Abstract
Purpose.
Multidrug resistance protein 4 (MRP4) effluxes a wide variety of endogenous compounds, including cyclic adenosine monophosphate (cAMP), and is exclusively expressed in vascular endothelial cells (ECs) of the retina. This study aimed to investigate the role of MRP4 in retinal vascular development.
Methods.
The retinal vascular phenotype of Mrp4−/− mice was examined by whole-mount immunohistochemistry at P3, P6, and P14. The retinas from P6 pups that received an intraperitoneal injection of either solvent control or forskolin, an inducer of intracellular cAMP formation, at P4 and P5 were analyzed in terms of their vascular formation (vascular length, vascular branching, vascular density, and the number of tip cells), cell proliferation and apoptosis, and vessel stability.
Results.
The Mrp4−/− mice exhibited no overt abnormalities in the development of the retinal vasculature, but retinal vascular development in the Mrp4−/− mice was suppressed in response to forskolin administration. There was a significant decrease in the vascular length, vascular branching, and vascular density, and inhibited tip cell formation at the vascular front. The forskolin-treated Mrp4−/− mice showed an increased number of Ki67-positive and cleaved caspase 3–positive ECs, a significant decrease in the amount of pericyte coverage, and a reduced number of empty sleeves. In pups exposed to hyperoxia (75% oxygen) from P7 to P12, the Mrp4−/− mice showed a significant increase in the unvascularized retinal area.
Conclusions.
Mrp4−/− mice exhibited suppressed retinal vascular development in response to forskolin treatment. Thus, Mrp4 might have protective roles in retinal vascular development by regulating the intracellular cAMP level.
The Mrp4−/− mice showed decreased retinal vascular density in response to forskolin treatment. Together with the increased apoptosis, decreased pericyte coverage, and enhanced vessel regression, Mrp4 might act protectively in retinal vascular development by regulating the intracellular cAMP level.
Introduction
Multidrug resistance protein 4 (MRP4, ABCC4) is a member of the ATP-binding cassette transporter family and acts as an energy-dependent transmembrane efflux transporter. MRP4 is extensively expressed in human tissues, including the kidney, liver, erythrocytes, adrenal gland, platelets, brain, and pancreas,1 and contributes to the maintenance of normal organ function by actively transporting a wide variety of xenobiotics and physiologic substrates, such as cyclic nucleotides.2–5 Previous experiments revealed that MRP4 inhibition prevents neointima formation after carotid artery injury and shows protective effects on hypoxia-induced pulmonary arterial hypertension by increasing the intracellular levels of cyclic nucleotides.6,7
Cyclic adenosine monophosphate (cAMP) is an important cyclic nucleotide that affects numerous cellular processes via the activation of various downstream targets, such as protein kinase A (PKA). Accumulated evidence indicates a relationship between cAMP signaling and vascular development: cAMP signaling plays an important role in ischemic neovascularization, mainly through the activation of the PKA/endothelial nitric oxide synthase pathway.8 Because MRP4 is reported to be expressed in retinal vascular endothelial cells (ECs)9,10 and involved in the efflux transport of cAMP,2–5 MRP4 is expected to play a critical role in vascular development. Using human retinal microvascular ECs, we previously conducted in vitro experiments to investigate the angiogenic properties of MRP4, demonstrating that MRP4 knockdown enhanced EC migration, decreased cell apoptosis, and resulted in a massive tubelike structure in a tube-formation assay.11 However, to the best of our knowledge, the in vivo role of Mrp4 in retinal vascular development remains unknown.
In the present study, we used Mrp4-knockout mice and forskolin, an inducer of intracellular cAMP, to investigate the functional importance of Mrp4 in the process of retinal vascular development. We provide the first in vivo evidence that MRP4 is involved in retinal vascular development.
Materials and Methods
Mice
C57BL/6J mice were purchased from a commercial supplier (CLEA Japan, Inc., Tokyo, Japan); Mrp4-knockout mice, which were established previously,12 were repeatedly backcrossed to the C57BL/6J mice. All of the mice were maintained under standard laboratory conditions (12/12 hours light/dark cycle and 20–24°C; food and water were provided without restriction). The Mrp4-knockout mice were crossed with wild-type (WT) or mutant mice, and their pups were genotyped by PCR before use in the experiments. All of the animal experiments were designed and conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the Kobe University Animal Experimentation Regulation. This study was approved by the Institutional Animal Care and Use Committee of Kobe University Graduate School of Medicine (Permission number: P101109).
