Abstract
A highly purified preparation of uridine 5′-diphosphate (UDP)-glucose (Glc) dehydrogenase (DH; EC 1.1.1.22) has been characterized from soybean (Glycine max L.) nodules. The enzyme had native and subunit molecular masses of approximately 272 and 50 kD, respectively. UDP-Glc DH displayed typical hyperbolic substrate kinetics and had Km values for UDP-Glc and NAD+ of 0.05 and 0.12 mm, respectively. Thymidine 5′-diphosphate-Glc and UDP-galactose could replace UDP-Glc as the sugar nucleotide substrate to some extent, but the enzyme had no activity with NADP+. Soybean nodule UDP-Glc DH was labile in the absence of NAD+ and was inhibited by a heat-stable, low-molecular-mass solute in crude extracts of soybean nodules. UDP-Glc DH was also isolated from developing soybean seeds and shoots of 5-d-old wheat and canola seedlings and was shown to have similar affinities for UDP-Glc and NAD+ as those of the soybean nodule enzyme. UDP-Glc DH from all of these sources was most active in young, rapidly growing tissues.
UDP-Glc DH (NAD+ 6-oxidoreductase; EC 1.1.1.22) catalyzes the oxidation of UDP-Glc to UDP-GlcUA with the concomitant reduction of two molecules of NAD+. The reaction is essentially irreversible and proceeds via a UDP-α-d-gluco-hexodialdose intermediate that remains tightly bound to the enzyme (Nelsestuen and Kirkwood, 1971). UDP-Glc DH has been studied extensively in animals, where it provides UDP-GlcUA for the synthesis of connective tissue glycosaminoglycans. In plants UDP-GlcUA is the donor of d-glucuronosyl units for the synthesis of several types of structural polysaccharides and is the precursor of other nucleotide sugars, including UDP-GalUA, UDP-Xyl, UDP-Ara, and UDP-d-apiose, which are incorporated into pectins and hemicelluloses (Ericson and Elbein, 1980; Brett and Waldron, 1990). However, UDP-Glc DH from plants has received little attention and is not well characterized biochemically. The widespread occurrence of this enzyme was questioned in early studies, which led to the suggestion that UDP-GlcUA may be formed in plants predominantly from the oxidation of myo-inositol (Loewus et al., 1973; Roberts and Cetorelli, 1973; Knee, 1978; Loewus and Loewus, 1980).
More recent investigations with radiolabeled Glc and myo-inositol in squash hypocotyls (Wakabayashi et al., 1989, 1991), the demonstration that UDP-Glc DH activity is induced in elicitor-stressed French bean cells (Robertson et al., 1995), and genetic evidence (Tenhaken and Thulke, 1996) all indicate that the oxidation of UDP-Glc may have a significant role in providing precursors of structural polysaccharides in plants. In this report we describe the properties of UDP-Glc DH from the host fraction of soybean root nodules. This tissue has enhanced expression of Suc synthase (a major source of UDP-Glc) and a very high turnover of Suc to support nodule development and symbiotic N fixation (Morell and Copeland, 1984; Thummler and Verma, 1987; Anthon and Emerich, 1990). Moreover, comparison of the kinetic properties of UDP-Glc DH with those of UDP-Glc pyrophosphorylase, which has been purified and characterized from the same tissue (Vella and Copeland, 1990), should provide an insight into how UDP-Glc is partitioned between divergent metabolic pathways. The presence of UDP-Glc DH in other plant tissues is also demonstrated.
MATERIALS AND METHODS
Seeds of soybean (Glycine max L. cv Alabaster) were obtained from Sydney Seeds (Sydney, NSW, Australia). Wheat (Triticum aestivum L. cv Vulcan), barley (Hordeum vulgare cv Clipper), and canola (Brassica napus L. cv Barossa) seeds were kindly donated by B.M. Halbisch (Narromine, NSW, Australia), Barrett Burston (Thornleigh, NSW, Australia), and Australian Seed and Vegetable Oils (Dubbo, NSW), respectively. Bradyrhizobium japonicum CB 1809 inoculum was a generous gift from Bio-Care Technology (Woy Woy, NSW, Australia). UDP-d-[U-14C]Glc was from Amersham and, unless otherwise indicated, all other chemicals and biochemicals were from Boehringer Mannheim or Sigma.
Growth of Plants
Seeds were surface sterilized in 0.4% (w/v) sodium hypochlorite for 5 min, washed with tap water for 15 min, and sown in a 1:1 sand:vermiculite mixture. Soybean seeds were coated with inoculum prior to sowing. Plants were grown in a greenhouse with minimum and maximum temperatures of approximately 20 and 28°C, respectively, and with supplementary lighting from Sylvania 400-W discharge lamps with a photon fluence of 200 μmol m−2 s−1 to provide a photoperiod of 16 h. Nodulated soybean plants were supplied weekly with the N-free nutrient solution of Evans et al. (1972) and tap water as required. Other plants were supplied with the same nutrient solution except that 5 mm NH4Cl and 5 mm KNO3 were added as an N source.
