Abstract
Objective:
The current study examined the relationship between severity of alcohol use disorders (AUDs) and the neural circuits that underlie response inhibition and error monitoring. In addition, we explored pre- and post-inhibition trial processes to determine the potential causal mechanisms responsible for disinhibition in AUDs.
Method:
One hundred sixty-four individuals with a range of drinking from non-treatment-seeking adults with problematic alcohol use to treatment-seeking adults with alcohol dependence completed a Go/NoGo task while undergoing functional magnetic resonance imaging.
Results:
Correlations between signal change during response inhibition and a composite measure of AUD severity revealed significant negative relationships in right insula/inferior frontal gyrus, pregenual anterior cingulate cortex, and inferior parietal lobe. Relationships with error monitoring-related response largely overlapped with that of correct inhibitions but also included rostral anterior cingulate cortex and left inferior frontal gyrus, such that more severe AUDs were associated with reduced response in these regions. Last, examination of pre- and postinhibition processes suggested that more severe AUDs are associated with greater engagement of motor response circuits before inhibition trials, suggesting greater pre-potent tendencies that may lead to disinhibition.
Conclusions:
The current results extend previous work by examining how variation in AUD severity is related to neural response during response inhibition and potential causal mechanisms responsible for impaired inhibitory control. More severe AUDs were associated with reduced engagement of neural circuits involved in behavioral control and enhanced pre-potent responding. This altered control may contribute to the progression of AUDs, as well as relapse after treatment.
Response inhibition, or the ability to withhold a Rpre-potent response, is one of the primary executive functions (Miyake et al., 2000) affected in individuals with alcohol and other substance use problems (Nigg et al., 2006). Prospective studies have revealed that poor behavioral inhibition during early adolescence predicts subsequent alcohol and substance use problems (Nigg et al., 2006; Wong et al., 2006), suggesting that impaired response inhibition may contribute to the development of alcohol use disorders (AUDs). Individuals with AUDs commit more errors on inhibition trials (Bjork et al., 2004; Goudriaan et al., 2005) and have longer stop signal reaction times (Lawrence et al., 2009).
To date, relatively few studies have specifically investigated the neural mechanisms underlying response inhibition and error monitoring problems in participants with alcohol dependence. Early studies using event-related potentials (ERPs) reported that alcohol-dependent (AD) subjects demonstrated reduced amplitudes during both Go trials and NoGo trials, suggesting reduced neural activity throughout the task (Rodriguez Holguin et al., 1999). Similarly, AD participants showed a smaller amplitude P300 response during Go trials and NoGo trials than did controls, and the difference between Go trials and NoGo trials is also reduced compared with healthy controls, further suggesting that activation of both responses and control over responses is impaired in AD patients (Kamarajan et al., 2005).
In addition to previous research using ERPs, recent studies have begun to use functional magnetic resonance imaging (fMRI) to investigate inhibition in AUDs. Comparison of AUD patients and controls during a stop signal task suggested reduced response in AUD patients in left dorsolateral prefrontal cortex (DLPFC) during successful inhibition trials and right DLPFC during post-error trials compared with controls (Li et al., 2009). In addition, AUD patients showed increased response in lateral and medial frontal cortex during errors compared with controls. Further, adolescents at increased risk for alcohol dependence (i.e., family history of alcoholism) show less activation of left DLPFC during inhibition compared with individuals who have no family history of alcoholism, despite similar behavioral performance (Schweinsburg et al., 2004). Last, among heavy drinkers, carriers of the variable tandem number repeat in DRD4 affected response in cingulate gyrus and precuneus during response inhibition, and a polymorphism in DRD2 influenced neural response in inferior frontal gyrus (IFG) during response inhibition (Filbey et al., 2011).
Although response inhibition is often the construct of interest in tasks such as the Go/NoGo and stop signal, error monitoring is also a key construct that contributes to behavioral control. Error monitoring is the ability to correctly assess whether a response for a given trial was consistent with task goals. Although most behavioral studies have not examined performance monitoring prospectively, there is some evidence that error monitoring is altered in AUDs. For example, AD individuals respond faster to Go trials after making an inhibition error, whereas control subjects show the expected post-error slowing of responses after making an error (Lawrence et al., 2009). In addition, one study of the event-related negativity (ERN) that is prominent during the experience of errors found that AD patients with comorbid anxiety had greater ERN amplitudes than controls, suggesting anxiety may play a moderating role on neural responses to errors (Schellekens et al., 2010). This finding mirrored an earlier combined ERP-fMRI study that found similar increased responses (blood oxygen level dependent [BOLD] and ERP amplitude) in AD individuals high in anxiety (Karch et al., 2008).
Two processes that may contribute to enhanced errors in withholding responses include inattention/increased prepotency and an inability to adjust behavior after errors are committed. Several studies in recent years have focused on the neural mechanisms of inattention, or the moments before critical trials in which lapses in attention occur and lead to increased error rates (Weissman et al., 2006). Examinations of the neural activity preceding errors in inhibition suggest increased activity within default mode networks, suggesting less focus on task relevant goals (Eichele et al., 2008). A related but potentially different explanation of increased errors is increased pre-potency of the dominant response. For example, participants who show relatively faster response times (RTs) before an inhibition trial may actually prime the motor system, thus making any withholding attempts even more difficult. An alternative process that may contribute to disinhibition in individuals with AUDs may be related to post-error processing, or the ability to make adjustments to behavior after the experience of an error (Botvinick et al., 2001). Recent studies of the stop signal task have shown some evidence of reduced post-error slowing in individuals with alcohol dependence (Lawrence et al., 2009; Li et al., 2009), suggesting a deficit in dynamic adjustments in behavioral control.
