This article shows that regulation of the ovule identity gene STK is dependent on the binding of a MADS domain protein containing repressor complex to its promoter and that BPC binding sites are essential for the recruitment of this complex. It provides evidence that this is probably a general mechanism by which BPCs regulate gene expression in plants.
Abstract
BASIC PENTACYSTEINE (BPC) transcription factors have been identified in a large variety of plant species. In Arabidopsis thaliana there are seven BPC genes, which, except for BPC5, are expressed ubiquitously. BPC genes are functionally redundant in a wide range of developmental processes. Recently, we reported that BPC1 binds to guanine and adenine (GA)–rich consensus sequences in the SEEDSTICK (STK) promoter in vitro and induces conformational changes. Here we show by chromatin immunoprecipitation experiments that in vivo BPCs also bind to the consensus boxes, and when these were mutated, expression from the STK promoter was derepressed, resulting in ectopic expression in the inflorescence. We also reveal that SHORT VEGETATIVE PHASE (SVP) is a direct regulator of STK. SVP is a floral meristem identity gene belonging to the MADS box gene family. The SVP-APETALA1 (AP1) dimer recruits the SEUSS (SEU)-LEUNIG (LUG) transcriptional cosuppressor to repress floral homeotic gene expression in the floral meristem. Interestingly, we found that GA consensus sequences in the STK promoter to which BPCs bind are essential for recruitment of the corepressor complex to this promoter. Our data suggest that we have identified a new regulatory mechanism controlling plant gene expression that is probably generally used, when considering BPCs’ wide expression profile and the frequent presence of consensus binding sites in plant promoters.
INTRODUCTION
Transcriptional regulation is still poorly understood in plants. Recently, a new class of transcription factors, named BASIC PENTACYSTEINE (BPC), was identified (Meister et al., 2004). In Arabidopsis thaliana, BPCs belong to a small gene family of seven members that encode activators and repressors of transcription (Meister et al., 2004; Monfared et al., 2011; Berger and Dubreucq, 2012). Based on their sequence similarity, they were divided into three classes: class I (containing BPC1 to BPC3), class II (containing BPC4 to BPC6), and class III (containing only BPC7) (Meister et al., 2004). All genes, except for BPC5, which is probably a pseudogene, are expressed ubiquitously. BPC genes belonging to these different classes were shown to be functionally redundant, and combining multiple bpc mutant alleles together results in a wide range of developmental defects (Monfared et al., 2011). Recently, we identified BPC1 as a regulator of the ovule identity gene SEEDSTICK (STK), which is specifically expressed in ovules (Rounsley et al., 1995; Pinyopich et al., 2003; Brambilla et al., 2007). BPC1 binds to the STK promoter at multiple guanine and adenine (GA)–rich boxes, which have the RGARAGRRA consensus sequence (Kooiker et al., 2005). Its cooperative binding was shown to induce conformational changes in the STK regulatory region, suggesting that multiple consensus sites are required for the regulation of STK. Furthermore, it was shown that STK expression was upregulated in the bpc1-1 single mutant, although the spatial expression profile of STK was not changed (Kooiker et al., 2005).
Here we describe the identification of the MADS domain factor SHORT VEGETATIVE PHASE (SVP) as another regulator of STK. This transcription factor was first identified as a repressor of the floral transition (Hartmann et al., 2000). By combining the strong ap1-10 allele with the agamous-like24 (agl24) svp double mutant, it was shown that, after the floral transition, SVP also acts as a floral meristem identity gene, because in this triple mutant, floral meristem identity was not specified, and inflorescence meristems developed in place of floral meristems, resulting in a phenotype that resembles a cauliflower curd-like structure (Gregis et al., 2008). During reproductive growth, SVP is expressed only in the floral meristem (stage 1 and 2 of flower development), which during this phase increases in size. In the floral meristem, SVP interacts with AP1 to recruit the LEUNIG (LUG)-SEUSS (SEU) corepressor (Gregis et al., 2006, 2009). This complex is important to repress homeotic gene expression during the first stages of flower development to prevent precocious differentiation of the floral meristem into floral organs. For instance, it was shown that SVP and AP1 directly bind and repress MADS box floral organ identity genes, such as APETALA3 (AP3), PISTILLATA (PI), SEPALLATA3 (SEP3), and AGAMOUS (AG) (Gregis et al., 2006; Gregis et al., 2009).
Here we show that the regulation of STK is dependent on the binding of a MADS domain protein–containing repressor complex to its promoter and that BPC binding sites are essential for the recruitment of this complex. We provide evidence that this is probably a general mechanism by which BPCs regulate gene expression in plants.
RESULTS
Class I BPC Proteins Bind the STK Promoter in Vivo
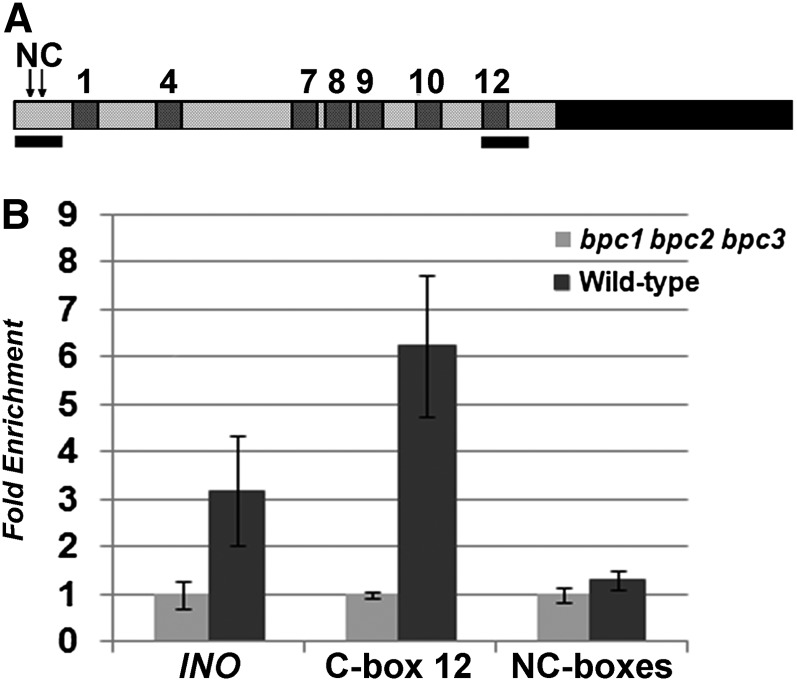
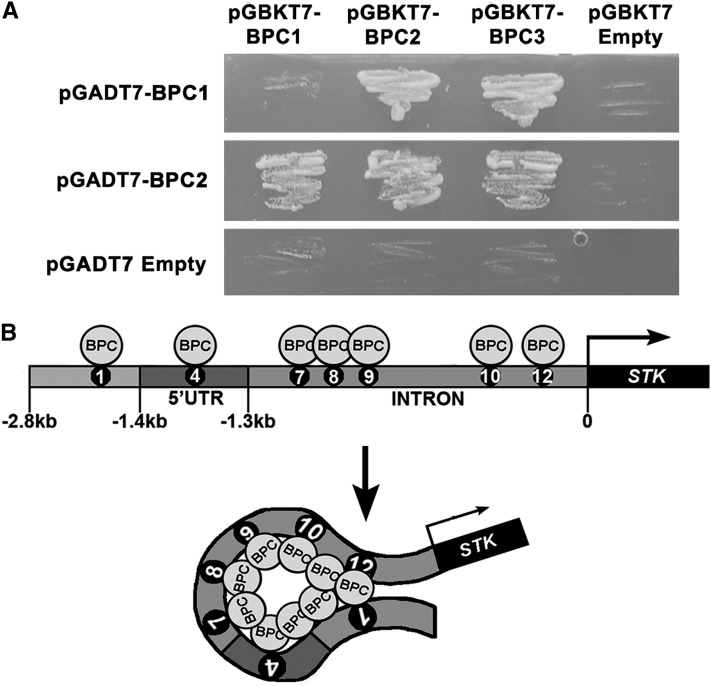
Electrophoretic mobility shift assay (EMSA) experiments using primers based on the STK promoter sequence predicted that BPC1 binds to seven out of 12 GAGA boxes (Kooiker et al., 2005). Based on these experiments, the RGARAGRRA consensus BPC1 binding sequence (consensus [C]-box) was proposed. The seven BPC1 binding sites (C-boxes) were numbered as 1, 4, 7, 8, 9, 10, and 12, based on their order in the STK promoter sequence (Figure 1A). In particular, C-boxes 4 and 12 showed the strongest binding.