Forskolin Treatment
Forskolin (Wako Biochemicals, Osaka, Japan) was dissolved in dimethyl sulfoxide (DMSO) and injected intraperitoneally into neonatal mice at postnatal days 4 (P4) and 5 (P5). Mice injected with DMSO served as the controls. The treated mice were euthanized at P6, and their retinas were isolated for whole-mount immunohistochemistry (IHC). We first tested the effect of different concentrations of forskolin on the survival rate and retinal vasculature and determined the optimal concentration, 1.0 μg/50 μL (0.3 mg/kg) at P4 and 1.5 μg/50 μL (0.5 mg/kg) at P5, used to compare the retinal vascular phenotypes between WT mice and Mrp4-deficient mice.
Oxygen-Induced Vasoobliteration Model in Mice
Oxygen-induced retinopathy was produced as previously reported.13 Litter-matched newborn mice with nursing mothers were maintained in a 75% oxygen chamber from P7 to P12. The chamber was closed during hyperoxia exposure. The unvascularized central retinal area was measured using commercial software (Photoshop CS; Adobe Systems, San Jose, CA) as previously reported, and the percentage of avascular area over the total retinal area was calculated.14
Immunohistochemistry
The immunostaining of whole-mount retinas was performed as previously described.10 In brief, after cardiac perfusion of 4% paraformaldehyde (PFA)/phosphate-buffered saline (PBS), the eyes were enucleated, and the retinal cups were isolated from the other parts of the eyes. The retinal cups were washed twice for 30 minutes with PBS and then incubated with 1% bovine serum albumin (BSA) in PBS containing 0.3% Triton X-100 (0.3% PBST) for 60 minutes at room temperature (RT) or overnight at 4°C to block any nonspecific binding. Next, the retinal cups were incubated with primary antibodies in 0.3% PBST containing 1% BSA overnight at 4°C, washed 5 times in 0.1% PBST for 30 minutes, and incubated with secondary antibodies overnight at 4°C. The cups were rinsed 5 times with 0.1% PBST, mounted in commercial antifade reagent (ProLong Gold Antifade Reagent; Invitrogen Molecular Probes, Carlsbad, CA), and covered with a coverslip. Fluorescence images were acquired and analyzed using a confocal laser scanning microscope (LSM-700; Carl Zeiss, Tokyo, Japan).
Quantitative Analysis
All of the quantifications were performed using the acquired confocal images of the flat-mount retinas. The vascular length, number of branch points, and percentage of area coverage by the ECs were calculated from the images in the proximal retina at ×100 magnification. The radial length of the vascular network was measured from the optic nerve head to the edge of the vascular network. The number of endothelial tip cells was quantified at the sprouting vascular front at ×200 magnification. The tip cells were defined as ECs extending numerous thin filopodia. The numbers of cleaved caspase 3–positive ECs and empty sleeves were evaluated in the proximal retina (×100 magnification), and the number of Ki67-positive ECs was counted at the vascular front. For quantification, 16 randomly selected fields from 4 mouse retinas (4 fields per retina) were analyzed using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsbweb.nih.gov/ij/index.html) or commercial analytical software (BZ-Analyzer software; Keyence, Osaka, Japan).
Antibodies
The primary antibodies used in this study were as follows: rat monoclonal anti-PECAM (clone MEC13.3; Santa Cruz Biotechnology, Inc., Tokyo, Japan), rabbit monoclonal anti-Ki67 (clone SP6; Thermo Scientific, Kanagawa, Japan), Cy3-conjugated monoclonal anti–α-smooth muscle actin (α-SMA) (clone 1A4; Sigma-Aldrich Japan, Tokyo, Japan), Cy3-conjugated rabbit polyclonal anti-NG2 (Millipore, Amsterdam, The Netherlands), rabbit polyclonal anti-cleaved caspase 3 (Cell Signaling, Tokyo, Japan), and rabbit polyclonal anti-type 4 collagen (Col4) (Cosmo Bio, Tokyo, Japan). The secondary antibodies used were Alexa Fluor 488–conjugated anti-rat IgG (Invitrogen) and Cy3-conjugated anti-rabbit IgG (Sigma-Aldrich).
