Abstract
Abnormal perinatal nutrition (APN) results in a predisposition to develop obesity and the metabolic syndrome and thus may contribute to the prevalence of these disorders. Obesity, including that which develops in organisms exposed to APN, has been associated with increased meal size. Vagal afferents of the gastrointestinal (GI) tract contribute to regulation of meal size by transmitting satiation signals from gut-to-brain. Consequently, APN could increase meal size by altering this signaling, possibly through changes in expression of factors that control vagal afferent development or function. Here two studies that addressed these possibilities are reviewed. First, meal patterns, meal microstructure, and the structure and density of vagal afferents that innervate the intestine were examined in mice that experienced early postnatal overnutrition (EPO). These studies provided little evidence for EPO effects on vagal afferents as it did not alter meal size or vagal afferent density or structure. However, these mice exhibited modest hyperphagia due to a satiety deficit. In parallel, the possibility that brain-derived neurotrophic factor (BDNF) could mediate APN effects on vagal afferent development was investigated. Brain-derived neurotrophic factor was a strong candidate because APN alters BDNF levels in some tissues and BDNF knockout disrupts development of vagal sensory innervation of the GI tract. Surprisingly, smooth muscle-specific BDNF knockout resulted in early-onset obesity and hyperphagia due to increases in meal size and frequency. Microstructure analysis revealed decreased decay of intake rate during a meal in knockouts, suggesting loss of vagal negative feedback contributed to their increase in meal size. However, meal-induced c-Fos activation within the dorsal vagal complex suggested this effect could be due to augmentation of vago-vagal reflexes. A model is proposed to explain how high-fat diet consumption produces increased obesity in organisms exposed to APN, and may be required to reveal effects of EPO on vagal function.
1. Introduction
1.1 General introduction and organization of review
Overeating and obesity have reached not only epidemic, but pandemic proportions [1]. A potential cause of obesity that has attracted recent interest involves exposure of an organism to abnormal nutrition in utero or shortly after birth [2, 3]. Such exposure is thought to disrupt development of tissues involved in the regulation of food intake and body weight, resulting in a predisposition to develop obesity. One change in feeding behavior that accompanies this obesity of developmental origin, as well as obesity in general, is the consumption of large meals [4–6]. A neural system that could play a role in this increase in meal size is the sensory component of the vagus nerve that innervates the gastrointestinal (GI) tract. This system reports to the brain on the status of food in the upper GI tract during a meal and this information contributes to meal termination, or satiation [7, 8]. One mechanism that could mediate the effects of abnormal perinatal nutrition (APN) on vagal sensory neurons would be altered expression of genes that control their development or function. A gene that is a strong candidate for this role is brain-derived neurotrophic factor (BDNF). Brain-derived neurotrophic factor expression is altered in both central and peripheral tissues by APN and related perinatal manipulations [9, 10]. Also, vagal afferents that innervate the GI tract are dependent on BDNF for normal development [11]. In the remainder of Section 1.0 (Sections 1.2 – 1.4), the anatomical and functional organization of the vagal sensory system and its integration with central nervous system (CNS) structures is described. Sections 2.0 and 3.0 review two sets of experiments presented at the 2011 SSIB meeting. The first set examined the effects of APN on patterns of food intake and development of vagal afferents that innervate the GI tract. The second set of experiments explored the ability of BDNF produced by the GI tract to regulate vagal afferent signaling and feeding behavior. Additionally, several implications of the findings of this study are considered.
1.2 Vagal afferents that innervate the GI tract
The vagus nerve innervates the viscera, including the GI tract, and therefore has a critical role in the regulation of food intake. Vagal afferents that innervate the wall of the GI tract transduce both chemical and mechanical stimulation produced by food as it is processed within the gut. For example, they increase their firing rate in response to nutrients such as glucose and lipids, and to distension of the gut wall [12–17]. The signals generated by these vagal afferents are transmitted directly to the brain, informing it about the amount, quality and location of food within the GI tract. This is possible because vagal sensory neurons project directly from peripheral organs to the brain [18–20]. The signals generated by vagal afferents provide the majority of the negative-feedback from the gut to the brain that lead to satiation or the end of a meal [7, 8]. Vagal sensory signals also contribute to satiety, which influences the length of the delay from one meal to the next, and they regulate digestive reflexes that can indirectly influence feeding [7, 8, 21].
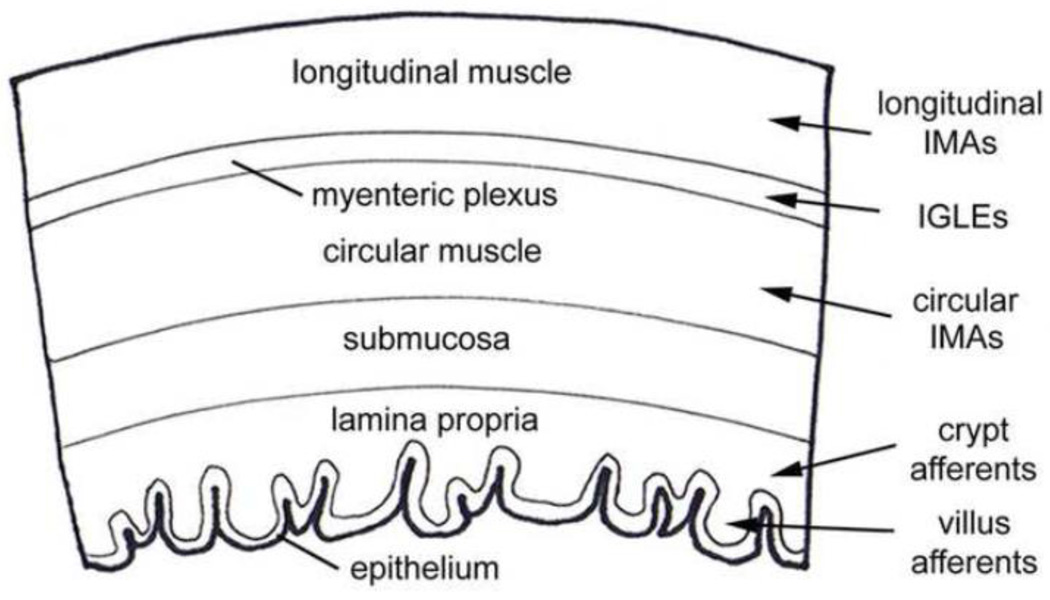
Vagal afferents have different functional specializations, achieved in part through innervation of specific tissue layers of the wall of the GI tract (Fig. 1). From the outermost serosal layer to the lumen, the layers of the GI wall include longitudinal smooth muscle, neurons and axons of the myenteric plexus, circular smooth muscle, submucosa, and the mucosa, which consists of lamina propria and epithelium. Four different classes of vagal afferents have been described, including villus afferents and crypt afferents that innervate the mucosa, intramuscular arrays (IMAs) that innervate the circular and longitudinal smooth muscle layers, and intraganglionic laminar endings (IGLEs) that innervate the myenteric plexus (Fig. 1). In the mucosa, villus afferents ramify within the lamina propria inside villi (a variant of this receptor, the antral gland afferent, terminates in the analogous tissue of the stomach antrum), and crypt afferents form rings around the crypts [22, 23]. Electrophysiological studies of GI mucosal innervation suggest it consists of both mechanoreceptive and chemoreceptive elements [15]. However, the relationship between these elements and the morphological classes of crypt and villus (or antral gland) afferents are unknown.
Figure 1.
This is a schematic drawing that illustrates the specific tissue layers of the GI tract wall and the types of vagal afferent receptors that innervate them. A wedge of a cross-section through the GI tract wall oriented with the outer serosal surface at the top and the inner lumen below is depicted here. This drawing represents a generalized set of tissue layers of the GI tract wall. Each tissue layer varies in thickness and structure from one GI organ compartment to another. The name of each tissue layer is indicated on the drawing. The different classes of vagal sensory receptors that innervate the GI tract are listed to the right of the drawing. Arrows point from each receptor type to the tissue layer(s) they innervate. Intramuscular arrays are only present in the longitudinal and circular muscle layers of the forestomach and the circular layer of the lower esophageal and pyloric sphincters. Crypt and villus afferents innervate the mucosa of the small intestine and antral gland afferents (not shown) supply a similar tissue as villus afferents, but within the mucosa of the stomach antrum.
The two classes of vagal afferents that innervate the outer muscle wall of the GI tract are mechanoreceptors (Figs. 1 and 2). Intramuscular arrays are thought to function as stretch receptors and IGLEs as tension receptors [24–27]. The long rectilinear terminals of IMAs are interconnected by cross-bridge fibers and run parallel to one-another and to smooth muscle fibers [24–27]. They also run parallel to, and in close apposition with the processes of intramuscular-interstitial cells of Cajal and form contacts with them [24–27]. Intramuscular arrays innervate the circular and longitudinal smooth muscle layers of the forestomach and the circular layer of the lower esophageal and pyloric sphincters (Figs. 1 and 2). These regions of the GI tract expand as food collects during a meal (forestomach), or as it passes from one compartment to the next (sphincters). Expansion of these regions would stretch IMAs, and thus may activate them [26–28]. In contrast, the terminals of an IGLE form numerous puncta that aggregate densely within a plane that covers a portion of a ganglion within the myenteric plexus [27, 29]. This plexus lies between the longitudinal and circular smooth muscle layers of the wall of the GI tract (Fig. 1). Intraganglionic laminar endings are distributed throughout the esophagus, stomach and intestines, but are not present in sphincters associated with these organs (Fig. 2) [26, 27, 30]. In addition to reporting on local muscle tension, IGLEs may be involved in coordinating motor patterns of the gut such as peristaltic contractions [27, 31].
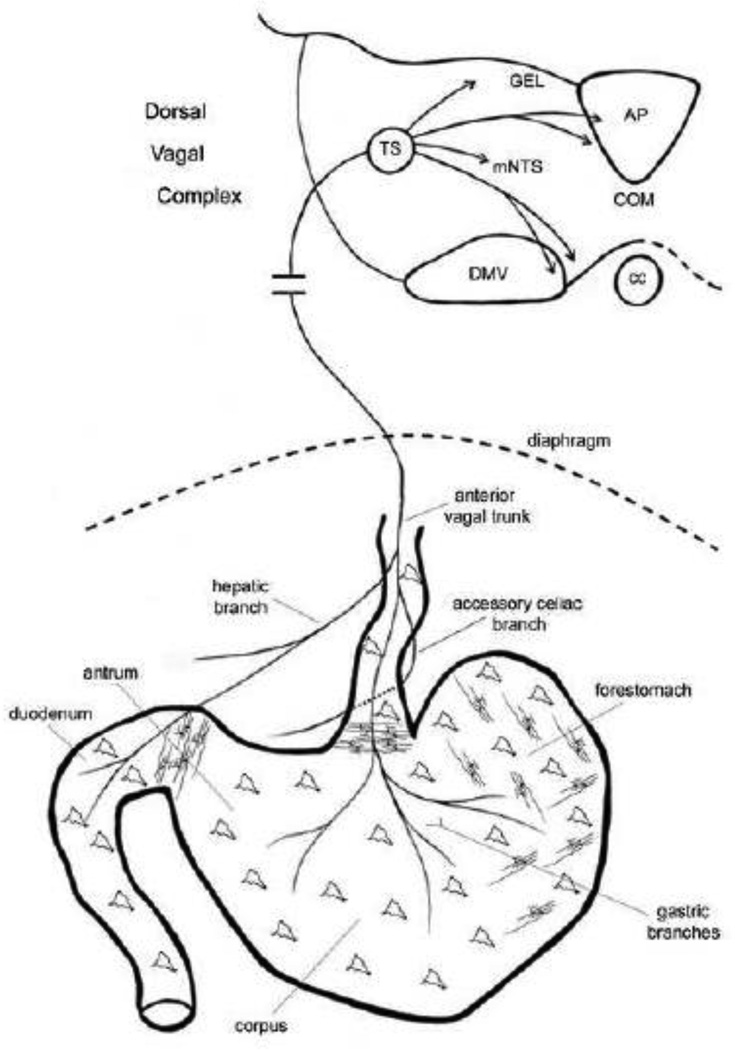
Figure 2.
Schematic drawing that illustrates the peripheral connections of vagal sensory neurons in the upper GI tract (bottom portion of schematic) as well as their central connections in left dorsal vagal complex of the brainstem (top part of schematic). For clarity, only the anterior vagal trunk and its branches are drawn, which arise mainly from the left cervical vagus nerve. From this ventral view the posterior trunk, which is not shown would lie behind the esophagus and give off gastric and celiac branches that innervate the dorsal stomach wall and intestine, respectively. The nodose ganglion, which houses the vagal sensory neurons and lies just outside the skull adjacent to the brainstem, is not shown. Also, the approximate distributions of the two main smooth muscle mechanoreceptor classes, IMAs and IGLEs, are indicated by parallel interconnected lines and amorphous shapes, respectively. See text for details. Abbreviations: AP, area postrema; cc, central canal; COM, commissural subnucleus; DMV, dorsal motor nucleus of the vagus; GEL, gelatinous subnucleus; mNTS, medial NTS; TS, solitary tract.