Figure 1.
Class I BPC Proteins Bind to C-Boxes in the STK Promoter.
(A) Schematic representation of the STK promoter indicating the C-boxes (dark gray numbered boxes) and the regions tested by ChIP (black bars). The two arrows indicate the position of the two NC-boxes that were tested.
(B) Real-time PCR analysis of ChIP experiments using chromatin extracted from wild-type plants (dark gray bars) and from the bpc1-3 bpc2 bpc3 triple mutant (light gray bars) testing the regions indicated in (A) and using the INO promoter as a control.
Previous analysis of the STK promoter sequence showed that apart from the seven C-boxes, there are five nonconsensus (NC) boxes (NC-boxes 2, 3, 5, 6, and 11), which have one mismatch with respect to the consensus sequence. These NC-boxes did not bind BPC1 in EMSA studies (Kooiker et al., 2005). We carefully reanalyzed the STK promoter region and discovered the presence of five additional NC-boxes (see Supplemental Figure 1 online).
To verify the ability of class I BPC proteins (BPC1, BPC2, and BPC3) to bind C- and NC-boxes in the STK promoter in vivo, three independent biological replicates of chromatin immunoprecipitation (ChIP) assays were performed using a polyclonal antibody that specifically recognizes class I BPC proteins (see Supplemental Figure 2 online). These experiments were conducted using chromatin extracted from wild-type inflorescences. Two different regions of the STK promoter of ∼250 bp were analyzed (Figure 1A), allowing us to distinguish between C-box and NC-box binding. One region was at the beginning of the promoter, containing two NC-boxes, and the other region was at the end of the promoter and contained only C-box 12, which showed strong binding in the EMSA experiments. As a positive control, binding to the promoter of INNER NO OUTER (INO) was tested (Meister et al., 2004), and as a negative control, the bpc1-3 bpc2 bpc3 triple mutant was used (Figure 1B).
ChIP analysis on the region containing C-box 12 showed high enrichment, confirming strong binding to this consensus site. When we tested the NC-boxes for BPC binding, we did not observe any enrichment, confirming the EMSA experiment and showing that the NC-boxes do not bind BPC1, BPC2, and BPC3 in vivo.
The C-Boxes Are Necessary for Correct Spatial and Temporal Regulation of STK Expression
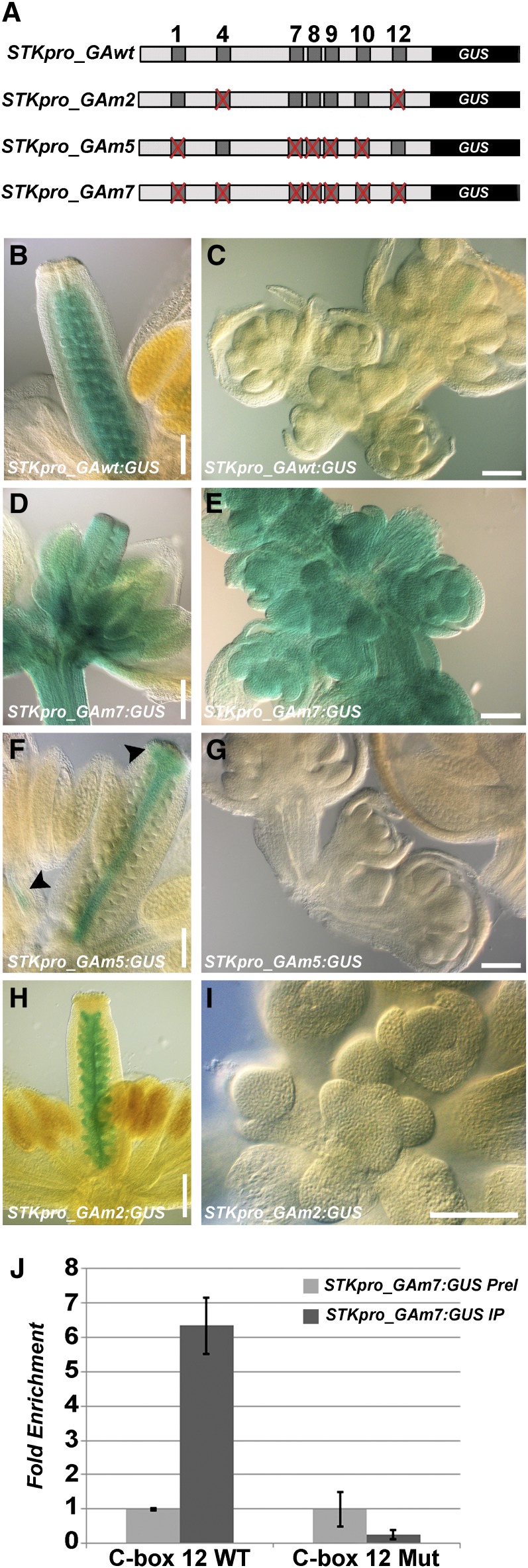
Recently, functional analysis of different classes of BPC genes in Arabidopsis showed that they have redundant functions in plant developmental processes (Monfared et al., 2011). Analysis of STK expression in the bpc1-1 mutant confirmed this, because STK was only slightly upregulated, but its expression profile was not affected (Kooiker et al., 2005). Therefore, considering redundancy between BPC factors and to avoid pleiotropic effects of higher-order bpc mutant combinations, we decided to investigate the role of BPC proteins in the regulation of STK by mutating the seven C-boxes in the STK promoter by changing in each box two purines essential for BPC1 binding into pyrimidine residues. This mutated promoter fragment of 2900 bp upstream of the STK translation start site was cloned in frame with the uidA reporter gene that encodes β-glucuronidase (GUS), and the resultant STKpro_GAm7:GUS construct (Figure 2A) was used for Arabidopsis transformation. As a positive control, we used the wild-type STK promoter (STKpro_GAwt:GUS; Figure 2A), which drives specific expression in the placenta and all stages of ovule development (Figure 2B; Pinyopich et al., 2003). Of the 28 independent transgenic lines that were transformed with the wild-type promoter construct, 89% showed the expected wild-type STK expression profile (Figures 2B and 2C), whereas in 11% of the lines, an aberrant profile was observed (see Supplemental Table 1 online). In these lines, expression was random in all parts of the plant. Those lines in which no GUS signal was detected (n = 3) were not considered for further analysis. Of the 125 independent lines transformed with the STKpro_GAm7:GUS construct, 94 plants (75%) showed strong deregulation of the GUS reporter gene. Strong blue staining was observed not only in all the floral organs but also in inflorescence and floral meristems (Figures 2D and 2E; see Supplemental Table 1 online), suggesting a pivotal role for the C-boxes in the regulation of spatial and temporal STK expression.
