Abstract
Background
Cirrhotic patients with acute kidney injury (AKI) admitted to intensive care units (ICUs) show extremely high mortality rates. We have proposed the MBRS scoring system, which can be used for assessing patients on the day of admission to the ICU; this new system involves determination of mean arterial pressure (MAP) and bilirubin level and assessment of respiratory failure and sepsis. We had used this scoring system to analyze the prognosis of ICU cirrhotic patients with AKI in 2008, and the current study was an external validation of this scoring system.
Methods
A total of 190 cirrhotic patients with AKI were admitted to the ICU between March 2008 and February 2011. We prospectively analyzed and recorded the data for 31 demographic parameters and some clinical characteristic variables on day 1 of admission to the ICU; these variables were considered as predictors of mortality.
Results
The overall in-hospital mortality rate was 73.2% (139/190), and the 6-month mortality rate was 83.2% (158/190). Hepatitis B viral infection (43%) was observed to be the cause of liver disease in most of the patients. Multiple logistic regression analysis indicated that the MBRS and Acute Physiology and Chronic Health Evaluation III (ACPACHE III) scores determined on the first day of admission to the ICU were independent predictors of in-hospital mortality in patients. In the analysis of the area under the receiver operating characteristic (AUROC) curves, the MBRS scores showed good discrimination (AUROC: 0.863±0.032, p<0.001) in predicting in-hospital mortality.
Conclusion
On the basis of the results of this external validation, we conclude that the MBRS scoring system is a reproducible, simple, easy-to-apply evaluation tool that can increase the prediction accuracy of short-term prognosis in critically ill cirrhotic patients with AKI.
Introduction
Liver cirrhosis is characterized by disturbances in the systemic circulation, including marked arterial vasodilation that occurs principally in the splanchnic circulation, reduces the total peripheral vascular resistance and arterial pressure, and causes a secondary increase in the cardiac output. These abnormalities are central to the development of several major complications in patients with cirrhosis, such as the hepatorenal syndrome, ascites, spontaneous bacterial peritonitis, dilutional hyponatremia, and hepatopulmonary syndrome. Renal failure is the most clinically relevant condition among these conditions because its appearance generally indicates a very poor prognosis [1]–[10].
We developed the MBRS scoring system, a simple prognostic model that includes determination of mean arterial pressure (MAP) and serum bilirubin level and assessment of acute respiratory failure and sepsis. These 4 variables are to be analyzed on day 1 of admission to the intensive care unit (ICU). We used this model to analyze and predict the in-hospital mortality in 111 critically ill cirrhotic patients with acute kidney injury (AKI) [11]. The MBRS score [calculated using the following predictors: MAP, <80 mmHg; serum bilirubin level, >80 µmol/L (4.7 mg/dl); acute respiratory failure, and sepsis] was defined as the sum of the values of the individual predictors, each value ranging from 0 to 4. This score has better discriminatory power than the other evaluation systems such as the Child-Pugh [12], model for end-stage liver disease (MELD) [13], Acute Physiology and Chronic Health Evaluation II and III (APACHE II & III) [14], [15], and sequential organ failure assessment (SOFA) system [16]. The area under the receiver operating characteristic curve (AUROC) values for the MBRS scores were significantly more than the AUROC values plotted for the Child-Pugh and APACHE II scores [11].
The prognostic value of MBRS scores for cirrhotic patients with AKI admitted to ICUs needs to be validated further through studies on different cohorts. Further confirmation is particularly important because we observed that, over time, the mortality rates of patients who showed the same characteristics at admission typically decreased. Possible causes that may not have affected the scoring variables, including improvements in therapies and management of bleeding, renal failure, respiratory failure, and sepsis, require additional testing in new study cohorts [2], [17]. To the best of our knowledge, no prospective clinical study has validated predictive power of MBRS scores on critically ill cirrhotics with AKI. We aimed to evaluate the reproducibility of the MBRS scoring system in predicting the in-hospital mortality rate by performing an external validation.
Materials and Methods
Ethics statement
This clinical study was conducted in full compliance with the ethical principles of the Declaration of Helsinki and was consistent with Good Clinical Practice guidelines and applicable local regulatory requirements. The local institutional review board of Chang Gung Memorial Hospital approved our study protocol. Patients meeting the inclusion criteria were invited to participate in this study on their first day of ICU admission. Trained physicians evaluated their mental status during the screening and informed consent procedure. Written informed consent was obtained from all mentally competent patients or next-of-kin of compromised ones prior to their participation.
Patient information and data collection
This study was performed between March 2008 and February 2011 in a 10-bed specialized ICU (hepatogastroenterology ICU) at a 2000-bed tertiary care referral hospital in Taiwan. In this study, we included 190 consecutive patients with hepatic cirrhosis and AKI requiring intensive monitoring and/or treatment that cannot be provided outside the ICU. We excluded patients who did not match the criteria of AKI (127 patients), patients who had previous end-stage renal disease patients undergoing regular renal replacement therapy (38 patients); patients whose hospital stay length <24 h (30 patients), patients who had received liver transplantation (16 patients), and patient who were readmitted (21 patients).
The following data were collected prospectively: demographic data; reason for admission to the ICU; immediate diagnosis; severity of the illness; MELD, SOFA, APACHE II, and APACHE III scores determined on the first day of ICU admission; the duration of hospitalization; and the treatment outcome. The primary study outcome was the in-hospital mortality rate. Follow-up examinations were performed 6 months after discharge of the patients from the hospital by analyzing the chart records.