1.3 Central nervous system pathways regulating feeding and body weight
Information about chemical and mechanical stimulation of the gut is carried to the medulla of the caudal hindbrain by vagal afferents where it converges on the dorsal vagal complex (DVC). This brain region is composed of the nucleus of the solitary tract (NTS), the dorsal motor nucleus of the vagus (DMV) and the area postrema (AP) (Fig. 2). All of these nuclei receive inputs from vagal afferents. However, these inputs are concentrated in specific subnuclei of the caudal NTS, including the medial (mNTS), commissural (COM), and gelatinous subnuclei (GEL) [19, 20]. After this information is processed in the NTS it is sent to the lateral parabrachial nucleus and from there it is further distributed along two main streams. One projects to midline, intralaminar, and ventromedial basal thalamic nuclei and ultimately to the cerebral cortex, including insular cortex [32, 33]. The other stream projects to limbic and hypothalamic nuclei, including the lateral hypothalamus, paraventricular nucleus of the hypothalamus (PVH), amygdala and bed nucleus of the stria terminalis. In turn, many of these, as well as other brain regions involved in regulating food intake send projections back to the DVC. These projections can modulate NTS input to the hypothalamus and forebrain, or to the DMV, which houses the vagal preganglionic neurons that form the efferent components of vago-vagal reflexes [21].
The system controlling feeding is widely distributed throughout the nervous system. However, the hypothalamus and DVC have garnered the most attention as key integrative sites for neural and hormonal controls of feeding [34]. Several hypothalamic nuclei are involved in detecting neural and blood-borne signals of energy status and organizing behavioral and metabolic responses to changes in these signals. These nuclei include the arcuate (ARH), PVH, dorsomedial (DMH), and ventromedial (VMH) nuclei as well as areas of the lateral hypothalamus. For example, the ARH has a high density of leptin receptors [7]. When leptin binds these receptors it activates pro-opiomelanocortin (POMC)/ cocaine- and amphetamine regulated transcript (CART) expressing neurons and inhibits neuropeptide Y (NPY)/agouti-related peptide (AGRP) expressing neurons [7]. These effects result in reduced food intake and increased energy expenditure mediated in part by ARH projections to other hypothalamic nuclei, including the VMH and PVH [7]. Interestingly, the nuclei of the DVC express many of the same neuropeptides and neuropeptide and hormone receptors as the hypothalamic nuclei [34]. Moreover, these signaling pathways produce many of the same effects on food intake and body weight in the DVC as they do in the hypothalamus [34].
1.4 Short-term and long-term controls of food intake
The controls of food intake and body weight are often separated into two major categories, short-term and long-term controls. Short-term controls of food intake regulate meal size and meal frequency. In contrast, long-term controls determine whether alterations in meal size or meal frequency result in changes in the amount of food consumed each day, and whether these changes lead to altered body weight. The dominant model of food intake regulation suggests that changes in levels of hunger or energy stores activate neural and hormonal signals that stimulate receptors in the forebrain and hypothalamus [7]. In response to this stimulation, these brain regions produce appropriate adjustments of meal size in large part by modulating responses of neurons in the NTS to vagal satiation signals [7]. In this manner, hypothalamic and forebrain structures control food intake and body weight over the long-term, whereas vagal afferents and their targets in the caudal NTS regulate short-term control of meal size. The evidence for this vagal sensory role includes the observations that branch-selective and sensory-selective abdominal vagotomy blocked the reduction in meal size produced by nutrient preloads infused into the GI tract [35–39], and sensory-selective vagotomy increased meal size [36].
2.0 The effects of abnormal perinatal nutrition on vagal afferents of the GI tract
Exposure to APN in both humans and animals can result in a predisposition to develop obesity and metabolic disorders in adulthood [2, 3]. Abnormal perinatal nutrition refers to a significant increase or decrease in exposure of the developing fetus or offspring to nutrients, or to a specific macronutrient. These changes may result from altered nutrition (e.g., obesity) or hormonal status (e.g., diabetes) of the mother during or after pregnancy [2, 40, 41]. Abnormal perinatal nutrition can also be achieved by manipulating early postnatal food intake, for example, through changes in litter size [3, 4, 42]. Predisposition to obesity is often defined as a greater-than-normal susceptibility to dietary obesity, often involving consumption of a high-energy (HE) diet (i.e. a diet with large proportions of fats and carbohydrates) [4, 43, 44]. This effect of APN is important because it may contribute to the prevalence of obesity in both adults and children [45–47]. Consequently, we utilized early postnatal overnutrition (EPO) to produce APN because it may most closely model the overeating of infants and children, which is thought to predispose them to obesity later in childhood, or in adulthood [48].
Early postnatal overnutrition affects many physiological systems that could contribute to the predisposition to develop obesity. In terms of the nervous system, the CNS is involved, especially the hypothalamus, which has exhibited both functional and anatomical alterations [49, 50]. Also, EPO resulted in increased sympathetic innervation of the pancreas, retroperitoneal fat, and intrascapular brown adipose tissue, whereas, sucrose-induced cardiac sympathetic activity was lost [51, 52]. It is not clear how these changes influence predisposition to obesity, but increased uptake and storage of glucose could be involved [52]. One major arm of the nervous system that would be among prime suspects for effects of EPO that could contribute to the predisposition to obesity is the vagal sensory innervation of the GI tract. Surprisingly, to our knowledge possible effects of EPO on this system had not been investigated. However, such effects could be important for development of predisposition to obesity. In particular, reduced signaling of vagal afferents of the GI tract can lead to increased meal size, which is one of the most common alterations to the feeding behavior of obese animals and people [5, 6].
There are at least two effects of EPO that could disrupt vagal development and consequently reduce vagal satiation signaling and increase meal size. First, at the ages postnatal overeating occurs, vagal sensory axon terminals in the wall of the GI tract are still developing into mechano- and chemoreceptors [53–55]. Therefore, these developing terminals would be exposed to excessive stimulation by food contents of the GI tract. Such overstimulation could decrease the sensitivity of these terminals to GI stimuli by altering development of their structural or functional components. Second, EPO could disrupt vagal afferent development by altering the early postnatal leptin surge. Changes in this surge have occurred in some models of APN [50, 56–60]. These alterations were thought to contribute to the reduced development of hypothalamic arcuate nucleus (ARH) projections, as well as to long-term changes in metabolism and body weight. Vagal afferents, like arcuate neurons, express leptin receptors and change their firing rate in response to leptin [50, 61, 62]. Therefore, APN may reduce vagal afferent development by altering the early leptin surge.
Since APN could disrupt development of vagal afferents of the GI tract by causing them to be stimulated excessively or by altering the leptin surge, we hypothesized that EPO would in fact reduce the development of these afferents. In particular, EPO would lead to a decrease in the size or number of vagal sensory receptors present in the GI tract, or to decreased sensory function of these receptors (e.g., increased threshold for detecting GI stimuli). We further proposed that these structural or functional disruptions of vagal afferents of the GI tract would result in reduced satiation signaling and increased meal size. Since these afferents also contribute to satiety signaling, if their function was reduced it could lead to a decrease in intermeal interval (IMI) and possibly to increased meal frequency.
2.1 Methodological considerations
To produce EPO, we raised mice in small litters (SL group). Early in adulthood we screened for altered development or function of vagal afferents that supply the GI tract. This was done by comparing the feeding behavior and vagal innervation of the intestine in the SL group to that of mice raised in normal-size litters (NL group). There were two key methodological considerations that distinguished this study from most APN studies. First, feeding behavior was examined utilizing meal pattern and microstructure analyses. Meal pattern analysis characterizes parameters that describe the pattern of meal taking, including meal size, frequency and duration, as well as the duration of intervals between meals. In contrast, meal microstructure characterizes the change in rate of feeding over the course of a meal. The value of meal pattern analysis is that changes in meal parameters can focus the search for mechanisms mediating hyperphagia. In a complementary manner, microstructural analysis can aid in distinguishing the relative contributions of vagal negative feedback and oropharyngeal positive feedback to changes in meal pattern parameters [63].
A second consideration that has often been overlooked in APN studies is that increased body weight could alter an animal’s feeding behavior and metabolism and thus mask the primary effects of APN on them. This is important because identifying the primary effects of APN could help determine the mechanisms underlying the predisposition to develop obesity. Therefore, effects on feeding behavior were studied prior to development of obesity. This was achieved by maintaining the SL and NL groups on a balanced diet after weaning and characterizing their feeding behavior at 3–4 months of age when their body weights were still in the normal range (Fig. 3A).
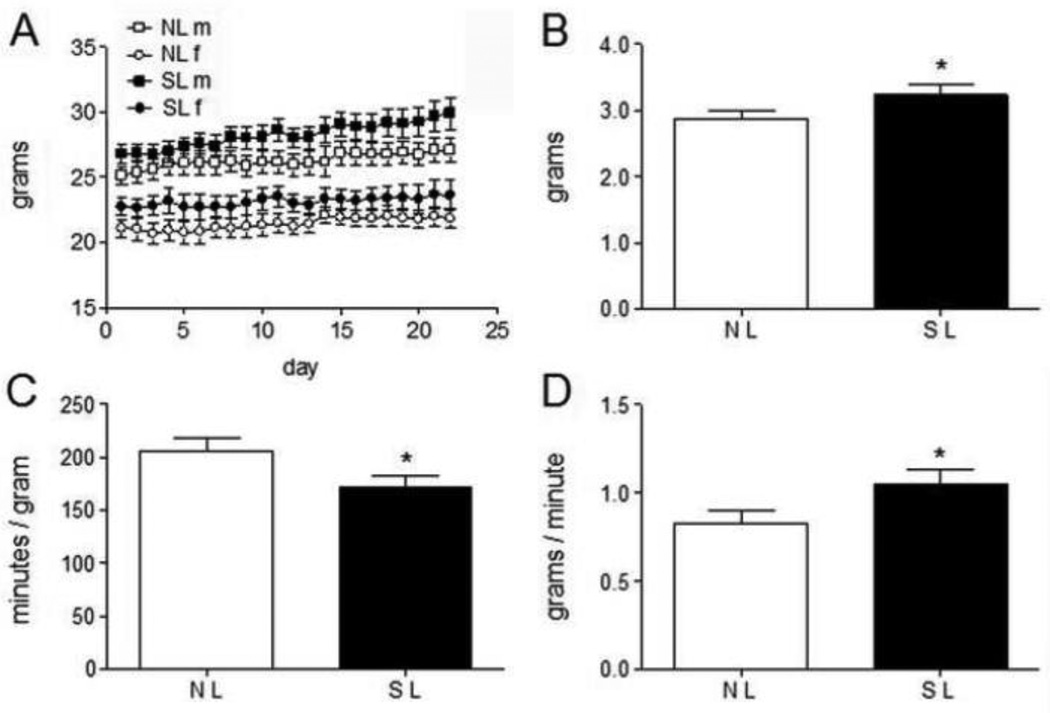
Figure 3.
Body weight and food intake variables derived from the meal pattern analysis conducted at 3–4 months of age that exhibited differences between SL and NL mice. A reduction in satiety ratio and an increase in first meal eating rate in SL as compared with NL mice implied that decreased satiety led to the modest increase in food intake by the SL group. A. Body weights are shown for each day meal pattern data were collected. B. Average daily food intake is plotted for SL and NL groups. C. Satiety ratio is plotted for SL and NL groups. D. Average eating rate during the first meal each day is plotted for SL and NL groups. This figure was previously published as Fig. 1B and Fig. 2A, F, and H [48], copyright Elsevier (2010).
Additionally, to determine whether EPO affected vagal afferent development, the density and structure of IGLEs that innervate the smooth muscle of the duodenum were compared in SL and NL mice. This experiment focused on the duodenum because it would experience excessive mechanical stimulation during early postnatal overeating.
2.2 Summary of results
We found no convincing evidence for EPO effects on vagal afferents: EPO had no significant effect on meal size or microstructure parameters (Fig. 2B and Table 3 in [48]). There was also no effect of EPO on the structure (Fig. 4 in [48]) or density of IGLEs (NL: 1308 ± 108/cm2, SL: 1388 ± 165/cm2) in the duodenum. We verified that the litter size manipulation successfully produced overeating in SL pups and a predisposition to obesity in SL adults: the body weight of SL mice was significantly increased compared to the NL group from postnatal day one to weaning (Fig. 1A in [48]). Also, adult fat pad weight was significantly increased in males of the SL group at 6–8 months of age (NL: 0.49 ± 0.07 g, SL: 1.29 ± 0.21 g). Females appear to be protected from some effects of APN [2].
