Abstract
Previously, we reported that overeating for only a few days markedly suppressed the secretion of growth hormone (GH). The purpose of the present study was to determine the role of this reduction in GH concentration on key metabolic adaptations that occur during 2 wk of overeating. Nine nonobese, healthy adults were admitted to the hospital for 2 wk, during which time they ate ∼4,000 kcal/day (70 kcal·kg fat-free mass−1·day−1; 50% carbohydrate, 35% fat, and 15% protein), and their plasma GH concentration was allowed to decline naturally (control). An additional eight subjects underwent the same overeating intervention and received exogenous GH treatment (GHT) administered in four daily injections to mimic physiological GH secretion throughout the 2-wk overeating period. We measured plasma insulin and glucose concentrations in the fasting and postprandial state as well as fasting lipolytic rate, proteolytic rate, and fractional synthetic rate (FSR) using stable-isotope tracer methods. GHT prevented the fall in plasma GH concentration, maintaining plasma GH concentration at baseline levels (1.2 ± 0.2 ng/ml), which increased fasting and postprandial assessments of insulin resistance (P < 0.05) and increased fasting lipidemia (all P < 0.05 vs. control). In addition, preventing the suppression in GH with overeating also blunted the increase in systemic proteolysis (P < 0.05 GHT vs. control). However, GHT did not alter lipolysis or FSR in response to overeating. In conclusion, our main findings suggest that the suppression in GH secretion that naturally occurs during the early stages of overeating may help attenuate the insulin resistance and hyperlipidemia that typically accompany overeating.
Keywords: obesity, lipolysis, proteolysis, muscle protein fractional synthetic rate, overfeeding
weight gain can only occur when energy intake exceeds energy expenditure (i.e., positive energy balance), and even a relatively modest positive energy balance can result in an individual becoming overweight and obese over time. Although the metabolic complications of obesity have been well described (16, 18, 31), far less is known about the dynamic metabolic adaptations that occur in response to overeating. Importantly, we reported previously that even just a few days of overeating profoundly suppressed plasma growth hormone (GH) concentration in nonobese adults (7). However, the metabolic consequences of this acute suppression in plasma GH concentration with overeating are not known.
GH has been identified as an important regulator of several metabolic processes. For example, we (39) and others (4, 9) found that the normal pulsatile pattern of GH secretion augments lipolytic rate. Additionally, GH has been reported to impair insulin sensitivity through direct inhibition of insulin signaling (1), as well as indirectly by increasing lipolysis (18), which in turn can alter insulin action via increasing fatty acid infiltration into insulin-responsive tissues. GH may also contribute to hyperlipidemia via alterations in the activity of lipoprotein lipase (30, 34, 40). GH has also garnered a great deal of attention for its role in regulating protein metabolism (10, 13, 48), but the direct effect of GH on muscle protein synthesis is controversial (32). Importantly, previous studies have found that overeating decreased insulin sensitivity (2, 41), exacerbated plasma triacylglyceride concentration (25), and altered protein metabolism (44), and the suppression of GH with overeating may be an important contributor to many of these responses. Therefore, the primary aim of this study was to examine how the suppression of GH with overeating might influence key metabolic adaptations that occur during 2 wk of overeating. To address these goals, we measured meal tolerance, lipolytic rate, proteolytic rate, and the rate of muscle protein synthesis before and after 2 wk of overeating while preventing the fall in plasma GH concentration via administration of exogenous GH. Given the important role of GH in the regulation of these metabolic processes, we hypothesized that preventing the suppression in GH with overeating would further impair glucose tolerance and augment the hyperlipidemia normally found with overeating.
MATERIALS AND METHODS
Subjects
A total of 22 young, healthy men (n = 18) and women (n = 4) participated in a 2-wk overeating protocol (initial body mass index 23.5 ± 0.3 kg/m2, age 24 ± 1 yr). Seven of these subjects also participated in our previously published study (7). Before the study, all subjects were weight stable, relatively sedentary (physical activity ≤2 h/wk), and not taking any medications. All procedures and protocols for this study were approved by the University of Michigan Institutional Review Board. Written, informed consent was obtained from all subjects before their participation in the study. This study was registered at ClinicalTrials.gov with the identifier no. NCT00355784.
General Study Design
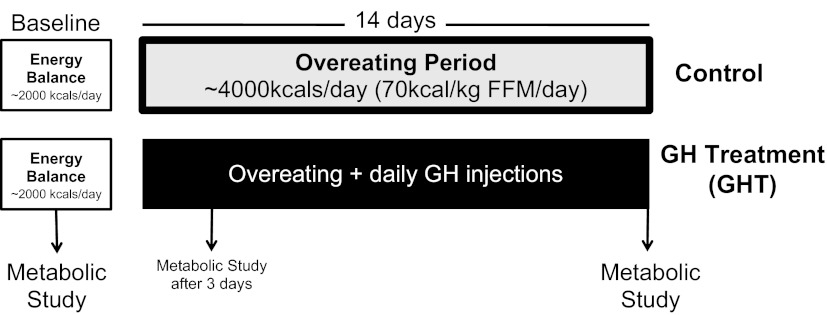
We performed a battery of metabolic measurements in our subjects before overeating, at 3–4 days of overeating (∼4,000 kcal/day), and again after 2 wk of overeating (Fig. 1; details of the diet and metabolic measurements are provided below). Our previous findings indicated that even just a few days of this same overeating protocol profoundly suppressed plasma GH secretion (7). Therefore, to assess the metabolic consequences of this suppression of GH in the present study, we administered 4 daily injections of exogenous GH to a cohort of eight subjects during the 2-wk overeating intervention (details of GH administration are provided below). The objective of these multiple GH injections each day during the overeating intervention was to mimic physiological GH secretion patterns typically found under “normal” eating conditions (i.e., energy balance) and to effectively prevent the reduction in plasma GH that naturally occurs with overeating. The metabolic responses in this cohort of subjects receiving GH treatment (GHT) were compared with a cohort of nine subjects that did not receive GH, and therefore, plasma GH concentration was allowed to fall naturally (control). In addition, as a secondary aim of this study, we also explored the effects of high GH concentrations during overeating in a separate, smaller cohort of five subjects that received a relatively high daily dose of GH (high GHT). In summary, unless otherwise indicated, all data presented herein are mean ± SE for n = 9 control subjects, n = 8 GHT subjects, and n = 5 high-GHT subjects.
Fig. 1.
Diagram of the basic study design for the baseline study and the 2-wk overeating period. After completing a 2-day “baseline” metabolic study, subjects were enrolled into either a “control” overeating group or a growth hormone (GH) treatment (GHT) group. All subjects ate 70 kcal·kg fat-free mass (FFM)−1·day−1 for 2 wk, and metabolic measurements were repeated after 3 days and after 2 wk of overeating. The only difference between the control and GHT groups was that subjects in the GHT groups received 4 daily intravenous injections of GH to prevent the suppression of GH that naturally occurs with overeating.
