Abstract
Protein kinase C theta (PKC-θ) is a key kinase in mediating T cell receptor (TCR) signals. PKC-θ activated by T cell receptor (TCR) engagement translocates to immunological synapses and regulates the activation of transcriptional factors NFκB, AP-1, and NFAT. These transcription factors then activate target genes such as IL-2. T cells deficient in PKC-θ display defects in T cell activation, survival, activation-induced cell death, and the differentiation into inflammatory T cells, such as Th2 and Th17 cells both in vitro and in vivo. Since these effector T helper cells are responsible for mediating autoimmunity, selective inhibition of PKC-θ is considered a treatment for prevention of autoimmune diseases and allograft rejection.
Keywords: PKC-θ, T cells, TCR signaling, autoimmunity, transplantation rejection
Introduction
PKC is family of serine/threonine kinases that can be divided into three subfamilies(1): conventional PKC, including PKCα β and γ, which are activated by Ca2+ and diacylglycerol (DAG); novel PKC, including PKCδ, θ, η, and ε, in which activation is dependent on DAG but independent of Ca2+; and atypical PKC, PKCζ and λ, in which activation is independent of both Ca2+ and DAG. PKC-θ is predominantly expressed in T cells and is activated through T cell receptor (TCR) signaling to regulate T cell function. Furthermore, PKC-θ−/− T cells display defects in proliferation, cytokine production, and differentiation(2). Given that it is a critical signaling molecule in the regulation of T cell function and T cell-dependent immunity, understanding the role of PKCθ will facilitate the development of treatments for T cell-mediated immune disorders. Here, we look into the role of PKCθ in T cell activation and differentiation.
PKC-θ mediates TCR signals required for T cell activation
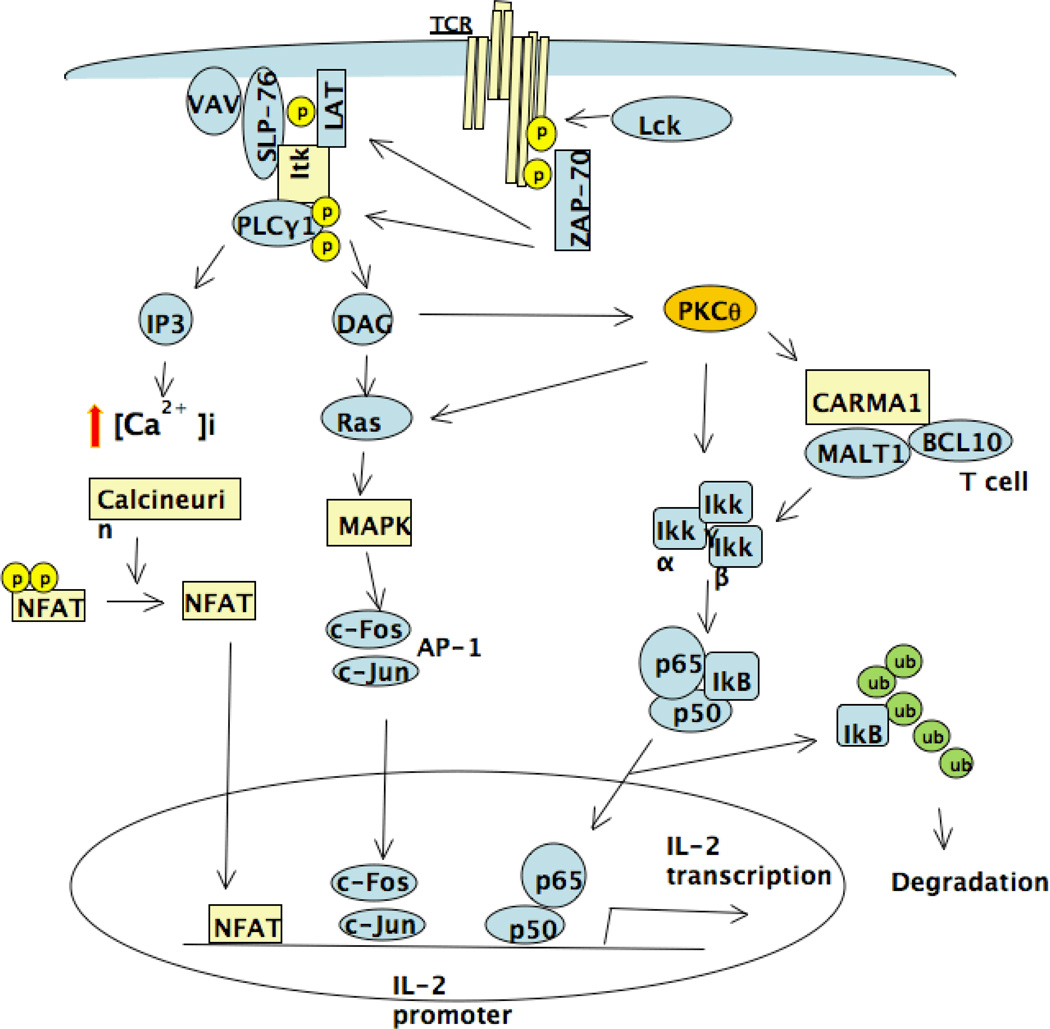
Once antigens are taken up by antigen presenting cells (APC), the antigens are fragmented to peptides and complexed with major histocompatibility complex (MHC) on the APC surface to present to TCRs (3). As a result of the engagement of MHC-peptide with TCR, T cells become activated via TCR-mediated signal pathways. The TCR signaling pathway is initiated by activation of Src family protein tyrosine kinase (PTK) LCK (4), which leads to recruitment of ZAP 70 and consequently adaptor proteins LAT, SLP76, and VAV. LAT recruits PLCγ1 (5, 6) which catalyzes the phosphatidylinositol 4,5-biphosphate into inositol triphosphate (IP3), a Ca2+ mobilizer, and diacylglycerol (DAG), the PKC-θ activator (4). Upon activation by DAG, PKC-θ mediates the activation of NF-κB (7). Several adaptor proteins play a critical role in mediating PKC-θ induced NF-κB activation including membrane-associated guanylate kinase (MAGUK), CARMA1, B-cell lymphoma 10 (Bcl 10), and mucosa-associated lymphoid tissue (MALT1). Together with PKC-θ, these