Abstract
Pattern separation, the process by which similar experiences can be stored as distinct memories, has been ascribed to the dentate gyrus (DG) of the hippocampus. The DG is the target of noradrenergic modulation directly and indirectly via the basolateral amygdala. We tested the hypothesis that noradrenergic activation (tested using salivary alpha-amylase) potentiates DG function, enhancing pattern separation, by showing participants fearful stimuli in a pre-training task and then testing their capacity for pattern separation in a later test. Consistent with our hypothesis, we found that increased levels of salivary alphaamylase were positively correlated with enhanced pattern separation performance even after accounting for general enhancements in recognition.
Keywords: Norepinephrine, Emotion, Pattern separation, Dentate gyrus
1. Introduction
Studies in rodents and humans have emphasized the role of the dentate gyrus (DG) subfield of the hippocampus in the process of pattern separation (orthogonalizing similar inputs to facilitate rapid storage; cf review by Yassa & Stark, 2011). Additionally, a large body of evidence strongly supports the notion that norepinephrine enhances the consolidation of new memories in animals and humans in a dose-dependent manner (Cahill, Prins, Weber, & McGaugh, 1994; McGaugh, 2000; McIntyre, Hatfield, & McGaugh, 2002; Segal & Cahill, 2009). The majority of this evidence stems from pharmacological experiments where post-training administration of adrenergic agonists and antagonists resulted in memory enhancement or impairment, respectively (Cahill & Alkire, 2003; Cahill et al., 1994; van Stegeren, Everaerd, Cahill, McGaugh, & Gooren, 1998; van Stegeren et al., 2005). Emotionally arousing encoding tasks result in memory enhancement, the extent of which is positively correlated with endogenous norepinephrine (NE) activation in animals and humans (McIntyre et al., 2002; Segal & Cahill, 2009). The purpose of the current experiment was to examine whether NE activation is associated with an enhanced ability to overcome interference across similar stimuli (i.e. engage in pattern separation).
The DG contains a high concentration of NE receptors and receives NE input via projections from the perforant path (PP) of the hippocampus (Young & Kuhar, 1980). The DG also receives strong modulation via direct NE projections from the locus coeruleus (LC) to the DG, and glutamatergic projections from the basolateral amygala (BLA), which is innervated by noradrenergic projections from the LC (McGaugh, 2002).
In addition to playing a role in long-term memory enhancement (McIntyre et al., 2003), long term potentiation (LTP) studies have shown that LC activation initiates a β-adrenergic and protein synthesis- dependent long-term, but not short-term increase in the synaptic strength of concurrently activated PP input to the DG (Brown, Walling, Milway, & Harley, 2005). It has been reported that NE release converts early phase frequency-induced LTP of PP input to an enduring late phase form (Harley, 2007). Based on studies showing that NE plays an integral role in memory enhancement, and synaptic plasticity of input to the PP/ DG of the hippocampus, we hypothesized that increasing endogenous NE via exposure to emotionally arousing stimuli would enhance the ability of the DG to discriminate among similar stimuli (i.e. facilitate pattern separation).
Salivary alpha amylase (sAA) is a well-established biomarker for NE (Chatterton, Vogelsong, Lu, Ellman, & Hudgens, 1996; Ehlert, Erni, Hebisch, & Nater, 2006). Secretions of sAA result primarily from NE released from sympathetic nerves that innervate acinar cells in the parotid gland (Castle & Castle, 1998; Ehlert et al., 2006; Whelton, 1996). NE binds to G-protein coupled receptors on the acinar cells of the parotid gland, activating cAMP, which results in the synthesis and secretion of alpha-amylase within approximately twenty seconds of NE binding to the receptors (Yoshimura, Fujita-Yoshigaki, Murakami, & Segawa, 2002).