Experimental Protocol
Before the overeating intervention (i.e., baseline) all subjects were admitted to the Michigan Clinical Research Unit at the University of Michigan Hospital for a 2-day experiment (Fig. 1). During the 1st day of this hospital visit they ate a weight-maintaining diet consisting of 39 kcal·kg fat-free mass (FFM) −1·day−1 (∼2,250 kcal/day; 50% carbohydrate, 35% fat, and 15% protein), and we collected blood samples every 20 min for 24 h to assess their 24-h plasma growth hormone and insulin profiles. In addition, at 0900 on this 1st day, we conducted a meal tolerance test (MTT), during which subjects ate 10 kcal/kg body wt (50% carbohydrate, 15% protein, and 35% fat), and we collected blood samples every 20 min for 2 h for assessment of postprandial plasma glucose and insulin concentrations. On the morning of the 2nd day of the trial, we performed our “metabolic study” after an overnight fast. During this metabolic study we performed a 6-h, primed, constant-rate infusion of [2H3]leucine, which was used to calculate whole body proteolytic rate [leucine rate of appearance (Ra) in plasma] and skeletal muscle protein synthesis [fractional synthetic rate (FSR)]. At 0900 we started a primed, constant-rate infusion of [2H5]glycerol for assessment of whole body lipolytic rate (glycerol Ra). Three arterialized blood samples were obtained at 1050, 1055, and 1100 for the determination of glycerol Ra and leucine Ra. Muscle biopsies were obtained from the vastus lateralis muscle at 0800 and 1300 to measure the incorporation of the infused [2H3]leucine into skeletal muscle. Muscle biopsies were performed under local anesthesia using a Bergstrom biopsy needle. Muscle samples were immediately cleaned with sterile saline, dried, frozen in liquid nitrogen, and stored at −80°C until later analysis. Blood samples were also collected at the time of the muscle biopsies to determine the isotope enrichment of the precursor pool [α-ketoisocaproate (α-KIC)].
On a separate occasion (often ∼1 wk after the baseline trial), subjects were admitted to the Clinical Research Unit for a 2-wk overeating intervention, and they remained as in-patients throughout the entire 2-wk period. During the 2-wk study, subjects ate 70 kcal·kg FFM−1·day−1 (∼4,000 kcal/day; 50% carbohydrate, 35% fat, and 15% protein). Each day, the subjects were provided three meals (at 0800, 1200, and 1900) and four snacks (at 1000, 1400, 1600, and 2200). Every morning of the 2-wk intervention we collected a blood sample after an overnight fast for measurements of plasma concentrations of insulin, glucose, nonesterified fatty acid (NEFA), and triacylglyceride, and we also measured plasma insulin-like growth factor I (IGF-I) in fasted plasma samples collected at baseline and at the end of the 2-wk overeating period. We reassessed 24-h plasma growth hormone and insulin profiles on days 3 and 4 and again at 2 wk of the overeating period. At 2 wk we also performed the same “metabolic study” as that performed during the baseline trial. Importantly, the meal provided for the MTT after 2 wk was exactly the same as that provided during the baseline trial (in terms of both energy content and macronutrients). Subjects were limited to 1,500 steps/day (as monitored by a pedometer). Body composition was measured using dual-energy X-ray absorptiometry during the baseline trial and at the end of the 2-wk overeating period.
GHT
Eight subjects (7 men and 1 woman) received exogenous GH (0.3 mg genotropin·m2·day−1; diluted in a 5% dextrose solution) in four intravenous injections each day of the 2-wk overeating intervention. This dose of GH was selected on the basis of previous findings from Jaffe et al. (17), who demonstrated that a daily dose of 0.5 mg·m2·day−1 in hypopituitary patients elevated their mean daily plasma GH levels to ∼2 ng/ml. Because our objective was to increase 24-h mean plasma GH concentration to the level we found in our previous study (∼1.2 ng/ml) (7), we used a dose 60% as great as that administered by Jaffe et al. (17). In an attempt to mimic the pattern of endogenous GH secretion (in which GH is typically secreted in a few relatively small pulses during the day, with a large nocturnal GH pulse), one-half of the daily exogenous GH dose was divided equally into three daytime bolus infusions, each lasting 20 min (at 0700, 1100, and 1600), and the other half of the daily GH dose was injected over 60 min starting at 2300. As noted above, the metabolic responses to overeating in this cohort of subjects that received exogenous GHT to prevent the suppression in plasma GH concentration were compared with the responses in nine subjects (7 men and 2 women) in whom plasma GH concentration was allowed to decline naturally during overeating (control). Control subjects received daily infusions of 5% dextrose to match the vehicle infusion in GHT. Seven of these control subjects also participated in our previously published study (7). The dose of GH administered to our GHT group was estimated/calculated to provide just enough GH to prevent the fall in plasma GH concentration with overeating (i.e., mimicking plasma GH profile found in nonovereating conditions). However, to better understand the physiological impact of the magnitude of GH exposure on these metabolic adaptations, a secondary aim of our study was to examine the effects of high plasma GH concentrations during overeating. To accomplish this goal, a separate, smaller cohort of five subjects (4 men and 1 woman) received a relatively high daily dose of GH (high GHT; 1 mg genotropin·m2·day−1) administered in the same manner as described above for GHT. This higher dose of GH was chosen to match doses used in previous studies examining GH administration in obesity (11, 36).
Analytical Procedures
Plasma concentrations of hormones and substrates.
Plasma GH concentration was determined by chemiluminescent assay on a Siemens Immulite System (Diagnostic Products, Los Angeles, CA), which measures both 20K and 22K GH isoforms. Plasma IGF-I concentration was determined via ELISA kit (Diagnostic Systems Laboratories, Brea, CA), and plasma insulin concentration was determined by radioimmunoassay (Millipore, Billerica, MA). Plasma concentrations of glucose (glucose oxidase; Thermo Fisher Scientific, Waltham, MA), NEFA (HR Series NEFA; Wako Chemicals USA, Richmond, VA), and triacylglyceride reagent (Sigma Aldrich, St. Louis, MO) were all determined by colorimetric assay.
Tracer/tracee ratios of [2H5]glycerol, [2H5]leucine, and [2H3]α-KIC in plasma.
Plasma samples (250 μl) were deproteinized in ice-cold acetone. For tracer/tracee ratio (TTR) measurements of [2H5]glycerol, the deproteinized samples were derivatized in pyridine and acetic anhydride, as reported previously (39). Glycerol TTR was determined using gas chromatography/mass spectrometry (GC-MS) with selectively monitored mass-to-charge ratios (m/z) of 145 and 148. For [2H3]leucine TTR, the deproteinized plasma samples were derivatized in 1:1 acetonitrile-N-methyl-N-tert(butyldimethylsilyl)trifluoroacetamide as described previously (15). Leucine TTR was determined using GC-MS with m/z of 202 and 203. For the plasma enrichment of the precursor pool α-KIC, 250 μl of plasma was deproteinized in ice-cold acetonitrile and derivatized with methoxyamine HCl after samples were titrated to pH 10 with KOH and incubated at 60°C for 1 h. Samples were dried, and the methoxyamine derivative of α-KIC was extracted using acetonitrile. Samples were again derivatized in 1:1 acetonitrile-N-methyl-N-tert(butyldimethylsilyl)trifluoroacetamide. TTR of α-KIC was determined using GC-MS with m/z of 216 and 219.
Skeletal muscle leucine TTR.
Leucine TTR in skeletal muscle was determined as described previously (15). Briefly, ∼20 mg of muscle tissue was homogenized in 1 ml of 3% trichloroacetic acid and centrifuged at 2,800 rfc for 20 min. The resulting pellet was washed in saline four times before incubation in 6 N HCl at 100°C for 24 h. Hydrolyzate was then dried under vacuum and derivatized in 1:1 acetonitrile-N-methyl-N-tert(butyldimethylsilyl)trifluoroacetamide. Muscle leucine TTR was determined using GC-MS with m/z of 202 and 203.
Skeletal muscle mTOR signaling.
We measured the protein content of the S6 ribosomal protein and phosphorylated S6 (Ser235/236), a downstream target of the mTOR TORC1 complex, using immunoblot analysis as described previously (21). Briefly, 30 μg of protein was separated on SDS-PAGE and transferred to nitrocellulose membrane. These membranes were incubated with anti-S6 ribosomal protein (no. 2,212; Cell Signaling Technology) and anti-phospho-S6 ribosomal protein (no. 2,211; Cell Signaling Technology). The blots were washed and developed via enhanced chemiluminescence (GE Healthcare) and quantified using AlphaEaseFC (AlphaInnotech, San Leandro, CA).
