Abstract
Objective:
Although hangover results from excessive alcohol consumption, the specific pathways through which hangover symptoms arise have not been elucidated. Research on predictors of hangover sensitivity may provide clues about such mechanisms. The present study investigated whether tobacco smoking on days of heavy drinking affects next-day hangover incidence and severity.
Method:
The study drew on diary data from a study on smoking and drinking among 113 students at a midwestern university in the United States. Participants completed a daily, web-based, 26-item survey for 8 weeks to assess prior-day alcohol and tobacco use as well as current-day hangover symptoms. Hierarchical linear modeling was used to test the hypothesis that amount of smoking is related to hangover, controlling for amount of alcohol consumed, sex, and other individual characteristics. Analyses were conducted after selecting only days with alcohol consumption levels that typically elicit hangover, then repeated on lighter drinking days for comparison. Validityof the hangover items was checked by comparing reports after such heavy drinking days with days of lighter drinking.
Results:
Across all possible person-days, 92% of daily reports were obtained. When selecting only events where an estimated blood alcohol concentration of 110 mg/dl was attained, smoking significantly increased the odds of hangover incidence and hangover severity while controlling for number of drinks consumed and sex. Additional analyses controlling for age first smoked regularly, frequency of drug use, type of drug involvement, or smoking status resulted in findings that were unchanged.
Conclusions:
Smoking more on heavy drinking days affects hangover sensitivity and severity, possibly because of acute pharmacological effects.
Hangover refers to the cluster of unpleasant symptoms of physical distress that occur as blood alcohol falls after an acute incident of drinking to intoxication. It is characterized predominantly by thirst, headache, nausea, and tiredness (Rohsenow et al., 2007) and is not to be confused with withdrawal, which occurs only after chronic administration and involves different neurological systems (Prat et al., 2009). The public health relevance of hangover is in its potential effects on occupational safety and performance (Howland et al., 2006; Rohsenow et al., 2006, 2010) and as a predictor of risk for drinking problems after the college years (Piasecki et al., 2005; Rohsenow et al., 2012). Mechanisms underlying hangover are not yet known, but proposed ones broadly include metabolic, fluid balance, hormonal changes, and toxicities resulting from metabolism of alcohol and beverage congeners (Penning et al., 2010).
Not all heavy drinkers appear to be susceptible to experiencing hangover, indicating that some drinkers have hangover insensitivity. A review of studies found that across various study designs, populations, and reference periods, around 23%-25% of people who drink enough to experience hangover report no hangover (Howland et al., 2008b). Attempts to predict who would experience hangover have had limited results so far. Predictors of frequency or severity of hangover found in some studies include having personality risk for alcoholism as measured by the MacAndrew Scale (Earleywine, 1993; MacAndrew, 1965), having a family history of alcoholism (Newlin and Pretorius, 1990; Piasecki et al., 2005), and having a certain alcohol dehydrogen-ase polymorphism (ADH1B*2) (Wall et al., 2005). Greater average quantity of drinking correlated with less intense hangovers in two studies (Rohsenow et al., 2012; Wall et al., 2000) and with more frequent hangovers in one study (Wall et al., 2005), whereas more frequent heavy drinking correlated with more frequent hangovers among women more than among men in another study (Piasecki et al., 2005). The only previous attempt to find individual difference predictors of hangover insensitivity combined data across three laboratory studies of heavy episodic drinkers in which participants achieved an estimated blood alcohol concentration (BAC) of 110 mg/dl (measured by breath alcohol analysis). That study found no relationship to sex, age, family history of drinking problems, or average daily drinking volume (Howland et al., 2008a).
Alcohol and tobacco use are positively correlated across a variety of studies, especially among heavy or problem drinkers (e.g., Burling and Ziff, 1988; Hurt et al., 1995; Kozlowski et al., 1986; Marks et al., 1997). Amounts of smoking and alcohol consumption vary on a daily basis, particularly among lighter smokers, who may drink and smoke more on social occasions (Jackson et al., 2010). Various mechanisms underlying the correlations of smoking with drinking have been proposed. Some predisposing individual differences that might result in smokers being more likely to drink more than nonsmokers include common genetic factors (Grucza and Bierut, 2006; Swan et al., 1997), negative af-fectivity (Hesselbrock and Hesselbrock, 2006), or childhood patterns of behavioral undercontrol that predispose both to smoking at an earlier age and to drinking more (e.g., Brown et al., 1996; Farrell et al., 1992; United States Department of Health and Human Services, 2000). In addition, smoking or nicotine use alters subjective responses to alcohol (e.g., Kouri et al., 2004; Madden et al., 1995; Piasecki et al., 2011). Some acute reasons for using nicotine with alcohol include additive effects on dopamine release in the meso-limbic system (Di Chiara and Imperato 1988; Funk et al., 2006; Pierce and Kumaresan, 2006) that could underlie the increased pleasure that is obtained from using both together (Piasecki et al., 2011; Rose et al., 2004) and the fact that nicotinic receptors are involved with effects of alcohol and may be the site of alcohol-tobacco interactions (Funk et al., 2006).
