Abstract
Liposomal nanocarriers modified with cell-penetrating peptide and a pH-sensitive PEG shield demonstrate simultaneously a better systemic circulation and site-specific exposure of the cell-penetrating peptide. PEG chains were incorporated into the liposome membrane via the PEG-attached phosphatidylethanolamine (PE) residue with PEG and PE being conjugated with the lowered pH-degradable hydrazone bond (PEG-HZ-PE), while cell-penetrating peptide (TATp) was added as TATp-PEG-PE conjugate. Under normal conditions, liposome-grafted PEG “shielded” liposome-attached TATp moieties, since the PEG spacer for TATp attachment (PEG(1000)) was shorter than protective PEG(2000). PEGylated liposomes accumulate in targets via the EPR effect, but inside the “acidified” tumor or ischemic tissues lose their PEG coating because of the lowered pH-induced hydrolysis of HZ and penetrate inside cells via the now-exposed TATp moieties. pH-responsive behavior of these constructs is successfully tested in cell cultures in vitro as well as in tumors in experimental mice in vivo. These nanocarriers also showed enhanced pGFP transfection efficiency upon intratumoral administration in mice, compared to control pH nonsensitive counterpart. These results can be considered as an important step in the development of tumor-specific stimuli-sensitive drug and gene delivery systems.
Keywords: pH-sensitive liposomes, Cell penetrating peptide, TATp, Hydrazone, PEG-PE, Enhanced permeability and retention
1. Introduction
Cancer chemotherapy is often complicated by serious systemic effects of anticancer actives. Therefore, despite new advances in the discovery of new potent anticancer agents, they still suffer the limitations in terms of dose regimen and usage in the patients. Site-specific release of drug from the long circulating carrier at the tumor site while maintaining minimal release during circulation, which leads to higher drug levels at tumor sites and less side effects, is of great interest in tumor chemotherapy. There are several approaches to this problem, including the use of stimuli-sensitive pharmaceutical nanocarriers, which is based on the fact that many pathological sites, including tumors, demonstrate hyperthermia or acidification (1–3). In general, environmentally sensitive carriers exhibit dramatic changes in their swelling behavior, network structure, permeability, or stability in response to changes in the pH or ionic strength of the surrounding fluid or temperature (4).
Researchers working in the area of the development of environment-responsive drug delivery systems have architectured numerous carriers or conjugate systems to selectively deliver actives to pathological sites. Kataoka’s group has prepared doxorubicin (DOX) loaded poly(beta-benzyl-L-aspartate) copolymer micelles and evaluated their pharmaceutical properties and biological significance (5). Accelerated DOX release was observed after lowering the surrounding pH from 7.4 to 5.0, suggesting a pH-sensitive release of DOX from the micelles. DOX loaded in the micelle showed a considerably higher antitumor activity compared to free DOX against mouse C26 tumor by i.v. injection, indicating a promising feature for PEG-PBLA pH-sensitive micelle as a long-circulating carrier system useful in modulated drug delivery.
Hydrophobically modified copolymers of N-isopropylacrylamide bearing a pH-sensitive moiety were investigated for the preparation of pH-responsive liposomes and polymeric micelles (6). The copolymers having the hydrophobic anchor randomly distributed within the polymeric chain were found to more efficiently destabilize egg phosphatidylcholine (EPC)/cholesterol liposomes than the alkyl-terminated polymers. Release of both a highly water-soluble fluorescent contents marker, pyranine, and an amphipathic cytotoxic anticancer drug, DOX, from copolymer-modified liposomes was shown to be dependent on pH. Also, polymeric micelles were studied as a delivery system for the photosensitizer aluminum chloride phthalocyanine, (AlClPc), currently evaluated in photodynamic therapy. pH-Responsive polymeric micelles loaded with AlClPc were found to exhibit increased cytotoxicity against EMT-6 mouse mammary cells in vitro than the control Cremophor EL formulation (7, 8). Drug carriers containing weak acids or bases can promote cytosolic delivery of macromolecules by exploiting the acidic pH of the endosome. Asokan et al. have prepared two pH-sensitive mono-stearoyl derivatives of morpholine, one with a (2-hydroxy)-propylene (ML1) linker and the other, an ethylene (ML2) linker. The pK(a) values of lipids ML1 and ML2, when incorporated into liposomes, are 6.12 and 5.91, respectively. Both lipids disrupt human erythrocytes at a pH equal to or below their pK(a) but show no such activity at pH 7.4. This group has also synthesized two Gemini surfactants or “bis-detergents” by cross-linking the headgroups of single-tailed, tertiary amine detergents through oxyethylene (BD1) or acid-labile acetal (BD2) moieties (9). As evidenced by thin-layer chromatography, BD2 was hydrolyzed under acidic conditions (pH 5.0) with an approximate half-life of 3 h at 37°C, while BD1 remained stable. Low pH-induced collapse of liposomes containing acid-labile BD2 into micelles was more facile than that of BD1. With BD1, the process appeared to be reversible in that aggregation of micelles was observed at basic pH. The irreversible lamellar-to-micellar transition observed with BD2-containing liposomes can possibly be attributed to acid-catalyzed hydrolysis of the acetal cross-linker, which generates two detergent monomers within the bilayer. Liposomes composed of 75 mol% bis-detergent and 25 mol% phosphatidylcholine were readily prepared and could entrap macromolecules such as polyanionic dextran of MW 40 kDa with moderate efficiency. The ability of BD2-containing liposomes to promote efficient cytosolic delivery of antisense oligonucleotides was confirmed by their diffuse intracellular distribution seen in fluorescence micrographs, and the up-regulation of luciferase in an antisense functional assay. Bae et al. formulated pH-sensitive polymeric mixed micelles composed of poly(L-histidine) (polyHis; M(w) 5000)/PEG (M(n) 2000) and poly(L-lactic acid) (PLLA) (M(n) 3000)/PEG (M(n) 2000) block copolymers with or without folate conjugation (10, 11). The polyHis/PEG micelles showed accelerated adriamycin release as the pH decreased from 8.0. In order to tailor the triggering pH of the polymeric micelles to the more acidic extracellular pH of tumors, while improving the micelle stability at pH 7.4, the PLLA/PEG block copolymer was blended with polyHis/PEG to form mixed micelles. Blending shifted the triggering pH to a lower value. Depending on the amount of PLLA/PEG, the mixed micelles were destabilized in the pH range of 7.2–6.6 (triggering pH for adriamycin release). When the mixed micelles were conjugated with folic acid, the in vitro results demonstrated that the micelles were more effective in tumor cell kill due to accelerated drug release and folate receptor-mediated tumor uptake. In addition, after internalization, polyHis was found to be effective for cytosolic ADR delivery by virtue of fusogenic activity. Certain pH-sensitive linkages have been popularly used to allow the drug release, protective “coat” removal, or new function appearance because of their fast degradation in acidified pathological sites (12–14). These include cis-aconityls (15, 16), electron-rich trityls (17), polyketals (18), acetals (19, 20), vinyl ethers (21, 22), hydrazones (23–25), poly(ortho-esters) (26), and thiopropionates (27). Such constructs may turn out to be useful for the site-specific delivery of drugs at the tumor sites(2), infarcts (28), inflammation zones (29) or cell cytoplasm or endosomes (30), since at these “acidic” sites, pH drops from the normal physiologic value of pH 7.4–6.0 and in the following section. A pH-sensitive cis-aconityl linkage has been used to make immunoconjugates of daunorubicin by Shen et al. (31) and Diener et al. (32) while DOX was conjugated to murine monoclonal antibodies (MoAb) raised against human breast tumor cells (33) or murine monoclonal antibody (MAb) developed against human pulmonary adenocarcinoma (34). The trityl group has been used in organic chemistry as an acid-cleavable protecting group for amino and hydroxyl groups. Patel group at Lilly Research Laboratories have established structure-stability relationship of different trityl-nucleoside derivatives by using NMR-spectroscopy (35). In general, the acid-sensitivity of these compounds increases with the electron-donating effects of the substituents (e.g., methoxy groups) that stabilize the intermediatory formed carbocation in the hydrolysis step. In vitro activity in a human colon carcinoma cell line showed that the antibody conjugates with the most pronounced acid lability exhibited the strongest inhibitory effects. However, the most stable conjugates were 20–30 times less active than the free nucleoside antimetabolite (36, 37). These structure-activity relationship also confirmed in animal experiments (35). Also, acetal linkages have the potential to be used as linkages for a range of alcohol functionalities, because their hydrolysis is generally first order relative to the hydronium ion, making the expected rate of hydrolysis 10 times faster with each unit of pH decrease (38) and, by altering their chemical structure, it is possible to tune their hydrolysis rate. In addition, acetals can be formed using a variety of types of hydroxyl groups including primary, secondary, tertiary and syn-1,2- and -1,3-diols, and the rate of hydrolysis can be tuned by varying the structure of the acetal. Gillies et al. synthesized a four different acetal-based conjugates using model drugs and PEO polymer (39). The hydrolysis kinetics of the conjugates had half-lives ranging from less than 1 min to several days at pH 5.0, with slower hydrolysis at pH 7.4 in all cases. Encrypted polymers containing pH-sensitive acetal linkage between either oligonucleotide or macromolecule and PEG showed direct vesicular escape and efficiently deliver oligonucleotides and macromolecules into the cytoplasm of hepatocytes (40). Acetal-based acid-degradable protein-loaded microgels also have showed promising results for the delivery of protein-based vaccines (41). Murthy group has introduced an acid-sensitive hydrophobic nanoparticle based on a new polymer, poly(1,4-phenyleneacetone dimethylene ketal) (PPADK), which complements existing biodegradable nanoparticle technologies (42). This polymer has