Calculations
Homeostatic model assessment of insulin resistance.
Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated by the equation HOMA-IR = (fasting plasma insulin × fasting plasma glucose)/k, where k represents a constant of 405 (24).
Area under the curve.
Area under the curve (AUC) for plasma glucose and insulin concentrations during the 2-h period after the test meal and 24-h insulin and GH AUCs were calculated using the trapezoidal rule.
Glycerol Ra and leucine Ra.
Glycerol Ra and leucine Ra were calculated by the steady-state Steele equation (37). Ra is equal to the rate of infusion of tracer divided by the average tracer/tracee ratio measured in the samples collected at steady state.
Skeletal muscle protein FSR.
FSR was calculated as the rate of [2H3]leucine tracer incorporated into skeletal muscle protein using the plasma α-KIC enrichment as the precursor pool (46) and the equation FSR (%/h) = [(E2 − E1) × 100]/[Ep × (t1 − t0)], where E2 and E1 represent muscle [2H3]leucine enrichments after 6 and 1 h, respectively, Ep represents the average plasma [2H3]αKIC enrichment, and (t1 − t0) represents the time between muscle biopsies (5 h). Unfortunately, because of tissue sample limitations we were not able to calculate muscle protein FSR in three of our control subjects (we did acquire adequate muscle samples from all of subjects in the GHT and high-GHT groups). Therefore, the FSR data presented in results were for six control subjects, eight GHT subjects, and five high-GHT subjects.
Statistical Analysis
Two-way repeated-measures ANOVAs with one factor repeated were used to determine significant differences with respect to day and treatment group. Tukey's post hoc analysis was then used to determine significant differences, with the α-level set to 0.05. All statistical analyses were performed using SigmaPlot software for Windows version 11.0. All data are presented as means ± SE.
RESULTS
Plasma Hormone and Substrate Concentrations
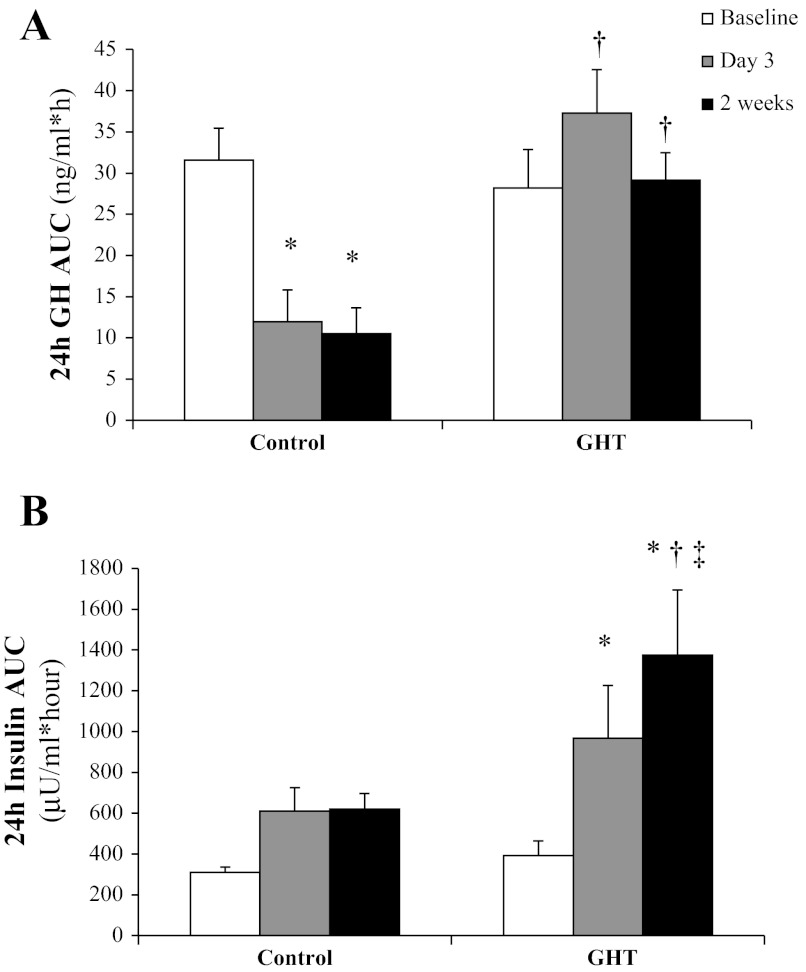
As we have reported previously (7), overeating suppressed 24-h plasma GH concentration by ∼70% (P < 0.05). As designed, GHT prevented the reduction in systemic GH concentration during overeating (Fig. 2A). Also, as designed, the separate group of subjects who received the higher daily doses of GH throughout the overeating intervention (high GHT) had markedly elevated 24-h average plasma GH concentrations (0.4 ± 0.1, 1.2 ± 0.1, and 4.1 ± 0.3 ng/ml at 2 wk for control, GHT, and high GHT, respectively, all P < 0.05). Because the major objective for this study was to examine how the suppression of GH that naturally occurs with overeating might underlie some of the key metabolic responses to overeating, our results (and figures) are focused primarily on the comparison between control vs. GHT. However, as a secondary comparison, we have also included data from the high GHT group for our main metabolic outcome measurements to give valuable insight into the impact that the magnitude of plasma GH exposure may have on these responses.
Fig. 2.
Twenty-four-hour area under the curve (AUC) for plasma GH concentration measured every 20 min for 24 h (A) and 24-h AUC plasma insulin concentration measured every 2 h for 24 h (B) before overeating, by day 3 of overeating, and by the end of the 2-wk overeating period in subjects who did not receive any GH treatment (control) and subjects who received low doses of GH daily (GHT). *Significantly different from baseline within treatment, P < 0.05. †Significantly different from control on the same day, P < 0.05. ‡Significantly greater than day 3, P < 0.05.
