Abstract
Arginine is derived from dietary protein intake, body protein breakdown, or endogenous de novo arginine production. The latter may be linked to the availability of citrulline, which is the immediate precursor of arginine and limiting factor for de novo arginine production. Arginine metabolism is highly compartmentalized due to the expression of the enzymes involved in arginine metabolism in various organs. A small fraction of arginine enters the NO synthase (NOS) pathway. Tetrahydrobiopterin (BH4) is an essential and rate-limiting cofactor for the production of NO. Depletion of BH4 in oxidative-stressed endothelial cells can result in so-called NOS3 “uncoupling,” resulting in production of superoxide instead of NO. Moreover, distribution of arginine between intracellular transporters and arginine-converting enzymes, as well as between the arginine-converting and arginine-synthesizing enzymes, determines the metabolic fate of arginine. Alternatively, NO can be derived from conversion of nitrite. Reduced arginine availability stemming from reduced de novo production and elevated arginase activity have been reported in various conditions of acute and chronic stress, which are often characterized by increased NOS2 and reduced NOS3 activity. Cardiovascular and pulmonary disorders such as atherosclerosis, diabetes, hypercholesterolemia, ischemic heart disease, and hypertension are characterized by NOS3 uncoupling. Therapeutic applications to influence (de novo) arginine and NO metabolism aim at increasing substrate availability or at influencing the metabolic fate of specific pathways related to NO bioavailability and prevention of NOS3 uncoupling. These include supplementation of arginine or citrulline, provision of NO donors including inhaled NO and nitrite (sources), NOS3 modulating agents, or the targeting of endogenous NOS inhibitors like asymmetric dimethylarginine.
Keywords: arginine metabolism, nitric oxide, therapy, citrulline, sepsis
the purpose of this review is to give an overview of and highlight recent developments on arginine metabolism and nitric oxide (NO) production in health and disease. The endogenous de novo production of arginine will be a special focus, and alterations in this pathway in disease and its relevance will be discussed. Finally, therapeutic applications to influence (de novo) arginine and NO metabolism will be summarized.
Physiology of Arginine and Arginine De Novo and NO Metabolism
Arginine is a conditionally essential amino acid in healthy adults, and therefore there is no specific nutritional requirement (11). However, dietary arginine is required in neonates, infants, and in certain conditions and diseases (171). In the 1980s, l-arginine was identified as the precursor for NO (127). Although first discovered in endothelial cells (116), NO appeared to be a ubiquitous molecule present in a variety of cells, including cells from the cardiovascular and nervous systems and also inflammatory cells. As such, NO has many physiological functions, and the relationship between arginine availability and NO production emphasizes the functional relevance of arginine.
Metabolic Pathways
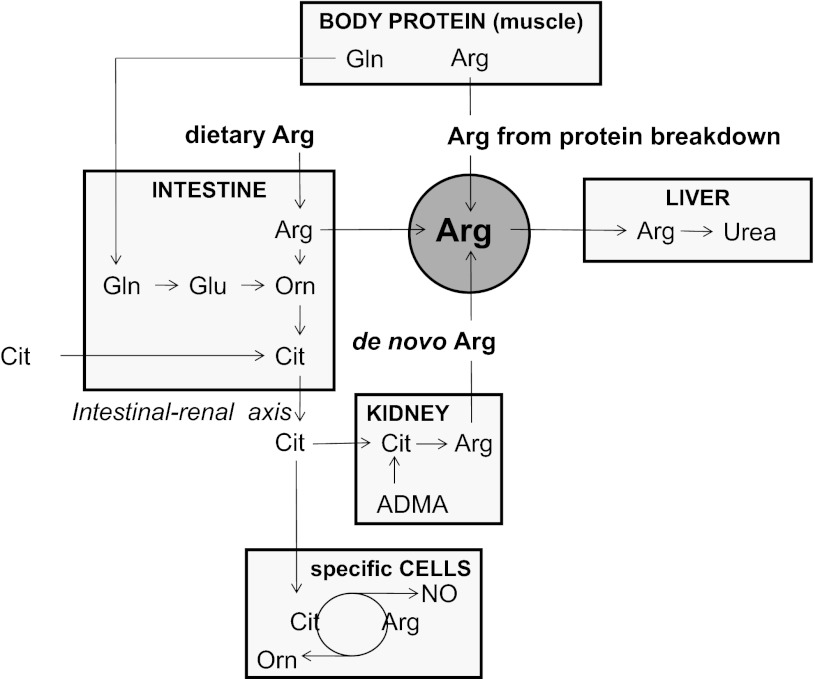
Arginine is derived from dietary intake, body protein breakdown, or endogenous de novo arginine production (Fig. 1). In the postabsorptive state, whole body arginine flux in healthy adults is ∼70–90 μmol·kg−1·h−1, which equals 15–20 g/day (31, 35; see Ref. 101 for review), while daily dietary arginine intake is about 4–6 g (76, 165). De novo arginine production, which contributes ∼10–15% to whole body arginine production under normal conditions (31, 53), involves the conversion of citrulline to arginine and is catalyzed by the enzymes argininosuccinate synthase (ASS) and argininosuccinate lyase (ASL) (54, 57). This conversion is part of the so-called intestinal-renal axis, with intestinal production of citrulline and renal synthesis of arginine (96, 160, 161, 170, 176). Citrulline availability is a limiting factor in this conversion (54).
Fig. 1.
Extrahepatic systemic arginine availability in healthy humans. ARG, arginine; CIT, citrulline; GLN, glutamine; GLU, glutamate; ORN, ornithine; ADMA, asymmetric dimethylarginine; NO, nitric oxide.
