Abstract
We have investigated two NADPH-cytochrome (Cyt) P450 reductase isoforms encoded by separate genes (AR1 and AR2) in Arabidopsis thaliana. We isolated AR1 and AR2 cDNAs using a mung bean (Phaseolus aureus L.) NADPH-Cyt P450 reductase cDNA as a probe. The recombinant AR1 and AR2 proteins produced using a baculovirus expression system showed similar Km values for Cyt c and NADPH, respectively. In the reconstitution system with a recombinant cinnamate 4-hydroxylase (CYP73A5), the recombinant AR1 and AR2 proteins gave the same level of cinnamate 4-hydroxylase activity (about 70 nmol min−1 nmol−1 P450). The AR2 gene expression was transiently induced by 4- and 3-fold within 1 h of wounding and light treatments, respectively, and the induction time course preceded those of CYP73A5 and a phenylalanine ammonia-lyase (PAL1) gene. On the contrary, the AR1 expression level did not change during the treatments. Analysis of the AR1 and AR2 gene structure revealed that only the AR2 promoter contained three putative sequence motifs (boxes P, A, and L), which are involved in the coordinated expression of CYP73A5 and other phenylpropanoid pathway genes. These results suggest the possibility that AR2 transcription may be functionally linked to the induced levels of phenylpropanoid pathway enzymes.
P450 reductase transfers two electrons from NADPH to P450s, which constitute the terminal electron acceptors in the microsomal electron transport system, catalyzing mono-oxygenation reactions with diverse endogenous and exogenous lipophilic substrates. More than 400 P450 genes have been identified in a wide range of organisms, from bacteria to animals (Nelson et al., 1996), and it has been suggested that a single form of P450 reductase is responsible for the electron transport to these diverse P450 isoforms in animals (Porter et al., 1990). In higher plants P450s are also involved in the biosynthesis of a variety of secondary metabolites, such as phenylpropanoids, terpenoids, sterols, fatty acids, and GAs (Donaldson and Luster, 1991; Bolwell et al., 1994). P450 reductase proteins and the corresponding cDNAs have been isolated from Vigna radiata (Shet et al., 1993) and also from Catharanthus roseus (Meijer et al., 1993), in which only a single-copy gene for P450 reductase has so far been detected. On the other hand, Benveniste et al. (1991) reported the purification of three isoforms of P450 reductase from the microsomal fractions of Helianthus tuberosus. However, it is not clear whether these three reductases were encoded by distinct genes or whether they were posttranslationally modified proteins encoded by a single-copy gene. Recently, two distinct P450 reductase cDNAs were isolated from Arabidopsis thaliana (D. Pompon, unpublished results; deposited in GenBank, accession nos. X66016 and X66017), and also from H. tuberosus (I. Benveniste, unpublished results; deposited in GenBank, accession nos. Z26250 and Z26251, respectively). These results indicate that, in contrast to mammals and yeast, at least some plant species contain a few isoforms of P450 reductase that are encoded by separate genes. The occurrence of multiple isoforms of P450 reductase in plants raises questions about physiological roles of the individual P450 reductase isoforms; however, characterization of these P450 reductase isoforms has not been reported.
In this paper we report the isolation and characterization of the cDNAs and the corresponding genes encoding two isoforms of P450 reductase of A. thaliana. We demonstrate by expressing the cDNAs in insect cells using a baculovirus expression system that these reductase genes encode functionally active P450 reductases. When used for reconstitution of the cinnamate 4-hydroxylase activity in a system containing the recombinant CYP73A5 (Mizutani et al., 1997), both recombinant Arabidopsis P450 reductases were as competent as the native P450 reductase from mung bean (Phaseolus aureus L.). Genomic organization of the two P450 reductase genes (AR1 and AR2) closely resembled each other with regard to the intron positions and exon sizes, whereas little similarity was observed in the DNA sequences of their promoter regions. RNA-blot analysis showed that AR1 was constitutively expressed throughout development, whereas AR2 expression was strongly induced by wounding and light treatments, and the induction time course of AR2 preceded those of PAL1 and CYP73A5, which encode PAL and cinnamate 4-hydroxylase, respectively. Possible regulation mechanisms that coordinate the expression of AR2 with those of PAL1 and CYP73A5 are discussed on the basis of sequence analyses of the AR1 and AR2 promoter regions.
MATERIALS AND METHODS
Plant Materials and Treatments
Arabidopsis thaliana ecotype Columbia (Col-0, Lehle Seeds, Tucson, AZ) seedlings were grown under sterile conditions on 0.8% agarose plates containing GM (Valvekens et al., 1988) in a growth chamber maintained at 22°C under continuous light.
For wounding treatment, leaves of 3-week-old plants were harvested, cut into 2-mm-wide strips, and incubated for 1 to 9 h in a Petri dish containing GM and 0.005% (w/v) chloramphenicol. For the 0-h time, sliced leaves were frozen immediately without further incubation.
For light treatment, 2-week-old plants grown in continuous light were placed in the dark for 2 d and then transferred to the light condition for 1 to 12 h.
Isolation of cDNA Clones of P450 Reductases
A cDNA of mung bean (Phaseolus aureus L.) P450 reductase was isolated using degenerate oligonucleotide probes designed from the partial amino acid sequences (MR1, RLVAVGLGDDDQ; MR2, LQYGVFGLGNRQYEHFNK; and MR3, LQMDGRYLRDV) determined from a purified mung bean P450 reductase (Mizutani et al., 1993a). A 1.5-kb fragment was obtained by PCR using a set of degenerate primers. A sense primer, SU18 (5′-CA[A/G]TA[T/C]GA[A/G]CA[T/C]TT[T/C]AA[T/C]AA-3′), was based on the peptide sequence QYEHFNK from MR2, and an antisense primer, SP69 (5′-TAIC[G/T]ICC[A/G]TCCAT[T/C]-3′, was derived from the peptide sequence QMDGRY from MR3. This PCR fragment was used as a hybridization probe to screen a total of 1,000,000 plaques from a mung bean cDNA library under hybridization conditions described previously (Mizutani et al., 1993b). Forty positive clones were isolated and the longest insert was completely sequenced. The cDNA consisted of a 161-bp 5′ untranslated region, a 371-bp 3′ noncoding region, and a 2073-bp open reading frame encoding a polypeptide of 691 amino acid residues.
Arabidopsis P450 reductase cDNAs were isolated from a cDNA library prepared from 7-d-old Arabidopsis seedlings (Mizutani et al., 1997) by using the full-length cDNA for mung bean P450 reductase as a probe under the following low-stringency conditions: hybridization for 16 h at 50°C in a hybridization buffer containing 1% BSA, 7% SDS, 50 mm sodium phosphate (pH 7.5), and 1 mm EDTA (Church and Gilbert, 1984); washing for 10 min in 6× SSC supplemented with 0.1% SDS at room temperature and for 20 min in 2× SSC with 0.1% SDS at 50°C. Twelve positive clones were isolated and divided into two groups according to their partial DNA sequences. The longest clones (AR1 and AR2) from the two groups were completely sequenced.
