Abstract
Burkholderia pseudomallei infections are fastidious to treat with conventional antibiotic therapy, often involving a combination of drugs and long-term regimes. Bacterial genetic determinants contribute to the resistance of B. pseudomallei to many classes of antibiotics. In addition, anaerobiosis and hypoxia in abscesses typical of melioidosis select for persistent populations of B. pseudomallei refractory to a broad spectrum of antibacterials. We tested the susceptibility of B. pseudomallei to the drugs hydroxyurea, spermine NONOate and DETA NONOate that release nitric oxide (NO). Our investigations indicate that B. pseudomallei are killed by NO in a concentration and time-dependent fashion. The cytoxicity of this diatomic radical against B. pseudomallei depends on both the culture medium and growth phase of the bacteria. Rapidly growing, but not stationary phase, B. pseudomallei are readily killed upon exposure to the NO donor spermine NONOate. NO also has excellent antimicrobial activity against anaerobic B. pseudomallei. In addition, persistent bacteria highly resistant to most conventional antibiotics are remarkably susceptible to NO. Sublethal concentrations of NO inhibited the enzymatic activity of [4Fe-4S]-cofactored aconitase of aerobic and anaerobic B. pseudomallei. The strong anti-B. pseudomallei activity of NO described herein merits further studies on the application of NO-based antibiotics for the treatment of melioidosis.
Keywords: antibiotics, antimicrobials, melioidosis, reactive nitrogen species, therapy, [4Fe-4S] clusters
Introduction
The Gram-negative, facultative intracellular pathogen B. pseudomallei is the causative agent of melioidosis. B. pseudomallei is a soil saprophyte widely distributed in Southeast Asia and Northern Australia. More recently, this microorganism has become an emerging opportunistic pathogen in Central and South America, and other tropical regions of the world (11). Humans and animals are often infected with B. pseudomallei through the respiratory tract upon contact with contaminated aerosols. Mammalian hosts can also be exposed to this microorganism through the oral mucosa after the ingestion of tainted food or water, or through skin abrasions and wounds (37).
The clinical presentations of B. pseudomallei infections in humans range from acute or chronic pneumonia to fatal septicemia. The progression of melioidosis is frequently associated with the development of pulmonary, hepatic and splenic abscesses that, for unclear reasons, involve prostatic tissue in Thai patients. Abscesses pose a physical barrier for the diffusion of antibiotics, and are consequently a major concern in the treatment of melioidosis (12). The extreme resistance of B. pseudomallei to multiple antibiotics further complicates antibiotic therapy of this fastidious infectious agent. The combination of microbial determinants and host physical barriers likely explain why lengthy regimes involving multiple classes of antibiotics are a common clinical practice in the therapy of melioidosis (7, 25). Despite extended treatments that last about 20 weeks, B. pseudomallei still relapse in 5-10% of the patients (8, 13, 26). Its intrinsic resistance to antibiotics, persistence in infected tissues, historical utilization as a bioweapon and the lack of a vaccine have led authorities in the United States to categorize B. pseudomallei as a category B select agent that requires BSL3 containment (9, 46, 50, 51).
The diatomic radical nitric oxide (NO) can be generated by NO synthase hemoproteins widely distributed among eukaryotes and present in some prokaryotic microorganisms. NO produced by the inducible NO synthase (iNOS) is a common component of the host arsenal against diverse pathogenic bacteria. For example, B. mallei and Pseudomonas aeruginosa are not only susceptible to iNOS-mediated cytotoxicity but they are also killed by a variety of reactive nitrogen species, including NO (4, 20, 21, 45, 53). The killing of these microorganisms by NO is quite remarkable, because most of the antimicrobial actions that this diatomic radical exerts against most microorganisms are manifested as cytostasis (14, 48). The iNOS hemoprotein appears, however, to be dispensable for host defense against B. pseudomallei in an experimental murine model of melioidosis (2, 3). The reason for the superfluous role of NO in the course of B. pseudomallei infection might reflect the capacity of this facultative intracellular pathogen to inhibit NO synthesis rather than the intrinsic resistance of these bacteria to reactive nitrogen species. Two mechanisms have been invoked by which B. pseudomallei minimize the production of NO by professional phagocytes. First, compared to lipopolysaccharide from enteric bacteria, the lipopolysaccharide of B. pseudomallei is hypostimulatory (1, 18). Second, although poorly characterized, B. pseudomallei downregulate NO synthesis by IFNγ-activated macrophages in an RpoS-depedent manner (45). The low levels of NO generated in response to B. pseudomallei infection probably confer a selective advantage to this microorganism. The concerted efforts of B. pseudomallei to inhibit NO synthesis may reflect hypersensitivity of this intracellular pathogen to NO oxidative and nitrosative congeners, offering unique opportunities for treating melioidosis. Given the NO-mediated killing of B. mallei and P. aeruginosa, we tested whether compounds that generate NO exert antimicrobial activity against B. pseudomallei. Our investigations indicate that several NO donors effectively kill aerobic, anaerobic and persistent populations of B. pseudomallei.
Materials and Methods
Bacterial strains and growth conditions
Strain K96243, a clinical isolate of B. pseudomallei, was grown in the BSL3 laboratory of the Department of Microbiology, University of Colorado School of Medicine, a facility certified by CDC for work with select agents. B. mallei strain ATCC 2344 and B. thailandensis E264 wild type strain were also used in the course of these investigations. The bacteria were grown overnight to stationary phase in Luria Bertani (LB) broth supplemented with 4% (w/v) glycerol (LBG) in a shaker incubator at 37°C and 315 RPM (New Brunswick Innova, Edison, NJ). Overnight cultures were diluted 1:100 in LBG broth or E-salts minimal medium supplemented with either 0.4% (w/v) glucose and 1% (w/v) yeast extract (EGYE), or 0.1% (w/v) casamino acids (ECA). Log phase bacteria were collected for experimentation at an OD600 of about 0.6. Bacterial cultures for anaerobic testing were pelleted by centrifugation and the samples moved into a Sheldon Bactron Anaerobic Chamber (Sheldon Manufacturing Inc., Cornelius, OR) containing a standard anaerobic gas mixure (5% H, 5% CO2, 90% N2). The B. pseudomallei bacterial pellets were resuspended in EGYE culture medium that had been deoxygenated in the anaerobic chamber for 24 h. The bacteria were adapted to the anaerobic environment for 2 h prior to challenge with the NO donors. Long-term anaerobiosis was achieved using shaking tubes in the “Wayne” model first described for work with Mycobacterium tuberculosis, and recently adapted for work with B. pseudomallei (16, 17). Hungate anaerobic glass tubes containing magnetic stir bars and filled with 14 ml of LBG broth were inoculated with log phase B. pseudomallei and sealed with Hungate stoppers and airtight screw caps. These cultures were stirred at 37°C for 31 days. Under these conditions, O2 and nutrients are gradually depleted from the culture medium and the bacteria are subjected to prolonged anaerobiosis.
