Abstract
Tight control of glucose uptake in skeletal muscles and adipocytes is crucial to glucose homeostasis and is mediated by regulating glucose transporter GLUT4 subcellular distribution. In cultured cells, Rab GAP AS160 controls GLUT4 intracellular retention and release to the cell surface and consequently regulates glucose uptake into cells. To determine AS160 function in GLUT4 trafficking in primary skeletal muscles and adipocytes and investigate its role in glucose homeostasis, we characterized AS160 knockout (AS160−/−) mice. We observed increased and normal basal glucose uptake in isolated AS160−/− adipocytes and soleus, respectively, while insulin-stimulated glucose uptake was impaired and GLUT4 expression decreased in both. No such abnormalities were found in isolated AS160−/− extensor digitorum longus muscles. In plasma membranes isolated from AS160−/− adipose tissue and gastrocnemius/quadriceps, relative GLUT4 levels were increased under basal conditions and remained the same after insulin treatment. Concomitantly, relative levels of cell surface-exposed GLUT4, determined with a glucose transporter photoaffinity label, were increased in AS160−/− adipocytes and normal in AS160−/− soleus under basal conditions. Insulin augmented cell surface-exposed GLUT4 in both. These observations suggest that AS160 is essential for GLUT4 intracellular retention and regulation of glucose uptake in adipocytes and skeletal muscles in which it is normally expressed. In vivo studies revealed impaired insulin tolerance in the presence of normal (male) and impaired (female) glucose tolerance. Concurrently, insulin-elicited increases in glucose disposal were abolished in all AS160−/− skeletal muscles and liver but not in AS160−/− adipose tissues. This suggests AS160 as a target for differential manipulation of glucose homeostasis.
Keywords: insulin signaling, membrane trafficking, glucose disposal
the regulation of the subcellular distribution of the glucose transporter GLUT4 in skeletal muscles and adipocytes is crucial to the maintenance of glucose homeostasis (39). Under fasting conditions, GLUT4 is predominantly sequestered in intracellular vesicles (GLUT4 vesicles) and is present at low levels at the cell surface. This limits glucose uptake into skeletal muscles and adipocytes, and circulating glucose is available to the brain to meet its energy needs. Insulin released after a meal stimulates, within minutes, the redistribution of GLUT4 to the cell surface by facilitating GLUT4 vesicle movement toward, and docking and fusion with, the plasma membrane (referred to as GLUT4 translocation). The resulting increase of GLUT4 at the cell surface leads to increased glucose uptake into skeletal muscles and adipocytes and disposal of 80–90% of ingested glucose. The molecular mechanisms by which GLUT4 is retained within primary adipocytes and skeletal muscles under fasting conditions, and by which insulin releases GLUT4 to the cell surface, are currently largely unknown. However, studies in cultured adipocytes and muscle cells expressing tagged GLUT4 to follow GLUT4 trafficking identified a role for the Rab GTPase-activating protein (Rab GAP) and Akt substrate of 160 kDa (AS160) in GLUT4 retention and release (11, 15, 27, 42, 47).
AS160 was discovered in 3T3-L1 adipocytes as a protein that was phosphorylated in response to insulin on multiple Akt recognition sites (17). A number of studies in cultured cells, predominantly 3T3-L1 adipocytes and L6 muscle cells, subsequently established the following model for AS160 function in GLUT4 trafficking (23). Under basal conditions, AS160 localizes to GLUT4 vesicles and is active as a Rab GAP. Consequently, AS160 keeps its Rab substrate, also in GLUT4 vesicles, inactive (GDP bound) and thus sustains GLUT4 intracellular retention. In response to insulin, AS160 is phosphorylated by Akt, and AS160 Rab GAP activity is inhibited. Its Rab substrate, now active (GTP bound), facilitates GLUT4 vesicle movement to and/or docking and fusion with the plasma membrane.
To determine the physiological role of AS160 in GLUT4 trafficking and thus the regulation of glucose uptake in primary adipocytes and skeletal muscles and control of glucose homeostasis, we used mice with a deletion of AS160 (AS160−/− mice). We found that AS160 is required for the proper regulation of GLUT4 trafficking and glucose uptake in adipocytes and skeletal muscles. Furthermore, we demonstrate that AS160 plays a crucial role in whole body glucose homeostasis.
MATERIALS AND METHODS
AS160−/− mice.
Mice with a deletion of AS160 were generated by Lexicon Genetics (Woodland, TX) by replacing exon 1 (containing the translation start site) and part of the intron downstream of exon 1 in the AS160 gene with a neo cassette. We obtained mice heterozygous for the AS160 deletion (AS160+/−) on a mixed 129 SvEvBrd and C57BL/6J albino background from Lexicon Genetics and back-crossed them onto a C57BL/6N background using microsatellite-assisted selection as described (18). Briefly, polymorphic microsatellite maps for 66 markers were obtained for five male AS160−/− mice on the mixed 129 SvEvBrd and C57BL/6 albino background. Microsatellite genotyping was performed using a standard protocol (9). Because of the mixed genetic background, we chose to initiate breeding of a (129 SvEvBrd × C57BL/6J) F1 male with the highest frequency of polymorphism to minimize the likelihood of transmitting hidden chromosome segments from the founding donor. The selected male was back-crossed to a female C57BL/6N (Taconic). Microsatellite markers in offspring were genotyped, and, using the same rationale as described above, again a male with the highest frequency of polymorphism was selected for back-crossing to a female C57BL/6N mouse. In the next round of back-crossing, male offspring with highest frequency of microsatellite markers for C57BL/6N were chosen for further breeding with female C57BL/6N mice to finally obtain offspring with all 66 microsatellite markers homozygous for C57BL/6N. These were intercrossed to expand the colony, and female offspring were then back-crossed to a male C57BL/6N mouse to introduce the C57BL/6N male Y chromosome into the colony. To maintain the C57BL/6N colony, offspring from these breeders were then intercrossed.
Genotyping.
Genotyping for the mutant AS160 locus was performed by PCR on genomic DNA isolated from mouse tail snips using the DirectPCR Lysis Reagent (Viagen Biotech). Forward primers used were 5′-AGTAGACTCAGAGTGGTCTTGG-3′ for the wild-type allele and 5′-GCAGCGCATCGCCTTCTATC-3′ for the knockout allele; the reverse primer common to both alleles was 5′-TCTGGAGAAATACTGGACACTAAGC-3′. Primers were obtained from Integrated DNA Technologies (IDT). PCR was performed using the HotStar HiFidelity Polymerase Kit (Qiagen) with a Mg2+ concentration of 1.75 mM in separate reactions for the wild-type and the AS160 knockout alleles. For PCR amplification, the following conditions were used: for the wild-type allele initial denaturation and enzyme activation step were at 95°C for 15 min followed by 10 cycles with denaturation at 94°C for 20 s, annealing at 60°C for 35% s in first cycle and then reduced by 1°C in each cycle to reach 50°C in the 10th cycle, and extension at 72°C for 60 s, followed by 35 cycles at 94°C for 20 s, annealing at 50°C for 30 s, and extension at 72°C for 60 s; for the AS160 knockout allele, initial denaturation and enzyme activation step were at 94°C for 15 min followed by 10 cycles with denaturation at 94°C for 20 s, annealing at 65°C for 35 s in first cycle and then reduced by 1°C in each cycle to reach 55°C in the 10th cycle, and extension at 72°C for 60 s, followed by 30 cycles at 94°C for 20 s, annealing at 55°C for 30 s, and extension at 72°C for 60 s. The PCR products with expected sizes of 491 bp for the wild-type and 393 bp for the AS160 knockout allele were separated on 1.2% agarose gels in Tris-borate-EDTA buffer (130 mM Tris base, 45 mM boric acid, and 2.5 mM EDTA). The PCR products were visualized with Sybr Gold (Molecular Probes). The DNA size standard used was the low-molecular-weight DNA ladder from New England Biolabs (NEB).
For experiments described below, mice homozygous for AS160 deletion (AS160−/−) and wild-type littermates (AS160+/+) were obtained predominantly from heterozygous (AS160+/−) parents. AS160−/− and AS160+/+ mice used in each experiment were matched for age and sex. Mice were housed under temperature- and humidity-controlled conditions under a constant 14:10-h light-dark cycle while being allowed free access to water and chow (7912 Teklad LM-485, Harlan Laboratories). All animal procedures were approved by the University of Virginia Institutional Animal Care and Use Committee.
Glucose uptake assays in isolated adipocytes and skeletal muscles.
Glucose uptake in isolated adipocytes was measured as described (29). The glucose uptake data were expressed in amoles per minute per cell by calculating cell numbers from lipid weights and adipocyte sizes as described (38). Glucose uptake in isolated soleus and extensor digitorum longus (EDL) muscles was performed as described (19), except that 2-deoxy-d-[1,2-3H]glucose (PerkinElmer cat. no. NET 549A250UC) at 0.1625 mCi/mmol was used and the uptake allowed to proceed for 20 min (40).
Subcellular fractionation of adipose tissue and skeletal muscle.
Mice fasted for 6 h (6 AM-12 PM) were anesthetized with Nembutal (50 μg/g body wt) and no insulin or insulin (Humulin R, Eli Lilly, 21 mU/g body wt) injected intraperitoneally. After 30 min, mice were euthanized by cervical dislocation, and epididymal or parametrial adipose tissue and gastrocnemius and quadriceps were dissected. Subcellular fractions were prepared (20, 22), and homogenates (adipose tissue), total membranes (gastrocnemius/quadriceps), and plasma membranes were immunoblotted for GLUT4.
Labeling of cell surface GLUT4 in adipocytes and soleus muscles.
Cell surface labeling was performed as described (26, 41, 49) with the biotinylated membrane-impermeant compound Bio-LC-ATB-BGPA (4,4-O-[2-[2-[2-[2-[2-[6-(biotinylamino)hexanolyl]-amino]ethoxy]exoxy]-ethoxy]-4–1-azi-2,2,2-trifluoroethyl)benzoyl]amino-1,3-propanediyl]bis-d-glucose) generously provided by Dr. Geoffrey Holman (University of Bath, Bath, UK). Streptavidin precipitates and aliquots of adipocyte lysates and solubilized soleus membranes were immunoblotted for GLUT4 as described below.
Glucose and insulin tolerance tests and analysis of blood glucose and plasma insulin.
Glucose and insulin tolerance tests were performed as described (20), except that for the glucose tolerance test, glucose (1 and 2 mg/g body wt) was administered intraperitoneally, and blood glucose levels were measured using a OneTouch Ultra glucometer (Lifescan). Random-fed and fasting glucose and insulin were determined in tail vein blood as described (12), except that plasma insulin was measured with an ultrasensitive mouse insulin ELISA kit (ALPCO Diagnostics, cat. no. 80-INSMSU). When insulin levels during a glucose tolerance test were determined, blood was collected from the tail vein at 0, 5, and 30 min after the injection of glucose, and blood glucose and plasma insulin were determined as described above.
Glucose disposal in adipose and skeletal muscle tissues and glucose incorporation into glycogen in liver in vivo.
Mice were fasted for 6 h (6 AM-12 PM) and then injected intraperitoneally with 2-deoxy-d-[1,2-3H]glucose (10 μCi) in either saline or glucose (2 g/kg body wt). Blood was collected at 0, 15, 30, 60, and 120 min after the injections for the determination of blood glucose and 2-deoxy-d-[1,2-3H]glucose, and tissues were dissected and processed at 120 min as described (52), except that insoluble material was pelleted from the tissue homogenates in water without perchloric acid treatment (16). Glucose uptake and glucose incorporation into glycogen were calculated as described (52) and were expressed in nanomoles of glucose per gram of tissue per minute.
