Abstract
Hemopexin (Hpx) is a liver-generated acute phase reactant that binds and neutralizes prooxidant free heme. This study tested whether acute kidney injury (AKI) triggers renal Hpx accumulation, potentially impacting heme Fe-mediated tubular injury. Mice were subjected to glycerol, cisplatin, ischemia-reperfusion (I/R), or endotoxemic [lipopolysaccharide (LPS)] AKI. In each instance, 3- to 30-fold renal cortical and isolated proximal tubule segment (PTS) Hpx increases resulted. Although renal cortex and PTS showed variable Hpx mRNA increases, due, in part, to increased mRNA stability, mRNA levels did not correlate with renal Hpx protein accumulation. Conversely, AKI evoked three- to fourfold increases in hepatic Hpx gene induction, which corresponded with three- to fourfold plasma Hpx increases. Renal immunohistochemistry, and increased urinary Hpx excretion, indicated that circulating Hpx gains tubule luminal/urinary access, followed by proximal tubule endocytic uptake. Paradoxically, in cultured renal cells (HK-2, HEK-293), Fe depletion, and not free heme excess, increased Hpx mRNA. LPS acutely increased HK-2 cell Hpx mRNA. This finding, coupled with observations that LPS evoked ∼30-fold greater renal Hpx mRNA increases than any other AKI model, suggests that inflammation, not heme exposure, activates the renal Hpx gene. Each form of AKI evoked early increases in circulating free heme, which subsequently fell to subnormal levels as plasma Hpx rose. In addition, purified Hpx blunted free Fe-mediated HK-2 cell death. In sum, these data indicated that AKI-associated hepatic stress generates Hpx, which gains renal tubule access. Given its ability to bind free heme and mitigate free Fe toxicity, Hpx loading can potentially confer cytoprotective effects.
Keywords: ischemia-reperfusion, endotoxemia, rhabdomyolysis, cisplatin
acute kidney injury (AKI) upregulates a variety of stress proteins that can confer a variety of cytoprotective effects. The heat shock family of proteins, e.g., heat shock protein (HSP)-70 and heme oxygenase 1 (HO-1; i.e., HSP-32), perhaps best exemplify this acute renal “stress response” (17, 18, 20, 29, 31). In a recent series of studies, we made the surprising observation that three proteins, i.e., albumin (30), alpha-fetoprotein (30), and haptoglobin (32), whose synthesis is classically ascribed only to liver, can also be acutely upregulated in renal cortex during ischemic or toxic renal damage. These findings led us to coin the term “renal hepatization” (30, 32), i.e., whereby genes that are normally suppressed in kidney are acutely “unmasked” and, hence, are expressed in the setting of AKI. In addition to their serving as potential AKI “biomarkers” due to their increased urinary excretion, these three proteins can potentially alter evolving renal damage. For example: 1) albumin can bind, and thus mitigate, the adverse effects of cytotoxic free fatty acids that are generated during AKI (6, 9); 2) haptoglobin can exert a variety of antioxidant actions (32); and 3) alpha-fetoprotein can exert immunomodulatory effects (8).
Hemopexin (Hpx) is another serum protein that is generally viewed as being exclusively synthesized in liver and inducible by systemic or hepatic stress (3, 5, 14, 25). Its classic role is as a scavenger of circulating free heme. Thus, during states of free heme release into the systemic circulation, most notably intravascular hemolysis, it binds free heme, or strips it from its weak albumin binding site, and then returns it to the liver where it is taken up via a heme-Hpx-specific receptor (2, 25). This has two well-recognized beneficial consequences: first, it salvages heme Fe by returning it to the liver, rather than its being lost due to urinary excretion. Second, as a consequence of heme binding, Hpx decreases heme Fe driven oxidative stress (3, 5, 14, 25). This latter property can mitigate tissue injury, e.g., during sepsis (10) or hemolytic states (26). It has recently become apparent that Hpx also possesses biologic activities that are independent of heme binding. For example, due to an intrinsic protease activity, Hpx can induce nephrin-dependent reorganization of the podocyte cytoskeleton, leading to proteinuria (11). In addition, recent evidence suggests that Hpx can exert potent anti-inflammatory effects (7, 12, 13, 23). As just one example, when macrophages are exposed to endotoxin in the presence of Hpx, decreased proinflammatory cytokine generation results (12).
Given Hpx's potential pleomorphic effects, we questioned whether the Hpx gene, normally silent in kidney, might be upregulated in response to AKI. If so, it would represent an additional example of the so called “renal hepatization” response. To gain insights into this issue, changes in renal Hpx expression were assessed in four different AKI models. To obtain proximal tubule specific results and mechanistic insights, in vitro correlates were sought using isolated mouse proximal tubule segments and cell culture (HEK 293; HK-2) experiments. The results of these experiments form the basis of this study.
METHODS
Animal Studies
All AKI models were conducted using male CD-1 mice (30–45 g; Charles River Laboratories, Wilmington, MA). They were housed under standard vivarium conditions with free food and water access. All protocols were approved by the Fred Hutchinson Cancer Research Center. Surgeries were performed while the animals were under deep pentobarbital anesthesia (40–50 mg/kg, administered by ip injection).
Ischemic AKI
Twelve mice were subjected to a midline laparotomy, and then either 30 min of bilateral (n = 6 mice) or left unilateral (n = 6) ischemic injury was imposed by application of microvascular clamp(s) to the renal pedicle(s). The clamps were then removed, uniform reperfusion was confirmed by the loss of kidney cyanosis, and the abdominal incisions were closed with sutures in two layers. Body temperature was maintained at 37°C throughout the ischemic period and until recovery from anesthesia with the use of an external heating source. Approximately 24 h postsurgery, the mice were reanesthetized, the abdominal incisions were reopened, a blood sample was obtained from the inferior vena cava, and then the postischemic kidneys, as well as the contralateral kidneys from the unilateral ischemia experiment, were resected. The kidneys were iced and thin cortical sections were cut with a razor blade. The samples were extracted for total protein and RNA (RNeasy; Qiagen). Plasma samples were used to determine blood urea nitrogen (BUN) concentrations as an index of renal injury severity. Plasma Hpx concentrations were determined by ELISA (Alpha Diagnostic International; no. 600–700-Hpx). The cortical RNA and protein extracts were assayed for Hpx mRNA and Hpx protein levels, respectively. Hpx mRNA was assayed by RT-PCR using the primers presented in Table 1. Results were expressed as a ratio to simultaneously measured GAPDH product. The above results were compared with those obtained from two sets of controls: 1) five mice subjected to the same surgical protocol described above but without inducing renal ischemia; and 2) six normal mice that had not been subjected to any prior surgical intervention. By comparing results from these two sets of controls, the impact of surgical stress, per se, independent of renal ischemia, could be assessed.
Table 1.
Primers used to detect mouse and human Hpx mRNA
| mRNA | Primer Sequences | Product Size |
|---|---|---|
| Human primers for RT-PCR | ||
| Hpx | 5′-TCTGCACTGACGTCTGACAACCAT-3′ | 307 bp |
| 5′-AGATAAAGGCCGCATCCACAGAGT-3′ | ||
| GAPDH | 5′-AAGGTCGGAGTCAACGGATTTGGT-3′ | 534 bp |
| 5′-AGTGATGGCATGGACTGTGGTCAT-3′ | ||
| Mouse primers for RT-PCR | ||
| Hpx | 5′-ACACTGCTTGGATACCTGGAGCTT-3′ | 854 bp |
| 5′-TGCCTCCCTTTGTCAGGAAGACAT-3′ | ||
| GAPDH | 5′-CTGCCATTTGCAGTGGCAAAGTGG-3′ | 437 bp |
| 5′-TTGTCATGGATGACCTTGGCCAGG-3′ |
Primer pairs that were employed to detect mouse hemopexin (Hpx) mRNA in renal cortical samples and human Hpx mRNA in RNA extracts from HEK 293 and HK-2 cells are presented. mRNAs were assessed by competitive RT-PCR, with the results being factored by simultaneously obtained GAPDH product.
Six mice were lightly anesthetized with isoflurane and then they were injected with 10 ml/kg of 50% glycerol, administered intramuscularly in equally divided dosages into each hindlimb. Six normal mice served as controls. Approximately 24 h postglycerol injection, the mice were deeply anesthetized, and then blood and renal cortical tissue samples were obtained and assayed for BUN, Hpx protein, and Hpx mRNA levels, as noted above.
Cisplatin Nephrotoxicity
Twelve mice received an intraperitoneal injection of cisplatin (30 mg/kg, in 1 ml of saline). As controls, 12 mice received an equal amount of saline vehicle. Either 1 or 3 days postcisplatin injection, six mice per group were anesthetized, and terminal plasma and renal cortical samples were obtained through an abdominal incision. The samples were assayed for Hpx, Hpx mRNA, and BUN levels, as noted above.
Endotoxin-Induced AKI
Ten mice were injected with 2 mg/kg of Escherichia coli lipopolysaccharide (LPS; 0111:B4; L-2630; Sigma Chemicals, St. Louis, MO; stock solution, 4 mg/ml saline). Either 4 or 24 h postinjection (n = 5 and 5 mice, respectively), the mice were anesthetized and terminal blood and renal cortical samples were obtained for Hpx protein/mRNA and BUN analyses, as above. Samples obtained from six normal mice that had received the LPS vehicle (saline) served as controls.
Assessment of Hepatic Hpx mRNA Levels Following Induction of AKI
To assess hepatic responses to the induction of AKI, hepatic RNA samples were obtained 24 h postglycerol injection, 72 h postcisplatin injection, 24 h postbilateral ischemia, or 24 h postsham surgery as used for the bilateral ischemia experiment (n = 5 each). Control hepatic RNA samples were obtained from five normal mice. All samples were then assayed for Hpx mRNA.