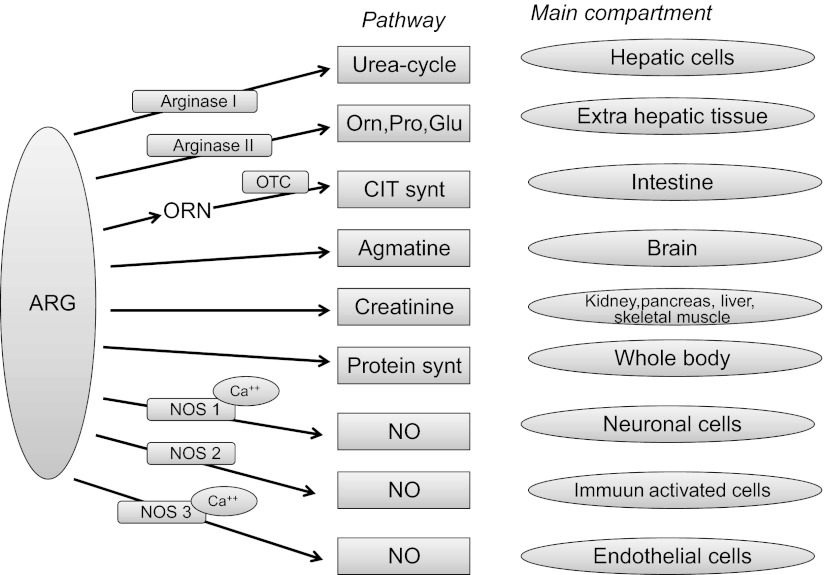
Arginine is a constituent for body protein synthesis, 80% of which is derived from recycling amino acids originating from protein breakdown. Moreover, arginine plays a key role in several other metabolic pathways catalyzed by various enzyme systems (see Refs. 48, 118, 171 for recent reviews) (Fig. 2). Arginine metabolism is highly compartmentalized within the body, since the enzymes involved in arginine metabolism are expressed in various organs, although to different extents. The only exceptional organ is the liver, which contains the complete urea cycle and its related enzymes. However, in healthy conditions, arginine produced in the liver urea cycle is not released to plasma (173). Due to compartmentalization, arginine metabolism and recycling are only partly in balance with plasma arginine concentration. This so-called “arginine paradox” explains that acute exogenous arginine provision can still increase NO production even though the intracellular arginine concentration far exceeds the Km of endothelial NO synthase (eNOS) (60). It is also likely that, once transported into the cell, arginine can no longer gain access to the membrane-bound eNOS. This makes intracellular arginine less useful as a reference point (147).
Fig. 2.
Compartments of arginine metabolism in healthy humans. ARG, arginine; ORN, ornithine; PRO, proline; GLU, glutamate; CIT, citrulline; NO, nitric oxide; NOS, nitric oxide synthase; Ca, calcium; OTC, ornithine transcarbamylase.
Arginine flux as measured with an intravenously infused stable isotope and subsequent dilution of this isotope in the plasma compartment reflects the whole body appearance of arginine in plasma. This plasma arginine flux does not account for hidden compartments (such as liver cells) in which arginine is produced without first being released into plasma. Of the plasma arginine flux, 15% enters the (extrahepatic) arginase pathway (31) that degrades arginine to ornithine and urea.
There are two isoforms of the enzyme arginase. Type I (cytosolic) arginase is predominantly expressed in the liver, as part of the urea cycle, but was also demonstrated at lower levels in various extrahepatic organs in rodents with a main role in production of ornithine for polyamine biosynthesis (175). Type II (mitochondrial) arginase is expressed in low levels in extrahepatic tissues and cells (such as brain, kidney, small intestine, red blood cells, and immune cells) and is mainly involved in the syntheses of ornithine, proline, and glutamate (81, 119). Based on the variability of arginase I and II among organs in rodents, organ-specific roles of arginase isoforms have been suggested (42). Under normal conditions, ∼40% of dietary arginine is extracted in the splanchnic area (33), which is likely due to the relatively high arginase activity in the intestinal mucosa. Arginine is a substrate for citrulline synthesis in the intestine through conversion by arginase II and ornithine transcarbamylase (OTC) metabolic pathways with interorgan exchange of ornithine (105, 107).
Arginine is substrate for creatine synthesis, which also requires glycine and methionine. Creatine synthesis consumes some 20–30% of arginine's amidino groups, whether provided in the diet or synthesized within the body, and therefore imposes an appreciable burden on the metabolism of arginine. Creatine is excreted from the body as urinary creatinine. This is a nonenzymatic and unregulated breakdown process that occurs at a rate of ∼1.7% of total body creatine and creatine phosphate per day (see Ref. 27 for review).