Preparation of tissues and cells for immunoblotting.
For experiments without insulin stimulation, tissues were isolated from random-fed mice, and homogenates were prepared as described (19). For the determination of GLUT1 expression in isolated white adipocytes, adipocytes were isolated by collagenase digestion as described (29), and lysates in SDS sample buffer were prepared from adipocytes after three washes in KRBH buffer without BSA. For the determination of GLUT1 expression in gastrocnemius/quadriceps, total membrane pellets obtained during subcellular fractionation (described above) were immunoblotted as described below. For the determination of Akt and Akt substrate phosphorylation, adipose tissue and gastrocnemius muscles were obtained from mice treated without or with insulin as described above for subcellular fractionations. Tissues were homogenized in homogenization buffer (HB; 25 mM HEPES, pH 7.4, 100 mM NaCl, 1 mM Na3VO4, 20 mM sodium pyrophosphate, 3 mM N-ethylmaleimide, 1 μg/ml pepstatin, 10 μM EP475, 10 μg/ml aprotinin, 0.2 mM PMSF, 10 mM β-glycerophosphate, and 10 mM sodium fluoride). Soleus and EDL muscles were isolated from anesthetized mice and preincubated for 1 h at 29°C in Krebs-Henseleit bicarbonate buffer with 0.1% BSA, 8 mM glucose, and 32 mM mannitol with shaking and under continuous gassing with 95% O2-5% CO2 as described (19). Muscles were then incubated for 15 min at 29°C with shaking and under continuous gassing in the above buffer without insulin or with insulin at concentrations that yielded half-maximal (0.42 nM insulin) and maximal (1.7 nM insulin) Akt phosphorylation. Muscles were then frozen in liquid nitrogen and homogenates prepared in HB. Adipocytes were isolated by collagenase digestion from epididymal and parametrial fat pads as described (29). After isolation, cells were washed three times in KRBH buffer containing BSA and three times in KRBH buffer without BSA. Cells were resuspended in KRBH buffer without BSA to yield a 10% cell suspension, and aliquots of the cell suspension were incubated with no insulin and increasing concentrations of insulin at 37°C for 15 min under continuous swirling. The treated cells were centrifuged at 300 g for 1 min to pack adipocytes on top of the buffer. The infranatant was removed, and the adipocytes were frozen in liquid nitrogen. Cell lysates were prepared by adding SDS sample buffer containing DTT. Protein concentrations were determined in SDS samples of homogenates, and cell lysates and equal amounts of protein were subjected to immunoblotting with phospho (p-)Akt substrate, Tbc1d1, p-Akt, and Akt antibodies.
Immunoblotting.
AS160 immunoblots were carried out as described (17) with antibodies raised against a GST fusion protein with a 250-amino acid (aa 577–826) portion of mouse AS160 (11) (see Fig. 1B) and antibodies raised against the carboxyl terminus of mouse AS160 (42) (see Fig. 5G). To detect abundant proteins cross-reacting with the AS160 antibodies, membranes were reversibly stained with Ponceau S (0.1% Ponceau S in 5% acetic acid) before incubation with antibodies. Immunoblots with p-Akt substrate (p-AS) antibodies (Cell Signaling Technology, cat. no. 9611, 1:1,000 diluted) and p-Akt antibodies (p-Akt Ser473-specific antibody antibody, Cell Signaling Technology, cat. no. 9271, 1:1,000 diluted) were carried out following the manufacturer's instructions. For the detection of p-AS, horseradish peroxidase (HRP)-coupled secondary antibodies and enhanced chemiluminescence was used as described below for GLUT1. For the detection of p-Akt, fluorescent secondary antibodies were used as described below for GLUT4. Immunoblots were stripped and reprobed for Tbc1d1 as described (5) and for total Akt using an Akt antibody (Cell Signaling Technology, cat. no. 9272, 1:1,000 diluted). GLUT4 immunoblots were performed as described (19), except that the secondary antibody was goat anti-rabbit IRDye 800 CW (LI-COR Biosciences, cat. no. 926-32211) diluted 1:15,000, blocking was performed in blocking buffer (LI-COR Biosciences, cat. no. 927-40000), primary and secondary antibodies were diluted in blocking buffer and washes were done in Tris-buffered saline containing 0.01% Tween 20. Fluorescent signals were detected and quantified using the Odyssey Infrared Imaging System (LI-COR Biosciences). For GLUT1 immunoblots, a polyclonal rabbit GLUT1 antiserum, generously provided by Dr. Samuel Cushman (National Institutes of Health), was used at a 1:1,000 dilution, and immunoblots were carried out as for GLUT4 with the exception that the secondary antibody used was goat anti-rabbit HRP (cat. no. 170-6515, Bio-Rad), and the detection method was enhanced chemiluminescence (Amersham ECL Plus, cat. no. RPN2132, GE Healthcare Life Sciences) as described (19).
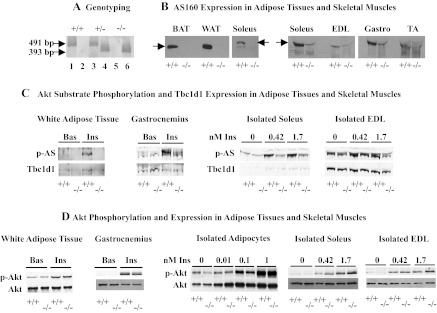
Fig. 1.
A: genotyping of AS160+/− offspring. PCR products of 491 and 393 bp for wild-type and AS160 knockout alleles, respectively, were obtained in reactions using genomic DNA isolated from an AS160+/+ (+/+; lanes 1 and 2), AS160+/− (+/−; lanes 3 and 4) and AS160−/− (−/−; lanes 5 and 6), lanes 1, 3, and 5 showing reactions with primers recognizing AS160 wild-type alleles and lanes 2, 4, and 6 reactions with primers recognizing the AS160 knockout alleles. B: AS160 expression in adipose tissues and skeletal muscles. Interscapular brown (BAT) and parametrial white adipose tissue (WAT) and soleus homogenates (50 μg total protein/lane) from 1-yr-old female AS160+/+ and AS160−/− mice, and soleus, extensor digitorum longus (EDL), gastrocnemius (Gastro), and tibialis anterior (TA) homogenates (100 μg total protein/lane) from 5- to 6-mo-old female AS160+/+ and AS160−/− mice were immunoblotted with AS160 antibodies against a GST fusion protein with a 250-amino acid (aa 577–826) portion of mouse AS160 (11). AS160 is absent from all tissues derived from AS160−/− mice. Faint band visible in all AS160−/− skeletal muscles with similar molecular weight as AS160 aligns with an abundant protein that stains with Ponceau S in all skeletal muscle tissues. BAT, WAT, and soleus samples shown on left were run on one gel; all skeletal samples on right were run together on another, allowing direct comparison of AS160 expression between BAT, WAT, and soleus on one side and the different skeletal muscle types on the other. Arrows point to AS160. C: Akt substrate phosphorylation and Tbc1d1 expression in adipose tissues and skeletal muscles. Epididymal adipose tissue and gastrocnemius were obtained from 2- to 3-mo-old male and female, respectively, AS160+/+ and AS160−/− mice treated without (Bas) or with insulin (Ins) (21 mU/g body wt) in vivo. Soleus and EDL were isolated from 10- to 12-wk-old female AS160−/− and AS160+/+ mice and incubated with no insulin or increasing concentrations of insulin as indicated. Homogenates (100 μg total protein) were immunoblotted for phosphorylated Akt substrates (p-AS; top). Blots were stripped and reprobed with Tbc1d1 antibodies (bottom). Immunoblots were repeated with ≥1 other set of samples, and similar results were obtained. Quantitations of Tbc1d1 signals and comparison of means ± SE (n = 3–4) for AS160−/− and AS160+/+ tissues with unpaired 2-tailed t-tests showed no significant differences (data not shown). D: Akt phosphorylation and expression in adipose tissues and skeletal muscles. WAT and gastrocnemius homogenates were obtained from 5- to 7-mo-old female and 8- to 9-wk-old male, respectively, AS160−/− and AS160+/+ mice treated without (Bas) or with insulin (21 mU/g body wt) in vivo. Parametrial adipocytes (isolated adipocytes), soleus, and EDL were obtained from 10- to 12-wk-old female AS160−/− and AS160+/+ mice and incubated with no insulin or increasing concentrations of insulin as indicated. Adipocyte lysates (20 or 30 μg total protein/lane), WAT (28 μg total protein/lane), gastrocnemius, soleus, and EDL homogenates (100 μg total protein/lane) were immunoblotted for p-Akt Ser473 (top) and total Akt (bottom). Experiments were repeated with ≥1 more set of samples, and similar results were obtained. Signals for p-Akt and Akt were quantified and normalized to AS160+/+ Bas (assigned a value of 1), and ratios for p-Akt/Akt were calculated. Means ± SE (n = 3–4) for AS160−/− and AS160+/+ for each condition were compared with unpaired 2-tailed t-tests; no significant differences (P < 0.05) were observed (data not shown). Experiments for isolated adipocytes, soleus, and EDL were repeated with 9- to 10-wk-old male AS160−/− and AS160+/+ mice, and similar results as shown for female were obtained.
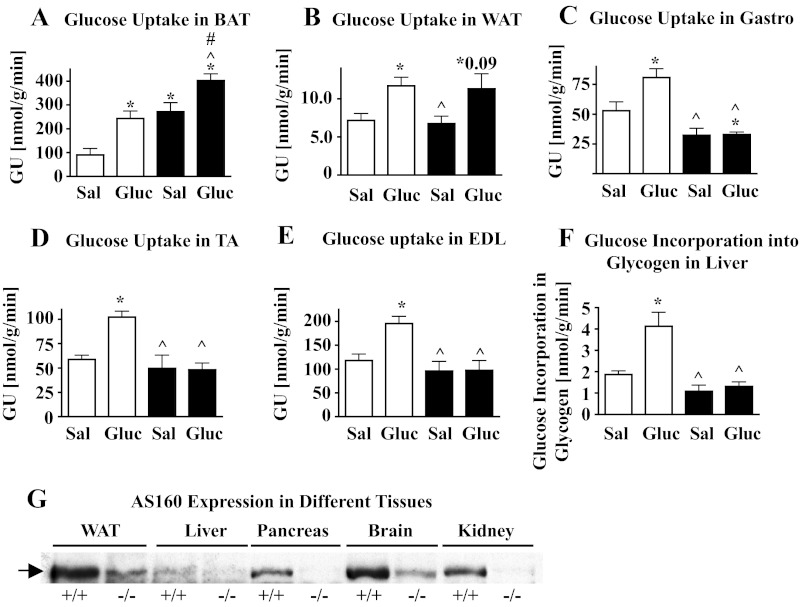
Fig. 5.
