Abstract
Leucine supplementation has been shown to prevent high-fat diet (HFD)-induced obesity, hyperglycemia, and dyslipidemia in animal models, but the underlying mechanisms are not fully understood. Recent studies suggest that activation of Sirtuin 1 (SIRT1) is an important mechanism to maintain energy and metabolic homeostasis. We therefore examined the involvement of SIRT1 in leucine supplementation-prevented obesity and insulin resistance. To accomplish this goal, male C57BL/6J mice were fed normal diet or HFD, supplemented with or without leucine. After 2 mo of treatment, alterations in SIRT1 expression, insulin signaling, and energy metabolism were analyzed. Eight weeks of HFD induced obesity, fatty liver, mitochondrial dysfunction, hyperglycemia, and insulin resistance in mice. Addition of leucine to HFD correlated with increased expression of SIRT1 and NAMPT (nicotinamide phosphoribosyltransferase) as well as higher intracellular NAD+ levels, which decreased acetylation of peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1α) and forkhead box O1 (FoxO1). The deacetylation of PGC1α may contribute to upregulation of genes controlling mitochondrial biogenesis and fatty acid oxidation, thereby improving mitochondrial function and preventing HFD-induced obesity in mice. Moreover, decreased acetylation of FoxO1 was accompanied by decreased expression of pseudokinase tribble 3 (TRB3) and reduced the association between TRB3 and Akt, which enhanced insulin sensitivity and improved glucose metabolism. Finally, transfection of dominant negative AMPK prevented activation of SIRT1 signaling in HFD-Leu mice. These data suggest that increased expression of SIRT1 after leucine supplementation may lead to reduced acetylation of PGC1α and FoxO1, which is associated with attenuation of HFD-induced mitochondrial dysfunction, insulin resistance, and obesity.
Keywords: leucine supplementation, obesity, SIRT1, PGC1α, FoxO1, insulin resistance
obesity, the most common nutritional disorder in Western countries, is primarily an imbalance between calorie intake and energy expenditure (17). Increasing evidence suggests that obesity and insulin resistance are associated with reduced mitochondrial biogenesis and impaired mitochondrial function (32), which can be triggered by adverse nutrition such as increased fatty acid exposure resulting from high-fat diet (HFD) or overfeeding. Impaired mitochondrial function leads to lipid accumulation in fat tissue, skeletal muscle, and liver, impairing insulin signaling and glucose metabolism (3). Interventions to increase mitochondrial biogenesis and improve mitochondrial function have been shown to correct insulin signaling and metabolic abnormalities (35).
Traditionally, strategies for preventing obesity have focused on dietary and lifestyle modifications such as caloric restriction and increasing physical activity. Although short-term weight loss can be achieved by various dietary approaches, sustainability of weight loss seems to be difficult (19, 22). Recently, several studies have shown that dietary manipulation of essential amino acids, including leucine, arginine, and glutamine, improves lipid and glucose metabolism (13, 31). Zhang et al. (50) reported that increasing dietary leucine intake improves glucose and cholesterol metabolism and prevents obesity in mice subjected to a HFD. However, the effects of dietary leucine on energy metabolism are controversial, because other groups found that dietary supplementation of leucine had no effects on those parameters (27) or found an increase in body weight (28). In addition, leucine has been shown to improve insulin signaling in adipose tissue (16). In other studies, however, administration of branched-chain amino acids (BCAA) enhanced insulin resistance in rats maintained on HFD (28). Thus, the effects of dietary leucine supplementation on energy metabolism and its underlying mechanisms remain to be determined.
Sirtuin 1 (SIRT1), an NAD+-dependent deacetylase, has been implicated in aging, metabolic regulation, and tolerance to oxidative stress. Increasing evidence suggests that activation of SIRT1 enhances glucose utilization, increases mitochondrial fatty acid oxidation (FAO), and promotes insulin sensitivity (18). In skeletal muscle, SIRT1 deacetylates peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1α) to induce the expression of genes controlling mitochondrial biogenesis (23) and FAO to maintain energy homeostasis (14). In the liver, SIRT1 stimulates gluconeogenesis through deacetylation of PGC1α and forkhead box O1 (FoxO1) (12, 36) under calorie restriction conditions. Moreover, activation of SIRT1 improves mitochondrial function and protects against diet-induced obesity and insulin resistance (21), suggesting that activation of SIRT1 may be an important mechanism to prevent metabolic disorders. In the present study, we have examined the involvement of SIRT1 in dietary leucine-prevented obesity and insulin resistance in HFD-induced obesity. Results from this study show that supplementation of leucine is associated with activation of SIRT1 signaling, suppression of insulin resistance, and prevention against metabolic disorders in a diet-induced obesity animal model.
MATERIALS AND METHODS
Experimental animals.
Male C57BL/6J mice from the Jackson Laboratories (Bar Harbor, ME) were used in the experiments. The mice were maintained on a 12:12-h light-dark cycle at 25°C and given free access to water and normal diet (ND) before the experiment. The animal protocol was reviewed and approved by the University of Oklahoma Institutional Animal Care and Use Committee. At 12 wk of age, the mice were fed ND (protein 20%, carbohydrate 70%, fat 10%; D12450B, Research Diet, New Brunswick, NJ) or HFD (protein 20%, carbohydrate 35%, fat 45%, total 5.7 kcal/g, D12451, Research Diet) supplemented with or without l-leucine (Sigma, Atlanta, GA; 1.5 g/100 ml in drink water). After 2 mo of treatment, the animals were euthanized, and the body weights were determined using an electronic balance. Total white adipose tissue including subcutaneous and visceral fat (both gonadal and perirenal) in the mice were collected and weighed using a balance. The livers, brown adipose tissues (BAT), and gastrocnemius muscles were collected for morphological, biochemical, and molecular biological analyses. To determine whether AMP-activated protein kinase (AMPK) mediates the effect of leucine on SIRT1 signaling, after 4 wk of HFD treatment the mice were randomly assigned to be transfected with adenovirus encoding GFP or dominant negative AMPK (DN-AMPK) (8) for 4 wk. The SIRT1 signaling was evaluated after the treatment.