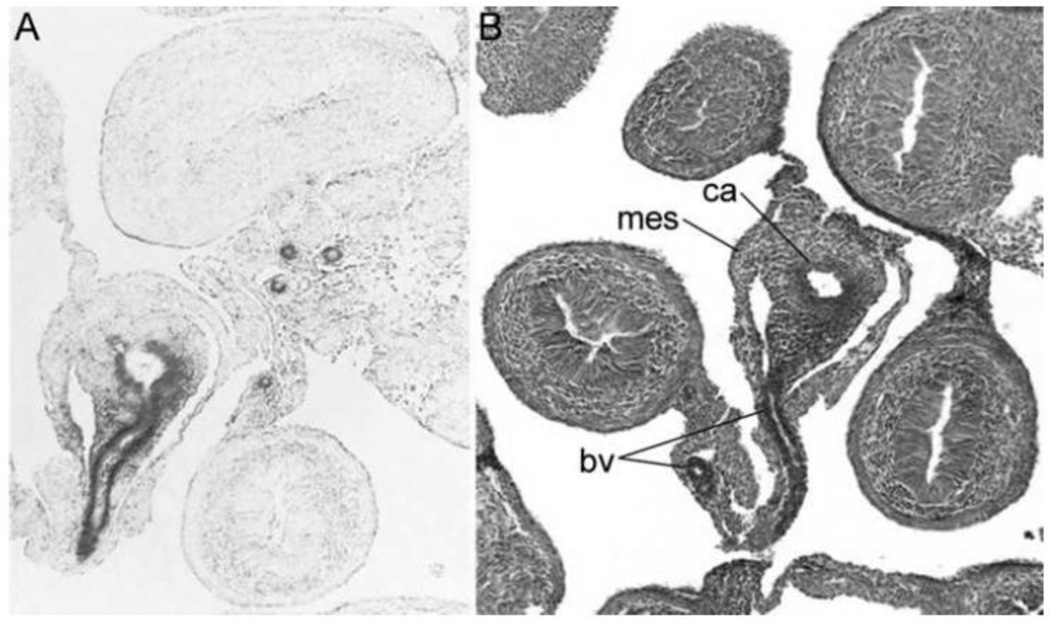
Figure 4.
Cre-mediated recombination in the GI tract of SM-BDNF KO embryos. Photomicrographs of X-gal-stained sections of embryonic intestines at E14 are shown. The sections shown in (A) and (B) were near-adjacent to each other and the one in (B) was counterstained with neutral red. The dark gray in (A) illustrates β-galactosidase expression in the intestines of SM-BDNF KO embryos, representing recombination of loxP sites and the loss of BDNF coding sequences. Cre-mediated recombination occurred mainly in vascular smooth muscle of GI blood vessels and also in some regions of the associated mesentery. BDNF expression normally occurs in these tissues at this age. Imaged at 100X magnification. Abbreviations: bv, blood vessel; ca, celiac artery; mes, mesentery.
Interestingly, SL mice did exhibit a small, but significant increase in daily food intake compared to the NL group (Fig. 3B). Also, satiety ratio was reduced in the SL group compared to the NL group (satiety ratio is the ratio of meal size to the subsequent IMI; Fig. 3C). This result indicated that a given amount of food was less effective at producing satiety in SL mice than in NL mice. It also suggested the increased food intake of the SL group was due to a modest satiety deficit. Additionally, the average consumption rate during the first meal, which followed mild food deprivation, was increased in the SL group and could have been consistent with a satiety deficit (Fig. 3D). Thus, it is possible that EPO altered satiety signaling by vagal afferents, although other pathways that signal satiety could have been affected. Importantly, the reduced satiety produced by EPO may be a primary effect that contributes to the predisposition to obesity.
2.3 Implications of results
Although we found no evidence for altered development of duodenal IGLEs, or vagal satiation signaling in mice that experienced EPO, it remains possible that development or function of other vagal afferents of the GI tract was altered. Such effects could have escaped detection if the remaining intact innervation compensated for them. In lieu of evidence for such effects, our results suggest that development and function of vagal afferents of the GI tract are largely resistant to the effects of overnutrition at early postnatal ages.
An additional possibility that might explain our failure to find EPO effects on vagal afferents, which is explored below, is that exposure to an HE diet, or the subsequent development of obesity – which we purposely avoided in this study - may be required to reveal a deficit in vagal satiation signaling.
3.0 Brain-derived neurotrophic factor, a candidate mediator of APN effects on vagal afferents of the GI tract
In parallel to examining effects of EPO on vagal afferents of the GI tract, we have been investigating a mechanism by which EPO or APN could affect their development. It is proposed that one way EPO or APN could accomplish this is through altering expression of genes involved in the development of vagal afferents that innervate the GI tract. We focused on the BDNF gene for several reasons. First, APN and related perinatal manipulations that affect adult health (e.g., maternal deprivation) alter expression levels of key feeding hormones, neuromodulators, and receptors (or receptor sensitivity), including BDNF and its receptor, trkB, in both CNS and peripheral tissues [9, 10, 40, 42, 64–70]. Second, BDNF is present in the developing and mature GI tract. In embryos BDNF is mainly expressed in smooth muscle of GI blood vessels, a portion of the gastric antrum-corpus and a portion of the intestine adjacent to the mesenteric attachment [53, 71]. BDNF is expressed throughout the smooth muscle wall of the GI tract in young adults [72]. Thus, BDNF can be secreted from cells in close proximity to developing vagal afferents as their axon terminals grow along blood vessels toward their target organs and form mechanoreceptors within smooth muscle. Third, vagal afferents produce trkB and thus can respond to BDNF released in the vicinity of their axon terminals [73, 74]. Fourth, BDNF is required for normal development of vagal afferents that innervate the smooth muscle of the stomach [11]. In this study a global knockout (KO) was employed and thus loss of BDNF from several sources could have contributed to its effects on vagal development, including the GI tract, vagal sensory neurons, and the DVC. Finally, regarding possible effects of BDNF on mature vagal sensory function, slowly adapting mechanoreceptors (SAMs) innervating the skin require BDNF for sensory transduction [75]. Since SAMs comprise a large proportion of vagal afferents of the GI tract involved in feeding [76, 77], it is possible BDNF also regulates sensory transduction of these vagal afferents.
Taken together, these findings prompted investigation of the ability of one of the possible sources of BDNF, the GI tract, to regulate the development or function of vagal afferents. Genetic manipulation was employed to reduce BDNF levels in the gut and an initial assessment of the effects on vagal sensory function was made utilizing meal pattern and microstructure analyses, and meal-induced c-Fos activation in the DVC. Methodological considerations and preliminary findings of this study are summarized below [78, 79].
3.1 Methodological considerations
The approach employed to reduce BDNF levels in the gut involved knocking out the BDNF gene. However, there were two issues that had to be addressed. The first was specificity of the effects of a BDNF knockout (KO). Brain-derived neurotrophic factor is expressed in several tissues [72, 80]. Therefore, a traditional BDNF KO, which would eliminate BDNF from all of these tissues, could affect the innervation of any of them in addition to the vagal afferents of the GI tract. Any “non-specific” effects that resulted could in turn influence feeding. Second, the BDNF gene KO results in death shortly after birth [81], precluding the study of its effects on mature vagal afferents or feeding behavior. One solution to overcome these obstacles is to use a conditional gene knockout that restricts the loss of BDNF to the GI tract. The consequent reduction in the number of tissues that lose BDNF improves animal viability and minimizes non-specific effects of the knockout that could affect feeding. Therefore, we targeted BDNF KO to smooth muscle and this did result in viable animals.
The cre-lox recombination method was utilized to selectively delete BDNF coding sequences from smooth muscle [81–85]. This was achieved by mating mice that express the cre recombinase in smooth muscle (SM22αcre mice) to mice lacking one BDNF allele (BDNFneo/+ mice) and mating their SM22αcre;BDNFneo/+ offspring to mice with a floxed BDNF allele (BDNF+/lox mice). Their SM22αcre/+;BDNFneo/lox offspring, designated “SM-BDNF KO” mice, had partial reduction of BDNF levels in all tissues, and complete or near-complete loss of BDNF from peripheral smooth muscle. Comparison groups studied were heterozygous BDNF global KO mice (BDNFneo/+) referred to as “BDNF +/−” mice, and controls, which included wild-type and SM22αcre mice. Cre-mediated recombination not only knocks out the floxed BDNF allele, but also activates expression of a lacZ reporter gene. Staining of the β-galactosidase protein produced by this expression was used to identify the tissues that experienced loss of BDNF [82].
This targeted gene knockout strategy exhibited a high efficiency at eliminating BDNF from the smooth muscle of blood vessels that supply the GI tract. This was established by the localization of β-galactosidase at embryonic day (E)14 –16 (Fig. 4) and the significant reduction of BDNF mRNA in the embryo esophagus-stomach (93%) and intestines (90%) compared to controls (not shown). Importantly, there was no β-galactosidase staining in the brain at these ages (not shown) and the SM22αcre transgene has not been reported to produce gene KO’s in the mature brain [84]. Moreover, the SM-BDNF KO occurred at an early enough age (by E14) to disrupt the development of vagal sensory axon terminals as they grow along blood vessels to reach their target GI tract organs and form receptors. Vagal axons first arrive at the stomach on E12 and a strong wave of axon ingrowth continues until E16 [54]. Vagal axon terminals begin to form mechanoreceptors on E16 [54]. Additionally, the SM22αcre transgene is efficient at producing gene KO’s in smooth muscle of the mature stomach and intestine and these tissues produce BDNF mRNA in mature mice [72, 84]. Therefore, BDNF expression in smooth muscle may also have been reduced in adult SM-BDNF KO mice and could have decreased the function of vagal mechanoreceptors that innervate this tissue.
3.2. Summary of results
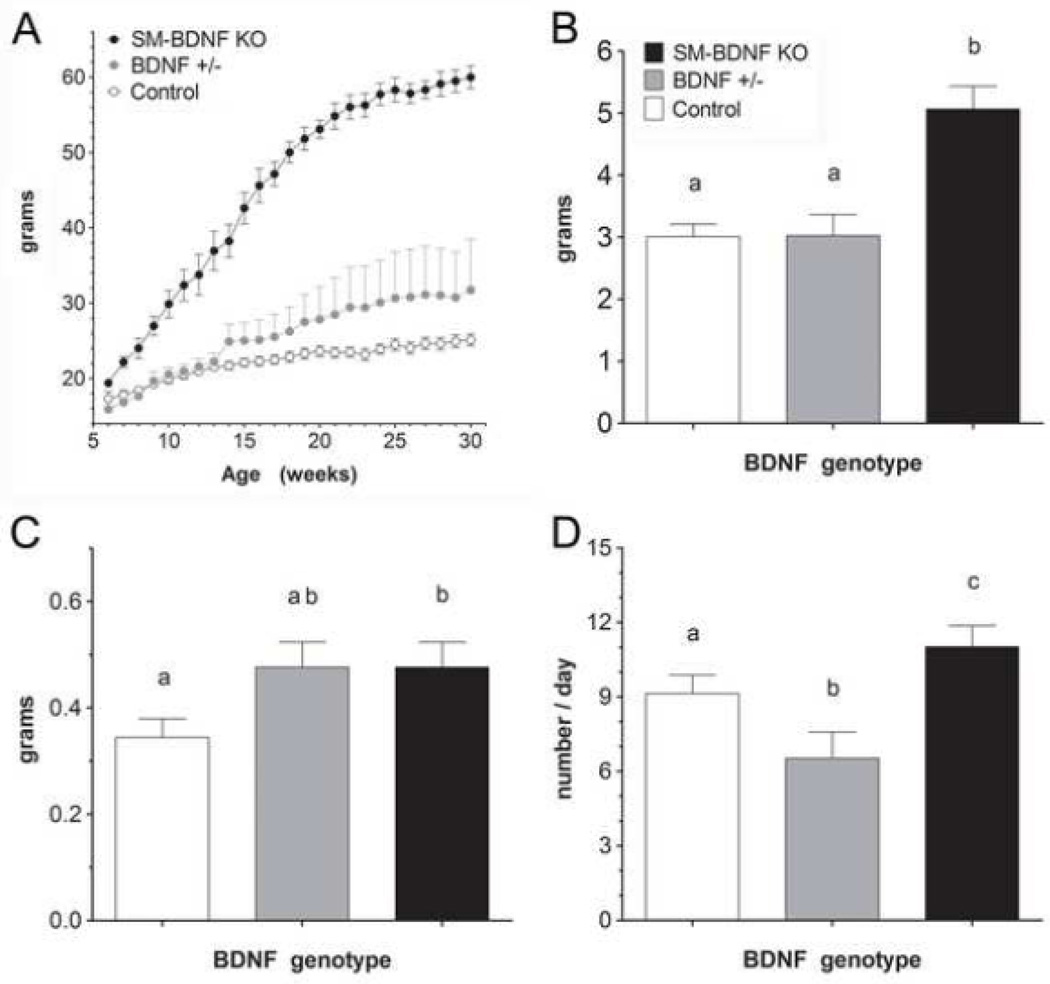
Surprisingly, SM-BDNF KO mice developed a dramatic, early-onset obesity (Fig. 5A). In contrast, BDNF+/− mice exhibited a trend toward a modest, late-onset obesity (Fig. 5A). Over weeks 20–30 when weight gain had largely tapered off, the average body weight was greater in the SM-BDNF KO group (53.6 ± 1.6 g) compared to either the BDNF+/− mice (30.1 ± 3.1 g) or controls (24.1 ± 1.9 g). The modest increase in body weight of BDNF+/− mice compared to controls was not significant. At 12–16 weeks of age, the average daily food intake of SM-BDNF KO mice was increased by 68% compared to BDNF+/− and control mice (Fig. 5B). Thus, at this age, early in the dynamic phase of weight gain, hyperphagia played a major role in the development of obesity in SM-BDNF KO mice. Since food intake was not measured prior to this age, it is not known whether overeating was also responsible for the initial increase in weight gain of SM-BDNF KO mice.
Figure 5.