A–F: Glucose uptake (GU) in different adipose tissues and skeletal muscles and glucose incorporation into glycogen in liver in vivo. GU and glucose incorporation into glycogen were determined in vivo in 12- to 14-wk-old male AS160+/+ (open bars) and AS160−/− mice (filled bars) fasted for 6 h and then injected with 2-deoxy-d-[1,2-3H]glucose in saline (Sal) or in glucose (Gluc; 2 g/kg body wt). After 120 min, mice were euthanized and adipose tissues, skeletal muscles and liver dissected. Amount of radioactive 2-deoxy-glucose phosphate, representing 2-deoxy-glucose taken up into cells, was determined, GU expressed in nmol·g tissue−1·min−1. Graphs show means ± SE (n = 3–5) for brown adipose tissue (BAT; A), white adipose tissue (WAT; B), gastrocnemius (Gastro; C), tibialis anterior (TA; D), and extensor digitorum longus muscles (EDL; E). Glucose incorporation into glycogen in liver was determined by measuring deoxy-d-[1,2-3H]glucose associated with liver glycogen and data (means ± SE, n = 4–5) plotted (F). Data were compared with unpaired 2-tailed t-tests, and significant differences (P < 0.05) are denoted as * comparing data with AS160+/+ Sal, ^ comparing data of AS160−/− Sal and AS160−/− Gluc to AS160+/+ Gluc, and # comparing data between AS160−/− Sal and AS160−/− Gluc by unpaired 2-tailed t-tests. G: AS160 expression in different tissues. Homogenates (50 μg total protein/lane) of liver, kidney, lung, pancreas, and brain together with WAT were obtained from 9-mo-old female AS160+/+ and AS160−/− mice and immunoblotted with AS160 antibodies against the carboxyl terminus of mouse AS160 (42). WAT was included for comparison of relative expression levels. Tissues derived from AS160−/− mice served as negative controls; bands detected by AS160 antibodies in AS160−/− tissues are proteins that are abundant in the respective tissues (confirmed by Ponceau S staining of immunoblots) and do not represent AS160. Arrow points to AS160.
RNA preparation and determination of GLUT4 mRNA.
Total RNA was isolated from epididymal and parametrial adipose tissues, and soleus and EDL muscles, using TRIzol Reagent according to the manufacturer's instructions (Life Technologies, cat. no. 15596-026). GLUT4 mRNA was then measured using a Quantitect Probe RT-PCR kit (Qiagen, cat. no. 204443). The Taqman probe for GLUT4 and the primers used were as described in Ref. 43. To determine the relative GLUT4 mRNA expression levels, a standard curve was obtained for one of the AS160+/+ samples by running reactions with four different amounts of total RNA (20, 10, 5, and 2.5 ng for adipose tissues and 10, 5, 2.5, and 1.25 ng for soleus and EDL) and plotting the threshold cycles vs. the log of total RNA input. Relative levels of GLUT4 mRNA in each sample were then derived from the standard curve.
Statistical analysis.
Statistical analysis was performed with GraphPad Prism software (GraphPad Software). One-sample t-tests with AS160+/+ basal assigned values of 100 or 1, unpaired two-tailed t-tests, and two-way ANOVA were used to compare data (means ± SE) as specified in the figure legends. Differences were considered significant for P values < 0.05.
RESULTS
Generation of whole body AS160−/− mice.
AS160−/− and AS160+/+ offspring of heterozygous (AS160+/−) breeders were identified by genotyping (Fig. 1A). The AS160−/− mice did not exhibit apparent developmental abnormalities, and their body weights were similar to those of wild types (Table 1). However, AS160−/− offspring were obtained at a lower than expected frequency from AS160+/− breeders, with 18.5% AS160−/−, 50.5% AS160+/−, and 31% AS160+/+. The lack of AS160 protein in AS160−/− mice was established for adipose tissues and skeletal muscles (Fig. 1B). But note, in these constitutive AS160−/− mice, AS160 is absent from all tissues. As shown for AS160+/+ tissues, AS160 is more abundant in white and brown adipose tissue than in soleus muscle (Fig. 1B, left). In addition, differentially spliced isoforms of AS160 are expressed in adipose and muscle tissues, with a shorter version expressed in the former and a longer version in the latter (37). Among the different skeletal muscles, AS160 is well expressed in soleus and gastrocnemius but is only barely detectable in extensor digitorum longus (EDL) and tibialis anterior (TA) muscles (Fig. 1B, right). The faint band visible in all AS160−/− skeletal muscles aligns with an abundant protein in skeletal muscle tissues that cross-reacts with the AS160 antibody.
Table 1.
Blood glucose and plasma insulin levels in random fed and fasting AS160−/− mice
| Mice | Random Fed |
After 6 h Fasting |
Random Fed |
||
|---|---|---|---|---|---|
| BG, mg/dl | Ins, ng/ml | BG, mg/dl | Ins, ng/ml | Body wt, g | |
| +/+ Young male (n = 5) | 164 ± 12.5 | 0.43 ± 0.09 | 151 ± 14.4 | 0.39 ± 0.03 | 20.8 ± 0.88 |
| −/− Young male (n = 6–7) | 148 ± 5.3 | 0.39 ± 0.05 | 109 ± 6.9* | 0.27 ± 0.02* | 19.9 ± 0.61 |
| +/+ Young female (n = 7) | 156 ± 10.3 | 0.38 ± 0.03 | 150 ± 9.3 | 0.29 ± 0.03 | 17.9 ± 0.55 |
| −/− Young female (n = 6–7) | 155 ± 16.2 | 0.39 ± 0.06 | 99 ± 3.9* | 0.30 ± 0.07 | 18.2 ± 0.40 |
| +/+ Older male (n = 5–10) | 166 ± 6.5 | 1.33 ± 0.19 | 171 ± 8.1 | 0.31 ± 0.03 | 30.6 ± 1.07 |
| −/− Older male (n = 6–12) | 124 ± 3.6* | 1.18 ± 0.28 | 101 ± 3.4* | 0.34 ± 0.01 | 29.8 ± 1.26 |
| +/+ Older female (n = 7) | 142 ± 10.3 | 0.28 ± 0.04 | 126 ± 8.0 | 0.33 ± 0.02 | 22.9 ± 0.56 |
| −/− Older female (n = 8–9) | 141 ± 8.7 | 0.29 ± 0.03 | 92 ± 5.1* | 0.28 ± 0.02 | 23.2 ± 0.81 |
Values are means ± SE. AS160, Akt substrate of 160 kDa. Blood glucose (BG) and plasma insulin (Ins) were measured in random-fed and fasted male and female AS160−/− (knockout, −/−) and AS160+/+ (wild type, +/+) mice at ages 7–10 wk (young male), 9–12 wk (young female), and 8–9 mo (older male and female). Body weights shown were measured after determination of blood glucose and insulin under random-fed conditions. Values for AS160−/− mice marked with asteriks (*) are statistically significantly different from respective values for AS160+/+ mice, P < 0.05 (unpaired 2-tailed t-tests).
Akt substrate phosphorylation and Tbc1d1 expression in AS160−/− adipose tissues and skeletal muscles.
AS160 (also designated Tbc1d4) (34) belongs to a family of proteins that are characterized by a TBC domain, a region that is found in the tre-2 oncogene and the yeast cell cycle regulators BUB2 and cdc16 (36) and corresponds to the Rab GAP domain. Among the TBC/Rab GAP domain-containing proteins, Tbc1d1 shares the highest resemblance to AS160; it has a similar size and structure, is 47% identical overall, and 79% identical in the carboxyl-terminal Rab GAP domain (37). Both AS160 and Tbc1d1 contain potential Akt phosphorylation sites and are phosphorylated by Akt when it is activated in response to insulin (6, 35, 42, 46). To evaluate whether Tbc1d1 compensated for AS160 in the AS160−/− mice, we determined phosphorylation of AS160 and Tbc1d1 in basal and insulin-treated AS160+/+ and AS160−/− adipose tissues and skeletal muscles in response to insulin by using p-Akt substrate antibodies and determined Tbc1d1 expression. In brown and white adipose tissues, soleus and gastrocnemius (Fig. 1C and data not shown), AS160 was the major Akt substrate of 160 kDa that was phosphorylated in response to insulin. Tbc1d1 was barely detectable in both AS160−/− and AS160+/+ tissues. Insulin increased the phosphorylation of an Akt substrate of 160 kDa in both AS160−/− and AS160+/+ EDL to similar extents. Immunoblotting of the same samples with Tbc1d1-specific antibodies identified this Akt substrate as Tbc1d1. Comparison of the levels of Tbc1d1 expression between AS160−/− and AS160+/+ tissues did not reveal any differences. Thus, Tbc1d1 does not compensate for the lack of AS160 by increased expression or insulin-elicited phosphorylation. To further evaluate whether alterations in signaling upstream of AS160/Tbc1d1 occurred in the AS160−/− mice, we determined basal and insulin-stimulated phosphorylation of Akt. There were no differences in phosphorylation or expression of Akt between AS160−/− and AS160+/+ mice in any of the adipose and muscle tissues under both in vivo and in vitro conditions (Fig. 1D).
Glucose uptake, GLUT4 expression and GLUT4 subcellular distribution in AS160−/− adipocytes.
Basal and insulin-stimulated glucose uptakes in primary adipocytes are predominantly mediated by GLUT4 (representing ∼90% of total glucose transporters in these cells) and correlate with GLUT4 cell surface expression (14). Thus, changes in GLUT4 expression and/or cell surface exposure would be expected to lead to changes in glucose uptake. When evaluating glucose uptake in isolated AS160−/− adipocytes, we found that basal glucose uptake was increased 1.7-fold and insulin-stimulated glucose uptake decreased by 35% compared with AS160+/+ adipocytes (Fig. 2A). Insulin still stimulated glucose uptake significantly in AS160−/− adipocytes, but the fold stimulation was lower than in AS160+/+ adipocytes (1.7-fold vs. 4.4-fold). Concomitantly, GLUT4 protein was decreased by 58% (Fig. 2B) and GLUT4 mRNA by 56% (AS160−/−/AS160+/+ = 0.44 ± 0.03, means ± SE, n = 4, P = 0.009) in AS160−/− adipocytes. The glucose transporter GLUT1, present at low levels in primary adipocytes, contributes little to glucose uptake but could augment basal glucose uptake when substantially increased. However, GLUT1 levels were normal in AS160−/− adipocytes (Fig. 2C). As shown in Fig. 1D, changes in glucose uptake in AS160−/− adipocytes were also not due to alterations in signaling upstream of AS160; there were no differences in phosphorylation or expression of Akt between AS160−/− and AS160+/+ adipocytes. Thus, abnormalities in glucose uptake in AS160−/− adipocytes could not be explained by changes in GLUT4 and GLUT1 expression or changes in signaling through Akt. However, consistent with the increased basal glucose uptake, we observed that parametrial and epididymal fat depots were significantly larger (29 and 23%) in young (6- to 7-wk-old male and 8- to 11-wk-old female) AS160−/− vs. AS160+/+ mice (male AS160−/− 205 ± 16.2 vs. AS160+/+ 159 ± 11.7 mg, n = 12, P = 0.03; female AS160−/− 221 ± 12.4 vs. AS160+/+ 180 ± 10.5 mg, n = 27–31, P = 0.015). Also, AS160−/− adipocytes, as determined for cells isolated from epididymal fat pads, were 25% larger (AS160−/− 4,006 ± 158 vs. AS160+/+ 3,088 ± 176 AU, n = 6, P = 0.003). Adipocyte number in epididymal fat pads (two per mouse) were not significantly different between AS160−/− and AS160+/+ (1.129 × 106 ± 1.156 × 105 vs. 1.008 × 106 ± 1.506 × 105, n = 6, P = 0.54). The changes in fat depot sizes were not reflected in body weights (male AS160+/+ 20.4 ± 0.57 vs. AS160−/− 20.4 ± 0.55 g, n = 12, P = 0.99; female AS160−/− 19.2 ± 0.24 vs. AS160+/+ 19.5 ± 0.25 g, n = 27–31, P = 0.43).
