Abstract
Progesterone (PROG) has been shown to protect the brain from traumatic injury and is now in Phase III clinical trials. Our work shows that PROG's beneficial effects can be reduced in vitamin D hormone (VDH) –deficient subjects. VDH can modulate neuronal apoptosis, trophic factors, inflammation, oxidative stress, excitotoxicity, and myelin and axon repair. We investigated whether VDH combined with PROG could improve behavioral outcomes more than PROG alone in VDH-sufficient rats given bilateral contusions of the medial frontal cortex. PROG and different doses of VDH (1 µg/kg, VDH1; 2.5 µg/kg, VDH2; 5 µg/kg, VDH3) were injected intraperitoneally 1 h post-injury. Eight additional doses of PROG were given subcutaneously over 8 days with tapering over the last two days. Neurobehavioral tests, necrotic cavity, neuronal death and activation of astrocytes were evaluated 21 days post-injury. We found that PROG and PROG+VDH preserve spatial memory processing. VDH1+PROG improved performance in acquisition more effectively than PROG alone, indicating that the low VDH dose is optimal for combination therapy. There were no significant differences in necrotic cavity size among the groups. The density of positive staining for reactive astrocytes (glial fibrillary acidic protein (GFAP)) increased and the cell bodies and processes of GFAP-positive cells were enlarged in the PROG+VDH1 group. Our data indicate that the combination of PROG and VDH is more effective than PROG alone in preserving spatial and reference memory, and that PROG plus low-dose VDH can activate GFAP reactions up to 21 days after injury. This effect may be one of the mechanisms underlying PROG's neuroprotective effects in combination with VDH.
Keywords: Traumatic brain injury, combination treatments, functional repair, progesterone, Vitamin D3 hormone
INTRODUCTION
Over 130 drugs have shown promise as protective agents in animal models of brain injury, but while dozens of these have been tested in Phase II and III clinical trials, to date all have failed to enhance CNS repair and recovery of function in humans. One reason for these failures is that brain injury is a complex disease that causes a cascade of molecular and anatomical events affecting not just the brain itself but multiple organ systems whose failures are often the cause of mortality or long-term morbidity. Drugs designed to act at a single receptor site or only in the brain may fail to address the more complex and manifold nature of the disease. In light of this problem, the National Institute of Neurological Disorders and Stroke (NINDS) has called for combination therapies with complementary targets or multi-potential drugs that would work more effectively to treat the systemic problems caused by traumatic brain injury (TBI) (Margulies and Hicks, 2009).
A number of pleiotropic agents are under pre-clinical investigation, but as of this writing only progesterone (PROG) is in Phase III trials, with nationwide and international studies underway. Two independent Phase II trials of PROG demonstrated more than a 50% reduction in mortality in severely injured patients and significantly enhanced functional outcome measures (Stein and Wright, 2010; Wright et al., 2007; Xiao et al., 2008). There are now over 147 preclinical studies demonstrating the beneficial effects of PROG in a variety of injury models that can cause inflammation, oxidative damage, cerebral edema and neuronal cell death (Gibson et al. 2011; Stein and Cekic, 2011; Stein, 2011; Stein and Hurn, 2009). Although a number of agents could be combined with PROG to increase its efficacy, we chose to study vitamin D hormone (VDH) for two reasons. First, the beneficial effects of PROG can be reduced, especially in the elderly, if the patient is VDH deficient (Cekic and Stein, 2010; Chatterjee, 2001; McCann and Ames, 2008). Second, VDH not only shares many mechanisms of neuroprotection with PROG, so that it may be able to amplify PROG's effects, it is also a hormone with its own additional mechanisms of action which have been shown to be neuroprotective in a number of injury models (Brewer et al., 2001; Buell and Dawson-Hughes, 2008; Chang et al., 2010; Lin et al., 2005), and thus may also be able to extend PROG's effects. One problem is that, according to the National Health and Nutrition Examination Survey, an average of 41% of Americans are VDH deficient. VDH deficiency is associated with a number of systemic conditions including hyperparathyroidism, metabolic syndrome, hypertension, obesity, diabetes mellitus, and stroke (Cekic et al., 2009).
Two recent experiments provide evidence that the combination of VDH with PROG can be more neuroprotective than PROG alone. One showed that the combination of the two hormones in cell culture was more effective at protecting primary cortical neurons from cytotoxic insult than either compound alone (Atif et al., 2009). Another experiment tested the combination in VDH-deficient aged rats (Cekic et al., 2011). Among the D-deficient groups, only those treated with VDH and PROG showed improved behavioral outcomes and reduced inflammatory proteins, demonstrating how deleterious VDH deficiency can be, even in rats treated with PROG.
In keeping with the NIH mandate, the current study sought to determine whether VDH combined with PROG could improve behavioral outcomes more effectively than PROG treatment alone in VDH-sufficient rats with bilateral contusion injury of the medial frontal cortex (MFC). We tested three different doses of VDH to determine which produced the best behavioral recovery in combination with PROG. Locomotor activity and tactile awareness (measures of habituation/hyperactivity and sensory neglect) were tested prior to injury and then throughout the experiment to monitor whether the treatments improved the behavioral outcomes. Necrotic cavity size, neuronal death and the reaction of astrocytes to the injury were also evaluated.
MATERIALS AND METHODS
Animals
Forty-six male Sprague–Dawley rats weighing 300–350g at the time of injury were used in this experiment. They were housed individually with unlimited access to food and water. Rats were placed under a 12:12-h reverse light–dark cycle(0800–2000 h) so that behavioral testing would occur during their active phase (Paulson and Robinson, 1994). After a 7-day acclimation to the colony, the rats were weighed daily for 4 days and handled as previously described (Cutler et al., 2007) in squads of 10. Baseline behavioral measures were taken one day after handling was complete (3 days before surgery). Rats were randomly assigned to the following groups: sham-vehicle (Sham-Veh; n=7), lesion-vehicle (CCI-Veh; n=7), lesion + PROG (PROG; n=9), PROG+VDH1 (n=8), PROG+VDH2 (n=8), and PROG+VDH3 (n=7). VDH1, VDH2 and VDH3 represent the low (1 µg/kg), medium (2.5 µg/kg) and high (5 µg/kg) doses of VDH, respectively. The rats were obtained from Charles River Laboratories, Wilmington, MA. The experiments reported here conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. Animal care and experimental protocols (164–2008) were approved by the Emory University Institutional Animal Care and Use Committee. Of the animals that started the experiment (n=52), six (one from each group, except for PROG+VDH3, which lost 2) did not complete the behavioral tests because of medical complications, so they were excluded from the data analyses, leaving a total of 46 rats to complete the experiment. Since the experimenters were blinded to the groups for behavioral testing, we did not add extra animals to compensate for the losses.
Induction of TBI
Isoflurane anesthesia was induced for 4 min at 5% and maintained at 2.5%. Using a SurgiVet pulse oximeter (model V3304; Waukesha, WI), blood SpO2 was monitored and maintained at levels of 90%. Body temperature was monitored throughout surgery (via rectal probe) and maintained at 36.5 – 37.5°C using a heating blanket (Harvard Apparatus, South Natick, MA). A midline incision was made along the scalp and the fascia cleared to expose the surface of the skull. Medial, lateral and dorsal stereotaxic coordinates were determined at bregma and a 5-mm diameter bilateral craniotomy was performed mid-sagittally, 3 mm anterior to bregma. MFC injury was created with an electromagnetic impactor device (3-mm diameter), over .5 ms with a velocity of 2.25 m/s and to a depth of 3.5 mm. Sutures were used to close the incision after all bleeding was controlled. Animals were placed on a heating pad until they regained consciousness and then returned to clean, individual home cages containing accessible, moistened food pellets. Sham animals were anesthetized, and an incision was made at the top of the head. The fascia was cleared to expose bregma, and then the incision was sutured closed.
PROG and VDH preparation and administration
PROG was administered in 2-hydroxyropyl-β-cyclodextran solution (HBC, 22.5% w/v solution in dH20) (vehicle) as the solvent. VDH (1, 25(OH)2 D3) was dissolved in 95% ethanol, aliquoted and stored at −80°C. On the day of surgery, the stock VDH was diluted in HBC stock solution and sterile water, resulting in a 22.5% HBC with 2% ethanol solution. Controls received a volume of vehicle equal to the volume of the PROG dose. Sixteen mg/kg PROG was administered (Cutler et al., 2007) intraperitoneally 1 h post-injury. This was followed by subcutaneous injections at 6, 24, 48, 72, 96, 120, 144 and 168 h post-contusion. Tapering was induced as halved dosages over the last two days of treatment to avoid PROG withdrawal (Cutler et al., 2006). We used 16 mg/kg of PROG because previous studies have shown this dose to be effective (Cutler et al., 2007; Goss et al., 2003) for reducing the effects of TBI. The rats were injected intraperitoneally with one dose of VDH (1, 2.5 or 5 µg/kg) 1 h post-injury.