SM-BDNF KO mice exhibited hyperphagia due to increases in meal size and frequency and early-onset obesity. A. Body weights plotted from 6–30 weeks of age. Groups plotted include SM-BDNF KO (n = 7), BDNF +/− (n = 5), and control (n = 8; SM22α–cre n = 5 plus wild type n = 3) mice. Group sizes at 6–12 weeks of age were smaller because the first SM-BDNF KO mice were not clearly obese until after 6 weeks of age, but their weights and those of some of their littermates of other genotypes are included in this graph. B. At 3–4 months of age, food intake averaged over the last 16 days of meal pattern collection (after meal pattern parameters had stabilized) was increased in SM-BDNF KO mice compared to control and BDNF +/− mice. C. SM-BDNF KO mice exhibited an increase in average meal size compared to the control group. The BDNF+/− group exhibited a similar trend, but it was not significant. D. SM-BDNF KO mice showed an increase in the average number of meals consumed daily compared to controls, whereas, BDNF +/− mice exhibited a reduction, which compensated for their large trend toward increased meal size. In panels B–D, the same lower case letters placed above histogram bars in each graph indicate no significant difference between groups, whereas different letters above histogram bars indicate a significant difference.
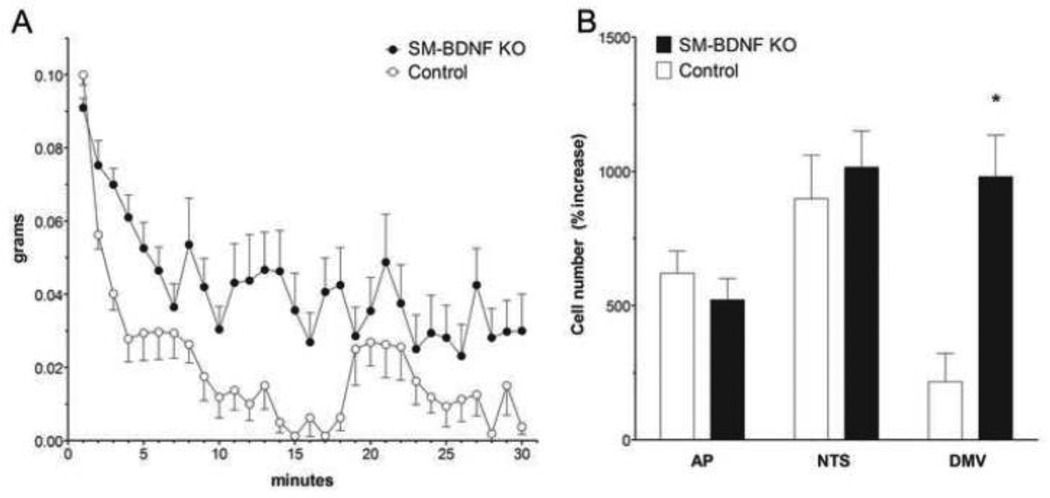
Meal pattern and microstructure analyses were carried out to gain insight into the mechanism that underlies overeating in SM-BDNF KO mice. To minimize possible confounds due to the effects of obesity on meal parameters, SM-BDNF KO mice (n = 7) were studied early in their phase of rapid weight gain (3 months of age). Their meal pattern parameters were compared to those of BDNF+/− (n = 4), and control (n = 7) mice. Mice with an SM-BDNF KO exhibited a 38% increase in average meal size compared to controls (Fig. 5C). They also showed a larger reduction in the rate of decay of intake during the first 30 min of eating each day than controls (Fig. 6A). The bulk of this rate of decay of eating has been attributed to vagal negative-feedback, or satiation, signaling [63]. Therefore, this finding suggested the increase in meal size of SM-BDNF KO mice was largely the result of a reduction in vagal satiation signaling.
Figure 6.
Meal microstructure and meal-induced c-Fos activation in the DVC were employed to aid determination of whether vagal negative feedback (satiation signaling) contributed to increased meal size in SM-BDNF KO mice. A. SM-BDNF KO mice exhibited changes in first meal microstructure consistent with a reduced contribution of vagal negative-feedback signaling to satiation. In particular, they showed a reduced rate of decay of eating compared to controls during rapid decay of intake rate (min 2–6) and gradual decay of intake rate (min 7–30). B. At two months of age, prior to the development of obesity, SM-BDNF KO mice exhibited greater meal-induced c-Fos activation in the DMV than did controls, whereas there were no differences in the NTS and AP. Although this finding is not consistent with reduced vagally-mediated negative-feedback signaling, it raises the possibility that vago-vagal reflexes such as receptive relaxation or gastric motility were augmented in SM-BDNF KO mice.
Mice with a SM-BDNF KO also demonstrated a 21% increase in the average number of meals consumed each day (Fig. 5D). This effect probably reflected a deficit in satiety as SM-BDNF KO mice exhibited a 33% decrease in satiety ratio compared to controls (SM-BDNF KO, BDNF+/−, and control = 167.97 ± 2.95, 217.6 ± 8.9, 249.4 ± 5.2 min/g, respectively). Also consistent with this interpretation, the average IMI of SM-BDNF KO mice was decreased by12% compared to controls even though they ate larger meals, albeit this difference was not significant (SM-BDNF KO, BDNF+/−, and control = 63.6 ± 3.6, 77.3 ± 6.8, and 71.8 ± 5.1 min, respectively). These findings raise the possibility that satiety signaling by vagal afferents was reduced in SM-BDNF KO mice, although alterations to other pathways that contribute to satiety could have occurred.
To more directly assess whether SM-BDNF KO mice had reduced vagal satiation or satiety signaling, c-Fos activation in the DVC was examined after consumption of a large meal [86]. Such a reduction would be represented by decreased activation of the DVC, especially in the NTS. To minimize possible confounds due to differences in body weight, mice were tested at 2–3 months of age before the body weight of SM-BDNF KO mice became significantly greater than for controls. There was a difference in baseline c-Fos activation (activation in non-fed mice) in the DMV of SM-BDNF KO and control mice that approached significance (SM-BDNF-KO: 1.56 ± 0.5 vs. control 8.97 ± 3.67 c-Fos-stained neuronal nuclei; p = 0.069). Therefore, c-Fos activation was assessed as the percent increase in the number of neuronal nuclei that showed c-Fos-like immunoreactivity in fed as compared to non-fed mice. In response to consumption of large meals of the same size, SM-BDNF KO mice exhibited a larger percent increase in c-Fos activation in the DMV as compared to controls (Fig. 6B). In contrast, meal-induced activation in the AP and NTS was similar in both groups. Thus, this data did not support a role for reduced vagal satiation or satiety signaling in the altered feeding of SM-BDNF KO mice.
3.3. Summary
In the present study, BDNF KO was targeted to smooth muscle to alter vagal afferent development or function and correlate these changes with effects of the KO on feeding behavior. This approach greatly reduced the number of tissues outside the GI tract that would experience reduced BDNF levels as compared with a global KO of BDNF, which had two benefits. It overcame the perinatal lethality associated with a global BDNF KO, permitting examination of the effects of reduced BNDF levels in GI smooth muscle on feeding behavior in mature animals. Also, it minimized the nonspecific effects (non-vagal or non-GI effects) of the KO on feeding behavior. Surprisingly, smooth muscle-specific KO of BDNF resulted in obesity. Moreover, food intake and meal pattern analyses at three months of age showed these mice were hyperphagic due to the cumulative effects of increased meal size and frequency. The findings of the meal pattern and microstructure analyses taken together were consistent with a role for reduced vagal afferent satiation or satiety signaling in the hyperphagia of SM-BDNF KO mice. However, meal-induced c-Fos activation in the DVC, which is a more direct measure of vagal sensory function, did not support this interpretation. In particular, increased activation of the DMV was observed rather than the predicted decrease in NTS activation.
3.4 Implications of results
3.4.1 Reconciling the meal pattern, microstructure, and meal-induced c-Fos activation results
The meal pattern and microstructure study and the meal-induced c- Fos activation experiment provided conflicting evidence regarding the role of reduced vagal afferent satiation and satiety signaling in the hyperphagia of SM-BDNF KO mice. One possible explanation for this pattern of results involves effects of the SM-BDNF KO on vagal afferents that mediate vago-vagal reflexes. The DMV contains the pre-motor neurons that form the efferent limb of vago-vagal digestive reflexes. Therefore, an increase in DMV activation could represent augmentation of these reflexes (e.g., gastric emptying, receptive relaxation) that could contribute to increased meal size. For example, increased receptive relaxation would produce greater-than-normal relaxation of the stomach muscle wall during a meal. This could lead to increased meal size by decreasing the increase in intragastric pressure produced by a given amount of ingesta. In turn, the vagal negative feedback produced by this amount of food would be decreased, and thus a larger quantity would have to be consumed to produce satiation. If the afferent limb of vago-vagal reflexes was altered by the SM-BDNF KO, it is possible the vagal afferent pathways that supply the intestine and inhibit gastric reflexes were lost or had reduced function [87, 88]. However, enhancement of esophageal-gastric vagal sensory pathways that activate these reflexes could also have occurred [89, 90].
It is also possible that the increased activation of the DMV in response to consumption of a large meal in SM-BDNF KO mice was due to reduced BDNF levels in tissues outside the GI tract. In particular, the levels of BDNF in the brains of SM-BDNF KO mice were reduced by about half (estimated based on the global loss of one BDNF allele). Therefore, altered activity in brain regions that normally express BDNF and regulate vago-vagal reflexes through axonal projections to the DVC could have contributed to the increased meal-induced response of DMV neurons in SM-BDNF KO mice [91–94].
There are also possible explanations for the altered feeding patterns of SM-BDNF KO mice, involving effects of decreased GI BDNF on peripheral neural systems other than the vagus. For instance, trkB receptor expression has been demonstrated in some myenteric neurons [72]. Therefore, reduced activation of these receptors in SM-BDNF KO mice could have altered the function of these neurons. Since the myenteric nervous system contributes to regulation of GI reflexes, including motility and exocrine and endocrine secretion, disruption of these activities could alter feeding patterns.
It will be important to determine whether the SM-BDNF KO does in fact alter GI reflexes, and if it does, which neural pathways are involved, and how these alterations contribute to hyperphagia and obesity. To date we have not succeeded in labeling vagal mechanoreceptors in the stomach and intestine utilizing our standard method for quantification, anterograde transport of wheat-germ agglutinin-horseradish peroxidase because SM-BDNF KO mice did not tolerate the surgery. We also failed to identify markers that labeled these receptors with the consistency required for quantification.
3.4.2 Evidence for a long-term role in regulation
The connectivity of vagal afferents with the CNS suggests that it has access to, and therefore could influence, CNS regions involved in long-term regulation of feeding behavior. Vagal sensory fibers are glutamatergic, or excitatory [95, 96]. Therefore, their activation excites second order sensory neurons of the NTS that project to several brain regions, forming chains of activation that ultimately lead to satiation and contribute to satiety. The brain regions excited downstream of vagal afferent activation include hypothalamic and forebrain nuclei considered important for long-term regulation of food intake and body weight [32]. This circuitry presents a conundrum: vagal afferents have fairly direct, strong excitatory connections to brain structures that appear to be involved in long-term regulation of food intake and body weight. This suggests that under at least some circumstances, they should be capable of influencing both the activity of this circuit and its regulation of long-term controls of feeding [7]. Surprisingly then, experimental manipulation of the vagal gut-brain axis in mammals typically has not revealed evidence consistent with an impact on long-term regulation [97]. Therefore, our finding that peripheral BDNF levels could influence long-term controls of feeding, possibly involving effects on vagal afferents of the GI tract may provide one of the few pieces of evidence in favor of a role these afferents in long-term control.
Despite the vast wealth of data suggesting vagal afferents of the GI tract contribute only to short-term controls of feeding, a small number of experiments have provided evidence consistent with a role in long-term regulation. For example, when intraperitoneal injections of CCK doses that produced no effect on body weight were combined with intracerebroventricular (ICV) infusions of leptin, they augmented weight loss produced by the ICV leptin [98–100]. Control experiments suggested the leptin effect was central and the CCK effect was peripheral, presumably involving activation of vagal afferents. Similarly, studies involving OLETF rats that lack CCK-1 receptors have implicated interactions between the CCK receptors in the dorsomedial hypothalamus and peripheral CCK receptors that influence vagal afferent signaling in the long-term regulation of food intake and body weight [101–103].
Another example of evidence for a role of vagal afferents that supply the gut in long-term regulation involved NT-4 KO mice [104]. They demonstrated a 90% loss of IGLEs in the upper intestine and exhibited several effects on feeding behavior that suggested vagal satiation signaling was reduced [104]. These effects included increased meal size on a liquid diet and increased meal duration and a trend toward increased meal size on a solid diet. But NT-4 KO mice compensated for these effects by trends toward decreased meal number and intake rate, which resulted in normal total daily intake and body weight. However, exposing NT-4 KO mice to daily alternation of an HE diet with chow overcame this compensation [105]. This diet regimen resulted in hyperphagia in both wild types and NT-4 KO mice on days they consumed the HE diet, but the magnitude of this hyperphagia was greater in NT-4 KO mice as compared to wild types. Since this increased overconsumption by NT-4 KO mice occurred over the course of one day, and involved the several meals consumed that day, technically it was a long-term change in food intake, although a temporary one. A contribution of NT-4 loss from tissues other than those associated with vagal development to this effect cannot be ruled out.