Figure 2.
Mutations in C-Boxes Abolish BPC Binding to the STK Promoter.
(A) Schematic representation of the STK promoter-GUS constructs used in this study: dark gray squares represent C-boxes, and the mutated ones are indicated with a red cross.
(B) to (I) GUS staining performed on inflorescences (at left, mature flowers; at right, inflorescence and floral meristems and young flowers) of STKpro_GAwt:GUS [(B) and (C)], STKpro_GAm7:GUS [(D) and (E)], STKpro_GAm5:GUS [(F) and (G)], and STKpro_GAm2:GUS [(H) and (I)] plants.
(J) ChIP assays using chromatin from plants containing the STKpro_GAm7:GUS construct. Real-time PCR analysis revealed that BPC proteins bind to C-box 12 in the endogenous wild-type (WT) STK promoter but not to the mutated (Mut) C-box 12. The graph represents the average of three replicates (n = 3), and the error bars show the sd.
IP, immunoprecipitation using antibodies against class I BPC proteins; PreI, immunoprecipitation using preimmune serum.
Bars in (B), (D), (F), and (H) = 200 μm; bars in (C), (E), (G), and (I) = 100 μm.
Kooiker et al. (2005) showed by tethered particle motion (TPM; Finzi and Dunlap, 2003) analysis that BPC1 is able to induce loops in the STK promoter and that prominent loop formation occurs between C-boxes 4 and 12, which were the strongest BPC1 binding sites in the EMSA assays. However, TPM experiments using a STK promoter fragment in which only C-boxes 4 and 12 were maintained and the other five were mutated showed that no conformational changes were introduced, suggesting that C-boxes 4 and 12 alone are not enough to induce looping. To investigate in more detail the importance of the C-boxes for STK regulation taking into consideration the previously performed TPM analysis, a STK promoter-GUS construct was prepared in which only C-boxes 4 and 12 were maintained and all five other C-boxes were mutated (STKpro_GAm5:GUS; Figure 2A). This STKpro_GAm5:GUS construct was introduced in Arabidopsis, and of the 71 independent transgenic lines that were analyzed, 27 plants (38%) showed deregulation of the GUS reporter (Figures 2F and 2G; see Supplemental Table 1 online), suggesting that C-boxes 4 and 12 are important for proper STK expression and that the mutation of five C-boxes led to more instability in the regulation of the expression of the reporter gene. It is important to notice that deregulation in these lines was observed only in the anthers and the style and stigma of the carpel (Figure 2F). The reporter line did not show expression in the inflorescence or floral meristem (Figure 2G).
To answer the question of whether C-boxes 4 and 12 are essential for proper STK expression, a promoter construct was generated in which only these two C-boxes were mutated (STKpro_GAm2:GUS; Figure 2A). Of the 57 independent transformants that were analyzed, only 14% showed deregulation of the GUS reporter gene (Figures 2H and 2I; see Supplemental Table 1 online), indicating that C-boxes 4 and 12 seem not to be essential for proper STK expression. These results showed that multiple C-boxes are important for the proper regulation of STK by BCP proteins.
To ensure that the mutations that were introduced in the C-boxes prevented BPC binding in vivo, three independent ChIP assays using BPC class I–specific antibodies were performed. The experiments were conducted using inflorescences of homozygous STKpro_GAm7:GUS lines. The wild-type endogenous C-box 12 was tested as a positive target sequence, and as a negative control, the preimmune serum was used. To discriminate between the endogenous wild-type C-box 12 and the mutated one located on the STKpro_GAm7:GUS construct, specific primers that differed in two 3′ nucleotides were used. In all three ChIP experiments, no enrichment was detected when binding to the mutated C-box 12 was tested (Figure 2J), whereas the endogenous C-box 12 was highly enriched. This result confirmed the efficacy of the mutations to abolish BPC binding in vivo and is consistent with the previous in vitro analysis (Kooiker et al., 2005). Furthermore, it supports the observation that deregulation of the STK promoter activity is caused by the absence of BPC binding to the mutated C-boxes.
Class I BPC Proteins Interact with Each Other
BPC proteins, at least the ones of class I, were shown to bind in vitro and in vivo to multiple C-boxes in the STK regulatory region. Furthermore, Kooiker et al. (2005) showed by TPM analysis that they induce conformational changes in the DNA by inducing loops between the different C-boxes. To investigate whether loops can be induced into the DNA through both the binding to the C-boxes and by direct interactions between these class I BPC proteins, we performed yeast two-hybrid assays. The open reading frames encoding BPC1, BPC2, and BPC3 were fused to the activation domain (AD) and binding domain (BD) and tested for interaction (Table 1). These assays showed that BPC1 interacted with BPC2 and BPC3; BPC2 interacted with BPC3 and also formed homodimers (Figure 3A). These data show that BPC proteins potentially can loop the DNA by binding to C-boxes and interacting with each other (Figure 3B).
Table 1. Class I BPC Interactions in Yeast.
| Plasmid | pGBKT7-BPC1 | pGBKT7-BPC2 | pGBKT7-BPC3 | pGBKT7 empty |
|---|---|---|---|---|
| pGADT7-BPC1 | – | ++ | ++ | – |
| pGADT7-BPC2 | ++ | ++ | ++ | – |
| pGADT7-BPC3a | ++ | ++ | ++ | ++ |
| pGADT7 empty | – | – | – | – |
– indicates no interaction; ++ indicates positive strong interaction.
BPC3 autoactivates as AD fusion.
Figure 3.
BPC Proteins of Class I Interact with Each Other.
(A) Yeast two-hybrid assays testing interactions between BPC proteins of class I using −W−L−H +5 mM of 3-AT selective media. The empty vectors were used as negative controls.
(B) Putative model of BPC interactions with the STK promoter: BPCs interact with each other and bind the STK promoter at multiple C-boxes; as a consequence, this might induce conformational changes in this region. UTR, untranslated region.