Definitions
Cirrhosis was diagnosed on the basis of the results of liver histology or a combination of physical signs and symptoms and findings from biochemical analysis and ultrasonography. Acute kidney injury was defined as a 50% increase in serum creatinine (SCr) level or an immediate requirement for renal replacement therapy. The measurement of SCr levels was repeated following the withdrawal of diuretics in the patients. A study stated that a 50% increase in SCr levels indicates acute renal dysfunction as per the RIFLE (risk of renal failure, injury to the kidney, failure of kidney function, loss of kidney function, and end-stage renal failure) classification system. In that study, the patient had RIFLE-R stage disease since the patient's SCr level had increased by a factor of 1.5 or more from the baseline [18]. Baseline SCr was the first value measured during hospitalization. The modification of diet in renal disease (MDRD) formula was used to estimate the baseline SCr levels in 15 patients because these patients had been admitted directly to the ICU and their previous SCr levels were unknown [18]. Respiratory failure was defined as a respiratory rate of ≤5/min or of ≥50/min, and/or requirement of mechanical ventilation for ≥3 days, and/or fraction of inspired oxygen (FiO2) of >0.4, and/or a positive end-expiratory pressure of >5 cm H2O [19]–[21]. Sepsis was defined as systemic inflammatory response syndrome (SIRS) plus suspected or proven infection. According to the guidelines of the American College of Chest Physician/Society of Critical Care Medicine (ACCP/SCCM) Consensus Conference, SIRS was defined as patients with more than one of the following clinical findings: body temperature, >38°C or <36°C; heart rate, >90 beats per minute; hyperventilation evidenced by a respiratory rate of >20 cycles per minute or a Paco2 of <32 mm Hg; and a white blood cell count of >12,000 cells per µL or <4,000 cells per µL [22].
The severity of the liver disease on admission to the ICU was determined by using the Child–Pugh and MELD scoring systems. Severity of the illness can also be assessed by using the SOFA, APACHE II, and APACHE III scoring systems. The MBRS score was based on 4 independent prognostic predictors: lower threshold of MAP, i.e., 80 mmHg (1 point); upper threshold cut-off of serum bilirubin, i.e., 80 µmol/L or 4.7 mg/dl (1 point); acute respiratory failure (1 point); and sepsis (1 point). Assessment of these predictors was performed on the day 1 of admission to the ICU [11]. The worst physiological and biochemical values determined on the first day of ICU admission were recorded. Clinical management of these patients was done by the method described elsewhere [11].
Clinical management
All patients received careful history taking, physical examination and a number of laboratory measurements. Potential nephrotoxins were discontinued. Renal ultrasound was arranged to exclude postrenal azotemia on the first day of ICU admission.
Patients who had a clear history of septic or hypovolemic shock, or a recent history of nephrotoxins exposure with high UNa (>40 mEq/L), high FENa (2%), and urine osmolality under 350 mOsm/kg were treated as intrinsic azotemia as further described. Patients with upper gastrointestinal bleeding from esophageal varices were initially treated with emergency sclerotherapy and administration of vasopressors. Patients with peptic ulcer, either with active bleeding, visible vessels or visible clots, were treated with sclerosing agents, followed by proton pump inhibitors. All patients received intravenous fluid depending on their fluid volume and electrolyte status. The decision to transfuse packed red blood cells (PRBC) was made according to the criteria of the attending physician or whenever a patient's hemoglobin level dropped below 8 g/dL [23]. Patients with bacterial infections on admission and patients who developed bacterial infections during hospitalization were treated with appropriate empiric antibiotic therapy according to culture results and the results of appropriate diagnostic methods. When acute renal failure was severe or progressive and measures to improve renal function had been unsuccessful, renal replacement therapy was performed [4].
In all other patients, diuretics, lactulose, and vasodilators were not given. Volume expansion therapy such as intravenous albumin (1 g/Kg QD or BID, up to a maximum of 100 g) and/or artificial plasma expanders were administrated to correct volume depletion and to keep central venous pressure over 10 cmH2O every 12 hrs for 2 days. Daily measurements of urine output and serum creatinine began on day 1 of ICU admission and continued for at least 2 days. Patients with volume-responsive serum creatinine improvement was treated as prerenal azotemia and kept receiving volume supply [4].
Patients without volume-responsive acute kidney injury who had no shock, recent nephrotoxin exposure nor evidence of parenchymal kidney disease history (by urinalysis and image) were treated as hepatorenal syndrome with terlipressin (0.5–2 mg iv every 4–6 hrs) plus albumin for at least 3 days. Others were treated as intrinsic azotemia as described above [4].
Statistical analysis
Descriptive statistics were expressed as mean and standard deviation values unless otherwise stated. In the primary analysis, we compared the number of hospital survivors with the number of nonsurvivors. Normal distribution of all the variables was analyzed using the Kolmogorov–Smirnov test. Student's t-test was used to compare the mean values of continuous variables and normally distributed data; in the case of the other data, the Mann–Whitney U test was used. Categorical data were analyzed using the χ2 test. The chi-square test for trends were used to assess categorical data associated with MBRS scores. Correlation of paired-group variables were assessed using linear regression and Pearson analysis. We assessed the risk factors for in-hospital mortality by using univariate analysis, and the variables that were found to be statistically significant (p<0.05) in the univariate analysis were included in the multivariate analysis. A multiple logistic regression model and forward elimination of data were used to analyze these variables.