To determine whether AKI might be associated with Hpx gene induction outside of liver and kidney, Hpx mRNA was measured in two additional organs (lung and spleen). To this end, lung and spleen mRNA samples were extracted from control mice, 24 h postglycerol mice, bilateral ischemia-reperfusion (I/R) mice, and sham surgical controls (n = 3 each) and assayed for Hpx mRNA. The values were contrasted to those observed in simultaneously obtained liver and kidney RNA samples.
Assessment of Hepatic vs. Renal Hpx mRNA Responses to Intravenous Fe Sucrose Injection
To further assess hepatic vs. renal responsiveness to Fe-mediated oxidative stress, four mice were injected with 2 mg of Fe sucrose (Venofer; Reagents Pharmaceuticals). Eighteen hours later, hepatic and renal cortical Hpx mRNA levels were assessed and contrasted with those seen in three normal mice.
Assessment of Urinary Hpx Levels
To ascertain whether the AKI-induced increases in plasma and renal cortical Hpx levels were associated with increased urinary Hpx excretion, urine samples were obtained from four mice at either 4 or 24 h postglycerol injection, four mice at 24 h post-LPS injection, and four mice at 2 days postcisplatin injection. They were assayed for Hpx by ELISA, and the results were expressed as either absolute concentrations (μg/ml) or as urinary Hpx-to-creatinine ratios. Urine samples from five normal mice established normal Hpx levels.
Hpx Immunohistochemistry
Four-micrometer frontal sections of formalin fixed kidneys were harvested from normal mice, 72 h postcisplatin mice, and 24 h postrenal I/R mice (n = 2 each). They were subjected to immunohistochemical detection of Hpx using a previously described technique (32). Hpx was detected with a rabbit anti-Hpx antibody (Abcam no. AB90947) at a 1:100 dilution (2 μg/ml), applied for 60 min. The antibody was detected with biotinylated sheep anti-rabbit IgG (Jackson ImmunoResearch), followed by application of streptavidin horseradish peroxidase. Staining was visualized with 3,3′-diaminobenzidine, and the sections were counterstained with hematoxylin. Concentration matched isotype control slides were run for each tissue sample (Jackson ImmunoResearch Laboratories).
Assessments of Hpx Protein and mRNA Levels in Isolated Proximal Tubules
The following experiment assessed whether whole renal cortical changes in Hpx mRNA and protein levels reflected, at least in part, changes within proximal tubules. Mice were injected with either LPS or cisplatin, as noted above. Either 24 h (LPS-injected mice; n = 5) or 48 h later (cisplatin-injected mice; n = 4), proximal tubules were isolated as previously described (16). The tubules were then extracted for both RNA and total protein and assayed for both Hpx mRNA and Hpx protein levels, as noted above.
Assessment of Hpx mRNA Stability
The above LPS experiment was repeated, but following tubule isolation, the tubules were incubated in the presence or absence of actinomycin D (2 μg/ml) to block de novo RNA synthesis, as previously described (16). By so doing, changes in Hpx mRNA levels reflect Hpx degradation rates. At the end of 90-min incubations, Hpx mRNA degradation was assessed by comparing the amount of Hpx mRNA in the samples incubated with and without actinomycin D and compared with baseline Hpx mRNA levels. For comparison, changes in a second mRNA, TNF-α, were assessed over the course of the same experiments.
Response of Cultured Cells to Fe-Mediated Oxidative Stress, Fe Depletion, and Endotoxin Exposure
Two cell lines were used for these experiments: human embryonic kidney (HEK 293) cells and the human proximal tubule cell line HK-2 (21). The HEK 293 cells were obtained from ATCC and were maintained in 10% FCS. HK-2 cells were grown in keratinocyte serum free medium supplemented with pituitary extract, as previously described (21). All cells were passaged in T75 flasks. For experimentation, the HEK 293 and HK-2 cells, recovered by trypsinization, were seeded into 6- or 12-well Costar plates, respectively, to assess mRNA responses. To determine Hpx or HO-1 protein responses, the cells were maintained in T75 flasks to permit sufficient protein recovery to permit detection by ELISA (Hpx: Abcam no. AB108860; HO-1: Enzo no. ADI-960–800). All experiments were performed in quadruplicate. In each of the following experiments, the test agents were added in sublethal dosages, as determined by a lack of lactate dehydrogenase release.
Responses to hemin.
Approximately 8 h following trypsinization and seeding into experimentation plates, the HEK 293 cells were challenged with either 0, 25, 50, or 100 μM hemin (solubilized in NH4OH, final concentration of 0.02%). In the case of HK-2 cells, a 0 or 2 μM hemin concentration was used. [Note: because the HK-2 cells were cultured in serum free medium (i.e., no Hpx), there was greater hemin sensitivity, and hence a lower hemin concentration was employed.] Eighteen 18 h later, RNA and protein were extracted and Hpx mRNA and protein levels were determined by RT-PCR and ELISA, respectively. The employed human Hpx primers are presented in Table 1. All PCR data were factored by GAPDH product. To confirm that the HEK 293 and HK-2 cells were able to respond to hemin, HO-1 mRNA (31) and protein levels (31) were also determined.
Fe sucrose challenge.
As a second Fe challenge, HK-2 cells were incubated overnight with 0 or 25 μg/ml of Fe sucrose (Venofer; American Regent Pharmaceuticals). Eighteen hours later, Hpx and HO-1 mRNA levels were assessed (n = 4 each).
To determine whether a longer Fe challenge might induce Hpx mRNA, HK-2 cells were incubated for 72 h with or without 25 μg/ml of Fe sucrose (n = 3 determinations each). Hpx mRNA levels were assessed at the completing of these prolonged incubations.
Fe depletion experiments.
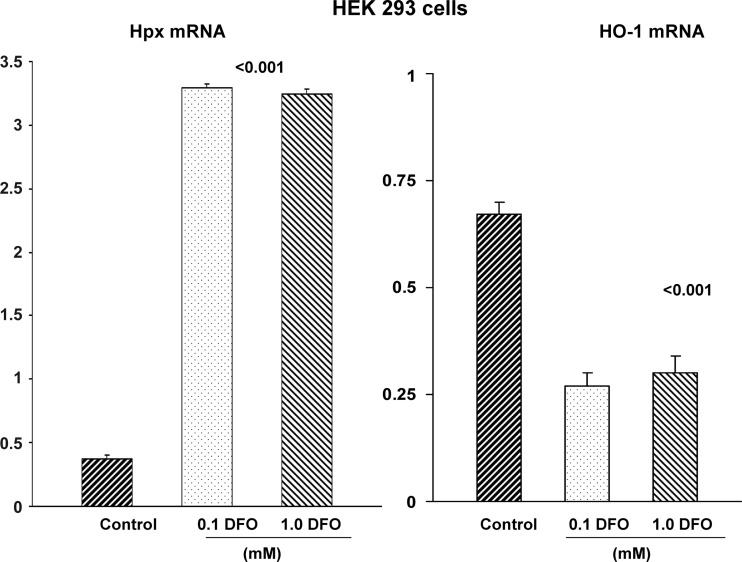
Given that Hpx is judged to have a role in maintaining systemic Fe balance (2, 3, 25), we assessed whether cells would respond to Fe depletion with an increase in Hpx mRNA. To this end, HEK 293 cells and HK-2 cells were incubated × 18 h with the Fe chelating agent deferoxamine (DFO) in doses of 0, 0.1, or 1 mM. After 18 h, Hpx and HO-1 mRNA levels were assessed by RT-PCR, as noted above.
LPS responsiveness.
To assess the impact of LPS on HK-2 cell Hpx expression, cells were incubated for 3 h without or with LPS (10 μg/ml). Hpx mRNA levels were then assessed.
Effect of purified Hpx on Fe-mediated injury.
Hpx is known to block heme-mediated toxicity via direct binding. However, we sought to test whether it might also possess antioxidant actions independent of heme binding effects. To this end, HK-2 cells were incubated with a nonheme Fe challenge (5 μM FeNH4SO4, complexed with equimolar hydroxyquinoline, “FeHQ,” to permit intracellular access) in the presence and absence 5 μM human Hpx (purified from plasma; Athens Research; cat no. 1616-086513). After an 18-h incubation, Fe-mediated cell injury was assessed by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (21). Concomitant control incubations in the presence or absence of Hpx assessed potential independent Hpx effects.
Free Heme Generation During AKI
Finally, we tested whether the four employed AKI models might release free heme into the systemic circulation, from where it could, via its potent prooxidant effects, potentially impact evolving renal and extrarenal tissue damage. If so, a concomitant Hpx increase might counteract this rising free heme burden by free heme binding. To test these hypotheses, plasma samples were collected at 4 and 24 h postbilateral I/R, 4 and 24 h postglycerol injection, 24 and 72 h postcisplatin injection, and 24 h post-LPS administration (n = 4–6 each). The samples were then assayed for free heme with a commercially available kit (BioVision, Milpitas, CA; no. 672-100).
Calculations and Statistics
All results are presented as means ± SE. Statistical comparisons were made by unpaired Student's t-test. If multiple comparisons were made, the Bonferroni correction was applied.