STK Is a Target of the MADS Domain Transcription Factor SVP
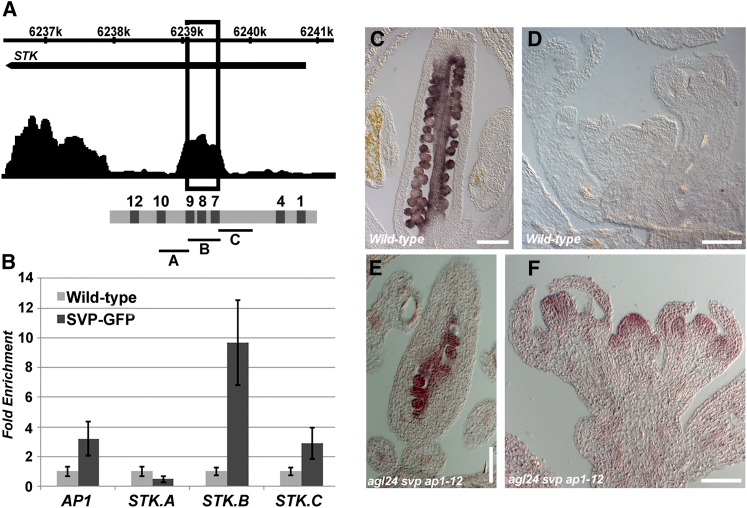
We recently performed a ChIP-sequencing assay to identify genome-wide targets for the floral meristem identity factor SVP, which resulted in the identification of STK (V. Gregis et al., unpublished results). This experiment showed that the MADS domain transcription factor SVP binds to various positions in the STK genomic region (Figure 4A). The direct interaction between SVP and the STK promoter was confirmed by three ChIP assays (independent biological replicates) using antibodies against green fluorescent protein (GFP) and chromatin extracted from inflorescences of svp homozygous mutant plants complemented with a SVPpro:SVP-GFP construct (Gregis et al., 2009). As a positive control, the enrichment of a region of the AP1 promoter known to interact with SVP was used (Grandi et al., 2011), whereas, as a negative control, inflorescences of wild-type plants were used. The STK promoter was divided into three parts (regions A, B, and C; Figure 4A) spanning all putative SVP promoter binding sites. This ChIP analysis revealed that the highest enrichment in all three replicates was in region B (Figures 4A and 4B), which contains three CArG boxes (MADS domain binding sites) surrounded by several C-boxes (Figure 4A; see Supplemental Figure 1 online).
Figure 4.
STK Is Target of SVP.
(A) Schematic representation of SVP binding to the STK locus as identified by ChIP-sequencing (V. Gregis, A. Sessa and M.M. Kater, unpublished results). The STK promoter contains few putative SVP binding sites localized close to the GAGA boxes 7, 8, and 9. The window at 6239k represents the region containing C-boxes 7 to 9, as shown in the diagram. A, B and C represent the STK regions that were tested by ChIP experiments.
(B) Real-time PCR analysis of ChIP assay using SVPpro:SVP-GFP svp and wild-type chromatin to confirm SVP binding to the STK regions A, B, and C. The AP1 promoter was used as positive control. The graph represents the average of three replicates (n = 3), and the error bars show the sd.
(C) to (F) In situ hybridization on wild-type [(C) and (D)] and agl24 svp ap1-12 triple mutant [(E) and (F)] inflorescences using a STK-specific antisense probe.
Bars in (C) and (E) = 200 μm; bars in (D) and (F) = 100 μm.
STK is closely related to AG, a gene regulating ovule, stamen, and carpel identity and floral meristem determinacy (Bowman et al., 1991). Recently, we showed that in the agl24 svp ap1-12 triple mutant, AG is ectopically expressed in floral meristems of stage 1 and 2 (Gregis et al., 2008), evidencing the redundant role of these three genes in the repression of AG at these very early stages of flower development. To investigate whether the binding of SVP to STK is, as previously observed for AG, important for the repression of STK in the floral meristem, we analyzed the expression of this gene in the agl24 svp ap1-12 triple mutant by in situ hybridization. In wild-type plants, STK is expressed in ovules and the placenta (Figure 4C; Pinyopich et al., 2003), and no expression was detectable in floral meristems and flowers at early stages of development (Figure 4D). Interestingly, in the agl24 svp ap1-12 triple mutant, STK expression in the carpel was like that in wild-type plants (Figure 4E); however, its expression was also observed in floral meristems and young flowers (Figure 4F). Taken together, these data suggest that SVP prevents STK expression in the floral meristem by direct binding to its promoter region.
BPC Factors Facilitate the Binding of the AP1-SVP-SEU-LUG Repressor Complex to the STK Promoter
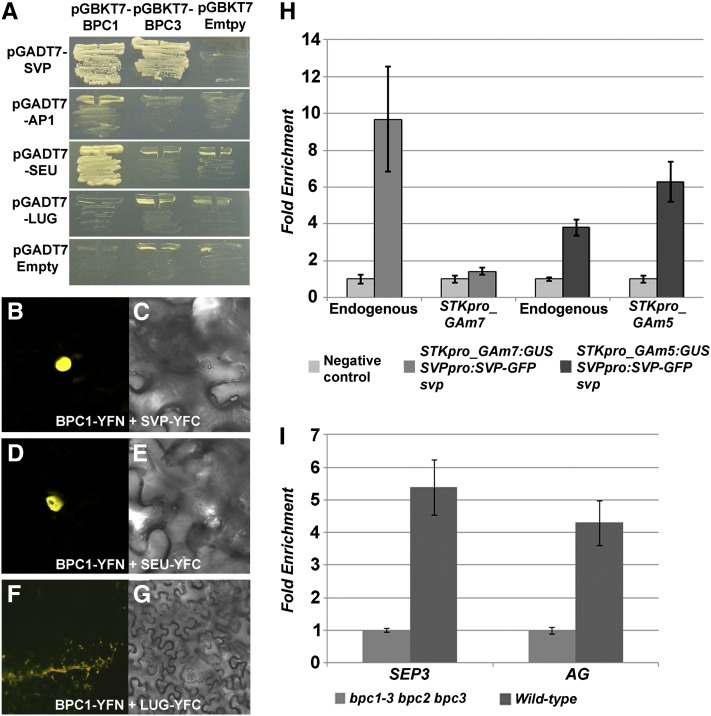
The region of the STK promoter (region B) to which SVP was shown to bind contains three CArG boxes, which are very close to C-boxes 7 and 8 (Figure 4A). This peculiar arrangement of BPC and MADS domain binding sites suggests that these factors might interact to facilitate binding to this promoter region. To investigate this further, we tested by yeast two-, three-, and four-hybrid experiments whether BPC proteins were able to interact with the AP1-SVP-SEU-LUG corepressor complex (Gregis et al., 2006). First, we tested by yeast two-hybrid assays the interactions between BPC1, BPC2, and BPC3, all cloned as BD fusions, with AP1, LUG, SEU, and SVP, all cloned as AD fusions. This analysis revealed that BPC1 strongly interacted with SEU and weakly with AP1 and SVP, whereas BPC3 interacted only weakly with SVP (Figure 5A). The strength of the interaction was tested by selecting for growth on medium without His and different concentrations of 3-amino-1,2,4triazole (3-AT). BPC2 did not interact with any of the proteins. The strength of the interactions was enhanced when three or four proteins were combined together in three- or four-hybrid assays, using SEU and LUG as bridging proteins and BPC1, BPC3, or SVP as BD or AD fusion proteins (see Supplemental Table 2 online).