Preparation of Crude Extracts
All steps were performed at 0 to 4°C. Plant tissues were homogenized with a mortar and pestle in 2 volumes of 40 mm Tris-HCl, pH 7.5, containing 0.5 mm NAD+, 1 mm EDTA, and 2.5 mm DTT (buffer A). The homogenate was filtered through Miracloth (Calbiochem) and centrifuged at 30,000g for 15 min. The supernatant was freed of low-molecular-mass solutes by passage through an Econo-PacI0 DG column (Bio-Rad) in buffer A prior to assaying for enzyme activity.
Purification of UDP-Glc DH from Soybean Nodules
Unless otherwise indicated, all steps were performed at 0 to 4°C. Nodules (20 g) were harvested from 20- to 30-d-old soybean plants and homogenized with a mortar and pestle in 40 mL of buffer A. A suspension of 10 g of insoluble PVP in 20 mL of buffer A was added to the homogenate, and after 5 min, the mixture was filtered through Miracloth and centrifuged at 30,000g for 15 min. The supernatant was fractionated by the addition of (NH4)2SO4, and the fraction that precipitated between 30 and 60% saturation was collected by centrifuging at 30,000g for 10 min. The precipitate was dissolved in 3 mL of buffer A and applied to a Fractogel TSK-HW 55 (F) column (2.2 × 50 cm) that had been equilibrated previously with 40 mm Tris-HCl, pH 7.5, containing 75 mm KCl, 0.5 mm NAD+, 1 mm EDTA, and 2.5 mm DTT (buffer B). The flow rate was 1 mL min−1 and 4-mL fractions were collected. Active fractions were pooled, (NH4)2SO4 (0.36 g mL−1) was added, and the precipitated proteins were collected by centrifugation at 30,000g for 10 min.
The pellet was dissolved in 3 mL of buffer B, exchanged into 25 mm Tris-HCl, pH 7.5, containing 0.5 mm NAD+, 1 mm EDTA, and 2.5 mm DTT (buffer C) using an Econo-Pac10 DG column, and applied to a 5-mL Econo-Pac Q column (Bio-Rad) that had been equilibrated previously with buffer C. The column was washed with 15 mL of buffer C at a flow rate of 2 mL min−1 and eluted with a gradient of 0 to 0.35 m KCl in a total volume of 40 mL of buffer C. Active fractions (2 mL) were pooled, concentrated to 3 mL using a microconcentrator (Amicon, Beverly, MA), exchanged into 25 mm Tris-HCl, pH 7.5, containing 0.5 mm NAD+ and 1 mm EDTA (buffer D), and applied to a Mono-Q HR 5/5 column (Pharmacia) that had been equilibrated previously with buffer D.
The column was washed with 5 mL of buffer D and eluted with a gradient of 0 to 0.35 m KCl in a total volume of 40 mL of buffer D using a flow rate of 1 mL min−1. Active fractions (2 mL) were pooled, concentrated, exchanged into buffer D and applied to a Mono-P column (Pharmacia) that had been equilibrated previously with buffer C. The column was eluted with 5% (v/v) Polybuffer 74 containing 0.5 mm NAD+ and 2.5 mm DTT at a flow rate of 1 mL min−1. Fractions of 1.5 mL were collected into tubes that contained 1 mL of 50 mm Tris-HCl, pH 8, 0.5 mm NAD+, 1 mm EDTA, and 2.5 mm DTT. Active fractions were pooled, exchanged into buffer C, concentrated to 1 mL, and 1% (w/v) BSA and 30% (v/v) glycerol were added. The enzyme lost less than 10% of its activity when stored at −20°C under these conditions for 1 week. Preparations of this type were free of phosphatase, UDP-Glc pyrophosphorylase, and NADH oxidase activities and were used to study the kinetic properties of UDP-Glc DH.
To purify UDP-Glc DH further, preparations obtained from the Mono-P step containing approximately 25 μg of protein in 100 μL were subjected to preparative electrophoresis in 4% low-melting-point agarose gels (FMC, Rockland, ME) at 4°C and 15 mA for 2 h. Following electrophoresis, thin strips cut from both sides of the gel were stained for UDP-Glc DH activity, as described by Gabriel and Gersten (1992), and were stained for protein using Coomassie brilliant blue R-250. UDP-Glc DH was recovered as denatured protein by freezing and thawing the unstained portion of the gel according to the manufacturer's instructions.
SDS-PAGE was performed at 20 to 22°C as described by Laemmli (1970), and nondenaturing PAGE was performed according to the method of Gabriel (1971). Gels were stained for carbohydrate using Schiff's fuchsin-sulfite reagent (Sigma) and stained for protein with silver reagent (Bio-Rad) according to the suppliers' instructions.
Partial Purification of UDP-Glc DH from Other Sources
UDP-Glc DH was purified as far as the Econo-Pac Q step from developing soybean seeds (20 g) and from shoots of 5-d-old wheat (15 g) and canola (40 g) seedlings according to the method described for soybean nodules. The partially purified enzyme was exchanged into buffer C, concentrated to approximately 1 mL, and stored at −20°C after the addition of 30% (v/v) glycerol.