The mechanisms underlying hangover are still speculative (Penning et al., 2010); therefore, the role that cigarette smoking might play in hangover is difficult to determine. Ethanol’s acute effects on the brain include complex central nervous system changes involving y-aminobutyric acid, dysregulated cytokine pathways, reduced acetylcholine, increased norepinephrine turnover, and other neurotransmitter changes (e.g., Kosten et al., 2005). Consequently, many brain regions may be affected by alcohol leaving the system. The effects of nicotine and alcohol on common neural systems in the brain might also increase residual effects as alcohol leaves the body. Smoking and nicotine withdrawal can acutely affect processes—such as sleep (e.g., Zhang et al., 2006), endocrine responses (Rohleder and Kirschbaum, 2006), and inflammatory responses (e.g., Fröhlich et al., 2003)—thought to play a role in hangover production or severity. Although the paucity of knowledge on neural or physiological changes that result in hangover means this area of investigation is still in the exploratory stage, the common mechanisms underlying alcohol and smoking co-occurrence suggest that it would be useful to investigate the role of smoking in hangover.
There has been little research investigating whether tobacco smoking can contribute to hangover incidence or severity. Hesse and Tutenges (2009) surveyed young adults about hangover severity and found that prior-day number of cigarettes smoked was not associated with hangover, nor was defining oneself as a “regular smoker.” Another report compared undergraduate smokers with nonsmokers and found no differences in likelihood of endorsing a liberally defined “hangover-like experience” (i.e., current hangover, “even just a little”) on waking when drinking frequency was controlled (Piasecki et al., 2010), but the effects of amount of smoking on hangover frequency or severity were not tested.
The present study drew on diary data from a study on smoking and drinking practices among university students (Jackson et al., 2010). Examination of the association between smoking and frequency of hangover must also consider consumption of alcohol in order to account for differences in both variables that might be attributable to confounds with amount of alcohol consumed, or sex differences in alcohol metabolism or in threshold for inducing hangover (Jackson, 2008). Therefore, the first aim of this study was to investigate the hypothesis that a greater amount of tobacco smoked would predict next-day sensitivity to hangover and a greater frequency of hangovers after controlling for variance in hangover that might be accounted for by prior-night number of drinks consumed and sex. Data were analyzed at the event level rather than just in aggregate. The second aim was to investigate whether the relationships are likely to be accounted for by common predisposing individual differences as outlined above. Although we could not directly assess childhood behavioral undercontrol or genetic factors, people who start smoking at an earlier age are more likely to have a pattern of behavioral undercontrol, to use alcohol earlier and more heavily, and to also use recreational drugs (e.g., Brown et al., 1996; Farrell et al., 1992). Therefore, the analyses were repeated while adding as covariates age at first daily smoking, then frequency of recreational drug use, then a categorical variable of no drug use versus only marijuana use versus other illicit drug use. Because inspection of the individual-level data from an early dose-response alcohol administration study (Chapman, 1970) shows that consistent reports of hangover require drinking to an estimated BAC of 110 mg/dl or higher (as described by Rohsenow et al., 2010, and in the review of hangover study methodology by Verster et al., 2010), analyses were conducted while selecting only nights for which estimated BAC was 110 mg/dl or greater, then on lighter drinking nights for comparison. Relationships were expected to be stronger when selecting data that involved drinking to the higher level.
Method
Participants and procedure
Participants were 113 college student drinkers enrolled in a daily diary study designed to examine alcohol use, tobacco use, and mood (Jackson et al., 2010). Participants had to have endorsed past-month drinking and to drink during the study; smokers were oversampled (reported 100 or more lifetime cigarettes). See Table 1 for characteristics of participants. All procedures were approved by the university institutional review board, and participants signed informed consent forms.
Table 1.
Characteristics of study participants (N= 113)
| Measure | M (SD) or n (%) |
| Male | 49 (43.4%) |
| Age, in yearsa | 18.5(0.67) |
| White | 108 (96.4%) |
| Perceived dependence on smokingb | 79 (70%) |
| Daily smokersc | 59 (53%) |
| Reported alcohol use on weekends only during study | 3 (3%) |
| Ever reached eBAC ≥ 110 mg/dl | 110(97%) |
| Reached eBAC ≥ 110 mg/dl more than once | 105 (93%) |
| Reported any hangover symptoms | 110(97%) |
| Reported hangover symptoms more than once | 106(94%) |
| Reported any hangover symptoms if eBAC ≥ 110 mg/dl | 108(96%) |
Notes: eBAC = estimated blood alcohol concentration.
90% were 18 or 19 years old;
endorsed the item “Have you ever felt that you needed tobacco or that you were dependent on it?”;
reported smoking “daily or almost daily” in the past month.
Participants completed a daily, web-based, 26-item survey for 8 weeks to assess prior-day alcohol and tobacco use as well as current-day hangover symptoms. Each day, participants received a morning email notice prompting them to log in and complete a survey. Paper-and-pencil surveys were available in the event that participants could not access the Internet. Initially, participants were brought into the lab and were given a brief orientation to the survey, including information on how to access to the survey as well as the standard definition of a drink. At this time, they were administered a baseline survey assessing substance use, smoking history, and other psychosocial constructs not used in the present study.
Measures
Drinking.
Respondents reported the number of standard drinks consumed on the prior day, ranging from 0 to 25 or more. We also assessed number of hours elapsed between first drink and last drink (in hourly intervals from 1 to 24, in addition to options for 30 minutes and 90 minutes).
Smoking.
Prior-day smoking was assessed by asking number of cigarettes smoked, ranging from zero to more than three packs (with an interval of one cigarette for one pack or less, and an interval of five cigarettes for more than one pack).