adaptors facilitate the activation of IKK, leading to phosphorylation, ubiquitination, and degradation of IκB and resulting in the release of NF-κB to the nucleus, where it participates in activation of target genes essential for T cell activation (8–10). Studies using PKC-θ deficient T cells or overexpression of constitutive active PKC-θ, or the catalytically inactive form of PKC-θ, demonstrated that PKC-θ also activates AP-1 signaling pathway in T cells (11). AP-1 is composed of c-Jun and c-Fos, which regulate many cellular events (12). Although the mechanism by which PKC-θ mediates activation of AP-1 is unclear, several studies have provided some insight into this process. Ras (13) and MAP Kinases: JNK, ERK, and P38, are involved in PKC-θ-mediated AP-1 activation (12). Li et al isolated PKC-θ interacting upstream MAP kinase, originally termed Ste20/SPS1-related proline and alanine-rich kinase(SPAK or PASK) (14, 15), and demonstrated that SPAK selectively interacts with PKC-θ and is involved in PKC-θ-mediated activation of AP-1, but not NF-κB. Furthermore, they showed that the activation of this kinase by TCR/CD28 stimulation was impaired in T cells from PKCθ−/− mice and that a SPAK-specific siRNA inhibited PKC-θ- mediated AP-1, but not NF-κB activation (16). Their data demonstrated PKC-θ was constitutively associated with Tec kinase in T cells and that the association increased during TCR signaling (17). Consistently, dominant negative Tec mutant blocked the PKC-θ-induced activation of an AP-1 reporter gene, but had no effect on NF-κB activation (18). Activation of T cells through TCR also leads to IP3-mediated elevation of cytosolic [Ca2+]i by inducing Ca2+ influx. Elevated intracellular Ca2+ ultimately leads to activation of phosphatase calcineurin, which dephosphorylates nuclear factor of activated T cells (NFAT), resulting in their translocation to the nucleus. Translocated NFAT cooperates with AP-1 to activate IL-2 expression. It was shown that, in the absence of AP-1, NFAT activation could lead to T cell anergy (19). Several studies have shown that PKC-θ enhances the activation of NFAT by stimulating Ca2+ influx, as TCR-induced Ca2+ influx and NFAT activation were defective in T cells from PKCθ−/− mice (20, 21). It is clear that PKC-θ regulates Ca2+ signal via PLCγ1, however, it is not clear how PKC-θ stimulates PLCγ1. Tek kinase family member Itk may be the missing link. Itk deficient T cells displayed defective Ca2+ influx and PLCγ1 activation (22). Furthermore, PLCγ1 activity was greatly stimulated Itk overexpression (17). Therefore, it is possible that PKC-θ regulates PLCγ1 activation via Itk. Altogether, PKC-θ-mediated TCR signals regulate multiple signaling pathways including NF-κB, AP1 and NFAT critical for T cell activation. Inhibition of PKC-θ is thus expected to prevent T cell activation by blocking these TCR signaling pathways.