Assay of Enzyme Activity
All enzyme assays were performed at 30°C. UDP-Glc DH activity was assayed radiochemically in reaction mixtures that contained, in a final volume of 10 μL, 0.75 μmol Tris-HCl, pH 8.4, 5 nmol UDP-[U-14C]Glc (6.8 nCi), and 10 nmol NAD+. The reaction was initiated by the addition of 4 μL of enzyme extract, and 3-μL aliquots were spotted onto Whatman no. 1 chromatography paper after 2, 10, and 20 min. Chromatograms were developed in 95% ethanol:0.1 m ammonium acetate and 2 mm EDTA, pH 7.0 (7:3), as described by Davies and Dickinson (1972a) and cut into lanes, the lanes were divided into 1-cm sections, and the radioactivity was counted by liquid scintillation spectrometry. Radioactive UDP-Glc and UDP-GlcUA were identified by comparison with unlabeled standards visualized under UV light.
UDP-Glc DH activity was measured spectrophotometrically in a continuous assay by monitoring the increase in A340 due to the reduction of NAD+. Reaction mixtures for the standard assay contained, in a final volume of 1 mL, 75 μmol Tris-HCl, pH 8.4, 3 μmol NAD+, and 1 μmol UDP-Glc. Reactions were initiated by the addition of enzyme, and under these conditions rates were linear for at least 10 min. Activity was calculated from linear initial rates on the basis that 2 mol of NADH were formed per mole of UDP-Glc oxidized. One unit of activity is defined as the amount of enzyme that catalyzed the formation of 1 μmol of UDP-GlcUA min−1. Kinetic parameters were determined by first establishing that double-reciprocal plots of activity versus substrate concentration were linear and then fitting the initial rate data to the Michaelis-Menten equation by nonlinear regression according to the method of Duggleby (1984). Activity of 3-hydroxybutyrate dehydrogenase was assayed as described by Wong and Evans (1971). Protein content was determined with Coomassie blue (Bio-Rad) or bichinchonic acid (Pierce) reagents according to the suppliers' instructions, using BSA as a standard. The leghemoglobin content of soybean nodules was determined by the hemochrome method as described by Appleby and Bergersen (1980).
RESULTS
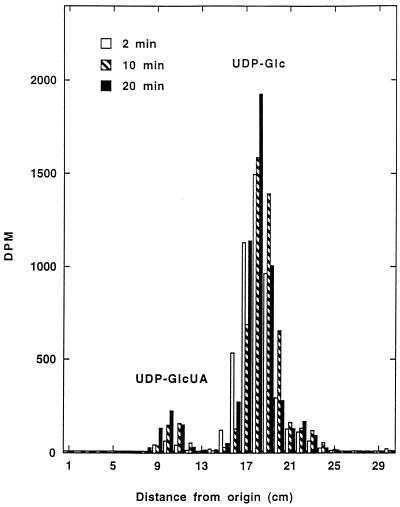
Before UDP-Glc DH activity was monitored routinely by spectrophotometric methods, the formation of UDP-GlcUA by a cytosolic extract from the host fraction of soybean nodules was demonstrated radiochemically. Increasing amounts of radioactivity corresponding to UDP-GlcUA were detected on paper chromatograms of samples taken at different intervals from a reaction mixture that contained UDP-[U-14C]Glc as the substrate (Fig. 1). UDP-Glc DH activity was measured spectrophotometrically in all subsequent experiments.
Figure 1.
Radiochemical assay of UDP-Glc DH from soybean nodules. Samples were taken at the times indicated from a reaction mixture that contained 5 nmol of UDP-[U14C]Glc (6.8 nCi), subjected to paper chromatography, and analyzed for radioactivity as described in Methods. The amounts of UDP-GlcUA that were formed after 2, 10, and 20 min were 0.06, 0.14, and 0.19 nmol, respectively. The data are from one of two duplicate experiments.
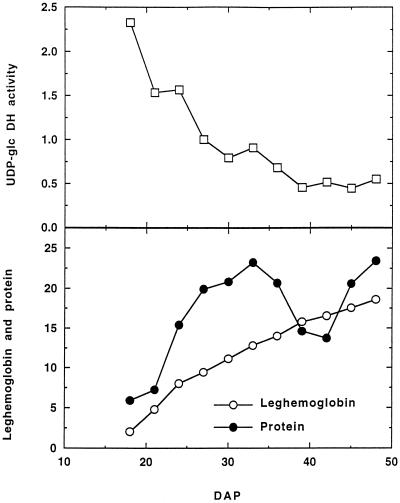
In a developmental study the specific activity of UDP-Glc DH was greatest in extracts of soybean nodules 18 DAP, when the nodules were first of sufficient size to harvest. Subsequently, activity declined gradually until 39 DAP and thereafter remained at a level that was approximately 20% of the initial value (Fig. 2). This pattern was also observed when UDP-Glc activity was expressed on a tissue fresh weight basis (results not shown). In comparison, the leghemoglobin content of soybean nodules increased from 18 DAP throughout nodule development, whereas the total soluble nodule protein content increased up to 32 DAP, declined until 42 DAP, and then increased again (Fig. 2). The decline in soluble protein content corresponded with the emergence of new nodules on secondary roots.
Figure 2.
Developmental changes in UDP-Glc DH activity, leghemoglobin, and soluble protein content of soybean nodules. UDP-Glc DH activity is in milliunits per milligram of protein; leghemoglobin is in micromoles per milligram of protein, and protein is in milligrams per gram fresh weight. At least seven plants were used to prepare the extract at each time. Data are from one of two duplicate experiments.