Morris water maze
The maze was a circular pool of water (115 cm diameter), made opaque with white paint, with a platform (11 × 11 cm) of the same color hidden 2 cm beneath the water’s surface. It was located in a lighted room with various visuospatial cues, such as a curtain, shelves, and a white wall. Nontoxic black marker was applied to the top of each rat’s head and neck area so they could be tracked by video. Water maze testing began 10 days after surgery. Rats received 2 trials per day over 7 consecutive days of testing. The platform remained in the same position relative to both the maze and the room throughout testing. A single trial consisted of placing a rat in the water from two different starting locations and letting it swim in the maze until it found and climbed onto the platform, where it then was permitted to remain for 20 sec. Rats that left the platform before 20 sec were returned to it promptly by the experimenter. If a rat did not locate the platform after 90 sec, it was guided to the platform, where it remained for the 20-sec period. Rats removed from the platform were then placed near a heater for 3 min before the second trial began.
For each trial, a computer connected to a video tracking system detected the contrast of the marked, dark head of each rat against the white opaque water. Path length, latency to reach the platform, and the percent time spent in the outer annulus of the pool were recorded. The percent time spent in the outer annulus was used as a measure of thigmotaxic behavior (Devan et al., 1999).
We used the second trial on each training day as a measure of short-term memory because of the short time period (5 min) between trials. On the eighth day of testing the platform was removed and a probe trial was performed. Each rat was placed in the water and allowed to swim for 30 sec. The purpose of this trial was to assess how well the rat remembered the platform location. The probe trial and the first trial of each day represent long-term memory because they are given 24 h after the last training session (Baldi et al., 2005). Latency to enter the platform quadrant and percent of time each rat spent in that quadrant were recorded.
Somatosensory neglect of the forepaws
Testing was conducted under red light in a quiet environment 3 days before surgery (baseline) and at 3, 8, 15 and 20 days post-surgery. Circular adhesive labels (1.3-cm diameter) were placed on the ventral left forepaw and the rat was placed in a clear Plexiglas testing box. Latency to contact the sticker (contact latency) and time required for each rat to remove the sticker with its mouth was recorded, with maximum test duration of 4 min. The testing box was cleaned with 70% ethanol and dried between trials.
Activity Testing
Testing for activity was done under red light in a quiet environment 3 days before surgery and at 3, 8, 15 and 20 days post-surgery. The purpose of testing prior to injury was to obtain a baseline for each animal that could be used to calculate percent change at post-injury time points. Two animals were tested simultaneously in individual boxes using the Digiscan Activity Monitoring System (AccuScan Instruments Inc., Columbus, OH). Rats were placed in the center of the activity box and the recording apparatus was turned on. After 10 min the computer stopped recording movements and animals were returned to their home cages. The activity boxes were cleaned with 70% ethanol and dried between trials.
Tissue preparation
Twenty-three days after surgery, rats were exposed to 5% isoflurane for 5 min. Once completely anesthetized, the rats were transcardially perfused with .05 M phosphate-buffered saline (PBS, 2 min) and fixed with 10% formalin buffer (PH=7.4). Brains were then extracted from the skull, post-fixed for 2 h at 4°C in the same fixative, and then placed in increasing amounts of 0.1 M phosphate-buffered sucrose (10%, 20%, 30%) each day. Brains were then dissected into anterior and posterior sections; the anterior section contained the entire lesion area. The anterior section was immersed in cryoprotectant and frozen using 2-methyl-butane chilled on dry ice. Sections were stored in cryoprotectant at −80°C. The frozen brain was then mounted in cryoprotectant, placed in a cryostat and cut into six series of 20-µm coronal sections.
Evaluation of necrotic cavity
Evaluation of the necrotic cavity was performed as previously described with slight modification (Wali et al., 2011). Briefly, slides from each level (from Bregma 1 mm to Bregma 5 mm) were selected for Nissl staining. After air drying, the slides were washed with PBS (PH=7.4), and stained in 0.1% cresyl violet solution for 5–10 min at 37°C. The slides then were rinsed quickly in distilled water, differentiated in 95% ethyl alcohol, dehydrated in 100% alcohol 2×5 min, cleared in xylene 2×5 min, and mounted with permanent mounting medium. The stained slides were scanned into computer and saved as digital images using PathScan Enabler IV (Meyer Instruments, Houston, TX). The necrotic cavity in each section was traced using image-J software from NIH and represented as the area (mm2). The volume of the necrotic cavity in each brain was calculated by the following formula: 1mm× (A1+A2)/2 + 1mm× (A2+A3)/2 + 1mm× (A3+A4)/2 + 1mm× (A4+A5)/2 1mm is the interval between the sections; A stands for area). The results were presented as the volume of tissue lost (mm3).
Fluoro-Jade C (F-Jc) staining
F-Jc was used to assess degenerating neurons in the brain (Ehara and Ueda, 2009). In this experiment, the procedures for F-Jc staining were performed as previously described (Ehara and Ueda, 2009). Briefly, after mounted slides had been air dried for 2 h, they were washed 3 times in PBS and then immersed in a basic alcohol solution consisting of 1% sodium hydroxide in 80% ethanol for 5 min, rinsed for 2 min with 70% ethanol, and then incubated in 0.06% potassium permanganate solution for 10 min. After rinsing with ddH2O, the slide-mounted tissues were stained with 0.0001% of F-Jc solution for 10 min, rinsed in distilled water 3×1 min, and covered with mounting medium with propidium iodide. The tissue was examined with a fluorescence microscope and pictures were taken of the peri-lesion cerebral cortex by ImagePlus software. The percentage of F-Jc positive cells was counted and calculated.
Immunohistochemical staining for GFAP
The slides were air-dried for 2 h, washed 3 times in PBS, incubated with blocking buffer (10% goat serum in PBS) at room temperature (RT) for 30 min, and then with rabbit anti-rat GFAP primary antibody (18-0063, Invitrogen, Carlsbad, CA) at 4°C overnight. The slides were then washed 3 times in PBS, and incubated with goat anti-rabbit IgG (H+L) labeled with Alexa Fluor 546 F(ab’) fragment (A-11071, Molecular Probes, Carlesbad, CA) for 1 h at RT. The slides were then rinsed with PBS for 3×5 min, and then covered with a mounting medium with 4',6-diamidino-2-phenylindole (DAPI). The tissue was examined with a fluorescence microscope and pictures taken from both sides of the peri-lesion cerebral cortex and subcortex by ImagePlus software. GFAP-positive cells were counted and the result calculated as a percentage of the positive cells/total counted cells.
Statistics
All results are expressed as the mean plus or minus the standard error of the mean. Statistical significance was set at p<0.05 for two-tailed tests. A repeated measures analysis of variance (ANOVA) was used to analyze MWM testing, locomotor activity, and somatosensory neglect of the forepaws followed by Tukey post-hoc individual comparisons. A one-way ANOVA and Tukey post-hoc comparisons were used for slope and probe trial analyses. The results from necrotic cavity size, F-Jc positive cells and GFAP positive cells were analyzed using one way ANOVA. For each outcome measure, we calculated the starting sample sizes and power needed to reject the null hypothesis with a p-value of 0.05. The results varied from a low of 0.81 (spatial learning probe trial) to a high of 0.99 (for measurement of learning rates in the Morris Water Maze). The number of group at these criteria was determined to be 6.