Hangover.
Current-day hangover symptoms were adapted empirically (based on factor loadings and inter-item correlations) from the Hangover Symptoms Scale by Slutske et al. (2003). To decrease response burden with repeated assessments, only five items were used. Although the subset does not include all valid hangover symptoms, high internal consistencies of the various published measures (e.g., α = .79 for this measure [Slutske et al., 2003] and α = .84 for an acute hangover measure [Rohsenow et al., 2007]) justify using a subset of items. For use in daily diaries, the questions were changed from asking about percentage of drinking occasions to “Have you felt_____today because of yesterday’s drinking” and included five symptoms: felt more tired than usual, had a headache, felt nauseated, felt very weak, and had difficulty concentrating on things. To provide a severity rating, item responses ranged from 1 (not at all) to 7 (extremely). A composite hangover scale was formed by taking the mean of the five items (Cronbach’s α across the time points = .92; Cronbach’s α aggregated across individuals = .94). We also created a dichotomous variable reflecting whether the respondent endorsed any hangover symptoms (nonzero mean score) to assess hangover insensitivity.
Blood alcohol estimation.
BAC was estimated for each day based on number of drinks, body weight, and the period over which the respondent consumed alcohol, recorded in hours (M = 6.46 hours, SD = 2.92). We used the Matthews and Miller (1979) formula: BAC estimate = [(standard drinks / 2) × (sex constant / weight)] − (0.017 × hours), where the sex constant is 9.0 for females and 7.5 for males.
Additional covariates.
Age first smoked was determined by asking respondents to indicate how old they were the first time they started smoking “regularly.” Past-year frequency of use of any drugs, measured on a seven-level ordinal scale ranging from 0 (never/not in the past year) to 7 (41 or more times in the past year), was summed across nine drugs: marijuana, 3,4-methylenedioxymethamphetamine (MDMA; Ecstasy), club drugs, inhalants, stimulants, crack, psychedelics, barbiturates, and heroin. To reflect the degree of involvement with illicit drugs, a three-level drug use variable was also computed that coded no past-year drug use as 0, past-year use of marijuana only as 1, and past-year use of any drugs other than marijuana as 2.
Data analysis approach
First, Pearson’s correlations were conducted among the variables to be entered into the models to show the univari-ate relationships. These were calculated using all data and repeated using only data from days where estimated BAC was 110 mg/dl or greater.
Because of the clustered nature of the data, with 56 time points per respondent, all subsequent data analysis was conducted using multilevel modeling (MLM; also called hierarchical linear modeling, HLM; Raudenbush and Bryk, 2002; Snijders and Bosker, 1999). These models permit varying numbers of observations and missing observations. Each “day” consisted of 1 day’s drinking and cigarette data and the next morning’s hangover data. We used HLM 6.06 (Raudenbush et al., 2004) to conduct the multilevel analyses. Level 1 variables were person-centered, and Level 2 variables were grand mean-centered. Level 1 corresponds to within-person time effects, and Level 2 corresponds to between-person data. Number of cigarettes and number of drinks served as time-varying Level 1 variables. We also controlled for sex at Level 2.
Second, the hangover items were tested for validity. Although three of our items had been found valid in experimental hangover induction studies (see Rohsenow et al., 2007), “felt very weak” and “difficulty concentrating” had not been tested to see if they are rated higher after a night of drinking to an estimated BAC of 110 mg/dl or more versus drinking less. Therefore, using MLM, we compared hangover ratings on days when estimated BAC was 110 mg/dl or more the night before with days when the previous night’s estimated BAC was greater than 0 but less than 110 mg/dl. Each hangover rating and the total score were entered into the analysis after controlling for sex.
Third, we tested the effects of smoking on hangover using MLM. The equations below correspond to a model predicting hangover total scores each day from number of cigarettes and number of drinks (Level 1). The model shows how sex was included as a covariate at Level 2.
The model that was fit was as follows:
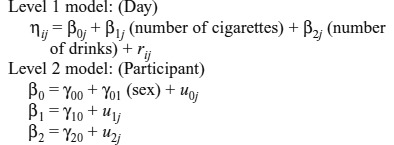 |
For analyses of any hangover versus no hangover (hangover sensitivity), we used a Bernoulli (unit-specific) model for binary data. Models were estimated with robust standard errors because of the nonnormality of the outcomes. The models were first conducted while selecting only days where estimated BAC was 110 mg/dl or more to eliminate data from days when hangover was unlikely to occur, and then, for comparison, repeated while selecting days of any lighter drinking (because nondrinking days are irrelevant).
Finally, in a series of parallel models, we added as control variables age first smoked daily, frequency of drug use, and the three-level drug use variable (in separate analyses) to determine whether the relationships between smoking and hangover were attributable to these indicators of an underlying predisposition. Then, given interest in whether hangover is greater among people who routinely smoke more heavily, we sought to determine whether effects of smoking on hangover were attributable to person-level smoking status, suggesting a heavier pattern of smoking rather than the quantity smoked on a given occasion. To address this, we conducted three additional models for each hangover variable, each controlling for one of three person-level smoking status variables: a binary variable reflecting whether they endorsed ever being a dependent smoker (because we had no measure of nicotine dependence), a binary variable indicating whether they reported smoking “daily or almost daily” in the past month, and a continuous variable reflecting the mean number of cigarettes smoked over the 8-week period.