Statistical Analysis
Nonparametric tests were used in the statistical analyses because the Shapiro–Wilk test revealed that some of the data deviated from a normal distribution. However, the descriptive statistics are provided as the mean ± SD, not as the median and range, so that the readers can easily understand our experimental data. The data were first analyzed using the Kruskal–Wallis test. Then, the Conover–Iman test with the Bonferroni adjustment was carried out for post hoc pairwise comparisons when the Kruskal–Wallis test showed a positive result (P < 0.05). The statistical analysis was performed using commercial statistical software (IBM/SPSS, version 19; IBM, Tokyo, Japan; or XLStat software; Addinsoft, Paris, France, as appropriate).
Results
Mrp4-Knockout Mice Exhibit Normal Retinal Vascular Development
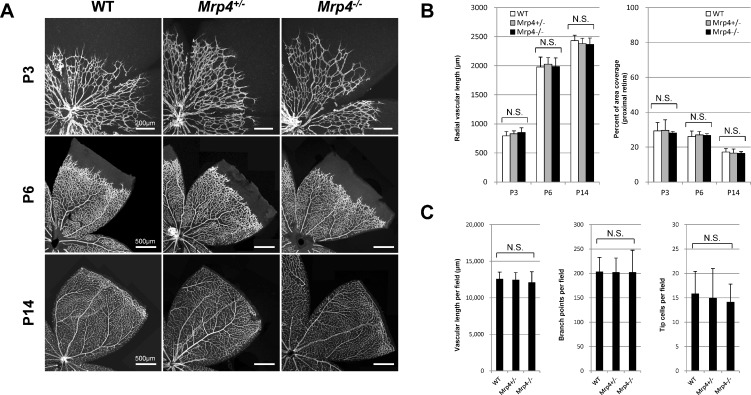
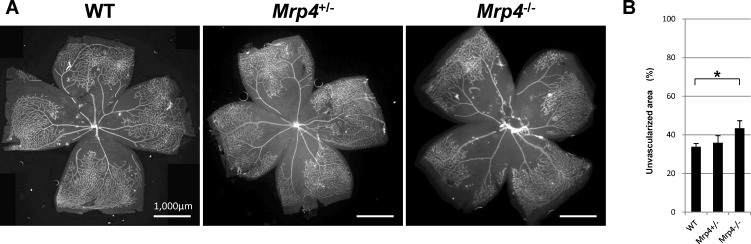
To identify the role of Mrp4 in retinal vascular development, we first investigated the postnatal retinal vasculature at different time points: P3, P6, and P14. Whole-mount immunostaining for PECAM, a well-known EC marker, showed normal retinal vascular development in the Mrp4−/− mice at all of the time points. We found no overt changes in vascular length, vascular branching, or vascular density in the Mrp4−/− mice. The Mrp4−/− mice exhibited normal radial vascular growth, and the number of tip cells at the angiogenic front, a hallmark of sprouting angiogenesis, was comparable between the Mrp4−/− mice and control mice (Fig. 1; see Supplementary Material and Supplementary Fig. S1, http://www.iovs.org/content/53/13/8029/suppl/DC1).
Figure 1. .
Retinal vascular development in Mrp4-knockout mice. (A) Whole-mount immunostaining for PECAM in retinas from WT, Mrp4+/−, and Mrp4−/− mice at different postnatal stages. (B) Quantification of the vascular parameters at P3, P6, and P14. (C) Quantification of the vascular parameters at P6. N.S., not significant. The data are provided as the mean ± SD.