Figure 5.
BPC Interaction with the AP1-SVP-SEU-LUG Repressor Complex and Recruitment of This Complex Depends on C-Boxes Present in the STK Promoter.
(A) Yeast two-hybrid assays testing interactions between AP1-AD, SVP-AD, SEU-AD, LUG-AD, and BPC1-BD, BPC3-BD on −W−L−H +5 mM of 3-AT selective media.
(B) to (G) Subcellular localization of reconstituted yellow fluorescent protein complexes in plant cells. Tobacco leaves were transiently transformed with the indicated YN and YC fusions. At left, yellow fluorescence; at right, bright field images.
(H) Real-time PCR analyses of the ChIP assays to analyze SVP binding to endogenous wild-type and mutated versions of fragment B of STKpro_GAm7:GUS and STKpro_GAm5:GUS promoters.
(I) Real-time PCR analyses of ChIP assays to analyze binding of class I BPCs to AG and SEP3 promoters using chromatin extracted from wild-type or bpc1-3 bpc2 bpc3 inflorescences.
(H) and (I) The graph represents the average of three replicates (n = 3), and the error bars show the sd.
To validate in planta the results obtained by the yeast interaction experiments, a bimolecular fluorescent complementation assay (BiFC; Walter et al., 2004) in tobacco (Nicotiana benthamiana) leaves was performed. The coding sequence of each gene was fused with a part of the yellow fluorescent protein, and then they were introduced into the cells through Agrobacterium tumefaciens–mediated transient transformation. As positive control, the SNF1 protein kinase was used (data not shown; Ferrando et al., 2001), whereas, as negative control, BPC1 and BPC3 interactions with LUG were used, because they did not show an interaction in yeast (Figures 5F and 5G). Using this system, both the interactions between BPC1 (or BPC3, data not shown) and SVP (Figures 5B and 5C) or SEU (Figures 5D and 5E) gave a clear nuclear fluorescent signal, supporting the interactions observed in yeast and the hypothesis that a multimeric complex of BPC proteins and AP1, SVP, SEU, and LUG can be formed in plants.
The protein interaction data supported the hypothesis that BPC proteins can facilitate and/or stabilize the binding of the AP1-SVP-SEU-LUG repressor complex to the CArG boxes in the STK promoter. To test this hypothesis, ChIP experiments using inflorescence tissue were performed. The svp SVPpro:SVP-GFP plants were crossed with plants containing the STKpro_GAm7:GUS construct in which all seven C-boxes are mutated. In subsequent generations, plants homozygous for the svp mutation and the two constructs were selected for ChIP experiments, and antibodies against GFP were used for these assays (Figure 5H). As a positive control, the endogenous STK promoter (region B) was used, whereas, as negative control, inflorescences of plants containing only the STKpro_GAm7:GUS construct were used. Furthermore, specific primers for the mutated C-boxes 7 and 8 were used to assay specifically binding to region B encoded by the STKpro_GAm7:GUS construct. In three out of three independent ChIP experiments, no binding of SVP to the mutated region B was observed, suggesting that BPC binding to C-boxes is necessary for binding of SVP to the CArG boxes in region B (Figure 5H).
The C-boxes and CArG boxes are very close to each other in region B (see Supplemental Figure 1 online); therefore, we could at this point not exclude the idea that the mutations in the C-boxes directly affected the affinity of SVP for the CArG binding site. To test this hypothesis, ChIP experiments were performed using inflorescences of svp mutant plants containing both the SVPpro:SVP-GFP and the STKpro_GAm5:GUS construct. In the latter construct, the C-boxes surrounding the SVP binding sites (C-boxes 7 and 8) are still mutated, and only the more distant boxes 4 and 12 are wild-type. As described above, these plants showed a low frequency of deregulation of GUS expression in stamens and carpels but no GUS expression in the floral meristem (Figures 2F and 2G). Interestingly, three independent ChIP experiments showed that SVP did bind to region B present in the STKpro_GAm5:GUS construct, confirming that the mutations that were introduced in the C-boxes close to the MADS domain binding sites did not influence the binding of SVP to this mutated STK promoter region. These experiments show clearly that C-box sequences to which BPC proteins bind are important to facilitate binding of the AP1-SVP-SEU-LUG repressor complex to the CArG boxes in the STK promoter.
BPCs Regulate Other Floral Homeotic Genes
The repressor complex SVP-AP1-SEU-LUG was shown to interact with the promoters of homeotic genes regulating floral organ identity, including the two MADS box transcription factors AGAMOUS (AG) and SEPALLATA3 (SEP3) (Gregis et al., 2009). The SVP repressor complex prevents expression of these genes at stages 1 and 2 of flower development. Analysis of the AG and SEP3 promoter regions revealed that several C-boxes are positioned within 100 bp from the CArG boxes that previously were shown to bind SVP in vivo (Gregis et al., 2009) (see Supplemental Figure 3 online). Three independent preparations of chromatin from wild-type inflorescences and bpc1-3 bpc2 bpc3 triple mutant inflorescences (used as negative control) were immunoprecipitated using antibodies against class I BPCs, confirming the binding of BPCs to these C-boxes (Figure 5I). These data indicate that the regulatory mechanism involving the BPC proteins is not restricted to STK, but rather may be a more general transcriptional regulatory mechanism common to many target genes.
STK Is Redundantly Regulated by BPC Proteins of Different Classes
To investigate whether STK regulation is dependent only on the class I BPC proteins (BPC1, BPC2, and BPC3), we introduced the STKpro_GAwt:GUS construct into the bpc1-3 bpc2 bpc3 triple mutant. This analysis revealed that in none of the single, double, or triple mutant combinations was GUS expression deregulated (see Supplemental Figure 4 online). This analysis shows that in addition to the class I BPC proteins, other BPC factors act redundantly in the regulation of STK.
DISCUSSION
The molecular mechanisms regulating the expression of homeotic floral genes are poorly understood in plants. The homeotic gene STK, which regulates ovule and seed development in Arabidopsis (Pinyopich et al., 2003; Brambilla et al., 2007), provides an excellent model for these studies, because, in addition to being a key regulator controlling seed production, its expression is precisely restricted to developing ovules (Pinyopich et al., 2003; Brambilla et al., 2007). Previously, we identified BPC1 as a direct regulator of STK, showing that it binds to the RGARAGRRA (C-box) consensus sequence, which is present in multiple copies in the STK promoter (Kooiker et al., 2005). Furthermore, in vitro single-molecule studies showed that BPC1 binding to these GA-rich elements causes DNA loop formation. These conformational changes might well be important for the regulation of STK. However, analysis of STK expression in the bpc1-1 single mutant showed that its expression was only mildly upregulated without changes in its expression profile. This is not surprising, considering that BPC1 shares sequence similarity to BPC2 and BPC3. Redundancy between these genes was further supported by the findings of Monfared et al. (2011), who showed that all the ubiquitously expressed BPC genes are likely to have overlapping functions. Furthermore, the analysis of the bpc1-3 bpc2 bpc3 triple mutant that we show here revealed that the profile of STK promoter activity in this triple mutant was the same as in wild-type plants, which further strengthens the idea that redundancy occurs between BPC genes of different classes.