There is also clinical evidence consistent with long-term effects of vagal afferents on body weight from studies involving chronic vagal stimulation in humans. Initially, chronic vagal stimulating electrodes were implanted in patients to reduce seizures that did not respond to pharmacological treatment [106]. Interestingly, some patients unexpectedly revealed other beneficial effects, including weight loss [107–109]. Vagal stimulation has also been found to improve satiety in binge eaters and to return them to more normal long-term eating patterns [110].
3.4.3 Possible mechanisms mediating long-term effects of altered vagal afferents on food intake and body weight in SM-BDNF-KO mice
Interestingly, the majority of the previous experiments that produced data consistent with long-term effects of vagal afferents involved manipulations of both the CNS feeding circuit and vagal afferents of the GI tract. Similarly, in our SM-BDNF KO mice, which exhibited hyperphagia and early-onset obesity, KO of BDNF from peripheral tissues employed to manipulate vagal afferents was combined with a partial reduction of BDNF levels in the CNS (due to the global loss of one BDNF allele in the BDNF+/− mice used to breed SM-BDNF KO mice). Thus, it may be necessary to alter the CNS feeding regulatory circuit to reveal long-term effects of vagal afferent manipulations.
Brain-derived neurotrophic factor and its high-affinity receptor, trkB, are expressed in several key brain regions involved in regulation of food intake and body weight and they play a significant role in both CNS plasticity and suppression of food intake [91, 111, 112]. These BDNF actions and its brain localization suggest two mechanisms by which partially reduced BDNF levels in the CNS might act on the feeding regulatory circuit to reveal long-term effects of vagal afferent manipulations. One possibility is that the CNS feeding regulatory circuit compensates for altered vagal sensory input and BDNF contributes to the neural plasticity involved in this process. In such an instance, partial reduction of BDNF levels in the brain could decrease plasticity and consequently interfere with the ability of the CNS circuit to compensate for loss of input from vagal afferents of the GI tract. Another possibility is that partial reduction of BDNF in the brain brings the animal closer to the threshold for overeating, thus making it more difficult for the CNS circuit to compensate.
3.4.4 Role of peripheral BDNF and other anorexigens in long-term regulation of food intake and body weight
In addition to the unexpected finding that vagal afferents may play a role in long-term regulation of feeding, it is intriguing that peripheral actions of anorexigens such as BDNF and CCK may also contribute, possibly by acting through their effects on vagal afferents. However, in the context of more recent views of the organization of the neurohormonal circuit regulating ingestive behavior, such a role for peripheral effects of anorexigens does not seem so far-fetched.
The neural system regulating feeding and body weight is distributed, involving numerous central and peripheral nervous system components [34]. Hormones and neurotransmitters act at all levels of this circuit to modulate its responsiveness or “tone”, including leptin, insulin, POMC, agouti-related peptide (AGRP), cocaine-and-amphetamine-regulated transcript (CART), NPY and BDNF [7, 113, 114]. The majority of research on the effects of these molecules on feeding behavior has focused on the hypothalamus and the DVC [7, 34]. But in actuality, it is probable that these molecules have effects at several levels of the feeding circuit, including the peripheral organs or tissues innervated by the peripheral extensions of this circuit. These broadly distributed effects of hormones and neurotransmitters provide opportunities for them to modulate several aspects of food intake regulation in a coordinated fashion, including those mediated by viscerosensory, motivational, affective and motor systems [34, 115]. For example, leptin receptors are expressed in peripheral tissues, peripheral nerves, and numerous CNS nuclei [116–120]. Moreover, leptin has been shown to have effects on food intake and body weight at many of these sites [121–132]. Consistent with this model of distributed hormone/neuromodulator effects, global KO of leptin produces dramatic hyperphagia and obesity, whereas, KO’s of leptin targeted to brain nuclei thought to be important for controlling feeding have produced more modest effects (e.g., ARH [133, 134]. Melanocortins, CCK, and BDNF are also anorexigens with widespread receptor distribution in the brain, peripheral nervous system and the viscera, and therefore, may also have broadly distributed effects on the feeding circuit [135–140].
This distributed view of the neurohormonal circuit regulating feeding behavior highlights how the actions of hormones and neuromodulators expressed in peripheral tissues have the opportunity to interact with effects of the same or different molecules expressed in the CNS. Moreover, through such interactions, the peripheral effects of hormones and neuromodulators could possibly contribute to long-term as well as short-term regulation of food intake and body weight.
3.4.5 A threshold hypothesis of predisposition to obesity caused by APN
The finding of hyperphagia and obesity in SM-BDNF KO mice provides a pillar upon which a hypothesis can be constructed that addresses questions about how an HE diet reveals the predisposition to obesity in animals that experienced APN.
Numerous organ and tissue systems of the body, including the nervous system, are impacted by APN [141, 142]. Further, these systemic effects of APN on development result in permanent alterations in an organism’s physiology and metabolism that favor development of obesity and the metabolic syndrome later in life (Ibid.). Given these widespread effects of APN on development, it is surprising that when raised on a balanced diet young adult animals often maintain relatively normal food intake and body weight for some period of time before gradually becoming obese [4]. This delay in the appearance of symptoms raises some important questions about organisms that experience APN, including: How do young adults maintain normal food intake and body weight? How does consumption of an HE diet accelerate the onset of hyperphagia and obesity and increase their magnitude?
Here a hypothesis is proposed – based essentially on a threshold model - to explain how consumption of an HE, or obesogenic diet reveals the susceptibility to develop obesity in organisms exposed to APN. This threshold model follows a similar principle to the threshold effect observed in degenerative disorders such as Parkinson’s disease. Simply put, overt behavioral symptoms typically do not become apparent until the gradual loss of substantia nigra neurons (and thus loss of dopamine) reaches a threshold of about 70–80% [143, 144].
The threshold model proposed here suggests the state of the feeding regulatory system must reach a critical threshold before hyperphagia is released. One cornerstone of this threshold hypothesis involves the widespread, but partial damage to the feeding regulatory system produced by APN. Another cornerstone is based on evidence reviewed below suggesting the contribution of HE diets to hyperphagia goes beyond their palatability. In particular, these diets appear to modify the neurohormonal regulatory system to favor, or even drive their own continued overconsumption. The threshold hypothesis has three parts. (1) APN produces widespread changes to the feeding regulatory system that alone are not always sufficient in degree to exceed the threshold for producing hyperphagia in young adults. (2) High-energy diets produce changes to the feeding regulatory system, involving reduced anorexigen levels in the brain that on their own are not always sufficient to cross the threshold for inducing overeating in young adults. (3) It is the sum or interaction of these changes produced by APN and HE diets that pushes the regulatory system across the threshold for hyperphagia, the occurrence of which leads to obesity and the metabolic syndrome.
3.4.6 Role of HE diets in revealing predisposition to obesity in organisms that experience APN/EPO
Several studies have demonstrated that diet or energy status can alter the levels of feeding regulatory hormones and neuromodulators, or their receptors in the brain, including melanocortin-4 receptors, AGRP, and POMC, as well as BDNF and its high affinity receptor, trkB [145–151]. For example, food deprivation increased BDNF mRNA expression and protein levels in the hippocampus and several other brains areas while decreasing it in the VMH and DVC [145, 146, 149]. In contrast, high-fat diets reduced BDNF expression in the hippocampus and ventral tegmental area (VTA) [152, 153]. Interestingly, a high-fat diet also reduced BDNF levels in the VMH and a high-fat diet or effects of the associated weight gain reduced trkB levels in the hypothalamus and nodose ganglion [150, 154]. Thus, in the VMH the direction of the change in BDNF levels driven by HE diet consumption paralleled that caused by food deprivation.
Further, peripheral as well as CNS sites may be impacted by the effects of HE diets (or effects of the subsequent weight gain) on anorexigen levels. For example, the effects of exogenous CCK on satiation, gastric emptying, and c-Fos activation of the DVC, which are mediated mainly by vagal afferents, are blunted in obese rats [155, 156]. This reduced responsiveness likely reflects the plasticity demonstrated by vagal sensory neurons in response to changes in energy status. Specifically, in diet-induced obese animals, vagal afferents exhibit reduced excitability and decreased electrophysiological and behavioral responsiveness to gastric loads and to anorexigens, including CCK [156–159]. They also show reduced expression of CCK-1 receptors [158]. Moreover, diet-induced obesity has also been associated with increased levels of orexigens or their receptors in vagal sensory neurons and in the CNS, including the CB1 endocannabinoid, orexin- 2 and growth hormone secretagogoue-1a receptors and neuropeptide Y (NPY) [147, 155, 156, 159].
Thus, a general picture has begun to take shape where consumption of an obesogenic diet may modify the “state” of the feeding regulatory circuitry by altering anorexigen and orexigen signaling so that a given amount of food is less satiating. Put in its strongest form, at least some obesogenic diets modify the “state” of the regulatory circuitry to mimic a food-deprived state. Consequently, the organism is fooled into thinking it is still hungry, or energy depleted, despite the concurrent consumption of a large number of calories. Two key examples of such possible effects of HE diets include decreased BDNF levels in the VMH [150, 154] and reduced POMC levels (and increased levels of the orexigen, NPY) in the ARH [147, 160].
3.4.7 A simpler model of predisposition to obesity: Late-onset obesity
If HE diets reduce anorexigen signaling in the brain and possibly the peripheral nervous system, why are animals that experienced APN more susceptible to these effects? An important clue came from a group of mutant mouse strains that exhibit a “late-onset” (or “middle-age”) obesity that results largely or entirely from hyperphagia. The mutations involved produce loss of function of the gene for the serotonin-2C receptor, the melanocortin-4 receptor, BDNF, agouti, or pro-opiomelanocortin (POMC) [113, 161–164]. In addition to exhibiting the same pattern of hyperphagia and weight gain as animals that experienced APN, mice with late-onset obesity show a similar predisposition to obesity. They become hyperphagic and gain excessive weight compared to wild types when offered an HE diet [162, 163, 165]. Also, mice with late-onset obesity develop insulin resistance [165]. Thus, late-onset obesity shares many of the defining characteristics of predisposition to obesity exhibited by animals that experienced APN.
The overconsumption of HE diets by mice with late-onset obesity is consistent with a threshold model. These mice have reduced levels of an anorexigen that may cause their feeding regulatory circuit to sit close to the threshold for hyperphagia. Consumption of an obesogenic diet could further reduce the levels of this anorexigen (and reduce the levels of other anorexigens) to push the animal over the threshold. However, the decrease in anorexigen levels produced by HE diets and the induction of hyperphagia are correlates. Consequently, it is possible that eating an obesogenic diet activates other mechanisms that favor hyperphagia and weight gain.
Thus, it is imperative to demonstrate that direct manipulation of anorexigen signaling can combine with the reduced anorexigen levels in mice with late-onset obesity to make them hyperphagic. Indeed, our findings in SM-BDNF KO mice may provide such a demonstration. These mice were created by combining a heterozygous BDNF(+/−) mutation that causes late-onset obesity with a targeted reduction of BDNF in peripheral smooth muscle (SM22αcre;BDNFlox/+). Thus, instead of feeding the obesogenic diet to the mice with late-onset obesity to further reduce BDNF levels, a targeted gene KO was used to decrease BDNF expression in their GI tract. This manipulation did result in a dramatic early-onset obesity and when examined at 12 – 16 weeks of age these mice were markedly hyperphagic. Consequently, if an HE diet produced reductions in BDNF levels (or the levels of other anorexigens) comparable to that produced by the targeted KO of BDNF in smooth muscle, it should push an animal that was already close to the threshold for eliciting hyperphagia over the threshold. An unexpected implication of the finding of early-onset obesity in SM-BDNF KO mice is that it may be possible for decreased anorexigen expression in peripheral tissues to combine with reduced anorexigen levels in the CNS to support overeating.
3.4.8 An obesogenic diet might be necessary to reveal effects of EPO on vagal afferent function
Based on the threshold hypothesis of APN, we speculate that consumption of an HE diet might be necessary for the effects of EPO on vagal afferents of the GI tract to increase meal size (see sections 2.2 and 2.3). The damage produced by EPO in our study was not sufficient to produce significant levels of hyperphagia. Therefore, its effects on vagal afferent development or function may have been subthreshold for increasing meal size. For instance, the Weibull parameter C of the microstructure analysis is influenced by vagal negative-feedback signaling [63]. Consequently, the near-significant trend we observed in this parameter is consistent with a near-threshold effect of EPO on vagal afferents of the GI tract [48]. If this interpretation is correct, then the effects of an HE diet-induced decrease in gut BDNF levels on vagal afferents could combine with the subthreshold effects of EPO on these afferents to increase meal size.
3.4.9 Implications of SM-BDNF KO findings for the APN hypothesis
There may be differences in the mechanisms underlying late-onset obesity and APN. However, the similarity in their susceptibility to HE diets suggests the possibility that the means by which these diets switch on hyperphagia may be similar in both instances. If obesogenic diets do act similarly in both of these models, then the decrease in anorexigen levels that results from consumption of these diets would combine with the reduced threshold for hyperphagia caused by APN to push the organism past this threshold. Interestingly, APN and other perinatal manipulations that affect adult health alter the expression of hormones, neuromodulators, or receptors (or receptor sensitivity) important for regulating feeding in both CNS and peripheral tissues, including BDNF and trkB [9, 10, 40, 42, 64–70]. Some of these changes in anorexigen and orexigen signaling produced by APN may combine with changes in the same (or other) signaling pathways produced by consumption of an HE diet to push an organism across the threshold for hyperphagia.