Calibration was assessed using the Hosmer–Lemeshow goodness-of-fit test to compare the number of observed deaths with the number of predicted deaths in the risk groups for the entire range of death probabilities. Discrimination was calculated using the AUROC values. The AUROC values were compared using a nonparametric approach. The AUROC analysis was also utilized to calculate the cut-off values, sensitivity, specificity, and overall correctness. Finally, cut-off points were calculated by calculating the best Youden index (sensitivity+specificity−1). Cumulative survival curves as a function of time were plotted using the Kaplan–Meier approach and were compared using the log rank test. All the statistical tests were 2-tailed. A p value of <0.05 was considered statistically significant. The data were analyzed using the Statistical Analysis for Social Sciences software, version 12.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Results
Subject characteristics
A total of 190 cirrhotic patients with AKI treated at the specialized hepatogastroenterology ICU were enrolled in the study between March 2008 and February 2011. The overall in-hospital mortality rate for the entire group was 73.2% (139/190), and the 6-month mortality rate was 83.2% (158/190). The demographic data and clinical characteristics of both the survivors and the nonsurvivors are listed in table 1. The median age of the patients was 58 years; 141 patients were men (74%), and 49 were women (26%). The median duration of stay in the ICU was 9 days. The causes of cirrhosis, the reasons for admission to the ICU, and presumptive etiologies of AKI are listed in table 2. Hepatitis B viral infection was observed to the cause of liver diseases in most of the patients. The most frequent reason for admission to the ICU was upper gastrointestinal bleeding. Patients who developed AKI tended to have a history of infection.
Table 1. Patients' demographic data and clinical characteristics.
| All patients (n = 190) | Survivors (n = 51) | Non-survivors (n = 139) | p-value | |
| Age (years) | 58±1 | 58±2 | 59±1 | NS (0.738) |
| Gender (M/F) | 141/49 | 41/10 | 100/39 | NS (0.238) |
| Length of ICU stay (days) | 9±9 | 6±4 | 10±11 | <0.001 |
| Length of hospital stay (days) | 25±25 | 32±33 | 23±21 | 0.067 |
| Serum Creatinine, ICU first day (mg/dL) | 3.2±2.4 | 2.5±2.2 | 3.6±2.4 | 0.005 |
| MAP, ICU admission (mmHg) | 73±18 | 86±16 | 69±17 | <0.001 |
| Glasgow coma scale, ICU admission | 9±5 | 10±5 | 9±5 | NS (0.104) |
| Leukocytes, ICU first day (g/dL) | 12.8±8.0 | 10.0±5.8 | 13.9±8.5 | 0.001 |
| Haemoglobin, ICU first day (g/dL) | 9.2±2.2 | 9.0±1.9 | 9.2±2.4 | NS (0.480) |
| Albumin, ICU first day (g/dL) | 2.5±0.5 | 2.6±0.6 | 2.4±0.5 | NS (0.130) |
| Sodium, ICU first day (mmol/L) | 135±17 | 138±9 | 134±19 | NS (0.151) |
| Bilirubin, ICU first day (umol/L) [median] | 11.2 [5.4] | 4.6 [3.1] | 13.6 [8.7] | <0.001 |
| Prothrombin time INR, ICU first day [median] | 2.8 [2.3] | 1.9 [1.6] | 3.1 [2.3] | 0.002 |
| AST, ICU first day (units/L) [median] | 530 [94] | 133 [67] | 678 [100] | 0.008 |
| ALT, ICU first day (units/L) [median] | 182 [45] | 56 [32] | 228 [53] | 0.002 |
| Platelets, ICU first day (×109/L) [median] | 95 [73] | 91 [73] | 97 [69] | NS (0.645) |
| DM (Yes/No) | 52/138 | 14/37 | 38/101 | NS (0.988) |
| Previous ascites (Yes/No) | 94/96 | 20/31 | 74/65 | NS (0.087) |
| Previous SBP (Yes/No) | 39/150 | 8/43 | 31/107 | NS (0.307) |
| Previous hepatic encephalopathy (Yes/No) | 116/74 | 29/22 | 87/52 | NS (0.473) |
| Previous EV bleeding (Yes/No) | 86/104 | 23/28 | 63/76 | NS (0.978) |
| Previous peptic ulcer bleeding (Yes/No) | 59/131 | 17/34 | 42/97 | NS (0.681) |
| Previous hepatoma (Yes/No) | 59/131 | 10/41 | 49/90 | 0.039 |
| Previous renal failure (Yes/No) | 57/133 | 17/34 | 40/99 | NS (0.544) |
| Respiratory failure, ICU first day (Yes/No) | 37/153 | 4/47 | 33/106 | 0.014 |
| Sepsis, ICU admission (\Yes/No) | 71/119 | 11/40 | 60/79 | 0.006 |
| Child-Pugh points (mean± SD) | 11.8±2.1 | 11.0±2.4 | 12.0±2.0 | 0.036 |
| MELD score (mean ± SD) | 33.2±1.1 | 24.7±8.8 | 35.8±11.3 | <0.001 |
| APACHE II (mean ± SD) | 25.5±0.77 | 20.9±6.9 | 26.9±8.5 | 0.001 |
| APACHE III (mean ± SD) | 106.0±3.19 | 77.9±29.1 | 114.7±32.6 | <0.001 |
| SOFA (mean ± SD) | 11.6±0.3 | 8.06±2.8 | 12.9±3.7 | <0.001 |
Abbreviation: M, male; F, female; ICU, intensive care unit; MAP, mean arterial pressure; INR, international normalized ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase; DM, diabetes mellitus; SBP, spontaneous bacterial peritonitis; EV, esophageal varices; SD, standard derivation; NS, not significant; MELD, model for end-stage liver disease; APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment.