Isolation of Genomic Clones for Two P450 Reductases
Genomic DNA clones were isolated using the full-length cDNAs of AR1 and AR2 as hybridization probes. A total of 100,000 plaques from a λEMBL3 (T7/SP6) library of Arabidopsis ecotype Col-0 genomic DNA (Clontech, Palo Alto, CA) were screened with either the AR1 or the AR2 probe under the following high-stringency conditions: hybridization for 16 h at 65°C in the hybridization buffer described above; washing for 10 min in 2× SSC with 0.1% SDS at room temperature and for 30 min in 0.1× SSC containing 0.1% SDS at 65°C. Six positive plaques for each AR1 and AR2 (AR1 clones and AR2 clones) were isolated through two additional rounds of screening, and λDNA was prepared from liquid lysates according to the method of Grossberger (1987). Genomic clones containing the longest inserts were identified by restriction-mapping analysis. The inserts were obtained from the AR1 and AR2 clones by digesting with XhoI and EcoRI, respectively, and were subcloned into pCRII vectors (Invitrogen, San Diego, CA) for DNA sequencing.
Heterologous Expression in Insect Cells
The two isoforms of P450 reductase were expressed using a baculovirus expression vector system, according to the method described previously (Summers and Smith, 1987), using a baculovirus transfer vector, pVL1392 (Invitrogen), Spodoptera furugiperda 21 (Sf21) cells (Invitrogen), and an infectious BaculoGold Baculovirus DNA (Pharmingen, San Diego, CA). Sf21 cells were maintained at 27°C as a monolayer culture in Grace's medium (Difco, Detroit, MI), supplemented with 0.33% TC yeastolate (Difco), 0.33% lactoalbumin, 10% fetal bovine serum, and 50 μg/mL gentamycin sulfate.
The expressed P450 reductases were purified from the infected Sf21 cells. The infected cells were sonicated and centrifuged at 100,000g for 1 h. The pellet was homogenized with buffer A (20 mm potassium phosphate [pH 7.25], 20% glycerol, and 1 mm DTT). For protein solubilization, buffer B was prepared by adding 1% Emulgen 913 (Kao Atlas, Tokyo, Japan) to buffer A. After solubilization in buffer B at 4°C for 3 h, the sample was centrifuged at 100,000g for 1 h, and the supernatant was applied to a DEAE-Sepharose column (5 × 10 cm) equilibrated with buffer B, and the protein was eluted with a linear KCl gradient (0–0.5 m) in buffer B. The pooled fractions containing the active P450 reductase were applied to a 2′,5′-ADP Sepharose column (1 × 7 cm) equilibrated with buffer B, and the protein was eluted from the column with 10 mm potassium phosphate buffer (pH 7.7) containing 20% glycerol, 1 mm EDTA, 0.1 mm DTT, and 0.5 mm NADP. The active fractions were further applied to a Mono-Q column equilibrated with buffer B, and the column was washed with buffer A until the absorption at 280 nm derived from Emulgen 913 disappeared from the eluted buffer. After the removal of the detergent the protein was eluted with a linear KCl gradient (0–0.5 m) in buffer A.
Assay Methods
P450 reductase was assayed by measuring its NADPH-Cyt c reductase activity, as described by Imai (1976). The rate of Cyt c reduction was calculated from the A550 change using an extinction coefficient (Σ = 21 mm−1 cm−1). trans-Cinnamic acid 4-hydroxylase activity was reconstituted using the purified recombinant CYP73A5 protein (Mizutani et al., 1997), according to the method described previously (Mizutani et al., 1993a). The reconstitution system consisted of the recombinant CYP73A5 (5 nm) and the purified recombinant P450 reductases (0.1 unit/mL) in 50 mm potassium phosphate buffer (pH 7.2) containing 0.01% (w/v) sodium cholate, 10 μg/mL dilauroylphosphatidylcholine (Funakoshi, Tokyo, Japan), and 0.4 mm trans-cinnamic acid. The reaction was started by adding 0.1 mm NADPH. p-Coumaric acid formation was determined by HPLC as described previously (Mizutani et al., 1993a). P450 was estimated from the CO-difference spectrum (Omura and Sato, 1964).
DNA Preparation and DNA-Blot Analysis
Genomic DNA was isolated from shoots of 3-week-old Arabidopsis seedlings and purified by ethidium bromide-CsCl density-gradient centrifugation as described by Ausubel et al. (1987). For Southern blots, genomic DNA was digested with the indicated restriction enzymes, separated by 0.7% agarose gel electrophoresis, and transferred to nylon membranes. The membranes were hybridized with either the full-length AR1 or AR2 cDNAs as a probe and washed under the high-stringency conditions described above. A 282-bp AR1 cDNA fragment was also prepared as a hybridization probe by digesting the AR1 cDNA with BamHI and HindIII. Since this probe contained the FMN-binding domain of P450 reductases (Porter and Kasper, 1986), it was expected to hybridize to all of the P450 reductase genes of Arabidopsis under the low-stringency conditions described above.
RNA Preparation and RNA-Blot Analysis
Total RNA was isolated by phenol/chloroform extraction followed by lithium chloride precipitation, as described by Lagrimini et al. (1987). Samples of total RNA (5 μg) were separated by 1.2% formaldehyde-agarose gel electrophoresis and blotted onto a nylon membrane. Hybridization and washing were carried out under the high-stringency conditions as described above. The hybridization signals were quantified using an imaging analyzer (BAS2000, Fuji Film, Tokyo, Japan). The hybridized DNA probes were stripped from the membranes by boiling in a buffer containing 0.01× SSC and 0.01% SDS for 20 min, and blots were rehybridized to an actin-1 probe (Nairn et al., 1988) for normalization.
DNA Sequencing and Analysis
DNA sequencing was performed using a Dye Deoxy Terminator Cycle Sequencing kit (ABI) and an automated DNA sequencer (model 373A, ABI). Sequences were analyzed using the software DNASIS, version 3.5 (Hitachi Software Engineering America, San Bruno, CA).