Hyperinsulinemic-euglycemic clamp and intraperitoneal glucose tolerance test.
Hyperinsulinemic-euglycemic clamps were performed as described previously (42). Mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (20 mg/kg). Heparin (200 U/ml) was intraperitoneally injected to prevent blood clotting. A polyethylene catheter was inserted into the right jugular vein for infusion of 20% glucose and insulin (200 mU/100 ml) in normal saline using a peristaltic pump. The left carotid artery was cannulated for blood sampling. Insulin (18 mU·kg−1·min−1) was infused through the jugular vein catheter from 0 to 120 min. During that period, blood glucose concentration was monitored every 5 min with a glucometer and clamped at euglycemic levels (5.0 ± 0.5 mmol/l) by a variable infusion of 20% glucose. Clamping was achieved by 90 min and maintained for 30 min. The mean glucose infusion rates (ml·min−1·kg−1) were calculated during the last 30 min of the clamp. To determine blood glucose levels, mice were fasted overnight and blood glucose was monitored by applying tail blood to the glucometer. Following an overnight fast, a glucose tolerance test was performed using an intraperitoneal injection of 1 g/kg body wt of 20% glucose in mice, and blood glucose levels were measured at 0, 30, 60, 90, and 120 min using the glucometer.
Measurement of cholesterol, triglyceride, and serum leucine concentration.
Serum cholesterol and triglyceride levels were measured enzymatically using reagents from Cayman Chemical (Ann Arbor, MI) according to the manufacturer's instructions. To determine liver triglycerides levels, liver lipids were extracted as described by Folch et al. (11). Triglyceride contents in lipid extracts were assayed using enzymatic kits (Cayman Chemical) according to the manufacturer's protocols. Serum leucine concentrations were analyzed by derivatization with phenylisothiocyanate and HPLC analysis as described previously (24).
Western blot analysis and immunoprecipitation.
Antibodies against PGC1α, Akt, phospho-Akt (Ser437), phospho-AMPK (Thr172), AMPK, phospho-acetyl coenzyme A carboxylase at Ser79 (P-ACC), TRB3, and FoxO1 were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against acetylated lysine and β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody against nicotinamide phosphoribosyltransferase (NAMPT) was from MBL International (Woburn, MA). Tissue homogenates were subjected to Western blot analysis as described previously (45, 46). The protein content was assayed by BCA protein assay reagent (Pierce, Rockford, IL). A total of 80–100 μg of protein was resolved by SDS-PAGE and then transferred to nitrocellulose membrane. Membrane was incubated with a 1:1,000 dilution of primary antibodies followed by a 1:5,000 dilution of horseradish peroxidase-conjugated secondary antibodies. Protein bands were visualized by ECL (GE Healthcare). The intensity of the individual bands on Western blots was measured by densitometry (model GS-700, imaging densitometer, Bio-Rad) (47). Immunoprecipitation was carried out using 0.5 mg of protein lysates, 1 μg of specific antibody, and protein A/G agarose.
RNA extraction and real-time PCR.
Total mRNA was extracted from liver, BAT, and gastrocnemius muscle with TRIzol reagent (Invitrogen). For reverse transcription, 1 μg of the total mRNA was converted to first-strand complementary DNA in 20-μl reactions using a cDNA synthesis kit (Promega). Quantitative RT-PCR reactions were performed as described (41). Calculations were performed by a comparative method (2−ΔΔCT) using GAPDH as an internal control (33). The primers used for the PCR are listed in Table 1.
Table 1.
Primers used for PCR analysis
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| PGC1α | 5′-ACTATGAATCAAGCCACTACAGAC-3′ | 5′-TTCATCCCTCTTGAGCCTTTCG-3′ |
| SIRT1 | 5′-ACGGTATCTATGCTCGCCTTG-3′ | 5′-GACACAGAGACGGCTGGAAC-3′ |
| NRF1 | 5′-ACAGATAGTCCTGTCTGGGGAAA-3′ | 5′-TGGT ACATGCTCACAGGGATCT-3′ |
| Ndufs8 | 5′-TGGCGGCAACGTACAAGTAT-3′ | 5′-CCTCGGATGAGTTCTGTCCA-3′ |
| mTFA | 5′-AAGACCTCGTTCAGCATATAACATT-3′ | 5′-TTTTCCAAGCCTCATTTACAAGC-3′ |
| CTP-1b | 5′-GGCAACAGTTGGTTCCAACTACT-3′ | 5′-CAGGAAGCTTAGGCATGTACGTT-3′ |
| PPARα | 5′-TCATACATGACATGGAGACCTTG-3′ | 5′-ACTGGCAGCAGTGGAAGAATC-3′ |
| MCAD | 5′-TGGCATATGGGTGTACAGGG-3′ | 5′-CCAAATACTTCTTCTTCTGTTGATCA-3′ |
See text for definitions.
Histological analysis of liver and adipose tissue.
Adipose and liver tissues were fixed in 4% paraformaldehyde for 3 days and were then paraffin embedded according to standard procedure. Adipose tissue sections (5 μm) were stained with hematoxylin and eosin as previously described (15). To detect lipid accumulation in the liver, a portion of each liver was embedded in Tissue-Tek O.C.T. Compound (Sakura Finetek USA, Torrance, CA), frozen on dry ice, and stored at −80°C for future sectioning. Subsequently, serial 7-μm-thick sections were collected on poly-d-lysine-coated slides. Sections were stained for 5 min with 0.5% Oil Red O in 60% aqueous triethylphosphate, followed by counterstaining with hemotoxylin.
Measurement of mitochondrial biogenesis and function.
Total DNA was extracted from the liver and BAT. The content of mtDNA was calculated using real-time PCR by measuring the threshold cycle ratio (ΔCT) of a mitochondrial encoded gene (cytochrome c oxidase 1: forward 5′-ACTATACTACTACTAACAGACCG-3′, reverse 5′-GGTTCTTTTTTTCCGGAGTA-3′) vs. nuclear-encoded gene (cyclophilin A: forward 5′-ACACGCCATAATGGCACTGG-3′, reverse 5′-CAGTCTTGGCAGTGCAGAT-3′). The ATP content in the cells was measured using the ATP determination kit from Molecular Probes (Eugene, OR) as described (41). Citrate synthase activity was measured spectrophotometrically in tissue extracts and expressed as nanomoles citrate produced per minute per milligram of protein, as described (41).