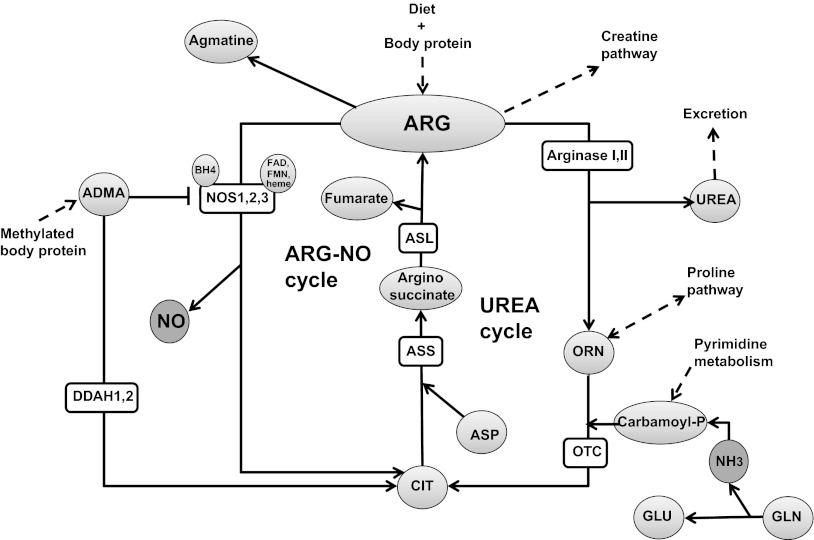
About 1.5% of arginine flux enters the NOS pathway (31) that converts arginine to NO and citrulline by either of three isoforms of the NOS enzyme (85, 114). NOS1 (neuronal NOS) and NOS3 (eNOS) are constitutive enzymes that are controlled by intracellular Ca2+/calmodulin. NOS2 is inducible at the level of gene transcription, Ca2+ independent, and expressed by macrophages and other tissues in response to (pro)inflammatory mediators. A mitochondrial NOS isoform (mtNOS) for production of NO in mitochondria has been proposed, but several studies have challenged the existence of a mitochondrial isoform (87). Several cofactors are known for NOS, of which tetrahydrobiopterin (BH4) is essential and rate limiting and is synthesized from guanosine triphosphate (GTP) via the GTP-cyclohydrolase-I (GTP-CH) pathway (see Ref. 43 for recent review). Other known cofactors are flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), and heme (reviewed in Ref. 51). Finally, arginine can be decarboxylated to agmatine, which acts as a cell-signaling molecule (118).
NO can also be derived from conversion of nitrite. The conversion of nitrite to NO can occur via simple nonenzymatic nitrite reduction under acidic conditions (e− + 2H+ + NO2− → NO− + H2O), resulting in NO production in the stomach (16). This NOS-independent NO production is controlled by oxygen tension, pH, reducing substrates, and nitrite levels (179). Production of NO from nitrite was first observed in heart tissue under conditions of ischemia with intracellular acidosis (180) and occurs primarily in tissues and not in blood (93). The level of nitrite-derived NO under ischemic conditions with acidosis is comparable to maximum constitutive NOS production, which makes this NOS-independent route a practical alternative pathway under ischemic conditions where NO production from NOS is compromised (179). Dietary nitrate, mainly from vegetables, is reduced to bioactive NO2− by facultative anaerobic bacteria in the saliva, and as such can serve as NO source. Arginine metabolic pathways and enzymes are summarized in Fig. 3.
Fig. 3.
Arginine metabolic pathways in healthy humans. ARG, arginine; ORN, ornithine; GLU, glutamate; CIT, citrulline; ASP, aspartate; GLN, glutamine; ADMA, asymmetric dimethylarginine; l-NMMA, NG-methyl-l-arginine; BH4, tetrahydrobiopterin; FAD, flavin adenine dinucleotide; FMN, flavin mononucleotide; ASS, argininosuccinate synthase; ASL, argininosuccinate lyase; DDAH, dimethylaminohydrolase.
Role of Arginine and NO in Normal Physiology
Arginine is a constituent of body proteins and is an intermediate in the urea cycle in the liver. The urea cycle is a series of five reactions, in which urea synthesis is the final step in the detoxification of ammonia. Moreover, the urea cycle has been considered a major pathway for the removal of metabolically generated bicarbonate, and as such a role for the liver in pH homeostasis (72). Other roles of arginine are related to arginine-derived metabolites. These include, among others that we will not further specify here, ornithine and derived polyamines (putrescine, spermine, and spermidine), which are important for cell growth and differentiation. Proline, which is hydroxylated to hydroxyproline posttranslationally, can also be derived from arginine and has a role in collagen formation, tissue repair, and wound healing. Creatine is also derived from arginine and plays a role in energy metabolism in muscle and neurons (see Ref. 173 for review). Apart from actions via its metabolites, arginine directly activates p70 S6 kinase and phosphorylation of 4E-BP1 through the mTOR signaling pathway (9) with stimulation of protein synthesis in a NO-independent way (14). NO has various roles in normal physiology, but we will not review all roles in full detail. NO derived from NOS1 and NOS3 acts as a neurotransmitter and a vasodilator, respectively (114). NO in the brain regulates many physiological processes affecting behavior and cognitive function, including synaptic plasticity. In addition, NO controls brain blood flow, promotes angiogenesis, and maintains cellular redox state, cell immunity, and neuronal survival. However, despite the many diverse roles of NO, regulation of the amount produced is important, as overproduction of NO may lead to neurodegeneration (115). NO is synthesized at high levels by NOS2 when activated during inflammatory processes by elevated circulating cytokine concentrations (mainly TNF-α and IL-1, IL-6, and IL-8) and/or microbial products like LPS (68, 85, 114, 120). This NO has immune regulatory functions, such as control or killing of infectious pathogens, modulation of cytokine production, and T-helper cell development. Moreover, NO can act as a free radical scavenger (157). Local NO responses are concentration and exposure time dependent (156). In general, at low concentrations NO promotes cell survival and proliferation and at high concentrations promotes cell cycle arrest, apoptosis, and senescence. As such, arginine has an indirect role in NO-mediated functions, including immune modulation (52, 139), or acts as immune response enhancing during immunological challenge (95).
Factors That Mediate Arginine and NO Metabolism and Availability
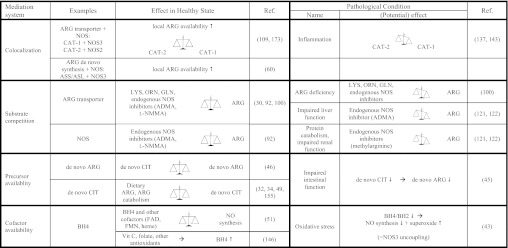
The level of dietary arginine intake and endogenous production on the one hand, and the extent of utilization or clearance on the other hand influence arginine metabolism and availability (Fig. 4).