RESULTS
Renal Cortical Hpx Levels
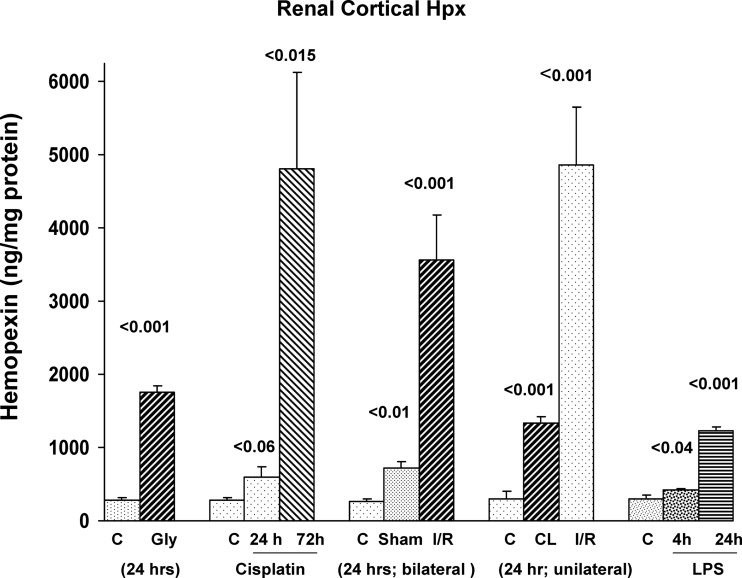
As depicted in Fig. 1, each of four experimental AKI models induced significant increases in renal cortical Hpx concentrations. However, the degree of increase varied considerably among the experimental groups. For example, whereas glycerol and LPS injection caused ∼3- to 4-fold Hpx increases, ∼10- to 30-fold increases were seen with cisplatin and I/R induced AKI. In the case of I/R, both unilateral and bilateral I/R models were studied, and comparable Hpx increases were observed. Of interest, the kidneys from sham surgical controls, used for the bilateral ischemic injury experiment, and the uninjured (contralateral) kidney from the unilateral I/R experiment, both had two- to fourfold increases in renal cortical Hpx concentrations vs. the values observed in the normal control mice. The degrees of renal insufficiency, as denoted by BUN concentrations, were as follows: glycerol-induced AKI, 114 ± 3 mg/dl; cisplatin AKI, 46 ± 10 and 135 ± 6 mg/dl (at 24 and 72 h, respectively); LPS, 58 ± 6 mg/dl (at 24 h); bilateral I/R, 135 ± 3 mg/dl; and unilateral I/R 25 ± 1 mg/dl. BUNs for control mice were 23 ± 3 mg/dl.
Fig. 1.
Renal cortical hemopexin (Hpx) protein levels rise following acute kidney injury (AKI). Renal cortical Hpx levels were measured following induction of glycerol AKI (gly; 24 h); cisplatin AKI (at 24 and 72 h); unilateral or bilateral ischemia-reperfusion (I/R)-induced AKI (24 h); or 4 or 24 h postendotoxin [lipopolysaccharide (LPS)] injection. Marked, but highly variable, renal cortical Hpx increases were observed compared with values seen in normal controls (C). Stepwise Hpx increases followed cisplatin and LPS injection. Notably, both sham surgery (used for the bilateral I/R experiment) and the contralateral (CL) kidneys from the unilateral ischemia experiment, were associated with elevated renal cortical Hpx levels vs. values observed in normal control mice.
Renal Cortical Hpx mRNA Levels
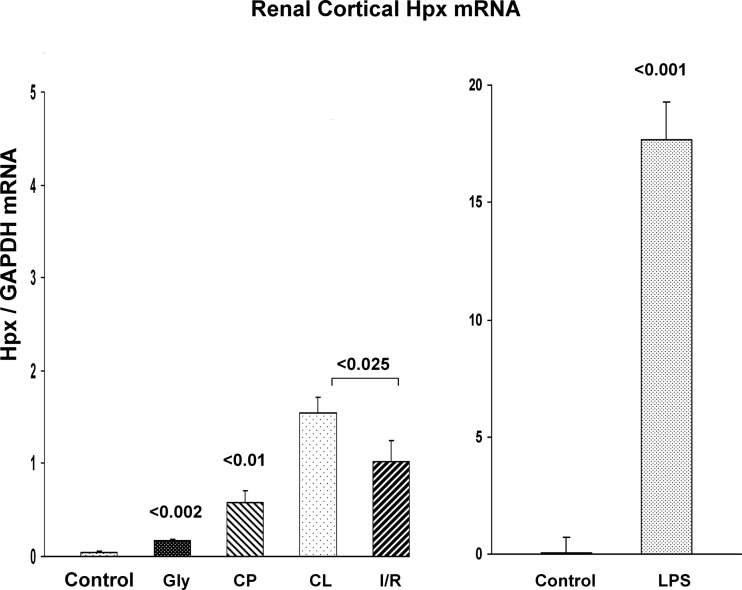
There was an apparent dissociation between the above noted renal cortical Hpx protein concentrations and the corresponding levels of Hpx mRNA. For example, the cisplatin and I/R models produced the most dramatic (∼30×) Hpx protein increases, and yet, there were relatively small increases in corresponding Hpx mRNA. Indeed, in the case of unilateral I/R, the contralateral (CL) kidneys manifested statistically greater increases in Hpx mRNA, compared with their postischemic counterparts. Conversely, LPS induced massive Hpx mRNA increases, reaching levels that were 10- to 30-fold higher than seen in any of the other AKI models (note the different scales in Fig. 2, left and right), and yet, the LPS model induced the smallest increase in renal cortical Hpx protein levels. Finally, although Hpx's major biologic role is thought to be as a free heme scavenger (suggesting the possibility that free heme would induce Hpx), the glycerol (heme-mediated) AKI model induced the smallest Hpx mRNA increase amongst the 4 AKI models. This implies that heme exposure, per se, is either a weak, or even not, an inducer of the Hpx gene (as also indicated by the cell culture experiments; to be described below).
Fig. 2.
Renal cortical Hpx mRNA levels following induction of AKI. Both glycerol and cisplatin (CP) caused significant increases in Hpx mRNA values within renal cortex. In the case of unilateral I/R injury, Hpx mRNA elevations were also observed. However, the values in the unilateral postischemic kidneys were actually lower than those seen in the CL uninjured kidneys. This suggests that the Hpx mRNA elevations following I/R injury were in response to systemic surgical stress, and not I/R injury, per se. In contrast to the relatively modest Hpx mRNA increases following glycerol, cisplatin, or I/R injury, LPS injection evoked massive Hpx mRNA increases, “dwarfing” those seen with the other models. Note the different scales at left vs. right.
Plasma Hpx Concentrations
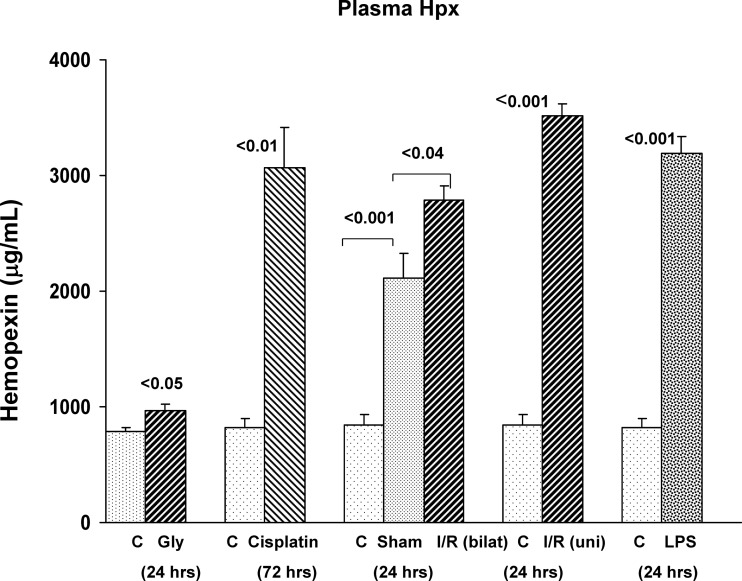
The cisplatin, I/R, and LPS models of AKI each evoked approximately three- to fourfold increases in plasma Hpx levels. In contrast, the glycerol AKI model caused at most, a trivial plasma Hpx protein increase, consistent with the relatively small increases in renal Hpx protein/mRNA levels, as noted above (Fig. 3). Of further note, the degrees of plasma Hpx elevations observed in the nonglycerol AKI models did not correlate with the levels of renal Hpx mRNA. For example, although LPS induced a ∼30-fold greater increase in renal cortical Hpx mRNA vs. the cisplatin and I/R models, all three of these models had comparable plasma Hpx levels. This implies that renal synthesis was not primarily responsible for the elevated plasma Hpx levels.
Fig. 3.
Plasma Hpx levels rise in response to AKI. CP, bilateral (bilat) I/R, unilateral (uni) I/R, and LPS injection each caused dramatic increases in plasma Hpx concentrations. Of note, sham surgery, used for the bilateral I/R experiment, also caused marked plasma Hpx elevations, compared with those seen in normal control mice. Only trivial, albeit statistically significant, plasma Hpx increases were observed following glycerol injection.
Hepatic Hpx mRNA Levels
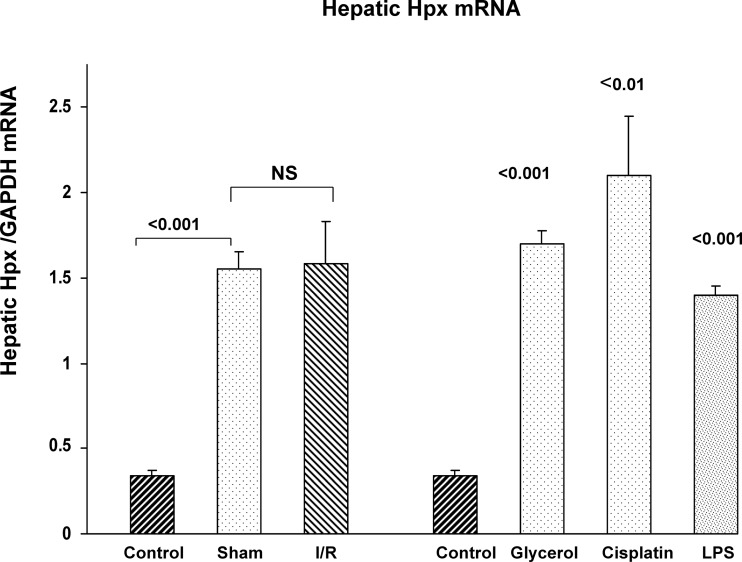
To gauge whether the liver was the likely origin of the plasma Hpx elevations, hepatic Hpx mRNA values were assessed. As shown in Fig. 4, the bilateral I/R protocol induced a dramatic increase in hepatic mRNA levels. However, this was due to the surgery, not the induction of AKI, given that sham surgery produced the same hepatic Hpx mRNA increase. The liver also mounted a marked Hpx mRNA response to each of the three nephrotoxic AKI models (glycerol, LPS, and cisplatin). Thus diverse forms of systemic stress induced hepatic Hpx mRNA expression. Of note, as shown in Table 2, unlike liver, the lung and spleen showed comparatively small changes in Hpx mRNA in test AKI models (values presented as ratios to values that were simultaneously obtained in organs from normal mice). This implies the dominance of the hepatic-Hpx mRNA response.