PKC-θ specifically translocates to immunological synapse
In contrast to other isoforms of PKC, PKC-θ is unique for its ability to translocate to the contact site of T cells and APCs, the immunological synapse (IS). IS is a clustering of specialized membrane microdomains where TCR signaling molecules, including TCR itself, are assembled (23). IS is believed to serve as a platform for delivering integrated signals essential for T cells activation (24). Therefore, IS components, organization and stability likely determine the outcome of TCR signals that instruct T cells to proliferation, cytokine production, differentiation, or even cell death (25). IS consists of three major compartments: central SMAC (cSMAC), peripheral SMAC (pSMAC) and distal SMAC (dSMAC) (26, 27). cSMAC was initially referred to as a signaling structure (28, 29). Outward from the center is the pSMAC, a ring of LFA-1/ICAM-1 co-localized with the cytoskeletal integrin linker talin (29). The outermost ring of IS is formed by dSMAC, a zone enriched in the tyrosine phosphatase CD45 (28). Recently, it was demonstrated that TCRs initially microcluster in the dSMAC, and continually move through the pSMAC to the cSMAC, which is believed to be critical for generation of continuous TCR signals required for T cell activation (30). PKC-θ co-localizes with TCR in the cSMAC (29, 31) or pSMAC in T cells (32). Intravital-microscopy observations showed that T cells expressing PKC-θ periodically broke open the pSMAC to create an asymmetric focal zone accumulation pattern and relocated to nearby areas where the pSMAC reformed (33). This periodic breaking of the symmetric pSMAC to form a polarized focal zone allows short bursts of migration, facilitating T cell interaction with multiple DCs (34). This observation is also consistent with the asymmetric cell divisions theory which suggests that IS leads to asymmetric cell division important for memory/effector differentiation of lymphocytes (35).
PKC-θ-mediated signals enhance T cell survival
An efficient adaptive immune system requires the capability of rapid expansion as well as reduction of immune cells. T cells meet such requirements as they can be induced towards proliferation or apoptosis dependent on the signals received via T cell receptor (TCR). Naïve T cells are activated to proliferate in response to foreign antigens, which is a critical step in adaptive immunity. Meanwhile, T cells are ready to undergo apoptosis when engaged with self-antigens, which is an important mechanism for self-tolerance. During T cell activation, survival of the T cells is enhanced by IL-2, which acts as an extrinsic survival factor. In addition, activated T cells substantially up-regulate Bcl-xL that intrinsically increases the ability of resistance to apoptosis (36–38). CD28, the costimulatory molecule, mediates the critical signals required for up-regulation of Bcl-xL during T cell activation (36, 37, 39). We have shown that in response to TCR stimulation, CD4+ PKC-θ−/− T cells failed to up-regulate Bcl-xL, and underwent accelerated apoptosis via a caspase and mitochondria-dependent pathway (21). Similar results were obtained when survival of the CD8+ T cells from PKC-θ−/− mice were examined (27). We further demonstrated that PKC-θ-mediated activation of NF-κB pathway was required for up-regulation of Bcl-xL (21). Therefore, the PKC-θ-mediated signals may function not only in the initial activation of naïve CD4+ T cells, but also in their survival during T cell activation by regulating Bcl-xL levels through NF-κB pathway. Consistent with this notion, we have also shown that PKC-θ-regulated survival is essential for cardiac allograft rejection in an adoptive transfer model as we discussed later (40). Since survival of the T cells determines the magnitude of immune responses, it is possible that immune responses can be controlled by manipulation of T cell survival via stimulation or inhibition of PKC-θ activity.
PKC-θ promotes activation-induced cell death (AICD)
To maintain homeostasis, the immune system has developed mechanisms to reduce the significantly expanded T cell population resulting from antigenic stimulation. Activation-induced cell death (AICD) is one of the mechanisms for eliminating clonally expanded T cells (41, 42). Fas (CD95) and Fas ligand (FasL, CD95L)-mediated apoptosis plays an important role in deleting effector T cells, as mice lacking Fas or FasL display defective deletion of peripheral T cells (43, 44), and eventually develop autoimmune disorders (45–47). In addition, FasL-induced apoptosis has been shown to be responsible for protecting immune privileged sites from cellular immune-mediated damage (48, 49). Our data showed that in the absence of PKC-θ, superantigen (SEB)-induced deletion of CD4+ T cells was defective in PKC-θ−/− mice (50). In response to SEB challenge, up-regulation of FasL, but not Fas, was significantly reduced in PKC-θ−/− mice. PKC-θ is thus required for maximum up-regulation of FasL in vivo. We further showed that stimulation of FasL expression depends on PKC-θ-mediated activation of NFAT pathway. In addition, PKC-θ−/− T cells displayed resistance to Fas-mediated apoptosis as well as activation-induced cell death (AICD). In the absence of PKC-θ, Fas-induced activation of apoptotic molecules such as caspase-8, caspase-3 and Bid was not efficient. However, AICD as well as Fas-mediated apoptosis of PKC-θ−/− T cells were restored in the presence of high concentration of IL-2, a critical factor required for potentiating T cells for AICD. PKC-θ is thus required for promoting FasL expression and for potentiating Fas-mediated apoptosis (50). Altogether, PKC-θ also plays a critical role in the elimination of expanded T cells that are no longer useful via AICD, which prepares immune system for subsequent antigen challenges by creating space for next immune cells expansion.