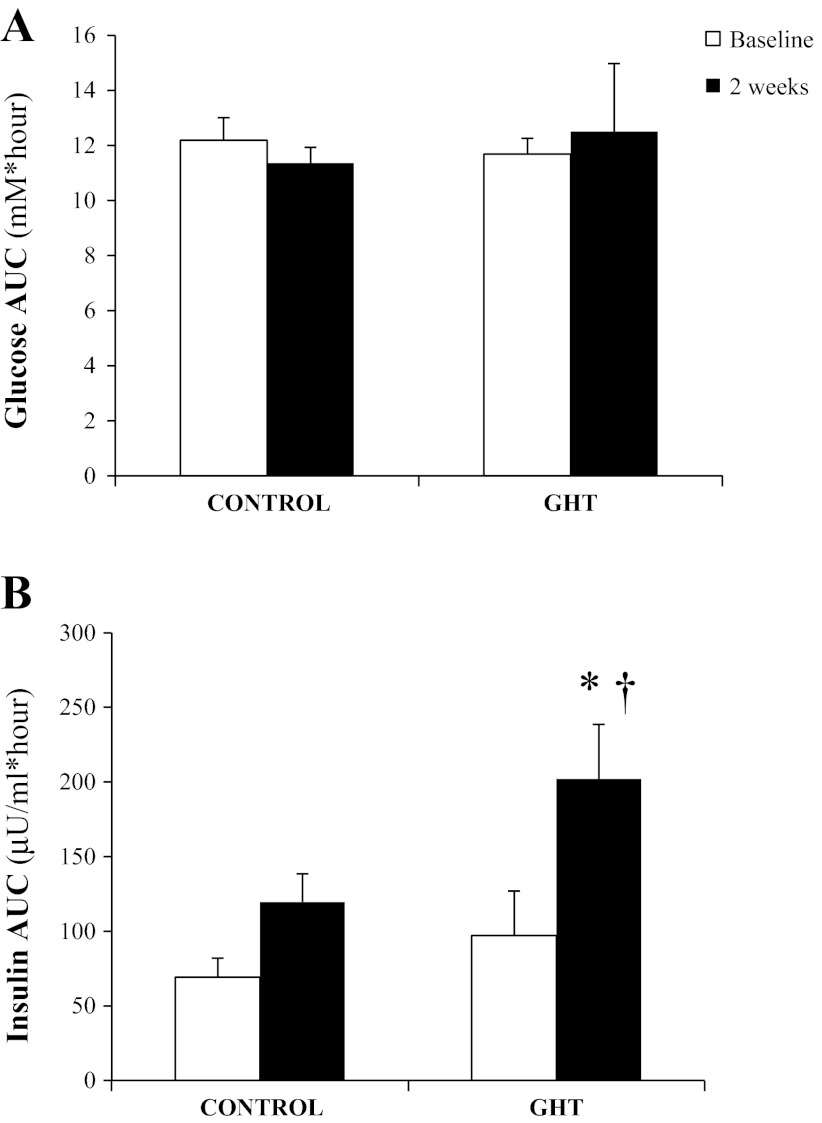
As expected, overeating elevated 24-h insulin concentration compared with baseline (Fig. 2B), and this elevated AUC during overeating was clearly a reflection of the greater total amount of insulin secreted in response to the larger and more frequent meals. Preventing the fall in GH during overeating resulted in an even greater elevation in 24-h plasma insulin at 2 wk in GHT compared with control (P < 0.05; Fig. 2B). During the MTT, the plasma glucose response to the meal was well maintained after 2 wk of overeating compared with baseline for both control and GHT (Fig. 3A). In contrast, plasma insulin response during the 2-h MTT was elevated at 2 wk of overeating (Fig. 3B). This increase in insulin AUC after the meal at 2 wk compared with baseline did not quite reach statistical significance in our control subjects (P = 0.09). However, the insulin response to the same meal doubled after 2 wk of overeating in our GHT cohort (P < 0.001), and insulin AUC was significantly greater at 2 wk in GHT compared with control (P < 0.02; Fig. 3B). Interestingly, insulin AUC in response to the MTT was similar in GHT and high GHT (248 ± 57 and 202 ± 37 μU·ml−1·h−1, respectively) despite nearly fourfold greater daily GH exposure during high GHT.
Fig. 3.
Plasma glucose AUC (A) and plasma insulin AUC (B) during the 2-h period immediately after the standardized test meal was ingested during the meal tolerance test (MTT) before overeating (baseline) and at 2 wk of overeating in subjects who did not receive any GH treatment (control) and subjects that received low doses of GH daily (GHT). The energy and macronutrient content of the MTT was identical before and during the overeating period. *Significantly greater than baseline within treatment, P < 0.05. †Significantly different from control at 2 wk, P < 0.05 (n = 8 subjects in each group; 1 control subject did not complete the MTT at 2 wk).
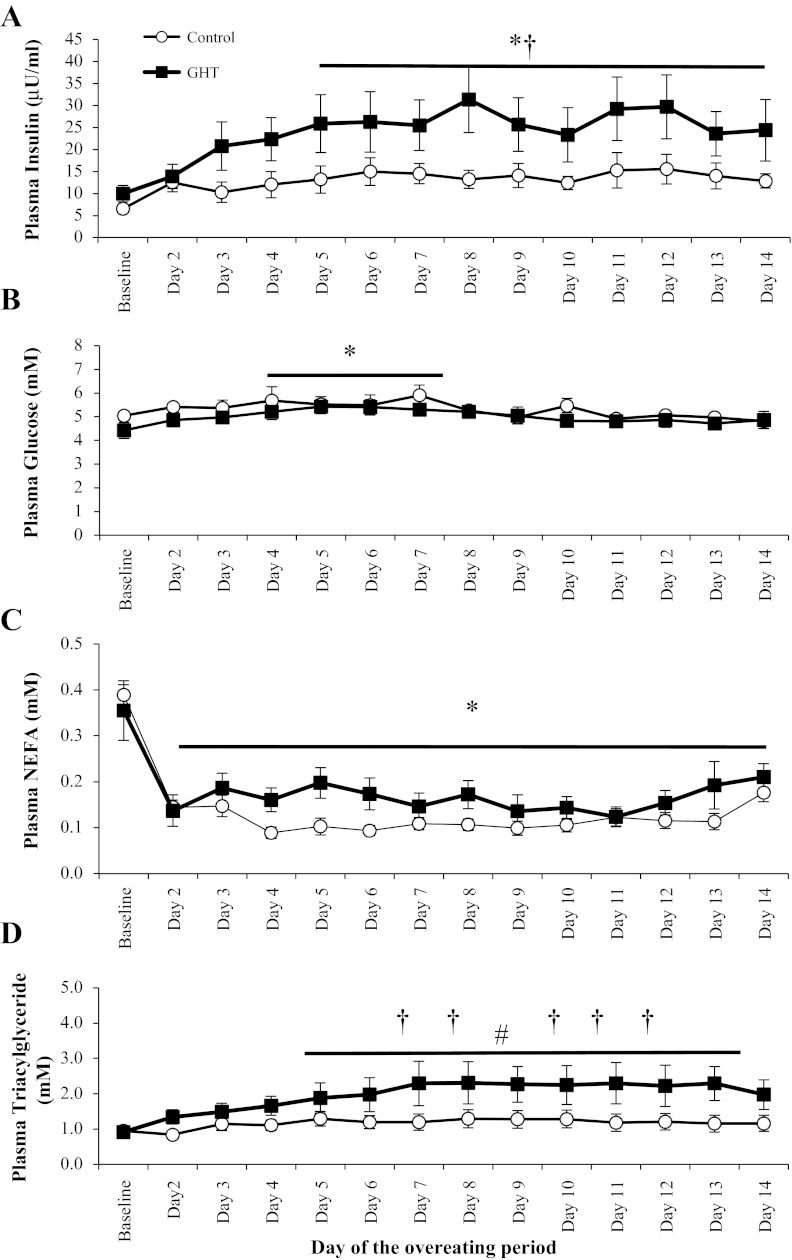
Fasting plasma insulin concentration was elevated above baseline levels by the 5th day of overeating (P < 0.05; Fig. 4A), and preventing the fall in GH nearly doubled fasting plasma insulin concentrations in GHT compared with control (P < 0.05; Fig. 4A). Despite the marked elevation in fasting plasma insulin concentration in GHT vs. control, fasting plasma glucose was identical between these two trials throughout the overeating period (Fig. 4B). These fasting plasma glucose and insulin concentrations can be used to calculate HOMA-IR, which provides some additional evidence to suggest that insulin resistance was greater after 2 wk of overeating in GHT compared with control (HOMA-IR: 5.40 ± 0.70 vs. 2.59 ± 0.74, respectively, P < 0.05).
Fig. 4.
Fasting plasma concentrations of insulin (A), glucose (B), nonesterified fatty acids (NEFA; C), and triacylglyceride (D) measured each morning after an overnight fast throughout the 2-wk overeating intervention. *Significantly different from baseline, P < 0.05. †GHT significantly different from control on the same day, P < 0.05. #Significantly different from baseline on days 5–14 in the GHT group, P < 0.05.