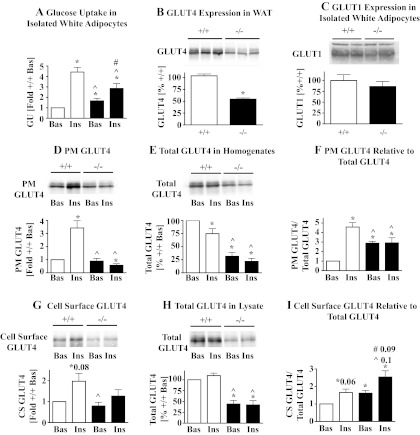
Fig. 2.
A: glucose uptake in isolated white adipocytes. Epididymal adipocytes were isolated from 6- to 7-wk-old male AS160−/− (filled bars) and AS160+/+ mice (open bars), and glucose uptake was measured in the absence (Bas) and presence of 10 nM insulin. Glucose uptake (GU) was calculated in amol glucose·cell−1·min−1 and data (means ± SE) plotted relative to AS160+/+ basal (+/+ Bas; n = 6). *P < 0.05 for comparisons of data with AS160+/+ Bas (1-sample t-tests with AS160+/+ Bas assigned a value of 1); ^P < 0.05 for comparison AS160−/− Bas and AS160−/− Ins with AS160+/+ Ins; #P < 0.05 for comparison between AS160−/− Bas and AS160−/− Ins using unpaired 2-tailed t-tests. Glucose uptake assays were repeated with female mice at 6–7 wk and with male and female mice at 5 mo, and similar results were obtained. B: GLUT4 expression in WAT. Homogenates prepared from epididymal fat pads (40 μg total protein/lane) from 6- to 7-wk-old male AS160−/− (filled bar) and AS160+/+ mice (open bar) were immunoblotted with GLUT4 antibodies. Top: GLUT4 immunoblot; bottom: quantitations of GLUT4 signals on immunoblots (means ± SE, n = 3) in AS160+/+ (%+/+, one of the AS160+/+ samples assigned a value of 100%). Data were compared with an unpaired 2-tailed t-test (*P < 0.05). Immunoblotting of WAT from 6- to 7-wk-old female and epididymal and parametrial adipose tissues from 5-mo-old male and female mice, respectively, yielded similar results (data not shown and Fig. 2E). C: GLUT1 expression in isolated adipocytes. Adipocytes were isolated from epididymal and parametrial adipose tissue of 14-wk-old male and female AS160−/− and AS160+/+ mice, and cell lysates (60 μg total protein/lane) immunoblotted for GLUT1. Representative immunoblot is shown in top. GLUT1 signals on immunoblots were quantified using TotalLab Quant, and means ± SE (n = 4) graphed in % of AS160+/+ with one of the AS160+/+ samples assigned a value of 100%. Data for AS160−/− and AS160+/+ were compared with unpaired 2-tailed t-tests; no significant difference (P < 0.05) was observed. D–F: plasma membrane (PM)-associated GLUT4. Female AS160+/+ (open bars) and AS160−/− (filled bars) 5- to 7-mo-old mice were fasted for 6 h and treated with no insulin (Bas) or insulin (21 mU/g body wt). WAT was dissected and subjected to subcellular fractionation. PMs (8 μg protein/lane; D: PM GLUT4) and homogenates (28 μg protein/lane; E: total GLUT4 in homogenates) were immunoblotted for GLUT4. D and E, top: representative GLUT4 immunoblots from one subcellular fractionation. PM GLUT4 and total GLUT4 were quantified and expressed as relative levels of AS160+/+ Bas, and means ± SE (n = 4) are shown in bar graphs below respective immunoblots. Ratios of normalized PM GLUT4 to total GLUT4 were calculated (PM GLUT4/Total GLUT4) and data (means ± SE, n = 4) plotted, F: PM GLUT4 relative to total GLUT4. *P < 0.05 for comparisons of data with AS160+/+ Bas (1-sample t-tests with AS160+/+ Bas assigned a value of 100 or 1); ^P < 0.05 for comparison AS160−/− Bas and AS160−/− Ins with AS160+/+ Ins; #P < 0.05 for comparison between AS160−/− Bas and AS160−/− Ins using unpaired 2-tailed t-tests. Preliminary studies with subcellular fractions from epididymal fat pads from male mice yielded similar results as shown for female. G–I: cell surface GLUT4. Adipocytes were isolated from parametrial fat depots of 8- to 11-wk-old female AS160+/+ (open bars) and AS160−/− mice (filled bars). Isolated adipocytes were incubated without (Bas) or with 20 nM insulin and processed for cell surface labeling with the biotinylated membrane-impermeable compound Bio-LC-ATB-BGPA. Representative immunoblots for labeled cell surface GLUT4 (in entire streptavidin precipitate) and total GLUT4 (in 15 μg adipocyte lysate protein/lane) are shown in G and H, respectively. Signals on immunoblots were quantified and corrected for total protein input, and AS160+/+ Ins, AS160−/− Bas, and AS160−/− Ins were expressed as fractions of AS160+/+ Bas and means ± SE (n = 4) plotted (bar graphs below respective immunoblots). Relative values for cell surface GLUT4 were divided by relative values for total GLUT4 to obtain ratios for cell surface GLUT4/total GLUT4 and means ± SE (n = 4) plotted, I: cell surface GLUT4 relative to total GLUT4. *P < 0.05 (unless indicated otherwise) comparing data to AS160+/+ Bas using 1-sample t-tests with AS160+/+ basal assigned a value of 100 or 1; ^P < 0.05 (unless indicated otherwise) comparing AS160−/− Bas and AS160−/− Ins with AS160+/+ Ins; #P < 0.05 (unless indicated otherwise) comparing data between AS160−/− Bas and AS160−/− Ins with unpaired 2-tailed t-tests.
To test the possibility that an increase in cell surface GLUT4 (despite decreased GLUT4 expression) was responsible for the augmented basal glucose uptake in AS160−/− adipocytes, we performed subcellular fractionation and cell surface labeling. Plasma membranes were isolated from adipose tissues of mice that were treated with or without insulin in vivo. We observed that, in plasma membranes obtained from non-insulin-treated mice, GLUT4 content was similar between AS160−/− and AS160+/+ (Fig. 2D). In response to insulin, GLUT4 increased 3.4-fold in the AS160+/+ plasma membranes but did not change in AS160−/− plasma membranes (Fig. 2D). In adipose tissue homogenates from which plasma membranes were derived, total GLUT4 was decreased by 25, 70, and 80% for AS160+/+ insulin, AS160−/− basal, and AS160−/− insulin, respectively, compared with AS160+/+ basal (Fig. 2E). Due to the decrease in total GLUT4, the relative subcellular distribution of GLUT4 is not well represented by the data for plasma membrane-associated GLUT4 in Fig. 2D. However, the proposed function for AS160 is to regulate GLUT4 subcellular distribution under basal and insulin-stimulated conditions (11, 42). Thus, to account for the changes in GLUT4 expression and obtain values for the relative distribution of GLUT4, we calculated the ratio for plasma membrane-associated GLUT4 and total GLUT4 (PM GLUT4/Total GLUT4; Fig. 2F). This revealed that the relative amount of GLUT4 in the plasma membrane was increased by a factor of 2.9 in basal AS160−/− adipocytes compared with basal AS160+/+. Insulin had no additional effect on GLUT4 content in AS160−/− plasma membranes, whereas insulin clearly increased relative GLUT4 content in AS160+/+ plasma membranes (4.6-fold). Under both basal and insulin-stimulated conditions, the relative amounts of GLUT4 in AS160−/− plasma membranes were significantly less than in AS160+/+ plasma membranes after insulin treatment. The lack of an insulin response in AS160−/− plasma membranes was not due to impaired insulin signaling upstream of AS160 under the in vivo conditions used for the experiment, as insulin treatment elicited a similar level of Akt phosphorylation in AS160−/− and AS160+/+ adipose tissue in the presence of similar Akt expression (Fig. 1D).
Glucose uptake occurs only after GLUT4 is exposed at the cell surface. Plasma membranes isolated by subcellular fractionation may, in addition to GLUT4 incorporated into the plasma membrane after vesicle fusion (cell surface-exposed GLUT4), contain docked GLUT4 vesicles. To label cell surface-exposed GLUT4 in isolated adipocytes under basal and insulin-stimulated conditions, we used a membrane-impermeant biotinylated photo-cross-linkable glucose analog (Bio-LC-ATB-BGPA) (26). We found that the amount of labeled GLUT4 under basal conditions, when corrected for total protein input, was similar for AS160−/− and AS160+/+ adipocytes (Fig. 2G). Labeled GLUT4 increased in response to insulin by 2- and 1.6-fold, respectively, in AS160+/+ and AS160−/− adipocytes (Fig. 2G). Considering that total GLUT4 in AS160−/− adipocyte lysates, from which cell surface GLUT4 was derived, was decreased by 57% (Fig. 2H), we calculated that the amount of cell surface GLUT4 as a fraction of total GLUT4 was increased by 1.6 in AS160−/− compared with basal AS160+/+ adipocytes and was similar to the amount observed in insulin-stimulated AS160+/+ adipocytes (Fig. 2I). Insulin stimulated a similar increase in the relative amounts of labeled GLUT4 in AS160−/− and AS160+/+ adipocytes (1.7- and 1.8-fold, respectively). Thus, relative amounts of labeled GLUT4 in insulin-stimulated AS160−/− adipocytes were 1.7-fold higher than in insulin-stimulated AS160+/+ adipocytes.
In summary, in AS160−/− adipocytes, basal glucose uptake was increased and insulin-stimulated glucose uptake decreased, whereas GLUT4 expression was downregulated and GLUT1 levels were normal. Relative levels of plasma membrane-associated GLUT4 and cell surface-labeled GLUT4 were increased under basal conditions in AS160−/− adipocytes. Although plasma membrane-associated GLUT4 in AS160−/− adipocytes did not further increase in response to insulin, cell surface-labeled GLUT4 and glucose uptake did. Although relative plasma membrane-associated and cell surface GLUT4 were increased, absolute respective GLUT4 levels were still lower than the glucose uptake data suggest.
Glucose uptake, GLUT4 expression, and GLUT4 subcellular distribution in different AS160−/− skeletal muscles.
AS160 is differentially expressed in different skeletal muscles (Fig. 1B), but glucose uptake is in all of them regulated by insulin. Also, in all of them GLUT4 is the predominant glucose transporter that, when at the cell surface, mediates glucose uptake (33). As representatives of muscles expressing high and low levels of AS160, respectively, we analyzed glucose uptake in isolated soleus and EDL muscles. As shown in Fig. 3, A and B, basal glucose uptake was normal in AS160−/− soleus and EDL. After insulin stimulation, glucose uptake was reduced by 31% in AS160−/− soleus but was normal in AS160−/− EDL. Concomitantly, GLUT4 protein levels were reduced by 35 in AS160−/− soleus (Fig. 3C) but were normal in AS160−/− EDL (Fig. 3D). In contrast to adipocytes, GLUT4 mRNA levels in AS160−/− soleus were not reduced (AS160−/−/AS160+/+ = 1.22 ± 0.25, mean ± SD, n = 2, with soleus pooled from 7–8 mice for each genotype, P > 0.05). GLUT1 protein expression was normal in AS160−/− soleus and EDL (data not shown). As shown in Fig. 1D, insulin signaling upstream of AS160 was normal, as Akt phosphorylation in response to increasing insulin concentrations was similar between AS160−/− and AS160+/+ soleus (Fig. 1D).