RESULTS
Isolation of Two Different cDNAs Encoding P450 Reductase of A. thaliana
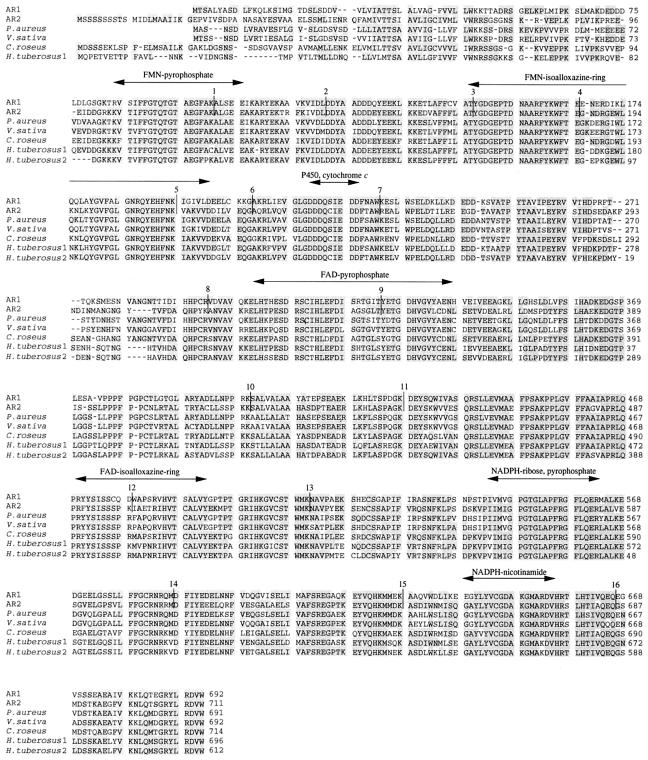
A P450 reductase cDNA was isolated from a P. aureus cDNA library and used for cloning of the cDNAs for two isoforms of Arabidopsis P450 reductases (AR1 and AR2). The amino acid sequence deduced from the P. aureus P450 reductase cDNA was 30 to 40% identical to those of mammalian P450 reductases (Fig. 1), and the three peptide sequences (MR1, MR2, and MR3) determined from the purified P. aureus P450 reductase were found within the predicted amino acid sequence in perfect agreement.
Figure 1.
Amino acid alignment of plant P450 reductases. The deduced amino acid sequences of AR1 and AR2 are aligned with those of P450 reductases from P. aureus, V. sativa, C. roseus, and H. tuberosus by the program Clustal W (Thompson et al., 1994). Identical amino acid residues are shadowed, and dashes were inserted to maximize the sequence homology. The putative functional regions involved in the interaction with FMN, FAD, NADPH, P450, and Cyt c (Porter and Kasper, 1986; Nisimoto, 1986; Yabusaki et al., 1988) are indicated above the sequences by an arrow. Intron positions are shown by vertical lines with the number of the position from the N terminus.
The amino acid sequences deduced from the cDNA clones (AR1 and AR2) from Arabidopsis seedlings were compared with those of the P450 reductases from other plant species (Fig. 1). The DNA sequences of AR1 and AR2 cDNAs were nearly identical to those of ATR1 and ATR2 cDNAs previously deposited by D. Pompon in GenBank (accession nos. X66016 and X66017, respectively). The small differences (data not shown) might be attributable to the ecotype difference between Columbia (AR1 and AR2) and Landsberg (ATR1 and ATR2).
The AR1 cDNA consists of a 2076-bp open reading frame, a 121-bp 5′ untranslated region, a 193-bp 3′ noncoding region, and a poly(A+) tail. The open reading frame encodes a polypeptide of 692 amino acid residues with a calculated molecular mass of 76,765 D. AR2 cDNA consists of a 2133-bp open reading frame, a 121-bp 5′ untranslated region, a 55-bp 3′ noncoding region, and a poly(A+) tail. Although a stop codon is not found within the 5′ untranslated region upstream, the first ATG codon in the AR2 cDNA sequence and several Met residues are seen downstream from the first Met, we assumed that the first ATG triplet was the start codon, according to the “first-AUG-rule” in which a first AUG serves as the initiator codon used in the translation of about 95% of the eukaryotic mRNAs (Kozak, 1987). Thus, the predicted open reading frame of AR2 cDNA encodes a polypeptide of 712 amino acid residues with a calculated molecular mass of 79,124 D.
Figure 1 shows the alignment of the deduced primary structures of AR1 and AR2 proteins with those of the reductases from other plant species. P450 reductases from animals and yeast consist of several functional domains, such as a hydrophobic membrane-anchoring region; FMN-, FAD-, and NADPH-binding regions; and the binding sites for P450 and Cyt c (Nisimoto, 1986; Porter and Kasper, 1986; Yabusaki et al., 1988). The amino acid sequences of these functional domains were well conserved between the two Arabidopsis reductases (Fig. 1), with the exception of less structural similarity in the N-terminal membrane-anchoring region. The deduced primary structures of AR1 and AR2 proteins are 63% identical to each other (Table I). It is interesting that the AR1 protein is more similar to the reductases of P. aureus and V. sativa than to AR2 (Table I). On the other hand, the AR2 protein is 73% identical to the C. roseus reductase and is 75 and 70% identical to HTR1 and HTR2 from H. tuberosus, respectively (Table I). Particularly, the N-terminal structures of AR1, P. aureus, and V. sativa reductases were shorter than those of AR2, C. roseus, and H. tuberosus 1 reductases (Fig. 1). Thus, plant P450 reductases can be divided into two groups: (a) AR1 type, AR1 and the P450 reductases of P. aureus and V. sativa; and (b) AR2 type, AR2, the C. roseus P450 reductase, and the two H. tuberosus reductases (HTR1 and HTR2). In contrast to the high homology among plant P450 reductases, AR1 and AR2 proteins were only 32 to 41% identical to the P450 reductases from other organisms (Table I), suggesting that the sequence variation among the plant P450 reductases had occurred after the divergence from other organisms.
Table I.
Identity of amino acid sequences of Arabidopsis P450 reductases to those of the reductases from various species
| Species | AR1 | AR2 | Accession No. |
|---|---|---|---|
| identity % | |||
| A. thaliana (AR1) | – | 63 | This study |
| A. thaliana (AR2) | 63 | – | This study |
| H. tuberosus (HTR1) | 67 | 75 | Z26250 |
| H. tuberosus (HTR2)a | 65 | 70 | Z26251 |
| V. sativa | 74 | 67 | Z26252 |
| P. aureus | 74 | 67 | This study |
| C. roseus | 63 | 73 | X69791 |
| Homo sapiens | 41 | 40 | S90469 |
| Rattus norvegicus | 40 | 41 | M12516 |
| Drosophila melanogaster | 41 | 42 | X93090 |
| Caenorhabditis elegans | 37 | 39 | U21322 |
| Saccharomyces cerevisiae | 37 | 37 | D13788 |
| Aspergillus niger | 38 | 38 | Z26938 |
| Bacillus megateriumb | 34 | 33 | J04832 |
HTR2 is a partial cDNA clone lacking the N-terminal region (Fig. 2).
In B. megaterium a gene encodes P450-BM-3, which consists of two domains corresponding to Cyt P450 and P450 reductase (Ruettinger et al., 1989), and, therefore, the region coding for the P450 reductase domain was used for the homology analysis.