Fig. 4.
Mediators of ARG-NO metabolism. NOS, nitric oxide synthase; ASS, argininosuccinate synthase; ASL, argininosuccinate lyase; CAT, cationic amino acid transporter; LYS, lysine; scales, in balance or unbalanced; BH4, tetrahydrobiopterin; FAD, flavin adenine dinucleotide; FMN, flavin mononucleotide; ADMA, asymmetric dimethylarginine.
Systemic and intracellular arginine availability.
Transporters for arginine uptake in the cell are often colocalized with arginine-converting enzymes and as such can modulate cellular arginine metabolism (173). For example, cationic amino acid transporter (CAT)-1 and NOS3 are colocalized in plasma membrane caveolae (109). This facilitates specific channeling of arginine to endothelial NO production without mixing with the total intracellular pool. This is in line with observations in vitro of extracellular rather than intracellular arginine being the major determinant for NO production in endothelial cells (147). Another example of the relation between arginine metabolism and its transporter is the upregulation by inflammatory cytokines of CAT-2 and downregulation of CAT-1 arginine transporters (137, 143) that result in increased availability of arginine to NOS2 and decreased availability to NOS3. Competition with lysine, ornithine, glutamine, and certain endogenous NOS inhibitors that use the same transporter as arginine may compromise intracellular arginine transport in conditions of low arginine (100). In addition to the link between arginine transport and intracellular arginine availability, the coupling between arginine-synthesizing and -converting enzymes or the competition between enzymes for arginine as a substrate determine its metabolic fate. For example, coupling between de novo arginine synthesis and NO production is supported by colocalization in endothelial cells of NOS3 and ASS/ASL (60). On the other hand, substrate competition between arginase and NOS reciprocally regulates NO levels in endothelial cells (10, 94, 173).
De novo arginine production from citrulline can be impaired by renal failure (24, 25, 39), but citrulline delivery to the kidney is the rate-limiting determinant of renal arginine production (39, 54). Impaired intestinal function is a major underlying reason for reduced citrulline availability (45; see Ref. 46 for review), supported by the observations that 80–90% of the citrulline is derived from conversion of glutamine to citrulline in the intestine (19, 20, 96, 97, 159).
Dietary arginine availability influences its own catabolism and that of other amino acids by controlling ureagenesis. Arginine is not only a substrate for ureagenesis, but also an activator of N-acetylglutamate synthetase, which is a key ureagenic enzyme (49). On a low-arginine diet, arginine catabolism (i.e., arginine hydroxylation with conversion to ornithine) is reduced with maintained de novo arginine production and reduced plasma arginine (32, 34, 155). This also applies when a low-protein (nitrogen) diet is fed and amino acids are more efficiently used for other processes than oxidation, and subsequently, less ammonia is present for detoxification in the liver urea cycle. However, a concept presented by the Cynober group states that, under the condition of low dietary protein intake, the intestinal conversion of arginine into citrulline by intestinal arginase and OTC is activated (47). The newly formed citrulline then bypasses liver metabolism and is converted back to arginine in the kidney (de novo arginine production), while at the same time ureagenesis is limited and arginine and other amino acids are spared for protein synthesis (50, 112).
Endogenous NOS inhibitors and enzymatic cofactors.
Methylarginines, such as asymmetric dimethylarginine (ADMA) and NG-methyl-l-arginine (l-NMMA), are the most powerful endogenous and competitive nonspecific NOS inhibitors that compete with l-arginine for the active site of NOS and for intracellular transport (30; see Ref. 92 for review). These methylarginines are derived from the catabolism of posttranslational modified proteins that contain methylated arginine residues. Methylarginines are eliminated from the body by a combination of renal excretion and metabolism through enzymatic degradation by dimethylaminohydrolase (DDAH) to citrulline and methylamines (126). Increased protein catabolism and impaired renal function can thus contribute to elevated levels of methylarginine. High hepatic expression of DDAH and uptake of ADMA make the liver important in the metabolism of ADMA, and hepatic dysfunction a prominent determinant of ADMA concentration (121, 122). Reference values (2.5th-97.5th %ile) for the l-arginine/ADMA ratio are between 74.3 and 225 (104).
Enzymatic cofactors for NO production, such as tetrahydrobiopterin (BH4), can be affected by several factors and can subsequently influence NO production and endothelial function. Factors that regulate BH4 activity are nutritional, therapeutic, and endothelium-derived factors. Vitamin C, folate, and other antioxidants enhance endothelial BH4 bioavailability through chemical stabilization or scavenging of reactive oxygen species (146). Depletion of BH4 by oxidation into 7,8-dihydrobiopterin (BH2) in oxidative-stressed endothelial cells can result in so-called NOS3 “uncoupling,” with production of superoxide instead of NO. It is now believed that the intracellular BH4-to-BH2 ratio, rather than absolute concentrations of BH4, is the key determinant of this NOS3 uncoupling (43).
Metabolic Alterations Related to Age, Sex, Animal Species and Strain Differences
In neonates, de novo arginine production via the interorgan intestinal-renal axis is not yet developed (171), but conversion of citrulline to arginine occurs in the intestine. The precursor of intestinal citrulline in this condition is proline. In addition, the absence of arginase in the placenta of the mother and in the neonatal intestines suggests a metabolic strategy to maximize the availability of arginine in the systemic circulation from mother to fetus and from maternal milk to neonate. In addition, polyamines and NO are essential for growth and angiogenesis. Therefore, limited de novo arginine production capacity makes arginine an essential amino acid in early life. In neonatal piglets, dietary arginine is conserved in times of deficiency by decreasing arginine hydroxylation and increasing recycling (158). Also, in a wide range of livestock reproduction processes, nonoptimum availability of arginine results in suppression or inhibition of arginine metabolism (171). NO bioavailability diminishes with aging and as such adds to the pathogenesis of disturbances in endothelium-dependent vasodilatation related to aging (153). This diminished NO bioavailability has been related to a decrease in BH4 and uncoupling of NOS3 with increased oxidative stress in aging (78, 149), but also to increased arginase activity with subsequently reduced arginine availability for NOS (see Ref. 142 for review). In the brain, the excitatory glutamate-NO-cGMP neurotransmission is normally in balance with the inhibitory GABA neurotransmission. In rats, an age-related regional imbalance of the glutamate/GABA balance was observed, caused by decreased glutamate levels. This is correlated with changes in levels of l-arginine and its metabolites in many brain regions (99).