Intraperitoneal Forskolin Administration Reduces Retinal Vascular Network Formation and Sprouting Angiogenesis in Mrp4-Knockout Mice
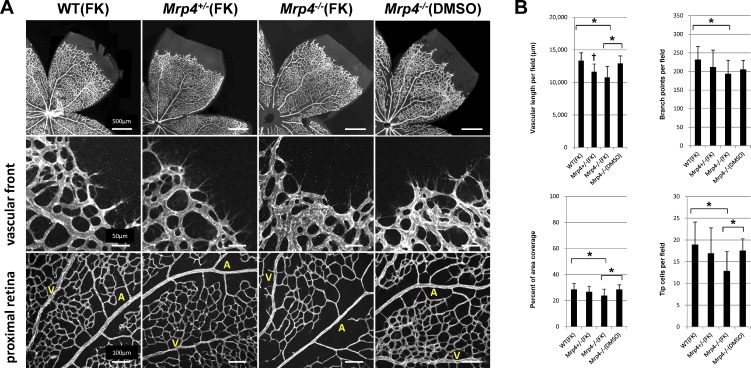
Because a normal retinal vasculature was observed in the Mrp4-knockout mice, we next addressed whether the enhanced elevation of the intracellular cAMP level can affect the retinal vascular phenotype in Mrp4-knockout mice. Following postnatal intraperitoneal forskolin administration, the retinas from P6 Mrp4−/− mice exhibited a significant decrease in vascular length, vascular branching, and vascular density. In addition, the tip cells were less abundant at the vascular front in Mrp4−/− mice than in control mice (Fig. 2). However, the Mrp4−/− mice showed no delay in retinal vascular extension following forskolin treatment (see Supplementary Material and Supplementary Fig. S2, http://www.iovs.org/content/53/13/8029/suppl/DC1).
Figure 2. .
Altered retinal vascular formation in forskolin-treated Mrp4-knockout mice. (A) Whole-mount immunostaining for PECAM in P6 retinas from WT, Mrp4+/−, and Mrp4−/− mice after intraperitoneal forskolin or DMSO administration from P4 to P5. (B) Quantification of the vascular parameters. P value is <0.01 (*). There is a significant difference between forskolin-treated Mrp4+/− mice and forskolin-treated WT mice or DMSO-treated Mrp4−/− mice (P < 0.01) (†). A, artery; V, vein. The data are provided as the mean ± SD.
Forskolin Treatment Enhances Retinal EC Proliferation and Apoptosis in Mrp4-Knockout Mice
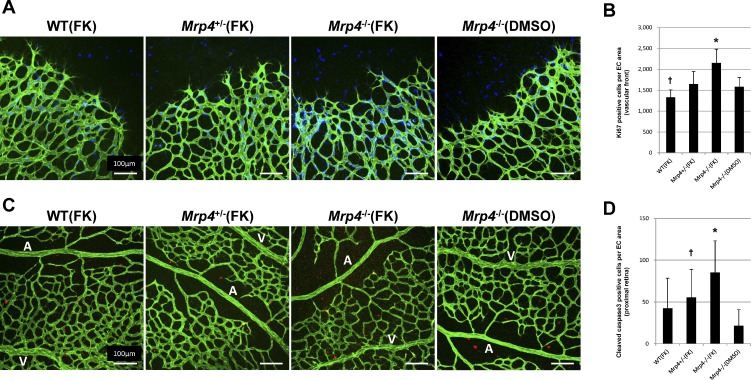
To determine the causes of the reduced retinal vascular network formation and sprouting angiogenesis in the forskolin-treated Mrp4-knockout mice, we investigated the effect of forskolin treatment on EC proliferation and apoptosis. Mrp4−/− mice exhibited an increased number of ECs positive for Ki67, a cellular marker for proliferation, at the vascular front. Apoptotic ECs that were double stained for PECAM and cleaved caspase 3, a marker for cells undergoing apoptosis, were more abundant in the proximal retina in the Mrp4−/− mice compared with control mice (Fig. 3). In contrast, nontreated animals showed the comparative number of cleaved caspase 3–positive ECs and Ki67-positive ECs between genotypes (see Supplementary Material and Supplementary Fig. S3, http://www.iovs.org/content/53/13/8029/suppl/DC1). There was no significant difference in the number of Ki67-positive ECs in the proximal retina or cleaved caspase 3–positive ECs in the distal retina (data not shown).
Figure 3. .