Biochemical Properties of AR1 and AR2 Proteins Expressed in Insect Cells
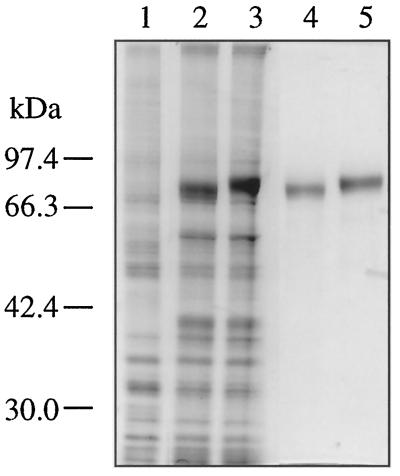
We expressed the AR1 and AR2 cDNAs in insect cells using a baculovirus expression vector system. SDS-PAGE analysis (Fig. 2) showed that new intense bands of 78 and 80 kD appeared in the microsomal fraction of the insect cells upon infection with the recombinant AR1 virus and the AR2 virus, respectively. These proteins were not found in the mock-infected cells (Fig. 2, lane 1). The difference in the apparent molecular masses of these newly appeared proteins reflected the molecular masses of AR1 (76,765 D) and AR2 (79,124 D) calculated from their deduced primary structures. The microsomal NADPH-Cyt c reductase activity of the recombinant-virus-infected cells was 1000-fold higher than that of the mock-infected cells. These recombinant AR1 and AR2 proteins were purified to homogeneity by three-step column chromatography (Fig. 2, lanes 4 and 5, respectively).
Figure 2.
Heterologous expression of recombinant AR1 and AR2 proteins in insect cells. SDS-PAGE was performed using 10% polyacrylamide slab gel, and proteins were visualized with Coomassie brilliant blue R-250. Lane 1, Solubilized fraction of microsomes of mock-infected Sf21 cells (10 μg of protein); lane 2, solubilized fraction of microsomes of Sf21 cells (10 μg of protein) infected with the recombinant virus containing AR1 cDNA; lane 3, solubilized fraction of microsomes of Sf21 cells (10 μg of protein) infected with the recombinant virus containing AR2 cDNA; lane 4, purified AR1 protein (200 μg of protein); and lane 5, purified AR2 protein (0.2 μg of protein). The migration of size standard is shown to the left of the gel.
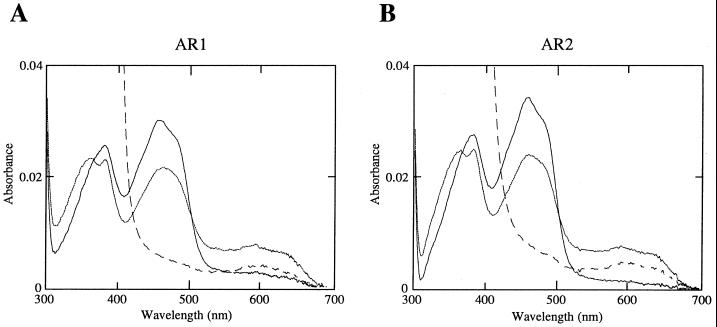
The recombinant AR1 and AR2 proteins showed similar absolute absorption spectra characteristic of flavoproteins (Fig. 3). Oxidized forms showed prominent peaks at 455 and 380 nm, typical of a flavoprotein, and the 455-nm peak disappeared when the enzymes were fully reduced by sodium dithionite. Aerobic treatment of the reductases with 50 μm NADPH produced a spectrum having a broad peak of approximately 600 nm, which is typical for the stable semiquinone form of flavoproteins. These spectral properties of the recombinant AR1 and AR2 proteins are very similar to that of the P450 reductase purified from rabbit microsomes (Iyanagi and Mason, 1973). The recombinant AR1 and AR2 proteins were also indistinguishable in terms of the Km values for NADPH and Cyt c (Table II), which were comparable to those of the reductases from P. aureus (22.1 and 24.8 μm for NADPH and Cyt c, respectively) and the H. tuberosus reductases (Benveniste et al., 1989). In the reconstitution system with either the AR1 or AR2 protein, the recombinant CYP73A5 protein (Mizutani et al., 1997) was able to catalyze the cinnamate 4-hydroxylase reaction at the same rate (Table. III). The addition of Cyt b5 to the reconstitution system did not enhance the level of the reconstituted cinnamate 4-hydroxylase activity under our experimental conditions (data not shown), although it is well known that Cyt b5 is required for maximum catalytic rate for certain species of mammalian P450s (Yamazaki et al., 1996). These results may suggest that both P450 reductases could be equally involved in the catalysis of CYP73A5 in vivo. However, it is possible that these two P450 reductases have different specificities toward individual P450s.
Figure 3.
Absolute absorption spectra of the purified recombinant AR1 and AR2 proteins. A, Purified recombinant AR1 protein; B, purified recombinant AR2. The one-electron reduced semiquinone forms were prepared by adding 1 mm NADPH to a final concentration of 25 μm, and the spectra were recorded after incubating for 10 min at 25°C. A few grains of sodium dithionite were added to completely reduce the reductases. [——], Oxidized form; [… . .], semiquinone form; and [– – – –], completely reduced form.
Table II.
The Km values for Cyt c and NADPH
Reactions were carried out in 0.3 m potassium phosphate (pH 7.7). After 2 min of preincubation at 28°C, reactions were initiated by addition of NADPH. Values are means ± sd of three separate determinations.
For the determination of the Km value for Cyt c, NADPH concentration was fixed at 50 μm, and the cytochrome c concentration was varied from 0.5 to 300 μm.
For the determination of the Km value for NADPH, Cyt c was added at 50 μm with varied NADPH concentrations (0.5–300 μm).
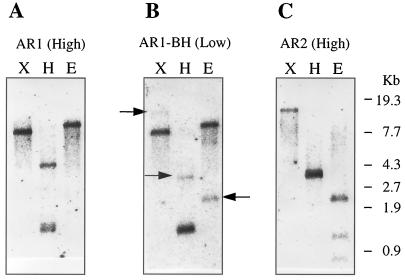
Genomic Southern-Blot Analysis
Genomic DNA was digested with EcoRI, HindIII, or XbaI, and hybridization was performed at the high-stringency conditions (65°C) using the full-length cDNAs for either AR1 or AR2 as a probe (Fig. 4, A and C). Neither EcoRI nor XbaI digestion cut the AR1 cDNA, and a single hybridization signal was observed when hybridized with the AR1 probe. Three fragments were found in the digestion with HindIII (Fig. 4A), which has two restriction sites in the AR1 cDNA sequence. There are two EcoRI sites, a HindIII site, and no XbaI site in the AR2 cDNA sequence. Therefore, three bands with the EcoRI digestion, two close bands with HindIII, and a single band with XbaI digestion were detected using the AR2 probe (Fig. 4C). The results indicated that each of the AR1 and AR2 cDNA probes hybridized with an independent single gene and did not cross-hybridize each other under the high-stringency conditions.