ketal linkages in its backbone and degrades via acid-catalyzed hydrolysis into low molecular weight compounds that can be easily excreted. PPADK forms micro- and nanoparticles, via an emulsion procedure, and can be used for the delivery of hydrophobic drugs and potentially proteins (43). Acid-labile polyethylene glycol (PEG) conjugated vinyl ether lipids were synthesized and used at low molar ratios to stabilize the nonlamellar, highly fusogenic lipid, dioleoylphosphatidyl ethanolamine, as unilamellar liposomes (22). Acid-catalyzed hydrolysis of the vinyl ether bond destabilized these liposomes by removal of the sterically stabilizing PEG layer, thereby promoting contents release on the hours timescale at pH < 5. pH-Sensitive amphiphilic hydrogels were synthesized by radiation copolymerization of ethylene glycol vinyl ether (EGVE), butyl vinyl ether (BVE) and acrylic acid (AA) in the presence of crosslinking agent, diethylene glycol divinyl ether (DEGDVE) (44, 45). The results of the swelling experiments indicated that the hydrogel which has 60:40:5 comonomer ratio (mol% of EGVE:BVE:AA in monomeric mixture) is pH-sensitive. While the hydrogel is in a fully hydrated form at pH > 6, it extensively dehydrates below pH 6. A two-stage volume phase transition was observed in the range of pH 6.0–7.0 and 7.5–8.0. In 1980, Hurwitz and co-workers reported for the first time that hydrazone-based polymer-daunorubicin conjugates have substantial cytotoxicity than the analogues containing noncleavable linkers between those conjugates which appeared to be completely inactive (46). In 1989, the Lilly labs reported the use of hydrazone linkages to target MoAb to potent cytotoxic DAVLB hydrazide (47). In vivo studies of antitumor activity showed that the efficiency and safety of the conjugate was increased over that of the unconjugated. The Kratz group has prepared trasnferin and albumin as carriers for targeting of chlorambusil, an anticancer active (48, 49). In vitro studies with both conjugates demonstrated them to be as active or more active than the free drug, whereas they had reduced toxicities. Toncheva et al. have prepared amphiphilic AB and ABA block copolymers from poly (ortho esters) and poly (ethylene glycol). The micelles formed by these co-block polymers were stable in PBS at pH 7.4 and 37°C for 3 days and in a citrate buffer at pH 5.5 and 37°C for 2 h (26). The remarkably enhanced gene silencing in hepatoma cells was achieved by assembling lactosylated-PEG-siRNA conjugates bearing acid-labile beta-thiopropionate linkages into polyion complex (PIC) micelles through the mixing with poly(L-lysine) (50). The PIC micelles with clustered lactose moieties on the periphery were successfully transported into hepatoma cells in a receptor-mediated manner, releasing hundreds of active siRNA molecules into the cellular interior responding to the pH decrease in the endosomal compartment. Eventually, almost 100 times enhancement in gene silencing activity compared to that of the free conjugate was achieved for the micelle system, facilitating the practical utility of siRNA therapeutics. Kataoka group (51) also architectured three types of newly engineered block copolymers forming polyplex micelles useful for oligonucleotides and siRNA delivery: (1) PEG-polycation diblock copolymers possessing diamine side-chain with distinctive pKa for siRNA encapsulation into polyplex micelles with high endosomal escaping ability, (2) Lactosylated PEG-(oligonucleotide or siRNA) conjugate through acid-labile beta-thiopropionate linkage to construct pH-sensitive PIC micelles, and (3) PEG-poly(methacrylic acid) block copolymer for the construction of organic/inorganic hybrid nanoparticles encapsulating siRNA. Recently, N-ethoxybenzylimidazoles (NEBI) linkers were introduced as potential pH-sensitive linkages. Kinetic analysis of eight derivatives of NEBIs showed that their rates of hydrolysis are accelerated in mild aqueous acidic solutions compared to in solutions at normal, physiological pH. A derivative of NEBI carrying DOX, a widely used anticancer agent, also showed an increased rate of hydrolysis under mild acid compared to that at normal physiological pH. The DOX analogue resulting from hydrolysis from the NEBI exhibited good cytotoxic activity when exposed to human ovarian cancer cells (52).
We have demonstrated the utility of highly pH-sensitive hydrazone bond-based PEG-PE conjugates in preparing double-targeted stimuli-sensitive pharmaceutical nanocarriers (53, 54). Two important temporal characteristics of such carriers include their sufficiently long life-time under normal physiological conditions and their sufficiently fast destabilization within the acidic target. Since real practical tasks may require different times for such carriers to stay in the blood and to release their contents (or “develop” an additional function) inside the target, we have synthesized a series of PEG-HZ-PE conjugates with different substituents at the hydra-zone bond and evaluated their hydrolytic stability at normal and slightly acidic pH values. These conjugates differed from each other with respect to the exact structure of groups forming the hydrazone linkage between phospholipid and PEG. The characterization of the in vitro behavior of these conjugates has provided important information useful for future design and development of pH-sensitive nanocarriers with controlled properties.
2. Materials
2.1. Chemicals
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, DOPE; 1,2-dipalmitoyl-sn-glycero-3-phosphothioethanolamine (Sodium Salt), DPPE-SH; 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (ammonium salt), Rh-PE (all from Avanti Polar Lipids).
(N-e-maleimidocaproic acid) hydrazide, EMCH; 4- (4-N-maleimidophenyl) butyric acid hydrazide hydrochloride, MPBH; N-(k-maleimidoundecanoic acid)hydrazide, KMUH; succinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate, SMCC (all from Pierce Biotechnology Inc., Rockford, IL).
2-acetamido-4-mecrcapto butanoic acid hydrazide, AMBH (Molecular Probes).
Methoxy poly(ethylene) glycol butyraldehyde (MW 2,000), mPEG-SH (MW 2,000) (all from Nektar Therapeutics, Huntsville, AL).
Triethylamine.
4-succinimidyl formylbenzoate (SFB) (Molbio, Boulder, Colorado).
Egg phosphatidylcholine (EPC), cholesterol (Ch), mPEG2000-DSPE, 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), Rhodamine-PE (Rh-PE), and phosphatidylthio-ethanolamine (DPPE-SH) (all from Avanti Polar Lipids).
mPEG2000-SH (Nektar Therapeutics, Huntsville, AL).
Maleimide-PEG1000-NHS (Quanta Biodesign, Powell, OH).
TATp-cysteine (Research Genetics, Huntsville, AL).
Succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate hydrazide (SMCCHz) (Molecular Biosciences Boulder, CO).
4-acetyl phenyl maleimide.
Sephadex G25m.
Sepharose CL4B.
Lewis Lung Carcinoma (LLC) cell line (ATCC, Rockville, MD).
Delbecco’s minimal essential medium, complete serum free medium and fetal bovine serum (Cellgro, Kansas City, MO).
2.2. Syntheses
All reactions are monitored by TLC using 0.25 mm × 7.5 cm silica plates with UV-indicator (Merck 60F-254), and mobile phase chloroform:methanol (80:20% v/v).
Phospholipid and PEG alone or their conjugates are visualized by phosphomolybdic acid and Dragendorff spray reagents (see Note 1).
Silica gel (240–360 μm) and size exclusion media, Sepharose CL4B (40–165 μm) and Sephadex G25m (Sigma-Aldrich) are used for silica column chromatography and size exclusion chromatography respectively (see Note 2).
2.3. Preparation of the TATp-Bearing, Rhodamine-Labeled Liposomal Formulations
The pH-sensitive or pH-insensitive, Rh-labeled, TATp-bearing liposomes are prepared by the lipid film hydration method.
A mixture of PC:Chol (7:3), TATp-PEG1000-PE, Rh-PE and either mPEG2000-HZ-PE (pH-sensitive) or mPEG2000-DSPE (pH-insensitive) at molar ratio 10:0.25:0.1:15 is evaporated under reduced pressure (see Note 3).
The dry lipid formed is hydrated with phosphate buffer saline, pH 7.4. The liposomal suspension is filtered through 0.2 μm polycarbonate filters and stored at 4°C until use.
The liposome particle mean size and size distribution are observed using a Coulter N4 Plus submicron particle analyzer.
2.4. Preparation of the TATp-Bearing, Rhodamine Labeled, pGFP Complexed Liposomal Formulations
The pH-sensitive or pH-insensitive, TATp-bearing pGFP-complexed liposomes are prepared by the spontaneous vesicle formation (SVF) method adopted from (55) with few modifications.
A plasmid solution is prepared by combining pGFP and 10 mM Tris-EDTA (TE) buffer, pH 7.4. A lipid solution in ethanol is prepared by dissolving EPC:Chol (7:3) in anhydrous ethanol, and then adding DOTAP, TATp-PEG1000-PE and either mPEG2000-HZ-PE (22, pH-sensitive) or mPEG2000-DSPE (pH-insensitive) at 10:0.25:15 molar ratio. The charge (±) ratio is 10:1.
The lipid and plasmid solutions are preheated to 37°C before mixing together. After mixing these solutions for 10 min, ethanol is evaporated under the reduced pressure. The samples are filtered through 0.2 μm polycarbonate filters and stored at 4°C until use.
The liposomal formulations are subjected to the agarose gel electrophoresis to test for the quantitative presence and intactness of the plasmid within the liposomes (56). In a typical case, the pGFP concentration is 3.22 μg/mg of total lipid.
The liposome particle mean size and size distribution are observed using a Coulter N4 Plus submicron particle analyzer.
3. Methods
3.1. Synthesis of Hydrazone-Based mPEG-HZ-PE Conjugates (54, 57)
3.1.1. Synthesis of Aliphatic Aldehyde-Derived Hydrazone-Based mPEG-HZ-PE Conjugates
Step 1: Synthesis of hydrazide-activated phospholipids
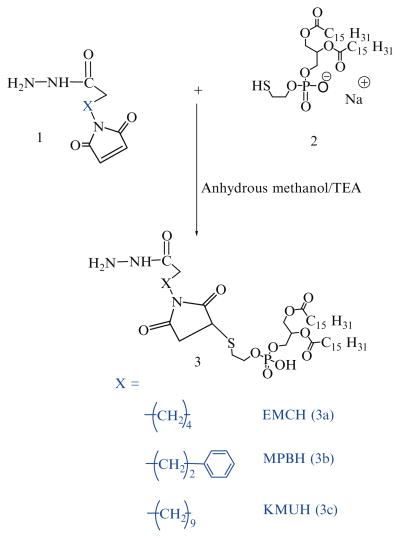
22 μmol of phosphatidylthioethanolamine, 2, are mixed with 1.5 molar excess of each acyl hydrazide linker (Table 1) in 3 mL anhydrous methanol containing 5 molar excess of tri-ethylamine over lipid (Scheme 1). The reaction is performed at 25°C under argon for 8 h (see Note 4).
Solvent is removed under reduced pressure, and the residue is dissolved in chloroform and applied to a 5-mL silica gel column which had been activated (150°C overnight) and pre-washed with 20 mL of chloroform. The column is equilibrated with an additional 15 mL of chloroform followed by 5 mL of each of the following chloroform:methanol mixtures 4:0.25, 4:0.5, 4:0.75, 4:1, 4:2 and, finally, with 6 mL of 4:3 v/v. The phosphate-containing fractions eluting in 4:l, 4:2 and 4:3 chloroform:methanol (v/v) are pooled and concentrated under reduced pressure. The product is stored in glass ampoules as chloroform solution under argon at −80°C.