Although a handful of studies have examined differences in response inhibition and error-monitoring mechanisms between controls and individuals with alcohol dependence, few studies have examined the relative impairment of these processes as a function of AUD severity. Thus, it is unknown whether those individuals with more severe alcohol problems show greater levels of executive dysfunction. Further, the cognitive and neural mechanisms by which behavioral control problems become worse over one’s drinking history are unclear, particularly with respect to whether disinhibitory problems are attributable to lack of attention or an inability to adjust one’s behavior after errors.
Thus, to address this important gap in knowledge, we examined response inhibition and error monitoring in a sample of individuals with a range of AUDs (problem drinking through alcohol dependence) to determine the degree to which AUD severity relates to executive dysfunction. We hypothesized that greater AUD severity would be associated with increased evidence of behavioral disinhibition. In addition, we hypothesized that, during response inhibition, individuals with more severe AUDs would engage regions within key behavioral control neural networks—including, for example, the right IFG, anterior cingulate cortex (ACC), subthalamic nucleus, and DLPFC—to a lesser degree than would those with less severe AUDs. We also hypothesized that AUD severity would be negatively correlated with response in anterior insula and rostral ACC during the experience of errors. Last, we expected that AUD severity would be related to greater deficits in post-error slowing, as indicated by previous research (Lawrence et al., 2009; Li et al. 2009).
Method
Participants
One hundred sixty-four individuals from a larger study on AUDs (Claus et al., 2011) participated in the present study. Demographic and drinking characteristics are presented in Table 1. Subjects were recruited into two separate studies from the greater Albuquerque metropolitan area through flyers, local newspaper advertisements, and Craigslist. The two studies recruited individuals across a range of AUDs from non-treatment-seeking adults with problematic alcohol use to treatment-seeking AD patients. The current study focused on the pre-treatment baseline assessment. To be invited for baseline assessment session, participants had to be between the ages of 21 and 55 years and report a recent history of drinking (three or more drinks per drinking episode for women, four or more for men) at least twice a week over the previous month, with no history of head injury or any neurological problems, no active or previous history of psychosis, no contraindications for participating in an MRI study, and no illicit drug use over the past month (excluding marijuana); also, female subjects could not be pregnant. Once enrolled, participants completed several questionnaires during a baseline assessment session. These included a demographics questionnaire, the Impaired Control Scale (ICS; Heather et al., 1993), a questionnaire that assessed age at first drink and years of regular drinking (Hutchison et al., 2002), the Alcohol Use Disorders Identification Test (AUDIT; Babor et al., 1992), the Alcohol Dependence Scale (Skinner and Horn, 1984), the alcohol abuse and dependence modules of the Structured Clinical Interview for the DSM-IV (SCID; First et al., 2002), the Beck Anxiety Inventory (BAI; Beck and Steer, 1990), and the Timeline Followback (Sobell and Sobell, 1992). Subjects were paid $125 for completing both the baseline and neuroimaging sessions. The study was approved by the University of New Mexico Health Science Center Human Research Review Committee.
Table 1.
Sample characteristics for participants included in and excluded from analyses
| Subjects included in analysis |
Excluded subjects |
|||
| Variable | M (SEM) | Range | M (SEM) | Range |
| n | 144 | – | 20 | – |
| ICS | 47.28(1.65) | 4–96 | 48.6 (3.97) | 17–83 |
| Yrs. drink | 14.17(0.83) | 1–39 | 15.3 (2.62) | 2–39 |
| AUDIT | 19.61 (0.65) | 7–38 | 19.1 (1.61) | 10–36 |
| ADS | 13.76(0.67) | 2–43 | 12.65(1.62) | 4–28 |
| Age | 32.64 (0.8) | 21–56 | 35.35 (2.8) | 21–55 |
| Prop. AA | 0.79 | – | 0.9 | – |
| Prop. AD | 0.61 | – | 0.65 | – |
| Prop. no AUD | 0.13 | – | 0.05 | – |
| Go pct. correct | 0.94 (0.01) | 0.71–1 | 0.65 (0.03) | 0.38–0.91 |
| NoGo pct. correct | 0.41 (0.01) | 0.075–0.825 | 0.4 (0.06) | 0.075–0.925 |
| d′ | 1.44(0.06) | 0.08–4.33 | 0.13 (0.09) | -0.46–1.29 |
| GoRT | 334.57 (4.9) | 135.11–457.89 | 276.36(21.53) | 114.24–480.47 |
| NoGo incorrect RT | 316.4(4.84) | 110.74–446.65 | 248.75 (20.94) | 106.64–451.46 |
Notes: ICS = Impaired Control Scale; yrs. drink = self-reported years of regular drinking; AUDIT = Alcohol Use Disorders Identification Test; ADS = Alcohol Dependence Scale; prop. AA = proportion of participants meeting criteria for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), alcohol abuse; prop. AD = proportion of participants meeting criteria for DSM-IV alcohol dependence; prop. no AUD = proportion of participants not meeting criteria for an alcohol use disorder according to DSM-IV criteria; Go pct. correct = percentage of correct responses Go trials; NoGo pct. correct = percentage of correct withholding of responses during NoGo trials; d′ = response sensitivity; Go RT = mean response time for correct Go trials; NoGo incorrect RT = mean response time for incorrect NoGo trials
Procedures
Participants were asked to abstain from alcohol the night before their baseline assessment session and scan session. Before completing their informed consent on their assessment day, all participants received breath alcohol analysis to ensure a breath alcohol concentration 0 mg/dl. In addition, all participants completed a urine drug screen, and female participants completed a pregnancy test. Subjects were placed in the scanner and a piece of tape was placed across the forehead to serve as feedback for movement reduction. The imaging session commenced and consisted of a 1-minute localizer, 8-second echo planar imaging (EPI) frequency adjustment, a 6-minute high-resolution T1-weighted magnetization prepared rapid acquisition gradient echo (MP-RAGE), two 6-minute EPI scans during the Go/NoGo task, and eight additional EPI scans lasting an additional 75 minutes. All scans were acquired with an eight-channel head coil, and EPI scans were acquired parallel to the ventral surface of a participant’s orbitofrontal cortex to reduce signal dropout and distortion in this region (Deichmann et al., 2003). Each volume acquired consisted of 33 axial slices (64 × 64 matrix, 3.75 × 3.75 mm2, 3.5 mm thickness, 1 mm gap). The high-resolution T1-weighted MP-RAGE anatomical image was also acquired (repetition time [TR] = 2,530 milleseconds [msec], echo time [TE] = 1.64 msec, flip angle = 7°, 192 sagittal slices, 256 × 256 matrix, slice thickness = 1 mm, no gap) for functional image registration.