NAD+/NADH measurements.
Nicotinamide adenonine dinucleotide (NAD+) content was measured using the Enzymchrom NAD+/NADH Assay Kit (Bioassay System) according to the manufacturer's protocol.
Statistics.
Data are expressed as means ± SE. Statistical analyses were performed with Student's t-test (2 groups) or one-way ANOVA with the Bonferroni procedure for multiple comparison tests (≥3 groups). P < 0.05 was considered statistically significant.
RESULTS
Leucine supplementation prevents HFD-induced obesity in mice.
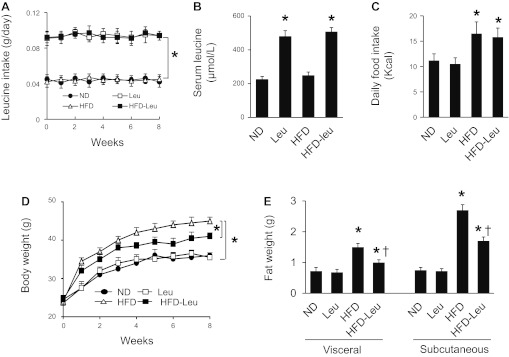
Male mice maintained on ND or HFD were supplemented with or without leucine via drinking water. The amounts of water consumed were not different between the mice supplemented with and without leucine. The leucine-supplemented mice ingested an additional ∼45 mg of leucine daily via water consumption, which nearly doubled the leucine intake (Fig. 1A) and serum leucine concentrations (Fig. 1B). Total caloric intake was increased by 17.6% in HFD mice compared with the mice maintained on ND, but leucine supplementation did not increase caloric intake in either ND or HFD mice (Fig. 1C). The mice on HFD gained significantly more weight, visceral fat, and subcutaneous fat than those fed a normal diet (Fig. 1, D and E). The increase in weight gain, visceral fat, and subcutaneous fat were significantly attenuated by leucine supplementation (Fig. 1, D and E). However, leucine supplementation had no effect on body weight and fat mass in mice maintained on a normal diet (Fig. 1, D and E).
Fig. 1.
Leucine supplementation prevents high-fat diet (HFD)-induced obesity. Mice were fed normal diet (ND) or HFD, supplemented with/without leucine (Leu, 1.5 g/100 ml in drinking water) for 8 wk. A: leucine intake was calculated from the amount of drinking water in individually caged mice; n = 30 in each group. *P < 0.05 vs. ND or HFD. B: serum leucine concentrations were analyzed as described in materials and methods; n = 10 in each group. *P < 0.05 vs. ND or HFD. C: daily caloric intake was calculated from the amount of ingested food in individually caged mice; n = 30 in each group. *P < 0.05 vs. ND or leucine. D: body weights were monitored at weeks indicated; n = 30 in each group. *P < 0.05. E: subcutaneous and visceral fat was measured after 8 wk of treatment; n = 10 in each group. *P < 0.05 vs. ND or Leu; †P < 0.05 HFD-Leu vs. HFD.
Effect of leucine supplementation on SIRT1 signaling in liver.
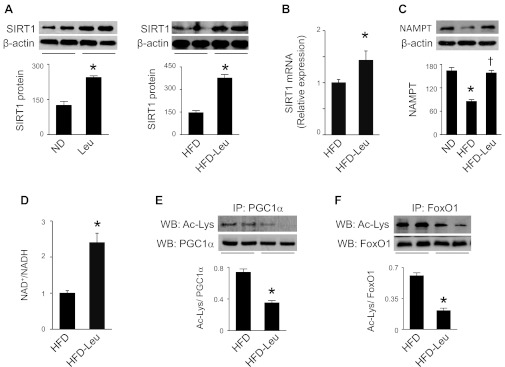
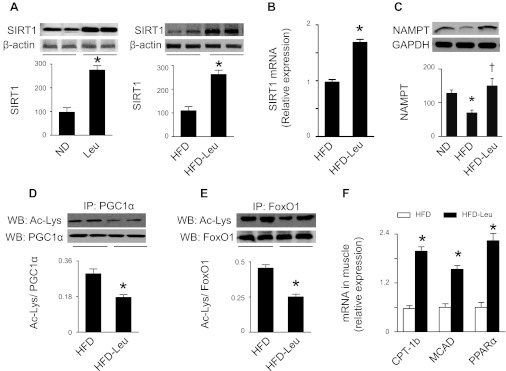
The facts that activation of SIRT1 enhances insulin sensitivity, lowers plasma glucose, and increases mitochondrial capacity in diet-induced obese mice (10, 21) prompted us to investigate whether leucine supplementation regulates SIRT1 in an HFD-induced obesity mouse model. As shown in Fig. 2, A and B, SIRT1 mRNA and protein levels were elevated in mice treated with leucine under ND and HFD conditions. Since SIRT1 deacetylation activity is driven by NAD+ levels, we determined whether dietary leucine could affect NAD+ biosynthesis. As expected, expression of NAMPT, an essential enzyme in regulation of NAD+ biosynthesis, was reduced in HFD mice. Addition of leucine to HFD restored NAMPT expression (Fig. 2C). In addition, increased NAD+/NADH ratio was observed in mice on HFD and leucine compared with HFD mice (Fig. 2D). These data suggest that leucine supplementation may enhance SIRT1 deacetylation activity in leucine-treated animals. To test this hypothesis, we determined the acetylation status of two well-known SIRT1 substrates, PGC1α and FoxO1 (49). Compared with HFD mice, acetylation of PGC1α (Fig. 2E) and FoxO1 was reduced in mice maintained on HFD and leucine (Fig. 2F), supporting that dietary leucine stimulates SIRT1 activity in the liver.
Fig. 2.