Importantly, our finding that SM-BDNF KO converts moderate late-onset obesity of BDNF+/− mutants to dramatic early-onset obesity is just a first step toward substantiating the threshold hypothesis. For example, it must be demonstrated that the effects of partial reduction of BDNF levels in smooth muscle of young adult organisms that experienced APN can induce hyperphagia and obesity. Further, it should be determined whether obesogenic diets reduce BDNF levels (and levels of other anorexigens, or levels of activity in their signaling pathways) in the GI tract. Finally, it should be determined whether blocking the effects of HE diet consumption on peripheral anorexigen signaling (or CNS anorexigen signaling) can prevent hyperphagia and obesity in young adult rodents that had experienced APN and were consuming an HE diet.
Highlights.
Early postnatal overnutrition (EPO) effect on vagal satiation signals was studied.
Also examined possible role of GI BDNF in EPO effects on vagal afferents.
EPO caused a satiety deficit that increased food intake; satiation remained normal.
Surprisingly, GI BDNF KO increased meal number and size causing obesity.
This finding suggests how high-energy diets may reveal predisposition to obesity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lieberman LS. Evolutionary and anthropological perspectives on optimal foraging in obesogenic environments. Appetite. 2006;47:3–9. doi: 10.1016/j.appet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Ferezou-Viala J, Roy AF, Serougne C, Gripois D, Parquet M, Bailleux V, et al. Long-term consequences of maternal high-fat feeding on hypothalamic leptin sensitivity and diet-induced obesity in the offspring. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1056–R1062. doi: 10.1152/ajpregu.00117.2007. [DOI] [PubMed] [Google Scholar]
- 3.Knittle JL, Hirsch J. Effect of early nutrition on the development of rat epididymal fat pads: cellularity and metabolism. J Clin Invest. 1968;47:2091–2098. doi: 10.1172/JCI105894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drewnowski A, Cohen AE, Faust IM, Grinker JA. Meal-taking behavior is related to predisposition to dietary obesity in the rat. Physiol Behav. 1984;32:61–67. doi: 10.1016/0031-9384(84)90071-4. [DOI] [PubMed] [Google Scholar]
- 5.Furnes MW, Zhao CM, Chen D. Development of obesity is associated with increased calories per meal rather than per day. A study of high-fat diet-induced obesity in young rats. Obes Surg. 2009;19:1430–1438. doi: 10.1007/s11695-009-9863-1. [DOI] [PubMed] [Google Scholar]
- 6.Strohmayer AJ, Smith GP. The meal pattern of genetically obese (ob/ob) mice. Appetite. 1987;8:111–123. doi: 10.1016/s0195-6663(87)80004-1. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 8.Smith GP. The direct and indirect controls of meal size. Neurosci Biobehav Rev. 1996;20:41–46. doi: 10.1016/0149-7634(95)00038-g. [DOI] [PubMed] [Google Scholar]
- 9.Coupe B, Dutriez-Casteloot I, Breton C, Lefevre F, Mairesse J, Dickes-Coopman A, et al. Perinatal undernutrition modifies cell proliferation and brain-derived neurotrophic factor levels during critical time-windows for hypothalamic and hippocampal development in the male rat. J Neuroendocrinol. 2009;21:40–48. doi: 10.1111/j.1365-2826.2008.01806.x. [DOI] [PubMed] [Google Scholar]
- 10.Germani E, Lesma E, Di Giulio AM, Gorio A. Progressive and selective changes in neurotrophic factor expression and substance p axonal transport induced by perinatal diabetes: protective action of antioxidant treatment. J Neurosci Res. 1999;57:521–528. [PubMed] [Google Scholar]
- 11.Murphy MC, Fox EA. Mice deficient in brain-derived neurotrophic factor have altered development of gastric vagal sensory innervation. J Comp Neurol. 2010;518:2934–2951. doi: 10.1002/cne.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathis C, Moran TH, Schwartz GJ. Load-sensitive rat gastric vagal afferents encode volume but not gastric nutrients. Am J Physiol. 1998;274:R280–R286. doi: 10.1152/ajpregu.1998.274.2.R280. [DOI] [PubMed] [Google Scholar]
- 13.Mei N. Recent studies on intestinal vagal afferent innervation. Functional implications. J Auton Nerv Syst. 1983;9:199–206. doi: 10.1016/0165-1838(83)90141-8. [DOI] [PubMed] [Google Scholar]
- 14.Melone J. Vagal receptors sensitive to lipids in the small intestine of the cat. J Auton Nerv Syst. 1986;17:231–241. doi: 10.1016/0165-1838(86)90060-3. [DOI] [PubMed] [Google Scholar]
- 15.Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol. 2002;87:2095–2103. doi: 10.1152/jn.00785.2001. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz GJ, Moran TH. Duodenal nutrient exposure elicits nutrient-specific gut motility and vagal afferent signals in rat. Am J Physiol. 1998;274:R1236–R1242. doi: 10.1152/ajpregu.1998.274.5.R1236. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Renehan WE, Fogel R. Neurons in the vagal complex of the rat respond to mechanical and chemical stimulation of the GI tract. Am J Physiol. 1998;274:G331–G341. doi: 10.1152/ajpgi.1998.274.2.G331. [DOI] [PubMed] [Google Scholar]
- 18.Altschuler SM, Escardo J, Lynn RB, Miselis RR. The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology. 1993;104:502–509. doi: 10.1016/0016-5085(93)90419-d. [DOI] [PubMed] [Google Scholar]
- 19.Norgren R, Smith GP. Central distribution of subdiaphragmatic vagal branches in the rat. J Comp Neurol. 1988;273:207–223. doi: 10.1002/cne.902730206. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro RE, Miselis RR. The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol. 1985;238:473–488. doi: 10.1002/cne.902380411. [DOI] [PubMed] [Google Scholar]
- 21.Rogers RC, McTigue DM, Hermann GE. Vagal control of digestion: modulation by central neural and peripheral endocrine factors. Neurosci Biobehav Rev. 1996;20:57–66. doi: 10.1016/0149-7634(95)00040-l. [DOI] [PubMed] [Google Scholar]
- 22.Berthoud HR, Kressel M, Raybould HE, Neuhuber WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl) 1995;191:203–212. doi: 10.1007/BF00187819. [DOI] [PubMed] [Google Scholar]
- 23.Powley TL, Spaulding RA, Haglof SA. Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. J Comp Neurol. 2011;519:644–660. doi: 10.1002/cne.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powley TL, Wang XY, Fox EA, Phillips RJ, Liu LW, Huizinga JD. Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol Motil. 2008;20:69–79. doi: 10.1111/j.1365-2982.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- 25.Berthoud HR, Powley TL. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol. 1992;319:261–276. doi: 10.1002/cne.903190206. [DOI] [PubMed] [Google Scholar]
- 26.Fox EA, Phillips RJ, Martinson FA, Baronowsky EA, Powley TL. Vagal afferent innervation of smooth muscle in the stomach and duodenum of the mouse: Morphology and topography. J Comp Neurol. 2000;428:558–576. doi: 10.1002/1096-9861(20001218)428:3<558::aid-cne11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 27.Wang FB, Powley TL. Topographic inventories of vagal afferents in gastrointestinal muscle. J Comp Neurol. 2000;421:302–324. [PubMed] [Google Scholar]
- 28.Fox EA, Phillips RJ, Martinson FA, Baronowsky EA, Powley TL. C-Kit Mutant Mice Have a Selective Loss of Vagal Intramuscular Mechanoreceptors that Innervate Gastric Smooth Muscle. Anat. Embryol. 2001a;204:11–26. doi: 10.1007/s004290100184. [DOI] [PubMed] [Google Scholar]
- 29.Neuhuber WL. Sensory vagal innervation of the rat esophagus and cardia: a light and electron microscopic anterograde tracing study. J Auton Nerv Syst. 1987;20:243–255. doi: 10.1016/0165-1838(87)90153-6. [DOI] [PubMed] [Google Scholar]
- 30.Berthoud HR, Patterson LM, Neumann F, Neuhuber WL. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol. 1997;195:183–191. doi: 10.1007/s004290050037. [DOI] [PubMed] [Google Scholar]
- 31.Neuhuber WL, Clerc N. Afferent innervation of the esophagus in cat and rat. In: Zenker W, Neuhuber WL, editors. The primary afferent neuron. A survey of recent morpho-functional aspects. New York and London: Plenum Press; 1990. pp. 93–107. [Google Scholar]
- 32.Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010;1350:18–34. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- 34.Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Front Neuroendocrinol. 2002;23:2–40. doi: 10.1006/frne.2001.0224. [DOI] [PubMed] [Google Scholar]
- 35.Phillips RJ, Powley TL. Gastric volume detection after selective vagotomies in rats. Am J Physiol. 1998;274:R1626–R1638. doi: 10.1152/ajpregu.1998.274.6.R1626. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz GJ, Salorio CF, Skoglund C, Moran TH. Gut vagal afferent lesions increase meal size but do not block gastric preload-induced feeding suppression. Am J Physiol. 1999;276:R1623–R1629. doi: 10.1152/ajpregu.1999.276.6.R1623. [DOI] [PubMed] [Google Scholar]
- 37.Walls EK, Phillips RJ, Wang FB, Holst MC, Powley TL. Suppression of meal size by intestinal nutrients is eliminated by celiac vagal deafferentation. Am J Physiol. 1995;269:R1410–R1419. doi: 10.1152/ajpregu.1995.269.6.R1410. [DOI] [PubMed] [Google Scholar]
- 38.Yox DP, Ritter RC. Capsaicin attenuates suppression of sham feeding induced by intestinal nutrients. Am J Physiol. 1988;255:R569–R574. doi: 10.1152/ajpregu.1988.255.4.R569. [DOI] [PubMed] [Google Scholar]
- 39.Yox DP, Stokesberry H, Ritter RC. Vagotomy attenuates suppression of sham feeding induced by intestinal nutrients. Am J Physiol. 1991;260:R503–R508. doi: 10.1152/ajpregu.1991.260.3.R503. [DOI] [PubMed] [Google Scholar]
- 40.Fahrenkrog S, Harder T, Stolaczyk E, Melchior K, Franke K, Dudenhausen JW, et al. Cross-fostering to diabetic rat dams affects early development of mediobasal hypothalamic nuclei regulating food intake, body weight, and metabolism. J Nutr. 2004;134:648–654. doi: 10.1093/jn/134.3.648. [DOI] [PubMed] [Google Scholar]
- 41.McCance DR, Pettitt DJ, Hanson RL, Jacobsson LT, Knowler WC, Bennett PH. Birth weight and non-insulin dependent diabetes: thrifty genotype, thrifty phenotype, or surviving small baby genotype? Bmj. 1994;308:942–945. doi: 10.1136/bmj.308.6934.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plagemann A, Harder T, Brunn M, Harder A, Roepke K, Wittrock-Staar M, et al. Hypothalamic proopiomelanocortin promoter methylation becomes altered by early overfeeding: an epigenetic model of obesity and the metabolic syndrome. J Physiol. 2009;587:4963–4976. doi: 10.1113/jphysiol.2009.176156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faust IM, Johnson PR, Hirsch J. Long-term effects of early nutritional experience on the development of obesity in the rat. J Nutr. 1980;110:2027–2034. doi: 10.1093/jn/110.10.2027. [DOI] [PubMed] [Google Scholar]
- 44.White CL, Purpera MN, Morrison CD. Maternal obesity is necessary for programming effect of high-fat diet on offspring. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1464–R1472. doi: 10.1152/ajpregu.91015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montague MC. The physiology of obesity. Abnf. J. 2003;14:56–60. [PubMed] [Google Scholar]
- 46.Rocchini AP. Childhood obesity and a diabetes epidemic. N Engl J Med. 2002;346:854–855. doi: 10.1056/NEJM200203143461112. [DOI] [PubMed] [Google Scholar]
- 47.Jeannot E, Mahler P, Duperrex O, Chastonay P. Evolution of overweight and obesity among elementary school children in Geneva. Swiss Med Wkly. 2010;140:w13040. doi: 10.4414/smw.2010.13040. [DOI] [PubMed] [Google Scholar]
- 48.Biddinger JE, Fox EA. Meal parameters and vagal gastrointestinal afferents in mice that experienced early postnatal overnutrition. Physiol Behav. 2010;101:184–191. doi: 10.1016/j.physbeh.2010.04.011. Epub 2010 Apr 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davidowa H, Li Y, Plagemann A. Altered responses to orexigenic (AGRP, MCH) and anorexigenic (alpha-MSH, CART) neuropeptides of paraventricular hypothalamic neurons in early postnatally overfed rats. Eur J Neurosci. 2003;18:613–621. doi: 10.1046/j.1460-9568.2003.02789.x. [DOI] [PubMed] [Google Scholar]
- 50.Simerly RB. Hypothalamic substrates of metabolic imprinting. Physiol Behav. 2008;94:79–89. doi: 10.1016/j.physbeh.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young JB. Effects of litter size on sympathetic activity in young adult rats. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1113–R1121. doi: 10.1152/ajpregu.00139.2001. [DOI] [PubMed] [Google Scholar]