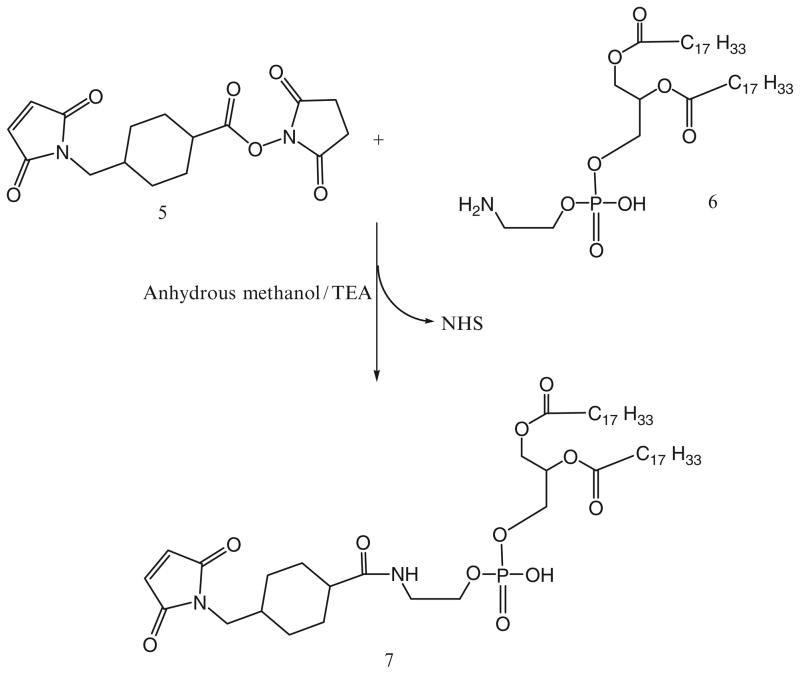
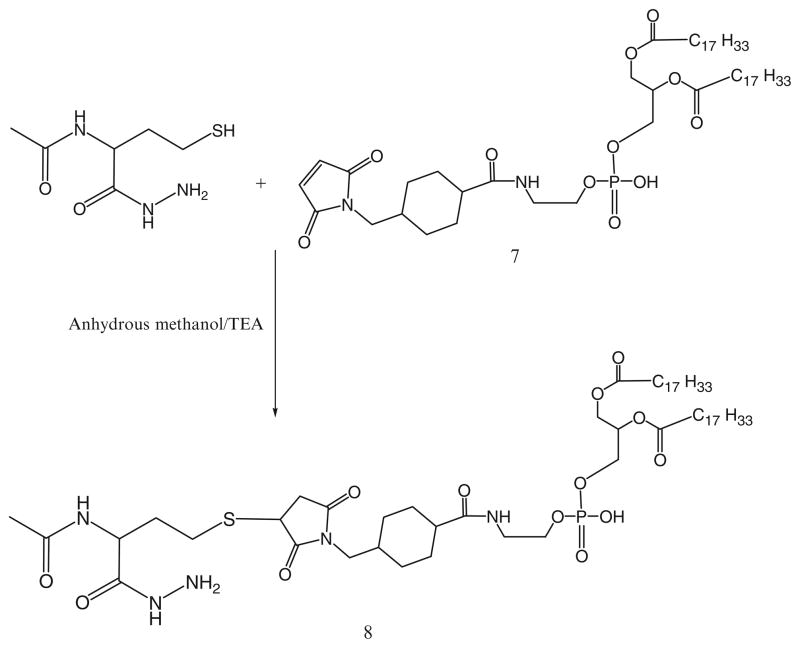
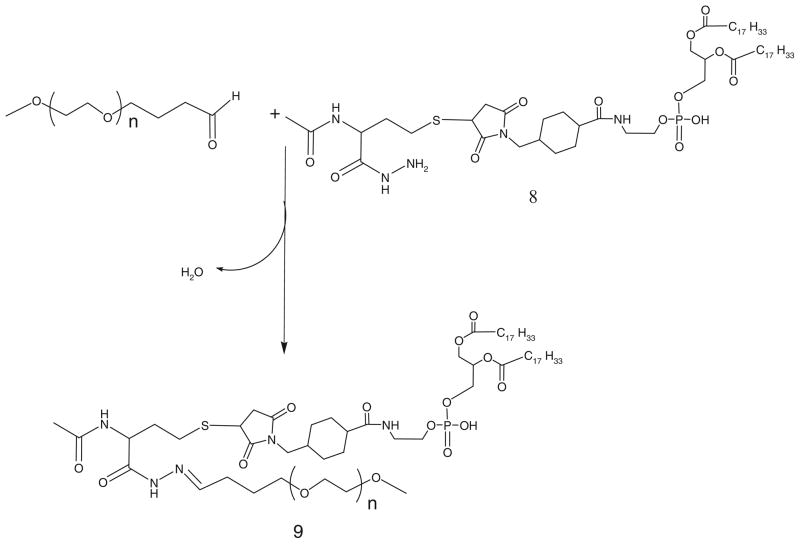
For the activation of phospholipid with AMBH, a maleimide derivative of phosphatidylethanolamine, 7, is prepared using SMCC (Scheme 2). In brief, phosphatidylethanolamine, 6, in chloroform was reacted with 1.5 molar excess of SMCC, 5, over lipid in presence of 5 molar excess of TEA under argon for 5 h. The maleimide-derivative is separated from excess SMCC on silica gel column using chloroform:methanol (4:0.2 v/v) mobile phase. The elution fractions containing Ninhydrin-negative and phosphorus-positive fractions are pooled and concentrated under reduced pressure. DOPE-maleimide is further used to synthesize AMBH-activated derivative of phospholipid, 8, by reacting with 1.5 molar excess of AMBH using TEA as catalyst (Scheme 3).
Table 1.
List of acyl hydrazide cross-linkers
| Linker used | Mol. wt. | Length of spacer arm |
|---|---|---|
| AMBH 2-acetamido-4-mercapto butanoic acid hydrazide |
191.25 | – |
| EMCH (N-e-maleimidocaproic acid) hydrazide |
225.24 | 11.8 Å |
| MPBH 4-(4-N-maleimidophenyl)butyric acid hydrazide |
309.5 | 17.9 Å |
| KMUH N-(k-maleimido undecanoic acid) hydrazide |
295.8 | 19.0 Å |
| SMCCH Succinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate hydrazide |
365.31 | – |
Scheme 1.
Synthesis of acyl hydrazide-activated phospholipids
Scheme 2.
Maleimide activation of phosphatidylethanolamine
Scheme 3.
AMBH-derivatized phospholipid via sulfhydryl-maleimide addition reaction
Step 2: Synthesis of mPEG-HZ-PE conjugates
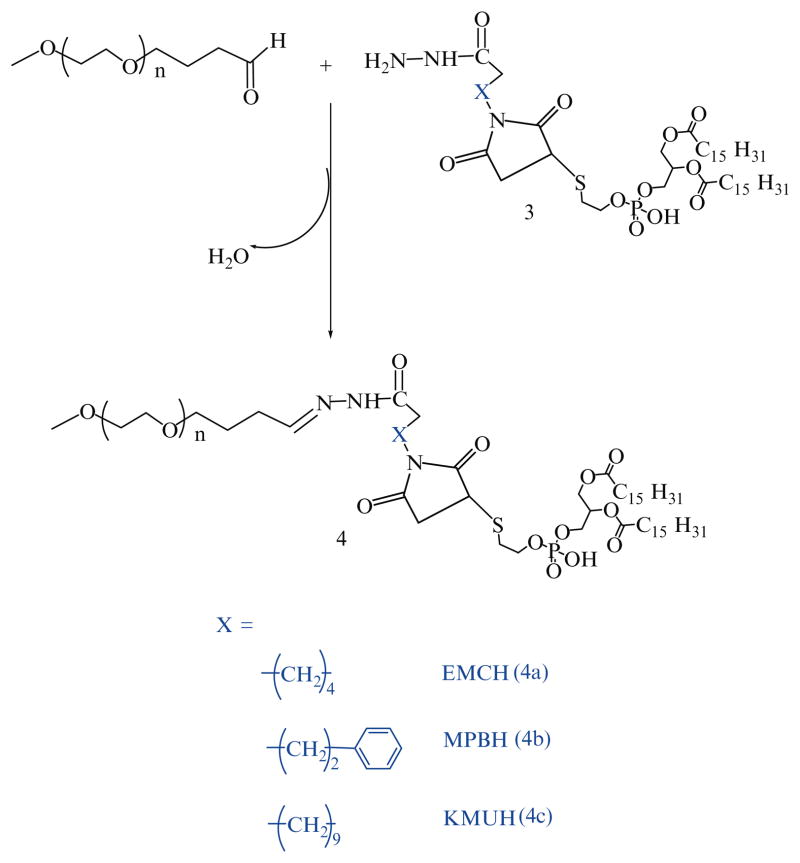
21 μmol of mPEG2000-butyraldehyde are reacted with 14 μmol of linker-activated phospholipid in 2 mL chloroform at 25°C in a tightly closed reaction vessel (Schemes 4 and 5).
After an overnight stirring, chloroform is evaporated under vacuum in rotary evaporator. The excess mPEG2000-butyraldehyde is separated from PEG-HZ-PE conjugates using gel filtration chromatography. The gel filtration chromatography is performed using sepharose-CL4B equilibrated overnight in pH 9–10 degassed ultra pure water (elution medium) in 1.5 × 30 cm glass column.
The thin film formed in round bottom flask after evaporating chloroform is hydrated with the elution medium and applied to the column. The micelles formed by PEG-HZ-PE conjugate are the first to elute from the column (see Note 5).
Micelle containing fractions are identified by Dragendorff spray reagent and pooled together, kept in freezer at −80°C overnight before subjecting to freeze drying.
The freeze dried PEG-HZ-PE conjugates are weighed and stored at −80°C as chloroform solution.
Scheme 4.
Synthesis of aliphatic aldehyde-based hydrazone-derived mPEG-HZ-PE
Scheme 5.