Fig. 4.
Hepatic Hpx mRNA levels rise in the setting of AKI. Each form of AKI evoked three- to fourfold increases in hepatic Hpx mRNA. Notably, sham surgery induced an almost identical increase in hepatic Hpx mRNA compared with bilateral I/R AKI. Thus surgical “stress,” and not the presence of I/R injury, was responsible for the hepatic Hpx mRNA elevations.
Table 2.
Ratios of liver, kidney, lung, and spleen Hpx mRNA levels in AKI, compared with control tissues (AKI values divided by control values)
| Liver | Kidney | Lung | Spleen | |
|---|---|---|---|---|
| Ischemia/reperfusion | 11.3 | 1.12 | 1.40 | 0 |
| Sham surgery | 8.0 | 1.4 | 0 | 0 |
| Glycerol | 6.17 | 1.25 | 0 | 1.12 |
Hpx mRNA levels were measured in liver, kidney, lung, and spleen 24 h postinduction of ischemic acute kidney injury (AKI), sham surgery, or glycerol injection and the values were contrasted to those observed in normal tissues Results are presented as ratios to the simultaneously observed control tissue values (e.g., postglycerol lung Hpx mRNA divided by normal lung Hpx mRNA). Compared with liver, the other tissues mounted a relatively trivial Hpx mRNA response.
Assessment of Hepatic vs. Renal Hpx mRNA Responses to Intravenous Fe Sucrose Injection
To further demonstrate relative hepatic vs. renal Hpx mRNA sensitivity to Fe-mediated oxidative stress, hepatic vs. renal cortical Hpx mRNA levels were assessed 18 h following intravenous Fe sucrose injection. Intravenous Fe sucrose raised hepatic Hpx mRNA from 0.51 ± 0.03 to 1.80 ± 0.27 (P = 0.01). In contrast, no significant change in renal cortical Hpx mRNA levels was observed (controls, 0.17 ± 0.02; Fe sucrose, 0.19 ± 0.03).
Urinary Hpx Protein Levels
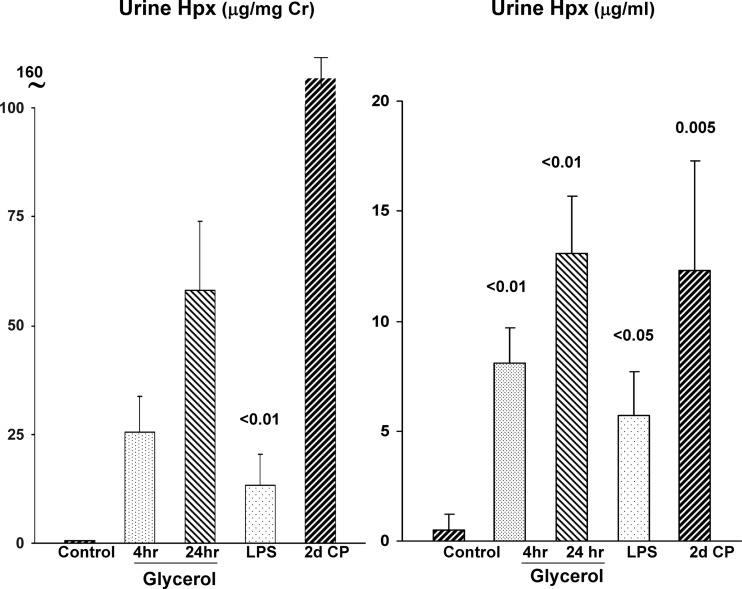
Given the dramatic plasma Hpx elevations that were documented in the setting of AKI, and given that Hpx can exert a proteinuric effect (11), we questioned whether it undergoes glomerular filtration, and hence, gains renal luminal/tubular cell access. Indeed, as depicted in Fig. 5, each of three tested AKI models (LPS; cisplatin nephrotoxicity; glycerol) manifested marked urinary Hpx increases, whether expressed as absolute concentrations (μg/ml) or as Hpx-to-urine creatinine ratios. Urine samples were assayed at 4 and 24 h postglycerol, 24 h post-LPS, and 2 days following cisplatin injection (n = 4–5 each).
Fig. 5.
Urinary Hpx excretion rises in response to AKI. Each form of AKI increased urinary Hpx excretion (data expressed as absolute concentrations and as urine Hpx-to-creatinine ratios). In the case of glycerol, marked increases were observed as early as 4 h postglycerol injection (d, day).
Immunohistochemistry
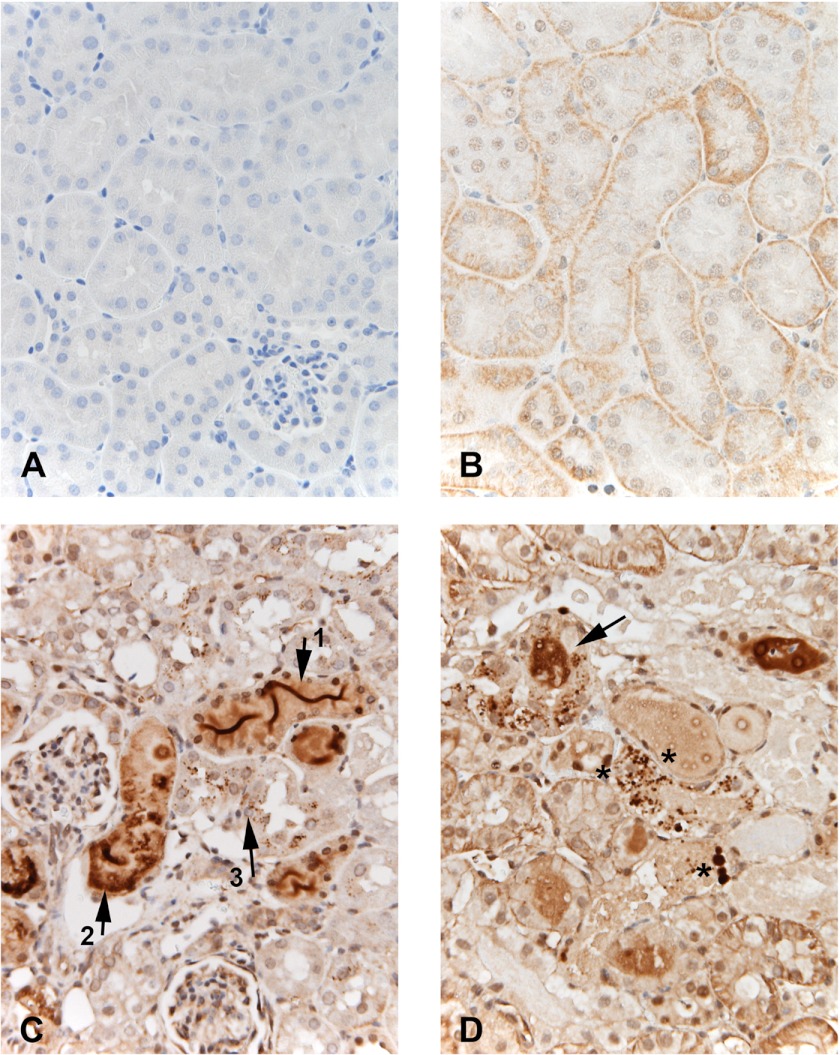
Faint Hpx staining, particularly at the basolateral membrane, was observed in normal kidney sections (compare isotype negative vs. Hpx antibody staining of normal kidney; Fig. 6, A and B, respectively). In contrast, following both cisplatin (Fig. 6C) and I/R-induced injury (Fig. 6D), prominent Hpx staining was observed, predominantly within proximal tubular lumina in cortex/medullary rays and outer medullary stripe. Modest to marked focal cytoplasmic Hpx staining was also apparent in proximal tubules. In addition, a notable finding was the presence of abundant Hpx stained reabsorption droplets within proximal tubule cells. This was particularly apparent adjacent to the luminal membranes, consistent with Hpx endocytic reabsorption. In contrast, whereas the inner medulla showed prominent Hpx stained casts, no evidence of tubular cell uptake/reabsorption droplets were observed (Fig. 7).
Fig. 6.
Immunohistochemical localization of Hpx following induction of CP and ischemic AKI. Compared with the isoytpe negative control (A), normal kidney (B) demonstrated trace Hpx staining with differing expression in different tubules. Staining appears predominantly at the basolateral membrane of proximal tubules. In contrast, the CP-treated kidney (C) demonstrated: 1) dense Hpx “ribbon like” staining within tubular lumina (arrow 1); 2) focal dense staining of tubule cytoplasm (arrow 2), and 3) numerous small Hpx containing reabsorption droplets within proximal tubules (arrow 3). Finally, the postischemic kidney (D) recapitulated the cisplatin findings with dense Hpx-stained casts (arrow) and prominent Hpx stained reabsorption droplets with proximal tubule cells (representative areas of increased uptake denoted by the asterisks). Lower asterisk denotes 4 prominent examples of this increased uptake.