- 52.Young JB. Developmental origins of obesity: a sympathoadrenal perspective. Int J Obes (Lond) 2006;30(Suppl 4):S41–S49. doi: 10.1038/sj.ijo.0803518. [DOI] [PubMed] [Google Scholar]
- 53.Fox EA, Murphy MC. Factors regulating vagal sensory development: potential role in obesities of developmental origin. Physiol Behav. 2008;94:90–104. doi: 10.1016/j.physbeh.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murphy MC, Fox EA. Anterograde tracing method using DiI to label vagal innervation of the embryonic and early postnatal mouse gastrointestinal tract. J Neurosci Methods. 2007;163:213–225. doi: 10.1016/j.jneumeth.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swithers SE, Baronowsky E, Powley TL. Vagal intraganglionic laminar endings and intramuscular arrays mature at different rates in pre-weanling rat stomach. Auton Neurosci. 2002;102:13–19. doi: 10.1016/s1566-0702(02)00172-8. [DOI] [PubMed] [Google Scholar]
- 56.Kirk SL, Samuelsson AM, Argenton M, Dhonye H, Kalamatianos T, Poston L, et al. Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS One. 2009;4:e5870. doi: 10.1371/journal.pone.0005870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, et al. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab. 2005;1:371–378. doi: 10.1016/j.cmet.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Delahaye F, Breton C, Risold PY, Enache M, Dutriez-Casteloot I, Laborie C, et al. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology. 2008;149:470–475. doi: 10.1210/en.2007-1263. [DOI] [PubMed] [Google Scholar]
- 59.Attig L, Solomon G, Ferezou J, Abdennebi-Najar L, Taouis M, Gertler A, et al. Early postnatal leptin blockage leads to a long-term leptin resistance and susceptibility to diet-induced obesity in rats. Int J Obes (Lond) 2008;32:1153–1160. doi: 10.1038/ijo.2008.39. [DOI] [PubMed] [Google Scholar]
- 60.Granado M, Garcia-Caceres C, Fuente-Martin E, Diaz F, Mela V, Viveros MP, et al. Effects of acute changes in neonatal leptin levels on food intake and long-term metabolic profiles in rats. Endocrinology. 2011;152:4116–4126. doi: 10.1210/en.2011-1233. [DOI] [PubMed] [Google Scholar]
- 61.Buyse M, Ovesjo ML, Goiot H, Guilmeau S, Peranzi G, Moizo L, et al. Expression and regulation of leptin receptor proteins in afferent and efferent neurons of the vagus nerve. Eur J Neurosci. 2001;14:64–72. doi: 10.1046/j.0953-816x.2001.01628.x. [DOI] [PubMed] [Google Scholar]
- 62.Wang YH, Tache Y, Sheibel AB, Go VL, Wei JY. Two types of leptin-responsive gastric vagal afferent terminals: an in vitro single-unit study in rats. Am J Physiol. 1997;273:R833–R837. doi: 10.1152/ajpregu.1997.273.2.R833. [DOI] [PubMed] [Google Scholar]
- 63.Davis JD. Morrison AR, Fluharty SJ. Progress in Psychobiology and Physiological Psychology. San Diego: Academic Press; 1998. "A model for the Control of Ingestion - 20 Years Later"; pp. 127–173. [Google Scholar]
- 64.Velkoska E, Cole TJ, Morris MJ. Early dietary intervention: long-term effects on blood pressure, brain neuropeptide Y, adiposity markers. Am J Physiol Endocrinol Metab. 2005;288:E1236–E1243. doi: 10.1152/ajpendo.00505.2004. [DOI] [PubMed] [Google Scholar]
- 65.Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28:12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Plagemann A, Roepke K, Harder T, Brunn M, Harder A, Wittrock-Staar M, et al. Epigenetic malprogramming of the insulin receptor promoter due to developmental overfeeding. J Perinat Med. 2010;38:393–400. doi: 10.1515/jpm.2010.051. [DOI] [PubMed] [Google Scholar]
- 67.Kuma H, Miki T, Matsumoto Y, Gu H, Li HP, Kusaka T, et al. Early maternal deprivation induces alterations in brain-derived neurotrophic factor expression in the developing rat hippocampus. Neurosci Lett. 2004;372:68–73. doi: 10.1016/j.neulet.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 68.Coupe B, Amarger V, Grit I, Benani A, Parnet P. Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology. 2010;151:702–713. doi: 10.1210/en.2009-0893. [DOI] [PubMed] [Google Scholar]
- 69.Plagemann A, Harder T, Rake A, Voits M, Fink H, Rohde W, et al. Perinatal elevation of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome x-like alterations in adulthood of neonatally overfed rats. Brain Res. 1999;836:146–155. doi: 10.1016/s0006-8993(99)01662-5. [DOI] [PubMed] [Google Scholar]
- 70.Mayeur S, Silhol M, Moitrot E, Barbaux S, Breton C, Gabory A, et al. Placental BDNF/TrkB signaling system is modulated by fetal growth disturbances in rat and human. Placenta. 2010;31:785–791. doi: 10.1016/j.placenta.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 71.Fox EA. A genetic approach for investigating vagal sensory roles in regulation of gastrointestinal function and food intake. Auton Neurosci. 2006;126–127:9–29. doi: 10.1016/j.autneu.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 72.Lommatzsch M, Braun A, Mannsfeldt A, Botchkarev VA, Botchkareva NV, Paus R, et al. Abundant production of brain-derived neurotrophic factor by adult visceral epithelia. Implications for paracrine and target-derived Neurotrophic functions. Am J Pathol. 1999;155:1183–1193. doi: 10.1016/S0002-9440(10)65221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ernfors P, Merlio JP, Persson H. Cells Expressing Messenger-Rna For Neurotrophins and Their Receptors During Embryonic Rat Development. Eur J Neurosci. 1992;4:1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 74.Zhuo H, Helke CJ. Presence and localization of neurotrophin receptor tyrosine kinase (TrkA, TrkB, TrkC) mRNAs in visceral afferent neurons of the nodose and petrosal ganglia. Brain Res Mol Brain Res. 1996;38:63–70. doi: 10.1016/0169-328x(95)00313-h. [DOI] [PubMed] [Google Scholar]
- 75.Carroll P, Lewin GR, Koltzenburg M, Toyka KV, Thoenen H. A role for BDNF in mechanosensation. Nat Neurosci. 1998;1:42–46. doi: 10.1038/242. [DOI] [PubMed] [Google Scholar]
- 76.Davison JS, Clarke GD. Mechanical properties and sensitivity to CCK of vagal gastric slowly adapting mechanoreceptors. Am J Physiol. 1988;255:G55–G61. doi: 10.1152/ajpgi.1988.255.1.G55. [DOI] [PubMed] [Google Scholar]
- 77.Zagorodnyuk VP, Chen BN, Brookes SJH. Intraganglionic laminar endings are mechanotransduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534.1:255–268. doi: 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fox EA, Biddinger JE, Jones KR, Worman A, McAdams J. Smooth muscle-specific knockout of brain-derived neurotrophic factor results in hyperphagia and obesity. Soc Neurosci Abstr. 2010 doi: 10.1016/j.neuroscience.2012.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Biddinger JE, Fox EA. Meal pattern and microstructure changes underlying hyperphagia and obesity in mice with smooth muscle-specific brain-derived neurotrophic factor knockout. Soc Neurosci Abstr. 2010 [Google Scholar]
- 80.Timmusk T, Belluardo N, Metsis M, Persson H. Widespread and developmentally regulated expression of neurotrophin-4 mRNA in rat brain and peripheral tissues. Eur J Neurosci. 1993;5:605–613. doi: 10.1111/j.1460-9568.1993.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 81.Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gorski JA, Zeiler SR, Tamowski S, Jones KR. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci. 2003;23:6856–6865. doi: 10.1523/JNEUROSCI.23-17-06856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, et al. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci U S A. 2002;99:7142–7147. doi: 10.1073/pnas.102650499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lepore JJ, Cheng L, Min Lu M, Mericko PA, Morrisey EE, Parmacek MS. High-efficiency somatic mutagenesis in smooth muscle cells and cardiac myocytes in SM22alpha-Cre transgenic mice. Genesis. 2005;41:179–184. doi: 10.1002/gene.20112. [DOI] [PubMed] [Google Scholar]
- 85.Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994a;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- 86.Rinaman L, Baker EA, Hoffman GE, Stricker EM, Verbalis JG. Medullary c-Fos activation in rats after ingestion of a satiating meal. Am J Physiol. 1998;275:R262–R268. doi: 10.1152/ajpregu.1998.275.1.R262. [DOI] [PubMed] [Google Scholar]
- 87.Glatzle J, Wang Y, Adelson DW, Kalogeris TJ, Zittel TT, Tso P, et al. Chylomicron components activate duodenal vagal afferents via a cholecystokinin A receptor-mediated pathway to inhibit gastric motor function in the rat. J Physiol. 2003;550:657–664. doi: 10.1113/jphysiol.2003.041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raybould HE, Glatzle J, Robin C, Meyer JH, Phan T, Wong H, et al. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2003;284:G367–G372. doi: 10.1152/ajpgi.00292.2001. [DOI] [PubMed] [Google Scholar]
- 89.Rogers RC, Hermann GE, Travagli RA. Brainstem pathways responsible for oesophageal control of gastric motility and tone in the rat. J Physiol. 1999;514:369–383. doi: 10.1111/j.1469-7793.1999.369ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wei JY, Wang YH, Go VL, Tache Y. Esophageal distension induced gastric relaxation is mediated in part by vagal peripheral reflex mechanism in rats. J Auton Nerv Syst. 1997;63:12–18. doi: 10.1016/s0165-1838(96)00126-9. [DOI] [PubMed] [Google Scholar]
- 91.Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gray TS, Magnuson DJ. Neuropeptide neuronal efferents from the bed nucleus of the stria terminalis and central amygdaloid nucleus to the dorsal vagal complex in the rat. J Comp Neurol. 1987;262:365–374. doi: 10.1002/cne.902620304. [DOI] [PubMed] [Google Scholar]
- 93.Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- 94.van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984;224:1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- 95.Andresen MC, Yang MY. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol. 1990;259:H1307–H1311. doi: 10.1152/ajpheart.1990.259.4.H1307. [DOI] [PubMed] [Google Scholar]
- 96.Hornby PJ. Receptors and transmission in the brain-gut axis. II. Excitatory amino acid receptors in the brain-gut axis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1055–G1060. doi: 10.1152/ajpgi.2001.280.6.G1055. [DOI] [PubMed] [Google Scholar]
- 97.Berthoud HR. The vagus nerve, food intake and obesity. Regul Pept. 2008;149:15–25. doi: 10.1016/j.regpep.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Matson CA, Reid DF, Cannon TA, Ritter RC. Cholecystokinin and leptin act synergistically to reduce body weight. Am J Physiol Regul Integr Comp Physiol. 2000;278:R882–R890. doi: 10.1152/ajpregu.2000.278.4.R882. [DOI] [PubMed] [Google Scholar]
- 99.Matson CA, Reid DF, Ritter RC. Daily CCK injection enhances reduction of body weight by chronic intracerebroventricular leptin infusion. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1368–R1373. doi: 10.1152/ajpregu.00080.2001. [DOI] [PubMed] [Google Scholar]
- 100.Matson CA, Ritter RC. Long-term CCK-leptin synergy suggests a role for CCK in the regulation of body weight. Am J Physiol. 1999;276:R1038–R1045. doi: 10.1152/ajpregu.1999.276.4.R1038. [DOI] [PubMed] [Google Scholar]
- 101.Bi S, Ladenheim EE, Schwartz GJ, Moran TH. A role for NPY overexpression in the dorsomedial hypothalamus in hyperphagia and obesity of OLETF rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R254–R260. doi: 10.1152/ajpregu.2001.281.1.R254. [DOI] [PubMed] [Google Scholar]
- 102.Bi S, Scott KA, Kopin AS, Moran TH. Differential roles for cholecystokinin a receptors in energy balance in rats and mice. Endocrinology. 2004;145:3873–3880. doi: 10.1210/en.2004-0284. [DOI] [PubMed] [Google Scholar]
- 103.Moran TH, Katz LF, Plata-Salaman CR, Schwartz GJ. Disordered food intake and obesity in rats lacking cholecystokinin A receptors. Am J Physiol. 1998;274:R618–R625. doi: 10.1152/ajpregu.1998.274.3.R618. [DOI] [PubMed] [Google Scholar]
- 104.Fox EA, Phillips RJ, Baronowsky EA, Byerly MS, Jones S, Powley TL. Neurotrophin-4 deficient mice have a loss of vagal intraganglionic mechanoreceptors from the small intestine and a disruption of short-term satiety. J. Neurosci. 2001b;21:8602–8615. doi: 10.1523/JNEUROSCI.21-21-08602.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Byerly MS, Fox EA. High-fat hyperphagia in neurotrophin-4 deficient mice reveals potential role of vagal intestinal sensory innervation in long-term controls of food intake. Neurosci Lett. 2006;400:240–245. doi: 10.1016/j.neulet.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 106.Amar AP, Heck CN, Levy ML, Smith T, DeGiorgio CM, Oviedo S, et al. An institutional experience with cervical vagus nerve trunk stimulation for medically refractory epilepsy: rationale, technique, and outcome. Neurosurgery. 1998;43:1265–1276. doi: 10.1097/00006123-199812000-00001. discussion 76-80. [DOI] [PubMed] [Google Scholar]