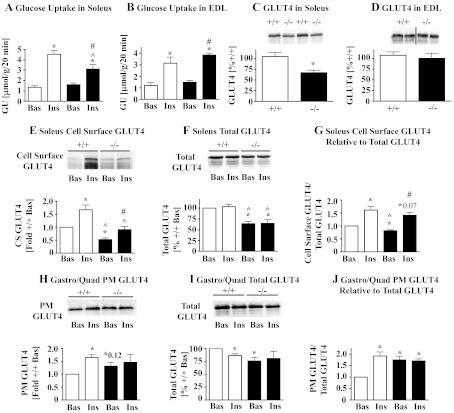
Fig. 3.
A and B: glucose uptake in soleus and EDL muscles. 2-Deoxyglucose uptake was determined in isolated soleus (A) and EDL muscles (B) isolated from 10-wk-old male AS160−/− (filled bars) and AS160+/+ mice (open bars) in the absence (Bas) and presence of 13 nM insulin (Ins). GU data expressed in μmol·g−1·20 min−1 represent means ± SE (n = 6). *P < 0.05 comparing data with AS160+/+ Bas; ^P < 0.05 comparing data for AS160+/+ Ins and AS160−/− Ins; #P < 0.05 comparing data for AS160−/− Bas and AS160−/− Ins using unpaired 2-tailed t-tests. C and D: GLUT4 expression in soleus and EDL muscles. GLUT4 expression was determined by immunoblotting of soleus (C) and EDL (D) homogenates obtained from 10 (C) and 6- to 7- (D) wk-old male AS160−/− and AS160+/+ mice. Top: representative results for 2 samples for each genotype from the same immunoblot; bottom: quantitations of GLUT4 signals on immunoblots (means ± SE, n = 3–4) in %+/+ (with one AS160+/+ sample assigned a value of 100%). *P < 0.05 compares AS160−/− with AS160+/+ data with unpaired 2-tailed t-tests. Experiments shown in A–D were also performed with 5-wk-old female and 20-wk-old male and female mice, and similar results were obtained. E–G: soleus cell surface GLUT4. Soleus muscles were isolated from 9-wk-old female AS160−/− (filled bars) and AS160+/+ mice (open bars), incubated without (Bas) or with 130 nM insulin and muscles processed and evaluated as described for Fig. 2, G–I. Representative immunoblots for cell surface GLUT4 (derived from entire streptavidin precipitates) and total GLUT4 (1/25 of total membrane pellet or ∼50 μg total membrane protein), respectively, are shown in E, top, soleus cell surface GLUT4 and F, soleus total GLUT4. Signals on immunoblots were quantified and corrected for protein input, and means ± SE (n = 3–4) were plotted (bar graphs below respective immunoblots). The ratios cell surface/total GLUT4 were calculated and data (means ± SE, n = 3–4) plotted (G: soleus cell surface GLUT4 relative to total GLUT4). *P < 0.05 (unless indicated otherwise) for comparisons of data with AS160+/+ Bas (1-sample t-tests with AS160+/+ Bas assigned a value of 100 or 1); ^P < 0.05 for comparison AS160−/− Bas and AS160−/− Ins with AS160+/+ Ins; #P < 0.05 for comparison between AS160−/− Bas and AS160−/− Ins using unpaired 2-tailed t-tests. Preliminary studies with soleus isolated from male mice yielded similar results as shown for female. H–J: Gastro/Quad PM-associated GLUT4. Experiment was carried out as described in Fig. 2, D–F, with 10- to 14-wk-old male AS160−/− (filled bars) and AS160+/+ mice (open bars) but gastrocnemius and quadriceps muscles were isolated, pooled (Gastro/Quad), and subjected to subcellular fractionation. Immunoblots for GLUT4 were performed on PM GLUT4 (5 μg protein/lane) and total membranes (total GLUT4)(10 μg protein/lane), and representative immunoblots for one subcellular fractionation are shown in H, Gastro/Quad PM GLUT4, and I, Gastro/Quad total GLUT4, respectively. Signals on immunoblots were quantified, and means ± SE (n = 4) were plotted (bar graphs below respective immunoblots). Ratios for PM GLUT4/Total GLUT4 (means ± SE, n = 4) were calculated as described in Fig. 2F and plotted (I: Gastro/Quad PM GLUT4 relative to total GLUT4). *P < 0.05 (unless indicated otherwise) comparing data to AS160+/+ Bas using 1-sample t-tests, with AS160+/+ Bas assigned a value of 100 or 1. Preliminary studies with subcellular fractions from Gastro/Quad isolated from female mice yielded similar results as shown for male.
To evaluate the effect of AS160 deletion on GLUT4 subcellular distribution in soleus, we determined GLUT4 cell surface exposure by cell surface labeling. As shown in Fig. 3E, cell surface GLUT4 in AS160−/− soleus was lower (by 47 and 46%, respectively) under both basal and insulin-stimulated conditions compared with respective AS160+/+ soleus. After correction for the 36% decrease in total GLUT4 (Fig. 3F), the relative amounts of labeled GLUT4 in basal AS160−/− soleus were 18% lower than in basal AS160+/+ soleus and not significantly different for insulin-stimulated AS160−/− and AS160+/+ soleus (Fig. 3G).
Due to its small size, soleus is not amenable to subcellular fractionation; the large skeletal muscles gastrocnemius and quadriceps were thus used for this analysis. As shown in Fig. 1, B and C, gastrocnemius is similar to soleus in that it also expresses high levels of AS160, AS160 is the predominant Akt substrate at 160 kDa phosphorylated in response to insulin, and Tbc1d1 is expressed at low levels. We found that GLUT4 was increased in AS160−/− plasma membranes derived from gastrocnemius/quadriceps of non-insulin-treated mice compared with AS160+/+. Insulin treatment did not elicit a further increase of GLUT4 in AS160−/− plasma membranes, whereas it triggered a significant increase of GLUT4 in AS160+/+ plasma membranes. Evaluation of total gastrocnemius/quadriceps membranes from which the plasma membrane fractions were derived showed 14, 24, and 19% decreases in GLUT4 for AS160+/+ insulin, AS160−/− basal and AS160−/− insulin, respectively, compared with AS160+/+ basal (Fig. 3I). After taking these changes in GLUT4 into account, we found that the amount of GLUT4 was increased 1.8-fold in AS160−/− plasma membranes under basal conditions and similar to AS160+/+ levels after insulin stimulation. There was no further increase in GLUT4 in response to insulin in AS160−/− plasma membranes, whereas in AS160+/+ plasma membranes there was a 1.9-fold increase (Fig. 3J). The lack of an insulin response was not due to impaired insulin signaling through Akt under the in vivo conditions used for insulin treatment before isolation of the muscles (Fig. 1D).
In summary, in AS160−/− soleus, insulin-stimulated glucose uptake was impaired proportionally to the decrease in GLUT4 expression. Although basal glucose uptake in soleus was normal, it was higher than expected based on total GLUT4 expression and GLUT4 cell surface exposure. GLUT1 expression was normal in AS160−/− soleus (data not shown). Relative levels of cell surface-labeled GLUT4 were similar for AS160−/− and AS160+/+ mice under both basal and insulin-stimulated conditions. In large skeletal muscles, plasma membrane-associated GLUT4 was increased under basal conditions and did not increase in response to insulin despite normal insulin signaling. Glucose uptake and GLUT4 expression were not affected in AS160−/− EDL.
Whole body glucose homeostasis and insulin sensitivity in AS160−/− mice.
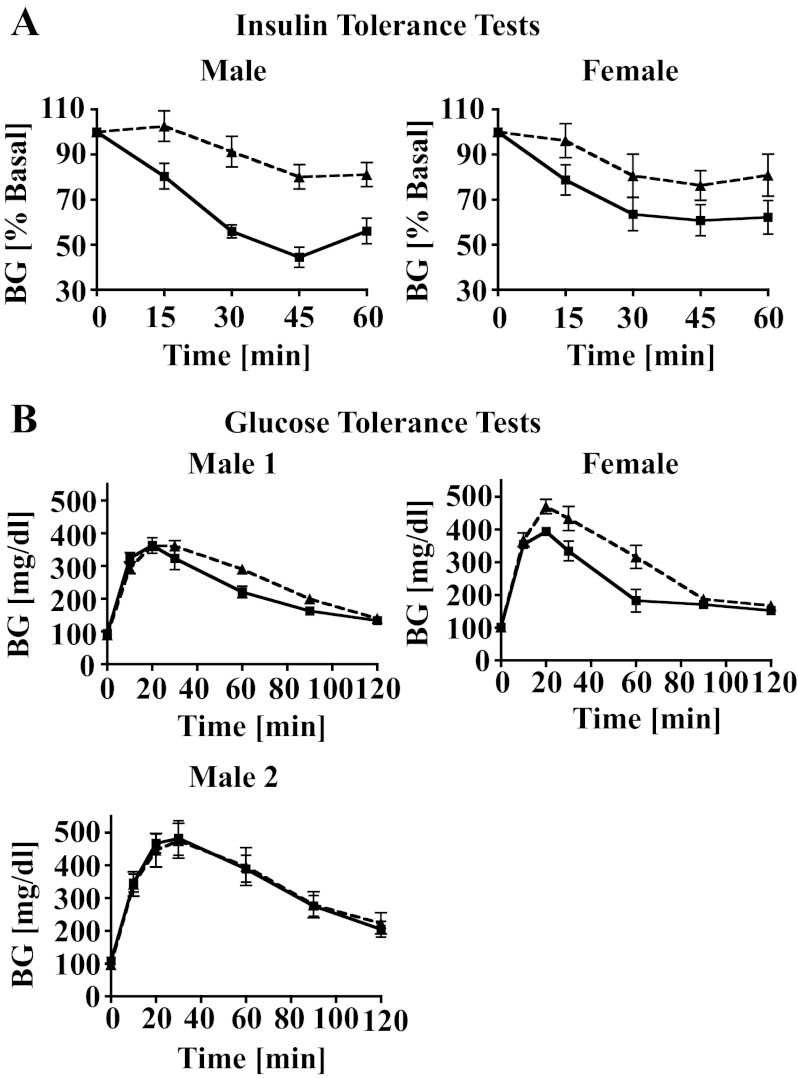
To determine how the deletion of AS160 affects whole body insulin sensitivity and glucose tolerance, we performed insulin and glucose tolerance tests. We found that insulin tolerance was impaired in both male and female AS160−/− mice (Fig. 4A), while glucose tolerance was impaired in female but not in male AS160−/− mice (Fig. 4B). The normal glucose tolerance in the presence of impaired insulin tolerance in the AS160−/− male was not due to increased insulin secretion, as insulin levels measured at 0, 5, and 30 min during a glucose tolerance test were the same for AS160−/− and AS160+/+ mice (data not shown). The results shown in Fig. 4 were obtained with young AS160−/− and AS160+/+ mice. However, glucose and insulin tolerance tests with 8- to 9-mo-old mice yielded similar results (data not shown). Random-fed and fasting blood glucose and plasma insulin levels for young (7–12 wk) and older (8–9 mo) AS160−/− and AS160+/+ male and female mice are listed in Table 1. Random-fed glucose and insulin levels were similar between AS160−/− and AS160+/+ mice for both males and females, with one exception: older male AS160−/− mice had lower glucose levels. However, after 6 h of fasting, AS160−/− mice of all ages and both sexes showed decreased blood glucose levels in the presence of normal or decreased (young male) insulin levels. In contrast, after overnight fasting, blood glucose levels between AS160−/− and AS160+/+ mice were similar for both males and females (data not shown).