The Go/NoGo task was based on a version of the task presented by Kaufman and colleagues (2003). Subjects viewed an alternating 1 Hz sequence of X’s and Y’s and responded with a button press on every trial in which the current stimulus differed from the previous stimulus (Go trials). However, if the letter was repeated (e.g., X preceded by an X) from the previous trial, subjects were instructed to withhold their response (NoGo trials). There were 312 trials in each of two runs, with 20 NoGo trials presented per run in a pseudorandom order to ensure that an equal number of NoGo trials occurred in each half of the 2-second TR. Each letter was presented for 700 ms and was followed by a 300 ms fixation cross. Each 2-second functional scan comprised two trials, with the position order of NoGo trials counterbalanced within a functional run (Kaufman et al., 2003). All stimuli were presented with E-Prime (PST; Pittsburgh) through a back-projected mirror system. Participants completed two runs of the task; each run was 5 minutes and 32 seconds in length.
Statistical analyses of demographics and behavior
Means and standard deviations were computed for continuous demographic variables, and counts were computed for all categorical variables. Rather than using a single severity scale for correlation analyses, we used a composite approach to determine severity, similar to our previous work (Liu et al., 2011). To compute a composite score of AUD severity, a principal component analysis was conducted on the following variables: AUDIT, Alcohol Dependence Scale, ICS, years of regular drinking, and average number of drinks per drinking day. The principal component analysis was conducted using the prcomp routine within the statistical package R (R Development Core Team, 2009).
For the Go/NoGo task, trials were binned according to trial type and response. Thus, Go trials were categorized as Correct if participants made a button press and Incorrect if participants failed to respond. NoGo trials were Correct if no button press was detected and Incorrect if participants responded with a button press. The mean reaction time for correct Go trials and incorrect NoGo trials was computed for each participant. In addition, proportion of correct Go trials and correct NoGo trials was computed. To compute d′, a measure of response sensitivity, hit rates [number of Go correct / (number of Go correct + number of Go incorrect)] and false alarms [NoGo incorrect / (NoGo incorrect + NoGo correct)] were determined and the difference of the z scores associated with each value was calculated [z (hit rate) − z (false alarm)]. Correlations between the AUD severity measure and the performance measures from the Go/NoGo task were determined using R (R Development Core Team, 2009).
In addition to standard RT analyses, we examined RTs of Go trials that occurred before and after NoGo trials to analyze pre- and post-trial processes that differ as a function of AUD severity. For each NoGo trial, the average RT of the two preceding Go trials and two Go trials following the NoGo trial was computed for correct and incorrect NoGo trials and averaged within each participant. The differences between the two preceding trial types (correct − incorrect NoGo) and two trial types following NoGo trials (correct − incorrect NoGo) were entered into a correlation with AUD severity.
Image analysis
All image analyses were completed using Functional Magnetic Resonance Imaging of the Brain (FMRIB)’s Software Library (FSL) Version 4.1.0 (Smith et al., 2004). The first three volumes of each functional run were discarded to allow the magnet to reach steady state. FSL’s Motion Correction using FMRIBs Linear Image Registration Tool (Jenkinson et al., 2002) was used to realign functional images within a given run to the middle volume within the run. Images were deskulled using FSL’s Brain Extraction Tool (Smith, 2002), spatially smoothed with a 5 mm full-width half-max Gaussian kernel, temporally filtered using a high-pass filter of 50 sec, pre-whitened using FMRIB’s Improved Linear Model (FILM), and grand mean intensity normalized; all of these steps were performed using FMRIB’s Expert Analysis Tool (FEAT) (Smith et al., 2004). In addition, motion parameters for each participant were entered as co-variates in the statistical model. The MP-RAGE anatomical image was deskulled with FSL’s Brain Extraction Tool and used for registration of the participant’s brain to the Montreal Neurological Institute (MNI) 152 template brain.
All analyses were run as part of a three-stage process. In the first stage, customized regressors were created for each participant for three conditions of interest within each functional run—Incorrect Go, Correct NoGo, Incorrect NoGo— and convolved with a double gamma hemodynamic response function. Note that Correct Go trials served as the baseline condition to which each regressor was compared (Kaufman et al., 2003). Statistical analyses were performed using the general linear model as implemented in FEAT. Time series analyses were conducted using FILM (Woolrich et al., 2004) with local autocorrelation estimation. This first-level analysis generated parameter estimates for each condition of interest. Because we were interested in the neural responses during successful withholding of responses, we examined the Correct NoGo regressor, and to examine unsuccessful inhibition trials (errors) monitoring, we examined the Incorrect NoGo regressor. In addition, to control for the novelty response associated with NoGo trials, we compared the Correct and Incorrect NoGo regressors to examine an additional measure of response inhibition and error monitoring.