Chatterton et al. (1996) measured sAA during various stress conditions, including aerobic exercise and written examinations, and found that sAA levels were tightly coupled to NE (and not epinephrine) levels. Pharmacological blockade of beta-adrenergic receptors using propranolol lowers sAA levels in situations where psychological stress (using emotional pictures) is used to increase arousal (van Stegeren, Rohleder, Everaerd, & Wolf, 2006). Further-more, pharmacological enhancement of NE using yohimbine results in increased sAA levels (Ehlert et al., 2006). Salivary alpha-amylase also appears to be more accurate measurement of central NE then blood plasma measurements of NE since the latter is also subject to spillover from the adrenal medulla (Ehlert et al., 2006). Taken together, these results demonstrate that sAA is a sensitive index for endogenous NE activity. Thus, we opted to assess NE activation using sAA levels before and after exposure to emotionally arousing stimuli.
2. Materials and methods
2.1. Participants
Thirty-three females (ages 18–35, mean = 22.8 years old) from the University of California, Irvine participated in this study in exchange for course credit. We restricted participation to females because they respond more reliably to the emotional pictures used to elicit the NE response (Segal & Cahill, 2009).
2.2. Experimental procedures
All experiments were conducted between the hours of 13:00 and 18:00 in order to control for the circadian rhythm of sAA. Participants were asked to fast, as well as not chew gum for two hours prior to the experiment. They were also asked to refrain from exercise and the consumption of alcohol and caffeine for 24 h prior to the experiment, so as to control for outside factors that could affect sAA levels. All experiments were presented and responses were collected using PsychToolbox running under the MATLAB 7.5 programming and runtime environment (The MathWorks, Natick, MA).
Fifteen minutes after the participant’s arrival to the laboratory a baseline salivary sample was taken (timepoint 1; see Fig. 1). Participants then viewed a slideshow of 138 randomly presented, emotionally arousing images taken from the International Affective Picture Set (IAPS). During the presentation of each picture, the participants rated the intensity of their personal emotional reaction to viewing that picture on a 9-point scale, ranging from 1 (not emotionally arousing) to 9 (extremely emotionally arousing), using standard keyboard button input. Each picture appeared on the screen for 5000 ms, followed by a fixation cross for 1000 ms. Only pictures with a negative valence were used in this study, since preliminary data suggests that these stimuli are much more effective in eliciting an NE response in female participants (Segal & Cahill, 2009). Immediately following the 15 min slide show, a second salivary sample was taken (timepoint 2).
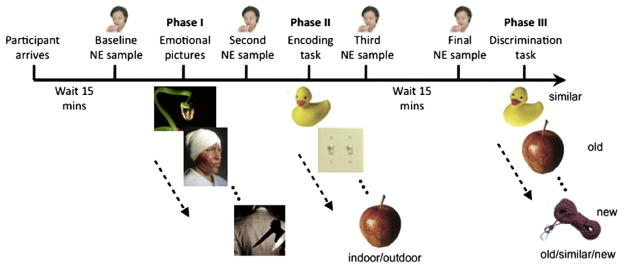
Fig. 1.
Overview of experimental procedures. Participants provided salivary samples at four time points: prior to encoding emotionally-arousing pictures, prior to incidental encoding of novel pictures, immediately following the encoding phase, and immediately prior to an old/similar/new discrimination task.
Participants then engaged in an incidental-encoding task previously used to assess pattern separation processing (Lacy, Yassa, Stark, Muftuler, & Stark, 2011), in which a set of 128 everyday items was presented in random order for 2000 ms with a 500 ms interstimulus-interval. Participants indicated whether the item was an “indoor” or an “outdoor” object by pressing keys on the keyboard (‘B’ for indoor, and ‘V’ for outdoor). Immediately following the incidental encoding task, a third salivary sample was taken (timepoint 3). Participants then experienced a 15 min delay, in which they engaged in non-arousing tasks (e.g. solving puzzles, word searches, etc.) to allow sAA levels to return to baseline and a fourth salivary sample was taken (timepoint 4).