Synthesis of PEG-HZ-PE conjugate using AMBH-activated phospholipid
3.1.2. Synthesis of Aromatic Aldehyde-Derived Hydrazone-Based mPEG-HZ-PE Conjugates
Step 1: Synthesis of hydrazide-activated PEG derivatives
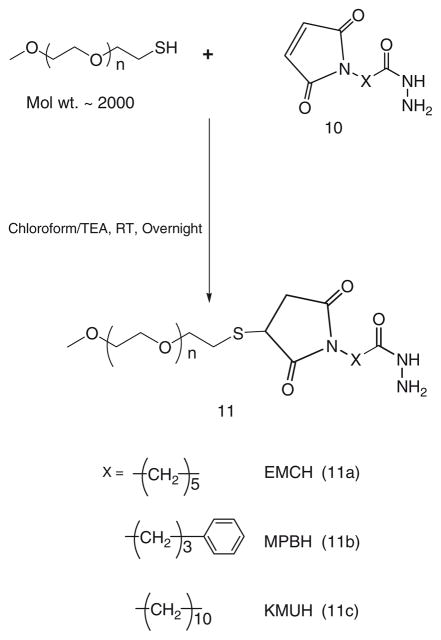
40 μmol of mPEG-SH in chloroform are mixed with two molar excess of acyl hydrazide cross-linkers: EMCH (10a), MPBH (10b), KMUH (10c) presence of 5 molar excess of triethylamine over lipid (see Note 5), (Scheme 6).
The excess EMCH is separated from the product by size exclusion chromatography using Sephadex G25m media.
The acyl hydrazide derivatives of PEG, (11a), (11b), (11c) are freeze dried and stored as chloroform solution at −80°C.
Scheme 6.
Synthesis of acyl hydrazide activated PEG
Step 2: Synthesis of aromatic aldehyde-activated phospholipid
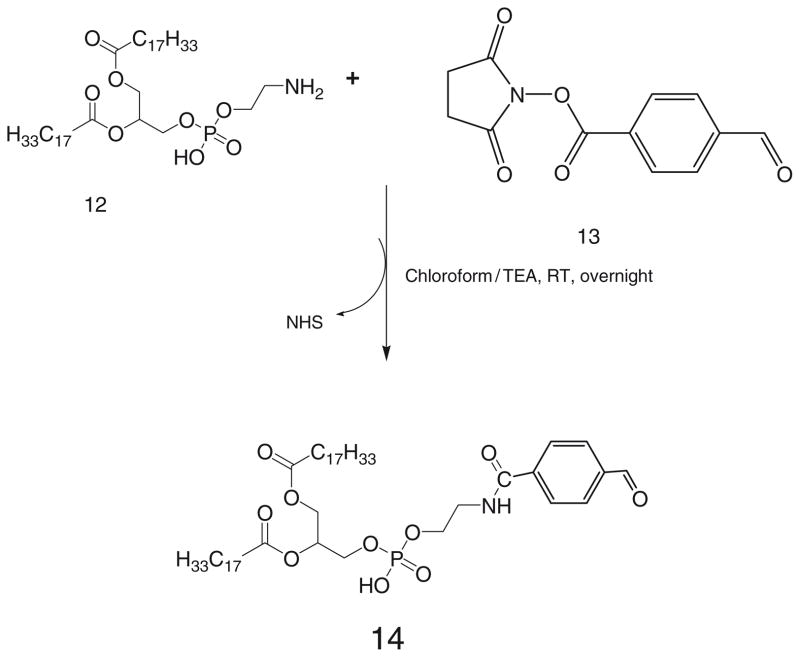
35 μmol of phosphatidylethanolamine, DOPE-NH2, 12, in chloroform are mixed with 2 molar excess of 4-succinimidyl-formyl benzoate, SFB, 13, in presence of 3 molar excess triethylamine over lipid (Scheme 7).
After stirring for 3 h, solvent is evaporated, residue is redissolved in chloroform and product is separated on silica gel column using acetonitrile:methanol mobile phases: 4:0, 4:0.25, 4:0.5, 4:0.75 and 4: 1 v/v.
The fractions containing product are identified by TLC analysis, pooled and concentrated. The product is stored as chloroform solution at −80°C.
Scheme 7.
SFB activation of phosphatidylethanolamine
Step 3: Synthesis of mPEG-HZ-PE conjugates
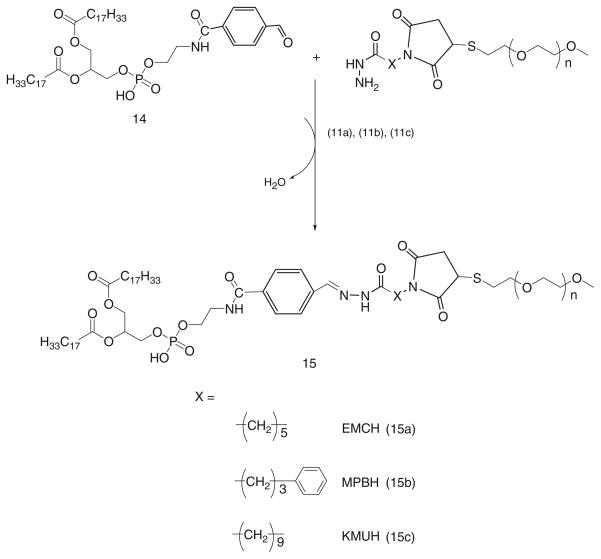
1.5 molar excess of SFB activated phospholipid, 14, are reacted with acyl hydrazide derivatized PEGs, 11a, 11b, and 11c respectively, in chloroform at room temperature (Scheme 8).
After overnight stirring, chloroform is evaporated under reduced pressure.
The PEG-HZ-PE conjugate is purified using size exclusion chromatography using Sepharose CL4B as described before.
Scheme 8.
Synthesis of PEG-HZ-PE conjugate
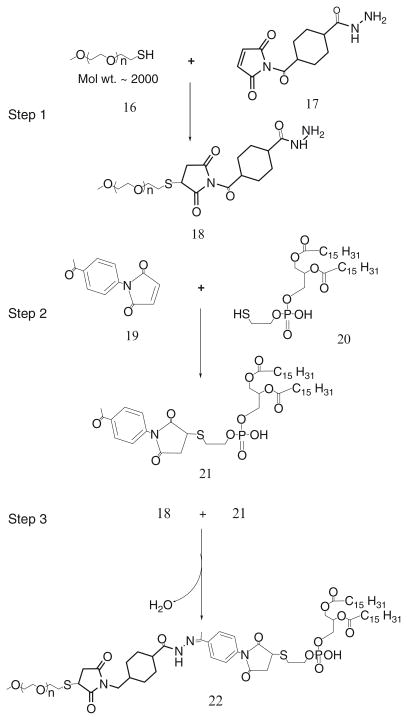
3.1.3. Synthesis of Aromatic Ketone-Derived Hydrazone-Based mPEG-HZ-PE Conjugates
Step 1: Synthesis of hydrazide derivative of PEG
mPEG-SH (MW 2000), 16, is reacted with 2 molar excess of SMCCHz, 17, in presence of triethylamine for 8 h in dry chloroform (Scheme 9).
Chloroform is evaporated, and the residue is dissolved in water.
The PEG-hydrazide derivative, 18, is separated and purified by the size exclusion gel chromatography using Sephadex G25m media.
The product is freeze dried and stored as chloroform solution at −80°C.
Scheme 9.
Synthesis of aromatic ketone-derived hydrazone based mPEG-HZ-PE
Step 2: Activation of phospholipid with 4-acetyl phenyl maleimide
40 μmol of 4-acetyl phenyl maleimide, 19, are reacted with 27 μmol of phosphatidylthioethanol (DPPE-SH), 20, in presence of triethylamine overnight with constant stirring under inert atmosphere of argon (Scheme 9).
The product, 21, is separated on a silica gel column using chloroform:methanol mobile phase (4:1 v/v).
The fractions containing product are identified by TLC analysis, pooled and concentrated.
Aromatic ketone-activated phospholipid is stored as chloroform solution at −80°C.
Step 3: Synthesis of mPEG-HZ-PE conjugate
Hydrazide-activated PEG derivative, 18, is reacted with 1.5 molar excess of the aromatic ketone-derivatized phospholipid, 21, overnight under the constant stirring at room temperature (Scheme 9).
The PEG-HZ-PE conjugate, 22, is separated and purified by size exclusion gel chromatography using Sepharose-CL4B media.
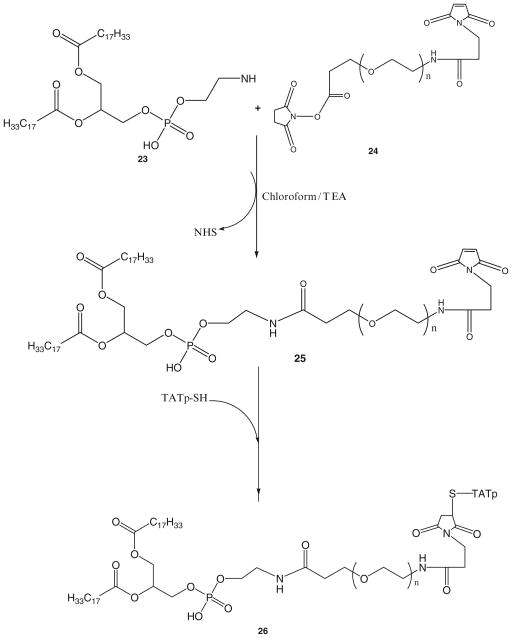
3.2. Synthesis of PE-PEG1000-TATp Conjugate (57)
Step 1: Synthesis of PE-PEG1000-maleimide
1.5 molar excess of DOPE-NH2, 23, is reacted with NHS-PEG1000-maleimide, 24, in chloroform under argon at room temperature in the presence of 3 molar excess triethylamine overnight with stirring.
The product PE-PEG1000-maleimide, 25, is separated on the Sephadex G25m column equilibrated overnight with the degassed double deionized water.
The product is freeze dried and stored under chloroform at −80°C.
Step 2: Synthesis of PE-PEG1000-TATp
Twofold molar excess of TATp-SH is mixed with PE-PEG1000-maleimide, 25, in chloroform under inert atmosphere with gentle shaking for 8 h.
The excess TATp-SH is separated from the product, 26, by gel filtration chromatography using Sephadex G25m media.
The freeze-dried product is stored under chloroform at −80°C until further use.