Unlike fasting plasma glucose concentration, which was reasonably well maintained throughout the overeating period, overeating suppressed fasting plasma NEFA concentration to low levels throughout the intervention in both groups (Fig. 4C). Overeating did not significantly elevate fasting plasma triacylglyceride concentrations in control, but preventing the suppression of GH with overeating augmented fasting lipidemia (Fig. 4D). Fasting plasma triacylglyceride concentrations during GHT were significantly greater than baseline levels by the 5th day of overeating as well as greater than control throughout much of the 2nd week of overeating (P < 0.05; Fig. 4D). Plasma total IGF-I concentration remained relatively stable throughout the 2-wk overeating period in control (152 ± 16 vs. 170 ± 15 ng/ml, baseline vs. 2 wk, respectively, P = 0.54), but GHT markedly increased plasma IGF-I concentration (139 ± 13 vs. 324 ± 28 ng/ml, baseline vs. 2 wk, respectively, P < 0.05). After 2 wk of overeating, we found no differences between subjects in the GHT group compared with those receiving the high GHT dose for plasma concentrations of insulin (22.4 ± 7.1 vs. 24.7 ± 5.2 μU/ml), glucose (4.9 ± 0.4 vs. 5.2 ± 0.25 mM), NEFA (0.21 ± 0.03 vs. 0.25 ± 0.05 mM), triacylglyceride (2.3 ± 0.48 vs. 2.1 ± 0.2 mM), and total IGF-I (325 ± 28 vs. 380 ± 21 ng/ml) despite the nearly fourfold greater GH exposure in the high-GHT group.
Body Composition
Our 2-wk overeating intervention increased body weight by ∼5%, and fat mass increased ∼10% in both groups (both P < 0.05; Table 1). We observed a trend (P = 0.1) for a greater weight gain when we prevented the reduction in GH with overeating (in our GHT group) compared with a relatively low GH concentration in control (Table 1). There was no difference in the amount of fat gained between the groups (Table 1), so the trend for a difference in weight gain between control and GHT appeared to be due to a subtle, yet significant (P < 0.05) difference in gain of FFM (Table 1). Subjects receiving the high GHT during the 2-wk overeating period increased their body weight by 3.1 ± 0.7 kg and increased fat mass by 1.1 ± 0.3 kg (both P < 0.05 vs. baseline), which was similar to the gains in body weight and fat mass in control and GHT. Also similar to GHT, the high-GHT group demonstrated a significant increase in FFM after 2 wk (2.3 ± 0.6 kg, P < 0.05 vs. baseline).
Table 1.
Changes in body weight, fat mass, and fat-free mass during the 2-wk overeating period in control and GHT
| Control |
GHT |
|||||
|---|---|---|---|---|---|---|
| Baseline | 2 wk | Δ | Baseline | 2 wk | Δ | |
| Body weight, kg | 75.0 ± 2.6 | 77.1 ± 2.7* | 2.4 ± 0.6 | 75.3 ± 3.3 | 79.1 ± 3.8* | 3.6 ± 0.6 |
| Fat mass, kg | 19.8 ± 2.4 | 21.2 ± 2.0* | 1.6 ± 0.3 | 16.8 ± 2.4 | 19.2 ± 2.6* | 1.7 ± 0.2 |
| Fat-free mass, kg | 55.2 ± 2.7 | 55.9 ± 2.8 | 0.7 ± 0.3 | 58.5 ± 3.0 | 59.9 ± 3.5* | 2.2 ± 0.3† |
Values are means ± SE; n = 9 control and n = 8 growth hormone treatment (GHT).
Significantly different from baseline, P < 0.05.
Significantly different from control, P < 0.05.
Lipolytic Rate (Glycerol Ra)
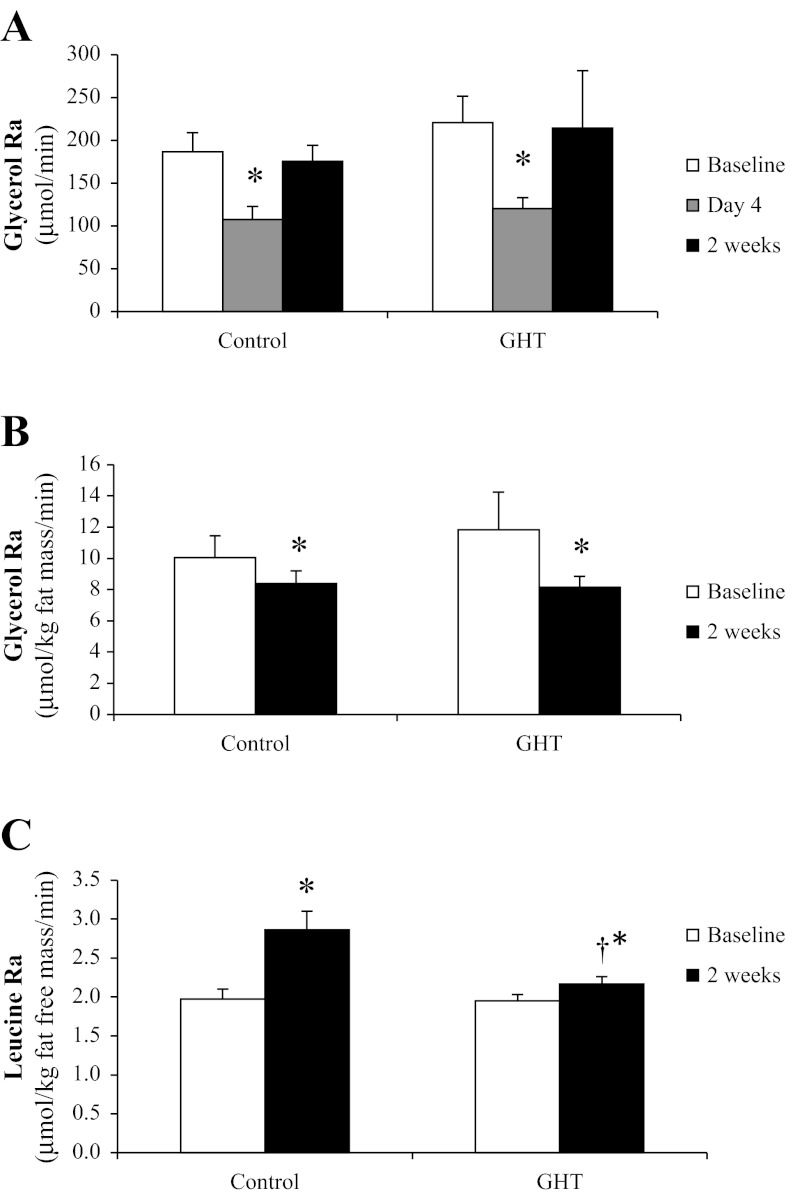
Fasting lipolysis was suppressed by the morning of the 4th day of overeating in both control and GHT (P < 0.05; Fig. 5A). However, by the end of the 2-wk overeating period, the absolute rate of lipolysis (μmol/min) had returned to baseline levels in both groups (Fig. 5A). Importantly, when expressed relative to fat mass, lipolytic rate was still suppressed by the end of the 2 wk (Fig. 5B). Therefore, the apparent “rebound” in the absolute lipolytic rate observed by the end of the 2-wk intervention in Fig. 5A was likely not a consequence of an intrinsic increase in the rate of lipolysis per unit fat mass but rather due to a greater absolute lipolytic rate stemming simply from the greater fat mass accrued from overeating. There were no differences in glycerol Ra between control and GHT. Similar to control and GHT, high GHT also demonstrated a suppressed absolute lipolytic rate on the morning of the 4th day of overeating and then rebounded by 2 wk of overeating (195 ± 25, 169 ± 22, and 192 ± 17 μmol/min at baseline, day 4, and 2 wk, respectively), and when expressed relative to fat mass, lipolytic rate remained suppressed at 2 wk (14.0 ± 3.0 vs. 11.8 ± 0.6 μmol·kg fat mass−1·min−1).
Fig. 5.
Lipolytic rate [glycerol rate of appearance (Ra)] measured after an overnight fast before and after the 2-wk overeating intervention expressed in absolute terms (μmol/min; A) and expressed relative to fat mass (μmol·kg fat mass−1·min−1; B) in both control and GHT. C: proteolytic rate (leucine Ra) measured after an overnight fast before and after the 2-wk overeating intervention in both control and GHT. *Significantly different from baseline within treatment, P < 0.05. †Significantly different from control at 2 wk, P < 0.05.