In mice, differences in arginine metabolism are described between sexes and strains, such as between C57BL6/J and FVB mice, as well as between B6 and ICR mice (102, 106). In female mice, plasma arginine was reported to be higher, and strain differences with regard to whole body de novo arginine production and portal-drained visceral (PDV) arginine metabolism were found (102).
Alterations of Arginine and NO Metabolism in Disease
Arginine metabolism is altered in disease states with regard to both its synthesis and its catabolism. This can result in a disruption of the normal homeostasis between metabolic pathways and the fasted blood arginine level. Tang et al. (154) proposed the global arginine bioavailability ratio (GABR), defined as plasma arginine divided by the sum of ornithine and citrulline, to account for arginine catabolic metabolites. This ratio was derived as a better index of reduced NO synthetic capacity than systemic arginine levels alone. Impaired intestinal absorption (64), impaired organ function such as intestinal dysfunction (47) or renal dysfunction (36), enzyme competition, and impaired cellular uptake may further compromise (de novo) arginine metabolism and (cellular) arginine availability. Subsequent functional consequences of altered arginine metabolism, such as altered endothelial function with hemodynamic changes on systemic (especially hypertension) and (micro)circulatory level, as well as immune alterations, are well known.
Metabolic Alterations of Arginine and Arginine De Novo and NO Under Stress Conditions
Normal fasted plasma arginine ranges between 80 and 100 μmol/l (162), whereas recent reference values (2.5th-97.5th %iles) from relatively healthy subjects from the Framingham Offspring Cohort were reported to be lower between 41 and 114 μmol/l (104). This could well be related to the fact that in that study the special treatment of plasma samples for arginine (immediately cooling and cooled spinning down within 30 min) was not done (162). When care is taken of proper treatment of blood samples, under stressed conditions plasma arginine is decreased (164) to levels as low as 50 μmol/l in patients with sepsis (62, 103, 110). The intestinal-renal pathway resulting in de novo arginine synthesis from citrulline is impaired in sepsis (103), which may be the results of limited citrulline availability due to intestinal failure (103, 129) and impaired glutamine-to-citrulline conversion (83), or limited arginine production due to renal failure (54, 134). Higher-level proteolysis can mask the decline in de novo synthesis of arginine, and as a consequence total arginine availability may be maintained (6, 103).
Increased utilization of arginine for the synthesis of proteins associated with the stress response, such as the acute-phase proteins, will reduce arginine availability in these conditions and may result in alterations in (isoform-specific) NOS enzyme activity. Increased NOS2 activity during sepsis coincides with the downregulation of the activity of other NOS isoforms (15, 71, 144). This reduces NO production enzyme specifically in conditions of overall NO production being either lowered or not different from healthy controls (84, 103). Increased plasma arginine clearance (84) due to enhanced arginase activity also reduces arginine availability for other catabolic pathways. Moreover, increased arginine oxidation is observed during sepsis in pediatric patients (6). Nutritional status also affects the metabolic response to endotoxemia, as demonstrated in a pig model of sepsis. A well-nourished condition before prolonged endotoxemia in this model resulted in a better ability to adapt to endotoxin-induced metabolic deterioration of arginine-NO metabolism compared with reduced caloric intake before endotoxemia (131).
Acute Conditions, Such as Trauma, Sepsis, and Acute Liver Failure
In sepsis, a reduced plasma arginine concentration was related to worse survival (62). Increased mortality in critically ill patients has also been related to elevated ADMA levels, which by substrate competition may be a causative factor in the development of multiple organ dysfunction (123). Sepsis, in particular septic shock, is characterized by elevated cardiac output and hypotension caused by vasodilatation that are associated with misdistribution of blood flow and low peripheral vascular resistance. These characteristics of sepsis have been attributed to increased NO production by NOS2 (4, 120). However, simultaneously decreased NOS3 expression may be related to microcirculatory shutdown and shunting, contributing to the reduced microvascular blood flow and impaired tissue oxygenation (79, 90, 128, 133, 140). Others have suggested that elevated NO production in critically ill patients impairs substrate and oxygen utilization by enhanced protein nitrosylation and inhibition of mitochondrial respiration (59, 132).
Increased NO production is likely also responsible for the hyperdynamic circulation found in patients with liver cirrhosis and may be an important mediator of the exaggerated circulatory abnormalities during acute systematic inflammation in so-called acute-on-chronic liver failure with increased cerebral blood flow (98). Upregulation of the NOS2 pathway in the endothelium with simultaneous downregulation of NOS3-mediated NO production was observed in critically ill cirrhotic patients after transjugular intrahepatic stent-shunt (TIPPS) placement through exacerbation of existing endotoxemia (80). Recently, in a pig model with early-phase acute liver failure, arginine deficiency, and increased ADMA did not limit whole body NO production. Arginine deficiency was caused by arginase-related arginine clearance. The stimulated intestinal-renal axis was insufficient to compensate the arginine deficiency (145).
