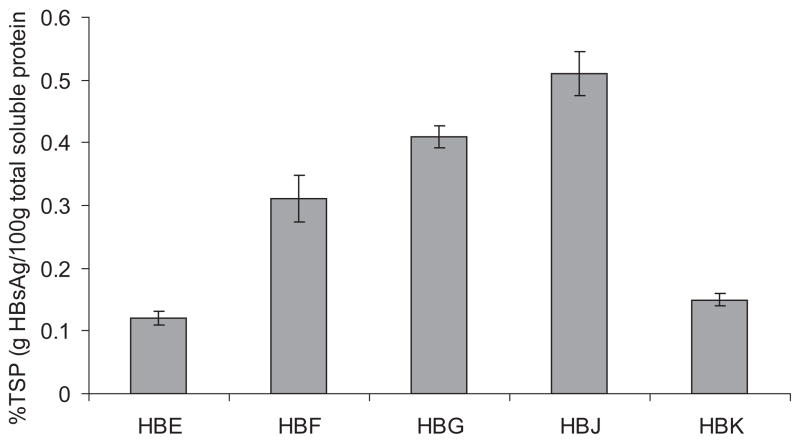
Constructs were introduced into HiII maize germplasm using Agrobacterium-mediated transformation, plants were backcrossed to non-transgenic HiII parents, and single seeds from this backcross (T1 seed) were analyzed by ELISA for HBsAg accumulation. Typically, single-insertion events with the highest concentration of HBsAg are selected for further breeding. Figure 2 depicts the difference in antigen accumulation between the various constructs of putative single-insertion, HBsAg-accumulating seed (top 10%). Encouragingly, all new constructs showed equivalent or improved mean accumulation over the previously engineered 1.4kb globulin1 promoter construct, HBE, based on the mean value for positive seeds. Comparison of the HBE and HBF lines revealed a 2.5-fold increase in accumulation when the 1.4kb promoter (0.12%TSP) was replaced by the 3kb extended globulin1 promoter (0.31%TSP).
Figure 2.
HBsAg accumulation in single seeds from the first generation (T1) after transformation, as determined by ELISA. Means of the top 10% of seeds were calculated for ears derived from putative single locus insertion events. Error bars represent standard error. Number of seeds included in the mean: n = 22, 24, 33, 24, and 23 for constructs HBE, HBF, HBG, HBJ, and HBK, respectively.
Doubling the 3kb globulin1 transcription unit (HBG) also enhanced mean HBsAg accumulation (0.41%TSP) over a single transcription unit (HBF), as expected. A further increase in accumulation was achieved by driving HBsAg expression with a 3x globulin1 promoter (HBJ; 0.51%TSP), exhibiting levels more than 4-fold higher in these lines than in the original HBE lines. The extended region of the 3kb globulin1 promoter and the tandemly repeated region of the 3x promoter contain putative RY-repeat/Sph and abscisic acid response elements (Hattori et al., 2002; Hattori et al., 1992), transcription factor binding sites which may contribute to increased transcription and subsequent protein expression and accumulation of HBsAg.
Subcellular targeting signal sequences also seemed to exert an effect on antigen accumulation. In the presence of a vacuolar targeting signal (HBK), accumulation was 2-fold lower (0.15%TSP) than cell wall-targeted antigen (HBF). The fusion of signal sequences to recombinant proteins has been shown to affect protein accumulation in a protein-specific manner (Hood et al., 2007; Streatfield et al., 2003) but to date it is not clear how signal sequences influence optimal accumulation. In the case of HBsAg, however, it is clear from empirical evidence presented here that the cell wall targeting signal is favorable for high accumulation of antigen.