Table 2. Causes of cirrhosis, reasons for ICU admission and presumptive causes of AKI.
| All patients (%) | Survivors (%) | Non-survivors (%) | p | |
| Causes of cirrhosis | ||||
| Alcoholic | 33 (17) | 15 (29) | 18 (13) | 0.005 |
| Hepatitis B | 60 (32) | 6 (12) | 54 (39) | <0.001 |
| Hepatitis C | 39 (20) | 11 (22) | 28 (20) | NS (0.716) |
| Alcoholic+Hepatitis B | 14 (7) | 8 (16) | 6 (4) | 0.006 |
| Alcoholic+Hepatitis C | 3 (2) | 1 (2) | 2 (1) | NS (0.771) |
| Hepatitis B+Hepatitis C | 5 (3) | 1 (2) | 4 (3) | NS (0.755) |
| Alcoholic+Hepatitis B+Hepatitis C | 1 (1) | 0 (0) | 1 (1) | NS (1.000) |
| Other causesa | 35 (17) | 9 (18) | 26 (19) | NS (0.868) |
| Primary ICU admission | ||||
| Severe UGI bleeding | 46 (24) | 18 (35) | 28 (20) | NS (0.031) |
| Severe sepsis | 34 (18) | 5 (10) | 29 (21) | NS (0.078) |
| Hepatic encephalopathy | 25 (13) | 11 (22) | 14 (10) | 0.038 |
| Respiratory failure | 10 (5) | 3 (6) | 7 (5) | NS (0.817) |
| AKI require renal replacement | 11 (6) | 2 (4) | 9 (6) | NS (0.504) |
| Othersb | 64 (35) | 12 (24) | 52 (37) | NS (0.073) |
| Presumptive etiology of AKI | ||||
| Pre-renal failure | 31 (16) | 13 (25) | 18 (13) | 0.038 |
| Infection-induced AKI | 51 (27) | 5 (10) | 46 (33) | 0.001 |
| Parenchymal renal diseases | 11 (6) | 5 (10) | 6 (4) | NS (0.151) |
| Acute tubular necrosis | 17 (9) | 3 (6) | 14 (10) | NS (0.370) |
| Nephrotoxic acute renal failure | 9 (5) | 6 (12) | 3 (2) | 0.006 |
| HRS type I/type II/total | 10/17/27 (14) | 1/2/3 (6) | 9/15/24 (17) | 0.046 |
| Othersc | 44 (23) | 16 (31) | 28 (20) | NS (0.104) |
Abbreviation: UGI, upper gastrointestinal; AKI, acute kidney injury; NS, not significant; ICU, intensive care unit; HRS, hepatorenal syndrome.
Primary biliary cirrhosis, autoimmune hepatitis, and other unknown causes.
Pancreatitis, hepatoma rupture, unknown cause, or multifactor related.
Mixed type, unknown cause, or multifactor related.
Risk factors for in-hospital mortality
The univariate analysis showed that 12 (Table 3) of the 31 variables (Table 1) were good prognostic indicators. On performing multivariate analysis, we identified that the MBRS and APACHE III scores determined on admission to the ICU have independent prognostic significance for assessing in-hospital mortality (Table 3). Regression coefficients of these variables were used to calculate the odds of death in each patient as follows:
Table 3. Variables showing prognostic significance.
| Parameter | Beta coefficient | Standard error | Odds ratios (95% CI) | p-value |
| Univariate logistic regression | ||||
| Length of ICU stay | 0.086 | 0.033 | 1.090(1.022–1.164) | 0.009 |
| Length of hospital stay | −0.013 | 0.006 | 0.987(0.975–0.999) | 0.038 |
| Serum Creatinine, ICU first day | 0.258 | 0.095 | 1.295(1.074–1.561) | 0.007 |
| MAP, ICU admission | −0.060 | 0.015 | 0.942(0.915–0.969) | <0.001 |
| Leukocytes, ICU first day | <0.001 | <0.001 | 1.000(1.000–1.000) | 0.004 |
| Bilirubin, ICU first day | 0.123 | 0.032 | 1.131(1.063–1.204) | <0.001 |
| Prothrombin time INR, ICU first day | 0.555 | 0.235 | 1.742(1.099–2.762) | 0.018 |
| AST, ICU first day | 0.002 | 0.001 | 1.002(1.000–1.003) | 0.053 |
| ALT, ICU first day | 0.005 | 0.002 | 1.005(1.000–1.010) | 0.041 |
| Previous hepatoma | 0.803 | 0.395 | 2.232(1.030–4.840) | 0.042 |
| Respiratory failure, ICU first day | 1.297 | 0.558 | 3.658(1.226–10.913) | 0.020 |
| Sepsis, ICU admission | 1.016 | 0.381 | 2.762(1.309–5.829) | 0.008 |
| Child-Pugh points | 0.203 | 0.099 | 1.225(1.009–1.486) | 0.040 |
| MELD | 0.119 | 0.029 | 1.127(1.065–1.192) | <0.001 |
| APACHE II | 0.099 | 0.031 | 1.104(1.038–1.174) | 0.002 |
| APACHE III | 0.040 | 0.009 | 1.040(1.022–1.059) | <0.001 |
| SOFA | 0.453 | 0.078 | 1.573(1.351–1.831) | <0.001 |
| Multivariate logistic regression | ||||
| MBRS score | 1.117 | 0.359 | 3.059(1.527–6.448) | 0.002 |
| APACHE III | 0.040 | 0.014 | 1.040(1.012–1.069) | 0.004 |
| Constant | −3.122 | 1.988 | 0.116 | 0.044 |
Abbreviation: ICU, intensive care unit; INR, international normalized ratio; MAP, mean arterial pressure; CI, confidence intervals; MELD, model for end-stage liver disease; APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment; MBRS, mean arterial pressure, bilirubin, respiratory failure and sepsis.