Participants were then given a surprise subsequent memory test, in which a set of 192 pictures was randomly presented (Fig. 2). The pictures included exact repetitions of the objects previously shown during the encoding phase (64 targets), slight variations of previously shown objects (64 lures), and objects that were not previously presented (64 foils). These stimuli have been previously developed and the “mnemonic similarity” of the lures has been calibrated (see Yassa, Lacy, et al., 2010; Yassa, Stark et al., 2010; Yassa, Muftuler, Stark, 2010 and Lacy et al., 2011 for details). The task itself is based on earlier work by Koutstaal and colleagues (1997). Participants were instructed to indicate by pressing a key whether the object was old (‘V’ key), similar (‘B’ key), or new (‘N’ key). Pictures remained on the screen until the participant pressed a response key and then advanced to the next trial.
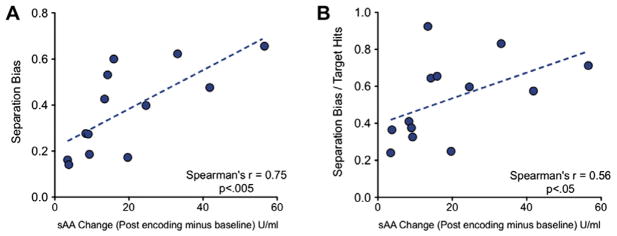
Fig. 2.
Relationship between change in salivary alpha amylase (sAA) and pattern separation. (A) Change in sAA, indexing the levels of endogenous NE, was correlated with separation bias scores (an index of pattern separation performance) (B) Even when overall memory performance is taken into account (dividing the separation bias by the target hit rate), the sAA change reliably predicts pattern separation performance. Both tests were conducted using nonparametric Spearman’s correlation.
3. Results and discussion
3.1. Salivary alpha-amylase response
Of the 33 participants, 13 responded reliably with an increase in their sAA levels (timepoint 3–timepoint 1), which is consistent with the 36% of positive responders reported previously (Segal & Cahill, 2009). Timepoint 3 reflects sAA levels immediately after encoding of the study objects, and timepoint 1 reflects baseline levels of sAA prior to the presentation of the emotional pictures (pre-training). This difference between these two reflects the elevated NE response during the encoding of the mnemonic similarity task. Participants who reliably showed this elevation will be referred to as responders. Eighteen participants who did not display an increase in sAA will be referred to as non-responders. Two participants were excluded from either group because their recognition behavioral performance was more than 2 standard deviations below the mean.
While it might be tempting to look for a difference in the bias for pattern separation in responders compared to non-responders, we are unable to use the non-responder group in this way. The non-responder group is operationally defined by no increase in sAA levels following the presentation of emotional stimuli (timepoint 3) from baseline (timepoint 1), which could occur for numerous reasons. An individual could have come into the study with high baseline levels, could be insensitive to the emotional pictures, or any number of other explanations. Thus, we opted to only use the responder group for this study. Since this group all showed a reliable change in sAA, we are also able to use the degree of change in sAA as a predictor.
In order to further examine sAA dynamics in the responder group, we compared timepoint 1 to timepoint 2 and found a significant increase in sAA (t12= 2.27, p < .05), suggesting that the IAPS stimuli were effective at raising endogenous NE levels. We also compared timepoint 3 to timepoint 1 and found a significant increase in sAA (t12= 4.44, p < .001), which is of course expected since we selected the group based on the criterion that timepoint 3 must be higher than timepoint 1. This analysis, though it may appear circular, is not intended to demonstrate anything aside from the fact that our selection criteria were effective. We found no significant difference between timepoint 2 and timepoint 3 (t12 = .34, p > .05), suggesting that NE levels were sustained at a high level throughout the encoding task. Finally, we found no significant difference between timepoint 4 and the baseline timepoint 1 (t12 = 2.16, p > .05) suggesting that NE levels at test had returned to baseline.