Intraperitoneal forskolin administration enhances both retinal EC proliferation and apoptosis in Mrp4-knockout mice. (A) Images of P6 retinas (at the vascular front) from forskolin- or DMSO-treated mice labeled for PECAM (green) and Ki67 (blue). (B) Quantification of the number of Ki67-positive cells per EC area. P value is < 0.01 in comparison between forskolin-treated Mrp4−/− mice and any other group (*). There is a significant difference between forskolin-treated WT mice and forskolin-treated Mrp4+/− mice or DMSO-treated Mrp4−/− mice (P < 0.01) (†). (C) Images of P6 retinas (in the proximal retina) from forskolin- or DMSO-treated mice labeled for PECAM (green) and cleaved caspase 3 (red). (D) Quantification of the number of cleaved caspase 3–positive cells per EC area. P value is <0.01 in comparison between forskolin-treated Mrp4−/− mice and forskolin-treated WT mice or DMSO-treated Mrp4−/− mice. There is a significant difference between forskolin-treated Mrp4+/− mice and DMSO-treated Mrp4−/− mice (P < 0.01) (†). A, artery; V, vein. The data are provided as the mean ± SD.
Decreased Vessel Stability in Mrp4-Knockout Mice following Forskolin Treatment
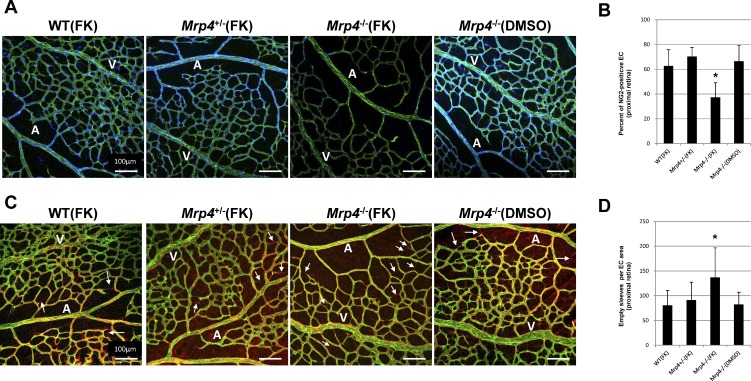
To determine whether vessel stability contributes to retinal vascular network formation following forskolin administration, we examined the amount of pericyte coverage and the number of empty basement membrane sleeves. Empty sleeves are immunoreactive for Col4 but not PECAM and are considered an indicator of vessel regression.15 There was a significant decrease in the amount of pericyte coverage, as determined by the percentage of ECs positive for a pericyte marker NG2 per field, in the proximal retina between the Mrp4−/− and control mice following forskolin treatment. In addition, an increased number of empty sleeves in the proximal capillary plexus was confirmed in the forskolin-treated Mrp4−/− mice (Fig. 4).
Figure 4. .
Forskolin treatment decreases vessel stability in Mrp4-knockout mice. (A) Whole-mount staining for PECAM (green) and NG2 (blue) showing the degree of pericyte coverage in the proximal retina of forskolin- or DMSO-treated P6 mice. (B) Quantification of the number of NG2-positive cells per EC area. P value is <0.01 in comparison between forskolin-treated Mrp4−/− mice and any other group (*). (C) Whole-mount staining of PECAM (green) and type 4 collagen (Col4) (red) in forskolin- or DMSO-treated P6 retinas. Empty sleeves (Col4+, PECAM−) are observed in the proximal vascular plexus (arrows). (D) Quantification of the number of empty sleeves per EC area. P value is <0.01 in comparison between forskolin-treated Mrp4−/− mice and any other group (*). A, artery; V, vein. The data are provided as the mean ± SD.
Hyperoxia Augments Retinal Vessel Regression in Mrp4-Knockout Mice
The increased number of empty sleeves in forskolin-treated Mrp4−/− mice implies that these mice have more unstable retinal vasculature than that of WT mice. To confirm this theory, we assessed the role of Mrp4 in oxygen-induced vasoobliteration. Mouse pups were exposed to continuous hyperoxia (75% oxygen) from P7 to P12, inducing widespread capillary obliteration in the proximal retina. The Mrp4−/− mice showed a significant increase in the unvascularized retinal area compared with the WT mice at P12 (Fig. 5).
Figure 5. .