Results
Descriptive information
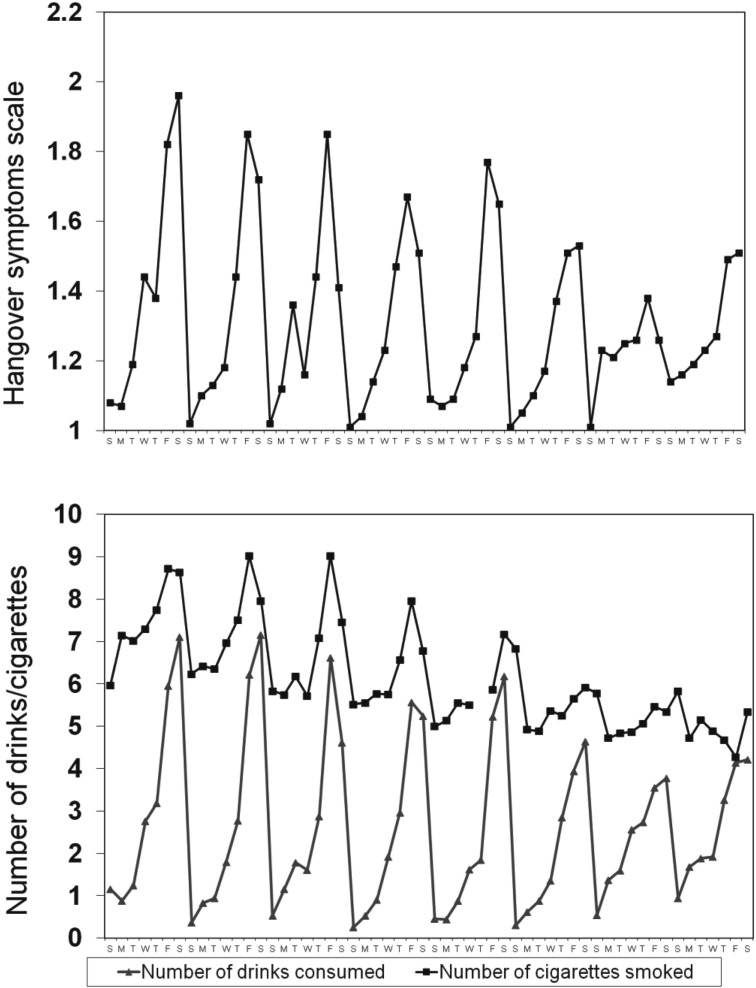
Across all possible person-days (56 days × 113 participants), 94% of daily drinking reports (5,930 / 6,328) were obtained. Daily participation rates, which declined over the 8-week interval, ranged from 100% to 73% (on the last survey day); the median daily retention rate was 95% (107 /113). Table 2 presents event-level characteristics; Figure 1 portrays the number of cigarettes, number of drinks, and degree of hangover reported over the 56-day interval. A strong 7-day pattern was evident for all measures.
Table 2.
Event-level characteristics across the 56-day period
| Measure | All study days M (SD) or n (%) N= 5,930 days | IfeBAC ≥ 110 mg/dl M (SD) or n (%) N= 1,272 days |
| No. of cigarettes per day | 7.16(6.67) | 10.13(7.40) |
| No. of drinks per day | 2.55 (4.74) | 10.42 (4.32) |
| No. of days eBAC ≥ 110 mg/dl reached | 1,272(21.5%) | 1,272(100%) |
| No. of days with any hangover | 1,144 (19.4%) | 998 (78.5%) |
| Degree of hangovera | 1.30(0.84) | 2.31(1.35) |
Notes: eBAC = estimated blood alcohol concentration.
Mean of five items rated from 1 (not at all) to 7 (extremely).
Figure 1.
Prevalence of hangover intensity (top panel) and number of drinks consumed and number of cigarettes smoked (bottom panel). Ns range from 82 to 113 across the 56 days.
Univariate correlations
Table 3 shows the bivariate associations between measures of drinking, smoking, and hangover. The lower diagonal shows the associations across the full study period, and the upper diagonal presents associations only for events where estimated BAC was 110 mg/dl or more. These correlations were computed at the within-subjects (daily) level, although very similar values were observed at the person-level with observations aggregated over the 8 weeks (be-tween-subjects association). Not surprisingly, the number of drinks was bivariately highly associated with any hangover and degree of hangover across all days; the magnitude of the relationship decreased when selecting days of high estimated BAC because of the restriction of range. The number of cigarettes showed small to moderate bivariate correlations with both number of drinks and degree of hangover. There appeared to be no association between sex and hangover despite the expected sex differences in the number of drinks consumed.
Table 3.
Correlations among measures of drinking, smoking, and hangover at the event level for the full study period (lower diagonal) and for events where estimated blood alcohol concentration was 110 mg/dl or greater (upper diagonal)
| Variable | No. of drinks | No. of cigarettes | Any hangover | Degree of hangover | Sex |
| No. of drinks | – | .10*** | .20*** | .23*** | .51*** |
| No. of cigarettes | .24*** | – | .13*** | .08*** | .00 |
| Any hangover | .79*** | .24*** | – | .51*** | -.03 |
| Degree of hangover | .65*** | .19*** | .74*** | – | -.06* |
| Sex | .12*** | -.01 | .00 | -.01 | – |
Notes: N ranges from 5,895 to 5,905 (lower diagonal), and N ranges from 1,270 to 1,272 (upper diagonal).
p <.05;
p <.001.