3.2. Pattern separation performance
We hypothesized that higher levels of endogenous NE could facilitate pattern separation on lure items. To evaluate pattern separation performance, we calculated a “separation bias” score based on the difference in the probability of “similar” responses to lure items from the probability of “similar” responses to foil items: p(“Similar”|Lure) – p(“Similar”|Foil). This metric allows us to index pattern separation ability, while correcting for any overall bias in participants’ probability of responding “similar”. We then calculated the correlation between the separation bias and the change in sAA levels (timepoint 3–timepoint 1) using a Spearman’s correlation (the sAA difference metric showed clear signs of violating the Gaussian assumption of parametric tests). We found that performance on the separation task was strongly positively correlated with increase of sAA, (Spearman’s r = 0.75, p < .005; Fig. 2A). As predicted, the correlation indicates a positive effect of NE activation on pattern separation performance.
One possibility is that this correlation was simply driven by increased memory performance overall, since noradrenergic activation at encoding results in subsequent memory enhancement (McGaugh, 2000, 2004; McIntyre et al., 2002; Segal & Cahill, 2009). To factor out such an effect of general memory enhancement, we calculated a new corrected metric that divided each participant’s separation bias by her overall hit rate (p(“Old”|Target). Accounting for hit rate, the separation bias still showed clear evidence of a correlation with change in sAA (Spearman’s r = 0.56; p < .05; Fig. 2B). These data indicate that pattern separation in particular is enhanced in individuals with greater sAA modulation above and beyond the general enhancement in recognition memory performance.
We selected the sAA responses at timepoint 3–timepoint 1 as the most appropriate contrast to capture the peak of the NE response to the emotional stimuli that was sustained throughout the exposure to the study task. In this way, we predicted that an increase in the NE response would result in greater pattern separation processing during the study task. In order to address whether an increase in the sAA response prior to the test session might have influenced pattern separation performance, we inspected the sAA response at timepoint 4 (just prior to the old/ similar/new test session) – timepoint 1 (initial baseline). We found no correlation between sAA response and separation bias (r = .15; p = .62), indicating that test performance is not modulated by NE activity. Likewise, we investigated the sAA response at timepoint 2 (just prior to study encoding) – timepoint 1 (initial baseline), and again did not observe a relationship with pattern separation performance at test (r =−.05; p = .86). These data suggest that only sAA modulation during exposure to the study images (timepoint 3–timepoint 1) predicts subsequent pattern separation performance, consistent with previous reports suggesting that the coincidence of NE and the training episode is required for NE modulation to be effective (McIntyre et al., 2002).
One interesting aspect of the study is the low NE arousal response rate (13 out of 33 participants), which has been demonstrated in the past. It is likely that there are individual differences in the amount of arousal generated by emotional stimuli. For example, it is possible that we needed more extreme emotional stimuli to trigger arousal in the non-responder group. If this was the case, one might predict that stimulus ratings might reveal such sensitivity. Unfortunately, when we compared the emotional ratings in the task between responders to non-responders we found no significant group difference. Nevertheless, subjective ratings may not be sensitive enough to detect differential propensity for arousal. Future work using additional measures of arousal (e.g. pupil dilation, galvanic skin response) that captures alterations in emotional arousal before and after viewing each slide could be helpful in this regard. In particular, pupil dilation, measured via eye tracking, has been established as a reliable way to capture arousal differences on a stimulus-by-stimulus basis (Nielsen, Ertman, Lakhani, & Cahill, 2011).