Severity of illness scoring systems
We have listed the results of goodness-of-fit as measured by the Hosmer-Lemeshow χ2 statistic denoting the predicted mortality risk, the predictive accuracy of the Child-Pugh points, MBRS, MELD, APACHE II, III, and SOFA scores in table 4. The comparison between discriminatory values of the 7 scoring systems has also been included in table 4. The AUROC analysis showed that the MBRS score has the best discriminatory power. The discriminatory powers of the RIFLE classification, Child-Pugh and the APACHE II scores were significantly lower than that of the MBRS score.
Table 4. Calibration and discrimination for the scoring methods in predicting hospital mortality.
| Calibration | Discrimination | |||||
| Goodness-of-fit (x2) | df | p | AUROC±SE | 95% CI | p | |
| RIFLE-R (n = 68) | ||||||
| MBRS | 3.349 | 3 | 0.341 | 0.810±0.077 | 0.660–0.961 | 0.001 |
| SOFA | 5.969 | 8 | 0.651 | 0.673±0.089 | 0.498–0.848 | 0.074 |
| MELD | 7.658 | 8 | 0.468 | 0.621±0.100 | 0.424–0.817 | 0.214 |
| RIFLE-I (n = 33) | ||||||
| MBRS | 0.466 | 3 | 0.926 | 0.873±0.103 | 0.670–1.000 | 0.020 |
| SOFA | 2.234 | 8 | 0.973 | 0.845±0.099 | 0.650–1.000 | 0.031 |
| MELD | 3.504 | 6 | 0.743 | 0.764±0.123 | 0.522–1.000 | 0.100 |
| RIFLE-F (n = 89) | ||||||
| MBRS | 1.193 | 2 | 0.551 | 0.933±0.031 | 0.872–0.994 | <0.001 |
| SOFA | 2.939 | 8 | 0.938 | 0.911±0.042 | 0.828–0.994 | <0.001 |
| MELD | 4.880 | 8 | 0.770 | 0.851±0.061 | 0.732–0.970 | <0.001 |
| Overall (n = 190) | ||||||
| MBRS | 1.160 | 3 | 0.763 | 0.863±0.032 | 0.801–0.925 | <0.001 |
| SOFA | 5.342 | 8 | 0.721 | 0.848±0.029 | 0.791–0.906 | <0.001 |
| MELD | 4.658 | 8 | 0.793 | 0.776±0.047 | 0.683–0.868 | <0.001 |
| Child-Pugh points | 7.740 | 5 | 0.171 | 0.622±0.065* | 0.496–0.749 | 0.047 |
| APACHE II | 4.574 | 8 | 0.802 | 0.686±0.053* | 0.583–0.789 | 0.003 |
| APACHE III | 12.531 | 8 | 0.129 | 0.793±0.045 | 0.705–0.881 | <0.001 |
| RIFLE | 0.329 | 1 | 0.566 | 0.679±0.043* | 0.679–0.764 | <0.001 |
Abbreviation: MBRS, mean arterial pressure, bilirubin, respiratory failure and sepsis; MELD, model for end-stage liver disease; APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment; df, degree of freedom; RIFLE, risk of renal failure, injury to kidney, failure of kidney function, loss of kidney function, and end-stage renal failure; AUROC, areas under the receiver operating characteristic curve; SE, standard error; CI, confidence intervals; NS, not significant.
p<0.05 versus MBRS score.
We examined the correlation between the scores determined by the Child-Pugh points, MBRS, MELD, APACHE II, III, and SOFA systems. The correlations between the scoring systems used on the first day of admission of the patients to the ICU have been listed in table 5. The MBRS score showed positive correlations with other scores in terms of the likelihood of in-hospital mortality (r>0.25, p<0.01) (Table 5).
Table 5. Correlation between scoring systems on the first day of ICU admission (Spearman rank correlation coefficients: r).
| Scores | MBRS | MELD | APACHE II | APACHE III | SOFA |
| Child-Pugh points | 0.308** | 0.436** | 0.048 | 0.231* | 0.357** |
| MBRS | - | 0.450** | 0.239** | 0.375** | 0.573** |
| MELD | - | 0.141 | 0.372** | 0.536** | |
| APACHE II | - | 0.682** | 0.530** | ||
| APACHE III | - | 0.693** |
Abbreviation: MBRS, mean arterial pressure, bilirubin, respiratory failure and sepsis; MELD, model for end-stage liver disease; APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment.
p<0.05;
p<0.01.
To assess the validity of the applied scoring methods, the sensitivity, specificity, and overall correctness of the prediction at selected cut-off points that provided the best Youden index were analyzed, and this data is listed in table 6. The MBRS score had the best Youden index and the highest overall correctness of prediction.
