Abstract
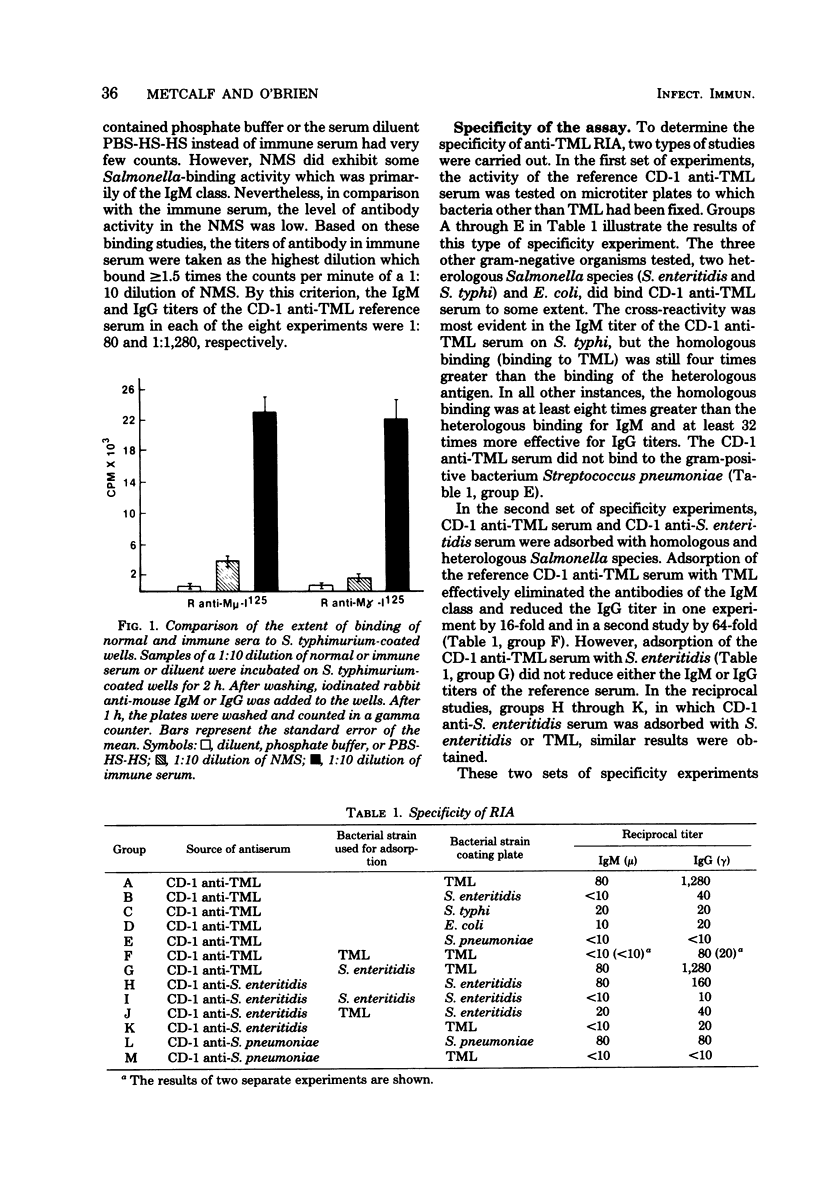
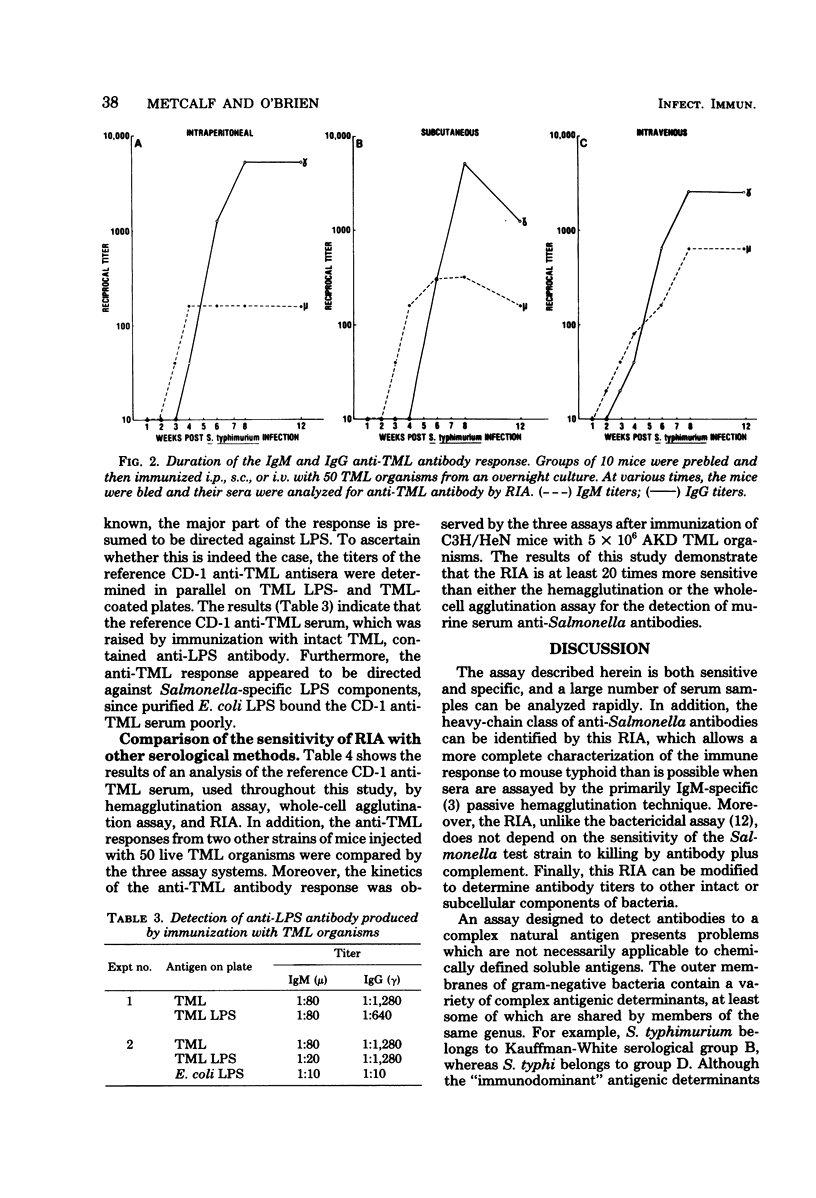
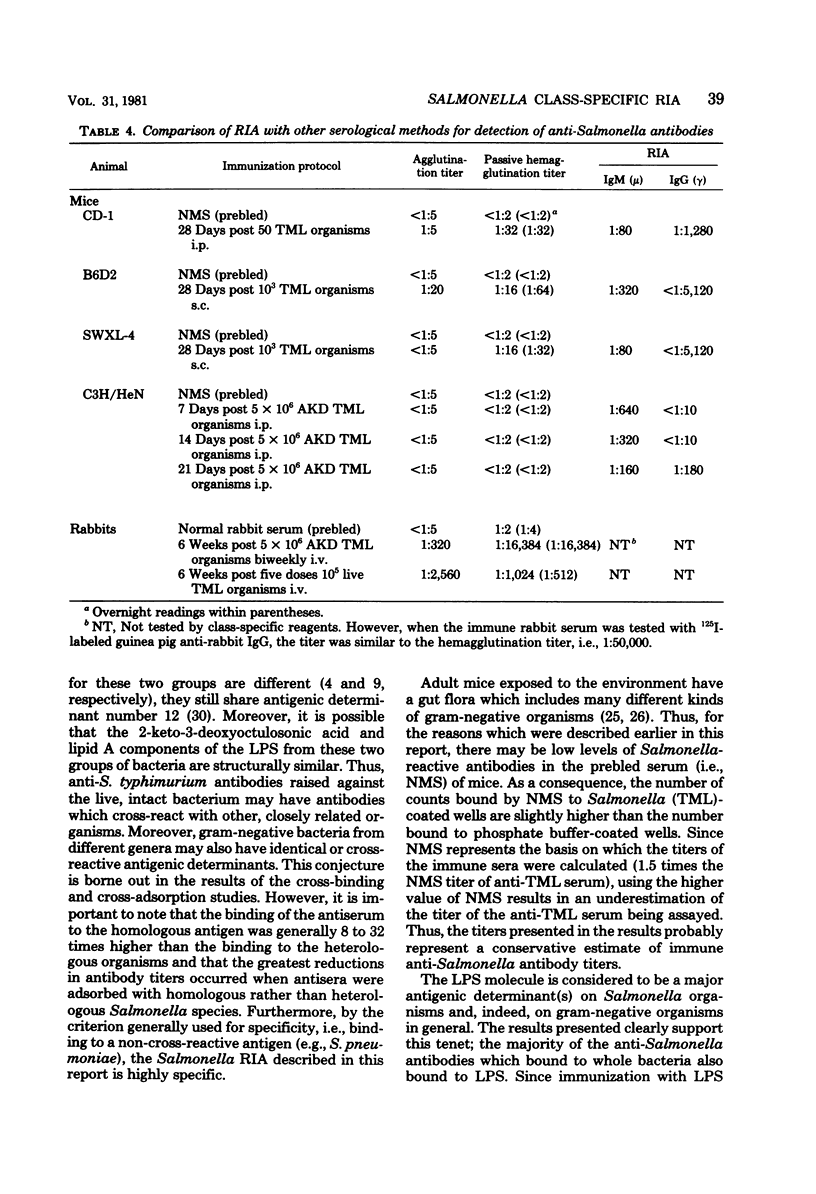
A heavy-chain class-specific, solid-phase radioimmunoassay was developed to characterize the murine antibody response to Salmonella typhimurium. The specificity of the assay was verified by quantitation of the extent of binding of anti-S. typhimurium antibodies to other bacterial genera and species and by cross-adsorption studies. The sensitivity of the procedure was also examined, and it was determined to be substantially more sensitive than either the passive hemagglutination or the whole-cell agglutination technique. The method was subsequently used to analyze th murine antibody response to S. typhimurium. Groups of mice were prebled and then immunized with live S. typhimurium via different routes. The animals were bled weekly for 12 weeks, and then sera were assayed for antibodies directed against whole bacteria or purified lipopolysaccharide. Anti-Salmonella antibodies of the immunoglobulin M class appeared in the serum approximately 2 to 3 weeks after immunization, and then immunoglobulin G anti-Salmonella antibodies appeared which constituted the major part of the long-term response. Immunoglobulin A was not a major component of the serum antibody response. The antibodies were primarily directed against the lipopolysaccharide determinants, but a small percentage of the response was directed against other cell surface components. Qualitatively and quantitatively similar anti-Salmonella antibody responses were observed in sera of outbred and inbred strains of mice.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Möller G., Sjöberg O. Selective induction of DNA synthesis in T and B lymphocytes. Cell Immunol. 1972 Aug;4(4):381–393. doi: 10.1016/0008-8749(72)90040-8. [DOI] [PubMed] [Google Scholar]
- Angerman C. R., Eisenstein T. K. Correlation of the duration and magnitude of protection against Salmonella infection afforded by various vaccines with antibody titers. Infect Immun. 1980 Feb;27(2):435–443. doi: 10.1128/iai.27.2.435-443.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson H. E., Lindberg A. A., Hammarström S. Titration of antibodies to salmonella O antigens by enzyme-linked immunosorbent assay. Infect Immun. 