We chose to only include women in this study because of the reduced variability in emotional responsiveness, however, there may be an important role for sex differences in emotional memory tasks. For example, there is some evidence that men and women process emotional information differently, where memory for the gist of an emotional story is enhanced in men, and memory for the details of an emotional story is enhanced in women (Cahill & van Stegeren, 2003; Cahill et al., 1994; Seidlitz & Diener, 1998). The beta-blocker, propranolol blocks norepinephrine and subsequently blocks the enhanced detail memory in women and enhanced gist memory in men (Cahill et al., 1994). Nielsen et al. (2011) reported enhanced detailed memory relative to the gist of an emotional story in naturally cycling young women, and enhanced gist memory for the same story in women on hormonal contraception. Memory for details vs. gist is thought is to be differentially facilitated by pattern separation (Yassa & Stark, 2011), thus investigating emotional pattern separation in men and women separately is an important future avenue of investigation.
A related finding is that pattern separation is compromised in healthy aging (Yassa, Lacy, et al., 2010; Lacy et al., 2011; Yassa, Mattfeld, Stark, & Stark, 2011) and individuals with amnestic Mild Cognitive Impairment (aMCI) (Yassa, Stark, et al., 2010), which may be closely linked to deterioration of the perforant (Kalus et al., 2006; Rogalski et al., 2009; Yassa, Muftuler, et al., 2010). Perhaps the deterioration of this pathway also decreases the noradrenergic input to the DG, therefore disrupting the DG’s ability to engage in pattern separation. Future experiments should examine varying pharmacological doses of noradrenergic agonists in both younger and older adults as well as aMCI patients to more fully understand the contribution of norepinephrine to pattern separation performance. Additionally, high resolution functional MRI combined with sAA measurement could allow for further examination of the extent to which norepinephrine plays a role in DG/CA3 activation and how this in turn modulates pattern separation in humans.
The findings reported here are the first to suggest that endogenous NE activation plays a role in pattern separation. Both human and animal studies have emphasized the role of the DG/CA3 in pattern separation (Yassa & Stark, 2011). Given the neuroanatomical evidence that there are direct noradrenergic projections from the perforant path to the DG (Young & Kuhar, 1980), it seems plausible that noradrenergic activation could play a critical role in modulating the DG’s ability to engage in pattern separation. While there is abundant evidence that increased NE enhances memory consolidation in animals and humans (Cahill et al., 1994; McGaugh, 2000; McIntyre et al., 2002; Segal & Cahill, 2009), the current findings suggest that NE also plays an integral role in pattern separation and that this could result in better memory for the details of an emotional situation.
Acknowledgments
This research was supported by a University of California at Irvine research fund awarded to C.S., a Johns Hopkins University research fund awarded to M.Y., and a grant from the National Institutes on Aging R03 AG032015. We thank Gianna O’Hara and Karen Wong for their assistance in data collection.
References
- Brown RA, Walling SG, Milway JS, Harley CW. Locus coeruleus activation suppresses feedforward interneurons and reduces beta-gamma electroencephalogram frequencies while it enhances theta frequencies in rat dentate gyrus. J Neurosci. 2005;25(8):1958–1991. doi: 10.1523/JNEUROSCI.4307-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill LF, Alkire MT. Epinephrine enhancement of human memory consolidation: Interaction with arousal at encoding. Neurobiol Learn Mem. 2003;79:194–198. doi: 10.1016/s1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Cahill LF, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Cahill L, van Stegeren A. Sex-related impairment of memory for emotional events with beta-adrenergic blockade. Neurobiol Learn Mem. 2003;79(10):81–88. doi: 10.1016/s1074-7427(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Castle D, Castle A. Intracellular transport and secretion of salivary proteins. Crit Rev Oral Biol Med. 1998;9(1):4–22. doi: 10.1177/10454411980090010301. [DOI] [PubMed] [Google Scholar]