Killing Assays
Aerobic bacteria grown to an OD600 of 0.6 were diluted 1:50 into the appropriate media and the samples aliquoted into round bottom 96-well plates (Evergreen Scientific, Los Angeles, CA). The bacterial cells were incubated with increasing concentrations of spermine NONOate (Cayman Chemical, Ann Arbor, MI) at 37°C for 2.5 h. For assays requiring anaerobic conditions, overnight cultures were diluted 1:50 into EGYE medium, and grown aerobically until they reached an OD600 of 0.6. Bacterial pellets were moved into an anaerobic chamber where they were resuspended in EGYE medium that had been depleted of O2 for 24 h. After 2 h of adaptation to the anaerobic environment, the bacteria were seeded into 96-well plates introduced into the anaerobic chamber 24 h earlier. The bacteria were then challenged with the NO donor spermine NONOate. Bacteria grown in the long-term “Wayne” anaerobic model of persistence were treated with the NO donor DETA NONOate (Cayman Chemical) during the last 24 h of culture. DETA NONOate and spermine NONOate were diluted to the desired concentrations in the indicated culture media from fresh 150 mM stocks prepared in 10 mM Tris-Cl buffer, pH 8.5. The half-lives of spermine NONOate and DETA NONOate at 37°C, pH 7.4, are 39 min and 20 h, respectively. At the indicated times, the surviving bacteria were diluted and plated on LB agar. The % of survival was calculated as (cfu tn/cfu t0) × 100.
Determination of MIC and MBC values
Standard minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations (MBC) were estimated in LBG broth inoculated with 106 CFU/ml of B. pseudomallei. Hydroxyurea (Sigma-Aldrich), spermine NONOate or DETA NONOate were tested at concentrations that ranged from 62.5 μg/ml to 1 mg/ml. The cultures were serially diluted and spotted on LB agar plates. The MIC was defined as the lowest concentration of the NO donor that visibly inhibited bacterial growth after 24 h of culture at 37°C with shaking, whereas the MBC was defined as the concentration of the NO donor that killed 99.9% of the original inoculum.
Aconitase enzymatic assay
Thirty-six ml of bacteria were grown in the presence of O2 in EGYE or ECA media to an OD600 of about 0.6. The bacterial cultures were treated with 100 μM spermine NONOate for 10 min. To study the effects of NO on the aconitase activity in the absence of O2, bacterial pellets were introduced into the anaerobic chamber and the cells resuspended in EGYE medium that had been deoxygenated in the anaerobic chamber for 24 h. After 2 h of adaptation in the anaerobic environment, the bacteria were challenged with 100 μM spermine NONOate for 10 min. Under these conditions, the viability of the bacteria was unaffected. The anaerobic cultures were removed from the chamber and the rest of the manipulations were conducted aerobically. The bacteria from the anaerobic and aerobic cultures were pelleted by centrifugation at 4000 RPM for 5 min. The pellets were washed in 4.5 ml of 50 mM Tris-HCl buffer, pH 7.4, and the bacteria were lysed after the addition of 1 ml of B-PER buffer (Pierce, Rockford, IL) while vortexing for 30 sec. Two milliliters of 50 mM Tris-HCl buffer, pH 7.4, were added to the suspension and the lysates clarified by centrifugation for 1 min at 14,000 RPM. The clear lysates were filter-sterilized twice and the surface of the tubes decontaminated in preparation for their removal from the BSL-3 facility. The samples were snap-frozen in liquid nitrogen and stored in liquid nitrogen until the enzymatic activity was assessed. Protein concentrations in the samples were normalized and the presence of aconitase activity tested in 30-60 μg protein as assessed by the formation of cis-aconitate in 50 mM Tris-HCl buffer, pH 7.4, containing 30 mM isocitrate. Aconitase activity is expressed as OD240/50 μg protein.
Results
Susceptibility of Burkholderia species to NO
We recently reported that B. mallei are highly susceptible to NO (21). In this study, we investigated the sensitivity of the related microorganisms B. mallei, B. pseudomallei and B. thailandensis to chemically generated NO. The NO donor spermine NONOate, which has a half-life of about 39 min and releases 2 moles of NO per mole of the compound, was used for most of these investigations. Bacteria grown to stationary phase were exposed to increasing concentrations of spermine NONOate (figure 1A). Stationary phase B. mallei subculture in fresh LB broth grew 3-fold during the 2.5 h of the experiment. As expected (21), B. mallei were highly susceptible to increasing concentrations of NO. The addition of 25 μM spermine NONOate killed over 99.9% of B. mallei after 2.5 h of treatment. Stationary phase B. pseudomallei and B. thailandensis were more resistant to NO than B. mallei. We also tested the susceptibility of rapidly growing Burkholderia species to NO. As seen with stationary phase bacteria, log phase B. mallei were readily killed by as little as 25 μM spermine NONOate (figure 1B). Although slightly less susceptible than B. mallei, log phase B. pseudomallei were also killed quite efficiently by spermine NONOate. In fact, 100 μM spermine NONOate reduced the number of B. pseudomallei 10,000-fold. In contrast to B. mallei and B. pseudomallei, log phase B. thailandensis were relatively resistant to spermine NONOate, since only about 10% of the population was killed by 100μM of the NO donor. The susceptibility of log phase bacteria to NO was studied in more detail in time kill curves (figure 1C). The addition of 100 μM spermine did not exert any measurable anti-Burkholderia activity 2h after exposure. In contrast, killing of log phase B. pseudomallei by 100μM spermine NONOate was linear for about 2 h, a time when approximately 99.9% of the original inoculum succumbed to the treatment. After 2 h, however, the surviving bacteria appear to be resistant to NO. These data suggest that the survivors express either innate or adaptive resistance to this diatomic radical. Alternatively, the survivors may represent persister cells in the population of B. pseudomallei. The anti-B. pseudomallei activity of spermine NONOate is dependent on the NO generating capacity of the drug, since the spermine control exerted no measurable antibiotic activity (not shown). Moreover, NO2- and NO3-, terminal oxidative products of NO, did not exert any appreciable killing of B. pseudomallei (not shown). Although B. mallei were slightly more susceptible to the cytotoxicity of NO, the time kill curves of B. mallei and B. pseudomallei followed similar kinetics. Early on, B. thailandensis were also efficiently killed by spermine NONOate; however, this microorganism resumed growth 1.5 h after challenge. The surviving bacteria after 2.5 h of 100 μM spermine NONOate treatment could represent a persistent population or a mutant population selected after NO treatment. While the former should become susceptible to NO after culture in fresh medium, the latter should remain resistant. To distinguish between these two possibilities, B. pseudomallei that survived challenge with 100 μM spermine NONOate were grown overnight to stationary phase in LB broth. Log phase bacteria grown from NO-treated controls were readily killed upon exposure to 100 μM spermine NONOate (figure 1D). These findings suggest that the fraction of bacteria that survive 2.5 h after NO challenge are a persistent population genetically susceptible to NO but in a physiological state refractory to NO cytotoxicity.