In addition to analyses of correct and incorrect response inhibition trials, we examined neural processes that preceded and followed correct and incorrect inhibition trials to investigate potential mechanisms that may lead to disinhibition. For pre-trial processing, we created explanatory variables that represented the 2 seconds before the onset of the inhibition trial, and separated the pre-trial events according to whether the inhibition trial was correct or incorrect. In the first-level analysis, these two additional regressors (pre-trial correct, pre-trial incorrect) were included with the regressors from the original model described above (correct NoGo, incorrect NoGo, and incorrect Go). It is important to note that the pre-trial regressors were orthogonalized with respect to the inhibition trials to reduce any shared variance in BOLD signal between the subsequent trials. The primary contrast of interest for the pre-trial analysis was the comparison of parameter estimates for the trials preceding correct inhibitions compared with those trials preceding incorrect inhibitions. The post-trial analysis was identical to the pre-trial analysis except that regressors were created for the 2 seconds following the correct/incorrect inhibition trials.
Contrast maps were then registered to the participant’s high-resolution anatomical image and the MNI 152 brain template using FMRIBs Linear Image Registration Tool (Jenkinson et al., 2002). Next, analyses from each run within a participant were combined using a fixed effects model in FEAT. This second-level analysis generated mean effects and within-subject variance estimates that were used in the third-level analysis. At the third level, we used FMRIB’s Local Analysis of Mixed Effects Stage 1 to analyze group differences in our contrasts of interest. All group-level maps were examined using a voxel size of 2 × 2 × 2 mm3. All main effects maps were thresholded using cluster-based thresholding based on Gaussian random field theory (cluster corrected p < .05) (Worsley et al., 1996). To determine the relationship between AUD severity and BOLD responses, a regression analysis was conducted with AUD severity scores entered as the primary predictor variable.
Correlational analyses within task relevant regions
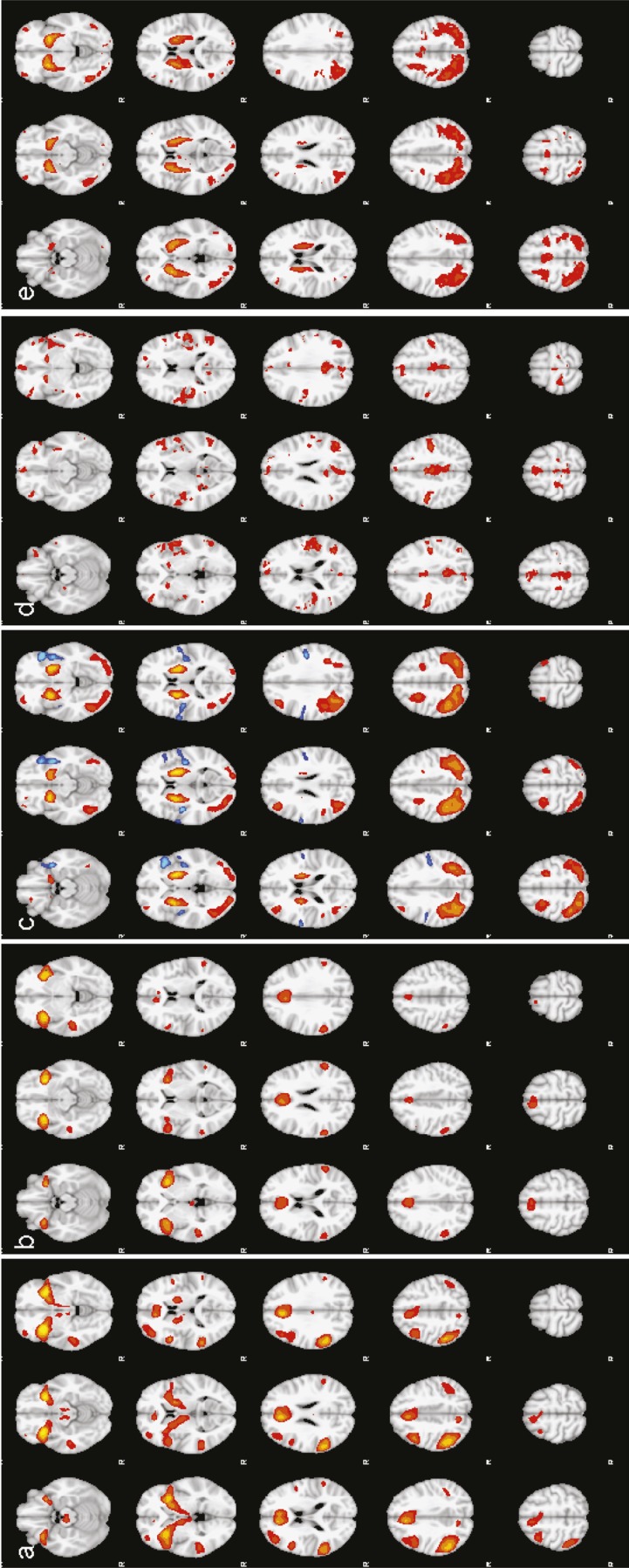
To examine the relationship between AUD severity and neural responses during response inhibition and error monitoring, we looked at the correlation between the AUD severity component score and BOLD response in brain regions that showed significant effects in the main effects analysis comparing correct response inhibition trials to correct go trials (thresholded at z > 10, cluster level p < .05), and incorrect inhibition trials to correct go trials (Figure 1).
Figure 1.