- 107.Abubakr A, Wambacq I. Long-term outcome of vagus nerve stimulation therapy in patients with refractory epilepsy. J Clin Neurosci. 2008;15:127–129. doi: 10.1016/j.jocn.2007.07.083. [DOI] [PubMed] [Google Scholar]
- 108.George MS, Sackeim HA, Rush AJ, Marangell LB, Nahas Z, Husain MM, et al. Vagus nerve stimulation: a new tool for brain research and therapy. Biol Psychiatry. 2000b;47:287–295. doi: 10.1016/s0006-3223(99)00308-x. [DOI] [PubMed] [Google Scholar]
- 109.Rush AJ, George MS, Sackeim HA, Marangell LB, Husain MM, Giller C, et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry. 2000;47:276–286. doi: 10.1016/s0006-3223(99)00304-2. [DOI] [PubMed] [Google Scholar]
- 110.Faris PL, Hofbauer RD, Daughters R, Vandenlangenberg E, Iversen L, Goodale RL, et al. De-stabilization of the positive vago-vagal reflex in bulimia nervosa. Physiol Behav. 2008;94:136–153. doi: 10.1016/j.physbeh.2007.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Noble EE, Billington CJ, Kotz CM, Wang C. The lighter side of BDNF. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1053–R1069. doi: 10.1152/ajpregu.00776.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yan Q, Radeke MJ, Matheson CR, Talvenheimo J, Welcher AA, Feinstein SC. Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J Comp Neurol. 1997;378:135–157. [PubMed] [Google Scholar]
- 113.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. Embo J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 115.Peters JH, Simasko SM, Ritter RC. Modulation of vagal afferent excitation and reduction of food intake by leptin and cholecystokinin. Physiol Behav. 2006;89:477–485. doi: 10.1016/j.physbeh.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 116.Hoggard N, Mercer JG, Rayner DV, Moar K, Trayhurn P, Williams LM. Localization of leptin receptor mRNA splice variants in murine peripheral tissues by RT-PCR and in situ hybridization. Biochem Biophys Res Commun. 1997;232:383–387. doi: 10.1006/bbrc.1997.6245. [DOI] [PubMed] [Google Scholar]
- 117.Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996;387:113–116. doi: 10.1016/0014-5793(96)00473-5. [DOI] [PubMed] [Google Scholar]
- 118.Mercer JG, Moar KM, Hoggard N. Localization of leptin receptor (Ob-R) messenger ribonucleic acid in the rodent hindbrain. Endocrinology. 1998;139:29–34. doi: 10.1210/endo.139.1.5685. [DOI] [PubMed] [Google Scholar]
- 119.Perello M, Scott MM, Sakata I, Lee CE, Chuang JC, Osborne-Lawrence S, et al. Functional implications of limited leptin receptor and ghrelin receptor co-expression in the brain. J Comp Neurol. 2011;14:22690. doi: 10.1002/cne.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Williams LM, Adam CL, Mercer JG, Moar KM, Slater D, Hunter L, et al. Leptin receptor and neuropeptide Y gene expression in the sheep brain. J Neuroendocrinol. 1999;11:165–169. doi: 10.1046/j.1365-2826.1999.00293.x. [DOI] [PubMed] [Google Scholar]
- 121.Paz-Filho G, Wong ML, Licinio J. The procognitive effects of leptin in the brain and their clinical implications. Int J Clin Pract. 2010;64:1808–1812. doi: 10.1111/j.1742-1241.2010.02536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kanoski SE, Hayes MR, Greenwald HS, Fortin SM, Gianessi CA, Gilbert JR, et al. Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology. 2011;36:1859–1870. doi: 10.1038/npp.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schloegl H, Percik R, Horstmann A, Villringer A, Stumvoll M. Peptide hormones regulating appetite--focus on neuroimaging studies in humans. Diabetes Metab Res Rev. 2011;27:104–112. doi: 10.1002/dmrr.1154. [DOI] [PubMed] [Google Scholar]
- 124.Felix B, Jean A, Roman C. Leptin inhibits swallowing in rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R657–R663. doi: 10.1152/ajpregu.00560.2005. [DOI] [PubMed] [Google Scholar]
- 125.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143:239–246. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- 126.Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, et al. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14:313–323. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Davis JF, Choi DL, Schurdak JD, Fitzgerald MF, Clegg DJ, Lipton JW, et al. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol Psychiatry. 2011;69:668–674. doi: 10.1016/j.biopsych.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.DiLeone RJ. The influence of leptin on the dopamine system and implications for ingestive behavior. Int J Obes (Lond) 2009;33(Suppl 2):S25–S29. doi: 10.1038/ijo.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ducroc R, Sakar Y, Fanjul C, Barber A, Bado A, Lostao MP. Luminal leptin inhibits L-glutamine transport in rat small intestine: involvement of ASCT2 and B0AT1. Am J Physiol Gastrointest Liver Physiol. 2010;299:G179–G185. doi: 10.1152/ajpgi.00048.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Iqbal J, Li X, Chang BH, Chan L, Schwartz GJ, Chua SC, Jr, et al. An intrinsic gut leptin-melanocortin pathway modulates intestinal microsomal triglyceride transfer protein and lipid absorption. J Lipid Res. 2010;51:1929–1942. doi: 10.1194/jlr.M005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yarandi SS, Hebbar G, Sauer CG, Cole CR, Ziegler TR. Diverse roles of leptin in the gastrointestinal tract: modulation of motility, absorption, growth, and inflammation. Nutrition. 2011;27:269–275. doi: 10.1016/j.nut.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhao Z, Sakata I, Okubo Y, Koike K, Kangawa K, Sakai T. Gastric leptin, but not estrogen and somatostatin, contributes to the elevation of ghrelin mRNA expression level in fasted rats. J Endocrinol. 2008;196:529–538. doi: 10.1677/JOE-07-0300. [DOI] [PubMed] [Google Scholar]
- 133.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 134.van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dietl MM, Palacios JM. The distribution of cholecystokinin receptors in the vertebrate brain: species differences studied by receptor autoradiography. J Chem Neuroanat. 1989;2:149–161. [PubMed] [Google Scholar]
- 136.Honda T, Wada E, Battey JF, Wank SA. Differential Gene Expression of CCK(A) and CCK(B) Receptors in the Rat Brain. Mol Cell Neurosci. 1993;4:143–154. doi: 10.1006/mcne.1993.1018. [DOI] [PubMed] [Google Scholar]
- 137.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 138.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 139.Patterson LM, Zheng H, Berthoud HR. Vagal afferents innervating the gastrointestinal tract and CCKA-receptor immunoreactivity. Anat Rec. 2002;266:10–20. doi: 10.1002/ar.10026. [DOI] [PubMed] [Google Scholar]
- 140.Smith GT, Moran TH, Coyle JT, Kuhar MJ, O'Donahue TL, McHugh PR. Anatomic localization of cholecystokinin receptors to the pyloric sphincter. Am J Physiol. 1984;246:R127–R130. doi: 10.1152/ajpregu.1984.246.1.R127. [DOI] [PubMed] [Google Scholar]
- 141.Fernandez-Twinn DS, Ozanne SE. Early life nutrition and metabolic programming. Ann N Y Acad Sci. 2010;1212:78–96. doi: 10.1111/j.1749-6632.2010.05798.x. [DOI] [PubMed] [Google Scholar]
- 142.Metges CC. Early nutrition and later obesity: animal models provide insights into mechanisms. Adv Exp Med Biol. 2009;646:105–112. doi: 10.1007/978-1-4020-9173-5_11. [DOI] [PubMed] [Google Scholar]
- 143.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 144.Marek K, Jennings D. Can we image premotor Parkinson disease? Neurology. 2009;72:S21–S26. doi: 10.1212/WNL.0b013e318198df97. [DOI] [PubMed] [Google Scholar]
- 145.Bariohay B, Lebrun B, Moyse E, Jean A. Brain-derived neurotrophic factor plays a role as an anorexigenic factor in the dorsal vagal complex. Endocrinology. 2005;146:5612–5620. doi: 10.1210/en.2005-0419. Epub 2005 Sep 15. [DOI] [PubMed] [Google Scholar]
- 146.Duan W, Lee J, Guo Z, Mattson MP. Dietary restriction stimulates BDNF production in the brain and thereby protects neurons against excitotoxic injury. J Mol Neurosci. 2001;16:1–12. doi: 10.1385/JMN:16:1:1. [DOI] [PubMed] [Google Scholar]
- 147.la Fleur SE, van Rozen AJ, Luijendijk MC, Groeneweg F, Adan RA. A free-choice high-fat high-sugar diet induces changes in arcuate neuropeptide expression that support hyperphagia. Int J Obes (Lond) 2010;34:537–546. doi: 10.1038/ijo.2009.257. [DOI] [PubMed] [Google Scholar]
- 148.Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gomez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–440. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 149.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zeeni N, Chaumontet C, Moyse E, Fromentin G, Tardivel C, Tome D, et al. A positive change in energy balance modulates TrkB expression in the hypothalamus and nodose ganglia of rats. Brain Res. 2009;1289:49–55. doi: 10.1016/j.brainres.2009.06.076. [DOI] [PubMed] [Google Scholar]
- 151.Huang XF, Han M, South T, Storlien L. Altered levels of POMC, AgRP and MC4-R mRNA expression in the hypothalamus and other parts of the limbic system of mice prone or resistant to chronic high-energy diet-induced obesity. Brain Res. 2003;992:9–19. doi: 10.1016/j.brainres.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 152.Cordeira JW, Frank L, Sena-Esteves M, Pothos EN, Rios M. Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J Neurosci. 2010;30:2533–2541. doi: 10.1523/JNEUROSCI.5768-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–814. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 154.Yu Y, Wang Q, Huang XF. Energy-restricted pair-feeding normalizes low levels of brain-derived neurotrophic factor/tyrosine kinase B mRNA expression in the hippocampus, but not ventromedial hypothalamic nucleus, in diet-induced obese mice. Neuroscience. 2009;160:295–306. doi: 10.1016/j.neuroscience.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 155.Covasa M, Grahn J, Ritter RC. High fat maintenance diet attenuates hindbrain neuronal response to CCK. Regul Pept. 2000;86:83–88. doi: 10.1016/s0167-0115(99)00084-1. [DOI] [PubMed] [Google Scholar]
- 156.Nefti W, Chaumontet C, Fromentin G, Tome D, Darcel N. A high-fat diet attenuates the central response to within-meal satiation signals and modifies the receptor expression of vagal afferents in mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1681–R1686. doi: 10.1152/ajpregu.90733.2008. [DOI] [PubMed] [Google Scholar]
- 157.Daly DM, Park SJ, Valinsky WC, Beyak MJ. Impaired intestinal afferent nerve satiety signaling and vagal afferent excitability in diet induced obesity in the mouse. J Physiol. 2011;21:21. doi: 10.1113/jphysiol.2010.204594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dockray GJ, Burdyga G. Plasticity in vagal afferent neurones during feeding and fasting: mechanisms and significance. Acta Physiol (Oxf) 2011;201:313–321. doi: 10.1111/j.1748-1716.2010.02219.x. [DOI] [PubMed] [Google Scholar]
- 159.Paulino G, Barbier de la Serre C, Knotts TA, Oort PJ, Newman JW, Adams SH, et al. Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats. Am J Physiol Endocrinol Metab. 2009;296:E898–E903. doi: 10.1152/ajpendo.90796.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.la Fleur SE, Vanderschuren LJ, Luijendijk MC, Kloeze BM, Tiesjema B, Adan RA. A reciprocal interaction between food-motivated behavior and diet-induced obesity. Int J Obes (Lond) 2007;31:1286–1294. doi: 10.1038/sj.ijo.0803570. [DOI] [PubMed] [Google Scholar]
- 161.Butler AA, Cone RD. The melanocortin receptors: lessons from knockout models. Neuropeptides. 2002;36:77–84. doi: 10.1054/npep.2002.0890. [DOI] [PubMed] [Google Scholar]
- 162.Butler AA, Cone RD. Knockout studies defining different roles for melanocortin receptors in energy homeostasis. Ann N Y Acad Sci. 2003;994:240–245. doi: 10.1111/j.1749-6632.2003.tb03186.x. [DOI] [PubMed] [Google Scholar]
- 163.Fox EA, Byerly MS. A mechanism underlying mature-onset obesity: evidence from the hyperphagic phenotype of brain-derived neurotrophic factor mutants. Am J Physiol Regul Integr Comp Physiol. 2004;286:R994–R1004. doi: 10.1152/ajpregu.00727.2003. [DOI] [PubMed] [Google Scholar]
- 164.Tecott LH, Abdallah L. Mouse genetic approaches to feeding regulation: serotonin 5-HT2C receptor mutant mice. CNS Spectr. 2003;8:584–588. [PubMed] [Google Scholar]
- 165.Nonogaki K, Strack AM, Dallman MF, Tecott LH. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med. 1998;4:1152–1156. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]