RESULTS
Weight and survival of rats
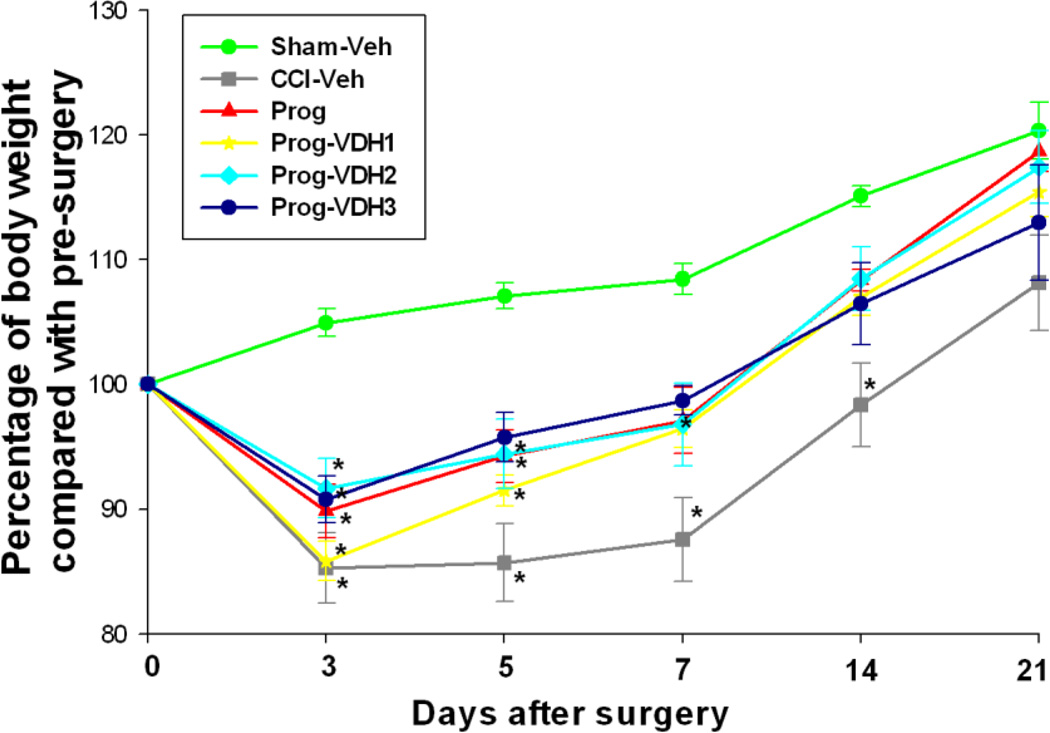
Body weight was presented as the percentage of body weight post-surgery to body weight pre-surgery. The results showed that all of the groups lost weight 3 days after TBI compared to sham controls. Fourteen days after TBI, body weight recovered (no significant difference compared to shams) in all treatment groups except CCI-Veh (Fig. 1).
Figure 1. Body weight changes over days post-surgery.
Body weight was presented as the percentage of body weight post-surgery to body weight pre-surgery. The results showed that all of the groups lost weight 3 days after TBI compared to sham controls. Fourteen days after TBI, body weights recovered (no significant difference compared to shams) in all treatment groups except CCI-Veh. *Compared with Sham-Veh, p<0.05.
Latency to find the platform
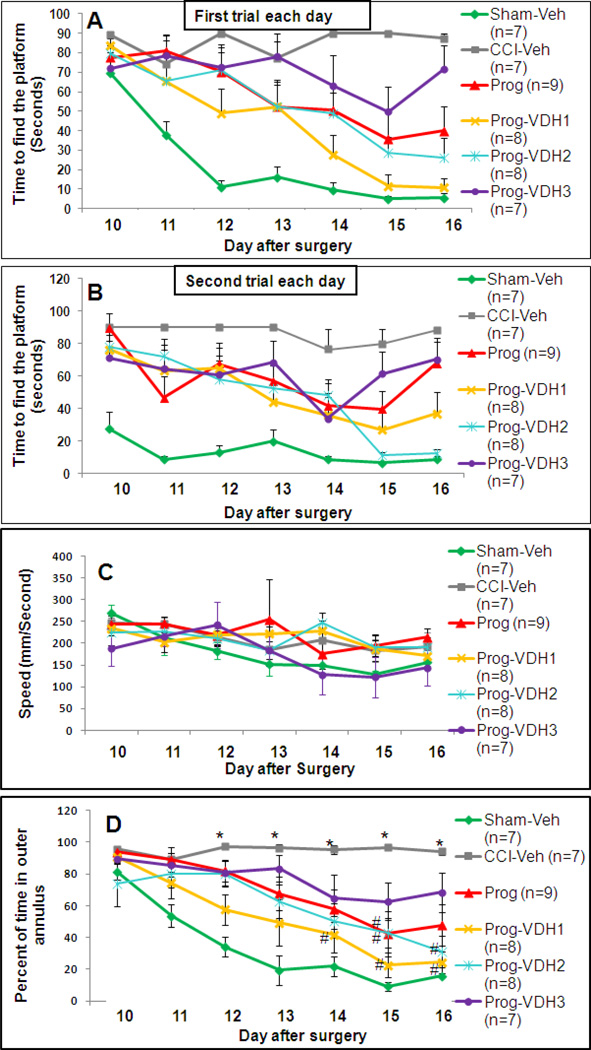
There were two trials for each testing day in the MWM. These trials were analyzed separately to differentiate between long- and short-term memory (Baldi et al., 2005; Prior et al., 1997). A repeated measures ANOVA of average latency to reach the platform showed a main effect of group for both the first (F (1, 5) = 8.9, p<0.01) and second (F (1, 5) = 5.47, p<0.01) trials. Post-hoc analysis (Tukey HSD) revealed that for the first trials on each day, all treatment groups except PROG+VDH3 showed improved acquisition of the task compared to CCI-Veh (p<0.05), and there were no significant differences among the treatment groups. Interestingly, compared with Sham-Veh, all the TBI groups except PROG+VDH1 showed impaired acquisition of the task (Fig. 2A). Post-hoc analyses (Tukey HSD) of the data from the second trial on each day showed that all groups were significantly different from Sham-Veh (p<0.05) except PROG and PROG+VDH2 (Fig. 2B). To evaluate improvement in learning and memory, we calculated the slope of the change in swim times over days. The results showed that the slope for CCI-Veh remained horizontal, demonstrating no improvement in performance (3.25±1.82), while the slope in the Sham-Veh group decreased sharply (−9.22±1.57, p<0.05). These findings indicate that CCI significantly impaired spatial learning performance. Compared with CCI-Veh, all treatment groups showed improvement in the rate/slope of learning (PROG: −8.07±2.87; PROG+VDH1: −12.41±1.54; PROG+VDH2: −9.09±0.99; PROG+VDH3: −7.93±2.35, p<0.05).
Figure 2. MWM Training trials.
A. Latency to find the platform in the first trials each day: All treatment groups except PROG+VDH3 showed improved acquisition of the task compared to CCI-Veh (p<0.05). There were no significant differences between any of the treatment groups. The only group that did not show a persistent impairment on the task compared to Sham-Veh was PROG+VDH1. B. Latency to find the platform in the second trials in each day: All groups were significantly worse than Sham-Veh (p<0.05) except PROG+VDH2. C: Swimming speed: There was no significant difference among the groups. D: Percentage of time spent in the outer annulus: Time in the outer annulus decreased over the training period in the Sham-Veh group. The percentages of time in the outer annulus in the CCI-Veh group remained flat over the training period and were significantly different from Sham-Veh from the 12th day after surgery (*p<0.05). Interestingly, compared with CCI-Veh, the percentage of time in the outer annulus decreased in the PROG group on the 15th day after surgery (#p<0.05), in PROG+VDH2 on the 15th and 16th days (#p<0.05), and in PROG+VDH1 from the 14th day to the end of the training period (#p<0.05). *Compared with Sham-Veh, p<0.05; #compared with CCI-Veh, p<0.05.
Swim speeds
We found no significant differences in swimming speed among the groups (Fig. 2C).
Thigmotaxis
Percent time spent in the outer annulus was calculated and used as a measure of thigmotactic behavior. As shown in Figure 2D, time spent in the outer annulus decreased over the training period in the Sham-Veh group. The percentages of time in the outer annulus in the CCI-Veh group remained flat over the training period and were significantly different from Sham-Veh from the 12th day after surgery (p<0.05). Interestingly, compared to CCI-Veh, the percentage of time in the outer annulus significantly decreased in the PROG group on the 15th day after surgery (p<0.05), in PROG+VDH2 on the 15th and 16th days (p<0.05), and in PROG+VDH1 from the 14th day to the end of the training period (p<0.05). Thigmotaxis can be taken as a sign of "anxiety" or "stress." Our data can be interpreted to suggest that PROG and VDH may reduce stress or anxiety-like behavior caused by TBI. The combination of PROG with low-dose VDH was better than PROG alone. The high dose combined with VDH was ineffective.