3.3. In vitro pH-Dependant Degradation of PEG-HZ-PE Conjugates
The time-dependant degradation of PEG-HZ-PE micelles incubated in buffer solutions (Phosphate buffer saline, pH 7.4 and 5.0) maintained at 37°C is followed by HPLC using Shodex KW-804 size exclusion column.
The elution buffer used is pH 7.0, Phosphate buffer (100 mM phosphate, 150 mM sodium sulfate), run at 1.0 mL/min. For fluorescent detection (Ex 550 nm/Em 590 nm) of micelle peak, Rh-PE (1 mol% of PEG-PE) is added to the PEG-PE conjugate in chloroform.
A film is prepared by evaporating the chloroform under argon stream and hydrated with the phosphate buffer saline, pH 7.4 or 5.0 (adjusted by pre-calculated quantity of 1 N HCl).
A peak that represents micelle population appears at the retention time between 9–10 min.
The degradation kinetics of micelles is assessed by following the area under micelle curve.
3.4. Avidin – Biotin Affinity Chromatography
To check the pH-sensitivity, biotin-containing micelles are formulated by mixing mPEG2000-HZ-PE (60% mol), PEG750-PE (37% mol), Rhodamine-PE (0.5% mol, fluorescent marker), and biotin-PE (2.5% mol, biotin component) in chloroform.
Chloroform is evaporated and a thin film is formed using rotary evaporator.
To test the binding of biotin-bearing Rh-PE-labeled, TATp-bearing liposomes before and after incubation at lowered pH values, the corresponding samples are kept for 3 h at pH 7.4 or 5.0 and then applied onto the Immobilized NeutrAvidin protein column (see Note 6).
The degree of the retention of the corresponding preparation on the column is estimated following the decrease in the sample rhodamine fluorescence at 550/590 nm after passing through the NeutrAvidin column (58).
3.5. In Vitro Cell Culture Studies
H9C2 rat embryonic cardiomyocytes in 10% fetal bovine serum DMEM are grown on coverslips in 6-well plates, then treated with various Rh-PE-labeled liposome samples (with and without preincubation for 3 h at pH 5.0) in serum-free medium (2 mL/well, 30 mg total lipid/mL).
After a 1 h incubation period, the media are removed and the plates washed with serum-free medium three times.
Individual coverslips are mounted cell-side down onto fresh glass slides with PBS (see Note 7). Cells are viewed with a Nikon Eclipse E400 microscope under bright light or under epifluorescence with rhodamine/TRITC filter (58) (see Note 8).
The images are analyzed using ImageJ 1.34I software (NIH) for integrated density comparison of red fluorescence between two groups (see Note 9).
3.6. In Vivo Studies
LLC tumors are grown in nu/nu mice (Charles River Breeding Laboratories, MA) by the s.c. injection of 8 × 104 LLC cells per mouse into the left flank (protocol # 05-1233R, approved by the Institutional Animal Care and Use Committee at Northeastern University, Boston).
When tumors reach 5–10 mm in diameter, they are injected at four to five different spots with 150 μL of Rh-labeled, TATp-bearing pH-sensitive or pH-insensitive liposomes in phosphate-buffered saline, pH 7.4 (see Note 10).
Mice are killed 6 h later by cervical dislocation, and excised tumors are cryo-fixed as described earlier.
Microtome cut sections are washed thoroughly with phosphate buffer saline (pH 7.4), dried and fixed on slides using Fluor Mounting medium.
These sections are observed under fluorescence microscopy using TRITC filter (59).
Further, the images are analyzed using ImageJ 1.34I software (NIH) for integrated density comparison of red fluorescence between pH-sensitive and pH-insensitive groups.
3.7. In Vivo Transfection with pGFP
LLC tumors are grown in nu/nu mice (Charles River Breeding Laboratories, MA) by the s.c. injection of 8 × 104 LLC cells per mouse into the left flank (protocol # 05-1233R, approved by the Institutional Animal Care and Use Committee at Northeastern University, Boston).
When tumors reach 5–10 mm in diameter, they are injected at four to five different spots with 150 μL of pGFP-loaded, TATp-bearing pH-sensitive or pH-insensitive liposomes in phosphate-buffered saline, pH 7.4.
Mice are killed 72 h later by cervical dislocation, and excised tumors are fixed in a 4% buffered paraformaldehyde overnight at 4°C, blotted dry of excess paraformaldehyde and kept in 20% sucrose in PBS overnight at 4°C.
Cryofixation is done by the immersion of tissues in ice-cold isopentane for 3 min followed by freezing at −80°C. Fixed, frozen tumors are mounted in Tissue-Tek OCT 4583 compound (Sakura Finetek, Torrance, CA) and sectioned on a Microtome Plus (TBS).
Sections are mounted on slides and analyzed by the fluorescence microscopy using FITC filter and with hematoxylineosin staining (see Note 11).
The images are analyzed using ImageJ 1.34I software (NIH) for integrated density comparison of green fluorescence between pH-sensitive and non-pH-sensitive groups.
4. Results and Discussion
4.1. Synthesis of Hydrazone-Based mPEG-HZ-PE Conjugates
Hydrazone-linkages have been very instrumental for the use as “pH-sensitive connections” because of their wide range of hydrolytic degradation kinetics strictly controlled by the nature of hydrazone bond formed. Hydrazones are much more stable than imines as a result of the delocalization of the π-electrons in the former. In fact, parent hydrazones are too stable for the application in drug delivery systems, and an electron withdrawing group has to be introduced to moderate the stability by somewhat disfavoring electron delocalization throughout the molecule as compared to the parent hydrazone. Hydrazones can be prepared from aldehydes or ketones and hydrazides under very mild conditions including aqueous solutions. Hydrazone bond formation can take place even in vivo from separate fragments which self-assemble under physiological conditions (60).
A set of different synthetic methods were designed based on the use of various aldehydes that can produce the hydrazone linkage between PEG and PE (54). Synthesis of aliphatic aldehyde-derived hydrazone containing PEG-PE conjugate was pursued in two steps. First, phospholipid was activated with four different acyl hydrazides. The sulfhydryl reactive group of phosphatidylthioethanolamine was reacted with maleimide end of maleimido acyl hydrazides (refer Table 1) through Michael addition, thus providing acyl hydrazide activated PE. mPEG-butyraldehyde, an aliphatic aldehyde, was then reacted with acyl hydrazide activated PE to get hydrazone based PEG-PE conjugate. To synthesize aromatic aldehyde-derived hydrazone, an aromatic aldehyde moiety was introduced into the phospholipid by reacting succinimidyl 4-formylbenzoate (SFB) with phosphatidylethanolamine under mild alkaline conditions. The acyl hydrazide-PEG derivatives were synthesized using mPEG-SH and maleimido acyl hydrazides (EMCH, MPBH, and KMUH). The SFB-activated phospholipid was then reacted with acyl hydrazide derivatized PEG. Aromatic ketone-derived hydrazone-based PEG-PE conjugates were synthesized by reacting aromatic ketone-activated phospholipids with acyl hydrazide-activated PEG (57).
4.2. Synthesis of PE-PEG1000-TATp Conjugate
TATp-SH was attached to the heterobifunctional PEG via the two step synthesis as shown in the Scheme 10. First, Mal-PEG-PE conjugate was synthesized by reacting DOPE-NH2 with the NHS end of heterobifunctional PEG derivative, 23, NHS-PEG1000-maleimide. PE-PEG1000-maleimide was then reacted with TATp-SH to form PE-PEG1000-TATp conjugate. The conjugate was separated by gel chromatography using the Sephadex G25m media.
Scheme 10.
Synthesis of PE-PEG-TATp conjugate
4.3. In Vitro pH-Dependent Degradation of PEG-HZ-PE Conjugates
All PEG-HZ-PE derivatives spontaneously form micelle in aqueous surroundings (61). The stability of hydrazone-based PEG-PE conjugates incubated at physiological pH 7.4 and acidic pH 5.0 in buffer solutions maintained at 37°C was investigated by HPLC. For this purpose, the area under the micelle peak of PEG-HZ-PE (Rt 9–10 min) was observed over a period of time. PEG-HZ-PE conjugates derived from an aliphatic aldehyde and different acyl hydrazides were found to be highly unstable under acidic conditions, with the micelle peak was completely disappearing within 2 min incubation at pH 5.0. At the same time, these conjugates were relatively stable at physiological pH: the PEG-HZ-PE conjugate, 9, with AMBH as cross-linker showed the half-life of 150 min followed by EMCH, 4a, (120 min), MPBH, 4b, (90 min), and KMUH, 4c, (20 min) (Table 2). The rate of hydrolysis among the aliphatic aldehyde-derived hydrazone-based PEG-PE conjugates (4a, 4b, 4c, and 9) at pH 7.4 seems to be dependent on carbon chain length of acyl hydrazide. The increase in number of carbon atoms in acyl hydrazide led to increase in rate of hydrolysis (PEG-PE conjugate 4c, acyl hydrazide with 10-C atoms >4a, acyl hydrazide with 5-C atoms >9, acyl hydrazide with 3-C atoms). Introducing an aromatic character within carbon chain of acyl hydrazide led to increase in hydrolysis as observed in case of 4b and 4a (rate of hydrolysis of 4b > 4a).
Table 2.
Half-lives of different hydrazone-based mPEG-HZ-PE conjugates incubated in phosphate buffered saline, pH 7.4 and pH 5.0 at 37°C over a period of time, h
| mPEG-HZ-PE Conjugate | Half-life (h) | |
|---|---|---|
| pH 7.4 | pH 5.0 | |
| 4a | 2 | <0.03 |
| 4b | 1.5 | <0.03 |
| 4c | 0.33 | <0.03 |
| 9 | 2.5 | <0.03 |
| 15a | >72 | >48 |
| 15b | >72 | >48 |
| 15c | >72 | >48 |
| 22 | 40 | 2.0 |
Alternatively, the PEG-HZ-PE conjugates derived from an aromatic aldehyde and acyl hydrazid es were found to be highly stable at pH 7.4 and 5.0 (Table 2). The half-life values were not attained at either of those pH values even at the end of incubation period of 72 h in pH 7.4 and 48 h in pH 5.0 buffer solutions maintained at 37°C. The resistance to hydrolysis exhibited by hydrazones derived from aromatic aldehydes can be attributed to the conjugation of the π bonds of –C = N– bond of the hydrazone with the π bonding benzene ring. Thus, it supports the finding that hydrazones formed from aromatic aldehydes are more stable to acidic hydrolysis than those formed from aliphatic ones (62, 63). The hydrazone hydrolysis involves the protonation of the –C = N nitrogen followed by the nucleophilic attack of water and cleavage of C–N bond of tetrahedran intermediate (64). Any of these steps is determining and dependant on the pH. The substituents on the carbonyl reaction partner influence the rate of hydrolysis through altering the pKa of the hydrazone with electron donating substituents facilitating protonation of the –C = N nitrogen (65).