Fig. 4.
A: insulin tolerance tests were performed on random-fed male (2 mo, n = 5–7) and female (4 mo, n = 7) AS160−/− (broken lines) and AS160+/+ mice (solid lines) at 2 PM. Blood glucose (BG) levels were measured before (time 0) and after ip injection of insulin at 15, 30, 45, and 60 min. Data (in % BG at time 0, means ± SE) were graphed. Data were statistically significantly different for male (P < 0.0001) and female mice (P = 0.003, 2-way ANOVA). B: glucose tolerance tests were performed with male (3–4 mo, n = 5–7) and female (4 mo, n = 7) AS160−/− (broken lines) and AS160+/+ mice (solid lines). BG levels were measured before (time 0) and after ip injection of glucose (1 and 2 mg/g body wt) at 10, 20, 30, 60, 90, and 120 min. Data (BG, means ± SE) were graphed. Data were statistically significantly different for female mice (P < 0.0001, 2-way ANOVA) but not for male mice (P > 0.05, 2-way ANOVA) using glucose at 1 (male 1) or 2 (male 2) mg/g body wt. When glucose at 2 mg/g body wt was used in female mice, several of the BG levels (8 for AS160+/+ and 14 for AS160−/−) were above the reading range of the glucometer (>600 mg/dl). Body weights for AS160+/+ and AS160−/− mice used in these tests were similar (data not shown). Tests were repeated with 8- to 9-mo-old male and female mice, and similar differences were observed between AS160−/− and AS160+/+ mice as shown for the younger mice.
To shed more light on the discrepancy between severely impaired insulin but normal glucose tolerance in male AS160−/− mice, we determined glucose uptake in different skeletal muscles and adipose tissues and glucose incorporation into glycogen in liver in vivo by injecting mice with radioactive 2-deoxyglucose together with either regular glucose or saline, as described (52). We found that, in brown adipose tissue of saline-injected mice, glucose uptake was increased threefold for AS160−/− compared with AS160+/+ mice and was thus similar to AS160+/+ glucose-injected mice (Fig. 5A). Glucose injection, stimulating insulin release, further increased glucose uptake 1.5-fold in AS160−/− brown adipose tissue, thus raising it above the level observed in glucose-injected AS160+/+ mice. GLUT4 is the major glucose transporter in brown adipocytes and predominantly determines basal and insulin-stimulated glucose uptake (44), but GLUT1 is also expressed. We found that in AS160−/− brown adipose tissue GLUT4 was decreased by 19% whereas GLUT1 was normal (data not shown). Glucose uptake in white adipose tissue was similar for AS160−/− and AS160+/+ mice injected with saline and glucose despite decreased expression of GLUT4 and normal expression of GLUT1 in AS160−/− adipose tissue and adipocytes (Fig. 2, B and C). The large mixed-fiber type skeletal muscles, represented by gastrocnemius and quadriceps muscles, are major contributors to glucose homeostasis. We found that glucose uptake was decreased by 39% in the AS160−/− gastrocnemius isolated from saline-injected mice (Fig. 5C). While after glucose injection glucose uptake increased 1.5-fold in AS160+/+ gastrocnemius, it had no effect in AS160−/− gastrocnemius (Fig. 5C). These findings could not be explained by major decreases in GLUT4 (Fig. 3I) or any changes in GLUT1 expression (data not shown). Furthermore, they were inconsistent with our data showing increased GLUT4 plasma membrane association in gastrocnemius/quadriceps under basal conditions (Fig. 3H). To evaluate whether the impaired glucose uptake in gastrocnemius was specific to muscles that normally express AS160 well (Fig. 1B), we also determined glucose uptake in TA and EDL muscles that normally express low levels of AS160 (Fig. 1B). As shown in Fig. 5, D and E, glucose uptake in TA and EDL was similarly affected as in gastrocnemius. Glucose uptake was normal in saline-injected AS160−/− TA and EDL. It did not increase after glucose injection in AS160−/− but increased 1.7-fold in AS160+/+ TA and EDL. GLUT4 expression was similar between AS160−/− and AS160+/+ TA (data not shown) and EDL (Fig. 3D). To evaluate whether the liver compensated for impaired glucose uptake in skeletal muscles, we measured glucose incorporation into glycogen, as described in Ref. 52. We found that, in saline-injected mice, glucose incorporation into glycogen was similar between AS160−/− and AS160+/+ liver. But while it increased 2.2-fold in AS160+/+ liver after glucose injection, it remained at basal levels in AS160−/− liver. AS160 is not expressed at detectable levels in liver but is abundant in tissues other than adipose and skeletal muscle tissues that play roles in the regulation of whole body glucose homeostasis: pancreas, brain, and kidney (Fig. 5G).
In summary, AS160−/− mice show an impaired ability to lower blood glucose levels in response to a bolus of insulin. Glucose tolerance is impaired in female but not male AS160−/− mice. Under random-fed conditions, insulin and glucose levels are normal. However, AS160−/− mice are hypoglycemic after 6 h of fasting in the presence of normal insulin levels. While glucose uptake is enhanced in brown adipose tissue of AS160−/− mice under both basal and insulin-stimulated conditions, it is similar to wild type in white adipose tissue. Glucose uptake in all evaluated AS160−/− skeletal muscles and glucose incorporation into glycogen in AS160−/− liver are normal or decreased (gastrocnemius) under basal conditions but do not respond to insulin.
DISCUSSION
The studies with isolated adipocytes and skeletal muscles presented herein establish that AS160 is required for proper regulation of glucose uptake and GLUT4 trafficking and expression in primary adipocytes and in skeletal muscles in which it is normally expressed. They further support the notion that there are differences in the regulation of glucose uptake and GLUT4 cell surface exposure between skeletal muscles and adipocytes. In vivo studies suggest that deletion of AS160 leads to conditions that allow differential regulation of glucose disposal into adipose tissues and skeletal muscles.
Key to the regulation of glucose uptake into adipocytes and skeletal muscles is GLUT4 intracellular sequestration under basal conditions. Previous studies in 3T3-L1 adipocytes in which AS160 was knocked down using shRNA GLUT4 amounts at the cell surface were increased in the basal state (11, 27). But the magnitude of the effect differed between studies, ranging from partial relocation of GLUT4 to the cell surface (3-fold increase) (11) to a full insulin-like effect (8-fold increase) (27). These mixed effects in 3T3-L1 adipocytes were most likely due to different extents of AS160 elimination (27). Our results with primary adipocytes show that complete knockout of AS160 increased relative GLUT4 plasma membrane association and cell surface expression under basal conditions to levels that were similar to those in insulin-stimulated wild type. Furthermore, basal glucose uptake was 1.7-fold higher in AS160−/− than in AS160+/+ adipocytes, even though total GLUT4 was reduced 58% in AS160−/− adipocytes. Thus, when basal glucose uptake in AS160−/− adipocytes is normalized to wild-type GLUT4 amounts, it is increased fourfold, which is comparable to the fivefold increase in glucose uptake that occurred in response to insulin in AS160+/+ adipocytes. In brown adipose tissue of AS160−/− mice, in vivo glucose uptake was threefold higher than in wild type under basal conditions and was thus the same as in insulin-stimulated wild type. Since AS160 knockout reduced the total amount of GLUT4 in brown adipose tissue by only 19%, normalization for the total amount of GLUT4 can be neglected. These findings thus support an indispensable role for AS160 in GLUT4 intracellular retention in basal primary adipocytes, preventing GLUT4 vesicles from docking and fusing with the plasma membrane. The lack of AS160 Rab GAP function in the AS160−/− adipocytes most likely leads to increased amounts of GTP-bound Rabs that in turn trigger unrestricted GLUT4 translocation to the plasma membrane.
There is good evidence that the rate-limiting step(s) controlled by insulin is/are the docking and/or fusion of the GLUT4 vesicles with the plasma membrane (1, 24, 30). Studies with 3T3-L1 adipocytes expressing AS160 phosphorylation site mutants (AS160-4A) placed the point of AS160 action upstream of GLUT4 vesicle fusion with the plasma membrane (51) and at the docking of GLUT4 vesicles to the plasma membrane (1). A recent in vitro study using GLUT4 vesicles and plasma membranes isolated from rat adipocytes further supports a role for AS160 in GLUT4 vesicle fusion with the plasma membrane (25). We observed that, although insulin did not further increase GLUT4 associated with the plasma membrane in AS160−/− adipocytes, it elicited a further increase in GLUT4 cell surface exposure and glucose uptake. This suggests that, while AS160 is the only factor restricting GLUT4 plasma membrane association, there are other insulin-mediated AS160-independent inputs regulating GLUT4 cell surface exposure and glucose uptake. Two possibilities are the Ral GAP-complex (8) and CDP138 (48). These two Akt substrates have been implicated in stimulating GLUT4 vesicle fusion with the plasma membrane upon phosphorylation. The fact that insulin-elicited (relative or absolute) cell surface exposure and glucose uptake were higher in AS160−/− white and brown adipocytes than in respective AS160+/+ adipocytes suggests that in wild-type cells the Rab GAP activity of AS160 is not completely suppressed by insulin.
Although the relative levels of GLUT4 at the cell surface were increased under both basal and insulin-stimulated conditions, the absolute levels of GLUT4 that were cell surface exposed were similar to wild type in basal AS160−/− adipocytes and decreased under insulin-stimulated conditions. While under insulin-stimulated conditions the decreased cell surface exposure was accompanied by similar relative decreases in glucose uptake, under basal conditions glucose uptake was higher than expected from cell surface-exposed GLUT4. A similar observation applies to isolated soleus, in which glucose uptake was higher than expected based on GLUT4 cell surface labeling data under basal and insulin-stimulated conditions. These discrepancies are not due to increased GLUT1 expression in adipocytes and soleus. Also, GLUT1 trafficking is not regulated by AS160 in 3T3-L1 adipocytes (11); therefore, differential GLUT1 distribution in AS160−/− cells most likely does not occur. Our observations thus imply that in AS160−/− adipocytes and soleus the intrinsic activity of cell surface GLUT4 is increased, pointing to a possible additional control point for AS160. A few studies have suggested activation of GLUT4 after incorporation into the plasma membrane (13). Alternatively, differential access of the cell surface label (glucose analog) and glucose to GLUT4 in AS160−/− and AS160+/+ cells may explain the discrepancy.