Percent time spent in the platform quadrant (Probe Trial)
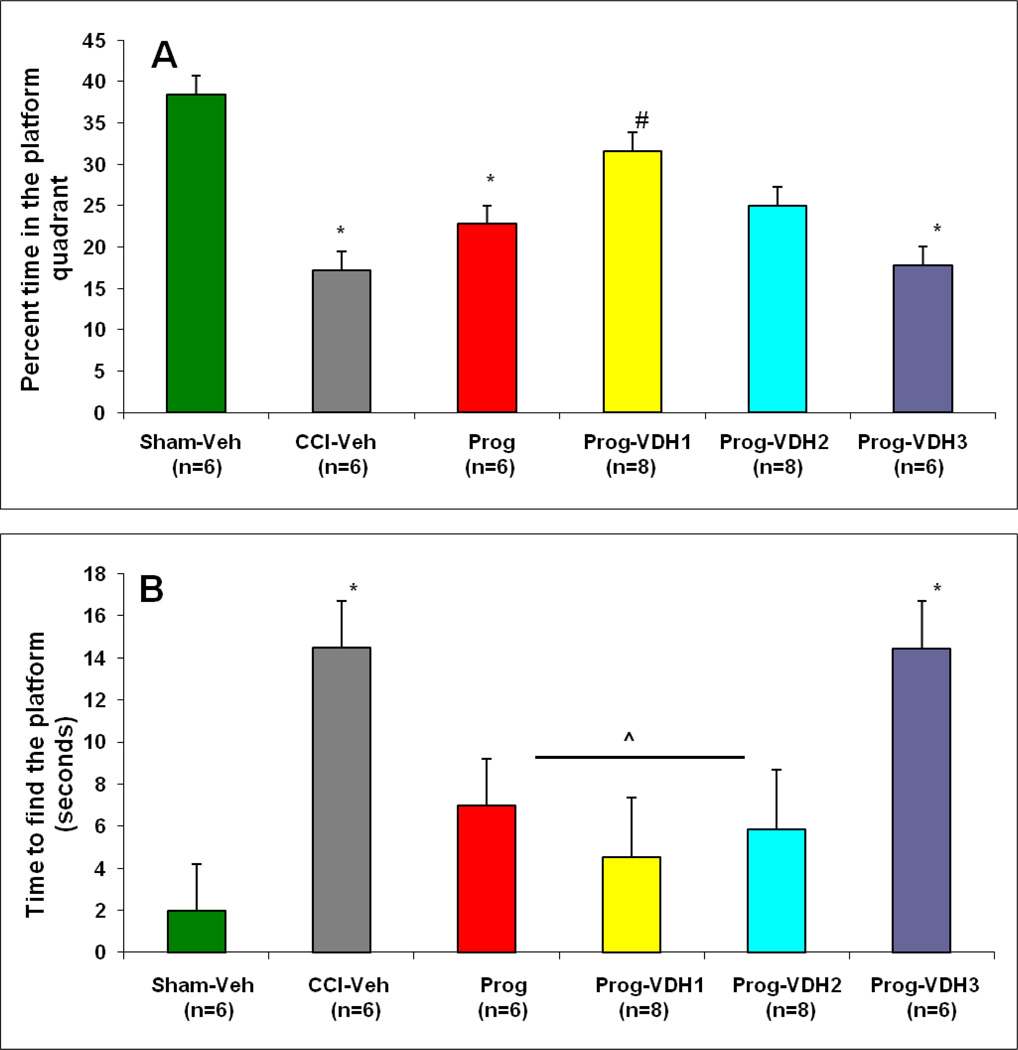
ANOVA showed a difference among the treatment groups (F (5, 37) = 3.5, p<0.01). Post-hoc analyses revealed that subjects in the PROG alone, CCI-Veh and PROG+VDH3 groups spent significantly less time in the platform quadrant compared to Sham-Veh (p<0.05). Interestingly, PROG+VDH1 rats spent more time in the correct quadrant than CCI-Veh or PROG-alone animals (p<0.05, Fig.3A). We interpret these results to demonstrate the advantages of PROG+VDH1 in improving spatial learning performance after TBI. Further, the combination of PROG plus low-dose VDH was more effective than PROG alone in preserving spatial and reference memory. In addition, all treatment groups except PROG+VDH3 showed a decreased latency compared to CCI-Veh (p<0.05), indicating that high-dose VDH failed to improve spatial learning performance after TBI.
Figure 3. Probe Trial.
A. Percent time spent in the platform quadrant: Average percent +/− SEM. PROG, CCI-Veh and PROG+VDH3 rats spent significantly less time in the platform quadrant than Sham-Veh (*p<0.05); PROG+VDH1 rats spent more time in the correct quadrant than CCI-Veh, PROG, and PROG+VDH3 animals (#p<0.05). B. Time to enter platform quadrant: Average time +/− SEM. CCI-Veh and PROG+VDH3 showed an increased latency compared to Sham-Veh (*p<0.05). All treatment groups except PROG+VDH3 showed a decreased latency compared to CCI-Veh (^p<0.05). * Compared with Sham-Veh, p<0.05; #: compared with CCI-Veh, PROG, and PROG+VHD3, p<0.05; ^compared with CCI-Veh, p<0.05.
Time to enter the platform quadrant (Probe Trial)
Results of ANOVA showed a difference among the treatment groups (F (5, 36) =2.48, p<0.01). Post-hoc analyses found that all TBI groups took more time to find the platform in the water maze than the sham controls (p<0.05, data not shown), and that all treatment groups except PROG+VDH3 showed a decreased latency compared to CCI-Veh (p<0.05). The results indicated that PROG+VDH3 is deleterious compared to PROG, PROG+VDH1 and PROG+VDH2 in the treatment of TBI (Fig. 3B).
Locomotor Activity
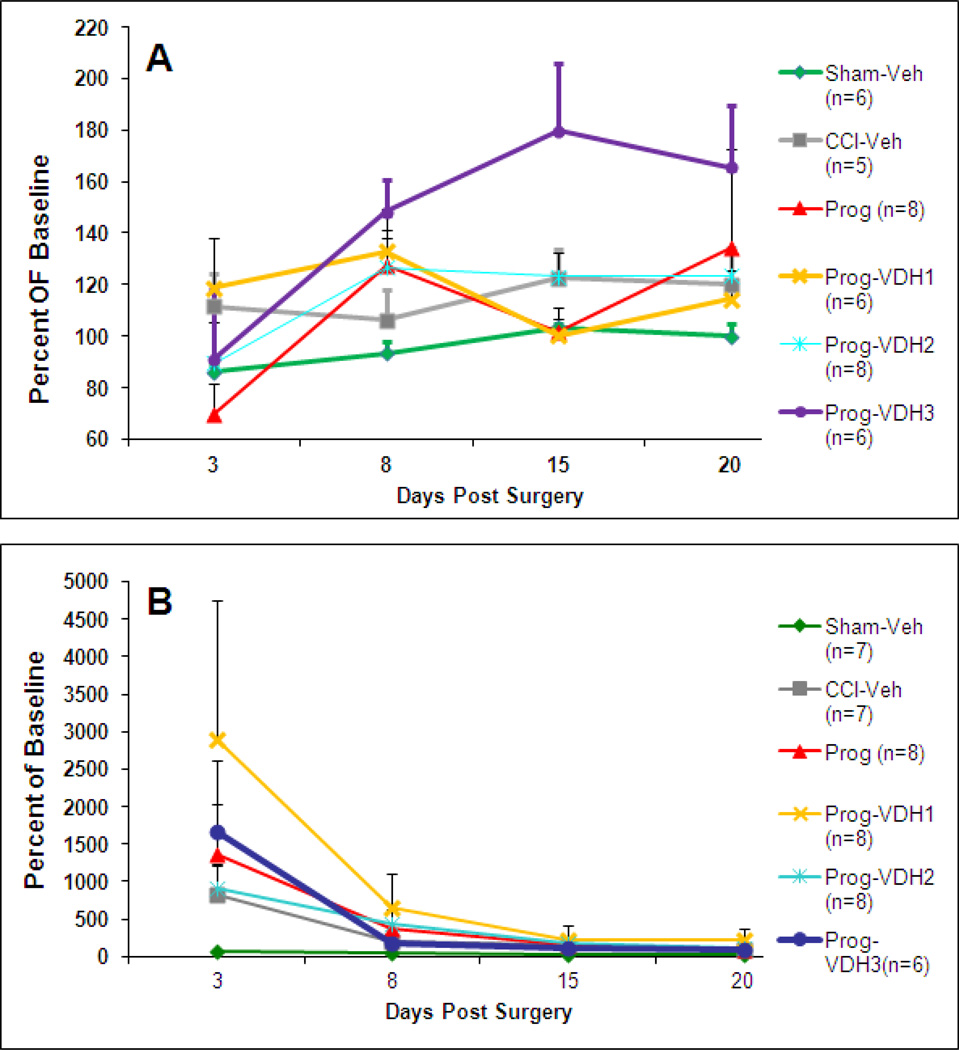
The average distance traveled at 3, 8, 15 and 20 days post-surgery did not differ among groups (Fig. 4A).
Figure 4. Locomotor Activity and Sticky Task.
A. Locomotor Activity: The average distance traveled at 3, 8, 15 and 20 days post-surgery did not differ among groups. B. Sticky Task: The average percent of baseline time at 3, 8, 15 and 20 days post-surgery did not show a significant difference among groups. (5000 percent represents roughly a 4 minute difference relative to pre-surgery baseline.)
Sticky Task
The average percent of baseline time at 3, 8, 15 and 20 days post-surgery did not differ among groups (Fig. 4B).