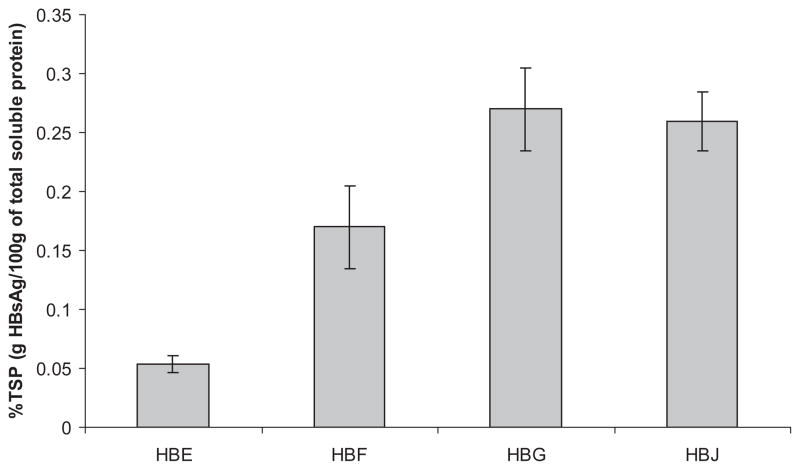
In order to initiate a backcrossing program into elite inbred lines, T1 seeds were grown and backcrossed to select parental lines. This T2 seed was collected and each ear was assayed as a 50-seed bulk. Because the transgene allelic frequency is diluted in the backcrossed T2 seed by non-transgenic seed included in the bulk, %TSP levels were roughly 2-fold lower in T2 bulks (Figure 3) compared to T1 single seeds (Figure 2), as expected. Most importantly, increases in HBsAg accumulation were maintained in the T2 generation relative to HBE, with 3-fold increases detected between HBE (0.05%TSP) and HBF (0.17%TSP) and 5-fold difference between HBE and HBG (0.27%TSP) or HBJ (0.26%TSP). Seeds from HBG and HBJ lines can now be used to produce homozygous, hybrid seed which should result in HBsAg levels well above that seen in T1 seed. Of note, HBG and HBJ lines destined for these breeding programs display single locus segregation ratios when backcrossed to parental inbreds (data not shown). Establishment of single locus status ensures that future maize line development will maintain or improve HBsAg levels since there are no multiple insertions to segregate and dilute allelic contributions from parents to progeny.
Figure 3.
HBsAg concentration in second generation (T2) ears with highest antigen accumulation, as determined by ELISA. Error bars represent standard error of two replicates.
Based on T1 and T2 seed data, HBsAg accumulation seems to be maximal in maize seed when it is fused to the BAASS signal sequence and when it is either driven by a 3kb globulin1 promoter in two tandem transcription units, or by an engineered 3x globulin1 promoter in a single transcription unit. HBsAg derived from HBE has previously been shown to dimerize and elicit a strong immunologic response in mice (Hayden et al, 2012), indicating that proteins with identical coding sequences produced in constructs HBE, HBF, HBG, and HBJ are also correctly folded and immunogenic. Production of the dimeric form has been confirmed in the HBG maize material (data not shown). Further breeding will be conducted to determine whether HBsAg accumulation differs between HBG and HBJ transgenic lines, as suggested by T1 seed data.
Effect of maize processing and temperature treatments
An ideal oral vaccine would maintain antigen integrity at ambient temperatures over long periods of time. Studies with commercialized parenteral HBsAg vaccines have elicited reduced antibody titers in human populations when the injected vaccine is exposed to 45°C for one week, 37°C for one month, or to ambient temperatures (not to exceed 49°C) for up to one month prior to administration (Hipgrave et al., 2006; Otto et al., 1999; Van Damme et al., 1992).
Cereal grains can stabilize proteins for several years at ambient temperatures (Fischer et al., 2004; Lamphear et al., 2002) but it is still unknown whether HBsAg in maize is stable under non-refrigerated conditions and whether it remains intact at ambient temperature extremes. The highest recorded temperature is 58°C, reached in El Azizia, Libya in 1922 (http://www.ncdc.noaa.gov/oa/climate/globalextremes.html). Therefore, HBsAg-expressing maize grain was ground and exposed for one week or one month to a range of temperatures between −20°C, and 80°C to span and exceed a normal ambient temperature range.