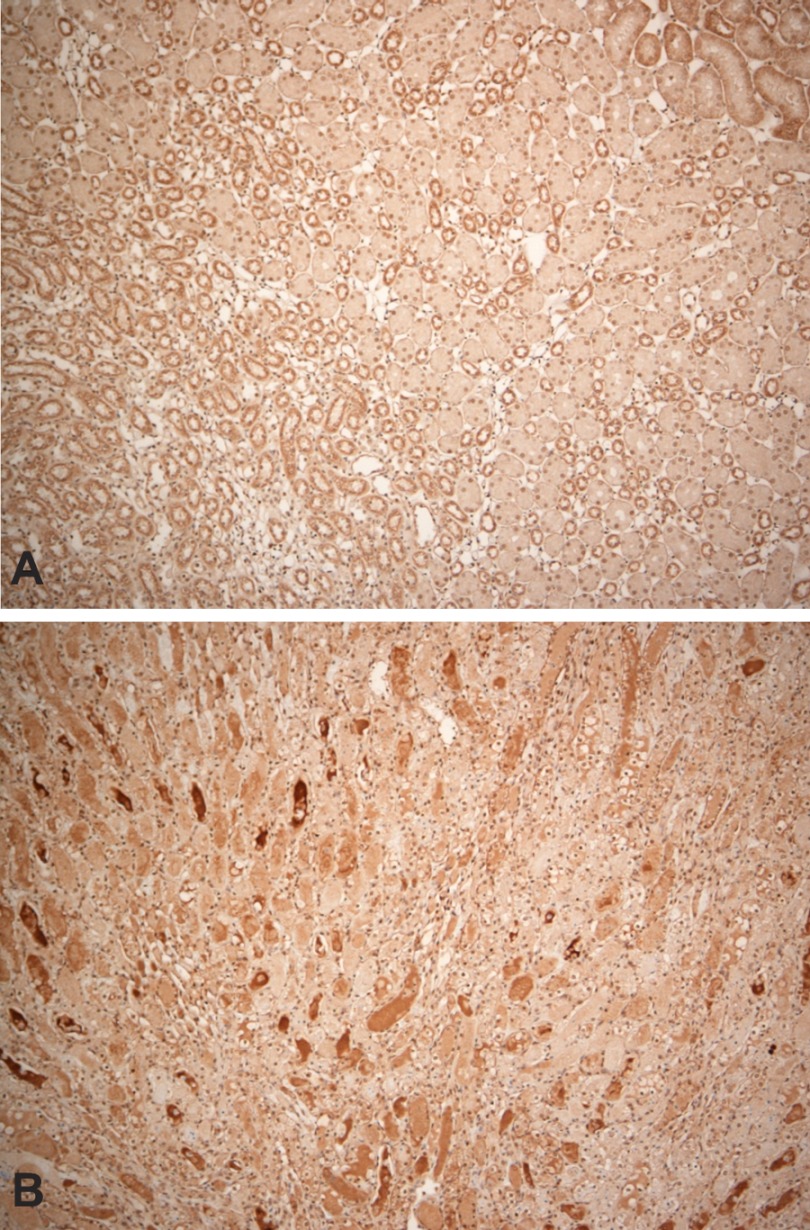
Fig. 7.
Immunohistochemical localization of Hpx within renal medulla in a control kidney (A) and in a kidney at 24 h postinduction of ischemic reperfusion injury. Compared with the control kidney (A), which shows minimal Hpx staining within the renal medulla, the postischemic kidney (B) manifests abundant Hpx staining casts. However, there was no increase in cytoplasmic Hpx staining and there was an absence of cellular Hpx-stained reabsorption droplets.
In sum, these findings were consistent with Hpx filtration and Hpx uptake, specifically by proximal tubule cells. In addition to the immunohistochemistry findings, both the I/R and cisplatin models were associated with prominent proximal tubule necrosis within the cortex and outer medullary stripe.
Isolated Proximal Tubule Hpx Protein and mRNA Levels
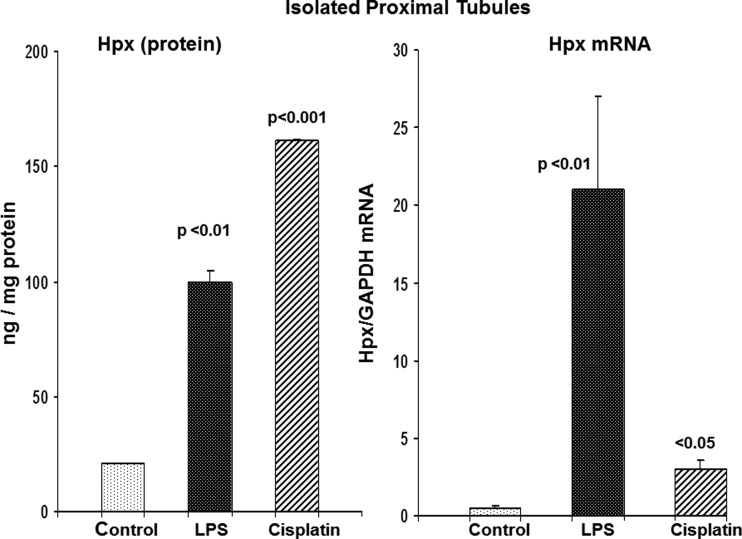
To confirm that AKI produced proximal tubule Hpx uptake, proximal tubule segments were isolated from control mice, and mice at either 24 h post-LPS or 48 h postcisplatin injection (Fig. 8). Immediately after isolation, they were assayed for Hpx protein (ELISA) as well as for Hpx mRNA. Both sets of AKI tubules manifested marked Hpx protein elevations, compared with control tubules. Both sets of AKI tubules also had statistically significant elevations in Hpx mRNA vs. controls. However, the mRNA values were ∼10-fold higher in the LPS vs. the cisplatin tubules, thereby recapitulating the whole renal cortical mRNA results. Finally, as with renal cortex, there was a relative dissociation between the degree of Hpx protein and mRNA elevations: the protein levels were higher in tubules from cisplatin vs. LPS-treated mice, despite the fact that Hpx mRNA levels were 10-fold lower in the cisplatin group.
Fig. 8.
Hpx protein and mRNA levels in isolated proximal tubules harvested from mice with AKI. Proximal tubules harvested from control mice and mice with 2 models of AKI (2 days postcisplatin injection; 24 h post-LPS injection) were analyzed for Hpx protein and mRNA. Both sets of AKI tubules had significant elevations in Hpx protein and mRNA. There was no apparent correlation between protein and mRNA levels: whereas the LPS tubules had massive elevations of Hpx mRNA, compared with cisplatin tubules, the cisplatin tubules had higher Hpx protein levels.
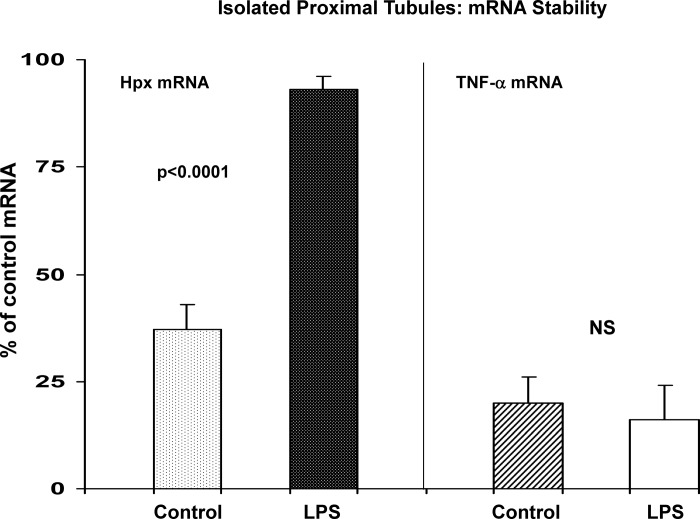
Hpx mRNA Stability Assessments
To assess whether increased mRNA stability, and not simply mRNA gene transcription rates, might impact tubule Hpx mRNA levels, isolated tubules were harvested from LPS-treated mice, and then Hpx mRNA stability was assessed by incubating them for 90 min in the presence of actinomycin D (blocking transcription and, thus, allowing assessment of mRNA degradation rates; Ref. 16). As shown in Fig. 9, control tubules manifested a 70% decline in Hpx mRNA content over 90-min actinomycin A incubations. Conversely, LPS-pretreated tubules only lost ∼10% of Hpx mRNA, indicating increased mRNA stability. To assess whether this reflected a generalized vs. a more specific change in mRNA stability, TNF-α mRNA degradation over these same incubations was assessed. As shown in Fig. 9, right, control and LPS-treated tubules manifested essentially identical reductions (∼80%) in TNF-α mRNA.
Fig. 9.
Hpx mRNA stability, as assessed in isolated proximal tubules. Isolated tubules were harvested from control and LPS treated mice and incubated for 90 min with actinomycin D to assess mRNA degradation rates. LPS induced marked Hpx mRNA stabilization, as indicated by ∼80% retention of control mRNA levels (vs. 35% retention in control tubules). For comparison, TNF-α mRNA was not stabilized by LPS pretreatment (right). This indicates that Hpx mRNA stabilization was not a completely nonspecific event.