Dietary leucine regulates sirtuin 1 (SIRT1) signaling and NAD+ levels in liver. A: liver homogenates were subjected to Western blot (WB) analysis using an anti-SIRT1 antibody (n = 5). *P < 0.05 vs. ND or HFD. B: real-time PCR analysis of SIRT1 mRNA in liver. C: liver homogenates were subjected to WB analysis using an anti-NAMPT (nicotinamide phosphoribosyltransferase) antibody (n = 5). *P < 0.05 vs. ND; †P < 0.05 vs. HFD. D: NAD+/NADH ratio in hepatic tissues was determined as described in materials and methods. E and F: liver homogenates were immunoprecipitated (IP) by anti-PGC1α (PPARγ coactivator 1α; E) or anti-FoxO1 (forkhead box O1; F) antibody, and acetylated lysine was detected by WB analysis (n = 5). *P < 0.05 vs. HFD.
Effects of leucine supplementation on mitochondrial biogenesis and lipid accumulation in liver.
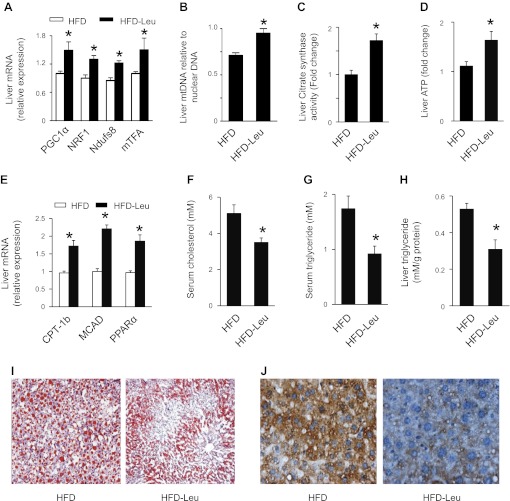
Reduced acetylation of PGC1α by SIRT1 has been shown to activate PGC1α and subsequently improve mitochondrial biogenesis and FAO (14). In agreement with these findings, in mice maintained on HFD and leucine there was an increase in expression of genes controlling mitochondrial biogenesis, including PGC1α, nuclear respiratory factor 1 (NRF1), mtDNA transcription factor A (mTFA), and NADH dehydrogenase [ubiquinone] iron-sulfur protein 8 (Ndufs8) (Fig. 3A). The elevated expression of mitochondrial biogenesis genes was associated with an increase in mtDNA content (Fig. 3B) and improvement of mitochondrial function, as evidenced by enhanced CS activity and ATP content (Fig. 3, C and D). Moreover, addition of leucine to HFD showed a significant increase in mRNA levels of PPARα, an important enzyme controlling fatty acid metabolism (Fig. 3E). The functional relevance of this induction was validated by increased expression of two important genes regulating FAO, including carnitine palmitoyltransferase-1b (CPT-1b) and medium-chain acyl-CoA dehydrogenase (MCAD) (Fig. 3E). Consistent with the increase in genes regulating FAO, addition of leucine to HFD decreased plasma levels of cholesterol (Fig. 3F) and triglyceride (Fig. 3G) and reduced lipid accumulation in the liver, as reflected by lower hepatic triglyceride levels (Fig. 3H) and fewer lipid droplets in the liver (Fig. 3I). We further studied the effect of leucine supplementation on oxidative stress by detecting the formation of 3-nitrotyrosine, a footprint of oxidative stress. The immunostaining of 3-nitrotyrosine was significantly reduced in HFD-Leu mouse liver compared with HFD liver (Fig. 3J). This result was consistent with the improvement of mitochondrial function, which could reduce the production of reactive oxygen species.
Fig. 3.
Effects of HFD and leucine on hepatic mitochondrial biogenesis and lipid accumulation. A: RNA was extracted from hepatic tissue, and mRNA levels of PGC1α, nuclear respiratory factor 1 (NRF1), NADH dehydrogenase [ubiquinone] iron-sulfur protein 8 (Ndufs8), and mitochondrial (mt)DNA transcription factor A (mTFA) were analyzed by quantitative real-time PCR. B: mtDNA/nuclear DNA ratio in liver was determined by quantitative real-time PCR. C: liver citrate synthase (CS) activity was assayed as described in materials and methods. D: liver ATP levels. E: expression of mRNA controlling fatty acid oxidation (FAO), including carnitine palmitoyltransferase-1b (CPT-1b), medium-chain acyl-CoA dehydrogenase (MCAD) and PPARα in liver was measured by real-time PCR. F and G: plasma cholesterol (F) and triglyceride (G) levels were measured after 16 h of fasting. H: liver lipids were extracted as described in materials and methods, and hepatic triglyceride levels were measured using a commercial kit; n = 5. *P < 0.05 vs. HFD. I: liver sample was embedded in Tissue-Tek O.C.T. compound, and frozen sections were prepared for Oil red O staining. Three mouse livers per group were analyzed. J: immunohistochemical analysis of 3-nitrotyrosine formation in liver from HFD and HFD-Leu mice. Three mouse livers per group were analyzed.
Effects of leucine supplementation on SIRT1 expression, mitochondrial biogenesis, and FAO gene expression in BAT.
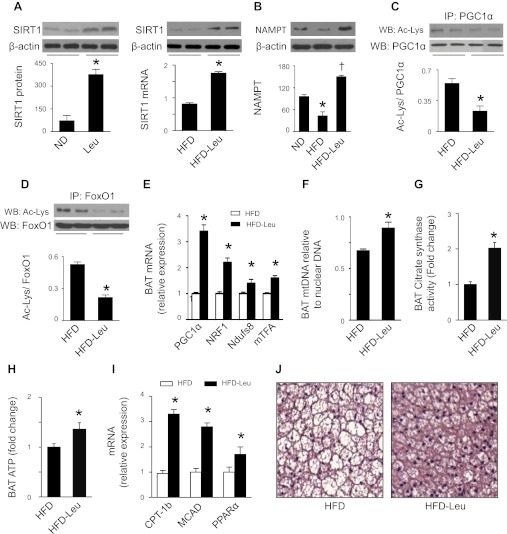
We next investigated whether dietary leucine regulates SIRT1 signaling in BAT, because it is a major tissue where energy is dissipated to maintain body temperature. Similar to alterations in liver, the BAT collected from mice on HFD and leucine exhibited increases in SIRT1 mRNA and protein (Fig. 4A) as well as NAMPT protein (Fig. 4B). Concomitantly, there was a reduction in acetylation of PGC1α (Fig. 4C) and FoxO1 (Fig. 4D) and an increase in expression of genes controlling mitochondrial biogenesis, including PGC1α, NRF1, mTFA, and Ndufs8 (Fig. 4E). The increase in mitochondrial biogenesis genes was associated with enhanced mtDNA content (Fig. 4F) and improved mitochondrial function, e.g., increased CS activity (Fig. 4G) and ATP contents (Fig. 4H). In addition, the expression of genes controlling FAO, including PPARα, CPT-1b, and MCAD, was elevated in mice subjected to HFD and leucine (Fig. 4I). Histological analysis revealed that HFD-Leu mice had smaller brown adipocytes (Fig. 4J).