Figure 4.
Southern-blot analysis of the P450 reductase genes. A. thaliana Columbia genomic DNA (1 μg) was digested with the restriction enzymes X (XbaI), H (HindIII), and E (EcoRI). The digested DNA was separated on 0.7% agarose gel, blotted onto a nylon membrane, and hybridized with a 32P-labeled probe. A, Full-length AR1 cDNA was used as a probe at high stringency; B, AR1 cDNA fragment obtained by digestion with BamHI and HindIII was used as a probe at low stringency. The weak-hybridization bands, which was ascribed to the AR2 gene, are indicated by arrows. C, Full-length AR2 cDNA was used as a probe under high stringency. The migration of size marker is shown to the right of the blots.
A 282-bp fragment was obtained from the AR1 cDNA by digesting with BamHI and HindIII and was used as a probe under the low-stringency conditions (50°C; Fig. 4B). This 282-bp fragment served as a universal probe to detect any P450 reductase genes in Arabidopsis (74.5% identity between AR1 and AR2 at the DNA level), since it encodes a domain containing the putative FMN-binding site conserved in all P450 reductases so far reported (Fig. 1; Porter, 1991). In each digestion two gene fragments were observed at strong and weak intensities: 8- and 15-kb fragments in EcoRI digestion, 1.5- and 3.8-kb fragments in HindIII digestion, and 11- and 2.4-kb fragments in XbaI digestion, respectively (Fig. 4B). When the hybridization patterns were compared (Fig. 4), these strong and weak hybridization signals detected under the low-stringency conditions could be ascribed to AR1 and AR2, respectively. In other words, the 282-bp fragment from the AR1 cDNA hybridized with only those DNA fragments derived from AR1 and AR2. Thus, there were two P450 reductase genes detected in Arabidopsis.
Gene Structure of AR1 and AR2
To characterize genomic organization of the two P450 reductase genes, we screened an Arabidopsis λEMBL3 genomic library using either the full-length AR1 or AR2 cDNA as a hybridization probe. After analysis of several positive clones by restriction endonuclease mapping and Southern hybridization, two genomic clones containing the entire coding region for each of AR1 and AR2, respectively, were selected for further investigation.
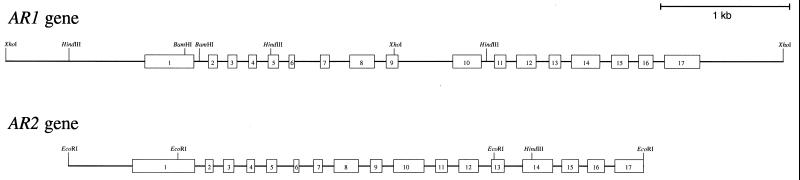
The physical maps of AR1 and AR2 gene organization are shown in Figure 5. Sequence comparison of AR1 and AR2 with those of the corresponding cDNAs showed that both AR1 and AR2 contain 17 exons and 16 introns (Figs. 1 and 5). All of the exon-intron boundaries are consistent with the proposed junction sequence “gt… ag” rule (Hanley and Schuler, 1988). It is interesting that the AR1 and AR2 coding sequences were divided at exactly the same position by introns (Fig. 1). This extensive conservation of intron placements suggest that the two reductase genes might have evolved by way of the duplication of a common ancestral gene. In contrast to the striking conservation of the intron positions, lengths of the corresponding introns vary between AR1 and AR2, and little sequence similarity was observed (data not shown). Furthermore, there was a correlation between the exon organization and the functional domains in both AR1 and AR2 (Fig. 1). Porter et al. (1990) proposed that the exon organization of the rat reductase gene correlated with the functional domains of the reductase. Comparison of the gene structures of Arabidopsis reductases with that of rat reductase showed that three intron positions (introns 9, 11, and 12) were consistent on their aligned amino acid sequences (data not shown).
Figure 5.
Gene organization of the AR1 and AR2 genes. Exons are indicated by boxes 1 to 17, and introns are indicated by solid lines.
The Promoter Regions of AR1 andAR2
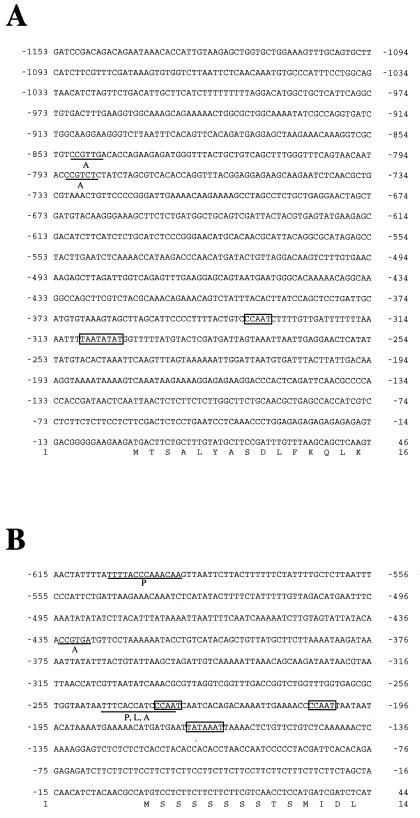
The DNA sequences of the 5′ flanking regions of AR1 and AR2 were also determined as shown in Figure 6. In a 1154-bp promoter region of AR1, both a putative TATA box and a putative CCAAT box were found 309 and 348 bp upstream of the ATG translation initiation codon, respectively (Fig. 6A, boxed). A 616-bp promoter region of AR2 also contained a putative TATA box at 171-bp and two putative CCAAT boxes 208 and 237 bp upstream of the ATG codon, respectively (Fig. 6B, boxed). O'Leary et al. (1994) reported that the promoter of the rat P450 reductase gene possesses neither a TATA nor a CCAAT box, but contains GC-rich consensus sequences for the transcription factor Sp1 and is similar to those of housekeeping genes. In contrast to the rat reductase promoter, both the TATA and CCAAT boxes, but no Sp1 consensus sequences, were found in the AR1 and AR2 promoters.
Figure 6.
The nucleotide sequences of the TATA-proximal regions of the AR1 and AR2 genes. A, AR1 promoter region; B, AR2 promoter region. The translation initiation codon ATG is located at +1. A putative TATA box and a CAAT box are boxed. Putative cis-acting elements homologous to sequence motifs for boxes P, A, and L (Logemann at al., 1995; Mizutani et al., 1997) are underlined. The deduced amino acid sequences of the coding regions are shown below the nucleotide sequences.
We previously demonstrated that the promoter of CYP73A5 encoding cinnamate 4-hydroxylase contains three sequence motifs (boxes P, A, and L) conserved among the genes for PAL (PAL) and 4-coumarate CoA:ligase (4CL) involved in the general phenylpropanoid pathway (Mizutani et al., 1997). These elements are thought to be important in controlling the coordinated expression of these genes under different environmental conditions (Logemann et al., 1995). It should be noted that, in the AR2 promoter region, there were two sequences similar to boxes P and A and a sequence homologous to box L (Fig. 6B, underlined). On the other hand, the AR1 promoter contained only two box A-like sequences but no sequences homologous to boxes P and L (Fig. 6A, underlined).