Figure 1. Cytotoxicity of NO against stationary and log phase Burkholderia species.
Stationary (A) and log (B) phase B. pseudomallei, B. thailandensis, and B. mallei were treated in EGYE (0.4% glucose, 1% yeast extract E salts medium) medium with increasing concentrations of the NO donor spermine NONOate. The fraction of bacteria surviving in the cultures was estimated 2.5 h after the addition of the NO donor. The survival of log phase bacteria was followed overtime after treatment with 100 μM spermine NONOate (sNO) (C). When expanded after overnight culture in LB broth, the apparently persistent population of B. pseudomallei (Bps) became susceptible to 100 μM sNO when grown in LB broth to log phase (log pers) (D). The data are the mean ± SD from 8-12 individual observations from 3 separate experiments.
Killing of B. pseudomallei by antibiotics that generate NO
In the following experiments, we determined the MIC and MBC values for 3 different NO donors (table 1). B. pseudomallei grown to an OD600 of 0.2 were seeded in 10 ml of LBG broth at a concentration of 106 CFU/ml. Increasing concentrations of the NO donors were added to the flasks and the MIC values recorded 24 h after challenge. Hydroxyurea showed the lowest MIC (i.e., 125 μg/ml) followed by DETA NONOate and spermine NONOate (i.e., 250μg/ml and >1 mg/ml, respectively). The anti-B. pseudomallei cytotoxicity of DETA NONOate appears to be associated with the NO generated by this drug, because the addition of 250 μg/ml polyamine DETA to LB broth did not inhibit bacterial growth after overnight culture. We calculated that 250 μg/ml of DETA NONOate generates a steady concentration of 1.5 μM NO in the media for the duration of the experiment. On the other hand, our estimates indicate that 1 mg/ml spermine NONOate generates 3.8 mM NO during the first hour, ever declining to about 1.5 μM and 90 nM after 7 h and 10 h, respectively. The MBC values for all the NO donors tested were higher than 1mg/ml. Further analysis indicated that 250 μg/ml of DETA NONOate did not reduce the viability of B. pseudomallei 2.5 h after challenge, whereas 1 mg/ml spermine NONOate killed about 99% of the bacterial cells (figure 2). Neither 250 μg/ml DETA nor 1 mg/ml spermine controls had any effects on bacterial viability 2.5 h after challenge. These data indicate that the anti-B. pseudomallei activity of DETA NONOate is likely due to the cytostasis exerted by the sustained fluxes of NO over an extended period of time. Despite the initial onslaught, the few survivors likely overcome the diminishing fluxes of NO generated by sperimine NONOate.
Table 1.
Susceptibility of B. pseudomallei to NO-dependent antibiotics
Minimal inhibitory concentration
Minimal bactericidal concentration
The data are from 3 independent experiments.
Figure 2. Comparison of the anti-B. pseudomallei activity of spermine NONOate and DETA NONOate.

The bacteria were exposed to 250 μg/ml DETA, 250 μg/ml DETA NONOate, 1 mg/ml spermine, or 1 mg/ml spermine NONOate as described for the experiments in table 1. Control represents the polyamine parent compound, whereas NONOate is the NO releasing derivative. The fraction of surviving bacteria was recorded 2.5 h after challenge. The data are the mean ± SD from 8 individual observations from 2 separate experiments.
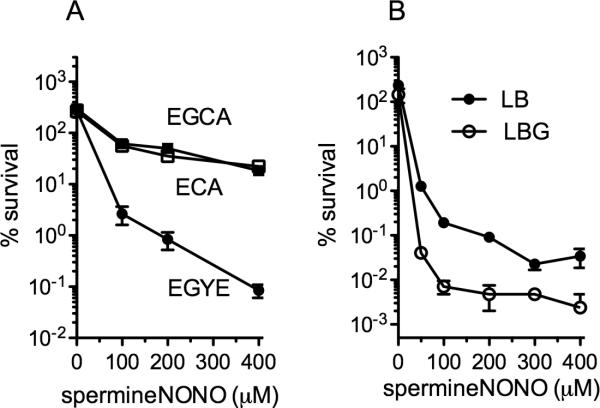
Influence of culture media on the susceptibility of B. pseudomallei to NO
B. pseudomallei tripled in numbers 2 h after culture, irrespectively of the media used in these experiments (figure 3A). As seen in figure 1, B. pseudomallei grown to log phase in EGYE medium were highly susceptible to increasing concentrations of NO (figure 3A). The NO-dependent killing of B. pseudomallei seen in EGYE medium depended on the presence of yeast extract but not glucose. Titration experiments revealed that supplementation of E salts with 0.1% (w/v) yeast extract supported the anti-B. pseudomallei activity of NO. In contrast, B. pseudomallei grown in E salts medium supplemented with 0.4% (w/v) glucose and/or 0.1% (w/v) casamino acids were relatively resistant to the cytotoxicity of spermine NONOate (figure 3). We considered whether the killing of B. pseudomallei by NO depended on vitamins and salts present in yeast extract. The addition of biotin, nicotinic acid, pyridoxin, riboflavin, thiamine, vitamin B, ZnCl, CaCl2 or CuSO4 to ECA medium did not, however, improve the killing of B. pseudomallei by spermine NONOate (not shown). These findings suggest that the cytotoxicity of NO against this opportunistic pathogen is best seen in complex medium. Accordingly, spermine NONOate exerted excellent anti-B. pseudomallei activity against bacteria grown in LB broth, especially if 4% glycerol (v/v) was present in the medium (figure 3B). Our investigations suggest that the metabolic state of B. pseudomallei affects the susceptibility of this opportunistic pathogen to NO.