Only a few studies have addressed AS160 function in muscle. In the L6 skeletal muscle cell line, AS160 knockdown and overexpression of the AS160-4A mutant affects GLUT4 subcellular distribution similarly to 3T3-L1 adipocytes (50). Studies with mouse tibialis anterior muscles transfected with wild type and mutant AS160 uncovered roles for AS160 in insulin- and exercise-mediated increases in glucose uptake (21). However, two recent studies with AS160 knockin mice (discussed in more detail below) suggest that, although AS160 is required for insulin-stimulated glucose uptake in all tested skeletal muscles (including soleus and EDL) (7), it is not involved in exercise-mediated glucose uptake (10). Our results with isolated soleus and EDL muscles show that the knockout of AS160 had quite different effects in different muscles. In EDL, AS160 knockout had no effect on glucose uptake or GLUT4 expression. In soleus, AS160 deletion caused a 35% reduction in the total GLUT4 amount. This reduction was associated with normal basal glucose uptake and a decrease of similar magnitude in insulin-stimulated glucose uptake. However, relative cell surface GLUT4 exposure in soleus was similar to wild type under both basal and insulin-stimulated conditions. This finding contrasts with the observations in adipocytes in which AS160 deletion increased basal and insulin-stimulated GLUT4 cell surface exposure. In adipocytes, this occurred together with increased GLUT4 plasma membrane association. Although GLUT4 plasma membrane association could not be determined in soleus, the findings for the large skeletal muscles showing that AS160 deletion increased GLUT4 in the plasma membrane in the basal state with no further change in response to insulin suggest that GLUT4 plasma membrane association in soleus may be similar to that in adipocytes. This in turn would imply differences in the regulation of GLUT4 cell surface exposure between soleus and adipocytes, being AS160 independent in the former and at least partially AS160 dependent in the latter. Clear differences in GLUT4 trafficking in primary adipocytes and skeletal muscles have been established by live microscopy (28, 30, 31). It is, however, also possible that adipocytes and skeletal muscles differentially respond to metabolic changes inflicted by AS160 deletion. This is supported by differential regulation of GLUT4 expression at the mRNA and protein levels, respectively, in adipocytes and soleus and by the in vivo data discussed below.
In AS160−/− adipocytes, both GLUT4 mRNA and GLUT4 protein levels are downregulated to a similar extent, but in soleus GLUT4 is decreased only at the protein level. While the data in adipocytes suggest changes in GLUT4 transcription, the data in soleus indicate increased GLUT4 protein turnover. At this point, the underlying mechanisms responsible for the changes in GLUT4 expression and the differences thereof between adipocytes and soleus are unknown. Observations in Tbc1d1-deficient (45) and in AS160 knockin mice (7) demonstrating down- and upregulation of GLUT4, respectively, in respective muscle tissues also only at the protein level suggest similar roles for AS160 and Tbc1d1 in GLUT4 regulation in muscles. It is possible that changes in GLUT4 trafficking resulting from the lack of AS160 and Tbc1d1 functions lead to increased degradation of GLUT4. This is, however, not supported by the finding that expression of the insulin-regulated aminopeptidase (IRAP), a protein that has the same trafficking characteristics as GLUT4 (19), is not changed in AS160−/− adipocytes and soleus [IRAP AS160−/−/IRAP AS160+/+ 0.97 ± 0.12 (n = 3) in adipose tissue and 1.16 ± 0.14 (n = 3) in soleus], whereas preliminary data from subcellular fractionations indicate that IRAP shows the same changes in subcellular distribution as GLUT4 (data not shown). IRAP expression is also not changed in Tbc1d1-deficient mice (45). Unless the turnovers of IRAP and GLUT4 are differently regulated, it is thus more likely that changes in GLUT4 expression are the consequence of metabolic changes that occur in response to modifications in GLUT4 trafficking inflicted by AS160 or Tbc1d1 deletion. Differential responses to metabolic changes that follow the different changes in GLUT4 trafficking and thus glucose uptake could also lead to differential regulation of GLUT4 in adipocytes and skeletal muscles of AS160−/− mice.
The disparate observations between soleus and EDL muscles most likely reflect differential AS160 expression; AS160 is well expressed in soleus and has low abundance in EDL (Fig. 1B and Ref. 46). Tbc1d1, in contrast, is well expressed in EDL but has low abundance in soleus (Fig. 1C and Ref. 46). These distributions suggest that AS160 and Tbc1d1 play similar but not redundant roles in the regulation of glucose uptake in different muscles. With regard to the similar roles of AS160 and Tbc1d1, in mice with a deletion in the Tbc1d1 gene and no detectable levels of Tbc1d1 protein, glucose uptake and GLUT4 expression are impaired in EDL but not in soleus (4, 45). In support of nonredundant roles for the two Rab GAPs, we found no upregulation of Tbc1d1 in any AS160−/− skeletal muscles and adipose tissues, and Szekeres et al. (45) found no upregulation of AS160 in Tbc1d1-deficient skeletal muscles.
Our in vivo studies demonstrate that AS160 plays a role in the regulation of glucose homeostasis. Alterations in glucose homeostasis in AS160−/− mice appeared under challenged conditions (fasting and glucose and insulin tolerance tests). The impaired lowering of blood glucose in response to insulin is most likely due to impaired glucose uptake by skeletal muscles and defective glycogen synthesis in liver, as demonstrated by the in vivo glucose disposal studies. The hypoglycemia observed in AS160−/− mice after 6 h of fasting may be the result of decreased glycogen reserve in the liver. However, the findings of normal (male) and only mildly impaired (female) glucose tolerance in AS160−/− mice are not compatible with severely impaired glucose disposal into all skeletal muscles and the liver; yet glucose uptake was increased in brown adipose tissue and normal in white adipose tissue. Skeletal muscle, due to its large mass, is the major contributor to glucose disposal. Although brown adipose tissue takes up the largest amount of glucose per gram of tissue, due to its small size its increased glucose uptake can make up for only a portion of the impaired glucose uptake into skeletal muscles. White adipose tissue contributes only little to glucose uptake (∼1/10th to 1/40th that of other tissues after saline and glucose injections, respectively), and in the mice used for the in vivo glucose uptake studies white adipose tissue mass was not significantly different for the AS160−/− mice. It is thus very likely that additional modifications contribute to the regulation of glucose homeostasis in AS160−/− mice. AS160 is not only found in skeletal muscles and adipocytes, it is also highly expressed in other tissues that play roles in the maintenance of glucose homeostasis, including pancreas (Fig. 5G) and pancreatic islets (2), brain (Fig. 5G, Ref. 17) and kidney (Fig. 5G, Ref. 32). Our insulin measurements do not support impaired β-cell function in AS160−/− mice. However, the role of AS160 in these other tissues awaits elucidation. The difference in glucose tolerance between male and female AS160−/− mice cannot be explained at this time. But different body compositions of male and female mice in general, with higher body fat content in male mice [25 ± 2.1 vs. 18 ± 0.4% in female (n = 4–6) at 19 wk of age, P = 0.004] and differential effects on glucose uptake between adipose and skeletal muscle tissues in AS160−/− mice may play a role in this.
The fact that glucose uptake was normal in isolated EDL but severely impaired in vivo, similar to other evaluated muscles, suggests that circulating factors/metabolites modified due to the absence of AS160 exert effects on glucose uptake. Preliminary data indicate that circulating free fatty acids are increased in the AS160−/− mice under the conditions used for the glucose disposal experiments (after 6 h of fasting and 2 h after glucose injection). Increased circulating free fatty acids can impair glucose disposal in skeletal muscle and liver (3). Interestingly, AS160−/− adipose tissue glucose uptake was unaffected by these changes. Also, note that this occurred in the presence of increased GLUT4 plasma membrane association in both gastrocnemius/quadriceps and adipose tissues. These findings thus further suggest differential regulation of cell surface exposure and/or GLUT4 activity between skeletal muscles and adipose tissues. The apparent difference in basal glucose uptake between isolated adipocytes and white adipose tissue in vivo of AS160−/− mice, increased and normal, respectively, is most likely due to differences in how glucose uptake is expressed, in the former as glucose taken up per adipocyte and in the latter as glucose taken up per gram of tissue. Considering that lipid stores in adipocytes determine to a large extent adipose tissue weight and adipocyte size, and adipocyte size on average was increased in AS160−/− adipose tissue, there are fewer cells per gram of adipose tissue in the AS160−/− than in the AS160+/+ mice. Thus, similar in vivo glucose uptake for AS160+/+ and AS160−/− adipose tissues per gram of tissue are compatible with increased glucose uptake per AS160−/− adipocyte. But note that no matter how the data are expressed, both in vivo and in vitro glucose uptake are higher than expected under both basal and insulin-stimulated conditions, when decreased GLUT4 expression is considered, and are consistent with relative increased cell surface exposure of GLUT4 in white adipocytes.
Tbc1d1-deficient mice also showed discrepancies between in vitro and in vivo glucose uptake (45); insulin-stimulated glucose uptake in EDL muscles was impaired in vitro but was increased in vivo. Clearly different from the AS160−/− mice, in which all skeletal muscles were affected, irrespective of whether they normally express high or low levels of AS160, in the Tbc1d1-deficient mice the increase in the in vivo glucose uptake was limited to EDL and tibialis anterior muscles, which normally express the highest levels of Tbc1d1. It is thus very likely that the mechanisms underlying the observations in Tbc1d1- and AS160-deficient mice are different.
Only one other study has addressed AS160 function in a physiological context (7). Chen et al. (7) generated AS160 knockin mice in which wild-type AS160 was replaced by AS160 with Thr649, a major site of insulin-stimulated Akt phosphorylation, mutated to Ala. In 3T3-L1 adipocytes, the same AS160 mutant reduced the insulin-stimulated increase in cell surface GLUT4 by 60%, most likely because Akt cannot phosphorylate the mutated site and the Rab GAP activity of AS160 is not suppressed by insulin (42). Indeed, in the AS160 knockin mice, insulin-stimulated glucose uptake in different muscles was inhibited and glucose tolerance impaired, although increased GLUT4 expression compensated, at least in part, for defects in GLUT4 translocation. Surprisingly, however, insulin-stimulated glucose uptake in adipocytes was not inhibited.
In conclusion, our studies establish AS160 as an important player in the regulation of GLUT4 trafficking and glucose uptake in vivo in primary adipocytes and skeletal muscles and in whole body glucose homeostasis. The significance of our observations to diseases associated with impaired glucose homeostasis, foremost diabetes, will need to be established. Elucidation of the molecular mechanisms underlying differential AS160 action in adipocytes and different skeletal muscles may identify novel targets for differential manipulation of glucose transport between skeletal muscles and adipocytes and thus of whole body glucose homeostasis.
GRANTS
We are also grateful to the grant agencies that supported the studies presented herein, the National Institutes of Health (R56 DK-081471 and R01 DK-81471 to S. R. Keller, F31 DK-084767 to M. N. Lansey) and the American Diabetes Association (7-08-RA-80 to S. R. Keller).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.N.L., N.N.W., S.R.H., J.R.S., and S.R.K. performed experiments; M.N.L., N.N.W., S.R.H., J.R.S., and S.R.K. analyzed data; M.N.L., N.N.W., S.R.H., J.R.S., and S.R.K. approved final version of manuscript; S.R.K. conception and design of research; S.R.K. interpreted results of experiments; S.R.K. prepared figures; S.R.K. drafted manuscript; S.R.K. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. Gustav Lienhard at Dartmouth Medical School (supported by the National Institutes of Health R01 DK-025336) for invaluable contributions to this study, providing the original AS160+/− breeders and AS160 antibodies and giving feedback on the manuscript. We thank Dr. Geoffrey Holman at the University of Bath in the UK for supplying the reagent Bio-LC-ATB-BGPA for GLUT4 cell surface labeling and protocols, Dr. Jason Chruma at the University of Virginia for allowing us to use his UV crosslinker for cell surface labeling experiments, Dr. Samuel Cushman at the National Institutes of Health for providing GLUT1 antibodies, and Caitlin Muse, Wendy Schmunk, Joshua Jolissaint, and Berhanemeskel Nida, undergraduate and medical students and laboratory assistants in the laboratory, for their contributions to this work.