Previous studies assessing protein stability in maize grain for other antigens were conducted using oil-extracted material by hexane treatment (Lamphear et al., 2002), therefore T2 seeds containing the HBG construct were oil-extracted by this method. In addition, supercritical fluid extraction (SFE) treatment has been shown to effectively remove oil from plant material (Brunner, 2005) therefore extracts were prepared by both methods and compared to full fat seed. Total soluble protein in these samples subjected to −20°C (typical long-term storage temperature), 55°C and 80°C for one week demonstrated that while soluble proteins are comparably abundant at −20°C and 55°C, they are significantly degraded at 80°C (Table 1). Proteins in general, therefore, are protected in the seed environment at temperatures up to 55°C. In addition, the oil-extracted seed environment provides additional protection against degradation at 80°C, as is evidenced by the four-fold decrease in total protein in full fat samples relative to oil-extracted samples (Table 1).
Table 1.
Total soluble protein and HBsAg protein content in HBsAg maize seed stored at −20°C, 55°C, and 80°C for one week.
| Total soluble protein (mg protein/g maize material ± std dev) | HBsAg (μg antigen/g maize material ± std dev) | |||||
|---|---|---|---|---|---|---|
| −20°C | 55°C | 80°C | −20C | 55C | 80C | |
| Full fat | 21.7 ± 1.7 | 21.1 ± 0.3 | 2.9 ± 0.6 | 55.8±5.5 | 27.8±12.2 | <0.1 |
|
| ||||||
| Hexane-treated | 20.2 ± 0.4 | 19.6 ± 0.2 | 12.4 ± 4.4 | 51.2±3.7 | 41.1±5.0 | 0.3±0.6 |
|
| ||||||
| SFE-treated | 20.4 ± 1.5 | 19.5 ± 0.7 | 14.9 ± 5.1 | 45.9±4.4 | 48.5±4.4 | 1.7±1.7 |
To determine whether HBsAg itself is stable in maize grain at these temperatures, ELISAs and immunoblots were performed to assess antigen concentrations. By ELISA, the maize material contained approximately 50μg HBsAg/g of maize material when stored at −20°C (Table 1). Antigen levels dropped drastically when exposed to 80°C for one week, mirroring the degradation of total soluble protein. Furthermore, oil extraction may have afforded some minimal protection at this temperature, especially in SFE-treated samples, but antigen levels were highly variable from sample to sample therefore the mean antigen concentration of four replicates was not statistically different from zero. Oil extraction seemed to more clearly protect HBsAg from degradation at 55°C, as evidenced by a lower antigen concentration in full fat maize (27.8μg/g) compared to hexane or SFE-treated maize (41.1μg/g and 48.5μg/g, respectively).
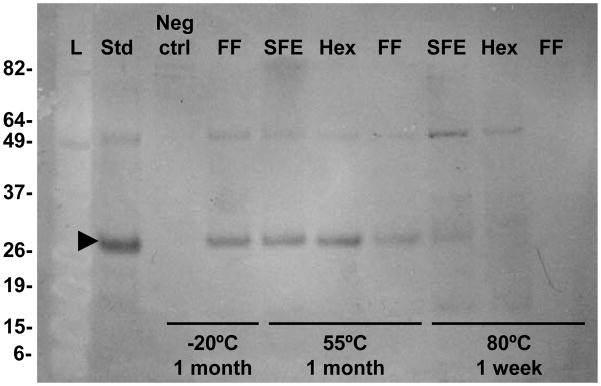
Immunoblot analysis demonstrated that maize samples either stored at −20°C or incubated at 55°C for one month contained the expected ~25kDa HBsAg band in an intact form (Figure 4). There was some decrease in concentration in the full fat seed samples incubated at 55°C but not in the oil-extracted samples. This suggests that, as with total soluble protein, HBsAg is more stable in oil-extracted maize seed and does not appreciably degrade in a 55°C environment for extended periods of time.
Figure 4.
Effect of oil extraction and temperature on maize-produced HBsAg, as determined by immunoblot. Arrowhead indicates monomeric form of HBsAg. L, prestained protein ladder; Std, 100ng yeast-derived rHBsAg standard; Neg ctrl, non-transgenic maize material; FF, full fat HBsAg maize; SFE, supercritical fluid extracted maize; Hex, hexane extracted maize. All maize samples were loaded at 1.5μg of total protein with the exception of the full fat sample treated at 80°C which was loaded at a maximum well volume (equivalent to 0.5μg). This corresponds to 0.2 to 0.3mg of starting maize material in lanes 3 through 9 and 0.5mg in lane 10.