Figure 3. Influence of growth media in the susceptibility of exponential phase B. pseudomallei to NO.
(A) B. pseudomallei were grown aerobically to log phase in EGYE (0.4% glucose, 1% yeast extract E salts medium), ECA (0.4% casamino acids E salts medium) or EGCA (0.4% glucose, 0.4% casamino acids E salts medium). Bacterial cells grown to OD600 of 0.6 were challenged with increasing concentrations of the NO donor spermine NONOate. The fraction of bacteria remaining in the wells was calculated 2.5 h after NO treatment. For the experiments in panel B, the bacteria were grown in LB broth or LBG (LB broth supplemented with 4% glycerol). The data are the mean ± SD from 4-12 individual observations from 2 separate experiments.
Influence of anaerobiosis on the susceptibility of B. pseudomallei to NO
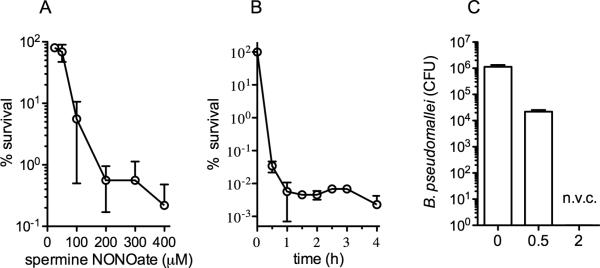
B. pseudomallei infections are quite resistant to several classes of antibiotics. Classically, the antibiotic resistance of B. pseudomallei has been associated with a variety of genetic or physiologic bacterial determinants (6, 23, 27, 32, 33, 41, 44, 49). Recently, we reported that anaerobiosis induces tolerance of B. pseudomallei to antibiotics frequently used for treating melioidosis (16). Since B. pseudomallei likely experience anaerobiosis or severe hypoxia in several cellular and anatomical compartments during the course of the infection, the antimicrobial activity of NO against anaerobic bacteria was tested. Figure 4A shows that B. pseudomallei were readily killed by increasing concentrations of spermine NONOate when the cultures had been pre-adapted for 2 h in the anaerobic environment. In fact, over 99.99% of the bacteria were killed by 1 h of exposure to 400 μM spermine NONOate (figure 4B). The number of bacteria did not change appreciably thereafter. The genome of B. pseudomallei encodes several nitrate reductases for growth in the absence of O2 using NO3- as a terminal electron acceptor. Neither the addition of NO3- nor NO2- to EGYE medium changed the excellent NO-dependent killing of anaerobic B. pseudomallei (not shown).
Figure 4. Susceptibility of anaerobic B. pseudomallei to chemically generated NO.
The bacteria were grown to the exponential phase in EGYE medium and adapted for 2 h in an anaerobic chamber. The bacterial survival was scored 2.5 h after the addition of increasing concentrations of spermine NONOate (A) or after the indicated times of exposure to 400 μM spermine NONOate (sNO) (B). The data are the mean ± SD from 5 individual observations from 2 separate experiments. For panel C, B. pseudomallei were grown in a modified “Wayne” model of anaerobiosis. Bacteria grown overnight in LB broth were subcultured 1:200 in LB broth to an OD600 of 0.6. Log phase bacteria were stirred in Hungate tubes for 31 days. The NO generator DETA NONOate was inoculated into the cultures during the last day of culture. The data the mean ± SD from 6 observations collected in 2 separate days. Non viable cultures (n.v.c.).
B. pseudomallei were gradually adapted to anaerobiosis in anaerobic Hungate tubes whereby the bacteria consume all available oxygen within 24 h at which point they survive under anaerobic conditions for several days (16). After several days under anaerobic conditions, most bacteria die leaving 0.1% of the anaerobic population that is stable and capable of persisting up to at least one year in a non-replicating state. At day 30 in the Hungate model, the persister population is tolerant to antibiotics effective against both aerobic as well as anaerobic B. pseudomallei (16). On day 30 of the Hungate model, the slow NO releaser DETA NONOate was inoculated into the cultures and the surviving bacteria recorded 24 h later. This method of adaptation to anaerobic conditions yielded bacteria highly susceptible to NO (figure 4C). In fact, no viable bacteria were recovered after overnight challenge with 2 mM DETA NONOate, a condition that steadily maintains about 2 μM NO in the culture medium. Thus, this persister population, which is entirely tolerant to conventional antibacterial treatment, appears to be highly susceptible to NO.
NO readily inactivates B. pseudomallei aconitase enzymatic activity
The anti-B. mallei activity of NO is associated with the inactivation of metalloproteins bearing [4Fe-4S] clusters (21). We therefore tested whether sublethal concentrations of spermine NONOate had any effect on the enzymatic activity of the [4Fe-4S]-cofactored aconitase. B. pseudomallei grown aerobically in EGYE medium to an OD600 of 0.6 had excellent aconitase activity (figure 5A). Having established the presence of aconitase activity in these cultures, we set out to determine whether NO had any effect on the enzymatic activity of this metalloprotein. To eliminate possible interpretation errors, we initially determined concentrations of spermine NONOate that did not affect bacterial viability under the culture conditions used for the aconitase assays. Screening of different NO concentrations showed that treatment of log phase B. pseudomallei with 100 μM spermine NONOate for 10 min dramatically decreased aconitase enzymatic activity (figure 5A). The aconitase activity of cell extracts recovered from NO-treated B. pseudomallei while growing in EGYE medium was similar to untreated controls grown in ECA medium (figure 5A). Interestingly, 100 μM spermine NONOate did not appear to affect the aconitase enzymatic activity of B. pseudomallei grown in ECA medium.
Figure 5. Effects of NO on the aconitase activity of aerobic and anaerobic B. pseudomallei.