When soybean nodule extracts were fractionated by differential centrifugation, UDP-Glc DH activity was present only in the 100,000g supernatant. No activity was detected in the 1,000g, 10,000g, or 100,000g pellets, and none was solubilized when these pellets were treated with buffer containing 5% (v/v) Triton X-100 or Tween 20. The addition of this concentration of Triton X-100 and Tween 20 to the standard reaction mixture had no effect on UDP-Glc DH activity. The absence of 3-hydroxybutyrate dehydrogenase activity indicated that the 100,000g supernatant was not contaminated with the soluble contents of the bacteroids. Extracts prepared as described by Copeland et al. (1989) from sonicated bacteroids contained less than 5% of the total UDP-Glc DH activity in soybean nodules.
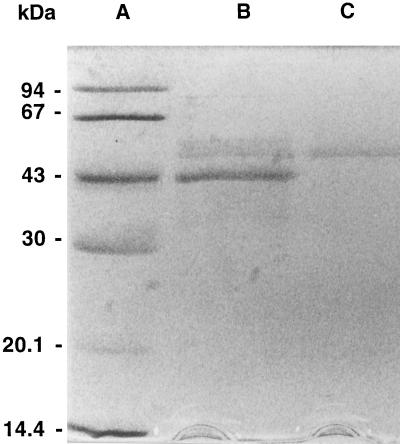
Soybean nodule UDP-Glc DH was purified approximately 60-fold from the host cytosolic fraction to a specific activity of 606 milliunits mg−1 protein, with an overall recovery of 4% (Table I). A single peak of activity was eluted in all of the chromatographic steps. UDP-Glc DH was eluted from the Mono-P column at pH 4.7, indicating that the pI was close to this value. The preparation was not homogenous after the Mono-P step, as shown by the presence of several polypeptide bands following SDS-PAGE (Fig. 3). The low abundance of UDP-Glc DH hampered further attempts to purify the enzyme chromatographically. Consequently, UDP-Glc DH obtained after the Mono-P step was electrophoresed in an agarose gel, and protein with the same mobility as the single band of UDP-Glc DH activity was recovered.
Table I.
Purification of UDP-Glc DH from the host cytosol of soybean nodules
| Purification Stage | Protein | Activity | Specific Activity | Recovery |
|---|---|---|---|---|
| mg | milliunits | milliunits mg−1 protein | % | |
| Crude extract | 108 | 1057 | 9.8 | 100 |
| (NH4)2SO4 fractionation | 62 | 841 | 14 | 80 |
| Fractogel | 17 | 453 | 27 | 43 |
| Econo-Pac Q | 5.1 | 203 | 40 | 19 |
| Mono-Q | 2.6 | 153 | 59 | 14 |
| Mono-P | 0.071 | 43 | 606 | 4 |
Nodules (20 g) were extracted as described in the text.
Figure 3.
Electrophoresis of soybean nodule UDP-Glc DH. Calibration proteins (A), the UDP-Glc DH preparation (4 μg of protein) obtained from the Mono-P step (B), and purified UDP Glc DH (approximately 0.3 μg of protein) recovered from a preparative agarose gel (C) were subjected to SDS-PAGE in a 12.6% gel that was then stained for protein with silver reagent as described in the text.
When this preparation was subjected to SDS-PAGE, a single polypeptide band with a molecular mass of approximately 50 kD was detected in gels stained with silver reagent (Fig. 3). A carbohydrate-positive band with the same mobility as the band of UDP-Glc DH activity was observed in nondenaturing gels that were stained with Schiff's reagent (not shown). The molecular mass of native UDP-Glc DH as determined by size-exclusion chromatography through a calibrated Superose 6 Prep Grade column (45 × 1 cm) was 272 ± 5 kD (mean ± se of two determinations).
After the Mono-P purification step, UDP-Glc DH was labile when stored in Tris-HCl, pH 7.5, at 4°C, with approximately 70% of activity being lost in 2 h. The addition of 0.5 mm NAD+ effectively stabilized the enzyme and resulted in essentially no activity being lost in 48 h at 4°C. UDP-Glc (0.5 mm) and Pi (50 mm) stabilized activity to a lesser extent, but Glc, Glc-1-P, and UDP (all at a concentration of 0.5 mm) and DTT (2.5 mm) were not effective. UDP-Glc DH was also very labile in crude extracts of soybean nodules that were prepared in the presence of 0.5 mm NAD+. At 4°C the losses of activity in 60 and 90 min were approximately 50 and 85%, respectively, but when these crude extracts were freed of low-molecular-mass solutes in an Econo-Pac10 DG desalting column equilibrated with buffer containing 0.5 mm NAD+, there was no loss of activity for at least 2 h. Further evidence indicating that crude nodule extracts contained a low-molecular-mass inhibitor of UDP-Glc DH was obtained by passing the crude extract through a Centricon 30 filter. Adding the filtrate back to the enzyme inhibited activity by approximately 25%, and a similar loss of activity occurred with filtrate that had been heated in a boiling water bath for 5 min.