Protein Metabolism
Whole body proteolytic rate (leucine Ra) in the overnight-fasted state increased significantly with overeating in control (P < 0.05; Fig. 5C). Interestingly, this increase in leucine Ra with overeating was not observed when we prevented the reduction in systemic GH during overfeeding with GHT (P < 0.05 vs. control at 2 wk; Fig. 5C). Similarly to our GHT group, high GHT also prevented the relatively large increase in proteolysis during overeating (leucine Ra in the high GHT group: 1.6 ± 0.1 vs. 1.9 ± 0.1 mmol·kg FFM−1·min−1 at baseline and 2 wk, respectively). There was a trend for 2 wk of overeating to increase the rate of skeletal muscle protein synthesis after an overnight fast in both control (FSR: 0.043 ± 0.009 to 0.063 ± 0.015%/h) and GHT (FSR: 0.047 ± 0.011 to 0.062 ± 0.018%/h), but these changes did not quite reach statistical significance (main effect for overeating: P = 0.09), which was likely a consequence of the relatively small sample size. Moreover, there was no difference in FSR between control, GHT, and high GHT. In support of our finding that GHT did not affect muscle protein synthesis, we also found the phosphorylation of the ribosomal protein S6 in skeletal muscle to be unaffected by overeating during control, GHT, or high GHT (P ≥ 0.4; data not shown). S6 is a downstream target of mTOR complex 1 and a key component in the regulation of protein synthesis.
DISCUSSION
Our previous findings provided the first evidence demonstrating the profound reduction in GH secretion with overeating (7). Our objective here was to examine the metabolic consequences of this marked suppression in GH during overeating by preventing the overeating-induced reduction in plasma GH via administration of exogenous GH throughout the overeating period in a manner that would mimic normal endogenous GH secretion (in terms of both magnitude and pulsatility). Major findings from this study indicate that preventing the fall in plasma GH during overeating impaired meal tolerance (as noted by a greater insulin excursion in response to the test meal) and elevated fasting plasma triacylglyceride concentration. We also found that overeating increased whole body proteolytic rate in the overnight-fasted state. Importantly, the suppression in plasma GH with overeating may underlie this increase in protein hydrolysis because preventing the reduction in plasma GH concentration during overeating blunted this increase in systemic proteolysis. Overall, our major findings suggest that the natural suppression in GH that occurs with overeating may help mitigate the rise of risk factors for metabolic and cardiovascular disease associated with overeating.
It was not surprising that overeating impaired meal tolerance, but our novel finding that plasma insulin response to a test meal at 2 wk was 70% greater in GHT subjects compared with control suggests the overeating-induced suppression in GH may actually help attenuate the overeating-induced impairment in meal tolerance. GH administration has been clearly linked with the development of insulin resistance (1, 28). However, because our GHT simply prevented the fall in plasma GH with overeating (rather than elevating GH to high levels), our novel findings indicate that the natural fall in GH secretion that occurs with overeating helps protect against impaired meal glucose tolerance. Our observation that the plasma insulin response to a test meal was similar in our GHT and high GHT groups suggests that, at least within the physiological range of plasma GH concentration, the magnitude of GH exposure may not be a key factor regulating insulin resistance. The link between GH and systemic insulin resistance has been attributed largely to effects of GH on the insulin-signaling pathway in liver and skeletal muscle. For example, GH-induced insulin resistance has been attributed to increased expression of suppressor of cytokine signaling-3 (28) and increased abundance of the regulatory subunit of phosphoinositol-3 kinase p85α (1) as well as increased phosphorylation and subsequent activation of c-Jun NH2-terminal kinase, a key inflammatory factor that can suppress insulin signaling (42). Therefore, the natural suppression in GH during conditions of caloric excess may help protect against further development of insulin resistance by attenuating GH-induced signaling events that normally disrupt insulin action.
Overeating per se did not significantly elevate fasting plasma triacylglyceride concentration in our control group. Previous studies have reported elevated fasting lipidemia with overeating, but typically, these overfeeding protocols contained a very high proportion of dietary carbohydrate (≥70% carbohydrate) (25, 26), which is known to accelerate hepatic de novo lipogenesis and increase fasting plasma triacylglyceride concentrations (25). The proportion of dietary carbohydrate to total energy intake in our study was 50%, which is relatively low compared with the high proportion of carbohydrate provided in these other studies (25, 26). In contrast to our control group, fasting plasma triacylglyceride concentration increased twofold above baseline levels with overeating when we prevented the fall in GH (with GHT). Although GH administration has been shown to increase plasma triacylglyceride concentration (30, 47), our study expands on these findings by demonstrating the reduction in GH that normally occurs when overeating attenuates hyperlipidemia, at least in the short term. The accumulation of plasma triacylglycerides in the overnight-fasted state is largely a balance between triacylglyceride production and the hydrolysis of the triacylglyceride within the circulation by lipoprotein lipase. In the overnight-fasted state, triacylglycerides are almost exclusively packaged within hepatically derived very low-density lipoproteins (VLDL) (23), and available evidence suggests that GH may either increase apolipoprotein B synthesis (6) or have a limited effect (19) on VLDL-triacylglyceride production. Conversely, GH has been reported to inhibit both adipose tissue and skeletal muscle lipoprotein lipase activity (30, 34, 40). Therefore, the effect of GH on plasma triacylglyceride accumulation may be primarily through its effect on inhibiting plasma triacylglyceride hydrolysis, with perhaps some contribution from an increase in VLDL production, thereby increasing fasting plasma triacylglyceride concentration. Because hyperlipidemia is a major risk factor for the development of cardiovascular disease, this effect of the suppression in GH on plasma triglyceride concentration during overeating may have important clinical relevance.
Although prolonged overeating is often found to induce at least a modest increase in FFM (8, 12, 43), the relatively small increase in FFM in our control group did not reach statistical significance likely because of the short duration of our intervention. In contrast, preventing the fall in GH throughout our brief, 2-wk overeating intervention did indeed induce a measurable increase in FFM. This finding is largely consistent with previous work reporting that exogenous GH administration for several weeks increased FFM in obese women (33) as well as in patients with growth hormone deficiency (38). However, the daily GH doses in these previous studies (33, 38) were four- to fivefold greater than our GHT, and they were provided as a single GH bolus in the evening (rather than as more physiological pulses throughout the day as in our study). Therefore, the effect of GH on FFM may be largely independent of the magnitude of the GH dose. This notion is further supported by our finding that the increase in FFM was identical in our GHT and high GHT groups despite a three- to fourfold greater exposure to GH in the latter. We must acknowledge that GH treatment can increase water retention (27), which could contribute to the measured increase in FFM. However, GH is known to increase FFM beyond its effects on hydration (10), yet the specific mechanisms underlying the effect of GH on FFM are not completely understood.
The accrual of FFM depends on the balance of proteolysis and protein synthesis. Our finding that GHT attenuated the proteolytic rate during overeating suggests that a GH-mediated reduction in the rate of protein breakdown may have contributed to the increase in FFM in this group. Putative mechanisms for the antiproteolytic effect of GH are not well described. GH may suppress proteolysis indirectly through the actions of the elevated plasma insulin and IGF-I concentrations (5, 14, 35) that we observed in the GHT group. Importantly, the increased FFM in our GHT groups was not a consequence of increased muscle protein synthesis. Despite the common misconception that GH administration induces a marked increase in muscle mass, many well-controlled studies have confirmed that GH does not augment skeletal muscle protein synthesis in humans (32, 45, 48). Although there is some evidence suggesting that plasma IGF-I, which is secreted by the liver in response to GH, may increase muscle protein synthesis in animal studies (22), we found no increase in skeletal muscle protein synthesis despite a threefold increase in plasma IGF-I concentration in our subjects, which supports previous findings in human subjects (48). Therefore, in our study, a GH-mediated reduction in systemic proteolytic rate, rather than increased skeletal muscle protein synthesis, likely contributed to the increased FFM we observed when we prevented the suppression in GH with overeating. Alternatively, our data also imply that the normal suppression in GH with overeating may augment the rate of protein degradation, at least in the short term.