Increased hyperoxia-induced retinal vessel regression in Mrp4-knockout mice. (A) Whole-mount PECAM staining of P12 retinas after 5 days of hyperoxia exposure. (B) Quantification of the unvascularized area. The data are provided as the mean ± SD. P value is <0.01 in comparison between Mrp4−/− mice and WT mice (*).
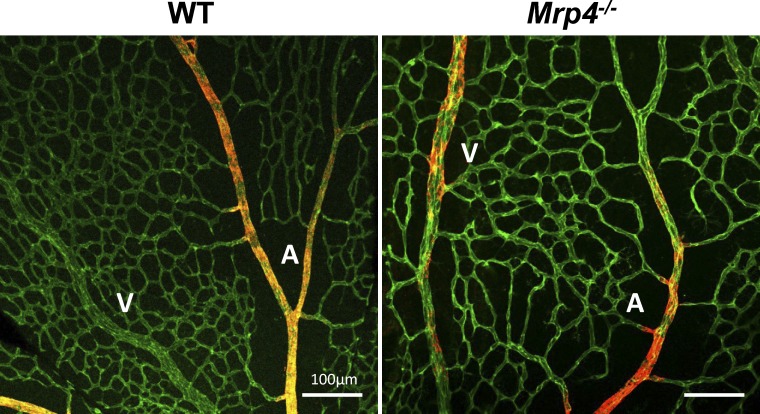
Altered Distribution of α-SMA–Positive ECs in P6 Retina from Forskolin-Treated Mrp4-Knockout Mice
We thought of arterialization of the retinal vasculature as another possible cause of the reduced retinal vascular density observed in the forskolin-treated Mrp4-knockout mice because artery is normally surrounded by a capillary-free zone by hyperoxygenation. To test this idea, we examined the spreading of ECs that were immunoreactive for α-SMA, a hallmark of an artery in the developing retina, and found an altered α-SMA distribution in forskolin-treated Mrp4−/− mice: α-SMA–positive ECs were visible on both the veins and the arteries (Fig. 6).
Figure 6. .
Altered distribution of α-SMA–positive retinal ECs in forskolin-treated Mrp4-knockout mice. Whole-mount PECAM (green) and α-SMA (red) staining images at P6 retinas are shown. α-SMA–positive ECs are observed on developing arteries of WT retina (left), whereas α-SMA–positive ECs are seen on veins as well as arteries of Mrp4−/− retina (right). A, artery; V, vein.
Discussion
Although MRP4 was first identified as a drug transporter, recent studies have shown that this protein mediates the cellular efflux of a wide range of other endogenous compounds, including cyclic nucleotides and prostaglandins.1 Because both cAMP and prostaglandin E2 (PGE2) are substrates for MRP4 and accumulated evidence shows that cAMP and PGE2 are involved in angiogenesis,8,16–18 we hypothesized that MRP4 plays an important role in vascular development in vivo. However, we found that Mrp4−/− mice display no overt abnormalities in the development of retinal vasculature, which is compatible with the fact that mice homozygous for an Mrp4 mutant allele are viable.12 Sassi and colleagues5 found a compensatory mechanism involved in intracellular cAMP homeostasis in cardiac myocytes, which could account for the normal retinal vascular phenotype observed in Mrp4−/− mice, although the mechanism of homeostasis has not been tested in vascular ECs to date.
In contrast to the normal retinal vascular development in Mrp4−/− mice, intraperitoneal forskolin administration suppressed postnatal retinal vascular formation: a decrease in vascular length, vascular branching, and vascular density and reduced tip cell formation at the vascular front were observed. Because forskolin is a commonly used biochemical that activates adenylyl cyclase and rapidly increases intracellular cAMP levels, we speculate that the abnormal retinal vascular phenotype observed in forskolin-treated Mrp4−/− mice could be caused by an increased intracellular cAMP level. This hypothesis is supported by previous studies reporting that MRP4 silencing affects intracellular cAMP concentrations only under conditions of enhanced cAMP formation.5 Although an overdose of forskolin appears to be a preferred method to obtain a more dramatic retinal vascular phenotype, it seems to be infeasible because forskolin overdose resulted in the death of Mrp4−/− mice (data not shown). The molecular mechanisms underlying the observed phenotype should be further investigated because cAMP is involved in numerous signal transduction pathways.