Table 6. Prediction of subsequent hospital mortality on the first day of ICU admission.
| Predictive factors | Cutoff point | Youden index | Sensitivity (%) | Specificity (%) | Overall correctness (%) |
| MAP (mmHg) | 80a | 0.41 | 62 | 79 | 71 |
| Bilirubin(umol/L) | 80a | 0.47 | 68 | 78 | 73 |
| Respiratory failure | Yesa | 0.16 | 24 | 92 | 58 |
| Sepsis | Yesa | 0.22 | 43 | 78 | 61 |
| MBRS score | 2a | 0.57 | 68 | 88 | 78 |
| Child-Pugh points | 11a | 0.29 | 67 | 62 | 65 |
| MELD score | 34a | 0.39 | 49 | 90 | 79 |
| APACHE II | 25a | 0.31 | 52 | 79 | 66 |
| APACHE III | 88a | 0.51 | 82 | 69 | 76 |
| SOFA | 9a | 0.53 | 82 | 71 | 76 |
Abbreviation: MAP, mean arterial pressure; ICU, intensive care unit; MBRS, mean arterial pressure, bilirubin, respiratory failure and sepsis; MELD, model for end-stage liver disease; APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment.
Value giving the best Youden index.
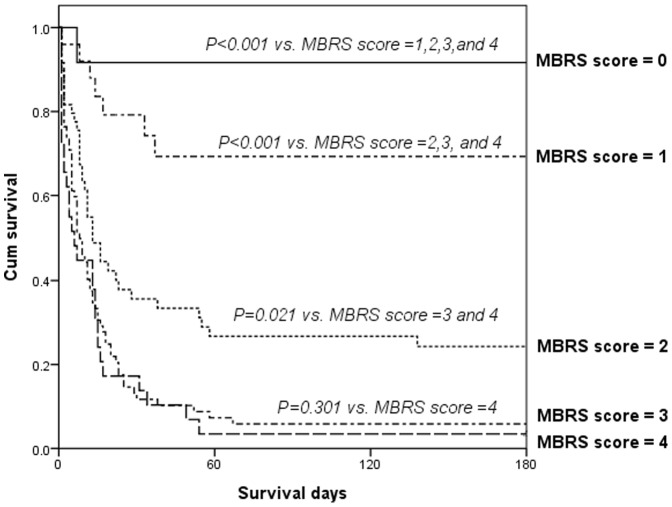
The patient number and the in-hospital mortality rate calculated as per the stratification data of the MBRS scores has been listed in table 7. The in-hospital mortality rate was 8%, 26%, 72%, 93%, and 97% for MBRS scores of 0, 1, 2, 3, and 4, respectively (χ2 for trend, p<0.001). A progressive and significant increase in the mortality rate was observed to correlate with the increasing MBRS scores of the patients. With reference to an MBRS score of 0, the odds ratios for different MBRS scores were as follows: odds ratio for MBRS score of 1 = 3.85; odds ratio for MBRS score of 2 = 28.286; odds ratio for MBRS score of 3 = 147.74; and odds ratio for MBRS score of 4 = 308. Cumulative survival rates differed significantly (p<0.05) for patients with MBRS score of 0 and patients with MBRS scores of 1, 2, 3, and 4. The comparisons between patients with MBRS score of 1 and those with MBRS scores of 2, 3, and 4 and between patients with MBRS score of 2 and those with MBRS scores of 3, and 4 has been depicted in Figure 1.
Table 7. MBRS score for critically ill cirrhotic patients with AKI.
| MBRS score | n | Hospital mortality (%) | Beta coefficient | Standard error | Odds rations (95%CI) | p |
| 0 | 12 | 8 | 0 | - | 1 (reference) | - |
| 1 | 27 | 26 | 1.348 | 1.133 | 3.850 (0.418–35.473) | NS (0.234) |
| 2 | 50 | 72 | 3.342 | 1.091 | 28.286 (3.334–239.974) | 0.002 |
| 3 | 72 | 93 | 4.993 | 1.143 | 147.740 (15.697–1384.178) | <0.001 |
| 4 | 29 | 97 | 5.730 | 1.458 | 308.000 (17.670–5368.505) | <0.001 |
| Constant | - | - | -2.398 | 1.044 | 0.091 | 0.022 |
Abbreviation: AKI, acute kidney injury; MBRS, mean arterial pressure, bilirubin, respiratory failure and sepsis; CI, confidence intervals; NS, notsignificant.
Figure 1. Survival Functions.
Cumulative survival in 190 critically ill cirrhotic patients with acute kidney injury according to their MBRS (mean arterial pressure, bilirubin, respiratory failure and sepsis) score after the first day of admission to a specialized hepatogastroenterology intensive care unit.
Discussion
In this study, the overall in-hospital mortality rate was 73.2%, which is consistent with the findings of previous reports and suggests that cirrhotic patients with AKI admitted to an ICU have an extremely poor prognosis [11], [24], [25]. This investigation showed that MBRS and APACHE III scores determined on the first day of admission to the ICU are significantly associated with in-hospital mortality in critically ill cirrhotic patients with AKI (Table 3). The MBRS score showed better discriminatory power than the Child-Pugh points, MELD, APACHE II, III, and SOFA scores (Table 4). The MBRS score had the best Youden index and the highest overall correctness of prediction (Table 6).