After one week at 80°C, the full fat samples have undetectable levels of HBsAg when examined by immunoblot analysis whereas SFE and hexane-treated samples show faint bands, further supporting an oil-extraction stabilization effect. This effect is corroborated by ELISAs (Table 1). The higher concentration of HBsAg in the SFE samples relative to hexane-treated samples may reflect differential protective effects based on oil extraction methods but longer-term studies will need to be conducted to confirm these observations. It is clear, however, that maize grain provides an extremely stable environment for HBsAg and can be used as a storage medium at locales where a cold-chain is not available.
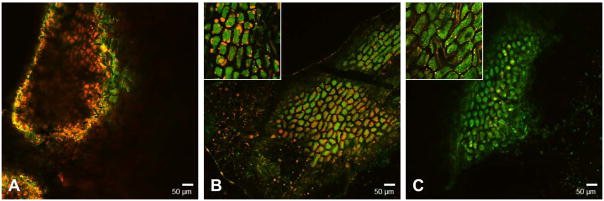
To begin to understand SFE and hexane treatment effects on protein stability, we sought to visualize the lipid removal efficiencies of both oil extraction methods. Because HBsAg is preferentially expressed in the embryo portion of the seed under the globulin1 promoter (Streatfield et al., 2010), embryos were dissected from whole seeds, ground, and imaged by confocal microscopy. As expected, oil-extracted samples had drastically reduced lipid content relative to the full fat samples while retaining large amounts of protein (Figure 5). SFE treatment seemed to extract oil more efficiently than hexane treatment, as was evidenced by the paucity of lipid staining (red color) in SFE samples. Differential oil extractions were confirmed by mass balance analyses which calculated 26% oil removal in SFE-treated samples and 24% oil removal in hexane-treated samples. Higher efficiency of lipid extraction by SFE could potentially explain the greater temperature protective effect afforded the SFE-treated samples relative to the hexane-treated samples in the ELISA assays and Western blot. In future studies, it will be imperative to test whether oil-extraction methods can affect the immunogenicity of maize material. These images, in conjunction with the improved protein integrity exhibited in the ELISAs and immunoblots, suggest that SFE-treated samples may produce the most immunogenic material when stored long-term at ambient temperatures.
Figure 5.
Presence of protein and lipids in A) full fat, B) hexane-treated, and C) SFE-treated maize samples as detected by confocal microscopy. Material was magnified to 40X in the main panels and 100X in the inset panels. Ground maize was stained with Nile Red to detect triglycerides, and Fast Green to detect protein.
To date, we have produced maize material that contains HBsAg at high concentrations and is highly resistant to temperature-mediated degradation, and therefore could be used as a practical oral delivery vehicle at a low cost in both industrialized countries and poorer nations around the globe. Further characterization and breeding of the material will be required to attain maximal HBsAg accumulation, and should be attainable in the near future. If comparable gains in antigen concentration are achieved in these new lines relative to gains achieved in the previously characterized HBE line (Hayden et al, 2012), production of hybrid embryo material will conservatively reach 3mg HBsAg per gram of material. This concentration is more than 350-fold higher than expression levels achieved in other edible plant systems and an increase of this magnitude will translate to a significant dosing advantage. One mg HBsAg doses could easily be administered in 300mg tablets or small wafers, dosing which is compatible with the pharmacy distribution system and is amenable to transportation into remote areas of the globe.