Aconitase enzymatic activity was determined in log phase bacteria grown to OD600 0.6 in EGYE or ECA media (A). Selected bacterial cultures were treated with 100 μM spermine NONOate (sNO) for 10 min. The effect that sNO has on the aconitase activity of anaerobic B. pseudomallei grown in EGYE medium is shown in B. The data are representative of 3 experiments performed on independent days.
There is some controversy regarding the identity of the reactive nitrogen species responsible for the NO-dependent inactivation of aconitase. Peroxynitrite (ONOO-), an product of the reaction of NO with O2-, readily oxidizes the Feαof the [4Fe-4S] cluster of aconitase, thereby inactivating the enzyme (5, 19). Some investigators, however, have contended that NO itself can inactivate aconitases through the nitrosylation of the solvent-exposed, electron withdrawing Feαatom (15). Given the potent antimicrobial activity of NO against anaerobic B. pseudomallei (a condition that precludes ONOO- formation), aconitase activity was examined in B. pseudomallei exposed to NO in the absence of O2. As described above, B. pseudomallei grown to an OD600 of 0.6 in EGYE medium and adapted for 2 h in an anaerobic chamber showed excellent aconitase enzymatic activity (figure 5B). Exposure of anaerobic B. pseudomallei to 100 μM spermine NONOate readily inhibited the aconitase enzymatic activity in these cultures. Remarkably, the aconitase activity of B. pseudomallei grown in an anaerobic environment appears to be even more susceptible to NO than controls grown aerobically. Cell lysates prepared, treated with NO and loaded into anaerobic cuvettes in the anaerobic chamber suffered a similar inhibition of aconitase activity to the one reported herein for NO-treated, live cultures (not shown).
Discussion
NO arrests the replication of phylogenetically diverse microorganisms such as Candida albicans, E. coli and Salmonella enterica (14, 38, 48). In contrast to these pathogens, B. pseudomallei belong to a select group of pathogenic bacteria, including B. mallei, B. cepacia, and P. aeruginosa, that are killed by NO (work herein and references 21, 52). B. pseudomallei appear to be more susceptible to NO than P. aeruginosa. Accordingly, reactive nitrogen species reduced the viability of B. pseudomallei about 10,000-fold, whereas these reactive species dropped the fraction of planktonic and mucoid P. aeruginosa about 10- to 100-fold (29, 31, 52). Consequently, as it has been proposed for P. aeruginosa (52), it might be worth evaluating the potential of NO-based antibiotics as therapeutic agents against melioidosis. NO was found to be quite efficient at killing aerobic and anaerobic B. pseudomallei. However, higher amounts of NO appear to be needed to kill the latter. This indicates that, even though NO kills B. pseudomallei, the presence of oxygen enhances the anti-Burkholderia activity of this diatomic radical.
The MIC and MBC values of the NONOates used in our studies define NO as a bacteriostatic drug against log phase B. pseudomallei. This seems surprising given the massive decline in bacterial viability that follows NO treatment. The expansion of a small population of survivors highly resistant to NO could explain the MIC and MBC values reported herein for spermine NONOate. NO does not appear to induce adaptive mutations in Burkholderia's genome, since expansion of the resistant population in fresh LB broth yields highly susceptible bacteria. The fraction of bacteria resistant to nitrosative stress most likely represent a persistent population in a physiological state refractory to the cytotoxicity of NO. The two NO donors tested exhibited different MIC values against B. pseudomallei, likely reflecting an inverse correlation with the half-lives of these compounds. Under the experimental conditions tested, DETA NONOate steadily releases about 2 μM NO in the media. The low NO fluxes generated by DETA NONOate inhibit B. pseudomallei growth for extended periods of time. In comparison, spermine NONOate gives rise to a 3 mM NO bolus shortly after challenge, gradually diminishing to low nanomolar concentrations 10 h later. Given the extreme susceptibility of B. pseudomallei to this diatomic radical, future efforts to develop NO-based antibacterials against B. pseudomallei should favor drugs that allow for long-lasting release of small NO fluxes rather than chemicals that provide a short burst of NO. Persistent B. pseudomallei were even more susceptible to NO than log phase bacteria. The high susceptibility of persistent B. pseudomallei to NO is quite remarkable, because this population is tolerant to conventional antibiotics used against aerobic and anaerobic bacteria (16).
Treatment of infectious diseases with prodrugs that release NO is an attractive alternative to more classical NO donors. For example, the antibiotic nitroimidazole kills anaerobic M. tuberculosis through the formation of NO (42). Similarly, NO generated from the nitro group of metronidazole is responsible for the compound's antimicrobial activity (30). Of relevance to our investigations, we recently reported excellent antimicrobial activity of metronidazole against anaerobic B. pseudomallei (16). H2O2, transition metals or hemoproteins bioactivate the production of NO from the prodrug hydroxyurea (24, 35). This antibiotic has been used in the clinic against human immunodeficiency virus, hepatitis C virus and Epstein-Barr virus (28, 34, 36). NO-mediated inactivation of ribonucleotide reductase and the consequent inhibition of deoxyribonucleotide biosynthesis are central to the mechanism of action of hydroxyurea. Interestingly, hydroxyurea exhibited the most profound anti-Burkholderia activity of all the NO-based antibiotics tested in the course of our studies. The widespread occurrence of anaerobic abscesses in B. pseudomallei-infected individuals warrants future evaluation of the therapeutic value of NO-dependent prodrugs in the treatment of these fastidious infections.
B. pseudomallei infections are remarkably fastidious to treat, often requiring long regimes and combined therapies. The expression of a variety of bacterial determinants is central to the antibiotic resistance of this opportunistic pathogen (23, 27, 32, 33, 41, 44, 49). Although much less studied, the complex lifecycle of B. pseudomallei within vertebrate hosts may also contribute to the lengthy regimes and frequent failure of the current therapy against melioidosis. Signals encountered during the course of the infection may select for persistent populations refractory to antibiotics that may otherwise be excellent choices when tested in vitro. For example, conditions mimicking the anaerobic environment of abscesses tolerize B. pseudomallei to a variety of antibiotics used in the clinic (16). Interestingly, our investigations show that persistent populations of B. pseudomallei selected in long-term anaerobic cultures become exquisitely sensitive to the antimicrobial actions of NO.