Our previous study showed the good discriminative power and independent predictive value of the MBRS scoring system in accurately predicting in-hospital mortality in critically ill cirrhotic patients with AKI [11]. The results of this study confirm these observations by showing that the MBRS score is a simple, reproducible, and easy-to-apply evaluation tool and has good prognostic value. This can help generate objective information for patients' families and physicians and supplement the judgments of clinical prognosis. Patients with cirrhosis are known to exhibit characteristic hyperdynamic circulation with secondary increase in heart rate and cardiac output and decrease in systemic vascular resistance, arterial blood pressure, and organ perfusion [26]–[28]. The fall in MAP resulted in glomerular blood flow decrease, and there was an autoregulation mechanism for keeping perfusion pressure which was achieved by pre-glomerular arteriole dilation until the cut-off point of MAP in 80 mmHg [29]. It was thought that MAP reflects not only the effective circulating volume caused by splanchnic vasodilation but also the instability of the hemodynamic system. Bilirubin level is a parameter reflecting both severity of an underlying liver illness and a superimposed liver injury caused by extrahepatic organ dysfunction [11]. Cirrhosis is associated with increased relative risk and death due to acute respiratory failure. In addition, cirrhotic patients requiring mechanical ventilation show an extremely poor prognosis [30]. Sepsis is a frequent cause of AKI and is associated with a poorer prognosis than that due to other causes. Patients with cirrhosis are susceptible to bacterial infections, which can lead to septic shock, metabolic acidosis, renal failure, hepatic encephalopathy, and decreased survival time [31]. The association of cirrhosis with such abnormalities makes the MBRS score an excellent tool for predicting in-hospital mortality in critically ill cirrhotic patients with AKI. Since no extrahepatic parameters are included in the determination of the Child-Pugh points, and no liver-specific prognostic factors are included in the determination of the APACHE II score, their discriminative powers are inferior to that of the MBRS score (Table 4).
This investigation has shown that APACHE III is an independent prognostic system for predicting in-hospital mortality in critically ill cirrhotic patients with AKI. The APACHE III system has been designed to increase the prediction accuracy of mortality in critically ill patients. A continuous weighing scheme for physiological variables, age, and comorbid conditions is used in this scoring system. However, the number of variables in this scoring system and their categorization has increased, and hence, enhancements in the statistical power increases the complexity of this system. Nevertheless, APACHE III is considered to be an economical scoring system to predict the severity of a disease and the probable mortality in patients [32].
In spite of the encouraging results observed in our study, several potential limitations in the study should also be considered. First, the study was conducted on patients from just 1 academic tertiary care medical center, which limits the generalization of our findings. Our results may be unsuitable for direct extrapolation to other hospitals with different patient populations. Second, the MBRS score is a specific scoring system developed only for cirrhotic patients with AKI who need intensive care support and not for the general ICU population. Third, we observed that hepatitis B viral infection (43%) was the leading cause of liver cirrhosis in patients and that a high proportion of patients had hepatoma (31%). This means that our results cannot be applied to the patients with liver disorders in North American and the European countries because liver diseases in these regions are largely because of hepatitis C viral infection or alcoholism. Fourth, the prognostic instruments were applied on patients already admitted to the specialized hepatogastroenterology ICU and were not used as a preadmission screening test; this may have skewed the results.
Fifth, defining baseline SCr as the first value measured during hospitalization might obscure the severity or even the presence of AKI. However, exactly true baseline SCr is not always available for all patients in clinical practice. Under various uncontrolled situation, choosing SCr established before admission as baseline value might run the risk to introduce some other biases and reduce the reproducibility of scoring systems. Due to above, many previous large studies also use admission SCr as baseline value to evaluate the impact of AKI on mortality in hospitalized patients [10], [25], [33]. As a matter of fact, cirrhotic patients who have stable renal function during hospitalization are thought to have a lower mortality rate, and such a relative low risk group is not our study target. Sixth, sequential measurement performed using these scoring systems (for example, daily, weekly) may reflect the dynamic aspects of the clinical diseases, and thus provide better information about the mortality risk in patients. Finally, the predictive accuracy of logistic regression models has its own limitations.
Conclusion
In conclusion, this study showed the grave prognosis in critically ill cirrhotic patient with AKI. The analytical data also showed that the MBRS and APACHE III scoring systems were independent predictors of short-term treatment outcome in critically ill patients. We confirmed that the MBRS scorings system is an accurate, simple, easy-to-apply, reproducible, and economical system capable of providing an improved prediction of prognosis along with objective information for clinical decision making for treating a homogenous group of patients. On the basis of the observed results, we feel that critically ill cirrhotic patients with AKI who show high MBRS scores (≥2 points) should be prioritized for liver transplantation, if they are suitable candidates for undergoing a surgery.