Group effects of contrasts in the Go/NoGo task, (a) NoGo correct > Go correct (z > 10, p < .05). (b) NoGo correct > Go correct (z > 10, p < .05). (c) NoGo correct > NoGo Incorrect (yellow/orange; z > 5, p < .05) and NoGo Incorrect > NoGo correct (blue; z > 3.09, p < .05). (d) Go trials before Error > Go trials before Correct Inhibition (z > 4.26, p < .05). (e) Go trials after Correct Inhibitions > Go trials after Errors (z > 7, p < .05). All images are in radiological convention.
In addition to correlations between alcohol use severity and inhibition-related response, we conducted each analysis controlling for total scores on the BAI (Beck and Steer, 1990). Because previous studies have shown evidence of the influence of trait-level anxiety on neural responses during response inhibition tasks (Karch et al., 2008; Schellekens et al., 2010), we included a proxy trait anxiety score that was measured with the BAI to determine whether the relationship between AUD severity and BOLD response was attenuated when including comorbid anxiety.
Results
Subject characteristics
Descriptive data for participants are described in Table 1. Overall, participants in the study had a wide range of AUD severity. Of note, there were no significant differences on alcohol-related variables between individuals excluded from analyses and those included in the final sample. One factor was significant in the principal component analysis, accounting for 79.3% of the variance in the drinking scores. The loadings for each measure on each component are presented in Table 2.
Table 2.
Standardized weightings of alcohol measures on alcohol use disorders severity component
| Measure | Loading weight |
| ICS | .876 |
| AUDIT | .299 |
| ADS | .277 |
| Avg. drinks/drinking day | .092 |
| Years of regular use | .240 |
Notes: ICS = Impaired Control Scale; AUDIT = Alcohol Use Disorders Identification Test; ADS = Alcohol Dependence Scale; avg. = average.
Go/NoGo performance measures
Summary statistics for all performance data are presented in Table 1. Twenty participants were dropped from subsequent analyses because of either poor performance on Go Correct trials (13 participants with <70% Correct Go) or poor response sensitivity as measured by d′ (7 participants with d′ < 0), leaving a final sample of 144 participants. To test the hypothesis that severity of problematic drinking was associated with increased errors and increased reaction times, we used correlation analyses between performance measures and alcohol use severity. Correlations between the AUD severity component and Go/NoGo performance measures (Go Correct RT, NoGo Incorrect RT, Go % Correct, NoGo % Correct, and d′) revealed no significant correlations (all rs <.15, p >.08).
Examination of pre- and post-NoGo performance revealed significant differences in RTs between Go trials that preceded NoGo trials, t(142) = 14.96, p <.001, and followed NoGo trials, t(142) = 2.68, p = .008. RTs for Go trials that preceded NoGo Incorrect trials (M =322 msec) were significantly faster than those that preceded correct inhibition trials (M = 378 msec). In contrast, RTs on Go trials after making an error were slowed (M =337 msec) compared with RTs after correct inhibition trials (M = 325 msec). When examining the relationship between AUD severity and difference scores for pre- and post-trial reaction times, we found a significant correlation with pre-trial processes, r(142) = -.18, p = .03, such that greater severity was associated with a reduced difference in pre-NoGo RTs. Whereas less severe AUDs were associated with faster RTs for errors than correct trials, more severe AUDs were associated with either no difference in RTs or faster responses during correct than error trials. Examination of post-trial processes revealed no significant relationships with AUD severity.
Functional magnetic resonance imaging results
Response inhibition.
As seen in Figure 1a, during successful inhibition (Correct NoGo > Correct Go), all participants showed significant activation in bilateral IFG, ACC, bilateral DLPFC, supplementary motor area (SMA), subthalamic nucleus, motor cortex, and posterior parietal cortex, as reported in previous studies (voxel level z > 10, corrected p < .05). In the contrast of Correct NoGo > Incorrect NoGo (Figure 1c), we found bilateral putamen/globus pallidus, bilateral middle frontal gyrus (MFG; Broca’s area [BA] 6), right superior frontal gyrus (BA 9), right frontal pole (BA 10), bilateral superior parietal lobe, and bilateral occipital gyrus.
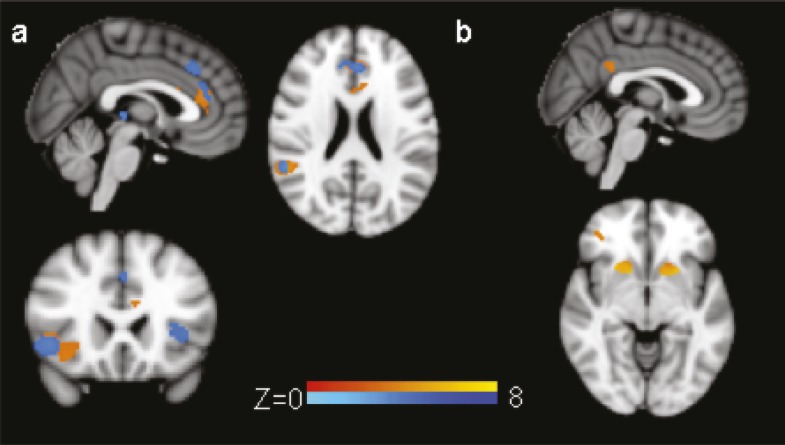
As seen in Figure 2a, increased AUD severity was associated with reduced neural response in pregenual ACC, right IFG/insula, and inferior parietal lobe (z > 3.09, corrected p < .05; Table 3). Including BAI scores did not alter the results; the relationships between each significant region and AUD severity scores all remained significant regardless of whether anxiety was incorporated into the model.
Figure 2.