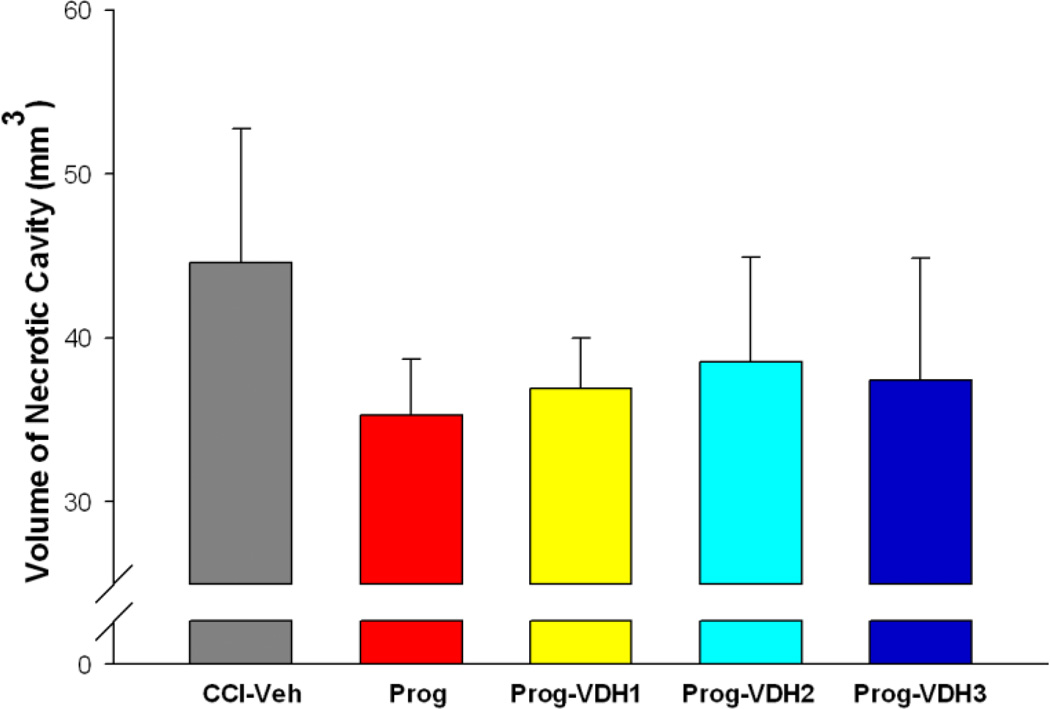
Necrotic cavity
CCI resulted in obvious brain damage, which formed a substantial cavity at 21 days after injury. However, there were no significant differences among the lesion groups (Fig. 5), so as measured 21 days after injury, the treatments were without effect in reducing necrosis caused by contusions of the frontal cortex.
Figure 5. Volume of necrotic cavity.
CCI resulted in obvious brain damage 21 days after injury as evidenced by a cavity in the site where the CCI was induced. The statistical results for volume of necrotic cavity did not show significant differences among the groups.
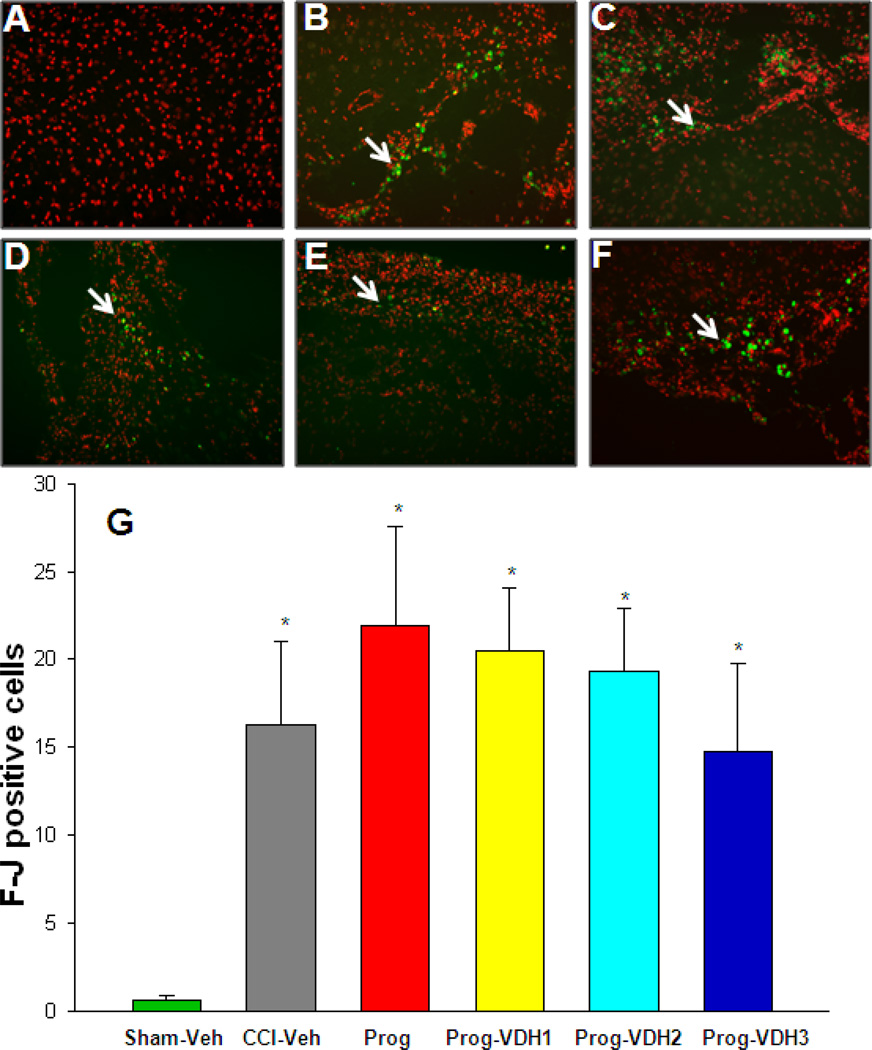
F-Jc positive cells
As shown in Fig. 6, TBI results in neurodegeneration as indicated by a significant increase in F-Jc positive cells (p<0.05, one way ANOVA). These cells were seen primarily on the edges of the necrotic cavity. Compared with Sham-Veh, F-Jc positive cells significantly increased in all CCI groups, but there were no significant differences between vehicle-treated, PROG, or PROG+VDH-treated rats.
Figure 6. Degenerative neurons (Fluro-Jade staining).
Slides were stained with Floro-Jade C (F-Jc) antibody, and counterstained with propidium iodide (PI). The F-Jc positive staining appears as green (indicated by white arrows), and the PI as red. F-Jc positive cells significantly increased in all the CCI groups compared to Sham-Veh (*p<0.05). However, there were no significant differences between CCI-Veh, PROG and PROG+VDH groups. *Compared with Sham-Veh, p<0.05.
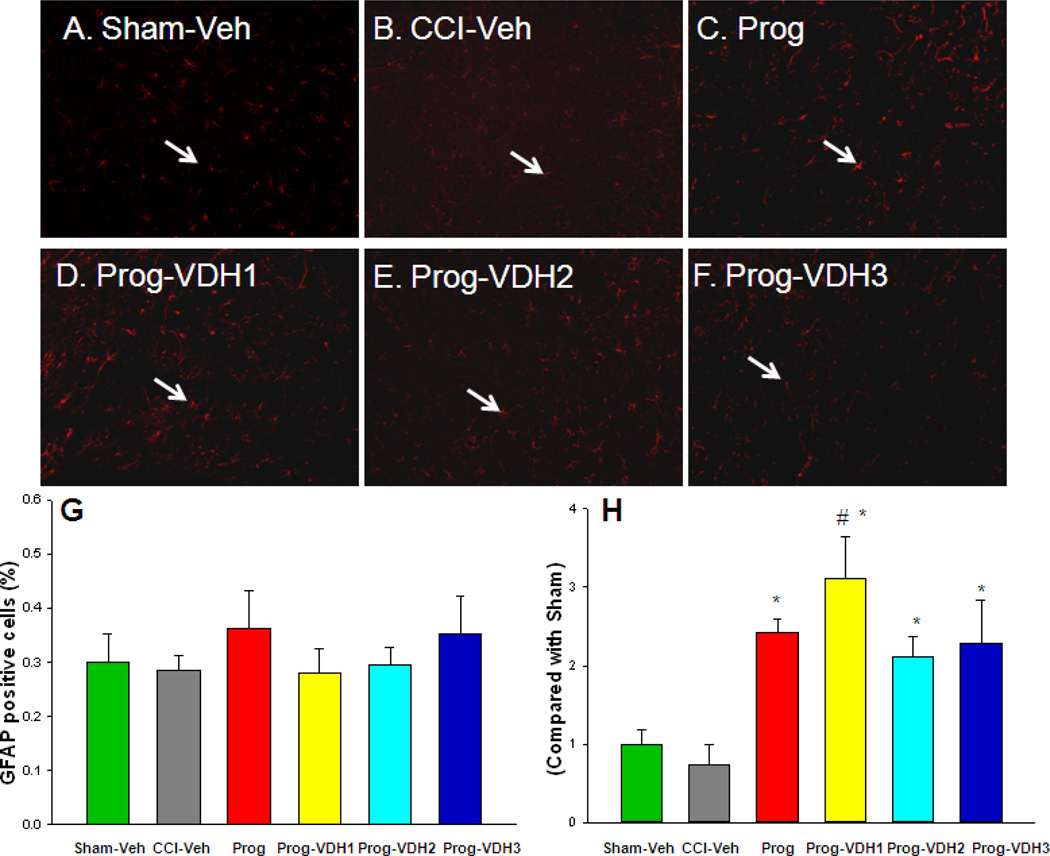
GFAP-positive cells
The percentage of GFAP-positive cells did not differ among the TBI groups (Fig. 7) as analyzed by one way ANOVA (p>0.05). Compared to the sham and CCI-Veh groups, the density of the positive staining increased, and the cell bodies and processes of the GFAP-positive cells were enlarged in the PROG+VDH1-treated group (Fig. 7, p<0.05).