Experimental Procedures
Construct design
The small form of the hepatitis B surface antigen (HBsAg) sequence (Genbank accession S62754.1) was codon optimized for expression in maize as described previously (Hayden et al, 2012; Streatfield et al., 2001). Constructs HBE and HBF were designed to drive expression of HBsAg using the 1.4kb and 3kb globulin1 promoter sequences (Hayden et al, 2012; Streatfield et al., 2010), respectively. In both constructs the HBsAg protein was fused at the N-terminus to a codon optimized type B barley alpha amylase signal sequence (BAASS; (Rogers, 1985). A third construct (HBG) contained the same plant transcription unit as HBF, but in duplicate, in a head-to-tail conformation. A fourth construct (HBJ) contained three copies of the 5′-most 1745bp of the 3kb globulin1 promoter in tandem, followed by the remaining promoter sequence. This promoter expressed the same BAASS:HBsAg fusion protein as in HBF. A fifth construct (HBK), contained a vacuolar targeting signal derived from barley aleurain (Holwerda et al., 1992) and was fused to the N-terminus of HBsAg. All constructs contained a potato protease inhibitor II (PinII) termination sequence (An et al., 1989). Following the PinII sequence a glufosinate resistance gene, the maize optimized Pat gene from Streptomyces viridiochromogenes, was used to screen for transformants as described previously (Streatfield et al., 2002).
Transformation into maize and propagation of seeds
Constructs were transferred first into Agrobacterium tumefaciens and were then used to transform maize as described previously (Hood et al., 2003). Transformation events were selected using bialaphos, and propagated as described previously (Streatfield et al., 2001; Streatfield et al., 2002). Individual seeds were analyzed for HBsAg expression and the lines showing the highest expression were chosen for backcrosses. The HBE lines were backcrossed as previously described (Hayden et al, 2012) and all other construct lines were backcrossed into elite inbred lines 16038 and MBS5411. Seed was collected from the second generation and 50 seeds were pooled and analyzed from each ear. The lines showing the highest levels of expression were used to repeat the backcrossing program in future generations.
Oil extraction
Seed from two T2 HBG lines were ground to cornmeal consistency, pooled, and subjected to oil extraction by supercritical CO2 or hexane. Supercritical fluid extraction (SFE) was performed at a pressure of 350 bar, a flowrate of 20–50g CO2/min, vessel (sample chamber) temperature of 35°C, and a cyclone (oil collection chamber) temperature of 50°C (see (Brunner, 2005) for review of SFE). Extraction was terminated when oil was no longer expelled from the sample. For hexane extractions, a total of 5mL of hexane over three extractions was used for every gram of ground maize material. Samples were vigorously mixed for 15 minutes during each extraction and filtered through No.1 Whatman filter paper using a Buchner funnel. Samples were dried in a fume hood to remove residual hexane.
Immunoblotting of maize material
Protein was extracted by three consecutive extractions from 100mg of ground seed. The first two extractions were done in 1mL of PBS+0.05%Tween, followed by a final extraction in 1mL PBS+1%TritonX-100. Extracts were prepared in NuPAGE LDS Sample Buffer (Invitrogen cat#NP0007) with 50mM DTT, heated for 10 minutes at 70°C, loaded onto a 10% Bis-Tris SDS PAGE gel, run in NuPAGE MES SDS running buffer, and transferred to a PVDF membrane using the iBlot system (Invitrogen). Rabbit anti-HBsAg (Genway#18-511-245179), AP-conjugated goat anti-rabbit IgG (Jackson#111-055-003), and BCIP/NBT liquid substrate (Sigma#B1911) were used to visualize HBsAg bands.
Antigen detection by ELISA
Protein extraction of ground maize material consisted of agitating either a single ground seed, or 100mg of ground 50-seed bulk, in 1mL of extraction buffer (PBS+1%TritonX-100). Six single seeds were assayed from each ear while 50-seed bulks were sampled in duplicate. Total soluble protein in the supernatant was determined using the Coomassie Brilliant Blue assay (Bradford, 1976). HBsAg was detected by a sandwich ELISA (Hayden et al, 2012) in which rabbit anti-HBsAg was used as a coating antibody (GenWay, cat# 18-511-245179), biotinylated anti-HBsAg (Meridian Life Science, cat# B65811B ) was used as a secondary antibody, and streptavidin-AP with pNPP were used to induce a chromophore response detected at 405nm. Washing buffer (PBS+0.05%Tween) was used between reagent steps, and blocking solution (3%BSA in PBS+0.05%Tween) after coating of the plates. Recombinant HBsAg (GenWay, cat# 10-663-45361) was used as a standard.