Correlations between Go/NoGo contrasts and alcohol use disorder severity. (a) Shows the negative correlation between alcohol use disorder (AUD) severity and the NoGo correct > Go correct contrast (orange regions; z > 3.09, p < .05) within functionally defined regions of interest including right inferior frontal gyrus (IFG)/insula, pregenual anterior cingulate cortex (ACC), and inferior parietal lobe. In addition, blue regions show significant relationships between AUD severity and responses in the NoGo incorrect > Go correct contrast (z > 3.09, p < .05). Although there is significant overlap with the correct inhibition correlations, errors were also associated with left IFG, dorsal ACC, and thalamus. (b) Shows the positive correlation between AUD severity and signal change in trials that preceded Incorrect NoGo trials compared with Correct NoGo trials. Individuals with more severe AUDs engaged bilateral striatum, posterior cingulate, and right middle frontal gyrus to a greater degree before incorrect inhibition trials compared with those individuals with less severe AUDs. All images are in radiological convention.
Table 3.
Clusters showing significant relationships with alcohol use disorder severity
| Contrast | Region | Voxels | Max. z | x | y | z |
| Correct NoGo > correct Go | Pregenual ACC | 426 | 4.44 | -4 | 34 | 16 |
| Right IFG/insula | 293 | 4.00 | 44 | 18 | -2 | |
| Right IPL | 274 | 4.37 | 58 | -44 | 18 | |
| Incorrect NoGo > correct Go | Rostral ACC | 321 | 3.77 | -4 | 36 | 24 |
| Right IFG/insula | 272 | 4.19 | 44 | 18 | -2 | |
| Left IFG/insula | 96 | 3.38 | -40 | 20 | 2 | |
| Right IPL | 84 | 4.17 | 56 | -44 | 20 | |
| Right MTG | 71 | 3.67 | 48 | -24 | -8 | |
| Thalamus | 32 | 3.72 | 2 | -28 | 4 | |
| Pre incorrect NoGo > pre correct NoGo | Left striatum | 139 | 5.67 | -24 | 12 | -4 |
| Right striatum | 133 | 6.08 | 22 | 16 | 2 | |
| Right MFG | 66 | 4.59 | 36 | 42 | 0 | |
| Posterior cingulate | 55 | 3.76 | 2 | -42 | 30 |
Notes: ACC = anterior cingulate cortex; IFG = inferior frontal gyrus; IPL = inferior parietal lobe; MTG = medial temporal gyrus; striatum = caudate and putamen; MFG = middle frontal gyrus. All clusters meet multiple comparisons corrections (z > 3.09, cluster p > .05).
Error monitoring.
To assess neural responses to errors, we examined trials in which subjects failed to withhold a response (NoGo Incorrect) compared with Go Correct trials. Significant differences in neural response were observed in widespread areas of cortex including bilateral IFG, ACC/SMA, bilateral insula, medial temporal gyrus, and angular/supramarginal gyrus (z > 10, cluster level p < .05; Figure 1b). In addition, when examining the contrast of NoGo Incorrect > NoGo Correct, we found a left later-alized cluster that included anterior and posterior insula, temporal pole, and pre-/post-central gyrus (z > 3.09, cluster level p < .05; Figure 1c).
Examination of correlations between AUD severity and error monitoring revealed significant correlations in bilateral IFG/insula, thalamus, right middle temporal gyrus, right IPL, and the rostral anterior cingulate zone (z > 3.09, cluster level p < .05; see Table 3 and Figure 2a). As with the case of the response inhibition contrast, the addition of BAI scores did not significantly influence the relationship between BOLD response and AUD severity.
Pre-inhibition processing.
Comparison of the two Go trials preceding response inhibition successes and errors revealed significant differences in several regions including bilateral caudate, SMA, posterior cingulate cortex, bilateral insula, and medial frontal cortex, with Go trials preceding errors showing greater response in these regions than Go trials preceding correct inhibitions (z > 6, p < .05 corrected; Figure 1d). Examination of the relationship between AUD severity and pre-trial processing revealed significant correlations in bilateral caudate/putamen, posterior cingulate, and right MFG (z > 3.09, p < .05 corrected; Table 3 and Figure 2b) such that more severe AUDs were associated with greater response in these regions before errors than before correct inhibitions.
Post-inhibition processing.
When comparing Go trials after successful and unsuccessful inhibition trials, we found that Go trials after successful inhibitions were associated with increased BOLD response in bilateral caudate/putamen, bilateral DLPFC (BA 46), thalamus, bilateral precentral gyrus, SMA, precuneus, lateral occipital cortex, and occipital fusiform compared with trials after unsuccessful inhibition trials (z > 6, p < .05 corrected; Figure 1e). No significant correlations emerged between AUD severity and post-inhibition trial response.
Discussion
In this study, we examined response inhibition and error monitoring in individuals with a wide range of AUD severity. We hypothesized that individuals with more severe AUDs would show reduced activity in IFG and presupplementary motor area during successful inhibition and in anterior cingulate cortex during error processing. We posited that these neural differences would be accompanied by differences in performance-based measures. Overall, our results suggest that more severe AUDs are associated with reduced engagement of frontal networks during response inhibition and error monitoring compared with less severe AUDs and that these relationships may result from inattention and/or reduced goal maintenance.