Expression of AR1 and AR2 in Arabidopsis
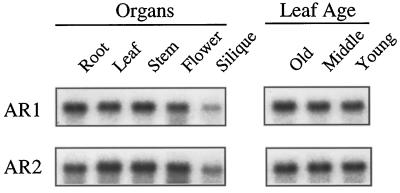
RNA gel-blot hybridization was performed to study expression of AR1 and AR2 using each of the full-length AR1 and AR2 cDNAs as gene-specific probes. The organ-specific and age-dependent expression patterns of the two reductases are shown in Figure 7. The AR1 mRNA was about 1.3-fold more abundant in roots and stems than in leaves and flowers. On the other hand, the AR2 expression was 2-fold higher in leaves, stems, and flowers than in roots. Both of the P450 reductase genes showed the least expression in siliques. Strong age dependency was not found in the expression levels of AR1 and AR2. This result contrasts with our previous observation that most of P450s isolated from Arabidopsis were more highly expressed in older leaves than in younger leaves (M. Mizutani, unpublished results).
Figure 7.
Tissue-specific expression of the AR1 and AR2 genes in Arabidopsis. A, Total RNA was isolated from the roots and leaves of 3-week-old plants, from inflorescence stems and flowers of 4-week-old plants, and from the siliques of 5-week-old plants. B, Total RNA was isolated from leaves of 3-week-old plants with 12 leaves. The first and second leaves represent the older (Old) leaves. The middle-aged leaves (Middle) were collected from the 4th and 5th positions, and the younger (Young) leaves were collected from the 9th and 10th positions, counted from the bottom. Plants were grown under continuous light. Five micrograms of total RNA was separated on formaldehyde agarose gels, transferred to nylon membranes, and hybridized to the indicated probes.
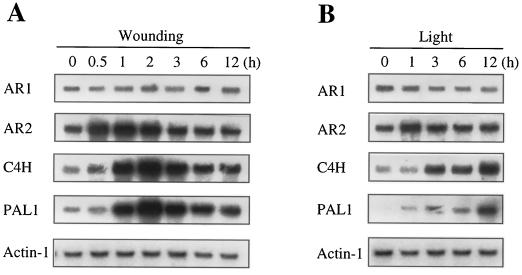
Expression of AR1 and AR2 in response to wounding and light treatments were also investigated (Fig. 8) and were compared with those of PAL (PAL1) and cinnamate 4-hydroxylase (CYP73A5). The strong and coordinated induction of PAL1 and CYP73A5 by wounding (Fig. 8A) was consistent with our previous observation (Mizutani et al., 1997). The AR2 expression level was significantly enhanced by wounding, and it reached a maximum (4-fold) within 1 h of the treatment and then gradually decreased to a basal level. On the other hand, the AR1 expression did not change during the treatment. The expression levels of PAL1 and CYP73A5 continually increased to 10-fold within 12 h of the light treatment (Fig. 8B). The AR2 expression also increased 3-fold as fast as 1 h of the onset of the light period. However, the induction was transient and the AR2 mRNA level gradually decreased to a basal level. On the other hand, the AR1 expression slightly decreased with light treatment. In summary, AR1 was constitutively expressed, whereas AR2 was induced in response to the wounding treatment and light. The AR2 induction was followed by those of PAL1 and CYP73A5, of which the magnitude of induction was greater than that of AR2.
Figure 8.
Effect of wounding and light treatment on expression levels of the AR1 and AR2 genes. A, Leaves were harvested from 3-week-old plants grown under continuous light. The harvested samples were cut into 2-mm-wide strips and incubated for 9 h under continuous light in a Petri dish containing GM. Total RNA was isolated at the times indicated after wounding and analyzed by RNA gel blotting (5 μg per lane) using the probes indicated. B, Two-week-old plants grown under continuous light were placed in the dark for 2 d and returned to the light condition. Total RNA was isolated from the leaves at the times indicated after the onset of the light period (0–9 h) and analyzed by RNA gel blotting (5 μg per lane) using the probes indicated.
DISCUSSION
A single form of P450 reductase is responsible for the electron transfer to a variety of different microsomal P450s in mammals and yeast (Yabusaki et al., 1988 Porter et al., 1990). In this paper we have described the isolation of two distinct cDNAs and the corresponding genomic clones encoding P450 reductase isoforms from A. thaliana. Genomic Southern-blot analysis under low-stringency conditions (Fig. 4B) demonstrated the existence of only two reductase genes in Arabidopsis, although the presence of additional reductase genes with very low sequence similarity cannot be ruled out. The occurrence of P450 reductase isoforms in higher plants has also been demonstrated from the isolation of two P450 reductase cDNAs from Arabidopsis (D. Pompon, unpublished data) and also from H. tuberosus (I. Benveniste, unpublished data). We have found by Southern-blot analysis under low-stringency conditions (data not shown) a few copies of the P450 reductase gene in P. aureus, whereas it has been reported that only a single-copy gene for P450 reductase has been detected under high-stringency hybridization conditions in V. radiata (Shet et al., 1993) and in C. roseus (Meijer et al., 1993). Benveniste et al. (1991) reported that two or three proteins were detected in the microsomes from all higher plants they tested by western-blot analysis using polyclonal antibodies prepared against the H. tuberosus reductase. We cannot rule out the possibility that some of these immunoreactive proteins might be posttranslationally modified proteins encoded by a single-copy gene. In fact, there are potential glycosylation sites in both AR1 protein (284-N) and AR2 protein (31-N and 358-N). Nonetheless, available results, as shown in Table I, suggest that the occurrence of the P450 reductase isoforms may be common in higher plants (Durst and Nelson, 1995).
We have also investigated the gene organization of AR1 and AR2 (Figs. 1 and 5). The highly conserved exon organization suggested that the two genes have evolved from a common ancestral gene. The most striking differences were observed in the region encoded by the first exon. Specifically, the N-terminal portion encompassing the signal anchor sequence of AR2 is significantly longer than that of AR1, and the amino acid sequences in this region are also very different between them. On the other hand, the sequence similarity was 68% between AR1 and AR2 proteins when calculated without the domain encoded by the first exon. Homology analysis of higher plant P450 reductases (Table I) indicated that plant P450 reductases may be divided into AR1 and AR2 types. For example, the P450 reductases from P. aureus and periwinkle were grouped into AR1 and AR2 types, respectively. It should be noted that the structural differences between these two types of P450 reductases were found primarily in the region encoded by the first exon.