Although GH has been characterized as a lipolytic hormone (9, 39), our findings suggest that the suppression in GH with overeating did not alter whole body lipolytic rate. Because our overfed subjects were hyperinsulinemic, it is likely that the potent antilipolytic action of insulin (3, 29) countered any lipolytic effects of GH (20). Along these lines, the suppression in lipolytic rate we found by the morning of the 4th day of overeating was accompanied by a twofold increase in fasting plasma insulin concentration. Interestingly, despite a persistent elevation in fasting insulin concentration by 2 wk of overeating, lipolysis returned to basal levels. This “rebound” in lipolytic rate by 2 wk of overeating may be due to the competing influences of hyperinsulinemia that suppresses lipolysis and the increased fat mass that augments total, whole body lipolytic rate (16). In support of this hypothesis, when we expressed lipolytic rate relative to fat mass to effectively remove the influence of fat mass on lipolysis, lipolytic rate remained suppressed at 2 wk of overeating, which may largely reflect the antilipolytic effect of the hyperinsulinemia.
In summary, the suppression of plasma GH concentration that naturally occurs with overeating may influence insulin action, plasma triacylglyceride concentration, and whole body proteolytic rate. The fall in GH concentration that occurs with overeating may act to mitigate the development of insulin resistance and hyperlipidemia that can arise with overeating. Alternatively, the suppression in GH may suppress the accrual of FFM that can occur with overeating by accelerating proteolytic rate. Additionally, the GH fall during overeating did not influence lipolysis, reflecting the limited role of GH in mediating lipolytic rate during overeating and suggesting that the suppression of lipolysis may be tied to the elevated fasting insulin concentration. It is important to acknowledge that most of our metabolic measurements here were performed in the postabsorptive state, so future experiments are warranted to assess metabolic consequences to the overeating-induced suppression in GH in the postprandial condition. However, comparing postprandial metabolic responses during overeating vs. measurements made before overeating presents the considerable challenge of distinguishing the acute metabolic effects stemming simply from a larger meal vs. metabolic adaptations that accrue as a result of more prolonged overeating. Overall, the key findings from our study indicate that the suppression of GH secretion that normally occurs during the early stages of overeating may help attenuate the insulin resistance and hyperlipidemia that are typically found with overeating.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-71955, with additional support from the Michigan Clinical Research Unit (NIH-UL1-RR-024986) and the Human Phenotyping Core of the Michigan Nutritional Obesity Research Center (P30-DK-089503).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.S.C., A.L.B., A.H., and J.F.H. performed the experiments; A.S.C., A.L.B., A.H., and J.F.H. analyzed the data; A.S.C., A.L.B., A.H., and J.F.H. interpreted the results of the experiments; A.S.C. and J.F.H. prepared the figures; A.S.C. and J.F.H. drafted the manuscript; A.S.C., A.L.B., and J.F.H. edited and revised the manuscript; A.S.C., A.L.B., A.H., and J.F.H. approved the final version of the manuscript; A.L.B. and J.F.H. did the conception and design of the research.
ACKNOWLEDGMENTS
We acknowledge the expert technical assistance of Kathleen Symons. We also acknowledge the efforts of our research dieticians and study coordinators Lisa Locke Michael and Suzette Howton.
REFERENCES
- 1. Barbour LA, Mizanoor Rahman S, Gurevich I, Leitner JW, Fischer SJ, Roper MD, Knotts TA, Vo Y, McCurdy CE, Yakar S, Leroith D, Kahn CR, Cantley LC, Friedman JE, Draznin B. Increased P85alpha is a potent negative regulator of skeletal muscle insulin signaling and induces in vivo insulin resistance associated with growth hormone excess. J Biol Chem 280: 37489– 37494, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Brons C, Jensen CB, Storgaard H, Hiscock NJ, White A, Appel JS, Jacobsen S, Nilsson E, Larsen CM, Astrup A, Quistorff B, Vaag A. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J Physiol 587: 2387– 2397, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Campbell PJ, Carlson MG, Hill JO, Nurjhan N. Regulation of free fatty acid metabolism by insulin in humans: role of lipolysis and reesterification. Am J Physiol 26: E1063– E1069, 1992 [DOI] [PubMed] [Google Scholar]
- 4. Cersosimo E, Danou F, Persson M, Miles JM. Effects of pulsatile delivery of basal growth hormone on lipolysis in humans. Am J Physiol Endocrinol Metab 271: E123– E126, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Chen Q, Li N, Zhu W, Li W, Tang S, Yu W, Gao T, Zhang J, Li J. Insulin alleviates degradation of skeletal muscle protein by inhibiting the ubiquitin-proteasome system in septic rats. J Inflamm (Lond) 8: 13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christ ER, Cummings MH, Albany E, Umpleby AM, Lumb PJ, Wierzbicki AS, Naoumova RP, Boroujerdi MA, Sönksen PH, Russell-Jones DL. Effects of growth hormone (GH) replacement therapy on very low density lipoprotein apolipoprotein B100 kinetics in patients with adult GH deficiency: a stable isotope study. J Clin Endocrinol Metab 84: 307– 316, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Cornford AS, Barkan AL, Horowitz JF. Rapid suppression of growth hormone concentration by overeating: potential mediation by hyperinsulinemia. J Clin Endocrinol Metab 96: 824– 830, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diaz EO, Prentice AM, Goldberg GR, Murgatroyd PR, Coward WA. Metabolic response to experimental overfeeding in lean and overweight healthy volunteers. Am J Clin Nutr 56: 641– 655, 1992 [DOI] [PubMed] [Google Scholar]
- 9. Dietz J, Schwartz J. Growth hormone alters lipolysis and hormone-sensitive lipase activity in 3T3-F442A adipocytes. Metabolism 40: 800– 806, 1991 [DOI] [PubMed] [Google Scholar]
- 10. Doessing S, Heinemeier KM, Holm L, Mackey AL, Schjerling P, Rennie M, Smith K, Reitelseder S, Kappelgaard AM, Rasmussen MH, Flyvbjerg A, Kjaer M. Growth hormone stimulates the collagen synthesis in human tendon and skeletal muscle without affecting myofibrillar protein synthesis. J Physiol 588: 341– 351, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drent ML, Wever LD, Adèr HJ, van der Veen EA. Growth hormone administration in addition to a very low calorie diet and an exercise program in obese subjects. Eur J Endocrinol 132: 565– 572, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Forbes GB, Brown MR, Welle SL, Lipinski BA. Deliberate overfeeding in women and men: energy cost and composition of the weight gain. Br J Nutr 56: 1– 9, 1986 [DOI] [PubMed] [Google Scholar]
- 13. Fryburg DA, Gelfand RA, Barrett EJ. Growth hormone acutely stimulates forearm muscle protein synthesis in normal humans. Am J Physiol Endocrinol Metab 260: E499– E504, 1991 [DOI] [PubMed] [Google Scholar]