Both the proliferation and apoptosis of vascular ECs play important roles in vascular network formation, and there are several lines of evidence that show that MRP4 is related to cell proliferation and apoptosis.6,7,11,19 We found a significant increase in cleaved caspase 3–positive ECs and Ki67-positive ECs in the retinas of forskolin-treated Mrp4−/− mice, which could partly explain the cellular mechanisms of the abnormal retinal vascular development. However, our previous in vitro experiments demonstrated that MRP4 knockdown does not alter cell proliferation but does reduce cell apoptosis, although forskolin treatment was not performed in these experiments.11 Furthermore, previous reports have shown that cAMP signaling enhances proliferation but inhibits apoptosis in vascular ECs.8,20,21 These discrepancies between our in vivo experimental results and previous reports might be due to the contribution of nonvascular retinal cells (e.g., pericytes) to the retinal EC fate or the effects of other MRP4 endogenous substrates.
In addition to increased cell apoptosis, decreased vessel stability might be responsible for the reduced retinal vascular density observed in the forskolin-treated Mrp4−/− mice. In the present study, significant differences were found in the degree of pericyte coverage and the number of empty sleeves between the Mrp4−/− and control mice, suggesting that the increased regression of established vessels devoid of pericytes is likely to cause reduced retinal vascular density. Our oxygen-induced vasoobliteration experiments reinforce the idea that the retinal vasculature is more vulnerable in Mrp4−/− mice than in WT mice. We hypothesized that another possible cause of the reduced retinal vascular density is arterialization of the retinal vasculature. Arterialization ameliorates ischemia and, thereby, results in a decrease in capillary density, and it was reported that cAMP signaling leads to an arterial fate for vascular progenitors.22 Our immunohistochemical data were concordant with this hypothesis. Expression of α-SMA, an arterial marker, was detected on the veins as well as on the arteries in forskolin-treated Mrp4−/− mice.
In conclusion, we identified the importance of Mrp4 in retinal vascular development in vivo. The result that the genetic ablation of Mrp4 suppresses retinal vascular development in combination with forskolin treatment suggests that an enhanced intracellular cAMP level could contribute to the formation of abnormal retinal vasculature; however, the molecular mechanisms remain elusive. Together with the increased EC proliferation and apoptosis, decreased pericyte coverage, and enhanced vessel regression observed in Mrp4−/− mice, Mrp4 might have protective roles in retinal vascular development through the regulation of the intracellular cAMP level. Because the mechanisms involved in postnatal retinal vascular development appear to share significant similarities to those in pathologic neovascular formation, preventing Mrp4 dysfunction might be a novel therapeutic approach for retinal vascular disorders.
Supplementary Material
Footnotes
Supported by Grants-in-Aid for Young Scientists (B) 22791660 and 24791854; the Ministry of Education, Culture, Sport, Science, and Technology (MEXT), Japan (SK); and National Institute of General Medical Sciences/National Institutes of Health Grant 2R01-GM60904 (JDS).