1972 Nov;6(5):703–708. doi: 10.1128/iai.6.5.703-708.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. L., Scher I., Mosier D. E. In vitro studies of the genetically determined unresponsiveness to thymus-independent antigens in CBA/N mice. J Immunol. 1976 Feb;116(2):301–304. [PubMed] [Google Scholar]
- Collins F. M. Effect of specific immune mouse serum on the growth of Salmonella enteritidis in nonvaccinated mice challenged by various routes. J Bacteriol. 1969 Feb;97(2):667–675. doi: 10.1128/jb.97.2.667-675.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974 Dec;38(4):371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K., Angerman C. R. Immunity to experimental Salmonella infection: studies on the protective capacity and immunogenicity of lipopolysaccharide, acetone-killed cells, and ribosome-rich extracts of Salmonella typhimurium in C3H/HeJ and CD-1 mice. J Immunol. 1978 Sep;121(3):1010–1014. [PubMed] [Google Scholar]
- Giannella R. A., Broitman S. A., Zamcheck N. Salmonella enteritis. II. Fulminant diarrhea in and effects on the small intestine. Am J Dig Dis. 1971 Nov;16(11):1007–1013. doi: 10.1007/BF02235013. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hunter K. W., Jr, Finkelman F. D., Strickland G. T., Sayles P. C., Scher I. Defective resistance to Plasmodium yoelii in CBA/N mice. J Immunol. 1979 Jul;123(1):133–137. [PubMed] [Google Scholar]
- Kenny K., Herzberg M. Antibody response and protection induced by immunization with smooth and rough strains in experimental salmonellosis. J Bacteriol. 1968 Feb;95(2):406–417. doi: 10.1128/jb.95.2.406-417.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny K., Herzberg M. Early antibody response in mice to either infection or immunization with Salmonella typhimurium. J Bacteriol. 1967 Mar;93(3):773–778. doi: 10.1128/jb.93.3.773-778.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman N. R., Taylor R. B. General methods for the study of cells and serum during the immune response: the response to dinitrophenyl in mice. Clin Exp Immunol. 1969 Apr;4(4):473–487. [PMC free article] [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Saxen H., Valtonen M., Mäkelä P. H. Immunization with major outer membrane proteins in experimental salmonellosis of mice. Infect Immun. 