This would support the fact that PEG-HZ-PE conjugates containing hydrazone bond derived from the aliphatic aldehyde are more prone to hydrolytic degradation. Aromatic aldehyde-derived hydrazone bond is too stable for the purpose of pH-triggered drug release. Careful selection of an aldehyde and an acyl hydrazide would be necessary for the application of the hydrazone-based chemistry for the development of pH-sensitive pharmaceutical nanocarriers.
As Scheme 9 shows, an aromatic ketone-derived hydrazone bond was introduced between PEG and PE. The presence of a methyl group (electron donating) on the carbonyl functional group would provide a sufficient lability of the hydrazone bond under mildly acidic conditions while an immediate aromatic ring (electron withdrawing) next to the hydrazone bond would offer the stability under acidic and neutral conditions. mPEG-HZ-PE conjugate, wherein the hydrazone bond is derived from an aromatic ketone, exhibited the half-lives of 2–3 h at slightly acidic pH values, and much higher stability (up to 40 h) at the physiological pH (Table 2).
4.4. Avidin–Biotin Affinity Chromatography
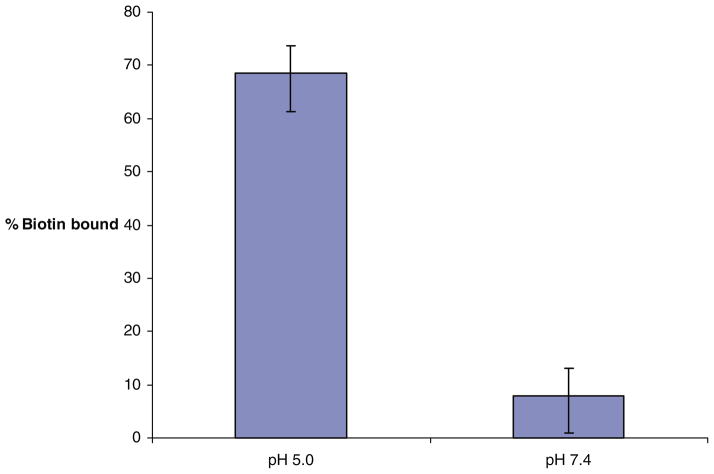
To determine the pH-sensitivity of mPEG-HZ-PE conjugates, biotin-embedded micelles shielded by cleavable mPEG2000-HZ-PE, were eluted through avidin immobilized gel media columns. The control micelle formulation (incubated at pH 7.4 at 37°C for 3 h) showed only a minimal biotin binding against 69% biotin binding of test micelle formulation (incubated at pH 5.0 at 37°C for 3 h), Fig. 1. This proves shielding effect of mPEG2000-HZ-PE conjugate under physiological pH condition and de-shielding after exposure to acidic environment.
Fig. 1.
Binding of pH-sensitive biotin-micelles to NeutrAvidin columns after the incubation at room temperature at pH 5.0 and 7.4
4.5. In Vitro Cell Culture Studies
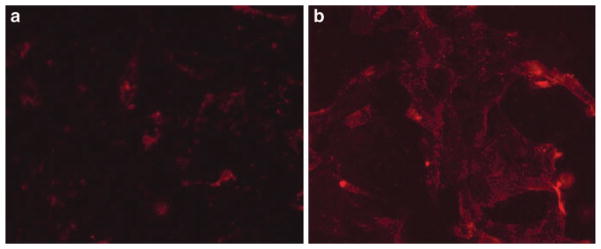
To study shielding/de-shielding effect of mPEG-HZ-PE under the influence of acidic pH, internalization of Rh-labeled, TATp-bearing, mPEG-HZ-PE-shielded liposomes pre-incubated at pH 7.4 and 5.0 was followed using H9C2 cells. As seen in Fig. 2a, b, Rh-labeled TATp-bearing, pH-sensitive liposomes incubated at pH 5.0 showed 2.5 times (ImageJ 1.34I data) more internalization than when incubated at pH 7.4 because of better accessibility of TATp for its action after detachment of pH-sensitive PEG corona from liposomal surface under the influence of “acidic” pH.
Fig. 2.
Fluorescence microscopy showing the internalization of Rh-PE-labeled TATp-modified pH-sensitive liposomes by H9C2 cells after the incubation at pH 7.4 2(a), and pH 5.0 2(b)
4.6. In Vivo Studies
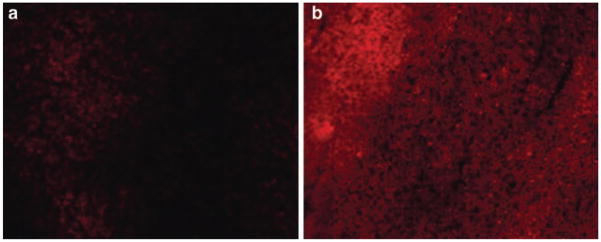
We attempted intratumoral injections of Rh-labeled, TATp-bearing pH-sensitive or pH-insensitive liposomes into LLC tumor-bearing mice to cover different physiological conditions. An “acidic” pH at the tumor site is a well-known fact which is of interest while developing physiology-based targeted delivery systems. Under the fluorescence microscope with TRITC filter, samples prepared 6 h post-injection from tumors injected with TATp-bearing, Rh-labeled, pH-sensitive liposomes demonstrated intensive and bright red fluorescence which was 4 times (as per ImageJ 1.34I data) more than that observed in the samples obtained from the tumors injected with TATp-bearing, Rh-labeled, pH-insensitive liposomes (Fig. 3a, b).
Fig. 3.
TRITC image of frozen tissue section treated with intratumoral injection of Rh-labeled/TATp/pH-insensitive liposome 3(a) or Rh-labeled/TATp/pH-sensitive liposome 3(b) into LLC tumor bearing mice
4.7. In Vivo pGFP Transfection Experiment
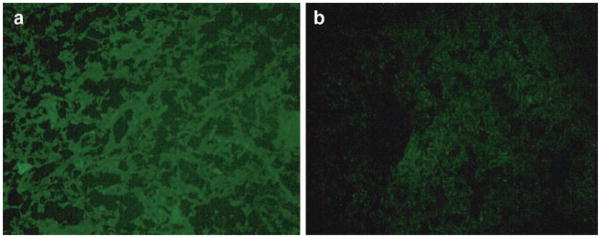
Also, we attempted a localized transfection of tumor cells by the direct intratumoral administration of sterically shielded with pH-sensitive (containing mPEG-HZ-PE, 25) or pH-insensitive (containing mPEG-DSPE) conjugates TATp-liposome-pGFP complexes into the tumor tissue by the intratumoral injections. Histologically, hematoxylin/eosin-stained tumor slices in animals injected with both preparations showed the identical typical pattern of poorly differentiated carcinoma (polymorphic cells with basophilic nuclei forming nests and sheets and containing multiple sites of neoangiogenesis). However, under the fluorescence microscope with FITC filter, samples prepared 72 h post-injection from tumors injected with pH-sensitive PEG-TATp-liposome-pGFP complexes demonstrated intensive and bright green fluorescence compared to only minimal GFP fluorescence observed in the samples obtained from the tumors injected with pH-insensitive PEG-TATp-liposome-pGFP complexes (Fig. 4a, b).
Fig. 4.
Fluorescence microscopy images of the LLC tumor sections from the tumors injected with pGFP-loaded TATp-bearing liposomes with the pH-cleavable PEG coat 4(a), and with the pH-non-cleavable PEG coat 4(b). Notice enhanced GFP expression in 4(a) case
The enhanced pGFP transfection by using pH-sensitive PEG-TATp-liposome-pGFP complexes is an ultimate result of the removal of mPEG-HZ-PE coat under the decreased pH of the tumor tissue, and better accessibility of de-shielded TATp moieties in TATp-liposome-pGFP complexes for internalization by the cancer cells allowing for the increased interactions of pGFP with cancer cell nuclei.
Owing to their physico-chemical properties, the long-circulating (PEGylated) liposomal carriers have the ability to accumulate inside the tumor tissue via the EPR effect, without further escape into undesired non-target sites. The pH at tumor sites is “acidic” (2, 3). Therefore, when TATp-pGFP-liposomes with an additional pH-sensitive PEG coating accumulate in the tumor tissue, the lowered pH-mediated removal of the protective PEG coat takes place, and TATp moieties become exposed and accessible for the interaction with cells. This leads to rapid pGFP pay-load delivery into the cancer cells as a result of the extensive TATp-mediated internalization of liposomes, and thereby enhanced transfection. The ImageJ analysis indicated a three times less transfection in the case of PEG-TATp-pGFP-pH-insensitive liposomes as non-detachable PEG coat interferes and sterically hinders the interactions between TATp and target cancer cells.
5. Conclusions
pH-sensitive mPEG-HZ-PE conjugates based on hydrazone bond chemistry were synthesized. The pH-dependant hydrolytic kinetics could be tuned using appropriate aldehyde or ketone and acyl hydrazide. These conjugates have immense applications in targeted drug delivery systems e.g., the development of the targeted drug carriers carrying the temporarily hidden function (e.g., cell penetrating peptide, TATp), and a detachable PEG-HZ-PE which, in addition to prolonging circulation half-life of carriers, can expose TATp function only under the action of certain local stimuli (such as lowered pH), represent a significant step on the way toward “smart” multifunctional pharmaceutical nanocarriers for target accumulation by EPR effect and intracellular penetration in a controlled fashion.
Acknowledgments
This work was supported by the NIH grants RO1 HL55519 and RO1 CA121838 to VPT.
Footnotes
Phosphomolybdic acid and Dragendorff are usually used for visualization of PEG and phospholipids respectively, but iodine fumes could be used as the universal developing agent for visualization of both and their conjugates as well on reversible basis without damaging the developed TLC plate.
If other grades of silica gel are intended for the separation purpose, then conduct a series of suitability experiments before use with respect to volume of media required, sample volume and typical elution profile.
While handling all materials stored in chloroform under nitrogen at −80°C, allow them stabilize at room temperature before opening and using them. This is good lab safety practice.
