Abstract
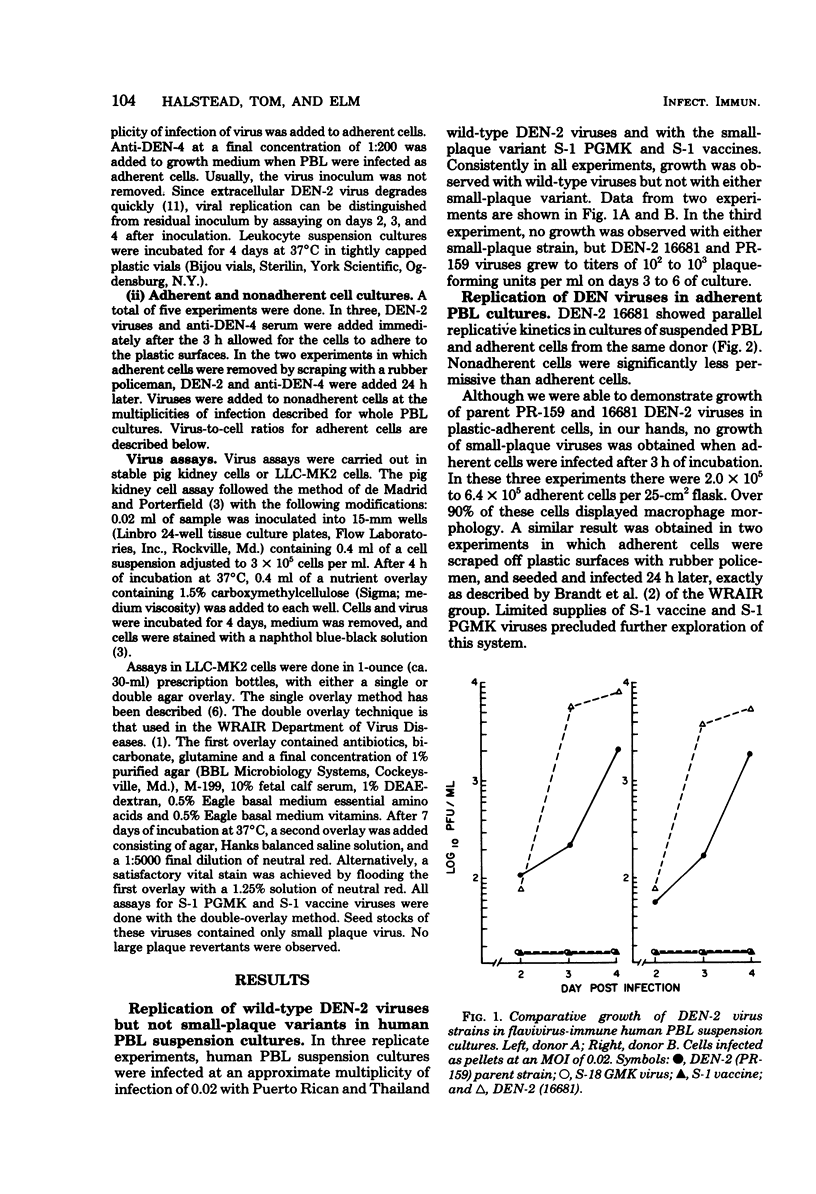
We wished to find a simple, biologically relevant method to evaluate the virulence of dengue viruses for human beings. Since cells of mononuclear phagocyte lineage may be important sites of dengue infection in primates, we evaluated the permissiveness of these cells to dengue virus as a correlate of virus virulence. Two wild-type, large-plaque, monkey-virulent dengue-2 virus strains and two small-plaque, monkey-avirulent dengue-2 virus strains were evaluated for their ability to replicate in human peripheral blood leukocyte cultures supplemented with enhancing antibody. One of the small-plaque strains was demonstrated to have reduced virulence for man. Wild-type dengue-2 viruses replicated readily in peripheral blood leukocyte suspension cultures, whereas small-plaque dengue-2 strains did not. Differences between our data and results obtained by other workers employing adherent peripheral blood leukocytes are discussed. Antibody-enhanced growth of dengue virus in suspension cultures of human peripheral blood leukocytes gives promise of being a simple in vitro system for characterizing dengue virus virulence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt W. E., McCown J. M., Top F. H., Jr, Bancroft W. H., Russell P. K. Effect of passage history on dengue-2 virus replication in subpopulations of human leukocytes. Infect Immun. 1979 Nov;26(2):534–541. doi: 10.1128/iai.26.2.534-541.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Madrid A. T., Porterfield J. S. The flaviviruses (group B arboviruses): a cross-neutralization study. J Gen Virol. 1974 Apr;23(1):91–96. doi: 10.1099/0022-1317-23-1-91. [DOI] [PubMed] [Google Scholar]
- Eckels K. H., Brandt W. E., Harrison V. R., McCown J. M., Russell P. K. Isolation of a temperature-sensitive dengue-2 virus under conditions suitable for vaccine development. Infect Immun. 1976 Nov;14(5):1221–1227. doi: 10.1128/iai.14.5.1221-1227.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckels K. H., Harrison V. R., Summers P. L., Russell P. K. Dengue-2 vaccine: preparation from a small-plaque virus clone. Infect Immun. 1980 Jan;27(1):175–180. doi: 10.1128/iai.27.1.175-180.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J., Allison A. C. Dengue viruses and mononuclear phagocytes. II. Identity of blood and tissue leukocytes supporting in vitro infection. J Exp Med. 1977 Jul 1;146(1):218–229. doi: 10.1084/jem.146.