Figure 7. GFAP-positive cells.
A–F. Representative figures showing GFAP-positive staining in red (Alexa Fluor 546 F(ab’) fragment, indicated by white arrows). G: The percentage of GFAP-positive cells did not differ among the TBI groups. H: Compared to Sham and CCI-Veh groups, the cell bodies and processes of the GFAP-positive cells were enlarged in the treatment groups (*p<0.05). This increase was significant in PROG+VDH1 compared with PROG (#p<0.05). *Compared with CCI-Veh, p<0.05; #compared with PROG, p<0.05.
DISCUSSION
Combination therapy and behavioral recovery
We assessed whether VDH supplementation combined with PROG could improve behavioral outcomes more effectively than PROG treatment alone in VDH-sufficient rats given bilateral injury of the MFC. Three different doses of VDH were tested in combination with the established best dose of PROG to determine which dose of VDH improved behavioral outcomes most effectively when given together with PROG. The doses were chosen to represent a low (1 µg/kg, VDH1), medium (2.5 µg/kg, VDH2) and high (5 µg/kg, VDH3) dose based on the effectiveness of VDH alone in other brain injury models (Lin et al., 2005; Losem-Heinrichs et al., 2005).
Although PROG showed evidence of neuroprotection in this animal model, we found that the combination treatment preserved spatial memory processing better than PROG alone. Two of the VDH doses, low and medium, in combination with PROG improved performance in acquisition trials and on the retention test, but only the low-dose combination group (VDH1) performed as well as sham animals in their rate of learning. We saw similar results in previous studies where combinational therapy was not simply additive; i.e. two higher doses do not necessarily lead to a two-fold better outcome and this is discussed in detail in (Cekic & Stein 2010).
In additional to the total "learning" scores we also looked at performance data for the MWM separately for the first and second trial of each day (odd vs. even trial graphs) to determine whether there were differences in performance on short- versus longer-term memory. The assumption was that rats use information from trial 1 to find the platform location in trial 2, which takes place 5 min after trial 1 (Morris, 1984).The low dose of VDH+PROG led to better performance in the odd trials (first trial of each day, representing long-term memory measured 24h later), while the medium dose showed improved performance on the even trials (second trial of each day, representing shorter-term memory). In addition, we found that thigmotactic behavior, a sign of "anxiety" or stress, was reduced by treatment with both PROG alone and PROG+ low-dose VDH. Less stressed animals tend to venture away from hugging the walls of the maze, thus increasing the chance of finding the submerged platform more rapidly. This observation is consistent with the known anxiolytic and GABAA agonistic effects of neurosteroids under a variety of stress-related conditions, so this is likely to be primarily an effect of the PROG (see for example Gunn et al., 2011). The specific role of VDH in response to stress has yet to be determined.
Damage to a variety of brain regions results in MWM deficits (Ferretti et al., 2007; Frielingsdorf et al. 2006). The VDH1+PROG group showed the best performance in the probe trial and the odd-numbered trials, indicating that it most effectively preserves long-term memory. However, the low-dose VDH+PROG combination did not result in any long-term behavioral changes on locomotor activity and sensory neglect tasks. This observation can be taken to suggest that the performance improvements were due to cognitive recovery rather than a reduction in injury-induced sensory or motor deficits. Previous work in our lab found that both PROG and VDH significantly (p<0.001) reduce neuronal loss induced by exposure to glutamate (0.5 micromol/L) toxicity, and that primary cortical cultures treated with VDH exhibited a U-shaped concentration-response curve (Atif et al., 2009). The lower dose of VDH (20 nmol/L) was most effective when used in combination with PROG (p < 0.01), while higher concentrations of VDH were either less protective or not protective at all. The mechanism behind this phenomenon might be a direct toxic effect of VDH on motor and hippocampal neurons at high concentrations. Another possibility is that higher doses inhibit receptor activation, produce receptor saturation, or prevent chaperone proteins from carrying the VDH into the cell. In addition, high-dose VDH may have side effects such as tiredness, muscle problems, and mental/mood changes, which affect behavior (Atif et al., 2009; Vieth, 1990; Jones, 2008). These are all interesting questions that can be pursued in further experiments.
Our finding that the combination of PROG and VDH supplementation is more effective is not surprising, since both drugs have been shown to be neuroprotective independently in a number of in vivo injury paradigms. PROG has been shown to provide protection against TBI (Cutler et al., 2007; Cutler et al., 2006; Grossman et al., 2004; Stein, 2008), stroke (Ishrat et al., 2010; Ishrat et al., 2009; Wang et al., 2010), and spinal cord injury (Garay et al., 2011).
VDH mechanisms and CNS repair
It is very important to note that VDH is not just a “vitamin” but also a steroid hormone that influences metabolic systems throughout the body through genomic and non-genomic pathways. The hormone is inert and requires a two-step enzymatic pathway for conversion to its active form 1, 25 (OH)2D3). Although long known for its role in regulating intestinal calcium and mineral homeostasis, VDH has been shown to modulate the immune system, the renin angiotensin system, and neuromuscular function, and to curtail the development of several diseases (Thacher and Clarke, 2011; Zhang and Naughton, 2010). VDH receptors are present throughout the brain in both neurons and glia, adding to the hormone's pleiotropic neuroprotective effects. Like PROG, VDH can modulate neuronal apoptosis, trophic factors, inflammation, oxidative stress, excitotoxicity, and myelin and axon repair (Garcion et al., 2002; McCann and Ames, 2008; Cekic et al., 2011; Stein and Cekic, 2011). Some of these VDH effects are brought about through mechanisms similar to PROG's, possibly leading to an additive effect. For example, if one receptor system becomes saturated or inhibited, limiting further action, the other could activate similar protective mechanisms through different signaling pathways. VDH also has many actions that may compensate for those PROG lacks. For example, an important function of VDH in TBI is modulating the rennin angiotensin system and blood pressure (Cekic and Stein, 2010) and downregulation of L-type calcium channel expression (Brewer et al., 2001). Other effects that may compensate for missing actions of PROG include potentiating axon regeneration, enhancing free radical scavenging, protecting against heme breakdown, and maintaining calcium levels (Cekic and Stein, 2010; Chatterjee, 2001; Cekic et al., 2009).
Combination therapy and calcium toxicity
As noted above, there are a number of interactions between VDH and PROG that could account for the improvements seen with the combination, including a reduction in excitotoxic cell death by moderating Ca2+. Increased cellular Ca2+ concentration is the final common step in the initiation of cell death in neurons, astrocytes and oligodendrocytes following TBI and other types of brain injury (Hazell, 2007). PROG (and its metabolites) and VDH are both protective against excitotoxic and ischemic cell death in their own right (Ciriza et al., 2004; Ciriza et al., 2006; Kajta et al., 2009; Stein, 2008). PROG is a competitive inhibitor for the σ1 receptor, through which it can reduce N-methyl-D-aspartate (NMDA) glutamate signaling and excitatory cholinergic signaling, and increase Ca2+ mobilization (Cai et al., 2008; Hu et al., 2009). It can also increase GABA and the GABAA receptor (through its 5a-reduced metabolite allopregnanolone (3a, 5a-tertrahydroprogesterone) (Gangisetty and Reddy, 2010)). Primary cortical neurons exposed to the best combination of PROG and VDH showed an additive effect in reducing excitotoxicity and upregulating MAPK in vitro (Atif et al., 2009).