PKC-θ function in T-mediated autoimmunity
Autoimmune disorders result from activation of self-reactive T cells which then differentiate into effectors and attack self-tissues. Naïve T cells can be differentiated into different types of T helper cells, which produce specific cytokines to regulate immune responses (51–53). Upon activation, naïve T cells differentiate into either inflammatory Th1, Th2, and Th17 cells or tolerant regulatory T cells (Treg). Each subset of T helpers produces specific cytokines. For example, Th1 cells produce IFNγ, Th2 cells produce IL-4, and IL-17 is produced by Th17 cells (54, 55). Differentiation of each T helper type is instructed by specific transcriptional factor such as T-bet for Th1, GATA3 for Th2, RORγt for Th17, and Foxp3 for Treg (54, 56–58). The function of PKC-θ in the differentiation of Th cells has been studied using PKCθ−/− mice, which showed severely impaired Th2-mediated airway hyperresponsiveness (AHR) in response to inhaled Ag or to Nippostongylus brasiliensis infection. However, Th1-mediated protection against Leishmania major infection was normal in PKCθ−/− mice (16). PKCθ−/− mice immunized with myelin oligodendrocyte glycoprotein (MOG) displayed impaired development of experimental autoimmune encephalitis (EAE) (59–61). Similarly, development collagen-induced arthritis (CIA) was also defective in PKCθ−/− mice (62). Prior to discovery of Th17 cells, both EAE and CIA were considered to be Th1-related autoimmunity. With the recent discovery of Th17, EAE and CIA have been associated with Th17-mediated diseases (63, 64). Indeed, defective development of EAE in PKCθ−/− mice was associated with impaired Th17 responses (60), suggesting crucial role of PKC-θ in Th17 differentiation. Our preliminary data also demonstrates that PKC-θ is required for Th17 formation in vitro. Altogether, our studies, and those of others, show PKC-θ is critical for generation of Th2 and Th17 responses. Therefore, selective inhibition of PKC-θ is considered a treatment for prevention of Th2 and Th17-mediated autoimmunity.
PKC-θ function in allograft rejection
Solid organ transplantation that benefits end-stage organ failure patients is severely limited by the occurrence of rejection. Alloreactive T cells are critical targets for tolerance induction since they mediate immune responses required for rejection. The alloreactive T cell pool is very large (65), which explains why immune responses against allografts are at least two orders of magnitude stronger than the immune responses against a specific antigen. Therefore, long-term tolerance to allografts is extremely difficult to establish. Alloreactive T cells can be tolerized through anergy, suppression and deletion. Anergy is the unresponsive state of T cells, which can be overcome by exogenous IL-2 (66). Suppression is usually mediated by suppressive cells such as Foxp3-expressing regulatory T cells (Tregs) (67). However, these two mechanisms do not likely change the size of alloreactive T cell pool. Similar problem exists for cyclosporin A (CsA), the most successful immunosuppressive drug used clinically so far. CsA prevents apoptosis of alloreactive T cells by inhibition of T cell activation (68), resulting in accumulation of large amounts of alloreactive T cells that can destroy allografts once immunosuppressive drugs are discontinued (69). Therefore, prevention of allograft rejection usually requires transplant recipients to take life-long immunosuppressive drugs, which can result in complications of infections and malignancy. In contrast, deletion induces tolerance by decreasing the number of alloreactive T cells via apoptosis. Deletion occurs both in the thymus and peripheral lymphoid tissues. In the periphery, activated T cells are deleted by two different forms of apoptosis: AICD and passive cell death. The former eliminates clonally expanded T cells via TNF family death receptors, such as Fas/FasL, which is largely independent of Bcl-xL (70), whereas the latter can be inhibited by Bcl-xL. Activated T cells up-regulate Bcl-xL which intrinsically enhances T cell survival (36, 39). CD28-mediated co-stimulatory signals are responsible for Bcl-xL up-regulation via activation of the NF-κB pathway (37, 71). Blockade of either co-stimulatory signals or the NF-κB pathway resulted in tolerance by induction of T cell apoptosis in wild type (WT) mice, but not in mice expressing the anti-apoptotic Bcl-xL transgene (72–75). These results demonstrated the critical role of passive T cell death in tolerance induction. It was proposed that apoptotic reduction of the extraordinary large pool of alloreactive T cells is required for tolerance induction (72). Because PKC-θ is a critical signaling molecule required for T cell activation and survival, we tested function of PKC-θ in allograft rejection using a cardiac rejection model (40). We demonstrated that Rag1−/− mice reconstituted with wild type (WT) T cells readily rejected fully mismatched cardiac allografts, whereas, Rag1−/− mice reconstituted with PKC-θ−/− T cells failed to promote rejection, suggesting that PKC-θ is required for T cell-mediated allograft rejection. Since PKC-θ is required for survival of activated T cells (21), we further tested the role of PKC-θ-regulated survival in cardiac allograft rejection, and demonstrated that Transgenic expression of Bcl-xL in PKC-θ−/− T cells was sufficient to restore cardiac allograft rejection (40). In contrast to adoptive transfer experiments, intact PKC-θ−/− mice displayed delayed, but successful cardiac allograft rejection, suggesting the potential compensation for PKC-θ function. Finally, a sub-therapeutic dose of anti-CD154 antibody or CTLA4-Ig, that was not sufficient to prevent cardiac allograft rejection in the WT mice, prevented heart rejection in the PKC-θ−/− mice. Thus, in combination with other treatments, inhibition of PKC-θ can achieving long-term survival of allografts (40, 76).