Validity of hangover ratings
The hangover validity analyses included data on 1,643 to 1,650 events, depending on the variable. Each hangover scale item was significantly higher on mornings after estimated BAC was 110 mg/dl or greater than on mornings after drinking to a lesser estimated BAC level, Fs (1, 111) from 119.20 to 350.81, parameter estimates (γ) range from 0.77 (nauseated) to 1.72 (tired), as was the total score, γ = 1.10, all ps < .001. Means and standard deviations for each item are displayed in Table 4.
Table 4.
Ratings of each hangover item for events where estimated blood alcohol concentration (eBAC) ≥ 110 mg/dl as opposed to events of drinking to a lesser eBAC: M (SD)
| eBAC the night before | More tired than usual | Nauseated | Headache | Feel very weak | Difficulty concentrating |
| ≥ 110 mg/dl | 3.15(1.84) | 1.87(1.47) | 2.13(1.62) | 2.24(1.53) | 2.16(1.56) |
| > 0 and < 110 mg/dl | 1.52(1.11) | 1.13(0.60) | 1.18(0.61) | 1.25(0.75) | 1.24(0.77) |
| Γ | 1.72 | 0.77 | 0.96 | 1.03 | 0.98 |
| F | 350.81 | 119.20 | 144.17 | 173.08 | 147.70 |
Notes: N= 1,657; df= 1, 111. All ps < .001. Response options ranged from 1 (not at all) to 7 (extremely).
Analyses of hypotheses
The multilevel models are shown in Table 5 (estimated BAC ≥ 110 mg/dl in the top panel). Number of drinks predicted the hangover variables, as expected. Sex did not predict hangover and, therefore, will not be discussed again. Controlling for these variables, smoking more heavily significantly increased the odds of any hangover and predicted degree of hangover only when drinking to an estimated BAC of 110 mg/dl or greater. We conducted ancillary analyses to explore the extent to which findings were similar when using a variable reflecting whether they were smoking on the drinking day; results indicated that any smoking predicted any hangover (OR = 2.37, 95% CI [1.49, 3.78], p < .001) and degree of hangover, γ = 0.34, F(1, 109) = 11.76, p < .001, for an estimated BAC of 110 mg/dl or greater but was not a significant predictor and showed no trend supporting such an association for an estimated BAC less than 110 mg/dl (and, in fact, the nonsignificant coefficient was in the opposite direction when predicting any drinking, γ = -0.01).
Table 5.
Multilevel models predicting hangover incidence and degree of hangover from prior-day number of cigarettes, number of drinks, and sex
| Any hangover |
Degree of hangover |
||||||
| Variable | γ | F | Significance | Odds ratio [95% CI] | γ | F | Significance |
| Events ≥ 110 mg/dl eBAC | |||||||
| Sex (male =1) | -0.16 | 0.27 | N.S. | 0.85 [0.47, 1.56] | 0.10 | 0.56 | N.S. |
| Number of drinks | 0.32 | 65.29 | p < .001 | 1.37 [1.27, 1.48] | 0.15 | 115.56 | p < .001 |
| Number of cigarettes | 0.10 | 10.37 | p < .01 | 1.10 [1.04, 1.17] | 0.05 | 26.11 | p < .001 |
| Events 0 < eBAC < 110 mg/dl | |||||||
| Sex (male =1) | -0.15 | 0.18 | N.S. | 0.89 [0.42, 1.88] | 0.07 | 2.50 | N.S. |
| Number of drinks | 0.68 | 66.59 | p < .001 | 1.98 [1.68, 2.34] | 0.10 | 28.62 | p < .001 |
| Number of cigarettes | 0.08 | 3.28 | p = .07 | 1.09 [0.99, 1.19] | 0.03 | 3.24 | p = .08 |
Notes: For events ≥ 110 mg/dl estimated blood alcohol concentration (eBAC), n = 1,272, approximate df= 1, 108 for sex, and approximate df= 1, 109 for number of drinks and number of cigarettes. For events where 0 < eBAC < 110 mg/dl, n = 385, approximate df= 1, 97 for sex, and approximate df= 1, 98 for number of drinks and number of cigarettes. n.s. = not significant.
In the models in which we added control variables—the age first smoked regularly, frequency of drug use, the three-level drug use variable, the endorsement of smoking dependence, or the average smoking rate of more than 8 weeks (in separate analyses)—gamma weights and ORs were virtually identical (data not presented).
Discussion
The number of cigarettes consumed on the day of a heavy drinking episode (estimated BAC ≥ 110 mg/dl) predicted both the presence and severity of hangover symptoms the following day, with heavier smoking predicting greater hangover. This is the first published study to examine daily variation in smoking as a predictor of hangover sensitivity or severity on days after very heavy drinking versus lighter drinking using event-level data. Smoking accounted for unique variance after controlling for the relationship of smoking rate to number of drinks and sex. This is important because it means the relationship is not just an artifact of the correlation between drinking rates and smoking rates each day along with the differences in drinking rates by sex. Although the relationships of drinking to hangover were significant regardless of whether data from very heavy drinking nights or lighter drinking nights were used, the effects of smoking on hangover were significant only after nights of drinking to an estimated BAC of at least 110 mg/dl.