When examining relationships between AUD severity and successful inhibition-related activity, we found reduced BOLD response in right IFG/insula, pregenual ACC, and right inferior parietal lobe in individuals with more severe AUDs. The right IFG/insula have received the most attention for a potential role in response inhibition (Aron, 2007; Aron et al., 2004). Comparisons of successful withholding of responses to unsuccessful attempts or to trials in which responses are required have consistently revealed IFG (Aron and Poldrack, 2006; Garavan et al., 1999; Liddle et al., 2001; Ridderinkhof et al., 2004). Although the available data have historically supported the role of IFG in response inhibition, recent data casts doubt on the role of this region in inhibition, instead suggesting that the IFG is particularly responsive to infrequent stimuli (Chatham et al., 2012; Hampshire et al., 2010; Sharp et al., 2010). Thus, the role of IFG in performance of tasks that purport to measure response inhibition is not disputed; however, determining what the exact mechanism is requires further evaluation. The reduced level of response of IFG in alcohol and other substance use disorders may suggest an impairment in maintaining task goals and using task relevant information in making subsequent responses. The pregenual ACC is typically observed in tasks in which evaluation of ongoing responses occurs (Banich, 2009; Kiehl et al., 2000; Orr and Weissman, 2009) and may act to signal the need for increased cognitive control to resolve conflict (Botvinick et al., 2004; Etkin et al., 2006, 2011). Garavan et al. (2002) have suggested that the cingulate is engaged in inhibition during difficult trials, a hypothesis that is consistent with its role—along with pre-supplementary motor area—in response competition and selection of appropriate motor responses. Last, the response in the inferior parietal lobe has been observed during the presentation of infrequent, task-relevant stimuli, suggesting an increase in attention (Clark et al., 2000; Garavan et al., 1999). The negative relationship with AUD severity further points to problems with allocating attentional resources in more severely dependent individuals.
As predicted, we found that greater AUD severity correlates with reduced signal change in response to errors in the anterior rostral cingulate zone, a region previously shown to be important for the processing of errors (Nee et al., 2011). This finding is consistent with Fein and Chang (2008), who found that density of family history of alcoholism was associated with reduced amplitude of the ERN in response to negative feedback. However, an earlier comparison of AD subjects and healthy control subjects revealed enhanced neural responses to errors in the AD group (Li et al., 2009), although the localization of cingulate/SMA effects was more anterior and dorsal than the anterior rostral cingulate ROI in the current study. In addition, Li et al. found group differences in the comparison of incorrect inhibition trials with correct inhibition trials, whereas the current study found a relationship with AUD severity only when comparing incorrect inhibitions with correct go trials. Last, Schellekens et al. (2010) found that the ERN was enhanced in individuals with alcohol dependence compared with controls, an effect that was larger for AD individuals with comorbid anxiety. Examination of anxiety in the current sample suggested that although anxiety and AUD severity were highly related, the addition of anxiety scores to the regression model did not negatively affect the findings. Because the current study did not include a group of healthy controls, it is difficult to know whether error monitoring is enhanced compared with controls.
Although many of the behavioral indices showed no significant relationships with AUD severity, we found that the difference in Go RTs before unsuccessful and successful NoGo trials was significantly associated with AUD severity. Specifically, those participants with less severe AUDs had faster responses before errors compared with correct inhibitions, suggesting increased vigilance/attention and increased pre-potency of responses. In contrast, individuals with more severe AUDs showed less of a difference in RTs, which may suggest overall reduced attention to the task. It is interesting to note that the significant correlations between severity and BOLD response differences between pre-error and pre-correct Go trials in putamen corroborate this behavioral observation. Previous studies have suggested that increased putamen response during successful inhibition compared with unsuccessful inhibition may result from differences in the speed at which the Go process is activated during each trial type, with the Go process activating more slowly during successful inhibitions and thus resulting in greater BOLD response in the putamen (Aron and Poldrack, 2006). The finding that some of the more severe AUD participants showed greater RTs on Go trials before making an error compared with Go trials before successfully inhibiting corresponds to greater difficulty activating Go processes, which would then be expected to result in greater putamen activity. These findings, along with the increased activation of the posterior cingulate, a region that is typically suppressed during effortful processing (Weissman et al., 2006), seem to be consistent with increased inattention in more severe AUDs.
Although the findings of the current study are compelling, it is important to note some potential limitations. First, although we analyzed severity that we assumed to result from chronic alcohol use, it would be useful to determine whether AUD severity is associated with response inhibition and error monitoring related activity in a sample that controlled exposure to alcohol. The current study suggests that executive dysfunction increases with alcohol dependence severity, but it is not clear if these differences contribute to the onset of AUDs or whether these differences emerge as one progresses from abuse to severe dependence. Future longitudinal studies are needed to determine the long-term effects of alcohol on neural responses. Also, the present study lacks a control group, thus limiting our ability to conclude that the observed regions contribute to problematic drinking specifically. For example, it is possible that BOLD response also varies widely among non-AUD samples and that variation in these regions does not depend on diagnostic status. Although evidence suggests that there are group differences between AD samples and social drinkers, future studies would nonetheless benefit from a control sample to further isolate specific effects that are attributable to AUDs.
Results from the current study suggest reduced engagement of frontal circuits important for behavioral control as a function of AUD severity, a finding that appeared to be driven by periods of inattention throughout the task. Degree of frontal engagement during inhibition could be particularly important for predicting treatment outcomes, as this ability may be associated with a better ability to overcome drinking-related urges. This type of finding would extend previous studies that show evidence of frontal white and gray matter metabolite concentrations predicting treatment outcomes (Durazzo et al., 2008).
Footnotes
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grants AA012238 and AA014886 (to Kent E. Hutchison).