Fig. 4.
Effects of dietary leucine on SIRT1 signaling and mitochondrial biogenesis in brown adipose tissue (BAT). A: BAT homogenates were subjected to WB analysis using an anti-SIRT1 antibody, total RNA was extracted from BAT, and mRNA levels of SIRT1 were analyzed by quantitative real-time PCR (n = 5). *P < 0.05 vs. ND or HFD. B: BAT homogenates were subjected to WB analysis using an anti-NAMPT antibody (n = 5). *P < 0.05 vs. ND; †P < 0.05 vs. HFD. C and D: PGC1α or FoxO1 was immunoprecipitated (IP), and WB was performed using indicated antibodies. E: expression of genes related to mitochondrial biogenesis in BAT. F: mtDNA/nuclear DNA ratio in BAT was determined by quantitative real-time PCR. G: BAT CS activity. H: ATP levels in BAT. I: expression of mRNA controlling FAO (n = 5). *P < 0.05 vs. HFD. J: BAT was formalin fixed and embedded in paraffin; sections were stained with hematoxylin and eosin. Three samples in each group were analyzed and showed similar results.
Effect of dietary leucine on SIRT1 expression in skeletal muscle.
We further assessed the effects of leucine supplementation on SIRT1 and FAO gene expression in skeletal muscle. In line with the findings in liver and BAT, expression of SIRT1 mRNA and protein (Fig. 5, A and B) as well as NAMPT protein (Fig. 5C) was increased in gastrocnemius muscles isolated from mice on HFD and leucine, which was accompanied by decreased acetylation of PGC1α (Fig. 5D) and FoxO1 (Fig. 5E). Notably, there was also a concomitant induction in genes controlling FAO, such as PPARα, CPT-1b, and MCAD (Fig. 5F).
Fig. 5.
Effects of HFD and leucine on SIRT1 signaling and expression of FAO genes in skeletal muscle. A: homogenates of gastrocnemius muscle were subjected to WB analysis using an anti-SIRT1 antibody (n = 5). *P < 0.05 vs. ND or HFD. B: RNA was extracted from gastrocnemius muscle, and quantitative real-time PCR was performed to determine SIRT1 mRNA levels. C: homogenates of gastrocnemius muscle were subjected to WB analysis to detect NAMPT expression (n = 5). *P < 0.05 vs. ND; †P < 0.05 vs. HFD. D: PGC1α was immunoprecipitated and Western blotted with an acetylated lysine antibody. E: muscle homogenates were immunoprecipitated by anti-FoxO1 antibody, and acetylated lysine was detected by WB analysis. F: relative mRNA expression levels of genes related to FAO (n = 5 in each group). *P < 0.05 vs. HFD.
Inhibition of AMPK prevents dietary leucine-activated SIRT1 signaling.
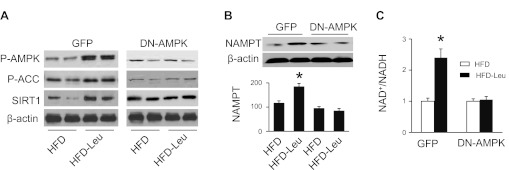
The AMP-activated protein kinase is an intracellular energy sensor and plays an important role in maintaining energy homeostasis (7). Activation of AMPK has been reported to increase SIRT1 protein levels in skeletal muscle (40) and to activate NAMPT, thereby increasing intracellular NAD+ levels (5). We therefore studied whether AMPK is involved in regulation of SIRT1 signaling by leucine. The mice subjected to 4 wk of HFD were assigned to be transfected with adenovirus encoding GFP or DN-AMPK for 4 wk, after the treatment SIRT1 signaling in the liver was analyzed. Addition of leucine to HFD increased the phosphorylation of AMPK (Thr172) and its downstream molecule ACC (Ser79) in mice transfected with GFP adenovirus. The increase in AMPK and ACC phosphorylation was abolished in mice transfected with adenovirus encoding DN-AMPK (Fig. 6A). At the same time, increased expression of SIRT1 was observed in HFD-Leu mice that were transfected with GFP adenovirus but not in HFD-Leu mice transfected with DN-AMPK adenovirus (Fig. 6A). Furthermore, addition of leucine to HFD enhanced NAMPT protein levels (Fig. 6B) and intracellular NAD+ levels (Fig. 6C) in mice transfected with GFP adenovirus, but these effects were absent in mice transfected with DN-AMPK adenovirus (Fig. 6, B and C). These results suggest that dietary leucine stimulates SIRT1 signaling through activation of AMPK.
Fig. 6.
Inhibition of AMPK prevents activation of SIRT1 signaling in HFD-Leu mice. Mice were fed HFD supplemented with or without leucine for 8 wk. After 4 wk of treatment, mice were randomly assigned to be transfected with adenovirus encoding GFP or dominant negative (DN)-AMPK. A: expressions of phospho-AMPK (Thr172), phospho-ACC (Ser79), and SIRT1 in liver were analyzed by WB. B: liver homogenates were subjected to WB to detect expression of NAMPT. C: NAD+/NADH ratio in hepatic tissues was determined as described in materials and methods (n = 5 in each group). *P < 0.05 vs. HFD.
Leucine supplementation promotes insulin signaling in HFD mice.