The results were not attributable to person-level variables associated with a predisposition to drink or use drugs more frequently because the effects were essentially the same when controlling for such variables. This suggests that the effects are more likely to be attributable to acute pharmacological effects of nicotine or other smoke constituents in the nervous system rather than being attributable to shared predisposing individual differences or traits (e.g., childhood pattern of behavioral undercontrol leading to earlier substance involvement). The results were also not attributable to a pattern of heavier smoking on average or to whether individuals smoked to a level of perceived nicotine dependence because controlling for these variables left the model results unaffected. This is consistent with others who failed to find hangover differences among smokers versus nonsmokers, although neither study modeled event-level smoking contemporaneously (Hesse and Tutenges, 2009; Piasecki et al., 2010). The results thus suggest that the amount of nicotine or smoke constituent exposure is important for affecting hangover sensitivity and severity. Although we do not know how many of the cigarettes were consumed while drinking, nicotine and other smoke constituents are well known to accumulate during the day and may play a pharmacological role while drinking. Future research would need to determine the neuropharmacological mechanisms by which smoking increases unpleasant residual effects of very heavy drinking and whether these same mechanisms play a role in harmful interactions, such as the adverse effects on brain structure, metabolites, cerebral blood flow, and neurocognition found among hazardous drinkers or alcoholics who also smoke (Durazzo et al., 2007).
Many surveys of hangover include items that have not been validated as hangover symptoms by comparing nights of drinking to a BAC of more than 110 mg/dl with nights without drinking to such levels (Rohsenow et al., 2007; Verster et al., 2010). Although three of the items in the measure we used had previously been validated in laboratory administration studies involving controlled heavy drinking, the other two items (feeling weak, trouble concentrating) were first validated in the present study, thus supporting preliminary evidence of these items by other studies in the field. These other studies indicated that feeling weak loaded highly on a hangover symptoms scale (Slutske et al., 2003) and was significantly elevated on mornings when college students endorsed a liberally defined hangover item in Piasecki et al. (2010). Both weakness and concentration problems were reported frequently as hangover symptoms by Dutch students (Penning et al., 2012). The ability to use event-level data to compare ratings the morning after drinking to an estimated BAC of 110 mg/dl with nights of lesser drinking makes an important methodological advance for validating hangover symptoms in the field rather than requiring controlled administration in the laboratory.
Strengths and limitations
The present study drew on a rich daily diary data set containing event-based data that permitted examination of episode-specific smoking and hangover. However, an important caveat is the restriction in temporal resolution when using daily data. With daily-level data, it was not possible to determine how much smoking occurred in the hours before reporting hangover symptoms the day after each heavy drinking episode, which could possibly influence symptom reports. However, in the natural environment, smokers will titrate to whatever nicotine level is comfortable at the time to avoid either withdrawal or too much nicotine. Therefore, this represents the naturalistic effects on any hangover rating. Interesting questions for future research may include how much smoking occurred subsequently, as a response to hangover, or as a prelude to or concomitant of additional drinking. Symptoms such as nausea might make a person tend to smoke less. Finally, it is possible that the results are not attributable to cigarettes per day per se but to some behavior correlated with higher smoking rates, such as poorer sleep.
These results are limited to a predominantly White sample of college students at one university and may be different among minority groups or among older adults with heavier smoking patterns. Smokers were oversampled in the diary study, which helps maximize variability in smoking in our analyses but somewhat reduces the generalizability of the sample.
Conclusions
These data add to what is known about interactions of smoking with alcohol use but leave questions about the mechanisms of these effects. We encourage researchers to replicate the findings reported in the present study using different research designs, measures, and populations, in addition to exploring the mechanisms underlying these interactions in future research.
Acknowledgments
Grateful appreciation is expressed to Dieter Meyerhoff, Dr.rer.nat., for his comments on an earlier version of this manuscript. Portions of this study were presented at the 12th Annual Meeting of the Society for Research on Nicotine & Tobacco, Bath, England, September 2010.
Footnotes
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grant K01 AA13938, by National Institute on Drug Abuse Grant 1 R01 DA023995, and by a Senior Research Career Scientist Award from the Department of Veterans Affairs.