Response of Cultured Cells to Heme Fe-Mediated Oxidative Stress
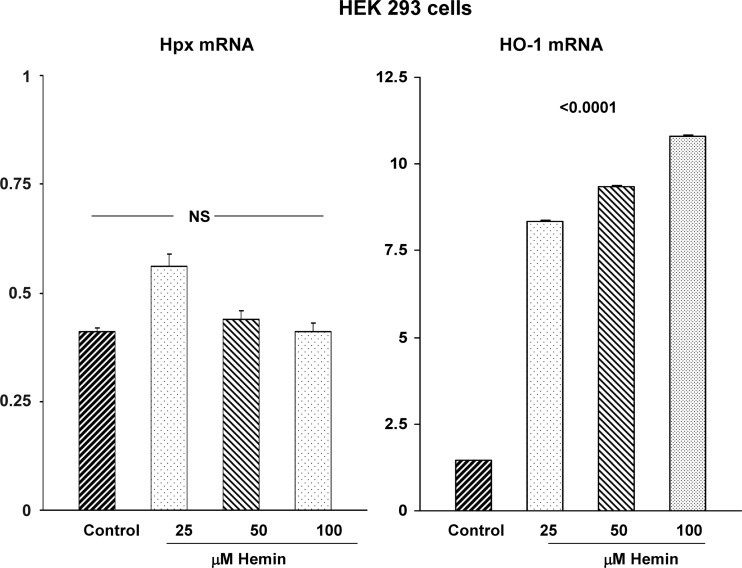
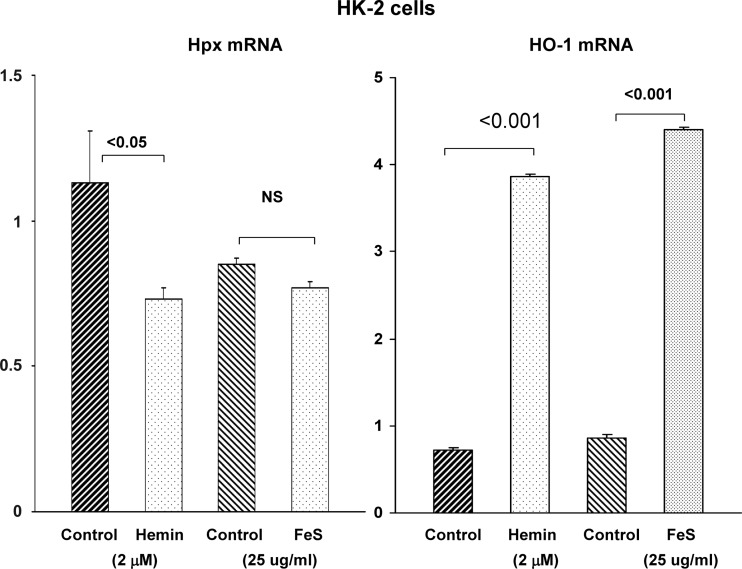
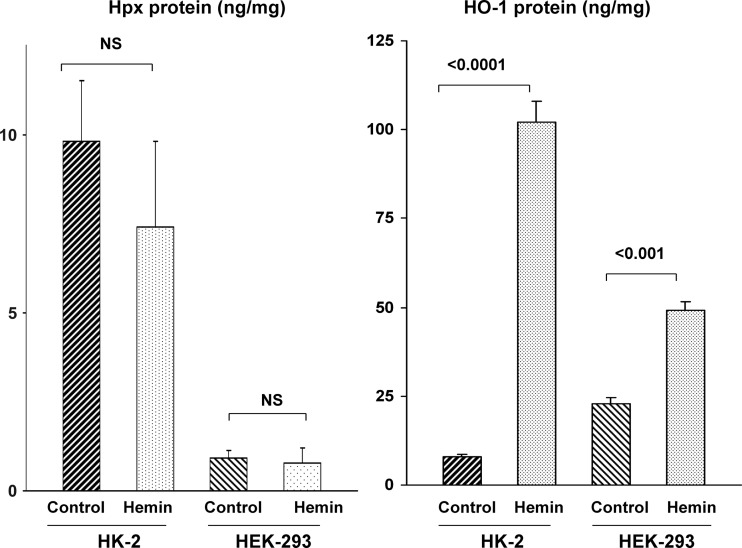
To more directly assess whether heme evokes changes in Hpx mRNA and protein expression, cultured HEK 293 cells and HK-2 proximal tubule cells were incubated with hemin (hemin concentrations are presented in the figures). Hemin did not elevate either Hpx mRNA (Figs. 10 and 11) or protein levels (Fig. 12) in either HEK-293 or HK-2 cells. In fact, hemin significantly lowered Hpx mRNA in HK-2 cells (Fig. 11). Conversely, hemin markedly increased both HO-1 protein and mRNA in both cell lines, confirming their heme responsiveness. As a second Fe challenge, HK-2 cells were incubated with Fe sucrose (FeS) for 18 h and no change in Hpx mRNA was observed despite the fact that Fe sucrose caused a fivefold increase in HO-1 mRNA (Fig. 11). Finally, incubating HK-2 cells with 25 μg/ml of Fe sucrose × 72 h tended to lower, not elevate, HK-2 cell Hpx mRNA levels (controls, 0.77 ± 18; Fe sucrose, 0.59 ± 011; P = 0.1). No lethal cell injury was observed as a result of these experiments (lactate dehydrogenase release: 10 ± 1% for controls, hemin-, and FeS-challenged cells).
Fig. 10.
HEK 293 cell responses to hemin. A dose titration of HEK 293 cells with hemin failed to induce a significant change in Hpx mRNA levels. Conversely, a direct dose-response relationship between heme oxygenase 1 (HO-1) mRNA and hemin was observed. This indicates that the failure of Hpx mRNA responsiveness was not due to an inability of these cells to respond to hemin driven oxidant stress.
Fig. 11.
HK-2 cell responses to hemin- and Fe sucrose (FeS)-induced oxidant stress. HK-2 cells responded to hemin with a significant reduction, not an increase, in Hpx mRNA. Furthermore, FeS-mediated oxidant stress failed to raise Hpx mRNA levels. In contrast, the cells responded to both the hemin and FeS challenges with significant increases in HO-1 mRNA, indicating an ability to respond to Fe-mediated oxidative stress.
Fig. 12.
HEK 293 and HK-2 cell Hpx and HO-1 protein levels following a hemin challenge. Incubation of either cell line with hemin failed to increase Hpx protein levels, as assessed in cell protein extracts. Conversely, both cell lines responded to hemin with significant increases in HO-1 protein, confirming that the cells were capable of synthesizing a heme-inducible protein.
Response of Cultured Cells to Fe Depletion
Because Hpx is a heme-conserving protein, we questioned whether cellular Fe depletion, rather than Fe overload, might upregulate Hpx gene expression. To test this hypothesis, HEK 293 and HK-2 cells were incubated with the Fe chelator DFO and 18 h later, Hpx mRNA levels were assessed. As shown in Fig. 13, DFO caused a sevenfold increase in Hpx mRNA. A reciprocal two-thirds reduction in HO-1 mRNA was observed (consistent with HO-1 being a Fe sensing gene).
Fig. 13.
HEK 293 cells respond to Fe depletion with increases in Hpx mRNA. Fe depletion, induced by two doses of deferoxamine, markedly increased Hpx mRNA. Conversely, Fe depletion decreased HO-1 mRNA, consistent with a decrease in oxidant stress.
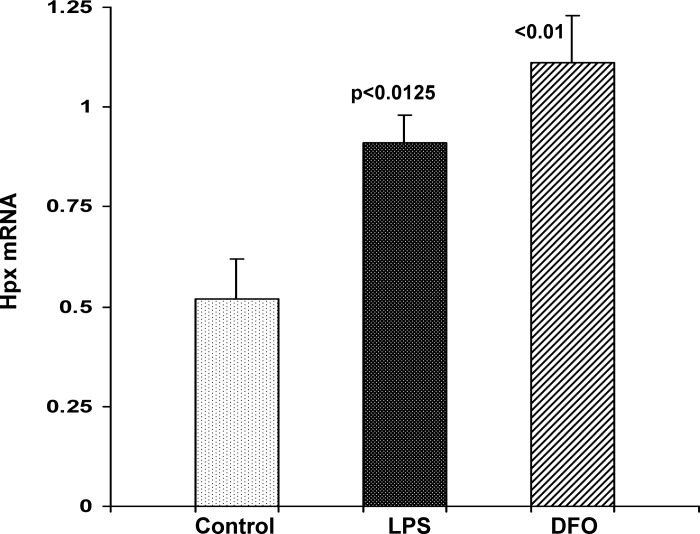
Response of HK-2 Cells to LPS
Given that LPS dramatically increased Hpx mRNA in vivo, a direct proximal tubule LPS effect was assessed by incubating HK-2 cells in the presence and absence of LPS. At 3 h post-LPS addition, an approximate doubling of Hpx mRNA was observed. Confirming the results obtained with DFO in HEK-293 cells, DFO also significantly increased HK-2 cell Hpx mRNA (Fig. 14).
Fig. 14.
HK-2 cells respond to LPS or Fe depletion with increases in Hpx mRNA. Cells were exposed to either 10 μg/ml of LPS or 0.1 mM deferoxamine (DFO), and 3 h later, Hpx mRNA was measured. Both challenges led to significant increases in Hpx mRNA.
Response of HK-2 Cells to FeHQ in the Absence and Presence of Hpx
Addition of purified Hpx had no independent effect on HK-2 MTT uptake. The 5-μM FeHQ challenge caused a 77 ± 1% reduction in MTT uptake. This was reduced to 62 ± 1% by concomitant Hpx addition. (P = 0.005).
Free Heme Generation During AKI
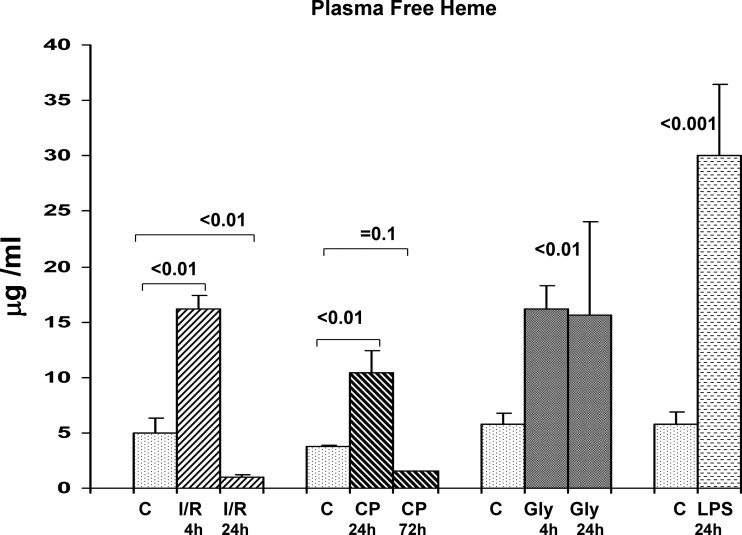
Within 4 h of inducing bilateral I/R injury, a threefold increase in plasma free heme was observed (Fig. 15). However, by 24 h post-I/R, free heme levels fell to subnormal values (coincident, and a likely consequence, of rising plasma Hpx levels). The same pattern was observed with cisplatin AKI: a threefold increase at 24 h and subnormal values at 72 h (again, consistent with rising Hpx levels). In the case of glycerol induced AKI, a two- to threefold increase in free heme levels was observed at 4 h, but these levels were maintained at the 24-h time point. This failure of free heme to have fallen at 24 h postglycerol injection is consistent with the fact that only a trivial increase in plasma Hpx levels was observed at this time point (see Fig. 3). Finally, LPS induced a fivefold increase in plasma free heme by 24 h (no time course was tested in this model).