1979 Sep;25(3):857–862. doi: 10.1128/iai.25.3.857-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDY M. Enhancement of the immunogenicity of typhoid vaccine by retention of the V1 antigen. Am J Hyg. 1953 Sep;58(2):148–164. doi: 10.1093/oxfordjournals.aje.a119596. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V., Collins F. M. Host-parasite relations in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):573–583. doi: 10.1084/jem.124.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond J. J., Lieberman R., Inman J. K., Mosier D. E., Paul W. E. Inability of mice with a defect in B-lymphocyte maturation to respond to phosphorycholine on immunogenic carriers. J Exp Med. 1977 Oct 1;146(4):1138–1142. doi: 10.1084/jem.146.4.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Leive L. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J Biol Chem. 1975 Apr 25;250(8):2911–2919. [PubMed] [Google Scholar]
- O'Brien A. D., Scher I., Campbell G. H., MacDermott R. P., Formal S. B. Susceptibility of CBA/N mice to infection with Salmonella typhimurium: influence of the X-linked gene controlling B lymphocyte function. J Immunol. 1979 Aug;123(2):720–724. [PubMed] [Google Scholar]
- Quintáns J., Kaplan R. B. Failure of CBA/N mice to respond to thymus-dependent and thymus-independent phosphorylcholine antigens. Cell Immunol. 1978 Jul;38(2):294–301. doi: 10.1016/0008-8749(78)90060-6. [DOI] [PubMed] [Google Scholar]
- Romeo D., Girard A., Rothfield L. Reconstitution of a functional membrane enzyme system in a monomolecular film. I. Formation of a mixed monolayer of lipopolysaccharide and phospholipid. J Mol Biol. 1970 Nov 14;53(3):475–490. doi: 10.1016/0022-2836(70)90078-1. [DOI] [PubMed] [Google Scholar]
- Rosenstreich D. L., Glode L. M. Difference in B cell mitogen responsiveness between closely related strains of mice. J Immunol. 1975 Sep;115(3):777–780. [PubMed] [Google Scholar]