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977 Jul 1;146(1):201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., Shotwell H., Casals J. Studies on the pathogenesis of dengue infection in monkeys. I. Clinical laboratory responses to primary infection. J Infect Dis. 1973 Jul;128(1):7–14. doi: 10.1093/infdis/128.1.7. [DOI] [PubMed] [Google Scholar]
- Halstead S. B., Simasthien P. Observations related to the pathogenesis of dengue hemorrhagic fever. II. Antigenic and biologic properties of dengue viruses and their association with disease response in the host. Yale J Biol Med. 1970 Apr;42(5):276–292. [PMC free article] [PubMed] [Google Scholar]
- Harrison V. R., Eckels K. H., Sagartz J. W., Russell P. K. Virulence and immunogenicity of a temperature-sensitive dengue-2 virus in lower primates. Infect Immun. 1977 Oct;18(1):151–156. doi: 10.1128/iai.18.1.151-156.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchette N. J., Halstead S. B., Chow J. S. Replication of dengue viruses in cultures of peripheral blood leukocytes from dengue-immune rhesus monkeys. J Infect Dis. 1976 Mar;133(3):274–282. doi: 10.1093/infdis/133.3.274. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M., DeStefano M. J. Macrophage spreading in vitro. I. Inducers of spreading. Exp Cell Res. 1973 Mar 15;77(1):323–334. doi: 10.1016/0014-4827(73)90584-3. [DOI] [PubMed] [Google Scholar]
- Scott R. M., Nisalak A., Cheamudon U., Seridhoranakul S., Nimmannitya S. Isolation of dengue viruses from peripheral blood leukocytes of patients with hemorrhagic fever. J Infect Dis. 1980 Jan;141(1):1–6. doi: 10.1093/infdis/141.1.1. [DOI] [PubMed] [Google Scholar]
- Scott R. M., Nisalak A., Eckels K. H., Tingpalapong M., Harrison V. R., Gould D. J., Chapple F. E., Russell P. K. Dengue-2 vaccine: viremia and immune responses in rhesus monkeys. Infect Immun. 1980 Jan;27(1):181–186. doi: 10.1128/iai.27.1.181-186.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector W. G., Lykke A. W. The cellular evolution of inflammatory granulomata. J Pathol Bacteriol. 1966 Jul;92(1):163–167. doi: 10.1002/path.1700920117. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Brandt W. E., Russell P. K., Dixon F. T. Replication of dengue-2 virus in cultured human lymphoblastoid cells and subpopulations of human peripheral leukocytes. J Immunol. 1976 Sep;117(3):953–961. [PubMed] [Google Scholar]