Soybean nodule UDP-Glc DH had optimum activity at pH 8.4 and activities of 90% or greater of the maximum between pH 8.0 and 9.0. The enzyme had no detectable activity with NADP+. UDP-Glc was the most effective nucleotide-sugar substrate, although TDP-Glc and UDP-Gal (both 1 mm) were utilized at 7% of the rate with 1 mm UDP-Glc. Activity with TDP-Glc and UDP-Gal did not appear to be due to contamination by UDP-Glc, since no UDP-Glc was detected when these nucleotide sugars were analyzed by paper chromatography as described by Davies and Dickinson (1972a). However, because of the low activity, kinetic parameters were not determined for these substrates. UDP-Man (1 mm) gave 0.5% of the activity with UDP-Glc, but no activity (i.e. NAD+ reduction) was detected with the following (all at a concentration of 1 mm): GDP-Glc, CDP-Glc, ADP-Glc, Glc, Glc-1-P, and Glc-6-P. The addition to standard reaction mixtures of the following had no effect on UDP-Glc DH activity: K+ and Na+ (final concentration, 50 mm); NH4+, Mg2+, Mn2+, Ca2+, Pi, SO42−, and NO3− (all at 5 mm); and Na2EDTA (10 mm).
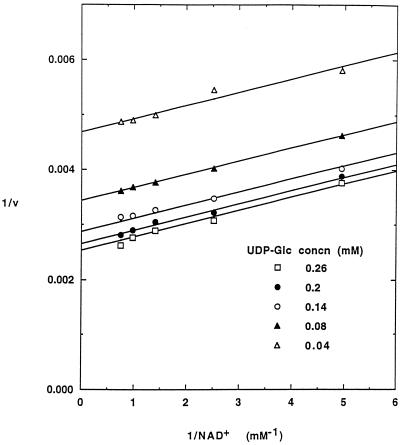
Soybean nodule UDP-Glc DH displayed typical hyperbolic kinetics with both UDP-Glc and NAD+ as the varied substrates. A parallel pattern of double-reciprocal plots was observed when the concentration of NAD+ was varied between 0.2 and 1.4 mm at different concentrations of UDP-Glc between 0.04 and 0.26 mm (Fig. 4). The data fit well to the initial rate equation for a substituted-enzyme mechanism, giving Km values of 0.051 ± 0.004 mm for UDP-Glc and 0.12 ± 0.01 mm for NAD+.
Figure 4.
The effect of NAD+ concentration on UDP-Glc DH activity. Reaction mixtures were of the composition described, except that the concentration of NAD+ was varied at the concentrations of UDP-Glc shown. Reaction rates (v) are in nanomoles of product per minute per milligram of protein, and the lines were drawn by fitting the data to the rate equation as described in Methods. Data are representative of three experiments.
Time-course studies with wheat and canola seedlings indicated that the specific activity of UDP-Glc DH in shoots was maximal after 4 to 5 d and then declined (results not shown). Using the procedure described for soybean nodules, we purified UDP-Glc DH between 4- and 10-fold, with approximately 20% recovery, from developing soybean seeds and wheat and canola shoots. The enzyme from all of these sources displayed typical hyperbolic kinetics with UDP-Glc and NAD+ as the varied substrates. The apparent Km values (mean ± se of duplicate experiments) for UDP-Glc and NAD+, respectively, were: developing soybean seeds, 0.10 ± 0.015 and 0.12 ± 0.03 mm; wheat shoots, 0.11 ± 0.01 and 0.20 ± 0.01 mm; and canola shoots, 0.08 ± 0.015 and 0.11 ± 0.02 mm. Activity of UDP-Glc DH was also detected in crude cytosolic extracts of 5-d-old barley and corn seedlings.
DISCUSSION
Developmental studies indicated that the activity of UDP-Glc DH (expressed on a per milligram of protein or per gram fresh weight basis) in soybean nodules and wheat and canola shoots was maximal in young, actively growing tissues. In other plant tissues, UDP-Glc DH has also been shown to be active when there is a demand for synthesis of structural polysaccharides (Rubery, 1972; Dalessandro and Northcote, 1977a, 1977b; Robertson et al., 1995; Tenhaken and Thulke, 1996). The soybean nodule enzyme was cytosolic, as it is in pea seedlings and animal tissues (Strominger and Mapson, 1957; Bdolah and Feingold, 1968a; Zalitis and Feingold, 1969).
Soybean nodule UDP-Glc DH had native and subunit molecular masses of 272 and 47 kD, respectively, indicating that the enzyme has a hexameric structure. In this regard, soybean nodule UDP-Glc DH is similar to the enzyme from bovine liver (Zalitis and Feingold, 1969) but differed from that of hen oviduct and microbial sources, which are suggested to be dimers (Bdolah and Feingold, 1968a, 1968b; Schiller et al., 1976). UDP-Glc DH from suspension-cultured Phaseolus vulgaris cells has a subunit molecular mass of 40 kD (Robertson et al., 1996), whereas the subunit size deduced from the cloned gene from soybean cell-suspension cultures is 50 to 52 kD (Tenhaken and Thulke, 1996). The detection with Schiff's reagent of a band in gels with the same mobility as UDP-Glc DH activity suggests that the soybean nodule enzyme may have had an associated polysaccharide group.