Keep the reaction vessel continuously flushed with inert gases like argon or nitrogen to remove headspace oxygen which might affect reaction rate or degrade the reaction vessel contents.
Use standardized suitable Sepharose CL4B gel filtration column for proper separation of micelles from excess PEG. Use degassed water (pH >8, adjusted with 0.1 N NaOH) as elution medium to protect “acid labile” PEG-PE conjugates.
Prepare Immobilized NeutrAvidin protein column well ahead so that it would stabilize as per manufacturer’s (Pierce) manual for at least 30 min of application of samples.
Formation of air bubbles is highly discouraged. Careful mounting of slide on stage is necessary.
This protocol can be used for other cell lines as well.
Quantification may be desirable for the interpretation of the results and can be performed in several different ways, we have used ImageJ software (NIH) for this purpose, but other techniques could be used.
If tumors do not reach the desired size, wait for more time as it takes longer to grow in some animals but 2–3 weeks is average time for tumor cell used in the current protocol.
Hemotoxylin-eosin staining protocol is used to observe histology of cancer tissue of treated and non-treated animals. Other parallel methods could be followed to reach the similar outcome.
References
- 1.Jayasundar R, Singh VP. In vivo temperature measurements in brain tumors using proton MR spectroscopy. Neurol India. 2002;50:436–439. [PubMed] [Google Scholar]
- 2.Engin K, Leeper DB, Cater JR, Thistlethwaite AJ, Tupchong L, McFarlane JD. Extracellular pH distribution in human tumours. Int J Hyperthermia. 1995;11:211–216. doi: 10.3109/02656739509022457. [DOI] [PubMed] [Google Scholar]
- 3.Ojugo AS, McSheehy PM, McIntyre DJ, McCoy C, Stubbs M, Leach MO, Judson IR, Griffiths JR. Measurement of the extracellular pH of solid tumours in mice by magnetic resonance spectroscopy: a comparison of exogenous (19)F and (31)P probes. NMR Biomed. 1999;12:495–504. doi: 10.1002/(sici)1099-1492(199912)12:8<495::aid-nbm594>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 4.Khare AR, Peppas NA. Release behavior of bioactive agents from pH-sensitive hydrogels. J Biomater Sci Polym Ed. 1993;4:275–289. doi: 10.1163/156856293x00564. [DOI] [PubMed] [Google Scholar]
- 5.Kataoka K, Matsumoto T, Yokoyama M, Okano T, Sakurai Y, Fukushima S, Okamoto K, Kwon GS. Doxorubicin-loaded poly(ethylene glycol)-poly(beta-benzyl-l-aspartate) copolymer micelles: their pharmaceutical characteristics and biological significance. J Control Release. 2000;64:143–153. doi: 10.1016/s0168-3659(99)00133-9. [DOI] [PubMed] [Google Scholar]
- 6.Leroux J, Roux E, Le Garrec D, Hong K, Drummond DC. N-isopropylacrylamide copolymers for the preparation of pH-sensitive liposomes and polymeric micelles. J Control Release. 2001;72:71–84. doi: 10.1016/s0168-3659(01)00263-2. [DOI] [PubMed] [Google Scholar]
- 7.Le Garrec D, Taillefer J, Van Lier JE, Lenaerts V, Leroux JC. Optimizing pH-responsive polymeric micelles for drug delivery in a cancer photodynamic therapy model. J Drug Target. 2002;10:429–437. doi: 10.1080/1061186021000001887. [DOI] [PubMed] [Google Scholar]
- 8.Taillefer J, Brasseur N, van Lier JE, Lenaerts V, Le Garrec D, Leroux JC. In-vitro and in-vivo evaluation of pH-responsive polymeric micelles in a photodynamic cancer therapy model. J Pharm Pharmacol. 2001;53:155–166. doi: 10.1211/0022357011775352. [DOI] [PubMed] [Google Scholar]
- 9.Asokan A, Cho MJ. Cytosolic delivery of macromolecules. 3. Synthesis and characterization of acid-sensitive bis-detergents. Bioconjug Chem. 2004;15:1166–1173. doi: 10.1021/bc049880n. [DOI] [PubMed] [Google Scholar]
- 10.Lee ES, Na K, Bae YH. Polymeric micelle for tumor pH and folate-mediated targeting. J Control Release. 2003;91:103–113. doi: 10.1016/s0168-3659(03)00239-6. [DOI] [PubMed] [Google Scholar]
- 11.Lee ES, Shin HJ, Na K, Bae YH. Poly(l-histidine)-PEG block copolymer micelles and pH-induced destabilization. J Control Release. 2003;90:363–374. doi: 10.1016/s0168-3659(03)00205-0. [DOI] [PubMed] [Google Scholar]
- 12.Braslawsky GR, Kadow K, Knipe J, McGoff K, Edson M, Kaneko T, Greenfield RS. Adriamycin(hydrazone)-antibody conjugates require internalization and intracellular acid hydrolysis for antitumor activity. Cancer Immunol Immunother. 1991;33:367–374. doi: 10.1007/BF01741596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo HS, Lee EA, Park TG. Doxorubicin-conjugated biodegradable polymeric micelles having acid-cleavable linkages. J Control Release. 2002;82:17–27. doi: 10.1016/s0168-3659(02)00088-3. [DOI] [PubMed] [Google Scholar]
- 14.Lee ES, Na K, Bae YH. Super pH-sensitive multifunctional polymeric micelle. Nano Lett. 2005;5:325–329. doi: 10.1021/nl0479987. [DOI] [PubMed] [Google Scholar]
- 15.Shen WC, Ryser HJ. cis-Aconityl spacer between daunomycin and macromolecular carriers: a model of pH-sensitive linkage releasing drug from a lysosomotropic conjugate. Biochem Biophys Res Commun. 1981;102:1048–1054. doi: 10.1016/0006-291x(81)91644-2. [DOI] [PubMed] [Google Scholar]
- 16.Ogden JR, Leung K, Kunda SA, Telander MW, Avner BP, Liao SK, Thurman GB, Oldham RK. Immunoconjugates of doxorubicin and murine antihuman breast carcinoma monoclonal antibodies prepared via an N-hydroxysuccinimide active ester intermediate of cis-aconityl-doxorubicin: preparation and in vitro cytotoxicity. Mol Biother. 1989;1:170–174. [PubMed] [Google Scholar]
- 17.Patel VF, Hardin JN, Mastro JM, Law KL, Zimmermann JL, Ehlhardt WJ, Woodland JM, Starling JJ. Novel acid labile COL1 trityl-linked difluoronucleoside immunoconjugates: synthesis, characterization, and biological activity. Bioconjugate Chem. 1996;7:497–510. doi: 10.1021/bc960038u. [DOI] [PubMed] [Google Scholar]
- 18.Heffernan MJ, Murthy N. Polyketal nanoparticles: a new pH-sensitive biodegradable drug delivery vehicle. Bioconjugate Chem. 2005;16:1340–1342. doi: 10.1021/bc050176w. [DOI] [PubMed] [Google Scholar]
- 19.Gillies ER, Frechet JM. pH-Responsive copolymer assemblies for controlled release of doxorubicin. Bioconjugate Chem. 2005;16:361–368. doi: 10.1021/bc049851c. [DOI] [PubMed] [Google Scholar]
- 20.Gillies ER, Jonsson TB, Frechet JM. Stimuli-responsive supramolecular assemblies of linear-dendritic copolymers. J Am Chem Soc. 2004;126:11936–11943. doi: 10.1021/ja0463738. [DOI] [PubMed] [Google Scholar]
- 21.Gumusderelioglu M, Kesgin D. Release kinetics of bovine serum albumin from pH-sensitive poly(vinyl ether) based hydrogels. Int J Pharm. 2005;288:273–279. doi: 10.1016/j.ijpharm.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Shin J, Shum P, Thompson DH. Acid-triggered release via dePEGylation of DOPE liposomes containing acid-labile vinyl ether PEG-lipids. J Control Release. 2003;91:187–200. doi: 10.1016/s0168-3659(03)00232-3. [DOI] [PubMed] [Google Scholar]
- 23.Kratz F, Beyer U, Roth T, Schutte MT, Unold A, Fiebig HH, Unger C. Albumin conjugates of the anticancer drug chlorambucil: synthesis, characterization, and in vitro efficacy. Arch Pharm (Weinheim) 1998;331:47–53. doi: 10.1002/(sici)1521-4184(199802)331:2<47::aid-ardp47>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 24.Beyer U, Roth T, Schumacher P, Maier G, Unold A, Frahm AW, Fiebig HH, Unger C, Kratz F. Synthesis and in vitro efficacy of transferrin conjugates of the anticancer drug chlorambucil. J Med Chem. 1998;41:2701–2708. doi: 10.1021/jm9704661. [DOI] [PubMed] [Google Scholar]
- 25.Di Stefano G, Lanza M, Kratz F, Merina L, Fiume L. A novel method for coupling doxorubicin to lactosaminated human albumin by an acid sensitive hydrazone bond: synthesis, characterization and preliminary biological properties of the conjugate. Eur J Pharm Sci. 2004;23:393–397. doi: 10.1016/j.ejps.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Toncheva V, Schacht E, Ng SY, Barr J, Heller J. Use of block copolymers of poly(ortho esters) and poly (ethylene glycol) micellar carriers as potential tumour targeting systems. J Drug Target. 2003;11:345–353. doi: 10.1080/10611860310001633839. [DOI] [PubMed] [Google Scholar]
- 27.Oishi M, Nagasaki Y, Itaka K, Nishiyama N, Kataoka K. Lactosylated poly(ethylene glycol)-siRNA conjugate through acid-labile beta-thiopropionate linkage to construct pH-sensitive polyion complex micelles achieving enhanced gene silencing in hepatoma cells. J Am Chem Soc. 2005;127:1624–1625. doi: 10.1021/ja044941d. [DOI] [PubMed] [Google Scholar]
- 28.Steenbergen C, Deleeuw G, Rich T, Williamson JR. Effects of acidosis and ischemia on contractility and intracellular pH of rat heart. Circ Res. 1977;41:849–858. doi: 10.1161/01.res.41.6.849. [DOI] [PubMed] [Google Scholar]
- 29.Frunder H. The pH changes of living tissue during activity and inflammation. Pharmazie. 1949;4:345–355. [Google Scholar]