To overcome the necessity of making an Arabidopsis mutant including all six bpc mutant alleles (BPC5 is considered to be a pseudogene), which based on their broad expression profile will probably also display severe phenotypic defects, we decided to mutate the consensus binding sites, which we called C-boxes, in the STK promoter to abolish BPC binding. This allowed us to study the function of BPC in the regulation of STK while preventing all kinds of pleiotropic effects caused by mutations in BPC genes. The GUS reporter lines that contain the STK promoter with the seven mutated consensus C-boxes showed strong deregulation of the GUS reporter gene throughout the flower during all stages of flower development. Promoter constructs in which only two strong BPC binding sites were mutated showed no deregulation. By contrast, plants that contained the reporter construct in which five C-boxes were mutated displayed ectopic expression in the carpel and stamens, suggesting that the number of BPC binding sites is important for correct gene expression. Our yeast interaction studies showed that BPC proteins of class I can interact with each other, and the fact that those of class II also interact between them and with those of class I (Wanke et al., 2011) further supports the idea that multiple DNA interactions combined with BPC protein–protein interactions will induce conformational changes into the STK promoter region, also corroborated by the previous reported in vitro TPM analysis (Kooiker et al., 2005).
An important outcome of our studies is that binding of BPC proteins to the STK promoter region is important for the repression of STK expression in the floral meristem. We showed that C-boxes to which BPC proteins bind are essential to facilitate binding of the SVP-AP1-SEU-LUG repressor complex to the STK promoter region. However, it is not yet completely clear how this mechanistically works. Do the conformational changes induced by BPC cause the exposure of the CArG boxes so that MADS domain proteins can bind to them, or are protein–protein interactions between BPC proteins and the repressor complex necessary to stabilize the MADS domain repressor complex on the STK promoter? Our protein interaction studies, which showed interactions between different members of the repressor complex and BPCs, strongly support the latter scenario, although we cannot exclude that BPC proteins are also important to make the CArG boxes available for MADS domain protein binding. Interesting in this respect is the analogy with the GAGA Associated Factor of Drosophila melanogaster (dGAF) (Berger and Dubreucq, 2012), which is involved in the regulation of a wide variety of processes, including the regulation of homeotic HOX genes (Botas, 1993; Graba et al., 1997; Lehmann, 2004). Although GAF proteins and BPCs do not show sequence homology similarity, they both bind to (GA)n or (CT)n sequences in promoter regions. Once dGAF binds DNA, it can both activate and repress gene transcription. This is the same for BPC proteins that are, for instance, involved in STK repression and INO activation (Meister et al., 2004). In case of activation, dGAF cooperates with the Trithorax-like group (Trx-G) complex (Poux et al., 2001), whereas, in case of repression, it forms a multimeric complex with Polycomb group (Pc-G) members (Horard et al., 2000). Apart from this, several findings suggest that the GAF should not be considered only as a factor involved in regulation of gene expression but also as a structural protein. Indeed, GAF can establish contact with subunits belonging to NURF and FACT complexes (Orphanides et al., 1998; Xiao et al., 2001; Shimojima et al., 2003), which are involved in the nucleosome spacing processes. BPCs share many similarities with GAF (Berger and Dubreucq, 2012); therefore, it is tempting to speculate that BPCs, apart from stabilizing the binding of the AP1-SVP-SEU-LUG repressor complex via protein–protein interactions on the STK promoter, also might play a role in the exposure of the CArG boxes by moving nucleosomes.
The regulation of STK expression is likely not always dependent on BPC binding to its promoter. The promoter constructs containing the mutated C-boxes showed deregulation only in floral tissues but not in vegetative tissues. This indicates that other regulatory mechanisms, independent of BPCs, are important to silence STK expression during vegetative development.
Furthermore, our analysis of the AG and SEP3 promoters showed that the function of BPCs in facilitating transcription complex binding to promoters, which we discovered using the STK promoter, probably accounts for many other genes. This is further strengthened by the following observations: (1) many genes contain C-boxes in their putative promoter regions, (2) plants having multiple bpc genes mutated show a broad range of phenotypic effects (Monfared et al., 2011), and (3) BPC genes are ubiquitously expressed in plants.
It is also important to notice that the regulatory functions of BPC factors as we describe here are likely not restricted to Arabidopsis, because BPC factors have also been identified in other plant species. In fact, this GAGA binding protein was first described in soybean (Glycine max) (GAGA Binding Protein [GBP]; Sangwan and O'Brian, 2002) and subsequently also in barley (Hordeum vulgare) (B Recombinant barley [BBR]; Santi et al., 2003) and rice (Oryza sativa) (Meister et al., 2004). Conservation of their function in different plant species is further suggested by the observation that in the promoter of the STK ortholog in rice, MADS13 (Dreni et al., 2007), C-box sequences are also present.
In conclusion, our data reveal important insights into molecular mechanisms controlling gene expression in plants. We show that a MADS domain repressor complex depends, for binding to a target promoter, on the binding of the ubiquitously expressed BPC factors to the same promoter region. These data therefore provide insight into the role of BPC factors in plant development. The fact that BPCs are functionally but not structurally related to the intensively studied dGAF factor of Drosophila is intriguing and makes BPC proteins particularly interesting for further studies.
METHODS
Plant Material and Growth Conditions
Arabidopsis thaliana ecotype Columbia was used in this study; the plants were directly sown on soil and kept under short-day conditions for 2 weeks (22°C, 8 h light and 16 h dark) and then moved to long-day conditions (22°C, 16 h light and 8 h dark). The agl24 svp ap1-12 triple mutant and the SVPpro:SVP-GFP line were previously described by Gregis et al. (2008) and Gregis et al. (2009), respectively. Genotyping of the bpc1-3 bpc2 bpc3 triple mutant was done according to Monfared et al. (2011).
STK Promoter Constructs and Plant Transformation
Each mutated version of the STK promoter (pSTK_GAm2, pSTK_GAm5, and pSTK_GAm7) was obtained by assembling together three, six, and eight fragments, respectively, which start and terminate with the appropriate mutated GAGA box replacing two purines (highlighted by the small font) by two pyrimidine residues: Box1, AGAAagAAA; Box4, AGAAAgaAAGAgaAGAGA; Box7, AGAAAgaAAGAgaAAGAAA; Box8, TCTCTTtcCTTTCT; Box9, TTCctCTCT; Box10, TCTctCTCT; and Box12, TTTCTCTctCC (for primers used, see Supplemental Table 3 online). All versions were recombined into the pDONOR207 vector (Life Technologies) and then recombined in the pBGWFS7 vector (Life Technologies), which already contains the GUS sequence. Arabidopsis was transformed with these constructs using the Agrobacterium tumefaciens–mediated floral dip method (Clough and Bent 1998).