Kinetic studies were performed with soybean nodule UDP-Glc DH after confirming the reaction radiochemically. The enzyme was specific for NAD+ as the pyridine nucleotide and was most active with UDP-Glc as the sugar nucleotide substrate. There was a small amount of activity with TDP-Glc and UDP-Gal, as has been reported for the enzyme from other plant, animal, and microbial sources (Strominger and Mapson, 1957; Ankel et al., 1966; Bdolah and Feingold, 1968b; Zalitis and Feingold, 1968, 1969; Davies and Dickinson, 1972b; Schiller et al., 1976). The nonintersecting pattern of double-reciprocal plots observed when the concentration of NAD+ was varied at fixed concentrations of UDP-Glc is consistent with the reaction mechanism having an irreversible step between the binding of NAD+ and UDP-Glc. In this respect, UDP-Glc DH from soybean nodules was similar to the bovine liver enzyme (Nelsestuen and Kirkwood, 1971).
The Km values of soybean nodule UDP-Glc DH for UDP-Glc and NAD+ were 0.05 and 0.12 mm, respectively. Similar values were determined for UDP-Glc DH from developing soybean seeds and wheat and canola shoots. The sugar-nucleotide affinities of UDP-Glc DH from the various sources examined in this study were comparable to that reported for the enzyme from animals and microorganisms (Strominger et al., 1954; Bdolah and Feingold, 1968a, 1968b; Gainey and Phelps, 1972). There is only limited information, obtained from relatively crude preparations, on the kinetic constants for UDP-Glc DH from other plant sources. The Km values of the enzyme from pea seedlings (0.07 and 0.11 mm for UDP-Glc and NAD+, respectively; Strominger and Mapson, 1957) are similar to those obtained in the present study, whereas UDP-Glc DH from lily pollen had lower affinity for UDP-Glc and NAD+, as indicated by Km values of 0.3 and 0.4 mm, respectively (Davies and Dickinson, 1972b). An enzyme preparation from suspension-cultured cells of P. vulgaris was reported to have a Km for UDP-Glc of 5.5 mm (Robertson et al., 1996). However, this enzyme, which had a significantly different subunit size of 40 kD and was co-purified with alcohol dehydrogenase activity, has since been suggested on the basis of genetic evidence not to be a UDP-Glc DH (Tenhaken and Thulke, 1996).
Analyses of several plant tissues indicate that the UDP-Glc content may range between 10 and 100 nmol g−1 fresh weight (ap Rees et al., 1984; Morrell and ap Rees, 1986; Gerhardt et al., 1987; MacRae et al., 1992, Schlüpmann et al., 1994). If we assume that UDP-Glc is confined to the cytosol and that the cytosol accounts for approximately 10% of the cellular volume, the UDP-Glc concentration in situ may be estimated to range between 0.1 and 1 mm. On the basis of the Km values determined for the soybean, wheat, and canola enzymes, concentrations of UDP-Glc in this range would be adequate for UDP-Glc DH activity.
UDP-Glc formed when Suc is cleaved by Suc synthase may be metabolized further in several ways. In addition to serving as a glucosyl donor in glucosylation reactions, UDP-Glc may be oxidized by UDP-Glc DH to UDP-GlcUA, or it may be converted by UDP-Glc pyrophosphorylase to Glc-1-P and incorporated into the glycolytic or pentose phosphate pathways. Highly purified preparations of UDP-Glc pyrophosphorylase and UDP-Glc dehydrogenase from the host cytosol of soybean nodules have similar affinity for UDP-Glc, as indicated by Km values of 0.07 and 0.05 mm, respectively (Vella and Copeland, 1990; this study). Since both enzymes display typical hyperbolic substrate kinetics, the partitioning of UDP-Glc between these reactions is unlikely to be regulated by the concentration of UDP-Glc. Conversely, the temporal variation in UDP-Glc DH activity indicates that factors regulating the amount of UDP-Glc DH protein may be important in directing UDP-Glc toward UDP-GlcUA formation. Thus, in soybean nodules, the activity of UDP-Glc DH (per gram fresh weight or per milligram of soluble nodule protein) was greatest during the initial stages of nodule growth and development. Activity subsequently declined to approximately one-third of the maximal level when the demand for C metabolism to provide energy and reductant for N fixation is greatest.
The failure in earlier studies to detect UDP-Glc DH activity in some plant species, including barley, corn, and Brassica spp. (Roberts and Cetorelli, 1973), may be attributed to the low abundance of the enzyme and its lability in the absence of NAD+. The presence of an inhibitor, as was found in crude extracts of soybean nodules, may also make detection of the enzyme difficult. Although the nature of this inhibitor remains to be identified, it may be relevant to note that UDP-Xyl strongly inhibits UDP-Glc DH from soybean nodules (D.C. Stewart and L. Copeland, unpublished results) and other sources (Feingold and Avigad, 1980; Robertson et al., 1996). It has been suggested that the oxidation of myo-inositol is a major source of UDP-GlcUA in plants and that UDP-Glc oxidation plays only a minor role in the production of precursors for structural polysaccharides (Feingold and Avigad, 1980; Loewus and Loewus, 1980). Our results suggest that the capacity to produce UDP-GlcUA in the reaction catalyzed by UDP-Glc DH may occur widely in plants, especially in young and actively growing tissues. The biochemical properties of this enzyme and its role in the provision of precursors for the synthesis of structural polysaccharides need to be further evaluated.