Conclusion
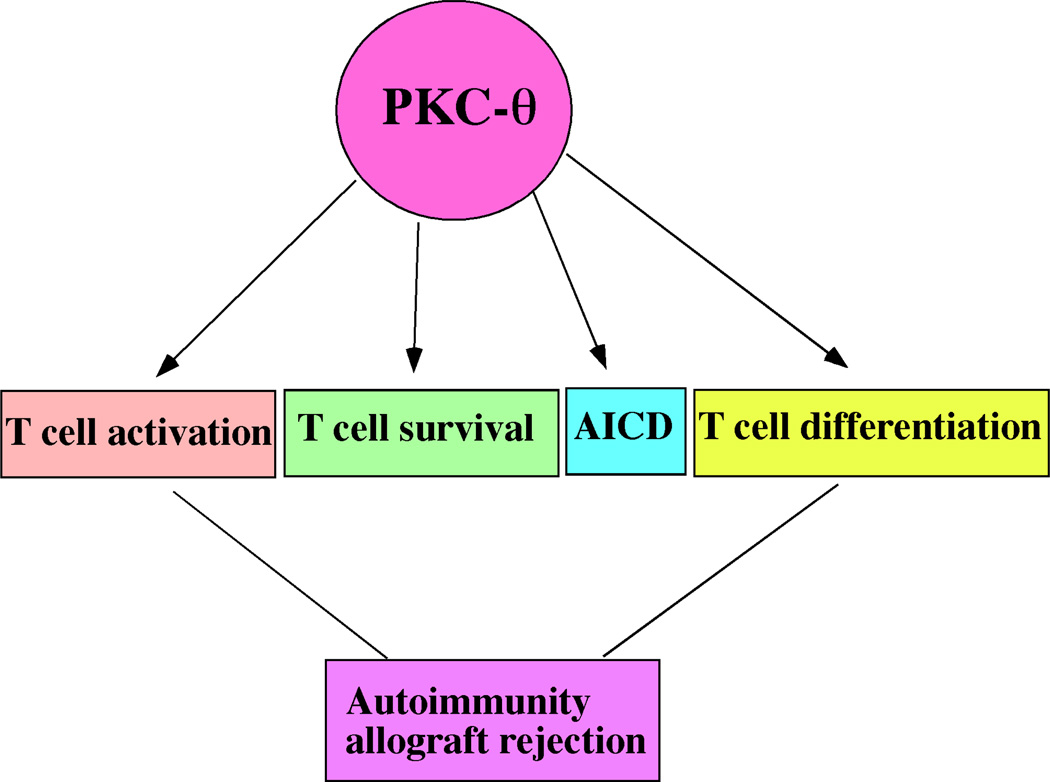
PKC-θ controls fundamental process in T cells that integrate TCR and CD28 signals leading to activation of transcriptional factor, NF-κB, AP-1 and NFAT essential for productive T cells activation and differentiation. Figure 1 illustrates the PKC-θ-regulated signaling pathways. Since PKC-θ regulates T cell-mediated immune responses in vivo, selective PKC-θ inhibitors are believed to have potential clinical application in the treatment of autoimmunity and prevention of allograft rejection. However, PKC-θ regulates multiple T cell differentiation processes which is illustrated in figure 2, and detailed mechanisms involved in each of these processes remain to be elucidated. Furthermore, questions needed to be addressed prior to clinical application of PKC-θ inhibitors include how PKC-θ coordinates multiple biological processes, and how inhibition of PKC-θ affects normal T cell function in vivo. It is encouraging to report that many pharmaceutical companies have developed selective PKC-θ inhibitors, and thus many of PKC-θ-regulated functions can be evaluated using these inhibitors instead of PKCθ−/− mice, which have potential developmental caveat. With the availability of PKC-θ inhibitors, it is now possible to test their efficacy in mouse models of human autoimmune diseases such as EAE and arthritis, which likely leads to clinical trial of PKC-θ-based treatments for human diseases.
Figure 1.
Schematic illustration of PKC-θ-regulated signaling pathways
Figure 2.
Multiple roles of PKC-θ in T cell function that regulates autoimmunity and allograft rejection
Acknowledgements
We thank Drs. Santhakumar Manicassamy, Sonal Gupta and Zhaofeng Huang for their significant contributions to the important results from my laboratory discussed in this review article. We also thank Silvia Da Costa for critically reading the manuscript and helpful discussion. Our work on PKC-θ is supported by Schweppe Foundation, American Cancer Society (Illinois) and NIH (AI53147).