Because T1 single seeds are highly variable in size and not routinely weighed before ELISA assays, %TSP is the only metric used to determine expression. In subsequent generations, seed was pooled, ground, and assayed per 100mg, giving both μg/g and %TSP levels of expression (Hayden et al, 2012).
Confocal microscopy
Germ samples from HBE lines (Hayden et al, 2012) were oil extracted as described above, dried to 6–15% moisture, ground, resuspended in PBS, and stained with Fast Green and Nile Red to detect protein and triglycerides, respectively. Nile Red was excited at 488nm and filtered images were collected between 500 and 600nm. Fast Green was excited at 633nm and filtered images were collected between 655 and 755nm.
Acknowledgments
This project was funded by NIH grant number 5R43AI068239-02 and 3R43AI068239-01A1S1.
References
- Ahishali E, Boztas G, Akyuz F, Ibrisim D, Poturoglu S, Pinarbasi B, Ozdil S, Mungan Z. Response to Hepatitis B Vaccination in Patients with Celiac Disease. Dig Dis Sci. 2008;53:2156–2159. doi: 10.1007/s10620-007-0128-3. [DOI] [PubMed] [Google Scholar]
- An G, Mitra A, Choi HK, Costa MA, An K, Thornburg RW, Ryan CA. Functional Analysis of the 3′ Control Region of the Potato Wound-Inducible Proteinase Inhibitor II Gene. Plant Cell. 1989;1:115–122. doi: 10.1105/tpc.1.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brunner G. Supercritical fluids: technology and application to food processing. J Food Eng. 2005;67:21–33. [Google Scholar]
- CDC. A Comprehensive Immunization Strategy to Eliminate Transmission of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: Immunization of Adults. MMWR. 2006;55(RR-16):1–33. [PubMed] [Google Scholar]
- Chaves SS, Daniels D, Cooper BW, Malo-Schlegel S, MacArthur S, Robbins KC, Kobetitsch JF, McDaniel A, D’Avella JF, Alter MJ. Immunogenicity of hepatitis B vaccine among hemodialysis patients: Effect of revaccination of non-responders and duration of protection. Vaccine. 2011;29:9618–9623. doi: 10.1016/j.vaccine.2011.10.057. [DOI] [PubMed] [Google Scholar]
- Daniell H, Singh ND, Mason H, Streatfield SJ. Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci. 2009;14:669–679. doi: 10.1016/j.tplants.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R, Stoger E, Schillberg S, Christou P, Twyman RM. Plant-based production of biopharmaceuticals. Curr Opin Plant Biol. 2004;7:152–158. doi: 10.1016/j.pbi.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Gao Y, Ma Y, Li M, Cheng T, Li SW, Zhang J, Xia NS. Oral immunization of animals with transgenic cherry tomatillo expressing HBsAg. World J Gastroenterol. 2003;9:996–1002. doi: 10.3748/wjg.v9.i5.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisshammer R. Understanding recombinant expression of membrane proteins. Curr Opin Biotech. 2006;17:337–340. doi: 10.1016/j.copbio.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Hattori T, Totsuka M, Hobo T, Kagaya Y, Yamamoto-Toyoda A. Experimentally determined sequence requirement of ACGT-containing abscisic acid response element. Plant Cell Physiol. 2002;43:136–140. doi: 10.1093/pcp/pcf014. [DOI] [PubMed] [Google Scholar]
- Hattori T, Vasil V, Rosenkrans L, Hannah LC, McCarty DR, Vasil IK. The Viviparous-1 gene and abscisic acid activate the C1 regulatory gene for anthocyanin biosynthesis during seed maturation in maize. Gene Dev. 1992;6:609–618. doi: 10.1101/gad.6.4.609. [DOI] [PubMed] [Google Scholar]