Abbreviations:
- DAP
days after planting
- UDP-Glc DH
UDP-d-Glc dehydrogenase
LITERATURE CITED
- Ankel H, Ankel E, Feingold DW. Biosynthesis of uridine diphosphate d-xylose. III. Uridine diphosphate d-glucose dehydrogenase of Cryptococcus laurentii. Biochemistry. 1966;5:1864–1869. doi: 10.1021/bi00870a012. [DOI] [PubMed] [Google Scholar]
- Anthon GE, Emerich DW. Developmental regulation of enzymes of sucrose and hexose metabolism in effective and ineffective soybean nodules. Plant Physiol. 1990;92:346–351. doi: 10.1104/pp.92.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ap Rees T, Leja M, Macdonald FD, Green JH. Nucleotide sugars and starch synthesis in spadix of Arum maculatum and suspension cultures of Glycine max. Phytochemistry. 1984;23:2463–2468. [Google Scholar]
- Appleby CA, Bergersen FJ. Preparation and experimental use of leghemoglobin. In: Bergersen FJ, editor. Methods for Evaluating Biological Nitrogen Fixation. J. Chichester, UK: Wiley; 1980. pp. 315–335. [Google Scholar]
- Bdolah A, Feingold DS. Uridine diphosphate-d-glucose dehydrogenase of hen oviduct. Biochim Biophys Acta. 1968a;159:176–178. doi: 10.1016/0005-2744(68)90256-8. [DOI] [PubMed] [Google Scholar]
- Bdolah A, Feingold DS. Uridine diphosphate-d-glucose dehydrogenase of Aerobacter aerogenes. J Bacteriol. 1968b;96:1144–1149. doi: 10.1128/jb.96.4.1144-1149.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett C, Waldron K (1990) Physiology and biochemistry of plant cell walls. In M Black, J Chapman, eds, Topics in Plant Physiology, Vol 2. Unwin Hyman, London, pp 4–57
- Copeland L, Quinnell RG, Day DA. Malic enzyme in bacteroids from soybean nodules. J Gen Microbiol. 1989;135:2005–2011. [Google Scholar]
- Dalessandro G, Northcote DH. Possible control sites of polysaccharide synthesis during cell wall growth and wall expansion of pea seedlings (Pisum sativum L.) Planta. 1977a;134:39–44. doi: 10.1007/BF00390092. [DOI] [PubMed] [Google Scholar]
- Dalessandro G, Northcote DH. Changes in enzymic activities of nucleoside diphosphate sugar interconversions during differentiation of cambium to xylem in sycamore and poplar. Biochem J. 1977b;162:267–279. doi: 10.1042/bj1620267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MD, Dickinson DB. Radiochemical assay for UDP-Glc dehydrogenase. Anal Biochem. 1972a;47:209–217. doi: 10.1016/0003-2697(72)90294-1. [DOI] [PubMed] [Google Scholar]
- Davies MD, Dickinson DB. Properties of uridine diphosphate dehydrogenase from pollen of Lilium longiforum. Arch Biochem Biophys. 1972b;152:53–61. doi: 10.1016/0003-9861(72)90192-0. [DOI] [PubMed] [Google Scholar]
- Duggleby RG. Regression analysis of non-linear Arrhenius plots: an empirical model and a computer program. Comput Biol Med. 1984;14:447–455. doi: 10.1016/0010-4825(84)90045-3. [DOI] [PubMed] [Google Scholar]
- Ericson MC, Elbein AD (1980) Biosynthesis of cell wall polysaccharides and glycoproteins. In PK Stumpf, EE Conn, eds, The Biochemistry of Plants, Vol 3. Academic Press, New York, pp 589–616
- Evans HJ, Koch B, Klucas K. Preparation of nitrogenase from nodules and separation into components. Methods Enzymol. 1972;24:470–476. doi: 10.1016/0076-6879(72)24092-7. [DOI] [PubMed] [Google Scholar]
- Feingold DS, Avigad G (1980) Sugar nucleotide transformation in plants. In PK Stumpf, EE Conn, eds, The Biochemistry of Plants, Vol 3. Academic Press, New York, pp 102–170
- Gabriel O. Analytical disc gel electrophoresis. Methods Enzymol. 1971;22:565–578. [Google Scholar]
- Gabriel O, Gersten DM. Staining for enzymic activity after gel electrophoresis. Anal Biochem. 1992;203:1–21. doi: 10.1016/0003-2697(92)90036-7. [DOI] [PubMed] [Google Scholar]
- Gainey PA, Phelps CF. Uridine diphosphate glucuronic acid production and utilization in various tissues actively synthesizing glycosaminoglycans. Biochem J. 1972;128:215–227. doi: 10.1042/bj1280215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt R, Stitt M, Heldt HW. Subcellular metabolite levels in spinach leaves. Regulation of sucrose synthesis during alterations in photosynthetic partitioning. Plant Physiol. 1987;83:398–407. doi: 10.1104/pp.83.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knee M. Metabolism of polymethylgalacturonate in apple fruit cortical tissue during ripening. Phytochemistry. 1978;17:1261–1264. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loewus F, Chen MS, Loewus ML (1973) The myo-inositol oxidation pathway to cell wall polysaccharides. In F Loewus, ed, Biogenesis of Plant Cell Wall Polysaccharides. Academic Press, New York, pp 1–28