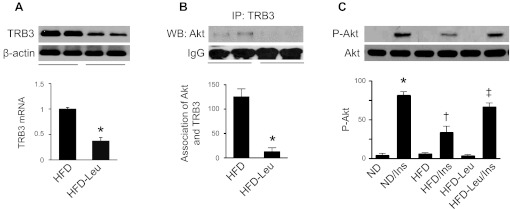
Activation of FoxO1 has been demonstrated to increase insulin sensitivity by repressing the pseudokinase TRB3 and promoting Akt phosphorylation. We therefore studied whether leucine supplementation regulates TRB3 expression and insulin signaling. As shown in Fig. 7A, the protein levels of TRB3 were lower in HFD-Leu mice relative to those in HFD mice. To determine the effect of dietary leucine on the interaction between Akt and TRB3, liver homogenates were immunoprecipitated with anti-TRB3 antibody and then blotted with anti-Akt antibody. Consistent with the lower protein levels of TRB3 in HFD-Leu mice, Akt/TRB3 association was significantly decreased in these mice (Fig. 7B). We further analyzed the effect of leucine on insulin-stimulated Akt phosphorylation in the animal. In mice maintained on ND, insulin increased Akt phosphorylation (Ser473). However, the effect of insulin on Akt phosphorylation was significantly inhibited in mice maintained on HFD (Fig. 7C). Addition of leucine to HFD restored insulin-stimulated Akt phosphorylation (Fig. 7C).
Fig. 7.
Leucine suppresses pseudokinase tribble 3 (TRB3) expression and improves insulin signaling in the liver. A: Expression of TRB3 in liver was analyzed by WB using an anti-TRB3 antibody (n = 5 per group). *P < 0.05 vs. HFD. B: liver homogenates were immunoprecipitated with anti-TRB3 antibody and then probed with an anti-Akt antibody by WB (n = 5 in each group). *P < 0.05 vs. HFD. C: liver homogenates were subjected to WB analysis to determine expression of total and phospho-Akt (Ser437; n = 5 per group). *P < 0.05 vs. ND; #P < 0.05 vs. ND/Ins; †P < 0.05 vs. HFD/Ins.
Leucine supplementation enhances insulin sensitivity in HFD mice.
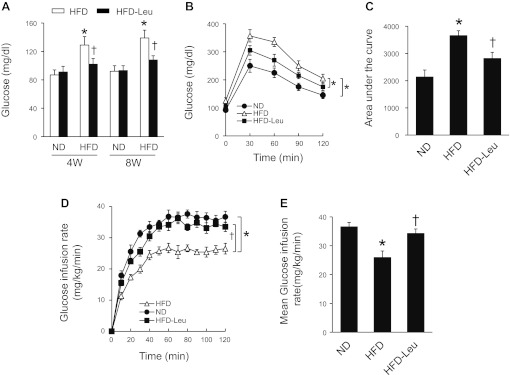
To validate that leucine supplementation promotes insulin signaling, we evaluated the effect of leucine on glucose metabolism in HFD mice. As expected, leucine supplementation normalized fasting blood glucose in HFD mice, and this effect persisted throughout the study period (Fig. 8A). Consistently, during the glucose tolerance test, there was a significant increase in blood glucose levels in HFD mice. Addition of leucine to the HFD reduced the blood glucose levels (Fig. 8B). The increased glucose tolerance by leucine was also illustrated with the area under the curve measurement (Fig. 8C), suggesting that leucine enhances insulin sensitivity. We therefore performed a hyperinsulinemic-euglycemic clamp study in these mice. In line with the fact that diet-induced obesity inhibits insulin sensitivity, a significant decrease in the glucose infusion rate was observed in HFD mice. Importantly, the glucose infusion rate in mice on HFD and leucine was significantly higher than that in HFD mice, indicating that dietary leucine enhances insulin sensitivity in a diet-induced obesity model (Fig. 8, D and E).
Fig. 8.
Leucine supplementation improves insulin sensitivity in mice subjected to HFD. A: fasting blood glucose levels were measured in tail vein blood sample using a glucometer (n = 8 in each group). *P < 0.05 vs. ND; †P < 0.05 vs. HFD. B: an intraperitoneal glucose tolerance test was performed in mice after an overnight fast (n = 5 per group). *P < 0.05. C: area under the curve (AUC) was calculated for each dietary condition (n = 5 per group). *P < 0.05 vs. ND; †P < 0.05 vs. HFD. D and E: hyperinsulinemic-euglycemic clamps were performed over a 120-min period as described in materials and methods. Insulin sensitivity was evaluated through the average glucose infusion rate at equilibrium in a hyperinsulinemic-euglycemic clamp (18 mU insulin·min−1·kg−1; n = 5 in each group). *P < 0.05 vs. ND; †P < 0.05 vs. HFD.
DISCUSSION
The present study demonstrates that dietary leucine prevents HFD-induced obesity, increases glucose infusion rate during a clamp, and reduces lipid accumulation, which could be consistent with elevated energy consumption. Addition of leucine to HFD correlates with increased SIRT1 expression, decreased PGC1α acetylation, and improved mitochondrial function and biogenesis. In addition, activation of SIRT1 leads to deacetylation of FoxO1, which reduces TRB3 expression and prevents the interaction between TRB3 and Akt, thereby enhancing insulin sensitivity.
Leucine has been implicated in the regulation of many cellular processes such as protein synthesis, cell growth, and energy metabolism through activation of the mammalian target of rapamycin (mTOR) (1). However, whether or not leucine alleviates the metabolic syndrome remains unproven (27), and relevant signaling mechanisms (39) are also incompletely understood. In the present study, we demonstrated that mice on HFD and leucine exhibited increases in expression of SIRT1 and NAMPT as well as intracellular NAD+ levels, which are required for SIRT1 to deacetylate lysine residues on target proteins (20). At the same time, there was a reduction in acetylation of two well-known SIRT1 substrates, PGC1α and FoxO1 (49). These data suggest an increase in SIRT1 deacetylation activity in mice treated with HFD and leucine. Since SIRT1 is a key regulator of energy and metabolic homeostasis, activation of SIRT1 may be an important mechanism by which leucine supplementation prevents HFD-induced obesity, insulin resistance, and metabolic disorders. In addition, we have demonstrated that overexpression of DN-AMPK prevents leucine-induced increases in SIRT1, NAMPT, and NAD+, suggesting that leucine supplementation may stimulate SIRT1 through activation of AMPK signaling.