An alternative to NO-based antibiotics is to engage host signaling pathways for the induction of endogenous NO synthesis. There are obvious advantages to this immunostimulatory approach. 1) The enzymatic production of NO allows for the sustained release of this antimicrobial overtime. The superb anti-B. pseudomallei activity of slow NO releasing drugs highlights the importance of the latter concept. 2) Enzymatic production delivers NO to the site of the infection, thereby increasing local concentrations of the antimicrobial. And 3) the enzymatic production of NO takes place as long as the pathogen is present in the infected tissue, but ceases with its eradication. The localized and transient enzymatic production of NO have the added advantage of minimizing the collateral damage associated with the reaction of this diatomic radical with host cell biomolecules. The immunostimulatory approach has already been used in treating some of the syndromes associated with acute B. pseudomallei infection. It remains to be investigated whether and to what extent granulocyte colony stimulating factor adjuvant therapy used in septic complications of melioidosis works through the production of NO (10, 22, 40).
B. mallei, B. pseudomallei, and B. thailandensis showed drastically different susceptibilities to reactive nitrogen species. For example, log and stationary phase B. mallei were readily killed by NO, whereas B. pseudomallei were preferentially killed when treated during the exponential phase of growth. The nonpathogenic, environmental microorganism B. thailandensis is as susceptible as B. pseudomallei to the early antimicrobial activity of NO. However, B. thailandensis resume growth shortly after exposure to the radical. The growth of B. thailandensis in the presence of NO resembles the gain in cell mass of Staphylococcus aureus following the utilization of NO-resistant metabolic pathways (39). The idea that metabolism is an important target of the antibacterial activity of NO is supported by the fact that the type of media greatly influenced the degree of anti-Burkholderia activity of NO. The expression of sensitive molecular targets could explain the high susceptibility of B. pseudomallei grown in EGYE medium to nitrosative stress. Accordingly, the composition of the media affected the differential susceptibility of aconitase activity of B. pseudomallei exposed to NO. In addition, selective expression of antinitrosative defenses such as the flavohemoprotein may have contributed to the differential susceptibility of log and stationary phase bacteria to NO-mediated killing.
Metalloproteins bearing [4Fe-4S] clusters such as aconitases are important targets of the NO-mediated cytotoxicity against B. mallei (21). Our current investigations indicate that the aconitase activity of B. pseudomallei is also exquisitely sensitive to the antimicrobial actions of NO. The NO-mediated inhibition of aconitase is more effective in anaerobic than aerobic bacteria. This observation is remarkable because NO itself, and not ONOO- arising from the interaction of NO and O2-, appears to be sufficient for the inhibition of aconitase enzymatic activity described herein. The mechanism of inhibition likely involves a rapid reversible phase followed by a long-lasting irreversible inactivation of the iron-nitrosyl [4Fe-4S] cluster (15, 43). The B. pseudomallei genome encodes two A-type aconitases, a class of aconitase that is resistant to oxidative stress (47). As discussed above, the aconitase activity of B. pseudomallei grown in EGYE medium, but not in ECA medium, was inactivated upon exposure to NO. These findings raise the possibility that the two A-type aconitases of B. pseudomallei are not only differentially expressed at different O2 tensions and in response to different carbon sources, but may also exhibit different reactivity with nitrosative and oxidative congeners of NO. These interesting possibilities await further investigation. B. pseudomallei aconitase activity was inhibited 10 min after exposure to NO, while a noticeable decline in viability was recorded 20 min later. In contrast to cell death associated with damage to the cell membrane, the NO-mediated inactivation of central metabolic pathways probably explains the slow onset of killing. The anti-B. pseudomallei activity of NO likely represents its damaging effect on multiple biomolecules, including aconitase. Enzymes with [4Fe-4S] clusters such as phosphogluconate dehydratase of the Entner-Doudoroff pathway could be inactivated upon exposure to NO. In addition, the oxidation of redox active thiols in enzymes such as glyceraldehyde-3-phosphate dehydrogenase of glycolysis or the inactivation of terminal cytochromes of the electron transport chain could contribute to the NO-dependent cytotoxicity against B. pseudomallei. Long-term nitrosylation of metal prosthetic centers of the electron transport chain may be of consequence to this strict aerobe. Metalloproteins containing zinc fingers and metal cofactors, and DNA molecules are also likely targets of the anti-B. pseudomallei activity of NO.
Highlights.
Burkholderia pseudomallei infections are fastidious to treat with conventional antibiotic therapy
B. pseudomallei are extraordinarily susceptible to a variety of NO donors
NO kills stationary and log phase, anaerobic and persistent B. pseudomallei
NO could be considered as potential treatment to treat melioidosis caused by B. pseudomallei infections
Acknowledgements
This research was supported by the Rocky Mountain Regional Center of Excellence grant number U54 AI-065357 and the Burroughs Wellcome Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arjcharoen S, Wikraiphat C, Pudla M, Limposuwan K, Woods DE, Sirisinha S, Utaisincharoen P. Fate of a Burkholderia pseudomallei lipopolysaccharide mutant in the mouse macrophage cell line RAW 264.7: possible role for the O-antigenic polysaccharide moiety of lipopolysaccharide in internalization and intracellular survival. Infect Immun. 2007;75:4298–304. doi: 10.1128/IAI.00285-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breitbach K, Klocke S, Tschernig T, van Rooijen N, Baumann U, Steinmetz I. Role of inducible nitric oxide synthase and NADPH oxidase in early control of Burkholderia pseudomallei infection in mice. Infect Immun. 2006;74:6300–9. doi: 10.1128/IAI.00966-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breitbach K, Wongprompitak P, Steinmetz I. Distinct roles for nitric oxide in resistant C57BL/6 and susceptible BALB/c mice to control Burkholderia pseudomallei infection. BMC Immunol. 2011;12:20. doi: 10.1186/1471-2172-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brett PJ, Burtnick MN, Su H, Nair V, Gherardini FC. iNOS activity is critical for the clearance of Burkholderia mallei from infected RAW 264.7 murine macrophages. Cell Microbiol. 2008;10:487–98. doi: 10.1111/j.1462-5822.2007.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro L, Rodriguez M, Radi R. Aconitase is readily inactivated by peroxynitrite, but not by its precursor, nitric oxide. J Biol Chem. 1994;269:29409–15. [PubMed] [Google Scholar]