Chronic Conditions, Such as Obesity, Diabetes, and Cardiovascular Diseases
Abnormalities in NO production and transport in vascular systems result in various cardiovascular pathologies, including hypertension, atherosclerosis, and angiogenesis-associated disorders (for recent review on the role of NO in the vasculature see Ref. 40). Reduced basal, NOS3-mediated NO synthesis or action leads to vasoconstriction, elevated blood pressure, or thrombus formation. On the other hand, NO overproduction by NOS2 leads to vasodilation, hypotension, vascular leakage, disruption of cell metabolism, and atherosclerosis, either directly or indirectly via the formation of NO adducts such as peroxynitrite (115). Cardiovascular and pulmonary disorders such as atherosclerosis, diabetes, hypercholesterolaemia, ischemic heart disease, and hypertension are characterized by NOS3 uncoupling with formation of superoxide instead of NO (see Refs. 43 and 66 for recent reviews). The lower NO production in these conditions due to a deficiency of BH4 also underlies the impaired action of insulin in the vasculature of obese and diabetic subjects (172). Diet-induced oxidative scavenging of NO and reduced NO bioavailability were also shown to accompany early diet-induced insulin resistance (18). Oxidative stress causing S-glutathionylation of NOS3 in endothelial cells with loss of NO and gain of superoxide is increased in hypertensive vessels (38). By inhibiting NO production, elevated cellular levels of endogenous methylarginines can impair vascular relaxation and are mediators of vascular dysfunction in disease. Elevated ADMA levels have been reported in hypercholesterolemia, atherosclerosis, hypertension, chronic heart failure, diabetes mellitus, and chronic renal failure (30, 148). The l-arginine:ADMA ratio was positively associated with the estimated glomerular filtration rate and diastolic blood pressure in a large cohort study (104) and may act as a clinical diagnostic tool for improved cardiovascular risk assessment (148). ADMA was also identified as an independent risk marker for mortality in ambulatory patients with peripheral arterial disease (23).
Neurological Diseases
In the brain, arginine as a precursor for NO is necessary for cerebrovascular homeostasis (NOS3) and is involved in learning and memory capacities via the glutamate-NO-cGMP pathway (NOS1). In neurological diseases, one or both of these routes are impaired. Also, high amounts of induced NO production by inflammatory factors (NOS2) contribute to oxygen stress and therefore can play a role in the severity of the diseases. In the onset of brain stroke, cerebrovascular disease, and Alzheimer's disease, hypoperfusion as an underlying cause of oxygen stress is one of the present avenues to understanding the initiation of these diseases (see Ref. 2 for review). A chronic imbalance of NOS in the brain is believed to be a key element.
In hepatic encephalopathy (HE), a neurocognitive disorder in which brain function is impaired and is associated with both acute and chronic liver dysfunction, hyperammonemia plays an important role in the pathophysiology. Alterations in the glutamate-NO-cGMP pathway are described, especially in acute HE and in relation with excessive glutamine production (ammonia detoxification by conversion of glutamate into glutamine) in the brain (1). Besides a direct effect on the glutamate neurotransmission cycle, glutamine can also limit the transport of arginine into neurons and astrocytes, because it competes with the glutamine transport (178). The implications are not clear yet and may differ in different stages of the disease.
Cancer
Humans with cancer have decreased systemic availability of arginine independent of the type of cancer, age, sex, or cachectic state (166). In mice, cancer affects de novo arginine production probably through diminished intestinal citrulline production (167). In addition, high arginase activity is observed via the myeloid suppressor cells in the microenvironment of tumors (177). Other research is focused on tumor growth and the arginine dependence of certain tumors that do not express ASS. Such tumors, such as melanoma and hepatocellular carcinoma, are sensitive to arginine depletion by arginine-degrading enzymes such as arginine deiminase (see Ref. 58 for review) or a recombinant form of human arginase I (88). A disturbed arginine metabolism could be a factor that is causing relative poor clinical outcome. NO is not only required for an adequate immune reaction during a surgical trauma after a tumor extraction but also contributes to cytotoxic-induced antitumor processes (77, 174).
Therapeutic Approaches to Influence Arginine and NO Metabolism
Reduced arginine intake in disease or malnutrition as well as increased metabolic needs can result in arginine deficiency or its increased requirement. Therapeutic approaches can aim at increasing substrate availability by supplementation or at influencing the metabolic fate of specific pathways related to NO bioavailability and prevention of NOS3 uncoupling.
Arginine Supplementation
Arginine supplementation varying between 3 and over 100 g/day has been used in clinical studies. Single doses of 3–8 g appear to be safe and rarely provoke adverse events (21), but single doses exceeding 9 g and especially when part of a dosing regimen of over 30 g/day have been associated with gastrointestinal discomfort, nausea, and (osmotic) diarrhea (67). Arginine has been used in supplemental nutrition for surgical patients, burn patients, and patients with sepsis and cancer to benefit regulation of blood pressure, wound healing, and immunomodulation or to serve as an anabolic stimulus. However, the benefits of arginine in these conditions are not uniformly proven and accepted.
Arginine supplementation in sepsis patients has been combined with a mixture of amino acids and other nutrients, referred to as immunonutrition (7, 17, 26, 63). Several reviews and opinion papers on its use have been published (65, 73–75, 86, 108, 152, 169), but conclusions regarding the benefits and potential use in sepsis are not uniform. Arginine treatment starting before endotoxemia in a pig model appeared beneficial by improved hepatosplanchnic perfusion and oxygenation during prolonged endotoxemia without causing deleterious systemic side effects. This is probably mediated through enhanced NO synthesis (130). Arginine supplementation has inconsistent effects on intestinal ischemia-reperfusion injury, and a recent publication with long-term intraduodenal arginine supplementation in a rat model of intestinal ischemia and reperfusion injury did not show observable benefits on intestinal morphology or on the inflammatory response (89). Remarkably, serum NOx was even decreased with arginine supplementation in this study, which suggests that arginine was probably not available for NO production due to substrate competition for arginine. Supplementing arginine in partial enteral feeding in neonatal pigs modestly increased intestinal mucosal growth through an NO-independent mechanism (135).