VDH itself can also modulate excessive calcium signaling by decreasing L-type voltage-sensitive Ca2+ channels (L-VSCCs) (Brewer et al., 2001) and inducing the synthesis of Ca2+-binding proteins, such as parvalbumin and calbindin (Chatterjee, 2001; de Viragh et al., 1989). While the two hormones diverge in the mechanisms through which they modulate excitotoxicity, they converge in modulating oxidative stress by decreasing lipid peroxidation and inducible nitric oxide synthase while increasing glutathione (Cekic and Stein, 2010; Cekic et al. 2009). Oxidative stress leads to further Ca2+ disturbances, which both hormones can then attack via divergent processes. These are just few examples of interactions between PROG and VDH in regulating excitotoxity, one of the many cytotoxic processes induced by TBI.
Combination therapy and necrosis
The present study also evaluated the size of the necrotic cavity caused by the contusion and resulting secondary neurodegeneration. As shown in Figure 5, there were no significant differences in the volume of the necrotic cavity among the groups subjected to TBI and hormone treatment. This observation is consistent with our previous studies (Wali et al., 2011) showing no significant difference in the extent of localized tissue damage in lesion alone compared to PROG-treated groups. This is especially the case when the histology is performed several weeks to months after the initial brain injury. Based on our observations, PROG-induced functional recovery cannot be attributed specifically to tissue sparing in the area of the lesion. If the beneficial effects of combination therapy occur at the molecular/cellular level, it may not be necessary for PROG to decrease tissue loss in the necrotic cavity in order to improve behavioral outcome. The recovery is more likely to be due to other hormone-induced compensatory molecular mechanisms, reported previously, that can enhance neuronal/glial activity in both proximal and distal tissue that survive the initial injury (Stein and Cekic, 2011; Stein and Wright, 2010).
We also found that F-Jc positive cells increased around the edge of the necrotic cavity compared to intact controls (Fig. 6). However, there was no significant difference in the number of F-Jc-positive degenerative neurons among the treated TBI groups. One possible reason for this observation is that 3 weeks post-surgery, the degenerating neurons were already cleared by glial cells and were more difficult to see with this histological technique. In a future study, it would be worthwhile to determine whether combinatorial or single treatment effects on neuronal loss could be seen in animals evaluated at an earlier stage after TBI.
Combination therapy and the glial response to injury
We have previously reported that TBI increased the expression of GFAP in the peri-contusion area at 72 h post-TBI, and that PROG treatment increased the expression of GFAP immediately surrounding the contusion and other brain areas (Guo et al., 2006). In this experiment, we detected the activation of astrocytes in brain 21 days after TBI. We found that the number of GFAP-positive cells in each group was not significantly different (Fig. 7). However, we observed that the density of the positive staining increased, and the cell bodies and processes of the GFAP-positive cells were enlarged in the PROG+VDH1 groups. This finding can be taken to indicate that PROG plus a low dose of VHD can affect the glial response to injury up to 21 days after injury. Since astrocytes are the most numerous cell type in the central nervous system, provide structural, trophic, and metabolic supports to neurons, and modulate synaptic activity, we suggest that the activation of astrocytes is one of mechanisms underlying the neuroprotective effects of PROG combined with a low dose of VDH (Chen and Swanson, 2003; De Keyser et al., 2008). This relatively long-term effect may be relevant to the question of whether in future studies, PROG should be given for longer times and even during the clinical rehabilitation stage of the injury/repair process.
CONCLUSION
The experiment reported here found that the combination of PROG and VDH is more effective than PROG alone in preserving spatial and reference memory. No long-lasting changes in behavior induced by the combination were observed in locomotor activity or a test of sensory neglect, suggesting that the primary behavioral effects were due to moderate improvements in stress and cognitive performance. Despite our positive findings, caution should be exercised in extrapolating to potential clinical application. Although comparable to many animal studies, our group sample sizes were not large enough to pick up on small differences between control and treatment groups, so there was a risk of accepting the null hypothesis when it should have been rejected. Although there are now more than 140 studies from 37 other laboratories showing beneficial effects of PROG in a variety of injury models, the number of papers reporting combined use of PROG and VDH (or any other neuroprotective agent) is still very small. As with PROG alone, the therapeutic and potential clinical benefits of combination therapy should be replicated in other laboratories before considering clinical trials with the combination of PROG and VDH. Our work is but a first step in that direction.
Highlights.
Progesterone (PROG) protects the brain from traumatic injury.
PROG’s beneficial effects can be reduced in Vitamin D (VDH) –deficient subjects.
PROG combined with VDH is better than PROG alone in preserving memory.
A low VDH dose is optimal for combination therapy.
ACKNOWLEDGMENTS
This work was supported by NIH 5R01NS048451 and 1R01HD061971 to DGS. A portion of this research was supported by a gift from BHR Pharma, AHA National Program SDG 0830481N to FH, and AHA 11SDG5430002 to IS. The authors thank Dr. Xiaobo Ma for his contribution to the evaluation of the necrotic cavity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
Along with Emory University, DGS is party to a licensing agreement with BHR Pharma, a privately held company, and may receive royalties and research funding from BHR Pharma related to PROG. DGS occasionally serves as consultant to BHR and receives compensation for these services. The terms of this arrangement have been reviewed and approved by Emory University, which receives the majority share of royalties and fees in accordance with its conflict of interest policies.
Contributor Information
Fang Hua, Email: fhua2@emory.edu.
Jenny I. Reiss, Email: jreiss44@gmail.com.
Huiling Tang, Email: htang9@emory.edu.
Jun Wang, Email: jwang26@emory.edu.
Xavier Fowler, Email: xfowler@emory.edu.
Iqbal Sayeed, Email: isayeed@emory.edu.
REFERENCES
- Atif F, Sayeed I, Ishrat T, Stein DG. Progesterone with Vitamin D affords better neuroprotection against excitotoxicity in cultured cortical neurons than progesterone alone. Mol Med. 2009;15:328–336. doi: 10.2119/molmed.2009.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi E, Efoudebe M, Lorenzini CA, Bucherelli C. Spatial navigation in the Morris water maze: working and long lasting reference memories. Neurosci Lett. 2005;378:176–180. doi: 10.1016/j.neulet.2004.12.029. [DOI] [PubMed] [Google Scholar]
- Brewer LD, Thibault V, Chen KC, Langub MC, Landfield PW, Porter NM. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci. 2001;21:98–108. doi: 10.1523/JNEUROSCI.21-01-00098.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: preventing "D"ecline? Mol Aspects Med. 2008;29:415–422. doi: 10.1016/j.mam.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Zhu Y, Furuya K, Li Z, Sokabe M, Chen L. Two different molecular mechanisms underlying progesterone neuroprotection against ischemic brain damage. Neuropharmacology. 2008;55:127–138. doi: 10.1016/j.neuropharm.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Cekic M, Cutler SM, VanLandingham JW, Stein DG. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol Aging. 2011;32(5):864–874. doi: 10.1016/j.neurobiolaging.2009.04.017. Epub 2009 May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekic M, Sayeed I, Stein DG. Combination treatment with progesterone and vitamin D hormone may be more effective than monotherapy for nervous system injury and disease. Front Neuroendocrinol. 2009;30:158–172. doi: 10.1016/j.yfrne.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekic M, Stein DG. Traumatic Brain Injury and Aging: Is a Combination of Progesterone and Vitamin D Hormone a Simple Solution to a Complex Problem? Neurotherapeutics. 2010;7:81–90. doi: 10.1016/j.nurt.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JH, Cha HR, Lee DS, Seo KY, Kweon MN. 1,25-Dihydroxyvitamin D3 inhibits the differentiation and migration of T(H)17 cells to protect against experimental autoimmune encephalomyelitis. PLoS One. 2010;5:e12925. doi: 10.1371/journal.pone.0012925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M. Vitamin D and genomic stability. Mutat Res. 2001;475:69–87. doi: 10.1016/s0027-5107(01)00080-x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- Ciriza I, Azcoitia I, Garcia-Segura LM. Reduced progesterone metabolites protect rat hippocampal neurones from kainic acid excitotoxicity in vivo. J Neuroendocrinol. 2004;16:58–63. doi: 10.1111/j.1365-2826.2004.01121.x. [DOI] [PubMed] [Google Scholar]