Multiple forms of P450s exist in Arabidopsis, and it is thought that they are involved in the metabolism of diverse secondary compounds (M. Mizutani, unpublished results). On the other hand, only two P450 reductase genes are present in Arabidopsis. In other words, two P450 reductase isoforms, AR1 and AR2, are transporting electrons to all P450 isoforms. AR1 and AR2 showed the different expression patterns such as tissue specificity (Fig. 7) and responsiveness to the wounding treatment and the light condition (Fig. 8), implicating different mechanisms for transcription activation. Wounding and light induced the expression of AR2, as well as those of PAL1 and CYP73A5 (Fig. 8). This induction pattern is consistent with the finding that only the AR2 promoter contained the consensus sequence motifs for the putative cis-acting elements (boxes P, A, and L) (Fig. 6B) involved in the coordinated expression of PAL and 4CL genes (Logemann et al., 1995). Conversely, the AR1 promoter does not contain sequence motifs for boxes P and L (Fig. 6A), steady-state levels of mRNA are unchanging, and, therefore, the AR1 gene appears to confer constitutive expression. We previously presented evidence that CYP73A5 in Arabidopsis is regulated together with the other phenylpropanoid pathway genes (i.e. PAL1 and 4CL) via a mechanism containing these three cis-acting elements (Mizutani et al., 1997). It has been also reported that PAL1 (Ohl et al., 1990) and CYP73A5 (Bell-Lelong et al., 1997) were strongly expressed in the vascular tissues of roots and leaves of Arabidopsis. Both PAL1 and CYP73A5 contain putative cis-elements, which were also found in the AR2 promoter. Whereas it has not been determined whether these cis-elements are involved in tissue-specific expression, it is possible that AR2 expression may be tissue specific and coordinated with the expression of PAL1 and CYP73A5 in controlling carbon flux through the phenylpropanoid pathway. However, the different induction time courses of AR2, PAL1, and CYP73A5 suggest that additional mechanism(s) contribute to the control of the AR2 mRNA levels in Arabidopsis.
We have shown that not only CYP73A5 but also several other P450 genes were induced by either wounding or light (M. Mizutani, unpublished results), indicating that the total number of P450 molecules in plants fluctuates in response to changes of environmental conditions. It has been proposed that about 10 to 15 molecules of P450s interact with one P450 reductase molecule in the microsomal membrane (Masters and Okita, 1982) and that the P450 reductase level should also vary in keeping an appropriate P450/P450 reductase ratio. It is therefore possible that plant P450 reductases are inducibly expressed in a manner coordinated with inducible P450s. However, it remains unclear why both the inducible type and the constitutive type of P450 reductases with indistinguishable enzymatic properties co-exist specifically in higher plants.
It is possible that the constitutive type (AR1) may be necessary to keep a basal P450 reductase level and that the expression of the inducible type (AR2) may synchronize with the fluctuation of the total P450 level, which should change dynamically in response to environmental conditions. The Km values for NADPH and Cyt c were very similar between the two P450 reductase isoforms (Table II), which was able to couple with CYP73A5 protein at essentially the same efficiency in the reconstitution system (Table III). However, it is not necessarily true that AR1 and AR2 proteins have similar affinity to individual P450s. Specifically, the different structures of the N-terminal portions between AR1 and AR2 (Fig. 1) may confer distinct physiological roles for each of the P450 reductase isoforms. Preparation of specific antibodies and promoter analysis of AR1 and AR2 could constitute interesting approaches for further investigation of physiological roles of the two reductases. Also, the inter- and intracellular localization of AR1 and AR2 should be studied together with those of various P450s in vivo. The production of transgenic plants, specifically loss-of-function mutants of AR1 and AR2, should also help to elucidate the relationship between the reductases and certain P450-dependent reactions.
Table III.
The reconstituted CYP73A5 activity
| Assay Condition | Cinnamate 4-Hydroxylase Activitya |
|---|---|
| nmol min−1 nmol−1 P450 | |
| Completeb | |
| AR1 | 63.6 ± 4.3 |
| AR2 | 70.2 ± 5.6 |
| Without NADPH | n.d.c |
| Without P450 reductase | n.d. |
| Without CYP73A5 | n.d. |
Reactions were carried out in the complete reaction mixture at 30°C and were initiated by addition of NADPH. Values are means ± sd of three separate determinations.
Complete reaction mixture contained 50 mm potassium phosphate (pH 7.25), 5 nm recombinant CYP73A5, 0.1 unit/mL recombinant P450 reductase (AR1 or AR2), 0.01% (w/v) sodium cholate, 10 μg/mL dilauroylphosphatidylcholine, 0.1 mm NADPH, and 0.4 mm trans-cinnamic acid.
n.d., Not detected.
ACKNOWLEDGMENTS
We wish to express our sincere gratitude to the late emeritus professor Ryo Sato for his invaluable advice and continuous encouragement. We also gratefully acknowledge Nobuko Uodome for care of the plants and Emi Ikui for DNA sequencing.