- Hayden CA, Streatfield SJ, Lamphear BJ, Fake GM, Keener TK, Walker JH, Clements JD, Turner DD, Tizard IR, Howard JA. Bioencapsulation of the hepatitis B surface antigen and its use as an effective immunogen. Vaccine. 2012;30:2937–2942. doi: 10.1016/j.vaccine.2012.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipgrave DB, Tran TNAM, Huong VUM, Do Tuan D, Nga NT, Long HT, Van NTHU, Maynard JE, Biggs BANN. Immunogenicity of a locally produced hepatitis B vaccine with the birth dose stored outside the cold chain in rural Vietnam. Am J Trop Med Hyg. 2006;74:255–260. [PubMed] [Google Scholar]
- Holwerda BC, Padgett HS, Rogers JC. Proaleurain vacuolar targeting is mediated by short contiguous peptide interactions. Plant Cell Online. 1992;4:307–318. doi: 10.1105/tpc.4.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood EE, Bailey MR, Beifuss K, Magallanes Lundback M, Horn ME, Callaway E, Drees C, Delaney DE, Clough R, Howard JA. Criteria for high level expression of a fungal laccase gene in transgenic maize. Plant Biotechnol J. 2003;1:129–140. doi: 10.1046/j.1467-7652.2003.00014.x. [DOI] [PubMed] [Google Scholar]
- Hood EE, Love R, Lane J, Bray J, Clough R, Pappu K, Drees C, Hood KR, Yoon S, Ahmad A. Subcellular targeting is a key condition for high-level accumulation of cellulase protein in transgenic maize seed. Plant Biotechnology Journal. 2007;5:709–719. doi: 10.1111/j.1467-7652.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- Kapusta J, Modelska A, Figlerowicz M, Pniewski T, Letellier M, Lisowa O, Yusibov V, Koprowski H, Plucienniczak A, Legocki A. A plant-derived edible vaccine against hepatitis B virus. FASEB J. 1999;13:1796–1799. doi: 10.1096/fasebj.13.13.1796. [DOI] [PubMed] [Google Scholar]
- Kumar GBS, Ganapathi T, Revathi C, Srinivas L, Bapat V. Expression of hepatitis B surface antigen in transgenic banana plants. Planta. 2005;222:484–493. doi: 10.1007/s00425-005-1556-y. [DOI] [PubMed] [Google Scholar]
- Lamphear BJ, Jilka JM, Kesl L, Welter M, Howard JA, Streatfield SJ. A corn-based delivery system for animal vaccines: an oral transmissible gastroenteritis virus vaccine boosts lactogenic immunity in swine. Vaccine. 2004;22:2420–2424. doi: 10.1016/j.vaccine.2003.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamphear BJ, Streatfield SJ, Jilka JM, Brooks CA, Barker DK, Turner DD, Delaney DE, Garcia M, Wiggins B, Woodard SL. Delivery of subunit vaccines in maize seed. J Control Release. 2002;85:169–180. doi: 10.1016/S0168-3659(02)00282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi S, Spina M, Spicuzza L, Rotolo N, La Rosa M. Hepatitis B vaccination failure in celiac disease: Is there a need to reassess current immunization strategies? Vaccine. 2009;27:6030–6033. doi: 10.1016/j.vaccine.2009.07.099. [DOI] [PubMed] [Google Scholar]
- Otto BF, Suarnawa I, Stewart T, Nelson C, Ruff TA, Widjaya A, Maynard JE. At-birth immunisation against hepatitis B using a novel pre-filled immunisation device stored outside the cold chain. Vaccine. 1999;18:498–502. doi: 10.1016/s0264-410x(99)00242-x. [DOI] [PubMed] [Google Scholar]
- Perez LV, Camacho FG, Sanchez VG, Flores EMI, Molina LC, Ruiz AC, Vega LCJyFDD. Eficacia de la vacuna contra el virus de la hepatitis B en pacientes con enfermedad inflamatoria intestinal. Med Clin (Barc) 2009;132:331–335. doi: 10.1016/j.medcli.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Qian B, Shen H, Liang W, Guo X, Zhang C, Wang Y, Li G, Wu A, Cao K, Zhang D. Immunogenicity of recombinant hepatitis B virus surface antigen fused with preS1 epitopes expressed in rice seeds. Transgenic Res. 2008;17:621–631. doi: 10.1007/s11248-007-9135-6. [DOI] [PubMed] [Google Scholar]