Beneficial effects of arginine supplementation were observed in patients with sickle cell disease suffering from pulmonary hypertension, as well as in the prevention of age-related glomerular injury, in reversing impaired vasodilatation in clinically asymptomatic hypercholesterolemic adults, and improving wound healing (12, 22, 70, 117, 138). A growing body of evidence indicates that arginine supplementation is beneficial in growth, health, and disease and may provide novel and effective therapies for obesity, diabetes, and metabolic syndrome (171). Malnourished head and neck cancer patients who received arginine-enriched nutrition perioperatively demonstrated better 10-year survival (29). Moreover, arginine was proposed as a therapy in hypertension, to interrupt the vicious cycle that initiates and maintains low NO (136). A recent meta-analysis concluded that short-term oral l-arginine supplementation is effective in improving the fasting vascular endothelial function, i.e., flow-mediated vasodilation (FMD), when the baseline FMD is low, and thus endothelial dysfunction can be restored (8). FMD is an early pathophysiological feature of cardiovascular disease and reflects local bioavailability of NO under physiological stimulation. Whether long-term arginine supplementation is beneficial is debated, since exogenous arginine also increases arginase with subsequent diversion of arginine from NOS and subsequent NO production (55).
Citrulline Supplementation
Citrulline supplementation as a single oral dose of 2, 5, 10, or 15 g is safe and well tolerated in healthy adults, with no effect on plasma levels of insulin and growth hormone and with urinary excretion of citrulline remaining low (<5%) even at high doses. Citrulline supplementation has proved to be an effective precursor for arginine and ornithine, but saturation of the renal conversion of citrulline into arginine probably occurred at the highest citrulline dose (15 g) (113). Citrulline-malate is an alternative citrulline source that is also applied as antiasthenia treatment and quickly lowers ammonia levels in hyperammonemia (47).
Citrulline supplementation likely restores the optimal balance between arginine production and metabolism as well as improving NO production and related functions. In an arginine-deprived in vitro model of macrophages, addition of citrulline restored NO production, while glutamine interfered with citrulline-mediated NO production (28). Therefore, in conditions of acute or chronic inflammation with arginine deficiency, citrulline supplementation is a potentially powerful approach to restoring NO production (50). In sickle cell disease, oral citrulline supplementation maintained elevated arginine levels and maintained nearly normal total leukocyte and neutrophil counts and has therefore been suggested as a useful palliative therapy in this condition (168). Citrulline supplementation ameliorated the development of pulmonary hypertension and increased NO production in piglets exposed to chronic hypoxia (3); this suggests that neonates exposed to prolonged periods of hypoxia from cardiac or pulmonary causes may potentially benefit from citrulline supplementation. In middle-aged men, citrulline supplementation improved arterial stiffness, which is considered a powerful predictor of cardiovascular disease (124). Citrulline supplementation restored nitrogen balance and generated large amounts of arginine in rats with short bowel syndrome (125).
NO Donors and NOS3 Modulating Agents
NO can also be derived from so-called NO donors, inhaled NO, and nitrite (sources). NO donors such as nitroglycerine are well known and used as vasodilators to treat heart conditions such as angina and chronic heart failure. In septic patients, nitroglycerine increased sublingual microvascular flow, even though arterial and central venous pressure dropped temporarily (151). The use of inhaled NO in the perioperative setting for the treatment of pulmonary hypertension in children is recommended (13). Short-term nitrite therapy reversed age-associated vascular endothelial dysfunction, large elastic artery stiffness, oxidative stress, and inflammation in old mice by restoring NO bioavailability through a NOS-independent conversion. Sindler et al. (150) therefore suggested that sodium nitrite may be a novel therapy for treating arterial aging in humans . Nitrite is also currently undergoing or being planned for clinical trials as a vasodilator drug in patients with cardiovascular diseases such as ischemic stress, sickle cell disease, coronary artery disease, and pulmonary hypertension (179). Nitrate-rich vegetable juice acutely increased nitrite (within 2.5 h) and reduced blood pressure as well as oxygen costs of moderate-intensity exercise in normotensive subjects. These effects were sustained during continuous juice intake over 15 days (163).
Other novel pharmacological approaches under development to increase NO bioavailability are targeted at preventing NOS3 uncoupling or enhancing NOS3 expression (see Refs. 43, 56, 61, 66 for recent reviews). Regarding the latter, BH4 or its synthetic versions may be a new therapeutic strategy to tackle myocardial and endothelial dysfunction (111). Other agents or therapies, e.g., statins, intravenous ascorbic acid administration, or exercise, act on preventing BH4 loss and on improving BH4 availability or on BH4 stability by scavenging superoxide; this functions by improved endothelial NOS coupling and vascular NO bioavailability (5, 61, 149). Local ascorbic acid infusion was demonstrated to improve NO-mediated muscle blood flow during exercise in elderly (44). While BH4 repletion only partly restored NOS activity and NO-dependent vasodilation, reversion of another redox-regulated mechanism controlling NOS function by thiol-specific reducing agents can restore vasodilation when NOS3 S-glutathionylation is increased (38). A pharmacological NOS3 enhancer (AVE3085) ameliorated endothelial dysfunction in db/db mice through increased NO bioavailability, which makes targeting NOS3 and NO a promising approach to combat diabetic vasculopathy (37).