References
- 1.Newton AC. Regulation of protein kinase C. Curr Opin Cell Biol. 1997;9:161–167. doi: 10.1016/s0955-0674(97)80058-0. [DOI] [PubMed] [Google Scholar]
- 2.Manicassamy S, Gupta S, Sun Z. Selective function of PKC-theta in T cells. Cell Mol Immunol. 2006;3:263–270. [PubMed] [Google Scholar]
- 3.Gay D, Maddon P, Sekaly R, Talle MA, Godfrey M, Long E, Goldstein G, Chess L, Axel R, Kappler J, et al. Functional interaction between human T-cell protein CD4 and the major histocompatibility complex HLA-DR antigen. Nature. 1987;328:626–629. doi: 10.1038/328626a0. [DOI] [PubMed] [Google Scholar]
- 4.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 5.Clements JL. Known and potential functions for the SLP-76 adapter protein in regulating T-cell activation and development. Immunol Rev. 2003;191:211–219. doi: 10.1034/j.1600-065x.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- 6.Berg LJ, Finkelstein LD, Lucas JA, Schwartzberg PL. Tec family kinases in T lymphocyte development and function. Annu Rev Immunol. 2005;23:549–600. doi: 10.1146/annurev.immunol.22.012703.104743. [DOI] [PubMed] [Google Scholar]
- 7.Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg PL, Littman DR. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 8.Weil R, Schwamborn K, Alcover A, Bessia C, Di Bartolo V, Israel A. Induction of the NF-kappaB cascade by recruitment of the scaffold molecule NEMO to the T cell receptor. Immunity. 2003;18:13–26. doi: 10.1016/s1074-7613(02)00506-x. [DOI] [PubMed] [Google Scholar]
- 9.Lin X, Wang D. The roles of CARMA1, Bcl10, and MALT1 in antigen receptor signaling. Semin Immunol. 2004;16:429–435. doi: 10.1016/j.smim.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Weil R, Israel A. T-cell-receptor- and B-cell-receptor-mediated activation of NF-kappaB in lymphocytes. Curr Opin Immunol. 2004;16:374–381. doi: 10.1016/j.coi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg PL, Littman DR. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 12.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 13.Baier-Bitterlich G, Uberall F, Bauer B, Fresser F, Wachter H, Grunicke H, Utermann G, Altman A, Baier G. Protein kinase C-theta isoenzyme selective stimulation of the transcription factor complex AP-1 in T lymphocytes. Mol Cell Biol. 1996;16:1842–1850. doi: 10.1128/mcb.16.4.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ushiro H, Tsutsumi T, Suzuki K, Kayahara T, Nakano K. Molecular cloning and characterization of a novel Ste20-related protein kinase enriched in neurons and transporting epithelia. Arch Biochem Biophys. 1998;355:233–240. doi: 10.1006/abbi.1998.0736. [DOI] [PubMed] [Google Scholar]
- 15.Johnston AM, Naselli G, Gonez LJ, Martin RM, Harrison LC, DeAizpurua HJ. SPAK, a STE20/SPS1-related kinase that activates the p38 pathway. Oncogene. 2000;19:4290–4297. doi: 10.1038/sj.onc.1203784. [DOI] [PubMed] [Google Scholar]
- 16.Marsland BJ, Soos TJ, Spath G, Littman DR, Kopf M. Protein kinase C theta is critical for the development of in vivo T helper (Th)2 cell but not Th1 cell responses. J Exp Med. 2004;200:181–189. doi: 10.1084/jem.20032229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomlinson MG, Kane LP, Su J, Kadlecek TA, Mollenauer MN, Weiss A. Expression and function of Tec, Itk, and Btk in lymphocytes: evidence for a unique role for Tec. Mol Cell Biol. 2004;24:2455–2466. doi: 10.1128/MCB.24.6.2455-2466.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altman A, Kaminski S, Busuttil V, Droin N, Hu J, Tadevosyan Y, Hipskind RA, Villalba M. Positive feedback regulation of PLCgamma1/Ca(2+) signaling by PKCtheta in restimulated T cells via a Tec kinase-dependent pathway. Eur J Immunol. 2004;34:2001–2011. doi: 10.1002/eji.200324625. [DOI] [PubMed] [Google Scholar]
- 19.Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 20.Pfeifhofer C, Kofler K, Gruber T, Tabrizi NG, Lutz C, Maly K, Leitges M, Baier G. Protein kinase C theta affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J Exp Med. 2003;197:1525–1535. doi: 10.1084/jem.20020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manicassamy S, Gupta S, Huang Z, Sun Z. Protein Kinase C-{theta}-Mediated Signals Enhance CD4+ T Cell Survival by Up-Regulating Bcl-xL. J Immunol. 2006;176:6709–6716. doi: 10.4049/jimmunol.176.11.6709. [DOI] [PubMed] [Google Scholar]
- 22.Liu KQ, Bunnell SC, Gurniak CB, Berg LJ. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J Exp Med. 1998;187:1721–1727. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 24.Moran M, Miceli MC. Engagement of GPI-linked CD48 contributes to TCR signals and cytoskeletal reorganization: a role for lipid rafts in T cell activation. Immunity. 1998;9:787–796. doi: 10.1016/s1074-7613(00)80644-5. [DOI] [PubMed] [Google Scholar]