Once activated, SIRT1 deacetylates and activates transcriptional factor PGC1α to induce genes regulating mitochondria biogenesis and FAO, thereby maintaining bioenergetic homeostasis. In our study, the mice on HFD and leucine showed decreased PGC1α acetylation, enhanced mitochondrial content, and improved mitochondrial function. In addition to increasing genes regulating mitochondrial biogenesis, deacetylation of PGC1α also enhanced the expression of genes regulating FAO, which activated the signaling that controls FAO. As a result, leucine supplementation normalized plasma lipid profile, reduced subcutaneous and visceral fat mass, and prevented lipid accumulation in the liver. Taken together, these results suggest a correlation between activation of SIRT1 and protection against metabolic disorders by dietary leucine in HFD mice.
FoxO1 has been shown to promote hepatic insulin sensitivity by regulating TRB3 expression, although it was originally identified as a negative regulator of insulin signaling (30). FoxO activity is regulated by several posttranslational modifications, including phosphorylation, acetylation, and ubiquitination (29). For example, SIRT1-mediated deacetylation inhibits FoxO1 (48) and FoxO3a (4) activity, suppressing the induction of proapoptotic genes. In agreement with these findings, the mice on HD and leucine exhibited activation of SIRT1 and deacetylation of FoxO1. Importantly, the reduction in acetylation of FoxO1 was accompanied by decreased TRB3 expression, suggesting that reduced acetylation of FoxO1 by SIRT1 may contribute to the downregulation of TRB3.
TRB3 is an endogenous inhibitor of Akt, which is a critical regulator in insulin signaling (9). The expression of TRB3 has been implicated in regulation of insulin signaling and glucose metabolism. For instance, diabetes increases expression of TRB3 at mRNA and protein levels in mouse liver (26). In cultured hepatocytes isolated from HFD mice, overexpression of TRB3 disrupts insulin signaling by directly binding to Akt and preventing Akt phosphorylation. In individuals susceptible to type 2 diabetes, TRB3 contributes to the development of insulin resistance by interfering with Akt activation (34). Furthermore, hepatic overexpression of TRB3 promotes hyperglycemia and glucose intolerance in mice (9). These data suggest that upregulation of TRB3 suppresses insulin sensitivity via Akt inhibition. In support of this hypothesis, we find that addition of leucine to HFD reduces the association of TRB3 and Akt and enhances insulin-stimulated Akt phosphorylation, which is accompanied by lower blood glucose and increased glucose infusion rate during a clamp in HFD mice. These studies suggest that leucine supplementation restores insulin sensitivity in HFD mice through suppressing TRB3 expression and interfering with the interaction between TRB3 and Akt.
The role of dietary leucine in regulation of energy metabolism remains controversial in the literature. Consistent with our findings, leucine supplementation has been demonstrated to enhance insulin sensitivity and to prevent dyslipidemia in obese animal models (25, 37, 50). By contrast, other groups reported that dietary supplementation of leucine has no effect on lipid metabolism and does not alter susceptibility to diet-induced obesity in mice (27). Moreover, supplemental BCAA (150% increase of valine, leucine, and isoleucine) worsens insulin resistance in rats subjected to HFD despite the fact that addition of BCAA reduces food intake and decreases body weight (28). It is likely that the effects of leucine on metabolism depend on the dietary context, species, and dose of the nutrients. Leucine supplementation increases leucine levels only in serum (2), whereas addition of BCAA enhances serum levels of valine, leucine, and isoleucine (28), suggesting that increased serum levels of valine and isoleucine may contribute to insulin resistance in the HFD animal. In the current study, compared with HFD mice there is a significant reduction in visceral fat in HFD-Leu mice, which may contribute to the beneficial effects of leucine supplementation in our model system because increased amount of visceral fat is associated with insulin resistance, type 2 diabetes, and dyslipidemia (6, 43, 44).
In summary, we have provided evidence that leucine supplementation correlates with increased expression of SIRT1 and deacetylation of PGC1α and FoxO1. These changes may contribute to the prevention of mitochondrial dysfunction, insulin resistance, and metabolic disorders in HFD mice. Our results provide a rationale for further exploring the role of leucine supplementation in the prevention and treatment of obesity and type 2 diabetes.
GRANTS
This study was supported by an National Institutes of Health grant (1P20 RR-024215-01 to Z. Xie), a Scientist Development Grant from the American Heart Association (Z. Xie), and a grant from Oklahoma Center for Advancement of Science and Technology (Z. Xie).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.L. and Z.X. conception and design of research; H.L., M.X., J.L., and C.H. performed experiments; H.L. and Z.X. analyzed data; H.L. and Z.X. prepared figures; H.L. and Z.X. drafted manuscript; Z.X. interpreted results of experiments; Z.X. edited and revised manuscript; Z.X. approved final version of manuscript.
ACKNOWLEDGMENTS
We are indebted to Dr. Ming-Hui Zou, Department of Medicine, University of Oklahoma Health Sciences Center, for helpful discussion.