GUS Staining and in Situ Hybridization
GUS assays were performed as described previously by Liljegren et al. (2000). The samples were mounted in 5% glycerol and subsequently observed using a Zeiss Axiophot D1 microscope equipped with differential interference contrast optics. Images were captured on an Axiocam MRc5 camera (Zeiss) using the Axiovision program (version 4.1).
In situ hybridization experiments were performed as described previously by Dreni et al. (2011) using as probe STK antisense RNA, which corresponds to nucleotides 455 to 818 (Brambilla et al., 2007).
ChIP Assay and Immunoblot Assay
ChIP and real-time PCR were performed as described by Gregis et al. (2009) using for SVP-GFP the commercial antibodies GFP:Living Colors_full-length (Clontech). The ChIP-sequencing experiment was performed using the same SVP-GFP line according to the protocol published by Kaufmann et al. (2009). The primers that were used for this analysis are reported in Supplemental Table 3 online. For BPCs, a polyclonal antibody raised against the full-length purified GST-tagged BPC1 was used (BPC1 coding sequence was cloned through the restriction sites BamHI/SalI into the pGEX-4T-1 vector). The antibody was tested on crude Escherichia coli protein extracts after induction of BPC1-GST, BPC2-GST, BPC3-GST, and BPC4 (BPC1, BPC2, and BPC3 were cloned as BamHI/SalI fragments into the pGEX-4T-1 vector, whereas pLYS-BPC4 was kindly provided by Charles Gasser). The antibody was used with a final concentration of 1:500 for immunoblot analysis and nondiluted for the ChIP assays (12 μL of antibody per 40 μL of Dynabeads protein A [Invitrogen]).
Yeast Two-, Three-, and Four-Hybrid Assays
The two- and three-hybrid assays were performed at 28°C in the yeast strain AH109 (Clontech), whereas the four-hybrid assay was performed at 28°C in strain PJ64-4A (Clontech) using the cotransformation technique (Egea-Cortines et al., 1999). The coding sequences of BPC1, BPC2, BPC3, SVP, SEU, LUG, and AP1 were cloned in the Gateway vector GAL4 system (pGADT7 and pGBKT7; Clontech) or pTFT1 Gateway and pRED Gateway (kindly provided by Richard Immink) passing through pDONOR207 (Life Technologies). Yeast two-and three-hybrid assays were tested on selective yeast synthetic dropout medium lacking Leu, Trp, adenine, and His supplemented with different concentrations of 3-aminotriazole (1, 2.5, and 5 mM of 3-AT). Four-hybrid interactions were assayed on selective yeast synthetic dropout medium lacking Leu, Trp, adenine, uracil, and His supplemented with different concentrations of 3-AT (1, 2.5, and 5 mM).
BiFC Assay
The BPC1, BPC2, BPC3, SVP, SEU, LUG, and AP1 coding sequences were first cloned into pDONR207 (Life Technologies) and subsequently by Gateway recombination transferred to the pYFPN43 and pYFPC43 vectors. SNF1 protein kinase in the same vectors was used as positive control (Ferrando et al., 2001). BiFC assays were performed in triplicate injecting Agrobacterium expressing viral suppressor p19/experimental constructs. The abaxial surfaces of infiltrated tobacco (Nicotiana benthamiana) leaves were imaged 3 d after inoculation.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: STK (At4G09960), BPC1 (AT2G01930), BPC2 (AT1G14685), BPC3 (AT1G68120), AGL24 (AT4G24540), SVP (AT2G22540), AP1 (AT1G69120), AG (AT4G18960), SEP3 (AT1G24260).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Position of C-, NC-, and CArG Boxes in the STK Promoter Region.
Supplemental Figure 2. BPC Proteins of Class I Are Specifically Recognized by a Polyclonal Antibody Serum Raised against BPC1.
Supplemental Figure 3. Position of C-Boxes and CArG Boxes in the AG and SEP3 Promoters.
Supplemental Figure 4. STK Expression Profile in Single, Double, and Triple Class I bpc Mutants.
Supplemental Table 1. GUS Activity in the STKpro:GAwt, STKpro:GAm2, STKpro:GAm5, and STKpro:GAm7 Lines.
Supplemental Table 2. Yeast Two-, Three-, and Four-Hybrid Assays to Test Interactions between BPC1, BPC2, and BPC3 and SVP, AP1, SEU, and LUG.
Supplemental Table 3. Primers Used in This Study.
Supplementary Material
Acknowledgments
We thank Charles Gasser for providing the bpc1-3 bpc2 bpc3 triple mutant and for the pLYS-BPC4 construct, Richard Immink for the pRED and pTFT gateway vectors, Frederico Valverde for supervision with BiFC assay, Alejandro Ferrando for BiFC positive control constructs, and Maarten Kooiker for BPC antibody production. This research was funded by the project FLOWERPOWER (AGRO-11 e Rif.n° 16976) from the Lombardy region and Fondo per gli Investimenti della Ricerca di Base–Futuro in Ricerca (RBFR08UG7J 001). S.S. and B.C. were funded by a PhD fellowship, and V.G. was funded by a postdoctoral fellowship from the Università degli Studi di Milano.
AUTHOR CONTRIBUTIONS
S.S., I.R.V., V.G., and B.C. performed the experiments, L.C. and M.K. designed the research strategy, and S.S. and M.K. did most of the writing.