- 14. Glass DJ. Signaling pathways perturbing muscle mass. Curr Opin Clin Nutr Metab Care 13: 225– 229, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Harber MP, Schenk S, Barkan AL, Horowitz JF. Effects of dietary carbohydrate restriction with high protein intake on protein metabolism and the somatotropic axis. J Clin Endocrinol Metab 90: 5175– 5181, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Horowitz JF, Coppack SW, Paramore D, Cryer PE, Zhao G, Klein S. Effect of short-term fasting on lipid kinetics in lean and obese women. Am J Physiol Endocrinol Metab 276: E278– E284, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Jaffe CA, Turgeon DK, Lown K, Demott-Friberg R, Watkins PB. Growth hormone secretion pattern is an independent regulator of growth hormone actions in humans. Am J Physiol Endocrinol Metab 283: E1008– E1015, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol Endocrinol Metab 277: E1130– E1141, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Krag MB, Gormsen LC, Guo Z, Christiansen JS, Jensen MD, Nielsen S, Jørgensen JO. Growth hormone-induced insulin resistance is associated with increased intramyocellular triglyceride content but unaltered VLDL-triglyceride kinetics. Am J Physiol Endocrinol Metab 292: E920– E927, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Krusenstjerna-Hafstrøm T, Madsen M, Vendelbo MH, Pedersen SB, Christiansen JS, Møller N, Jessen N, Jørgensen JO. Insulin and GH signaling in human skeletal muscle in vivo following exogenous GH exposure: impact of an oral glucose load. PLoS One 6: e19392, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li M, Paran C, Wolins NE, Horowitz JF. High muscle lipid content in obesity is not due to enhanced activation of key triglyceride esterification enzymes or the suppression of lipolytic proteins. Am J Physiol Endocrinol Metab 300: E699– E707, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loughna PT, Mason P, Bates PC. Regulation of insulin-like growth factor 1 gene expression in skeletal muscle. Symp Soc Exp Biol 46: 319– 330, 1992 [PubMed] [Google Scholar]
- 23. Magkos F, Sidossis LS. Measuring very low density lipoprotein-triglyceride kinetics in man in vivo: how different the various methods really are. Curr Opin Clin Nutr Metab Care 7: 547– 555, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412– 419, 1985 [DOI] [PubMed] [Google Scholar]
- 25. Minehira K, Bettschart V, Vidal H, Vega N, Di Vetta V, Rey V, Schneiter P, Tappy L. Effect of carbohydrate overfeeding on whole body and adipose tissue metabolism in humans. Obes Res 11: 1096– 1103, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Mittendorfer B, Sidossis LS. Mechanism for the increase in plasma triacylglycerol concentrations after consumption of short-term, high-carbohydrate diets. Am J Clin Nutr 73: 892– 899, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Moller J, Nielsen S, Hansen TK. Growth hormone and fluid retention. Horm Res 51, Suppl 3: 116–120, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Nielsen C, Gormsen LC, Jessen N, Pedersen SB, Møller N, Lund S, Jørgensen JO. Growth hormone signaling in vivo in human muscle and adipose tissue: impact of insulin, substrate background, and growth hormone receptor blockade. J Clin Endocrinol Metab 93: 2842– 2850, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Nurjhan N, Campbell PJ, Kennedy FP, Miles JM, Gerich JE. Insulin dose-response characteristics for suppression of glycerol release and conversion to glucose in humans. Diabetes 35: 1326– 1331, 1986 [DOI] [PubMed] [Google Scholar]
- 30. Oscarsson J, Ottosson M, Johansson JO, Wiklund O, Mårin P, Björntorp P, Bengtsson BA. Two weeks of daily injections and continuous infusion of recombinant human growth hormone (GH) in GH-deficient adults. II. Effects on serum lipoproteins and lipoprotein and hepatic lipase activity. Metabolism 45: 370– 377, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 46: 983– 988, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Rennie MJ. Claims for the anabolic effects of growth hormone: a case of the emperor's new clothes? Br J Sports Med 37: 100– 105, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Richelsen B, Pedersen SB, Børglum JD, Møller-Pedersen T, Jørgensen J, Jørgensen JO. Growth hormone treatment of obese women for 5 wk: effect on body composition and adipose tissue LPL activity. Am J Physiol Endocrinol Metab 266: E211– E216, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Richelsen B, Pedersen SB, Kristensen K, Børglum JD, Nørrelund H, Christiansen JS, Jørgensen JO. Regulation of lipoprotein lipase and hormone-sensitive lipase activity and gene expression in adipose and muscle tissue by growth hormone treatment during weight loss in obese patients. Metabolism 49: 906– 911, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab 287: E591– E601, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Skaggs SR, Crist DM. Exogenous human growth hormone reduces body fat in obese women. Horm Res 35: 19– 24, 1991 [DOI] [PubMed] [Google Scholar]
- 37. Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann NY Acad Sci 82: 420– 430, 1959 [DOI] [PubMed] [Google Scholar]
- 38. Stenlöf K, Sjöström L, Lönn L, Bosaeus I, Kvist H, Tölli J, Lindstedt G, Bengtsson BA. Effects of recombinant human growth hormone on basal metabolic rate in adults with pituitary deficiency. Metabolism 44: 67– 74, 1995 [DOI] [PubMed] [Google Scholar]
- 39. Surya S, Horowitz JF, Goldenberg N, Sakharova A, Harber M, Cornford AS, Symons K, Barkan AL. The pattern of growth hormone delivery to peripheral tissues determines insulin-like growth factor-1 and lipolytic responses in obese subjects. J Clin Endocrinol Metab 94: 2828– 2834, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takahashi S, Satozawa N. The 20-kD human growth hormone reduces body fat by increasing lipolysis and decreasing lipoprotein lipase activity. Horm Res 58: 157– 164, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Tam CS, Viardot A, Clément K, Tordjman J, Tonks K, Greenfield JR, Campbell LV, Samocha-Bonet D, Heilbronn LK. Short-term overfeeding may induce peripheral insulin resistance without altering subcutaneous adipose tissue macrophages in humans. Diabetes 59: 2164– 2170, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Taniguchi CM, Aleman JO, Ueki K, Luo J, Asano T, Kaneto H, Stephanopoulos G, Cantley LC, Kahn CR. The p85alpha regulatory subunit of phosphoinositide 3-kinase potentiates c-Jun N-terminal kinase-mediated insulin resistance. Mol Cell Biol 27: 2830– 2840, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tremblay A, Despres JP, Theriault G, Fournier G, Bouchard C. Overfeeding and energy expenditure in humans. Am J Clin Nutr 56: 857– 862, 1992 [DOI] [PubMed] [Google Scholar]
- 44. Welle S, Matthews DE, Campbell RG, Nair KS. Stimulation of protein turnover by carbohydrate overfeeding in men. Am J Physiol Endocrinol Metab 257: E413– E417, 1989 [DOI] [PubMed] [Google Scholar]
- 45. West DW, Burd NA, Tang JE, Moore DR, Staples AW, Holwerda AM, Baker SK, Phillips SM. Elevations in ostensibly anabolic hormones with resistance exercise enhance neither training-induced muscle hypertrophy nor strength of the elbow flexors. J Appl Physiol 108: 60– 67, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wolfe RR, Goodenough RD, Wolfe MH, Royle GT, Nadel ER. Isotopic analysis of leucine and urea metabolism in exercising humans. J Appl Physiol 52: 458– 466, 1982 [DOI] [PubMed] [Google Scholar]
- 47. Wolthers T, Lemming L, Grøfte T, Møller N, Christiansen JS, Klausen IC, Jørgensen JO. Effects of growth hormone on serum lipids and lipoproteins: possible significance of increased peripheral conversion of thyroxine to triiodothyronine. Metabolism 45: 1016– 1020, 1996 [DOI] [PubMed] [Google Scholar]
- 48. Yarasheski KE, Campbell JA, Smith K, Rennie MJ, Holloszy JO, Bier DM. Effect of growth hormone and resistance exercise on muscle growth in young men. Am J Physiol Endocrinol Metab 262: E261– E267, 1992 [DOI] [PubMed] [Google Scholar]