Disclosure: W. Matsumiya, None; S. Kusuhara, None; K. Hayashibe, None; K. Maruyama, None; H. Kusuhara, None; M. Tagami, None; J.D. Schuetz, None; A. Negi, None
References
- 1.Borst P, de Wolf C, van de Wetering K. Multidrug resistance-associated proteins 3, 4, and 5. Pflügers Arch. 2007;453:661–673 [DOI] [PubMed] [Google Scholar]
- 2.Ritter CA, Jedlitschky G, Meyer zu Schwabedissen H, Grube M, Kock K, Kroemer HK. Cellular export of drugs and signaling molecules by the ATP-binding cassette transporters MRP4 (ABCC4) and MRP5 (ABCC5). Drug Metab Rev. 2005;37:253–278 [DOI] [PubMed] [Google Scholar]
- 3.Russel FG, Koenderink JB, Masereeuw R. Multidrug resistance protein 4 (MRP4/ABCC4): a versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol Sci. 2008;29:200–207 [DOI] [PubMed] [Google Scholar]
- 4.Ose A, Ito M, Kusuhara H, et al. Limited brain distribution of [3R,4R,5S]-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate phosphate (Ro 64-0802), a pharmacologically active form of oseltamivir, by active efflux across the blood-brain barrier mediated by organic anion transporter 3 (Oat3/Slc22a8) and multidrug resistance-associated protein 4 (Mrp4/Abcc4). Drug Metab Dispos. 2009;37:315–321 [DOI] [PubMed] [Google Scholar]
- 5.Sassi Y, Abi-Gerges A, Fauconnier J, et al. Regulation of cAMP homeostasis by the efflux protein MRP4 in cardiac myocytes. FASEB J. 2012;26:1009–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sassi Y, Lipskaia L, Vandecasteele G, et al. Multidrug resistance-associated protein 4 regulates cAMP-dependent signaling pathways and controls human and rat SMC proliferation. J Clin Invest. 2008;118:2747–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hara Y, Sassi Y, Guibert C, et al. Inhibition of MRP4 prevents and reverses pulmonary hypertension in mice. J Clin Invest. 2011;121:2888–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bir SC, Xiong Y, Kevil CG, Luo J. Emerging role of PKA/eNOS pathway in therapeutic angiogenesis for ischaemic tissue diseases. Cardiovasc Res. 2012;95:7–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tachikawa M, Toki H, Tomi M, Hosoya K. Gene expression profiles of ATP-binding cassette transporter A and C subfamilies in mouse retinal vascular endothelial cells. Microvasc Res. 2008;75:68–72 [DOI] [PubMed] [Google Scholar]
- 10.Tagami M, Kusuhara S, Honda S, Tsukahara Y, Negi A. Expression of ATP-binding cassette transporters at the inner blood-retinal barrier in a neonatal mouse model of oxygen-induced retinopathy. Brain Res. 2009;1283:186–193 [DOI] [PubMed] [Google Scholar]
- 11.Tagami M, Kusuhara S, Imai H, et al. MRP4 knockdown enhances migration, suppresses apoptosis, and produces aggregated morphology in human retinal vascular endothelial cells. Biochem Biophys Res Commun. 2010;400:593–598 [DOI] [PubMed] [Google Scholar]
- 12.Leggas M, Adachi M, Scheffer GL, et al. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol. 2004;24:7612–7621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith LE, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111 [PubMed] [Google Scholar]
- 14.Stahl A, Connor KM, Sapieha P, et al. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010;51:2813–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2003;163:1801–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamiyama M, Pozzi A, Yang L, DeBusk LM, Breyer RM, Lin PC. EP2, a receptor for PGE2, regulates tumor angiogenesis through direct effects on endothelial cell motility and survival. Oncogene. 2006;25:7019–7028 [DOI] [PubMed] [Google Scholar]
- 17.Rao R, Redha R, Macias-Perez I, et al. Prostaglandin E2-EP4 receptor promotes endothelial cell migration via ERK activation and angiogenesis in vivo. J Biol Chem. 2007;282:16959–16968 [DOI] [PubMed] [Google Scholar]
- 18.Yanni SE, Barnett JM, Clark ML, Penn JS. The role of PGE2 receptor EP4 in pathologic ocular angiogenesis. Invest Ophthalmol Vis Sci. 2009;50:5479–5486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Copsel S, Garcia C, Diez F, et al. Multidrug resistance protein 4 (MRP4/ABCC4) regulates cAMP cellular levels and controls human leukemia cell proliferation and differentiation. J Biol Chem. 2011;286:6979–6988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisslthaler B, Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res. 2009;105:114–127 [DOI] [PubMed] [Google Scholar]
- 21.Torella D, Gasparri C, Ellison GM, et al. Differential regulation of vascular smooth muscle and endothelial cell proliferation in vitro and in vivo by cAMP/PKA-activated p85alphaPI3K. Am J Physiol Heart Circ Physiol. 2009;297:H2015–H2025 [DOI] [PubMed] [Google Scholar]
- 22.Yamamizu K, Yamashita JK. Roles of cyclic adenosine monophosphate signaling in endothelial cell differentiation and arterial-venous specification during vascular development. Circ J. 2011;75:253–260 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.