References
- Aron AR. The neural basis of inhibition in cognitive control. The Neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. The Journal of Neuroscience. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. The Journal of Neuroscience. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Babor T, de la Fuente J, Saunders J, Grant M. The Alcohol Use Disorders Identification Test: Guidelines for use in primary health care. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- Banich MT. Executive function: The search for an integrated account. Current Directions in Psychological Science. 2009;18:89–94. [Google Scholar]
- Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio, TX: The Psychological Corporation; 1990. [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: Relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34:133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Chatham CH, Claus ED, Kim A, Curran T, Banich MT, Mu-nakata Y. Cognitive control reflects context monitoring, not motoric stopping, in response inhibition. PLoS ONE. 2012;7(2):e31546. doi: 10.1371/journal.pone.0031546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP, Fannon S, Lai S, Benson R, Bauer L. Responses to rare visual target and distractor stimuli using event-related fMRI. Journal of Neurophysiology. 2000;83:3133–3139. doi: 10.1152/jn.2000.83.5.3133. [DOI] [PubMed] [Google Scholar]
- Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 2011;36:2086–2096. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. NeuroImage. 2003;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Yeh P-H, Meyerhoff DJ. Combined neuroimaging, neurocognitive and psychiatric factors to predict alcohol consumption following treatment for alcohol dependence. Alcohol and Alcoholism. 2008;43:683–691. doi: 10.1093/alcalc/agn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichele T, Debener S, Calhoun VD, Specht K, Engel AK, Hug-dahl K, Ullsperger M. Prediction of human errors by maladaptive changes in event-related brain networks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6173–6178. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Fein G, Chang M. Smaller feedback ERN amplitudes during the BART are associated with a greater family history density of alcohol problems in treatment-naïve alcoholics. Drug and Alcohol Dependence. 2008;92:141–148. doi: 10.1016/j.drugalcdep.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Claus ED, Morgan M, Forester GR, Hutchison K. Dopaminergic genes modulate response inhibition in alcohol abusing adults. Addiction Biology. 2011 doi: 10.1111/j.1369-1600.2011.00328.x. Advance online publication. doi:10.1111/j.1369-1600.2011.00328.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. Washington, DC: American Psychiatric Press; 2002. [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RAP, Stein EA. Dissociable executive functions in the dynamic control of behavior: Inhibition, error detection, and correction. NeuroImage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: An event-related functional MRI study. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W. Decision making in pathological gambling: A comparison between pathological gamblers, alcohol dependents, persons with Tourette syndrome, and normal controls. Cognitive Brain Research. 2005;23:137–151. doi: 10.1016/j.cogbrainres.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: Inhibition and attentional control. NeuroImage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heather N, Tebbutt JS, Mattick RP, Zamir R. Development of a scale for measuring impaired control over alcohol consumption: A preliminary report. Journal of Studies on Alcohol. 1993;54:700–709. doi: 10.15288/jsa.1993.54.700. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, McGeary J, Smolen A, Bryan A, Swift RM. The DRD4 VNTR polymorphism moderates craving after alcohol consumption. Health Psychology. 2002;21:139–146. [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padma-nabhapillai A, Begleiter H. Alcoholism is a disinhibitory disorder: Neurophysiological evidence from a Go/No-Go task. Biological Psychology. 2005;69:353–373. doi: 10.1016/j.biopsycho.2004.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch S, Jäger L, Karamatskos E, Graz C, Stammel A, Flatz W, Mulert C. Influence of trait anxiety on inhibitory control in alcohol-dependent patients: Simultaneous acquisition of ERPs and BOLD responses. Journal of Psychiatric Research. 2008;42:734–745. doi: 10.1016/j.jpsychires.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. Journal of Neuroscience. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: An event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, Clark L. Impulsivity and response inhibition in alcohol dependence and problem gambling. Psychopharmacology. 2009;207:163–172. doi: 10.1007/s00213-009-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Luo X, Yan P, Bergquist K, Sinha R. Altered impulse control in alcohol dependence: Neural measures of stop signal performance. Alcoholism: Clinical and Experimental Research. 2009;33:740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Human Brain Mapping. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Calhoun VD, Chen J, Claus ED, Hutchison KE. Effect of homozygous deletions at 22q13.1 on alcohol dependence severity and cue-elicited BOLD response in the precuneus. Addiction Biology. 2011 doi: 10.1111/j.1369-1600.2011.00393.x. Advance online publication. doi:10.1111/j.1369-1600.2011.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Nee DE, Kastner S, Brown JW. Functional heterogeneity of conflict, error, task-switching, and unexpectedness effects within medial prefrontal cortex. NeuroImage. 2011;54:528–540. doi: 10.1016/j.neuroimage.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Orr JM, Weissman DH. Anterior cingulate cortex makes 2 contributions to minimizing distraction. Cerebral Cortex. 2009;19:703–711. doi: 10.1093/cercor/bhn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Rodriguez Holguin S, Porjesz B, Chorlian DB, Polich J, Begleiter H. Visual P3a in male alcoholics and controls. Alcoholism: Clinical and Experimental Research. 1999;23:582–591. [PubMed] [Google Scholar]
- Schellekens AFA, de Bruijn ERA, van Lankveld CAA, Hulstijn W, Buitelaar JK, de Jong CAJ, Verkes RJ. Alcohol dependence and anxiety increase error-related brain activity. Addiction. 2010;105:1928–1934. doi: 10.1111/j.1360-0443.2010.03065.x. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Tapert SF. An fMRI study of response inhibition in youths with a family history of alcoholism. Annals of the New York Academy of Sciences. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, Mehta MA. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner H, Horn J. Alcohol Dependence Scale: Users Guide. Toronto, Ontario: Addiction Research Foundation; 1984. [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Supplement 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. New York, NY: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wong MM, Nigg JT, Zucker RA, Puttler LI, Fitzgerald HE, Jester JM, Adams K. Behavioral control and resiliency in the onset of alcohol and illicit drug use: A prospective study from preschool to adolescence. Child Development. 2006;77:1016–1033. doi: 10.1111/j.1467-8624.2006.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for fMRI group analysis using Bayesian inference. NeuroImage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]