REFERENCES
- 1.Bai L, Wang Y, Fan J, Chen Y, Ji W, Qu A, Xu P, James DE, Xu T. Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab 5: 47–57, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Bouzakri K, Ribaux P, Tomas A, Parnaud G, Rickenbach K, Halban PA. Rab GTPase-activating protein AS160 is a major downstream effector of protein kinase B/Akt signaling in pancreatic beta-cells. Diabetes 57: 1195–1204, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Campello RS, Alves-Wagner AB, Abdulkader F, Mori RC, Machado UF. Carbohydrate- and lipid-enriched meals acutely disrupt glycemic homeostasis by inducing transient insulin resistance in rats. Can J Physiol Pharmacol 90: 537–545, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Chadt A, Leicht K, Deshmukh A, Jiang LQ, Scherneck S, Bernhardt U, Dreja T, Vogel H, Schmolz K, Kluge R, Zierath JR, Hultschig C, Hoeben RC, Schurmann A, Joost HG, Al-Hasani H. Tbc1d1 mutation in lean mouse strain confers leanness and protects from diet-induced obesity. Nat Genet 40: 1354–1359, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Chavez JA, Roach WG, Keller SR, Lane WS, Lienhard GE. Inhibition of GLUT4 translocation by Tbc1d1, a Rab GTPase-activating protein abundant in skeletal muscle, is partially relieved by AMP-activated protein kinase activation. J Biol Chem 283: 9187–9195, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, Mackintosh C. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J 409: 449–459, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Chen S, Wasserman DH, MacKintosh C, Sakamoto K. Mice with AS160/TBC1D4-Thr649Ala knockin mutation are glucose intolerant with reduced insulin sensitivity and altered GLUT4 trafficking. Cell Metab 13: 68–79, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen XW, Leto D, Xiao J, Goss J, Wang Q, Shavit JA, Xiong T, Yu G, Ginsburg D, Toomre D, Xu Z, Saltiel AR. Exocyst function is regulated by effector phosphorylation. Nat Cell Biol 13: 580–588, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietrich W, Katz H, Lincoln SE, Shin HS, Friedman J, Dracopoli NC, Lander ES. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics 131: 423–447, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ducommun S, Wang HY, Sakamoto K, MacKintosh C, Chen S. Thr649Ala-AS160 knock-in mutation does not impair contraction/AICAR-induced glucose transport in mouse muscle. Am J Physiol Endocrinol Metab 302: E1036–E1043, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eguez L, Lee A, Chavez JA, Miinea CP, Kane S, Lienhard GE, McGraw TE. Full intracellular retention of GLUT4 requires AS160 rab GTPase activating protein. Cell Metab 2: 263–272, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Fantin VR, Wang Q, Lienhard GE, Keller SR. Mice lacking insulin receptor substrate 4 exhibit mild defects in growth, reproduction, and glucose homeostasis. Am J Physiol Endocrinol Metab 278: E127–E133, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Furtado ML, Poon V, Klip A. GLUT4 activation: thoughts on possible mechanisms. Acta Physiol Scand 178: 287–296, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Holman GD, Kozka IJ, Clark AE, Flower CJ, Saltis J, Habberfield AD, Simpson IA, Cushman SW. Cell surface labeling of glucose transporter isoform GLUT4 by bis-mannose photolabel. J Biol Chem 265: 18172–18179, 1990 [PubMed] [Google Scholar]
- 15.Ishikura S, Klip A. Muscle cells engage Rab8A and myosin Vb in insulin-dependent GLUT4 translocation. Am J Physiol Cell Physiol 295: C1016–C1025, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Jenkins AB, Furler SM, Kraegen EW. 2-Deoxy-d-glucose metabolism in individual tissues of the rat in vivo. Int J Biochem 18: 311–318, 1986 [DOI] [PubMed] [Google Scholar]
- 17.Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, Lienhard GE. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a rab GTPase-activating protein (GAP) domain. J Biol Chem 277: 22115–22118, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Khromov AS, Wang H, Choudhury N, McDuffie M, Herring BP, Nakamoto R, Owens GK, Somlyo AP, Somlyo AV. Smooth muscle of telokin-deficient mice exhibits increased sensitivity to Ca2+ and decreased cGMP-induced relaxation. Proc Natl Acad Sci USA 103: 2440–2445, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keller SR, Davis AC, Clairmont KB. Mice deficient in the insulin-regulated membrane aminopeptidase show substantial decreases in glucose transporter GLUT4 levels but maintain normal glucose homeostasis. J Biol Chem 277: 17677–17686, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Klip A, Ramlal T, Young DA, Holloszy JO. Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett 224: 224–230, 1987 [DOI] [PubMed] [Google Scholar]
- 21.Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem 281: 31478–31485, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Kurlawalla-Martinez C, Stiles B, Wang Y, Devaskar SU, Kahn BB, Wu H. Insulin hypersensitivity and resistance to streptozotocin-induced diabetes in mice lacking PTEN in adipose tissue. Mol Cell Biol 25: 2498–2510, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koumanov F, Holman GD. Thrifty Tbc1d1 and Tbc1d4 proteins link signalling and membrane trafficking pathways. Biochem J 403: e9–e11, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koumanov F, Jin B, Yang J, Holman GD. Insulin signaling meets vesicle traffic of GLUT4 at a plasma membrane-activated fusion step. Cell Metab 2: 179–189, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Koumanov F, Richardson JD, Murrow BA, Holman GD. AS160 phosphotyrosine-binding domain constructs inhibit insulin-stimulated GLUT4 vesicle fusion with the plasma membrane. J Biol Chem 286: 16574–16582, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koumanov F, Yang J, Jones AE, Hatanaka Y, Holman GD. Cell-surface biotinylation of GLUT4 using bis-mannose photolabels. Biochem J 330: 1209–1215, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larance M, Ramm G, Stockli J, van Dam EM, Winata S, Wasinger V, Simpson F, Graham M, Junutula JR, Guilhaus M, James DE. Characterization of the role of the rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J Biol Chem 280: 37803–37813, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Lauritzen HP, Galbo H, Brandauer J, Goodyear LJ, Ploug T. Large GLUT4 vesicles are stationary while locally and reversibly depleted during transient insulin stimulation of skeletal muscle of living mice: imaging analysis of GLUT4-enhanced green fluorescent protein vesicle dynamics. Diabetes 57: 315–324, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Liu SC, Wang Q, Lienhard GE, Keller SR. Insulin receptor substrate 3 is not essential for growth or glucose homeostasis. J Biol Chem 274: 18093–18099, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Lizunov VA, Matsumoto H, Zimmerberg J, Cushman SW, Frolov VA. Insulin stimulates the halting, tethering, and fusion of mobile GLUT4 vesicles in rat adipose cells. J Cell Biol 169: 481–489, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lizunov VA, Stenkula KG, Lisinski I, Gavrilova O, Yver DR, Chadt A, Al-Hasani H, Zimmerberg J, Cushman SW. Insulin stimulates fusion, but not tethering, of GLUT4 vesicles in skeletal muscle of HA-GLUT4-GFP transgenic mice. Am J Physiol Endocrinol Metab 302: E950–E960, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lier N, Gresko N, Di Chiara M, Loffing-Cueni D, Loffing J. Immunofluorescent localization of the Rab-GAP protein TBC1D4 (AS160) in mouse kidney. Histochem Cell Biol 138: 101–112, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Lund S, Holman GD, Zierath JR, Rincon J, Nolte LA, Clark AE, Schmitz O, Pedersen O, Wallberg-Henriksson H. Effect of insulin on GLUT4 cell surface content and turnover rate in human skeletal muscle as measured by the exofacial bis-mannose photolabeling technique. Diabetes 46: 1965–1969, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto Y, Imai Y, Lu Yoshida N, Sugita Y, Tanaka T, Tsujimoto G, Saito H, Oshida T. Upregulation of the transcript level of GTPase activating protein KIAA0603 in T cells from patients with atopic dermatitis. FEBS Lett 572: 135–140, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Peck GR, Chavez JA, Roach WG, Budnik BA, Lane WS, Karlsson HK, Zierath JR, Lienhard GE. Insulin-stimulated phosphorylation of the rab GTPase-activating protein TBC1D1 regulates GLUT4 translocation. J Biol Chem 284: 30016–30023, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson PM, Zon LI. Molecular cloning of a cDNA with a novel domain present in the tre-2 oncogene and the yeast cell cycle regulators BUB2 and cdc16. Oncogene 11: 1139–1148, 1995 [PubMed] [Google Scholar]
- 37.Roach WG, Chavez JA, Miinea CP, Lienhard GE. Substrate specificity and effect on GLUT4 translocation of the rab GTPase-activating protein Tbc1d1. Biochem J 403: 353–358, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roland AV, Nunemaker CS, Keller SR, Moenter SM. Prenatal androgen exposure programs metabolic dysfunction in female mice. J Endocrinol 207: 213–223, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rowland AF, Fazakerley DJ, James DE. Mapping insulin/GLUT4 circuitry. Traffic 12: 672–681, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Roy D, Perreault M, Marette A. Insulin stimulation of glucose uptake in skeletal muscles and adipose tissues in vivo is NO dependent. Am J Physiol Endocrinol Metab 274: E692–E699, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Ryder JW, Yang J, Galuska D, Rincon J, Bjornholm M, Krook A, Lund S, Pedersen O, Wallberg-Henriksson H, Zierath JR, Holman GD. Use of a novel impermeable biotinylated photolabeling reagent to assess insulin- and hypoxia-stimulated cell surface GLUT4 content in skeletal muscle from type 2 diabetic patients. Diabetes 49: 647–654, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278: 14599–14602, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Shimokawa T, Kato M, Ezaki O, Hashimoto S. Transcriptional regulation of muscle-specific genes during myoblast differentiation. Biochem Biophys Res Commun 246: 287–292, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Slot JW, Geuze HJ, Gigengack S, Lienhard GE, James DE. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol 113: 123–135, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szekeres F, Chadt A, Tom RZ, Deshmukh AS, Chibalin AV, Biörnholm M, Al-Hasani H, Zierath JR. The Rab-GTPase activating protein TBC1D1 regulates skeletal muscle glucose metabolism. Am J Physiol Endocrinol Metab 303: E524–E533, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Taylor EB, An D, Kramer HF, Yu H, Fujii NL, Roeckl KS, Bowles N, Hirshman MF, Xie J, Feener EP, Goodyear LJ. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem 283: 9787–9796, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thong FS, Bilan PJ, Klip A. The rab GTPase-activating protein AS160 integrates akt, protein kinase C, and AMP-activated protein kinase signals regulating GLUT4 traffic. Diabetes 56: 414–423, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Xie X, Gong Z, Mansuy-Aubert V, Zhou QL, Tatulian SA, Sehrt D, Gnad F, Brill LM, Motamedchaboki K, Chen Y, Czech MP, Mann M, Kruger M, Jiang ZY. C2 domain-containing phosphoprotein CDP138 regulates GLUT4 insertion into the plasma membrane. Cell Metab 14: 378–389, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J, Hodel A, Holman GD. Insulin and isoproterenol have opposing roles in the maintenance of cytosol pH and optimal fusion of GLUT4 vesicles with the plasma membrane. J Biol Chem 277: 6559–6566, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Zaid H, Antonescu CN, Randhawa VK, Klip A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem J 413: 201–215, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Zeigerer A, McBrayer MK, McGraw TE. Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160. Mol Biol Cell 15: 4406–4415, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zisman A, Peroni OD, Abel ED, Michael MD, Mauvais-Jarvis F, Lowell BB, Wojtaszewski JF, Hirshman MF, Virkamaki A, Goodyear LJ, Kahn CR, Kahn BB. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med 6: 924–928, 2000 [DOI] [PubMed] [Google Scholar]