- Loewus FA, Loewus MW (1980) myo-Inositol: biosynthesis and metabolism. In PK Stumpf, EE Conn, eds, The Biochemistry of Plants, Vol 3. Academic Press, New York, pp 43–76
- MacRae E, Quick WP, Benker C, Stitt M. Carbohydrate metabolism during postharvest ripening of kiwifruit. Planta. 1992;188:314–323. doi: 10.1007/BF00192797. [DOI] [PubMed] [Google Scholar]
- Morell MK, Copeland L. Enzymes of sucrose breakdown in soybean nodules. Alkaline invertase. Plant Physiol. 1984;74:1030–1034. doi: 10.1104/pp.74.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell S, ap Rees T. Sugar metabolism in developing tubers of Solanum tuberosum. Phytochemistry. 1986;25:1579–1585. [Google Scholar]
- Nelsestuen GL, Kirkwood S. The mechanism of action of uridine diphosphoglucose dehydrogenase. J Biol Chem. 1971;246:3828–3834. [PubMed] [Google Scholar]
- Roberts RM, Cetorelli JJ (1973) UDP-d-glucuronic acid pyrophosphorylase and the formation of UDP-d-glucuronic acid in plants. In F Loewus, ed, Biogenesis of Plant Cell Wall Polysaccharides. Academic Press, New York, pp 49–68
- Robertson D, McCormack BA, Bolwell GP. Cell wall polysaccharide biosynthesis and related metabolism in elicitor-stressed cells of French bean (Phaseolus vulgaris L.) Biochem J. 1995;306:745–750. doi: 10.1042/bj3060745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Smith C, Bolwell GP. Inducible UDP-glucose dehydrogenase of French bean (Phaseolus vulgaris L.) locates to vascular tissue and has alcohol dehydrogenase activity. Biochem J. 1996;313:311–317. doi: 10.1042/bj3130311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubery PH. The activity of uridine diphosphate-d-glucose: nicotinamide-adenine dinucleotide oxidoreductase in cambial tissue and differentiating xylem isolated from sycamore trees. Planta. 1972;103:188–192. doi: 10.1007/BF00387370. [DOI] [PubMed] [Google Scholar]
- Schiller JG, Lamy F, Frazier R, Feingold DS. UDP-glucose dehydrogenase from E. coli. Purification and subunit structure. Biochim Biophys Acta. 1976;453:418–425. doi: 10.1016/0005-2795(76)90137-9. [DOI] [PubMed] [Google Scholar]
- Schlüpmann H, Bacic A, Read SM. Uridine diphosphate glucose metabolism and callose synthesis in cultured pollen tubes of Nicotianaalata Link et Otto. Plant Physiol. 1994;105:659–670. doi: 10.1104/pp.105.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger JL, Kalckar HM, Axelrod J, Maxwell ES. Enzymic oxidation of uridine diphosphate glucose to uridine diphosphate glucuronic acid. J Am Chem Soc. 1954;76:6411–6412. [Google Scholar]
- Strominger JL, Mapson LW. Uridine diphosphoglucose dehydrogenase of pea seedlings. Biochem J. 1957;66:567–572. doi: 10.1042/bj0660567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhaken R, Thulke O. Cloning of an enzyme that synthesizes a key nucleotide-sugar precursor of hemicellulose biosynthesis from soybean: UDP-glucose dehydrogenase. Plant Physiol. 1996;112:1127. doi: 10.1104/pp.112.3.1127. 1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummler F, Verma DPS. Nodulin-100 of soybean is the subunit of sucrose synthase regulated by the availability of free heme in nodules. J Biol Chem. 1987;262:14730–14736. [PubMed] [Google Scholar]
- Vella J, Copeland L. UDP-glucose pyrophosphorylase from the plant fraction of nitrogen-fixing soybean nodules. Physiol Plant. 1990;78:140–146. [Google Scholar]
- Wakabayashi K, Sakurai N, Kuraishi S. Effects of ABA on hypocotyl. I. Changes in incorporation of glucose and myo-inositol into cell-wall components. Plant Cell Physiol. 1989;30:99–105. [Google Scholar]
- Wakabayashi K, Sakurai N, Kuraishi S. Effects of abscisic acid on the synthesis of cell wall polysaccharides in segments of etiolated squash hypocotyl. II. Levels of UDP-neutral sugars. Plant Cell Physiol. 1991;32:427–432. [Google Scholar]
- Wong PP, Evans HJ. Poly-β-hydroxybutyrate utilization by soybean (Glycine max Merr.) nodules and assessment of its role in maintenance of nitrogenase activity. Plant Physiol. 1971;47:750–755. doi: 10.1104/pp.47.6.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalitis J, Feingold DS. The mechanism of action of UDPG dehydrogenase. Biochem Biophys Res Commun. 1968;31:693–698. doi: 10.1016/0006-291x(68)90617-7. [DOI] [PubMed] [Google Scholar]
- Zalitis J, Feingold DS. Purification and properties of UDPG dehydrogenase from beef liver. Arch Biochem Biophys. 1969;132:457–465. doi: 10.1016/0003-9861(69)90389-0. [DOI] [PubMed] [Google Scholar]