References
- Brown RA, Lewinsohn PM, Seeley JR, Wagner EF. Cigarette smoking, major depression, and other psychiatric disorders among adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:1602–1610. doi: 10.1097/00004583-199612000-00011. [DOI] [PubMed] [Google Scholar]
- Burling TA, Ziff DC. Tobacco smoking: A comparison between alcohol and drug abuse inpatients. Addictive Behaviors. 1988;13:185–190. doi: 10.1016/0306-4603(88)90010-x. [DOI] [PubMed] [Google Scholar]
- Chapman LF. Experimental induction of hangover. Quarterly Journal of Studies on Alcohol, Supplement. 1970;5:67–86. [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Meyerhoff DJ. The neurobio-logical and neurocognitive consequences of chronic cigarette smoking in alcohol use disorders. Alcohol and Alcoholism. 2007;42:174–185. doi: 10.1093/alcalc/agm020. [DOI] [PubMed] [Google Scholar]
- Earleywine M. Personality risk for alcoholism covaries with hangover symptoms. Addictive Behaviors. 1993;18:415–420. doi: 10.1016/0306-4603(93)90058-h. [DOI] [PubMed] [Google Scholar]
- Farrell AD, Danish SJ, Howard CW. Relationship between drug use and other problem behaviors in urban adolescents. Journal of Consulting and Clinical Psychology. 1992;60:705–712. doi: 10.1037//0022-006x.60.5.705. [DOI] [PubMed] [Google Scholar]
- Fröhlich M, Sund M, Löwel H, Imhof A, Hoffmeister A, Koenig W. Independent association of various smoking characteristics with markers of systemic inflammation in men: Results from a representative sample of the general population (MONICA Augsburg Survey 1994/95) European Heart Journal. 2003;24:1365–1372. doi: 10.1016/s0195-668x(03)00260-4. [DOI] [PubMed] [Google Scholar]
- Funk D, Marinelli PW, Lê AD. Biological processes underlying co-use of alcohol and nicotine: Neuronal mechanisms, cross-tolerance, and genetic factors. Alcohol Research & Health. 2006;29:186–192. [PMC free article] [PubMed] [Google Scholar]
- Grucza RA, Bierut LJ. Co-occurring risk factors for alcohol dependence and habitual smoking: Update on findings from the Collaborative Study on the Genetics of Alcoholism. Alcohol Research & Health. 2006;29:172–178. [PMC free article] [PubMed] [Google Scholar]
- Hesse M, Tutenges S. Evening experiences versus drinking indicators as predictors of hangover on a summer holiday. American Journal on Addictions. 2009;18:130–134. doi: 10.1080/10550490802544367. [DOI] [PubMed] [Google Scholar]
- Hesselbrock VM, Hesselbrock MM. Developmental perspectives on the risk for developing substance abuse problems. In: Miller WR, Carroll KM, editors. Rethinking substance abuse: What the science shows and what we should do about it. New York, NY: Guilford Press; 2006. pp. 97–114. [Google Scholar]
- Howland J, Almeida AB, Rohsenow DJ, Minsky SJ, Greece JA. How safe are federal regulations on occupational alcohol use? Journal of Public Health Policy. 2006;27:389–404. doi: 10.1057/palgrave.jphp.3200104. [DOI] [PubMed] [Google Scholar]
- Howland J, Rohsenow DJ, Allensworth-Davies D, Greece J, Almeida A, Minsky SJ, Hermos J. The incidence and severity of hangover the morning after moderate alcohol intoxication. Addiction. 2008a;103:758–765. doi: 10.1111/j.1360-0443.2008.02181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland J, Rohsenow DJ, Edwards EM. Are some drinkers resistant to hangover? A literature review. Current Drug Abuse Reviews. 2008b;1:42–46. doi: 10.2174/1874473710801010042. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Dale LC, Offord KP, Croghan IT, Hays JT, Gomez-Dahl L. Nicotine patch therapy for smoking cessation in recovering alcoholics. Addiction. 1995;90:1541–1546. doi: 10.1046/j.1360-0443.1995.9011154112.x. [DOI] [PubMed] [Google Scholar]
- Jackson KM. Heavy episodic drinking: Determining the predictive utility of five or more drinks. Psychology of Addictive Behaviors. 2008;22:68–77. doi: 10.1037/0893-164X.22.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KM, Colby SM, Sher KJ. Daily patterns of conjoint smoking and drinking in college student smokers. Psychology of Addictive Behaviors. 2010;24:424–435. doi: 10.1037/a0019793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, George TP, Kleber HD. The neurobiology of substance dependence: Implications for treatment. In: Frances RJ, Miller SI, Mack AH, editors. Clinical textbook of addictive disorders. 3rd ed. New York, NY: Guilford Press; 2005. pp. 3–15. [Google Scholar]
- Kouri EM, McCarthy EM, Faust AH, Lukas SE. Pre-treatment with transdermal nicotine enhances some of ethanol’s acute effects in men. Drug and Alcohol Dependence. 2004;75:55–65. doi: 10.1016/j.drugalcdep.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Jelinek LC, Pope MA. Cigarette smoking among alcohol abusers: A continuing and neglected problem. Canadian Journal of Public Health. 1986;77:205–207. [PubMed] [Google Scholar]
- MacAndrew C. The differentiation of male alcoholic outpatients from nonalcoholic psychiatric outpatients by means of the MMPI. Quarterly Journal of Studies on Alcohol. 1965;26:238–246. [PubMed] [Google Scholar]
- Madden PAF, Heath AC, Starmer GA, Whitfield JB, Martin NG. Alcohol sensitivity and smoking history in men and women. Alcoholism: Clinical and Experimental Research. 1995;19:1111–1120. doi: 10.1111/j.1530-0277.1995.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Marks JL, Hill EM, Pomerleau CS, Mudd SA, Blow FC. Nicotine dependence and withdrawal in alcoholic and nonalcoholic ever-smokers. Journal of Substance Abuse Treatment. 1997;14:521–527. doi: 10.1016/s0740-5472(97)00049-4. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Miller WR. Estimating blood alcohol concentration: Two computer programs and their applications in therapy and research. Addictive Behaviors. 1979;4:55–60. doi: 10.1016/0306-4603(79)90021-2. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Pretorius MB. Sons of alcoholics report greater hangover symptoms than sons of nonalcoholics: A pilot study. Alcoholism: Clinical and Experimental Research. 1990;14:713–716. doi: 10.1111/j.1530-0277.1990.tb01231.x. [DOI] [PubMed] [Google Scholar]