- 25.Dustin ML. Hunter to gatherer and back: immunological synapses and kinapses as variations on the theme of amoeboid locomotion. Annu Rev Cell Dev Biol. 2008;24:577–596. doi: 10.1146/annurev.cellbio.24.110707.175226. [DOI] [PubMed] [Google Scholar]
- 26.Dustin ML. Modular design of immunological synapses and kinapses. Cold Spring Harbor Perspect Biol. 2009;1:a002873. doi: 10.1101/cshperspect.a002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barouch-Bentov R, Lemmens EE, Hu J, Janssen EM, Droin NM, Song J, Schoenberger SP, Altman A. Protein kinase C-theta is an early survival factor required for differentiation of effector CD8+ T cells. J Immunol. 2005;175:5126–5134. doi: 10.4049/jimmunol.175.8.5126. [DOI] [PubMed] [Google Scholar]
- 28.Freiberg BA, Kupfer H, Maslanik W, Delli J, Kappler J, Zaller DM, Kupfer A. Staging and resetting T cell activation in SMACs. Nat Immunol. 2002;3:911–917. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- 29.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 30.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monks CR, Kupfer H, Tamir I, Barlow A, Kupfer A. Selective modulation of protein kinase C-theta during T-cell activation. Nature. 1997;385:83–86. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- 32.Somersalo K, Anikeeva N, Sims TN, Thomas VK, Strong RK, Spies T, Lebedeva T, Sykulev Y, Dustin ML. Cytotoxic T lymphocytes form an antigen-independent ring junction. J Clin Invest. 2004;113:49–57. doi: 10.1172/JCI200419337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sims TN, Soos TJ, Xenias HS, Dubin-Thaler B, Hofman JM, Waite JC, Cameron TO, Thomas VK, Varma R, Wiggins CH, Sheetz MP, Littman DR, Dustin ML. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 34.Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, Nussenzweig MC. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 35.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, Killeen N, Orange JS, Russell SM, Weninger W, Reiner SL. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 36.Van Parijs L, Ibraghimov A, Abbas AK. The roles of costimulation and Fas in T cell apoptosis and peripheral tolerance. Immunity. 1996;4:321–328. doi: 10.1016/s1074-7613(00)80440-9. [DOI] [PubMed] [Google Scholar]
- 37.Radvanyi LG, Shi Y, Vaziri H, Sharma A, Dhala R, Mills GB, Miller RG. CD28 costimulation inhibits TCR-induced apoptosis during a primary T cell response. J Immunol. 1996;156:1788–1798. [PubMed] [Google Scholar]
- 38.Noel PJ, Boise LH, Green JM, Thompson CB. CD28 costimulation prevents cell death during primary T cell activation. J Immunol. 1996;157:636–642. [PubMed] [Google Scholar]
- 39.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 40.Manicassamy S, Yin D, Zhang Z, Molinero LL, Alegre ML, Sun Z. A critical role for protein kinase C-theta-mediated T cell survival in cardiac allograft rejection. J Immunol. 2008;181:513–520. doi: 10.4049/jimmunol.181.1.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. 2002;109(Suppl):S97–S107. doi: 10.1016/s0092-8674(02)00704-3. [DOI] [PubMed] [Google Scholar]
- 42.Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 43.Singer GG, Abbas AK. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994;1:365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 44.Singer GG, Carrera AC, Marshak-Rothstein A, Martinez C, Abbas AK. Apoptosis, Fas and systemic autoimmunity: the MRL-lpr/lpr model. Curr Opin Immunol. 1994;6:913–920. doi: 10.1016/0952-7915(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 45.Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, Strober W, Lenardo MJ, Puck JM. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 46.Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A, de Villartay JP. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 48.Griffith TS, Yu X, Herndon JM, Green DR, Ferguson TA. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity. 1996;5:7–16. doi: 10.1016/s1074-7613(00)80305-2. [DOI] [PubMed] [Google Scholar]
- 49.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 50.Manicassamy S, Sun Z. The critical role of protein kinase C-theta in Fas/Fas ligand-mediated apoptosis. J Immunol. 2007;178:312–319. doi: 10.4049/jimmunol.178.1.312. [DOI] [PubMed] [Google Scholar]