Glossary
- C
consensus
- NC
nonconsensus
- ChIP
chromatin immunoprecipitation
- EMSA
electrophoretic mobility shift assay
- GUS
β-glucuronidase
- TPM
tethered particle motion
- AD
activation domain
- BD
binding domain
- GFP
green fluorescent protein
- 3-AT
3-amino-1,2,4-triazole
- BiFC
bimolecular fluorescent complementation assay
References
- Berger N., Dubreucq B. (2012). Evolution goes GAGA: GAGA binding proteins across kingdoms. Biochim. Biophys. Acta 1819: 863–868 [DOI] [PubMed] [Google Scholar]
- Botas J. (1993). Control of morphogenesis and differentiation by HOM/Hox genes. Curr. Opin. Cell Biol. 5: 1015–1022 [DOI] [PubMed] [Google Scholar]
- Bowman J.L., Drews G.N., Meyerowitz E.M. (1991). Expression of the Arabidopsis floral homeotic gene AGAMOUS is restricted to specific cell types late in flower development. Plant Cell 3: 749–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla V., Battaglia R., Colombo M., Masiero S., Bencivenga S., Kater M.M., Colombo L. (2007). Genetic and molecular interactions between BELL1 and MADS box factors support ovule development in Arabidopsis. Plant Cell 19: 2544–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Dreni L., Jacchia S., Fornara F., Fornari M., Ouwerkerk P.B., An G., Colombo L., Kater M.M. (2007). The D-lineage MADS-box gene OsMADS13 controls ovule identity in rice. Plant J. 52: 690–699 [DOI] [PubMed] [Google Scholar]
- Dreni L., Pilatone A., Yun D., Erreni S., Pajoro A., Caporali E., Zhang D., Kater M.M. (2011). Functional analysis of all AGAMOUS subfamily members in rice reveals their roles in reproductive organ identity determination and meristem determinacy. Plant Cell 23: 2850–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea-Cortines M., Saedler H., Sommer H. (1999). Ternary complex formation between the MADS-box proteins SQUAMOSA, DEFICIENS and GLOBOSA is involved in the control of floral architecture in Antirrhinum majus. EMBO J. 18: 5370–5379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando A., Koncz-Kálmán Z., Farràs R., Tiburcio A., Schell J., Koncz C. (2001). Detection of in vivo protein interactions between Snf1-related kinase subunits with intron-tagged epitope-labelling in plants cells. Nucleic Acids Res. 29: 3685–3693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi L., Dunlap D. (2003). Single-molecule studies of DNA architectural changes induced by regulatory proteins. Methods Enzymol. 370: 369–378 [DOI] [PubMed] [Google Scholar]
- Graba Y., Aragnol D., Pradel J. (1997). Drosophila Hox complex downstream targets and the function of homeotic genes. Bioessays 19: 379–388 [DOI] [PubMed] [Google Scholar]
- Grandi V., Gregis V., Kater M.M. (2011). Uncovering genetic and molecular interactions among floral meristem identity genes in Arabidopsis thaliana. Plant J. 69: 881–893 [DOI] [PubMed] [Google Scholar]
- Gregis V., Sessa A., Colombo L., Kater M.M. (2006). AGL24, SHORT VEGETATIVE PHASE, and APETALA1 redundantly control AGAMOUS during early stages of flower development in Arabidopsis. Plant Cell 18: 1373–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregis V., Sessa A., Colombo L., Kater M.M. (2008). AGAMOUS-LIKE24 and SHORT VEGETATIVE PHASE determine floral meristem identity in Arabidopsis. Plant J. 56: 891–902 [DOI] [PubMed] [Google Scholar]
- Gregis V., Sessa A., Dorca-Fornell C., Kater M.M. (2009). The Arabidopsis floral meristem identity genes AP1, AGL24 and SVP directly repress class B and C floral homeotic genes. Plant J. 60: 626–637 [DOI] [PubMed] [Google Scholar]
- Hartmann U., Höhmann S., Nettesheim K., Wisman E., Saedler H., Huijser P. (2000). Molecular cloning of SVP: A negative regulator of the floral transition in Arabidopsis. Plant J. 21: 351–360 [DOI] [PubMed] [Google Scholar]
- Horard B., Tatout C., Poux S., Pirrotta V. (2000). Structure of a polycomb response element and in vitro binding of polycomb group complexes containing GAGA factor. Mol. Cell. Biol. 20: 3187–3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann K., Muiño J.M., Jauregui R., Airoldi C.A., Smaczniak C., Krajewski P., Angenent G.C. (2009). Target genes of the MADS transcription factor SEPALLATA3: Integration of developmental and hormonal pathways in the Arabidopsis flower. PLoS Biol. 7: e1000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooiker M., Airoldi C.A., Losa A., Manzotti P.S., Finzi L., Kater M.M., Colombo L. (2005). BASIC PENTACYSTEINE1, a GA binding protein that induces conformational changes in the regulatory region of the homeotic Arabidopsis gene SEEDSTICK. Plant Cell 17: 722–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M. (2004). Anything else but GAGA: A nonhistone protein complex reshapes chromatin structure. Trends Genet. 20: 15–22 [DOI] [PubMed] [Google Scholar]
- Liljegren S.J., Ditta G.S., Eshed Y., Savidge B., Bowman J.L., Yanofsky M.F. (2000). SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 404: 766–770 [DOI] [PubMed] [Google Scholar]
- Meister R.J., Williams L.A., Monfared M.M., Gallagher T.L., Kraft E.A., Nelson C.G., Gasser C.S. (2004). Definition and interactions of a positive regulatory element of the Arabidopsis INNER NO OUTER promoter. Plant J. 37: 426–438 [DOI] [PubMed] [Google Scholar]
- Monfared M.M., Simon M.K., Meister R.J., Roig-Villanova I., Kooiker M., Colombo L., Fletcher J.C., Gasser C.S. (2011). Overlapping and antagonistic activities of BASIC PENTACYSTEINE genes affect a range of developmental processes in Arabidopsis. Plant J. 66: 1020–1031 [DOI] [PubMed] [Google Scholar]
- Orphanides G., LeRoy G., Chang C.H., Luse D.S., Reinberg D. (1998). FACT, a factor that facilitates transcript elongation through nucleosomes. Cell 92: 105–116 [DOI] [PubMed] [Google Scholar]
- Pinyopich A., Ditta G.S., Savidge B., Liljegren S.J., Baumann E., Wisman E., Yanofsky M.F. (2003). Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature 424: 85–88 [DOI] [PubMed] [Google Scholar]
- Poux S., Melfi R., Pirrotta V. (2001). Establishment of Polycomb silencing requires a transient interaction between PC and ESC. Genes Dev. 15: 2509–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounsley S.D., Ditta G.S., Yanofsky M.F. (1995). Diverse roles for MADS box genes in Arabidopsis development. Plant Cell 7: 1259–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan I., O’Brian M.R. (2002). Identification of a soybean protein that interacts with GAGA element dinucleotide repeat DNA. Plant Physiol. 129: 1788–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi L., Wang Y., Stile M.R., Berendzen K., Wanke D., Roig C., Pozzi C., Müller K., Müller J., Rohde W., Salamini F. (2003). The GA octodinucleotide repeat binding factor BBR participates in the transcriptional regulation of the homeobox gene Bkn3. Plant J. 34: 813–826 [DOI] [PubMed] [Google Scholar]
- Shimojima T., Okada M., Nakayama T., Ueda H., Okawa K., Iwamatsu A., Handa H., Hirose S. (2003). Drosophila FACT contributes to Hox gene expression through physical and functional interactions with GAGA factor. Genes Dev. 17: 1605–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M., Chaban C., Schütze K., Batistic O., Weckermann K., Näke C., Blazevic D., Grefen C., Schumacher K., Oecking C., Harter K., Kudla J. (2004). Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40: 428–438 [DOI] [PubMed] [Google Scholar]
- Wanke D., Hohenstatt M.L., Dynowski M., Bloss U., Hecker A., Elgass K., Hummel S., Hahn A., Caesar K., Schleifenbaum F., Harter K., Berendzen K.W. (2011). Alanine zipper-like coiled-coil domains are necessary for homotypic dimerization of plant GAGA-factors in the nucleus and nucleolus. PLoS ONE 6: e16070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H., Sandaltzopoulos R., Wang H.M., Hamiche A., Ranallo R., Lee K.M., Fu D., Wu C. (2001). Dual functions of largest NURF subunit NURF301 in nucleosome sliding and transcription factor interactions. Mol. Cell 8: 531–543 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.