- Penning R, McKinney A, Verster JC. Alcohol hangover symptoms and their contribution to the overall hangover severity. Alcohol and Alcoholism. 2012;47:248–252. doi: 10.1093/alcalc/ags029. [DOI] [PubMed] [Google Scholar]
- Penning R, van Nuland M, Fliervoet LAL, Olivier B, Verster JC. The pathology of alcohol hangover. Current Drug Abuse Reviews. 2010;3:68–75. doi: 10.2174/1874473711003020068. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jahng S, Wood PK, Robertson BM, Epler AJ, Cronk NJ, Sher KJ. The subjective effects of alcohol-tobacco co-use: An ecological momentary assessment investigation. Journal of Abnormal Psychology. 2011;120:557–571. doi: 10.1037/a0023033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Sher KJ, Slutske WS, Jackson KM. Hangover frequency and risk for alcohol use disorders: Evidence from a longitudinal high-risk study. Journal of Abnormal Psychology. 2005;114:223–234. doi: 10.1037/0021-843X.114.2.223. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Slutske WS, Wood PK, Hunt-Carter EE. Frequency and correlates of diary-measured hangoverlike experiences in a college sample. Psychology of Addictive Behaviors. 2010;24:163–169. doi: 10.1037/a0017148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse? Neuroscience & Biobehavioral Reviews. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Prat G, Adan A, Sánchez-Turet M. Alcohol hangover: A critical review of explanatory factors. Human Psychopharmacology: Clinical & Experimental. 2009;24:259–267. doi: 10.1002/hup.1023. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, editors. Hierarchical linear models: Applications and data analysis methods. 2nd ed. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Raudenbush SW, Bryk AS, Cheong YF, Congdon R, editors. HLM 6: Hierarchical linear and nonlinear modeling. Chicago, IL: Scientific Software International; 2004. [Google Scholar]
- Rohleder N, Kirschbaum C. The hypothalamic-pituitary-adrenal (HPA) axis in habitual smokers. International Journal of Psychophysiology. 2006;59:236–243. doi: 10.1016/j.ijpsycho.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Howland J, Arnedt JT, Almeida AB, Greece J, Min-sky S, Sales S. Intoxication with bourbon versus vodka: Effects on hangover, sleep, and next-day neurocognitive performance in young adults. Alcoholism: Clinical and Experimental Research. 2010;34:509–518. doi: 10.1111/j.1530-0277.2009.01116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Howland J, Minsky SJ, Arnedt JT. Effects of heavy drinking by maritime academy cadets on hangover, perceived sleep, and next-day ship power plant operation. Journal of Studies on Alcohol. 2006;67:406–415. doi: 10.15288/jsa.2006.67.406. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Howland J, Minsky SJ, Greece J, Almeida A, Roehrs TA. The Acute Hangover Scale: A new measure of immediate hangover symptoms. Addictive Behaviors. 2007;32:1314–1320. doi: 10.1016/j.addbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Howland J, Winter M, Bliss CA, Littlefield CA, Heeren TC, Calise TV. Hangover sensitivity after controlled alcohol administration as predictor of post-college drinking. Journal of Abnormal Psychology. 2012;121:270–275. doi: 10.1037/a0024706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Brauer LH, Behm FM, Cramblett M, Calkins K, Lawhon D. Psychopharmacological interactions between nicotine and ethanol. Nicotine & Tobacco Research. 2004;6:133–144. doi: 10.1080/14622200310001656957. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Piasecki TM, Hunt-Carter EE. Development and initial validation of the Hangover Symptoms Scale: Prevalence and correlates of hangover symptoms in college students. Alcoholism: Clinical and Experimental Research. 2003;27:1442–1450. doi: 10.1097/01.ALC.0000085585.81711.AE. [DOI] [PubMed] [Google Scholar]
- Snijders T, Bosker R, editors. Multilevel analysis: An introduction to basic and advanced multilevel modeling. Thousand Oaks, CA: Sage; 1999. [Google Scholar]
- Swan GE, Carmelli D, Cardon LR. Heavy consumption of cigarettes, alcohol and coffee in male twins. Journal of Studies on Alcohol. 1997;58:182–190. doi: 10.15288/jsa.1997.58.182. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services. Treating tobacco use and dependence: Clinical practice guideline. Washington, DC: Public Health Service; 2000. [Google Scholar]
- Verster JC, Stephens R, Penning R, Rohsenow D, McGeary J, Levy D, Young M. The alcohol hangover research group consensus statement on best practice in alcohol hangover research. Current Drug Abuse Reviews. 2010;3:116–126. doi: 10.2174/1874473711003020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall TL, Horn SM, Johnson ML, Smith TL, Carr LG. Hangover symptoms in Asian Americans with variations in the aldehyde dehydrogenase (ALDH2) gene. Journal of Studies on Alcohol. 2000;61:13–17. doi: 10.15288/jsa.2000.61.13. [DOI] [PubMed] [Google Scholar]
- Wall TL, Shea SH, Luczak SE, Cook TA, Carr LG. Genetic associations of alcohol dehydrogenase with alcohol use disorders and endophenotypes in white college students. Journal of Abnormal Psychology. 2005;114:456–465. doi: 10.1037/0021-843X.114.3.456. [DOI] [PubMed] [Google Scholar]
- Zhang L, Samet J, Caffo B, Punjabi NM. Cigarette smoking and nocturnal sleep architecture. American Journal of Epidemiology. 2006;164:529–537. doi: 10.1093/aje/kwj231. [DOI] [PubMed] [Google Scholar]