- Chatterton RT, Jr, Vogelsong KM, Lu YC, Ellman AB, Hudgens GA. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clin Physiol. 1996;16(4):433–448. doi: 10.1111/j.1475-097x.1996.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Erni K, Hebisch G, Nater U. Salivary alpha-amylase levels after yohimbine challenge in healthy men. J Clin Endocrinol Metab. 2006;91(12):5130–5133. doi: 10.1210/jc.2006-0461. [DOI] [PubMed] [Google Scholar]
- Harley CW. Norepineprhine and the dentate gyrus. Prog Brain Res. 2007;163:299–318. doi: 10.1016/S0079-6123(07)63018-0. [DOI] [PubMed] [Google Scholar]
- Kalus P, Slotboom J, Gallinat J, Mahlberg R, Cattapan-Ludewig K, Wiest R, et al. Examining the gateway to the limbic system with diffusion tensor imaging: The perforant pathway in dementia. Neuroimage. 2006;1:123–131. doi: 10.1016/j.neuroimage.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Schacter DL. Gist-based false recognition of pictures in older and younger adults. J Mem Lang. 1997;37:555–583. [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler TL, Stark CEL. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn Memory. 2011;18:15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Memory – a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory consolidation and the amygdala: A systems perspective. Trends Neurosci. 2002;25(9):456–461. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Ann Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Hatfield T, McGaugh JL. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. Eur J Neurosci. 2002;16(7):1223–1226. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- Nielsen SE, Ertman N, Lakhani S, Cahill LF. Hormonal contraception usage is associated with altered memory for an emotional story. Neurobiol Learn Mem. 2011;96:378–384. doi: 10.1016/j.nlm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski EJ, Murphy CM, de Toledo-Morrell L, Shah RC, Moseley ME, Bammer R, et al. Changes in the parahippocampal white matter integrity in amnestic mild cognitive impairment: A diffusion tensor imaging study. Behav Neurol. 2009;21(1):51–61. doi: 10.3233/BEN-2009-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal SK, Cahill LF. Endogenous noradrenergic activation and memory for emotional material in men and women. Psychoneuroendocrinology. 2009;34:1263–1271. doi: 10.1016/j.psyneuen.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Seidlitz L, Diener E. Sex differences in the recall of affective experiences. J Pers Soc Psychol. 1998;74(1):262–271. doi: 10.1037//0022-3514.74.1.262. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Goekoop R, Everaerd W, Scheltens P, Barkhof F, Kuijer J, Rombouts S. Noradrenaline mediates amygdala activation in men and women during encoding of emotional material. Neuroimage. 2005;24:898–909. doi: 10.1016/j.neuroimage.2004.09.011. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Everaerd W, Cahill LF, McGaugh JL, Gooren LJG. Memory for emotional events: Differential effects of centrally versus peripherally acting beta-blocking agents. Psychopharmacology. 1998;138:305–310. doi: 10.1007/s002130050675. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Rohleder N, Everaerd W, Wolf OT. Salivary alpha-amylase as a marker for adrenergic activity during stress: Effect of betablockade. Psychoneuroendocrinology. 2006;31:137–141. doi: 10.1016/j.psyneuen.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Whelton H. Introduction: the anatomy and physiology of the salivary glands. In: Edgar WM, O’Mullane DM, editors. Saliva and oral health. 3. London: British Dental Association; 1996. pp. 1–8. [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2010:1–12. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CEL. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci. 2011;108(21):8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Muftuler TL, Stark CEL. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proc Natl Acad Sci USA. 2010;107(28):12687–12691. doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. Pattern separation in the hippocampus. Trends Neurosci. 2011;34(10):515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CEL. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. Neuroimage. 2010;51:1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K, Fujita-Yoshigaki J, Murakami M, Segawa A. Cyclic AMP has distinct effects from Ca2+ in evoking priming and fusion/exocytosis in parotid amylase secretion. Eur J Physiol. 2002;444:586–596. doi: 10.1007/s00424-002-0844-7. [DOI] [PubMed] [Google Scholar]
- Young WS, 3rd, Kuhar MJ. Noradrenergic alpha 1 and alpha 2 receptors: light microscopic autoradiographic localization. Proc Natl Acad Sci USA. 1980;77(33):1696–700. doi: 10.1073/pnas.77.3.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]