- 30.Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 31.Shen WC, Ryser HJ. cis-Aconityl spacer between daunomycin and macromolecular carriers: a model of pH-sensitive linkage releasing drug from a lysosomotropic conjugate. Biochem Biophys Res Commun. 1981;102:1048–1054. doi: 10.1016/0006-291x(81)91644-2. [DOI] [PubMed] [Google Scholar]
- 32.Diener E, Diner UE, Sinha A, Xie S, Vergidis R. Specific immunosuppression by immunotoxins containing daunomycin. Science. 1986;231:148–150. doi: 10.1126/science.3484557. [DOI] [PubMed] [Google Scholar]
- 33.Ogden JR, Leung K, Kunda SA, Telander MW, Avner BP, Liao SK, Thurman GB, Oldham RK. Immunoconjugates of doxorubicin and murine antihuman breast carcinoma monoclonal antibodies prepared via an N-hydroxysuccinimide active ester intermediate of cis-aconityl-doxorubicin: preparation and in vitro cytotoxicity. Mol Biother. 1989;1:170–174. [PubMed] [Google Scholar]
- 34.Sinkule JA, Rosen ST, Radosevich JA. Monoclonal antibody 44–3A6 doxorubicin immunoconjugates: comparative in vitro anti-tumor efficacy of different conjugation methods. Tumour Biol. 1991;12:198–206. doi: 10.1159/000217705. [DOI] [PubMed] [Google Scholar]
- 35.Patel VF, Hardin JN, Mastro JM, Law KL, Zimmermann JL, Ehlhardt WJ, Woodland JM, Starling JJ. Novel acid labile COL1 trityl-linked difluoronucleoside immunoconjugates: synthesis, characterization, and biological activity. Bioconjug Chem. 1996;7:497–510. doi: 10.1021/bc960038u. [DOI] [PubMed] [Google Scholar]
- 36.Patel VF, Hardin JN, Starling JJ, Mastro JM. Novel trityl linked drug immunoconjugates for cancer therapy. Bioorg Med Chem Lett. 1995;5:507–512. [Google Scholar]
- 37.Patel VF, Hardin JN, Grindey GB, Schultz RM. Tritylated oncolytics as prodrugs. Bioorg Med Chem Lett. 1995;5:513–518. [Google Scholar]
- 38.Fife T, Jao L. Substituent effects in acetal hydrolysis. J Org Chem. 1965;30:1492–1495. [Google Scholar]
- 39.Gillies ER, Goodwin AP, Frechet JM. Acetals as pH-sensitive linkages for drug delivery. Bioconjug Chem. 2004;15:1254–1263. doi: 10.1021/bc049853x. [DOI] [PubMed] [Google Scholar]
- 40.Murthy N, Campbell J, Fausto N, Hoffman AS, Stayton PS. Design and synthesis of pH-responsive polymeric carriers that target uptake and enhance the intracellular delivery of oligonucleotides. J Control Release. 2003;89:365–374. doi: 10.1016/s0168-3659(03)00099-3. [DOI] [PubMed] [Google Scholar]
- 41.Murthy N, Xu M, Schuck S, Kunisawa J, Shastri N, Frechet JM. A macromolecular delivery vehicle for protein-based vaccines: acid-degradable protein-loaded microgels. Proc Natl Acad Sci U S A. 2003;100:4995–5000. doi: 10.1073/pnas.0930644100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heffernan MJ, Murthy N. Polyketal nanoparticles: a new pH-sensitive biodegradable drug delivery vehicle. Bioconjug Chem. 2005;16:1340–1342. doi: 10.1021/bc050176w. [DOI] [PubMed] [Google Scholar]
- 43.Lee S, Yang SC, Heffernan MJ, Taylor WR, Murthy N. Polyketal microparticles: a new delivery vehicle for superoxide dismutase. Bioconjug Chem. 2007;18:4–7. doi: 10.1021/bc060259s. [DOI] [PubMed] [Google Scholar]
- 44.Gümüsderelioglu M, Kesgin D. Release kinetics of bovine serum albumin from pH-sensitive poly(vinyl ether) based hydrogels. Int J Pharm. 2005;288:273–279. doi: 10.1016/j.ijpharm.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 45.Gümüsderelioglu M, Topal IU. Vinyl ether/acrylic acid terpolymer hydrogels synthesized by [gamma]-radiation: characterization, thermosensitivity and pH-sensitivity. Radiat Phys Chem. 2005;73:272–279. [Google Scholar]
- 46.Hurwitz E, Wilchek M, Pitha J. Soluble macromolecules as carriers for daunorubicin. J Appl Biochem. 1980;2:25. [Google Scholar]
- 47.Laguzza BC, Nichols CL, Briggs SL, Cullinan GJ, Johnson DA, Starling JJ, Baker AL, Bumol TF, Corvalan JR. New antitumor monoclonal antibody-vinca conjugates LY203725 and related compounds: design, preparation, and representative in vivo activity. J Med Chem. 1989;32:548–555. doi: 10.1021/jm00123a007. [DOI] [PubMed] [Google Scholar]
- 48.Beyer U, Roth T, Schumacher P, Maier G, Unold A, Frahm AW, Fiebig HH, Unger C, Kratz F. Synthesis and in vitro efficacy of transferrin conjugates of the anticancer drug chlorambucil. J Med Chem. 1998;41:2701–2708. doi: 10.1021/jm9704661. [DOI] [PubMed] [Google Scholar]
- 49.Kratz F, Beyer U, Roth T, Schutte MT, Unold A, Fiebig HH, Unger C. Albumin conjugates of the anticancer drug chlorambucil: synthesis, characterization, and in vitro efficacy. Arch Pharm (Weinheim) 1998;331:47–53. doi: 10.1002/(sici)1521-4184(199802)331:2<47::aid-ardp47>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 50.Oishi M, Nagasaki Y, Itaka K, Nishiyama N, Kataoka K. Lactosylated poly(ethylene glycol)-siRNA conjugate through acid-labile beta-thiopropionate linkage to construct pH-sensitive polyion complex micelles achieving enhanced gene silencing in hepatoma cells. J Am Chem Soc. 2005;127:1624–1625. doi: 10.1021/ja044941d. [DOI] [PubMed] [Google Scholar]
- 51.Kataoka K, Itaka K, Nishiyama N, Yamasaki Y, Oishi M, Nagasaki Y. Smart polymeric micelles as nanocarriers for oligonucleotides and siRNA delivery. Nucleic Acids Symp Ser (Oxf) 2005;49:17–18. doi: 10.1093/nass/49.1.17. [DOI] [PubMed] [Google Scholar]
- 52.Kong SD, Luong A, Manorek G, Howell SB, Yang J. Acidic hydrolysis of N-Ethoxybenzylimidazoles (NEBIs): potential applications as pH-sensitive linkers for drug delivery. Bioconjug Chem. 2007;18:293–296. doi: 10.1021/bc060224s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sawant RM, Hurley JP, Salmaso S, Kale AA, Tolcheva E, Levchenko T, Torchilin VP. “Smart” drug delivery systems: double-targeted pH-responsive pharmaceutical nanocarriers. Bioconjugate Chem. 2006;17:943–949. doi: 10.1021/bc060080h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kale AA, Torchilin VP. Design, synthesis, and characterization of pH-sensitive PEG-PE conjugates for stimuli-sensitive pharmaceutical nanocarriers: the effect of substitutes at the hydrazone linkage on the ph stability of PEG-PE conjugates. Bioconjug Chem. 2007;18:363–370. doi: 10.1021/bc060228x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeffs LB, Palmer LR, Ambegia EG, Giesbrecht C, Ewanick S, MacLachlan I. A scalable, extrusion-free method for efficient liposomal encapsulation of plasmid DNA. Pharm Res. 2005;22:362–372. doi: 10.1007/s11095-004-1873-z. [DOI] [PubMed] [Google Scholar]
- 56.Torchilin VP, Levchenko TS, Rammohan R, Volodina N, Papahadjopoulos-Sternberg B, D’Souza Gerard GM. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proc Natl Acad Sci U S A. 2003;100:1972–1977. doi: 10.1073/pnas.0435906100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kale AA, Torchilin VP. Enhanced transfection of tumor cells in vivo using “Smart” pH-sensitive TAT-modified pegy-lated liposomes. J Drug Target. 2007;15:538–545. doi: 10.1080/10611860701498203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sawant RM, Hurley JP, Salmaso S, Kale AA, Tolcheva E, Levchenko T, Torchilin VP. “Smart” drug delivery systems: double-targeted pH-responsive pharmaceutical nanocarriers. Bioconjug Chem. 2006;17:943–949. doi: 10.1021/bc060080h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Torchilin VP, Levchenko TS, Rammohan R, Volodina N, Papahadjopoulos-Sternberg B, D’Souza GGM. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proc Natl Acad Sci U S A. 2003;100:1972–1977. doi: 10.1073/pnas.0435906100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rideout D. Self-assembling drugs: a new approach to biochemical modulation in cancer chemotherapy. Cancer Invest. 1994;12:189–202. doi: 10.3109/07357909409024874. discussion 268–269. [DOI] [PubMed] [Google Scholar]
- 61.Lukyanov AN, Gao Z, Torchilin VP. Micelles from polyethylene glycol/phosphati-dylethanolamine conjugates for tumor drug delivery. J Control Release. 2003;91:97–102. doi: 10.1016/s0168-3659(03)00217-7. [DOI] [PubMed] [Google Scholar]
- 62.Apelgren LD, Bailey DL, Briggs SL, Barton RL, Guttman-Carlisle D, Koppel GA, Nichols CL, Scott WL, Lindstrom TD, Baker AL, et al. Chemoimmunoconjugate development for ovarian carcinoma therapy: preclinical studies with vinca alkaloid-monoclonal antibody constructs. Bioconjugate Chem. 1993;4:121–126. doi: 10.1021/bc00020a003. [DOI] [PubMed] [Google Scholar]
- 63.Baker MA, Gray BD, Ohlsson-Wilhelm BM, Carpenter DC, Muirhead KA. Zyn-Linked colchicines: controlled-release lipophilic prodrugs with enhanced antitumor efficacy. J Control Release. 1996;40:89–100. [Google Scholar]
- 64.Cordes EH, Jencks WP. The Mechanism of hydrolysis of schiff’s bases derived from aliphatic amines. J Am Chem Soc. 1963;85:2843–2848. [Google Scholar]
- 65.Harnsberger HF, Cochran EL, Szmant HH. The basicity of hydrazones. J Am Chem Soc. 1955;77:5048–5050. [Google Scholar]