Fig. 15.
Plasma free heme concentrations following induction of AKI. During the initiation phases of I/R, glycerol, or cisplatin induced AKI (4, 4, and 24 h, respectively) an ∼3-fold increase in plasma free heme was observed, compared with values observed in control mouse plasma. Upon reaching the so called maintenance phase of I/R or cisplatin AKI (24 h and 72 h respectively), the plasma free heme levels fell, reaching values that were approximately two-thirds lower than were observed in normal mouse plasma. These falling free heme levels corresponded to rising plasma Hpx levels. In contrast, free heme concentrations did not fall between 4 by 24 h postglycerol injection. This was likely due to a relative failure of plasma Hpx upregulation during this period (see Fig. 3). Finally, LPS injection led to a 6-fold increase in free heme by 24 h postinjection.
DISCUSSION
This study demonstrates for the first time that diverse forms of AKI evoke dramatic Hpx accumulation within injured renal cortex. The proximal tubule is a dominant participant in this response, given that 5- to 10-fold Hpx protein increases were documented in isolated proximal tubule segments extracted from mice with AKI, vs. normal controls. Thus, like haptoglobin (Hp) and alpha fetoprotein (AFP), Hpx can be considered to be a component of the so-called acute “renal hepatization” response.
In the case of Hp and AFP, increased renal synthesis appears to be the dominant underlying mechanism for their renal cortical accumulation, based on observations that the extent of their increases are closely paralleled by the levels of their respective mRNAs (30, 32). However, in the case of Hpx, no such correlation between protein and mRNA levels exists. For example, the most dramatic Hpx mRNA increases among the four AKI models was induced by LPS, yet LPS evoked the smallest Hpx protein elevations. Conversely, cisplatin and I/R injury caused the greatest renal cortical Hpx protein increases, and yet, they induced comparatively small increases in Hpx mRNA. The results obtained with the unilateral I/R model are particularly instructive in this regard. Although unilateral I/R evoked marked renal Hpx protein levels compared with those found in the contralateral kidney, the latter had higher levels of Hpx mRNA. In sum, these findings strongly suggest that increased gene transcription was not the dominant mechanism driving renal Hpx accumulation.
It is notable that Hpx (like Hp and AFP) is a hepatic “acute phase reactant,” and as such, the Hpx gene is readily induced in liver in response to diverse forms of “systemic stress.” For example, it has previously been demonstrated that even sham abdominal surgery in rodents (as used as a control for partial hepatectomy) markedly increases Hpx mRNA (1). This culminates in increased Hpx synthesis and secretion into the systemic circulation. Given the apparent dissociation between renal cortical Hpx protein and Hpx mRNA levels, we questioned whether AKI might also initiate hepatic Hpx synthesis, culminating in increased plasma Hpx levels and, ultimately, glomerular filtration with increased renal tubular uptake. To explore this possibility, Hpx mRNA levels were measured in liver following induction of each AKI model, as well as sham abdominal surgery. In each instance, approximately three- to fourfold hepatic mRNA elevations were observed. Of note, relatively small, if any increases, were observed in two nonhepatic tissues (lung and spleen), suggesting the dominance of the hepatic Hpx response.
Given Hpx's size of ∼57–65 kDa, a normal glomerular capillary wall might classically be expected to prevent Hpx filtration and hence tubular luminal access. However, two points are noteworthy in this regard. First, it has become increasingly apparent that comparably sized proteins, e.g., albumin (69 kDa), do in fact undergo substantial glomerular filtration. For example, in humans, it has been estimated that at least 3 g/day of albumin are filtered and subsequently reabsorbed, largely by the proximal tubule (24). Second, recent studies suggest that Hpx can directly increase glomerular capillary permeability to proteins, perhaps as a function of an inherent protease activity (11). This property has led to suggestions that Hpx may be one of the serum factors that contribute to proteinuria in minimal change disease nephrotic syndrome (15). Given these two considerations, we performed immunohistochemistry on renal sections from control mice and mice with AKI with two goals in mind: first, to confirm that Hpx was reaching tubular lumina, and second, to assess whether proximal tubule reabsorption occurs. If so, then a case for so-called “reverse organ cross talk” in AKI would exist whereby: 1) systemic stress, initiated by either surgery or different forms of AKI, induces hepatic Hpx synthesis; 2) elevated plasma Hpx levels result; 3) increased amounts of Hpx are then filtered by the glomerulus; and 4) with subsequent endocytic reabsorption, proximal tubule Hpx loading results. Indeed, the immunohistochemistry findings support this concept, given that the AKI mice, but not the control mice, manifested clear evidence of Hpx within tubular lumina. Furthermore, abundant Hpx-containing proximal tubule reabsorption droplets, indicative of endocytosis, were readily observed. While Hpx-stained casts were seen in renal inner medulla, no evidence of cellular Hpx-containing reabsorption droplets were observed (consistent with the fact that proximal, but not distal nephrons, participate in megalin-cubulin-mediated endocytosis). Finally, that up to 100-fold increases in urinary Hpx levels were documented following AKI and that proximal tubules harvested from AKI mice manifested ∼5- to 10-fold Hpx increases provide additional support for the concept that increased Hpx filtration and tubule uptake had occurred.
Given that each of the AKI models induced at least some, albeit highly variable, increases in renal cortical Hpx mRNA, it seems likely that Hpx synthesis within proximal tubules played at least some role in elevating renal Hpx protein levels. Of note, within the liver, the “stress response” induces not only Hpx gene transcription but, even more importantly, a marked stabilization of newly synthesized Hpx mRNA (1). To determine whether AKI might induce Hpx mRNA stabilization within proximal tubules, Hpx mRNA degradation rates were assessed in tubules harvested from control and LPS-pretreated mice. Indeed, the LPS-pretreated tubules manifested only 10% Hpx mRNA degradation over the course of 90-min actinomycin D incubations, compared with 70% Hpx mRNA degradation in control tubules. Thus these findings indicate that the results of any increase in AKI-initiated Hpx mRNA production would be magnified by a decrease in Hpx mRNA degradation rates. That no change in TNF-α mRNA stability was noted during these experiments indicates that the Hpx mRNA results were not simply due to a completely nonspecific change in mRNA degradation rates.
To address potential initiators of renal Hpx gene transcription in AKI, extensive experiments were undertaken in cultured HEK 293 and HK-2 proximal tubule cells. Given that the primary role of Hpx is as a free heme binder, we explored the hypothesis that an increase in cellular free heme, a known consequence of AKI (4, 19, 27), might drive Hpx gene transcription. However, this appeared not to be the case, given that neither hemin nor Fe sucrose exposure increased Hpx mRNA or protein levels in these two cell culture systems. That both hemin and Fe sucrose evoked brisk HO-1 mRNA and protein increases shows that they serve as “internal controls,” i.e., by indicating that both cell lines were clearly capable of responding to heme Fe-driven oxidative stress. Supporting these in vitro observations was that intravenous Fe sucrose, while inducing an approximate tripling of hepatic Hpx mRNA, failed to change renal cortical mRNA levels. A second function of Hpx as a free heme scavenger is to prevent urinary heme Fe loss and, hence, the development of Fe deficiency in states such as paroxysmal nocturnal hemoglobinuria. Hence, Hpx is considered to play a potentially important role in maintaining Fe homeostasis (2, 3, 25). Thus we next tested the converse to the heme Fe-Hpx induction hypothesis, i.e., that Fe deficiency, and not heme excess, drives renal Hpx gene transcription. Indeed, this was the case, given that deferoxamine treatment abruptly increased Hpx mRNA levels in both HEK 293 and HK-2 cells. However, intratubular free heme Fe surplus, and not deficiency, develops during AKI (4, 19, 27). Thus Fe deficiency cannot readily explain why AKI triggered increases in renal Hpx mRNA. This conundrum led us to consider a third hypothesis: that intrarenal or systemic inflammation, well-recognized secondary consequences of AKI, was responsible for activating the Hpx gene. Two pieces of information provide support for this third hypothesis. First, LPS injection caused profound renal Hpx mRNA accumulation, which was ∼30-fold higher than seen in any other AKI model. and second, LPS addition to HK-2 cells abruptly doubled Hpx mRNA. Thus we posit that, in the setting of AKI, the renal Hpx gene likely responds to an inflammatory stimulus and that this, and not predominantly changes in heme/Fe balance, initiates renal Hpx gene transcription. Given the great complexity of AKI-initiated inflammation, much additional study would be required to dissect out the specific initiators, and “downstream” inflammatory pathways, that may be involved.