- 6.Chan YY, Tan TM, Ong YM, Chua KL. BpeAB-OprB, a multidrug efflux pump in Burkholderia pseudomallei. Antimicrob Agents Chemother. 2004;48:1128–35. doi: 10.1128/AAC.48.4.1128-1135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaowagul W. Recent advances in the treatment of severe melioidosis. Acta Trop. 2000;74:133–7. doi: 10.1016/s0001-706x(99)00062-5. [DOI] [PubMed] [Google Scholar]
- 8.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng AC, Dance DA, Currie BJ. Bioterrorism, Glanders and melioidosis. Euro Surveill. 2005;10:E1–2. [PubMed] [Google Scholar]
- 10.Cheng AC, Stephens DP, Anstey NM, Currie BJ. Adjunctive granulocyte colony-stimulating factor for treatment of septic shock due to melioidosis. Clin Infect Dis. 2004;38:32–7. doi: 10.1086/380456. [DOI] [PubMed] [Google Scholar]
- 11.Currie BJ, Dance DA, Cheng AC. The global distribution of Burkholderia pseudomallei and melioidosis: an update. Trans R Soc Trop Med Hyg. 2008;102(Suppl 1):S1–4. doi: 10.1016/S0035-9203(08)70002-6. [DOI] [PubMed] [Google Scholar]
- 12.Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year darwin prospective study. PLoS Negl Trop Dis. 2010;4:e900. doi: 10.1371/journal.pntd.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74:417–33. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Groote MA, Granger D, Xu Y, Campbell G, Prince R, Fang FC. Genetic and redox determinants of nitric oxide cytotoxicity in a Salmonella typhimurium model. Proc Natl Acad Sci U S A. 1995;92:6399–403. doi: 10.1073/pnas.92.14.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner PR, Costantino G, Szabo C, Salzman AL. Nitric oxide sensitivity of the aconitases. J Biol Chem. 1997;272:25071–6. doi: 10.1074/jbc.272.40.25071. [DOI] [PubMed] [Google Scholar]
- 16.Hamad MA, Austin CR, Stewart AL, Higgins M, Vazquez-Torres A, Voskuil MI. Adaptation and antibiotic tolerance of anaerobic Burkholderia pseudomallei. Antimicrob Agents Chemother. 2011;55:3313–23. doi: 10.1128/AAC.00953-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamad MA, Zajdowicz SL, Holmes RK, Voskuil MI. An allelic exchange system for compliant genetic manipulation of the select agents Burkholderia pseudomallei and Burkholderia mallei. Gene. 2009;430:123–31. doi: 10.1016/j.gene.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hara Y, Mohamed R, Nathan S. Immunogenic Burkholderia pseudomallei outer membrane proteins as potential candidate vaccine targets. PLoS One. 2009;4:e6496. doi: 10.1371/journal.pone.0006496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hausladen A, Fridovich I. Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. J Biol Chem. 1994;269:29405–8. [PubMed] [Google Scholar]
- 20.Hetrick EM, Shin JH, Paul HS, Schoenfisch MH. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials. 2009;30:2782–9. doi: 10.1016/j.biomaterials.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones-Carson J, Laughlin J, Hamad MA, Stewart AL, Voskuil MI, Vazquez-Torres A. Inactivation of [Fe-S] metalloproteins mediates nitric oxide-dependent killing of Burkholderia mallei. PLoS One. 2008;3:e1976. doi: 10.1371/journal.pone.0001976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorens PG, van Overveld FJ, Bult H, Vermeire PA, Herman AG. Muramyldipeptide and granulocyte-macrophage colony-stimulating factor enhance interferon-γ-induced nitric oxide production by rat alveolar macrophages. Agents Actions. 1993;38:100–5. doi: 10.1007/BF02027220. [DOI] [PubMed] [Google Scholar]
- 23.Kumar A, Chua KL, Schweizer HP. Method for regulated expression of single-copy efflux pump genes in a surrogate Pseudomonas aeruginosa strain: identification of the BpeEF-OprC chloramphenicol and trimethoprim efflux pump of Burkholderia pseudomallei 1026b. Antimicrob Agents Chemother. 2006;50:3460–3. doi: 10.1128/AAC.00440-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon NS, Stuehr DJ, Nathan CF. Inhibition of tumor cell ribonucleotide reductase by macrophage-derived nitric oxide. J Exp Med. 1991;174:761–7. doi: 10.1084/jem.174.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10:S122–9. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 26.Limmathurotsakul D, Chaowagul W, Chierakul W, Stepniewska K, Maharjan B, Wuthiekanun V, White NJ, Day NP, Peacock SJ. Risk factors for recurrent melioidosis in northeast Thailand. Clin Infect Dis. 2006;43:979–86. doi: 10.1086/507632. [DOI] [PubMed] [Google Scholar]
- 27.Livermore DM, Chau PY, Wong AI, Leung YK. beta-Lactamase of Pseudomonas pseudomallei and its contribution to antibiotic resistance. J Antimicrob Chemother. 1987;20:313–21. doi: 10.1093/jac/20.3.313. [DOI] [PubMed] [Google Scholar]
- 28.Lori F, Lisziewicz J. Hydroxyurea: overview of clinical data and antiretroviral and immunomodulatory effects. Antivir Ther. 1999;4(Suppl 3):101–8. [PubMed] [Google Scholar]
- 29.Major TA, Panmanee W, Mortensen JE, Gray LD, Hoglen N, Hassett DJ. Sodium nitrite-mediated killing of the major cystic fibrosis pathogens Pseudomonas aeruginosa, Staphylococcus aureus, and Burkholderia cepacia under anaerobic planktonic and biofilm conditions. Antimicrob Agents Chemother. 2010;54:4671–7. doi: 10.1128/AAC.00379-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mason RP, Josephy PD. An electron spin resonance investigation of the iron-catalyzed reaction of metronidazole with cysteine. J Inorg Biochem. 1985;24:161–5. doi: 10.1016/0162-0134(85)80008-8. [DOI] [PubMed] [Google Scholar]
- 31.McCollister BD, Hoffman M, Husain M, Vazquez-Torres A. Nitric oxide protects bacteria from aminoglycosides by blocking the energy-dependent phases of drug uptake. Antimicrob Agents Chemother. 2011;55:2189–96. doi: 10.1128/AAC.01203-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mima T, Schweizer HP. The BpeAB-OprB efflux pump of Burkholderia pseudomallei 1026b does not play a role in quorum sensing, virulence factor production, or extrusion of aminoglycosides but is a broad-spectrum drug efflux system. Antimicrob Agents Chemother. 2010;54:3113–20. doi: 10.1128/AAC.01803-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore RA, DeShazer D, Reckseidler S, Weissman A, Woods DE. Efflux-mediated aminoglycoside and macrolide resistance in Burkholderia pseudomallei. Antimicrob Agents Chemother. 1999;43:465–70. doi: 10.1128/aac.43.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nozaki A, Numata K, Morimoto M, Kondo M, Sugimori K, Morita S, Miyajima E, Ikeda M, Kato N, Maeda S, Tanaka K. Hydroxyurea suppresses HCV replication in humans: a Phase I trial of oral hydroxyurea in chronic hepatitis C patients. Antivir Ther. 2010;15:1179–83. doi: 10.3851/IMP1668. [DOI] [PubMed] [Google Scholar]
- 35.Pacelli R, Taira J, Cook JA, Wink DA, Krishna MC. Hydroxyurea reacts with heme proteins to generate nitric oxide. Lancet. 1996;347:900. doi: 10.1016/s0140-6736(96)91378-1. [DOI] [PubMed] [Google Scholar]
- 36.Pakakasama S, Eames GM, Morriss MC, Huls MH, Rooney CM, Heslop HE, Krance RA. Treatment of Epstein-Barr virus lymphoproliferative disease after hematopoietic stem-cell transplantation with hydroxyurea and cytotoxic T-cell lymphocytes. Transplantation. 2004;78:755–7. doi: 10.1097/01.tp.0000129813.54517.25. [DOI] [PubMed] [Google Scholar]
- 37.Prevention C. f. D. C. a. Melioidosis: General information. National Center for Zootic, Vector-Borne, and Enteric Diseases. [Online.] 2011. posting date.