- Ciriza I, Carrero P, Frye CA, Garcia-Segura LM. Reduced metabolites mediate neuroprotective effects of progesterone in the adult rat hippocampus. The synthetic progestin medroxyprogesterone acetate (Provera) is not neuroprotective. J Neurobiol. 2006;66:916–928. doi: 10.1002/neu.20293. [DOI] [PubMed] [Google Scholar]
- Cutler SM, Cekic M, Miller DM, Wali B, VanLandingham JW, Stein DG. Progesterone improves acute recovery after traumatic brain injury in the aged rat. J Neurotrauma. 2007;24:1475–1486. doi: 10.1089/neu.2007.0294. [DOI] [PubMed] [Google Scholar]
- Cutler SM, Vanlandingham JW, Stein DG. Tapered progesterone withdrawal promotes long-term recovery following brain trauma. Exp Neurol. 2006;200:378–385. doi: 10.1016/j.expneurol.2006.02.137. [DOI] [PubMed] [Google Scholar]
- De Keyser J, Mostert JP, Koch MW. Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J Neurol Sci. 2008;267:3–16. doi: 10.1016/j.jns.2007.08.044. [DOI] [PubMed] [Google Scholar]
- de Viragh PA, Haglid KG, Celio MR. Parvalbumin increases in the caudate putamen of rats with vitamin D hypervitaminosis. Proc Natl Acad Sci U S A. 1989;86:3887–3890. doi: 10.1073/pnas.86.10.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denson DD, Gordon AB, Wald MM, Gupta S, Hoffman SW, Stein DG. ProTECT: A Randomized Clinical Trial of Progesterone for Acute Traumatic Brain Injury. Ann Emerg Med. 2007;49:391–402. doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]
- Devan BD, McDonald RJ, White NM. Effects of medial and lateral caudate-putamen lesions on place- and cue-guided behaviors in the water maze: relation to thigmotaxis. Behav Brain Res. 1999;100:5–14. doi: 10.1016/s0166-4328(98)00107-7. [DOI] [PubMed] [Google Scholar]
- Ehara A, Ueda S. Application of Fluoro-Jade C in acute and chronic neurodegeneration models: utilities and staining differences. Acta Histochem Cytochem. 2009;42:171–179. doi: 10.1267/ahc.09018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti V, Sargolini F, Oliverio A, Mele A, Roullet P. Effects of intra-accumbens NMDA and AMPA receptor antagonists on short-term spatial learning in the Morris water maze task. Behav Brain Res. 2007;179:43–49. doi: 10.1016/j.bbr.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Frielingsdorf H, Thal LJ, Pizzo DP. The septohippocampal cholinergic system and spatial working memory in the Morris water maze. Behav Brain Res. 2006;168:37–46. doi: 10.1016/j.bbr.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Gangisetty O, Reddy DS. Neurosteroid withdrawal regulates GABA-A receptor alpha4-subunit expression and seizure susceptibility by activation of progesterone receptor-independent early growth response factor-3 pathway. Neuroscience. 2010;170:865–880. doi: 10.1016/j.neuroscience.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay L, Tungler V, Deniselle MC, Lima A, Roig P, De Nicola AF. Progesterone attenuates demyelination and microglial reaction in the lysolecithin-injured spinal cord. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.06.065. e-pub. 2011/07/09. [DOI] [PubMed] [Google Scholar]
- Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13:100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Coomber B, Murphy SP. Progesterone is neuroprotective following cerebral ischaemia in reproductively ageing female mice. Brain. 2011;134:2125–2133. doi: 10.1093/brain/awr132. [DOI] [PubMed] [Google Scholar]
- Goss CW, Hoffman SW, Stein DG. Behavioral effects and anatomic correlates after brain injury: a progesterone dose-response study. Pharmacol Biochem Behav. 2003;76:231–242. doi: 10.1016/j.pbb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Grossman KJ, Goss CW, Stein DG. Effects of progesterone on the inflammatory response to brain injury in the rat. Brain Res. 2004;1008:29–39. doi: 10.1016/j.brainres.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Gunn BG, Brown AR, Lambert JJ, Belelli D. Neurosteroids and GABA(A) receptor interactions: A focus on stress. Front Neurosci. 2011;5:131. doi: 10.3389/fnins.2011.00131. Epub 2011 Dec 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Sayeed I, Baronne LM, Hoffman SW, Guennoun R, Stein DG. Progesterone administration modulates AQP4 expression and edema after traumatic brain injury in male rats. Exp Neurol. 2006;198:469–478. doi: 10.1016/j.expneurol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Hazell AS. Excitotoxic mechanisms in stroke: an update of concepts and treatment strategies. Neurochem Int. 2007;50:941–953. doi: 10.1016/j.neuint.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Hu Z, Li Y, Fang M, Wai MS, Yew DT. Exogenous progesterone: a potential therapeutic candidate in CNS injury and neurodegeneration. Curr Med Chem. 2009;16:1418–1425. doi: 10.2174/092986709787846523. [DOI] [PubMed] [Google Scholar]
- Ishrat T, Sayeed I, Atif F, Hua F, Stein DG. Progesterone and allopregnanolone attenuate blood-brain barrier dysfunction following permanent focal ischemia by regulating the expression of matrix metalloproteinases. Exp Neurol. 2010;226:183–190. doi: 10.1016/j.expneurol.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T, Sayeed I, Atif F, Stein DG. Effects of progesterone administration on infarct volume and functional deficits following permanent focal cerebral ischemia in rats. Brain Res. 2009;1257:94–101. doi: 10.1016/j.brainres.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582S–586S. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- Kajta M, Makarewicz D, Zieminska E, Jantas D, Domin H, Lason W, Kutner A, Lazarewicz JW. Neuroprotection by co-treatment and post-treating with calcitriol following the ischemic and excitotoxic insult in vivo and in vitro. Neurochem Int. 2009;55:265–274. doi: 10.1016/j.neuint.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Lin AM, Chen KB, Chao PL. Antioxidative effect of vitamin D3 on zinc-induced oxidative stress in CNS. Ann N Y Acad Sci. 2005;1053:319–329. doi: 10.1196/annals.1344.028. [DOI] [PubMed] [Google Scholar]
- Losem-Heinrichs E, Gorg B, Redecker C, Schleicher A, Witte OW, Zilles K, Bidmon HJ. 1alpha,25-dihydroxy-vitamin D3 in combination with 17beta-estradiol lowers the cortical expression of heat shock protein-27 following experimentally induced focal cortical ischemia in rats. Arch Biochem Biophys. 2005;439:70–79. doi: 10.1016/j.abb.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Margulies S, Hicks R. Combination therapies for traumatic brain injury: prospective considerations. J Neurotrauma. 2009;26:925–939. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22:982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Relationship between circadian changes in spontaneous motor activity and dorsal versus ventral striatal dopamine neurotransmission assessed with on-line microdialysis. Behav Neurosci. 1994;108:624–635. doi: 10.1037//0735-7044.108.3.624. [DOI] [PubMed] [Google Scholar]
- Stein DG. Progesterone exerts neuroprotective effects after brain injury. Brain Research Reviews. 2008;57:386–397. doi: 10.1016/j.brainresrev.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DG. Is progesterone a worthy candidate as a novel therapy for traumatic brain injury? Dialogues in Clinical Neuroscience. 2011;13 doi: 10.31887/DCNS.2011.13.2/dstein. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DG, Cekic MM. Progesterone and vitamin d hormone as a biologic treatment of traumatic brain injury in the aged. PMR. 2011;3(Suppl 1)(6):S100–S110. doi: 10.1016/j.pmrj.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DG, Hurn PD. Effects of Sex Steroids on Damaged Neural Systems. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. 2 ed. Oxford: Elsevier; 2009. pp. 2223–2258. [Google Scholar]
- Stein DG, Wright DW. Progesterone in the clinical treatment of acute traumatic brain injury. Expert Opin Investig Drugs. 2010;19:847–857. doi: 10.1517/13543784.2010.489549. [DOI] [PubMed] [Google Scholar]
- Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86:50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieth R. The mechanisms of vitamin D toxicity. Bone Miner. 1990;11:267–272. doi: 10.1016/0169-6009(90)90023-9. [DOI] [PubMed] [Google Scholar]
- Wali B, Sayeed I, Stein DG. Improved behavioral outcomes after progesterone administration in aged male rats with traumatic brain injury. Restor Neurol Neurosci. 2011;29:61–71. doi: 10.3233/RNN-2011-0579. [DOI] [PubMed] [Google Scholar]
- Wang J, Jiang C, Liu C, Li X, Chen N, Hao Y. Neuroprotective effects of progesterone following stroke in aged rats. Behav Brain Res. 2010;209:119–122. doi: 10.1016/j.bbr.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, Salomone JP, Dent LL, Harris OA, Ander DS, Lowery DW, Patel MM, Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit Care. 2008;12:R61. doi: 10.1186/cc6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Naughton DP. Vitamin D in health and disease: current perspectives. Nutr J. 2010;9:65. doi: 10.1186/1475-2891-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]