Funding Statement
This work was supported by the National Science Council of Taiwan (NSC 99-2314-B-182A-009-MY3). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wehler M, Kokoska J, Reulbach U, Hahn EG, Strauss R (2001) Short-term prognosis in critically ill patients with cirrhosis assessed by prognostic scoring systems. Hepatology 34: 255–261. [DOI] [PubMed] [Google Scholar]
- 2. Cholongitas E, Senzolo M, Patch D, Shaw S, Hui C, et al. (2006) Scoring systems for assessing prognosis in critically ill adult cirrhotics. Aliment Pharmacol Ther 24: 453–464. [DOI] [PubMed] [Google Scholar]
- 3. Jenq CC, Tsai MH, Tian YC, Lin CY, Yang C, et al. (2007) RIFLE classification can predict short-term prognosis in critically ill cirrhotic patients. Intensive Care Med 33: 1921–1930. [DOI] [PubMed] [Google Scholar]
- 4. Garcia-Tsao G, Parikh CR, Viola A (2008) Acute kidney injury in cirrhosis. Hepatology 48: 2064–2077. [DOI] [PubMed] [Google Scholar]
- 5. Ginès P, Schrier RW (2009) Renal failure in cirrhosis. N Engl J Med 361: 1279–1290. [DOI] [PubMed] [Google Scholar]
- 6. Cholongitas E, Senzolo M, Patch D, Shaw S, O'Beirne J, et al. (2009) Cirrhotics admitted to intensive care unit: the impact of acute renal failure on mortality. Eur J Gastroenterol Hepatol 21: 744–750. [DOI] [PubMed] [Google Scholar]
- 7. Slack A, Yeoman A, Wendon J (2010) Renal dysfunction in chronic liver disease. Crit Care 14: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tandon P, Garcia-Tsao G (2011) Renal dysfunction is the most important predictorof mortality in cirrhotic patients with spontaneous bacterial peritonitis. Clin Gastroenterol Hepatol 9: 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong F, Nadim MK, Kellum JA, Salerno F, Bellomo R, et al. (2011) Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut 60: 702–709. [DOI] [PubMed] [Google Scholar]
- 10. Belcher JM, Garcia-Tsao G, Sanyal AJ, Bhogal H, Lim JK, et al. (2012) Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology doi:10.1002/hep.25735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fang JT, Tsai MH, Tian YC, Jenq CC, Lin CY, et al. (2008) Outcome predictors and new score of critically ill cirrhotic patients with acute renal failure. Nephrol Dial Transplant 23: 1961–1969. [DOI] [PubMed] [Google Scholar]
- 12. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R (1973) Transection of the esophagus for bleeding esophageal varices. Br J Surg 60: 646–649. [DOI] [PubMed] [Google Scholar]
- 13. Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, et al. (2001) A model to predict survival in patients with end-stage liver disease. Hepatology 33: 464–470. [DOI] [PubMed] [Google Scholar]
- 14. Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13: 818–824. [PubMed] [Google Scholar]
- 15. Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, et al. (1991) The APACHE III prognostic system: risk prediction of hospital mortality for critically ill hospitalized adults. Chest 100: 1619–1636. [DOI] [PubMed] [Google Scholar]
- 16. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, et al. (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22: 707–710. [DOI] [PubMed] [Google Scholar]
- 17. Cholongitas E, Marelli L, Shusang V, Senzolo M, Rolles K, et al. (2006) A systematic review of the performance of the model for end-stage liver disease (MELD) in the setting of liver transplantation. Liver Transpl 12: 1049–1061. [DOI] [PubMed] [Google Scholar]
- 18. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P (2004) Acute Dialysis Quality Initiative workgroup: Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) Prognosis in acute organ system failure. Ann Surg 202: 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tran DD, Groeneveld ABJ, van der Meulen J, Nauta JJ, Strack van Schijndel RJ, et al. (1990) Age, chronic disease, sepsis, organ system failure, and mortality in a medical intensive care unit. Crit Care Med 18: 474–479. [DOI] [PubMed] [Google Scholar]
- 21. Tsai MH, Chen YC, Ho YP, Fang JT, Lien JM, et al. (2003) Organ system failure scoring system can predict hospital mortality in critically ill cirrhotic patients. J Clin Gastroenterol 37: 251–257. [DOI] [PubMed] [Google Scholar]
- 22. American College of Chest Physicians/Society of Critical Care MedicineConsensus Conference (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20: 864–874. [PubMed] [Google Scholar]
- 23. Cardenas A, Gines P, Uriz J, Bessa X, Salmeron JM, et al. (2001) Renal failure after upper gastrointestinal bleeding in cirrhosis: Incidence, clinical course, predictive factors, and short-term prognosis. Hepatology 34: 671–676. [DOI] [PubMed] [Google Scholar]
- 24. Chen YC, Tsai MH, Ho YP, Hsu CW, Lin HH, et al. (2004) Comparison of the severity of illness scoring systems for critically ill cirrhotic patients with renal failure. Clin Nephrol 61: 111–118. [DOI] [PubMed] [Google Scholar]
- 25. Tu KH, Jenq CC, Tsai MH, Hsu HH, Chang MY, et al. (2011) Outcome scoringsystems for short-term prognosis in critically ill cirrhotic patients. Shock 36: 445–450. [DOI] [PubMed] [Google Scholar]
- 26. Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH (1988) Peripheral arterial vasodilation hypothesis: a proposal for the initiation of sodium and water retention in cirrhosis. Hepatology 8: 1151–1157. [DOI] [PubMed] [Google Scholar]
- 27. Groszmann R, Kotelanski B, Cohn JN, Khatri IM (1972) Quantitation of portasystemic shunting from the splenic and mesenteric beds in alcoholic liver disease. Am J Med 53: 715–722. [DOI] [PubMed] [Google Scholar]
- 28. Iwakiri Y, Groszmann RJ (2006) The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology 43: S121–S131. [DOI] [PubMed] [Google Scholar]
- 29. Short A, Cumming A (1999) ABC of intensive care. Renal support. BMJ 319: 41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shellman RG, Fulkerson WJ, DeLong E, Piantadosi CA (1988) Prognosis of patients with cirrhosis and chronic liver disease admitted to the medical intensive care unit. Crit Care Med 16: 671–678. [DOI] [PubMed] [Google Scholar]
- 31. Tsai MH, Chen YC, Lien JM, Tian YC, Peng YS, et al. (2008) Hemodynamics and metabolic studies on septic shock in patients with acute liver failure. J Crit Care 23: 468–472. [DOI] [PubMed] [Google Scholar]
- 32. Chen YC, Tian YC, Liu NJ, Ho YP, Yang C, et al. (2006) Prospective cohort study comparing sequential organ failure assessment and acute physiology, age, chronic health evaluation III scoring systems for hospital mortality prediction in critically ill cirrhotic patients. Int J Clin Pract 60: 160–166. [DOI] [PubMed] [Google Scholar]
- 33. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW (2005) Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370. [DOI] [PubMed] [Google Scholar]