- 38.Ren B, Zhang N, Yang J, Ding H. Nitric oxide-induced bacteriostasis and modification of iron-sulphur proteins in Escherichia coli. Mol Microbiol. 2008;70:953–64. doi: 10.1111/j.1365-2958.2008.06464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richardson AR, Libby SJ, Fang FC. A nitric oxide-inducible lactate dehydrogenase enables Staphylococcus aureus to resist innate immunity. Science. 2008;319:1672–6. doi: 10.1126/science.1155207. [DOI] [PubMed] [Google Scholar]
- 40.Sanders SP, Kim J, Connolly KR, Porter JD, Siekierski ES, Proud D. Nitric oxide inhibits rhinovirus-induced granulocyte macrophage colony-stimulating factor production in bronchial epithelial cells. Am J Respir Cell Mol Biol. 2001;24:317–25. doi: 10.1165/ajrcmb.24.3.4131. [DOI] [PubMed] [Google Scholar]
- 41.Sawasdidoln C, Taweechaisupapong S, Sermswan RW, Tattawasart U, Tungpradabkul S, Wongratanacheewin S. Growing Burkholderia pseudomallei in biofilm stimulating conditions significantly induces antimicrobial resistance. PLoS One. 2010;5:e9196. doi: 10.1371/journal.pone.0009196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh R, Manjunatha U, Boshoff HI, Ha YH, Niyomrattanakit P, Ledwidge R, Dowd CS, Lee IY, Kim P, Zhang L, Kang S, Keller TH, Jiricek J, Barry CE., 3rd PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science. 2008;322:1392–5. doi: 10.1126/science.1164571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tortora V, Quijano C, Freeman B, Radi R, Castro L. Mitochondrial aconitase reaction with nitric oxide, S-nitrosoglutathione, and peroxynitrite: mechanisms and relative contributions to aconitase inactivation. Free Radic Biol Med. 2007;42:1075–88. doi: 10.1016/j.freeradbiomed.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Tribuddharat C, Moore RA, Baker P, Woods DE. Burkholderia pseudomallei class a β-lactamase mutations that confer selective resistance against ceftazidime or clavulanic acid inhibition. Antimicrob Agents Chemother. 2003;47:2082–7. doi: 10.1128/AAC.47.7.2082-2087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Utaisincharoen P, Arjcharoen S, Limposuwan K, Tungpradabkul S, Sirisinha S. Burkholderia pseudomallei RpoS regulates multinucleated giant cell formation and inducible nitric oxide synthase expression in mouse macrophage cell line (RAW 264.7). Microb Pathog. 2006;40:184–9. doi: 10.1016/j.micpath.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Vanaporn M, Vattanaviboon P, Thongboonkerd V, Korbsrisate S. The rpoE operon regulates heat stress response in Burkholderia pseudomallei. FEMS Microbiol Lett. 2008;284:191–6. doi: 10.1111/j.1574-6968.2008.01216.x. [DOI] [PubMed] [Google Scholar]
- 47.Varghese S, Tang Y, Imlay JA. Contrasting sensitivities of Escherichia coli aconitases A and B to oxidation and iron depletion. J Bacteriol. 2003;185:221–30. doi: 10.1128/JB.185.1.221-230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vazquez-Torres A, Jones-Carson J, Balish E. Nitric oxide production does not directly increase macrophage candidacidal activity. Infect Immun. 1995;63:1142–4. doi: 10.1128/iai.63.3.1142-1144.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vorachit M, Lam K, Jayanetra P, Costerton JW. Resistance of Pseudomonas pseudomallei growing as a biofilm on silastic discs to ceftazidime and cotrimoxazole. Antimicrob Agents Chemother. 1993;37:2000–2. doi: 10.1128/aac.37.9.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiersinga WJ, van der Poll T. Sepsis: new insights into its pathogenesis and treatment. Ned Tijdschr Geneeskd. 2010;154:A1130. [PubMed] [Google Scholar]
- 51.Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol. 2006;4:272–82. doi: 10.1038/nrmicro1385. [DOI] [PubMed] [Google Scholar]
- 52.Yoon SS, Coakley R, Lau GW, Lymar SV, Gaston B, Karabulut AC, Hennigan RF, Hwang SH, Buettner G, Schurr MJ, Mortensen JE, Burns JL, Speert D, Boucher RC, Hassett DJ. Anaerobic killing of mucoid Pseudomonas aeruginosa by acidified nitrite derivatives under cystic fibrosis airway conditions. J Clin Invest. 2006;116:436–46. doi: 10.1172/JCI24684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu H, Nasr SZ, Deretic V. Innate lung defenses and compromised Pseudomonas aeruginosa clearance in the malnourished mouse model of respiratory infections in cystic fibrosis. Infect Immun. 2000;68:2142–7. doi: 10.1128/iai.68.4.2142-2147.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]