REFERENCES
- 1. Anthony JC, Anthony TG, Kimball SR, Jefferson LS. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J Nutr 131: 856S– 860S, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Boucher J, Macotela Y, Bezy O, Mori MA, Kriauciunas K, Kahn CR. A kinase-independent role for unoccupied insulin and IGF-1 receptors in the control of apoptosis. Sci Signal 3: ra87, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bournat JC, Brown CW. Mitochondrial dysfunction in obesity. Curr Opin Endocrinol Diabetes Obes 17: 446– 452, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303: 2011– 2015, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458: 1056– 1060, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carey VJ, Walters EE, Colditz GA, Solomon CG, Willett WC, Rosner BA, Speizer FE, Manson JE. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses' Health Study. Am J Epidemiol 145: 614– 619, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Carling D. The AMP-activated protein kinase cascade—a unifying system for energy control. Trends Biochem Sci 29: 18– 24, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 55: 496– 505, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 300: 1574– 1577, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 8: 347– 358, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226: 497– 509, 1957 [PubMed] [Google Scholar]
- 12. Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem 280: 20589– 20595, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, Carroll RJ, Meininger CJ, Wu G. Dietary l-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J Nutr 135: 714– 721, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J 26: 1913– 1923, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He C, Choi HC, Xie Z. Enhanced tyrosine nitration of prostacyclin synthase is associated with increased inflammation in atherosclerotic carotid arteries from type 2 diabetic patients. Am J Pathol 176: 2542– 2549, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hinault C, Mothe-Satney I, Gautier N, Lawrence JC, Jr, Van OE. Amino acids and leucine allow insulin activation of the PKB/mTOR pathway in normal adipocytes treated with wortmannin and in adipocytes from db/db mice. FASEB J 18: 1894– 1896, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Hofbauer KG. Molecular pathways to obesity. Int J Obes Relat Metab Disord 26, Suppl 2: S18–S27, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Jiang WJ. Sirtuins: novel targets for metabolic disease in drug development. Biochem Biophys Res Commun 373: 341– 344, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Katz DL. Competing dietary claims for weight loss: finding the forest through truculent trees. Annu Rev Public Health 26: 61– 88, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Knight JR, Milner J. SIRT1, metabolism and cancer (Review). Curr Opin Oncol 24: 68– 75, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127: 1109– 1122, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 332: 621– 628, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Li L, Pan R, Li R, Niemann B, Aurich AC, Chen Y, Rohrbach S. Mitochondrial biogenesis and peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) deacetylation by physical activity: intact adipocytokine signaling is required. Diabetes 60: 157– 167, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. MacLean DA, Spriet LL, Hultman E, Graham TE. Plasma and muscle amino acid and ammonia responses during prolonged exercise in humans. J Appl Physiol 70: 2095– 2103, 1991 [DOI] [PubMed] [Google Scholar]
- 25. Macotela Y, Emanuelli B, Bang AM, Espinoza DO, Boucher J, Beebe K, Gall W, Kahn CR. Dietary leucine—an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One 6: e21187, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matsushima R, Harada N, Webster NJ, Tsutsumi YM, Nakaya Y. Effect of TRB3 on insulin and nutrient-stimulated hepatic p70 S6 kinase activity. J Biol Chem 281: 29719– 29729, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Nairizi A, She P, Vary TC, Lynch CJ. Leucine supplementation of drinking water does not alter susceptibility to diet-induced obesity in mice. J Nutr 139: 715– 719, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS, Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9: 311– 326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Obsil T, Obsilova V. Structure/function relationships underlying regulation of FOXO transcription factors. Oncogene 27: 2263– 2275, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans Nature 389: 994– 999, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Opara EC, Petro A, Tevrizian A, Feinglos MN, Surwit RS. L-glutamine supplementation of a high fat diet reduces body weight and attenuates hyperglycemia and hyperinsulinemia in C57BL/6J mice. J Nutr 126: 273– 279, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350: 664– 671, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prudente S, Hribal ML, Flex E, Turchi F, Morini E, De CS, Bacci S, Tassi V, Cardellini M, Lauro R, Sesti G, Dallapiccola B, Trischitta V. The functional Q84R polymorphism of mammalian Tribbles homolog TRB3 is associated with insulin resistance and related cardiovascular risk in Caucasians from Italy. Diabetes 54: 2807– 2811, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Ren J, Pulakat L, Whaley-Connell A, Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med 88: 993– 1001, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434: 113– 118, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Ropelle ER, Pauli JR, Fernandes MF, Rocco SA, Marin RM, Morari J, Souza KK, Dias MM, Gomes-Marcondes MC, Gontijo JA, Franchini KG, Velloso LA, Saad MJ, Carvalheira JB. A central role for neuronal AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) in high-protein diet-induced weight loss. Diabetes 57: 594– 605, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Schwarzer R, Dames S, Tondera D, Klippel A, Kaufmann J. TRB3 is a PI 3-kinase dependent indicator for nutrient starvation. Cell Signal 18: 899– 909, 2006 [DOI] [PubMed] [Google Scholar]
- 39. She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab 6: 181– 194, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suwa M, Nakano H, Radak Z, Kumagai S. Short-term adenosine monophosphate-activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside treatment increases the sirtuin 1 protein expression in skeletal muscle. Metabolism 60: 394– 403, 2011 [DOI] [PubMed] [Google Scholar]
- 41. Tedesco L, Valerio A, Cervino C, Cardile A, Pagano C, Vettor R, Pasquali R, Carruba MO, Marsicano G, Lutz B, Pagotto U, Nisoli E. Cannabinoid type 1 receptor blockade promotes mitochondrial biogenesis through endothelial nitric oxide synthase expression in white adipocytes. Diabetes 57: 2028– 2036, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tran TT, Gupta N, Goh T, Naigamwalla D, Chia MC, Koohestani N, Mehrotra S, McKeown-Eyssen G, Giacca A, Bruce WR. Direct measure of insulin sensitivity with the hyperinsulinemic-euglycemic clamp and surrogate measures of insulin sensitivity with the oral glucose tolerance test: correlations with aberrant crypt foci promotion in rats. Cancer Epidemiol Biomarkers Prev 12: 47– 56, 2003 [PubMed] [Google Scholar]
- 43. Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 7: 410– 420, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr 81: 555– 563, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Xie Z, Dong Y, Scholz R, Neumann D, Zou MH. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation 117: 952– 962, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xie Z, Dong Y, Zhang J, Scholz R, Neumann D, Zou MH. Identification of the serine 307 of LKB1 as a novel phosphorylation site essential for its nucleocytoplasmic transport and endothelial cell angiogenesis. Mol Cell Biol 29: 3582– 3596, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, Zou MH. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes 60: 1770– 1778, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang Y, Hou H, Haller EM, Nicosia SV, Bai W. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J 24: 1021– 1032, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yu J, Auwerx J. Protein deacetylation by SIRT1: an emerging key post-translational modification in metabolic regulation. Pharmacol Res 62: 35– 41, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 56: 1647– 1654, 2007 [DOI] [PubMed] [Google Scholar]