- Rosenstreich D. L., Vogel S. N., Jacques A., Wahl L. M., Scher I., Mergenhagen S. E. Differential endotoxin sensitivity of lymphocytes and macrophages from mice with an X-linked defect in B cell maturation. J Immunol. 1978 Aug;121(2):685–690. [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R., COSTELLO R. THE DEVELOPMENT OF THE BACTERIAL FLORA IN THE GASTROINTESTINAL TRACT OF MICE. J Exp Med. 1965 Jul 1;122:59–66. doi: 10.1084/jem.122.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., McAllister J. S., Davis C. P. Anaerobic bacteria on the mucosal epithelium of the murine large bowel. Infect Immun. 1971 Oct;4(4):492–502. doi: 10.1128/iai.4.4.492-502.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher I., Berning A. K., Asofsky R. X-linked B lymphocyte defect in CBA/N mice. IV. Cellular and environmental influences on the thymus dependent IgG anti-sheep red blood cell response. J Immunol. 1979 Jul;123(1):477–486. [PubMed] [Google Scholar]
- Scher I., Steinberg A. D., Berning A. K., Paul W. E. X-linked B-lymphocyte immune defect in CBA/N mice. II. Studies of the mechanisms underlying the immune defect. J Exp Med. 1975 Sep 1;142(3):637–650. doi: 10.1084/jem.142.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal G. P., Klinman N. R. Defining the heterogeneity of anti-tumor antibody responses. J Immunol. 1976 Jun;116(6):1539–1546. [PubMed] [Google Scholar]
- Sultzer B. M., Goodman G. W. Endotoxin protein: a B-cell mitogen and polyclonal activator of C3H/HeJ lymphocytes. J Exp Med. 1976 Sep 1;144(3):821–827. doi: 10.1084/jem.144.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M., Nilsson B. S. PPD tuberculin--a B-cell mitogen. Nat New Biol. 1972 Dec 13;240(102):198–200. doi: 10.1038/newbio240198a0. [DOI] [PubMed] [Google Scholar]
- Svenson S. B., Nurminen M., Lindberg A. A. Artificial Salmonella vaccines: O-antigenic oligosaccharide-protein conjugates induce protection against infection with Salmonella typhimurium. Infect Immun. 1979 Sep;25(3):863–872. doi: 10.1128/iai.25.3.863-872.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. I. Evidence for a single gene that influences mitogenic and immunogenic respones to lipopolysaccharides. J Exp Med. 1974 Nov 1;140(5):1147–1161. doi: 10.1084/jem.140.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Trenkner E., Cohn M. The use of bacterial lipopolysaccharides to show that two signals are required for the induction of antibody synthesis. J Exp Med. 1973 Sep 1;138(3):699–714. doi: 10.1084/jem.138.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]