- Richter LJ, Thanavala Y, Arntzen CJ, Mason HS. Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat Biotechnol. 2000;18:1167–1171. doi: 10.1038/81153. [DOI] [PubMed] [Google Scholar]
- Rogers JC. Two barley alpha-amylase gene families are regulated differently in aleurone cells. J Biol Chem. 1985;260:3731–3738. [PubMed] [Google Scholar]
- Roome AJ, Walsh SJ, Cartter ML, Hadler JL. Hepatitis B vaccine responsiveness in Connecticut public safety personnel. JAMA. 1993;270:2931–2934. [PubMed] [Google Scholar]
- Rybicki EP. Plant-produced vaccines: promise and reality. Drug Discov Today. 2009;14:16–24. doi: 10.1016/j.drudis.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B Virus Infection: Epidemiology and Vaccination. Epidemiol Rev. 2006;28:112–125. doi: 10.1093/epirev/mxj009. [DOI] [PubMed] [Google Scholar]
- Streatfield SJ, Bray J, Love RT, Horn ME, Lane JR, Drees CF, Egelkrout EM, Howard JA. Identification of maize embryo-preferred promoters suitable for high-level heterologous protein production. GM crops. 2010;1:162–172. doi: 10.4161/gmcr.1.3.12816. [DOI] [PubMed] [Google Scholar]
- Streatfield SJ, Jilka JM, Hood EE, Turner DD, Bailey MR, Mayor JM, Woodard SL, Beifuss KK, Horn ME, Delaney DE. Plant-based vaccines: unique advantages. Vaccine. 2001;19:2742–2748. doi: 10.1016/S0264-410X(00)00512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streatfield SJ, Lane JR, Brooks CA, Barker DK, Poage ML, Mayor JM, Lamphear BJ, Drees CF, Jilka JM, Hood EE. Corn as a production system for human and animal vaccines. Vaccine. 2003;21:812–815. doi: 10.1016/s0264-410x(02)00605-9. [DOI] [PubMed] [Google Scholar]
- Streatfield SJ, Mayor JM, Barker DK, Brooks C, Lamphear BJ, Woodard SL, Beifuss KK, Vicuna DV, Massey LA, Horn ME. Development of an edible subunit vaccine in corn against enterotoxigenic strains of Escherichia coli. In Vitro Cell Dev Biol Plant. 2002;38:11–17. [Google Scholar]
- Tacket CO, Pasetti MF, Edelman R, Howard JA, Streatfield S. Immunogenicity of recombinant LT-B delivered orally to humans in transgenic corn. Vaccine. 2004;22:4385–4389. doi: 10.1016/j.vaccine.2004.01.073. [DOI] [PubMed] [Google Scholar]
- Thanavala Y, Mahoney M, Pal S, Scott A, Richter L, Natarajan N, Goodwin P, Arntzen CJ, Mason HS. Immunogenicity in humans of an edible vaccine for hepatitis B. Proc Natl Acad Sci USA. 2005;102:3378–3382. doi: 10.1073/pnas.0409899102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohme RA, Awosika-Olumo D, Nielsen C, Khuwaja S, Scott J, Xing J, Drobeniuc J, Hu DJ, Turner C, Wafeeg T. Evaluation of hepatitis B vaccine immunogenicity among older adults during an outbreak response in assisted living facilities. Vaccine. 2011;29:9316–9320. doi: 10.1016/j.vaccine.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme P, Cramm M, Safary A, Vandepapeliere P, Meheus A. Heat stability of a recombinant DNA hepatitis B vaccine. Vaccine. 1992;10:366–367. doi: 10.1016/0264-410x(92)90064-q. [DOI] [PubMed] [Google Scholar]
- van den Berg R, van Hoogstraten I, van Agtmael M. Non-Responsiveness to Hepatitis B Vaccination in HIV Seropositive Patients; Possible Causes and Solutions. AIDS Rev. 2009;11:157–164. [PubMed] [Google Scholar]