- 51.Carbone FR, Kurts C, Bennett SR, Miller JF, Heath WR. Cross-presentation: a general mechanism for CTL immunity and tolerance. Immunol Today. 1998;19:368–373. doi: 10.1016/s0167-5699(98)01301-2. [DOI] [PubMed] [Google Scholar]
- 52.Kagi D, Ledermann B, Burki K, Zinkernagel RM, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol. 1996;14:207–232. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- 53.Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu Rev Immunol. 1997;15:235–270. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- 54.Mosmann TR, Coffman RL. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 55.Openshaw P, Murphy EE, Hosken NA, Maino V, Davis K, Murphy K, O'Garra A. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong C, Flavell RA. Control of T helper cell differentiation--in search of master genes. Sci STKE. 2000;2000:pe1. doi: 10.1126/stke.2000.49.pe1. [DOI] [PubMed] [Google Scholar]
- 57.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 58.Zhou L, Lopes JE, Chong, Ivanov MM, II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salek-Ardakani S, So T, Halteman BS, Altman A, Croft M. Protein kinase Ctheta controls Th1 cells in experimental autoimmune encephalomyelitis. J Immunol. 2005;175:7635–7641. doi: 10.4049/jimmunol.175.11.7635. [DOI] [PubMed] [Google Scholar]
- 60.Tan SL, Zhao J, Bi C, Chen XC, Hepburn DL, Wang J, Sedgwick JD, Chintalacharuvu SR, Na S. Resistance to experimental autoimmune encephalomyelitis and impaired IL-17 production in protein kinase C theta-deficient mice. J Immunol. 2006;176:2872–2879. doi: 10.4049/jimmunol.176.5.2872. [DOI] [PubMed] [Google Scholar]
- 61.Anderson K, Fitzgerald M, Dupont M, Wang T, Paz N, Dorsch M, Healy A, Xu Y, Ocain T, Schopf L, Jaffee B, Picarella D. Mice deficient in PKC theta demonstrate impaired in vivo T cell activation and protection from T cell-mediated inflammatory diseases. Autoimmunity. 2006;39:469–478. doi: 10.1080/08916930600907954. [DOI] [PubMed] [Google Scholar]
- 62.Healy AM, Izmailova E, Fitzgerald M, Walker R, Hattersley M, Silva M, Siebert E, Terkelsen J, Picarella D, Pickard MD, LeClair B, Chandra S, Jaffee B. PKC-theta-deficient mice are protected from Th1-dependent antigen-induced arthritis. J Immunol. 2006;177:1886–1893. doi: 10.4049/jimmunol.177.3.1886. [DOI] [PubMed] [Google Scholar]
- 63.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18:349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 64.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 65.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166:973–981. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 66.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 67.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 68.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 69.Li XC, Strom TB, Turka LA, Wells AD. T cell death and transplantation tolerance. Immunity. 2001;14:407–416. doi: 10.1016/s1074-7613(01)00121-2. [DOI] [PubMed] [Google Scholar]
- 70.Van Parijs L, Abbas AK. Role of Fas-mediated cell death in the regulation of immune responses. Curr Opin Immunol. 1996;8:355–361. doi: 10.1016/s0952-7915(96)80125-7. [DOI] [PubMed] [Google Scholar]
- 71.Jones RG, Parsons M, Bonnard M, Chan VS, Yeh WC, Woodgett JR, Ohashi PS. Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-X(L) levels in vivo. J Exp Med. 2000;191:1721–1734. doi: 10.1084/jem.191.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wells AD, Li XC, Li Y, Walsh MC, Zheng XX, Wu Z, Nunez G, Tang A, Sayegh M, Hancock WW, Strom TB, Turka LA. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 73.Bertolino P, Heath WR, Hardy CL, Morahan G, Miller JF. Peripheral deletion of autoreactive CD8+ T cells in transgenic mice expressing H-2Kb in the liver. Eur J Immunol. 1995;25:1932–1942. doi: 10.1002/eji.1830250721. [DOI] [PubMed] [Google Scholar]
- 74.Wells AD, Li XC, Strom TB, Turka LA. The role of peripheral T-cell deletion in transplantation tolerance. Philos Trans R Soc Lond B Biol Sci. 2001;356:617–623. doi: 10.1098/rstb.2001.0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou P, Balin SJ, Mashayekhi M, Hwang KW, Palucki DA, Alegre ML. Transplantation tolerance in NF-kappaB-impaired mice is not due to regulation but is prevented by transgenic expression of Bcl-xL. J Immunol. 2005;174:3447–3453. doi: 10.4049/jimmunol.174.6.3447. [DOI] [PubMed] [Google Scholar]
- 76.Wang L, Xiang Z, Ma LL, Chen Z, Gao X, Sun Z, Williams P, Chari RS, Yin DP. Deficiency of protein kinase C-theta facilitates tolerance induction. Transplantation. 2009;87:507–516. doi: 10.1097/TP.0b013e318195fd36. [DOI] [PubMed] [Google Scholar]