Abbreviations:
- GM
germination medium
- P450
Cyt P450
- P450 reductase
NADPH-Cyt P450 reductase
- PAL
Phe ammonia-lyase
LITERATURE CITED
- Ausubel FM, Brent R, Kingston RE, Moore DD, Siedman JG, Smith JA, Struhl K (1987) Current Protocols in Molecular Biology. John Wiley, New York
- Bell-Lelong DA, Cusumano JC, Meyer K, Chapple C. Cinnamate 4-hydroxylase expression in Arabidopsis. Regulation in response to development and the environment. Plant Physiol. 1997;113:729–738. doi: 10.1104/pp.113.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste I, Lesot A, Hasenfratz MP, Durst F. Immunocharacterization of NADPH-cytochrome P450 reductase from Jerusalem artichoke and other higher plants. Biochem J. 1989;259:847–853. doi: 10.1042/bj2590847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste I, Lesot A, Hasenfratz MP, Kochs G, Durst F. Multiple forms of NADPH-cytochrome P450 reductase in higher plants. Biochem Biophys Res Commun. 1991;177:105–112. doi: 10.1016/0006-291x(91)91954-b. [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Bozak K, Zimmerlin A. Plant cytochrome P450. Phytochemistry. 1994;37:1491–1506. doi: 10.1016/s0031-9422(00)89567-9. [DOI] [PubMed] [Google Scholar]
- Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson RP, Luster DG. Multiple forms of plant cytochromes P450. Plant Physiol. 1991;96:669–674. doi: 10.1104/pp.96.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durst N, Nelson DR. Diversity and evolution of plant P450 and P450-reductases. Drug Metab Drug Interact. 1995;12:189–206. doi: 10.1515/dmdi.1995.12.3-4.189. [DOI] [PubMed] [Google Scholar]
- Grossberger D. Minipreps of DNA from bacteriophage lambda. Nucleic Acids Res. 1987;15:6737. doi: 10.1093/nar/15.16.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley BA, Schuler MA. Plant intron sequences: evidence for distinct groups of introns. Nucleic Acids Res. 1988;16:7159–7174. doi: 10.1093/nar/16.14.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y. The use of 8-aminooctyl Sepharose for the separation of some components of hepatic microsomal electron transfer system. J Biochem. 1976;80:267–276. doi: 10.1093/oxfordjournals.jbchem.a131273. [DOI] [PubMed] [Google Scholar]
- Iyanagi T, Mason HS. Some properties of hepatic reduced nicotinamide adenine dinucleotide phosphate-cytochrome c reductase. Biochemistry. 1973;12:2297–2308. doi: 10.1021/bi00736a018. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrimini LM, Burkhart W, Moyer M, Rothstein S. Molecular cloning of complementary DNA encoding the lignin-forming peroxidase from tobacco: molecular analysis and tissue-specific expression. Proc Natl Acad Sci USA. 1987;84:7542–7546. doi: 10.1073/pnas.84.21.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann E, Parniske M, Hahlbrock K. Modes of expression and common structural features of the complete phenylalanine ammonia-lyase gene family in parsley. Proc Natl Acad Sci USA. 1995;92:5905–5909. doi: 10.1073/pnas.92.13.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters BSS, Okita RT. The history, properties and function of NADPH-cytochrome P450 reductase. In: Schenkman JB, Kupfer D, editors. Hepatic Cytochrome P450 Monooxygenase System. Oxford, UK: Pergamon Press; 1982. pp. 342–360. [Google Scholar]
- Meijer AH, Cardoso MIL, Voskuilen JT, de Waal A, Verpoorte R, Hoge JHC. Isolation and characterization of a cDNA clone from Catharanthus roseus encoding NADPH-cytochrome P450 reductase, an enzyme essential for reactions catalyzed by cytochrome P450 monooxygenases in plants. Plant J. 1993;4:47–60. doi: 10.1046/j.1365-313x.1993.04010047.x. [DOI] [PubMed] [Google Scholar]
- Mizutani M, Ohta D, Sato R. Purification and characterization of a cytochrome P450 (trans-cinnamic acid 4-hydrox- ylase) from etiolated mung bean seedlings. Plant Cell Physiol. 1993a;34:481–488. [Google Scholar]
- Mizutani M, Ohta D, Sato R. Isolation of a cDNA and a genomic clone encoding cinnamate 4-hydroxylase from Arabidopsis and its expression manner in planta. Plant Physiol. 1997;113:755–763. doi: 10.1104/pp.113.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani M, Ward E, DiMaio J, Ohta D, Ryals J, Sato R. Molecular cloning and sequencing of a cDNA encoding mung bean cytochrome P450 (P450C4H) possessing cinnamate 4-hydroxylase activity. Biochem Biophys Res Commun. 1993b;190:875–880. doi: 10.1006/bbrc.1993.1130. [DOI] [PubMed] [Google Scholar]
- Nairn CJ, Winsett L, Ferl RJ. Nucleotide sequence of an actin gene from Arabidopsis thaliana. Gene. 1988;65:247–257. doi: 10.1016/0378-1119(88)90461-1. [DOI] [PubMed] [Google Scholar]
- Negruk V, Yang P, Subramanian M, McNevin JP, Lemieux B. Molecular cloning and characterization of the CER2 gene of Arabidopsis thaliana. Plant J. 1996;9:137–145. doi: 10.1046/j.1365-313x.1996.09020137.x. [DOI] [PubMed] [Google Scholar]
- Nelson DR, Koymans L, Kamataki T, Stegeman JJ, Feyereisen R, Waxman DJ, Waterman MR, Gotoh O, Coon MJ, Estabrook RW and others. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics. 1996;6:1–42. doi: 10.1097/00008571-199602000-00002. [DOI] [PubMed] [Google Scholar]
- Nisimoto Y. Localization of cytochrome c-binding domain on NADPH-cytochrome P450 reductase. J Biol Chem. 1986;261:14232–14239. [PubMed] [Google Scholar]
- Ohl S, Hedrich S, Chory J, Lamb CJ. Functional properties of phenylalanine ammonia-lyase promoter from Arabidopsis. Plant Cell. 1990;2:837–848. doi: 10.1105/tpc.2.9.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary KA, Beck TW, Kasper CB. NADPH-cytochrome P450 oxidoreductase gene: identification and characterization of the promoter region. Arch Biochem Biophys. 1994;310:452–459. doi: 10.1006/abbi.1994.1192. [DOI] [PubMed] [Google Scholar]
- Omura T, Sato R. The carbon monooxide binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- Porter TD. An unusual yet strongly conserved flavoprotein reductase in bacteria and mammals. Trends Biochem Sci. 1991;16:154–158. doi: 10.1016/0968-0004(91)90059-5. [DOI] [PubMed] [Google Scholar]
- Porter TD, Kasper CB. NADPH-cytochrome P450 oxidoreductase: flavin mononucleotide and flavin adenine dinucleotide domains evolved from different flavoproteins. Biochemistry. 1986;25:1682–1687. doi: 10.1021/bi00355a036. [DOI] [PubMed] [Google Scholar]
- Porter TD, Thomas WB, Kasper CB. NADPH-cytochrome P450 oxidoreductase gene organization correlates with structural domains of the protein. Biochemistry. 1990;29:9814–9818. doi: 10.1021/bi00494a009. [DOI] [PubMed] [Google Scholar]
- Ruettinger RT, Wen LP, Fulco AJ. J Biol Chem. 1989;264:10987–10995. [PubMed] [Google Scholar]
- Shet MS, Sathasivan K, Arlotto MA, Mehdy MC, Estabrook RW. Purification, characterization, and cDNA cloning of an NADPH-cytochrome P450 reductase from mung bean. Proc Natl Acad Sci USA. 1993;90:2890–2894. doi: 10.1073/pnas.90.7.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers MD, Smith GE (1987) A Manual of Methods for Baculovirus Vectors and Insect Cell Culture Procedures, bulletin no. 1555. Texas Agricultural Experiment Station and Texas A & M University, College Station
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvekens D, Montagu MV, Lusebettens MV. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci USA. 1988;85:5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabusaki Y, Murakami H, Ohkawa H. Primary structure of Saccharomyces cerevisiae NADPH-cytochrome P450 reductase deduced from nucleotide sequence of its cloned gene. J Biochem. 1988;103:1004–1010. doi: 10.1093/oxfordjournals.jbchem.a122370. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Johnson WW, Ueng YF, Shimada T, Guengerich FP. Lack of electron transfer from cytochrome b5 in stimulation of catalytic activities of cytochrome P4503A4. Characterization of a reconstituted cytochrome P450 3A4/NADPH cytochrome P450 reductase system and studies with apo-cytochrome b5. J Biol Chem. 1996;271:27438–27444. doi: 10.1074/jbc.271.44.27438. [DOI] [PubMed] [Google Scholar]