Finally, we questioned the potential functional significance of the AKI-associated increases in plasma and renal Hpx protein expression that occur in response to AKI. Towards this end, we tested the hypothesis that AKI, or associated extra-renal tissue injury, might release free heme into the systemic circulation from where it might induce extrarenal or renal damage. Indeed, we observed that plasma free heme levels did, in fact, increase approximately three- to fivefold over normal values during the so called initiation phase of each form of AKI. Of further interest was that in the case of I/R- and cisplatin-induced injury, the free heme elevations were relatively transient, given that they plummeted to subnormal values by 1 and 3 days, respectively. Given that Hpx levels rose dramatically over these same time intervals, it seems highly plausible that these free heme reductions stemmed from countervailing plasma Hpx increases. The free heme data obtained from the glycerol model are consistent with this hypothesis. By 4 h postglycerol injection, a threefold plasma free heme increase was observed and this persisted unabated for 24 h. In addition, during this time, only a miniscule change in plasma Hpx levels occurred. Thus the failure of free heme to fall postglycerol injection may well have reflected, at least in part, a relative failure of plasma Hpx upregulation. Given these data, we advance the following concept: 1) diverse forms of AKI cause systemic release of prooxidant heme from injured renal and/or extrarenal tissues; 2) after a time delay, plasma Hpx levels rise and bind this excess free heme, even lowering them to subnormal values; 3) plasma heme-Hpx binding is known to decrease heme-mediated renal oxidant stress (26); and 4) because tubule injury, per se, releases intracellular heme from cytochromes and respiratory enzymes (4, 19, 26), increased intratubular cell Hpx levels would be expected to bind this liberated heme, and thus confer a beneficial effect. A study by Vogt et al. (28) illustrates the potential functional significance of these events. These investigators reported that LPS pretreatment protects against subsequent glycerol-induced acute renal failure (28). Although part of this protection resulted from an LPS-mediated upregulation of HO-1 (28), it seems likely that LPS also increased circulating and intrarenal Hpx, which then counterbalanced glycerol-mediated cytotoxic free heme release. A further mechanistic link between Hpx and cytoprotection is underscored by the fact that the Hpx-heme complex is a potent inducer of the HO-1 gene (22). Finally, our current observation that Hpx reduced Fe (HQ) toxicity in HK-2 cells indicates an ability to mitigate oxidant injury, even in the absence of free heme.
In conclusion, this study provides the following new observations and concepts: 1) diverse forms of AKI cause, or are associated with, striking increases in renal cortical and proximal tubule Hpx levels. 2) This change stems, at least in part, from an acute increase in hepatic Hpx production, which leads to increased circulating Hpx levels, Hpx filtration, and proximal tubule Hpx uptake. 3) To variable degrees, increased intrarenal Hpx synthesis may also contribute to renal Hpx accumulation during AKI. This likely stems from at least some increase in Hpx mRNA production and, importantly, from a marked stabilization of Hpx mRNA, prolonging its half life. 4) Despite its role as a free heme binder, the renal tubular cell Hpx gene can be triggered by Fe deficiency and not necessarily by Fe (e.g., Fe sucrose) or free heme. This underscores Hpx's role as a regulator of systemic Fe homeostasis. 5) LPS triggers a massive increase in renal cortical Hpx gene transcription and in cultured HK-2 cells. This suggests that inflammation, a concomitant of virtually all forms of AKI, and not alterations in intrarenal heme/Fe balance, per se, is a likely trigger for renal Hpx transcription in the setting of acute renal failure. 6) We show, for the first time, that diverse forms of AKI are associated with free heme release into the systemic circulation. Given its marked prooxidant capacity, it may then contribute to both evolving renal or extrarenal tissue damage. However, the latter can potentially be counterbalanced by rising plasma Hpx increases which can bind the excess free heme, and lower it to even subnormal values; and 7) To the degree that AKI releases free heme within tubular cells, concomitant renal tubular Hpx accumulation would be expected to exert a countervailing cytoprotection actions. This could occur either by 1) free heme binding, 2) heme-Hpx induction of cytoprotective HO-1 (22), or 3) by exerting a direct antioxidant effect, e.g., as noted above. Despite this multiplicity of actions, as well as its ability to impact tissue inflammation (7, 12, 13, 23), the ultimate impact of renal Hpx accumulation on the evolution of AKI remains to be defined. However, based on all of the above, it does seem highly likely that pathophysiologic links between the acute hepatic stress response, Hpx generation, and kidney injury exist.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-38432 and DK-083310.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.A.Z. conception and design of research; R.A.Z., A.C.J., and K.B. analyzed data; R.A.Z., A.C.J., and K.B. interpreted results of experiments; R.A.Z. prepared figures; R.A.Z. drafted manuscript; R.A.Z., A.C.J., and K.B. edited and revised manuscript; R.A.Z. and A.C.J. approved final version of manuscript; A.C.J. and K.B. performed experiments.
REFERENCES
- 1.Albrecht JH, Muller-Eberhard U, Kren BT, Steer C. Influence of transcriptional regulation and mRNA stability on hemopexin gene expression in regenerating liver. Arch Biochem Biophys 314: 229–233, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Ascenzi P, Bocedi A, Visca P, Altruda F, Tolosano E, Beringhelli T, Fasano M. Hemoglobin and heme scavenging. Life 57: 749–759, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Baker HM, Anderson BF, Baker EN. Dealing with iron: common structural principles in proteins that transport iron and heme. Proc Natl Acad Sci USA 100: 3579–3583, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baliga R, Zhang Z, Baliga M, Shah SV. Evidence for cytochrome P-450 as a source of catalytic iron in myoglobinuric acute renal failure. Kidney Int 49: 362–369, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Delanghe JR, Langlois MR. Hemopexin: a review of biological aspects and the role in laboratory medicine. Clin Chim Acta 312: 13–23, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Aspects Med 33: 209–290, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Fink MP. Hemopexin: newest member of the anti-inflammatory mediator club. J Leukoc Biol 86: 203–204, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Irony-Tur-Sinai M, Grigoriadis N, Tsiantoulas D, Touloumi O, Abramsky O, Brenner T. Immunomodulation of EAE by alpha-fetoprotein involves elevation of immune cell apoptosis markers and the transcription factor FoxP3. J Neurol Sci 279: 80–87, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Kanno S, Kurauchi K, Tomizawa A, Yomogida S, Ishikawa M. Albumin modulates docosahexaenoic acid-induced cytotoxicity in human hepatocellular carcinoma cell lines. Toxicology 200: 154–161, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Larsen R, Gozzelino R, Jeney V, Tokaji L, Bozza FA, Japiassú AM, Bonaparte D, Cavalcante MM, Chora A, Ferreira A, Marguti I, Cardoso S, Sepúlveda N, Smith A, Soares MP. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med 29: 51–71, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Lennon R, Singh A, Welsh GI, Coward RJ, Satchell S, Ni L, Mathieson PW, Bakker WW, Saleem MA. Hemopexin induces nephrin-dependent reorganization of the actin cytoskeleton in podocytes. J Am Soc Nephrol 11: 2140–2149, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang X, Lin T, Sun G, Beasley-Topliffe L, Cavaillon JM, Warren HS. Hemopexin down-regulates LPS-induced proinflammatory cytokines from macrophages. J Leukoc Biol 86: 229–235, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin T, Sammy F, Yang H, Thundivalappil S, Hellman J, Tracey KJ, Warren HS. Identification of hemopexin as an anti-inflammatory factor that inhibits synergy of hemoglobin with HMGB1 in sterile and infectious inflammation. J Immunol 189: 2017–2022, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mauk MR, Smith A, Mauk AG. An alternative view of the proposed alternative activities of hemopexin. Protein Sci 20: 791–805, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarthy ET, Sharma M, Savin VJ. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 5: 2115–2121, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Naito M, Bomsztyk K, Zager RA. Endotoxin mediates recruitment of RNA polymerase II to target genes in acute renal failure. J Am Soc Nephrol 19: 1321–1330, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int 70: 432–443, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Nath KA. Human AKI and heme oxygenase-1. J Am Soc Nephrol 23: 971–974, 1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paller MS, Jacob HS. Cytochrome P450 mediates tissue-damaging hydroxyl radical formation during reoxygenation of the kidney. Proc Natl Acad Sci USA 91: 7002–7006, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riordan M, Sreedharan R, Kashgarian M, Siegel NJ. Modulation of renal cell injury by heat shock proteins: lessons learned from the immature kidney. Nat Clin Pract Nephrol 2: 149–156, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK2 an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int 45: 48–57, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Smith A. Links between cell-surface events involving redox-active copper and gene regulation in the hemopexin heme transport system. Antioxid Redox Signal 2: 157–175, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Spiller F, Costa C, Souto FO, Vinchi F, Mestriner FL, Laure HJ, Alves-Filho JC, Freitas A, Rosa JC, Ferreira SH, Altruda F, Hirsch E, Greene LJ, Tolosano E, Cunha FQ. Inhibition of neutrophil migration by hemopexin leads to increased mortality due to sepsis in mice. Am J Respir Crit Care Med 183: 922–31, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Tojo A, Kinugasa S. Mechanisms of glomerular albumin filtration and tubular reabsorption. Int J Nephrol 2012: 481520, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolosano E, Fagoonee S, Morello N, Vinchi F, Fiorito V. Heme scavenging and the other facets of hemopexin. Antioxid Redox Signal 12: 305–320, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Tolosano E, Hirsch E, Patrucco E, Camaschella C, Navone R, Silengo L, Altruda F. Defective recovery and severe renal damage after acute hemolysis in hemopexin-deficient mice. Blood 94: 3906–3914, 1999 [PubMed] [Google Scholar]
- 27.Tracz MJ, Alam J, Nath KA. Physiology and pathophysiology of heme: implications for kidney disease. Am Soc Nephrol 18: 414–420, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Vogt BA, Alam J, Croatt AJ, Vercellotti GM, Nath KA. Acquired resistance to acute oxidative stress. Possible role of heme oxygenase and ferritin. Lab Invest 72: 474–483, 1995 [PubMed] [Google Scholar]
- 29.Wang Z, Gall JM, Bonegio RG, Havasi A, Hunt CR, Sherman MY, Schwartz JH, Borkan SC. Induction of heat shock protein 70 inhibits ischemic renal injury. Kidney Int 79: 861–870, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ware LB, Johnson AC, Zager RA. Renal cortical albumin gene induction and urinary albumin excretion in response to acute kidney injury. Am J Physiol Renal Physiol 300: F628–F638, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zager RA, Johnson AC, Becker K. Plasma and urinary heme oxygenase-1 in AKI. J Am Soc Nephrol 23: 1048–1057, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zager RA, Vijayan A, Johnson AC. Proximal tubule haptoglobin gene activation is an integral component of the acute kidney injury “stress response”. Am J Physiol Renal Physiol 303: F139–F1348, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]