Targeting Endogenous NO Inhibitors
An alternative approach to increase NO bioavailability is via targeting endogenous inhibitors of NO synthesis such as ADMA or arginase. Pharmacological modification of dimethylarginine dimethylaminohydrolase (DDAH) enzymes that metabolize ADMA (91) or treatment with the arginase inhibitor N(ω)-hydroxy-nor-l-arginine (nor-NOHA) are options (69, 82). Nor-NOHA restored microvascular coronary artery function in type 2 diabetic rats and caused cardioprotection against myocardial ischemia-reperfusion injury in rats by a mechanism with increased utilization of arginine by NOS and increased NO availability (69, 82). Recently published reference levels for the l-arginine:ADMA ratio may be helpful for evaluation of the effects of l-arginine supplementation in participants with an impaired l-arginine/NO pathway (104).
Therapies That Influence NO-Mediated Effects
Patients receiving IL-2 cytokine treatment for advanced malignancy demonstrate increased endogenous nitrate synthesis (77), whereas NOS3 knockout mice were resistant to IL-2-induced hypotension and vascular leak. Methylene blue, by inhibiting guanylate cyclase and cGMP, could inhibit this NOS3-mediated vascular leak (141). Selective inhibition of p38 mitogen-activated protein kinase (MAPK), a mediator of vascular inflammation and activated by oxidized low-density lipoproteins, improved NO-mediated vasodilation in patients with hypercholesterolemia (41). Those authors suggested that p38 MAPK could therefore be a novel target for patients with cardiovascular disease.
Summary, Conclusion, and Future Research
While NO production is dependent on arginine availability as its precursor, the odd circumstance is that only a small percentage of arginine is used for NO synthesis and that either too much or too little NO is detrimental. This suggests that the relation between arginine availability and NO production is not simply a case of precursor availability; rather, it is more likely the combination of the availability of arginine along with cofactors and rate-limiting enzymes that determine the rate of production of NO. The compartmentalization of arginine metabolism plays a role here in what is also referred to as the “arginine paradox.”
During the past few years, it has become recognized that endothelial NOS uncoupling and NOS3-dependent superoxide generation, induced by stress, are key mediators in the pathogenesis of cardiovascular and pulmonary diseases. Local arginine deficiency, which can be the result of arginine catabolism via arginase or competition with methylarginines, results in endothelial NOS uncoupling. Modulating NOS uncoupling and targeting NOS3-dependent ROS formation are recent developments that require further clinical testing. Arginine seems to have a critical and dual role here, both as a substrate for NOS and as a radical scavenger. Since arginine released from local protein breakdown may not be available for NOS, coupling of the enzymes for de novo arginine and NOS3 could make citrulline a good and maybe even better source for NO. The antioxidant action of citrulline could further contribute to preventing NOS uncoupling, but this is not yet known. Specific drugs that act on increasing local arginine availability for NO production, or those that mediate or provide cofactors for NO production, are also considered useful.
In conclusion, the complex regulation of NO synthesis and intracellular availability of arginine as its precursor probably require an approach beyond the primary provision of extra arginine. A multi-target approach addressing substrate competition, precursor availability, and cofactor availability may be useful, and future research could focus on developing such strategies that can optimize NO bioavailability. This can be applied to conditions of compromised or unbalanced NO production, such as those of endothelial dysfunction in various acute and chronic diseases.
GRANTS
The work of this article was supported in part by National Institute of General Medicinal Sciences Grant R01 GM-084447. The content is solely the responsibility of the authors and does not necessarily represent official views of the National institute of General Medicinal Sciences or the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.C.L. and G.A.T.H. drafted manuscript; Y.C.L., G.A.T.H., R.R.W., and N.E.D. approved final version of manuscript; G.A.T.H. prepared figures; R.R.W. and N.E.D. edited and revised manuscript.
ACKNOWLEDGMENTS
Y. C. Luiking is an employee of Danone Research, Centre for Specialized Nutrition, The Netherlands.
REFERENCES
- 1. Albrecht J, Zielinska M, Norenberg MD. Glutamine as a mediator of ammonia neurotoxicity: A critical appraisal. Biochem Pharmacol 80: 1303–1308, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aliev G, Palacios HH, Lipsitt AE, Fischbach K, Lamb BT, Obrenovich ME, Morales L, Gasimov E, Bragin V. Nitric oxide as an initiator of brain lesions during the development of Alzheimer disease. Neurotox Res 16: 293–305, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Ananthakrishnan M, Barr FE, Summar ML, Smith HA, Kaplowitz M, Cunningham G, Magarik J, Zhang Y, Fike CD. l-Citrulline ameliorates chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol 297: L506–L511, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Annane D, Sanquer S, Sebille V, Faye A, Djuranovic D, Raphael JC, Gajdos P, Bellissant E. Compartmentalised inducible nitric-oxide synthase activity in septic shock. Lancet 355: 1143–1148, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Antoniades C, Bakogiannis C, Leeson P, Guzik TJ, Zhang MH, Tousoulis D, Antonopoulos AS, Demosthenous M, Marinou K, Hale A, Paschalis A, Psarros C, Triantafyllou C, Bendall J, Casadei B, Stefanadis C, Channon KM. Rapid, direct effects of statin treatment on arterial redox state and nitric oxide bioavailability in human atherosclerosis via tetrahydrobiopterin-mediated endothelial nitric oxide synthase coupling. Circulation 124: 335–345, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Argaman Z, Young VR, Noviski N, Castillo-Rosas L, Lu XM, Zurakowski D, Cooper M, Davison C, Tharakan JF, Ajami A, Castillo L. Arginine and nitric oxide metabolism in critically ill septic pediatric patients. Crit Care Med 31: 591–597, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Atkinson S, Sieffert E, Bihari D. A prospective, randomized, double-blind, controlled clinical trial of enteral immunonutrition in the critically ill. Guy's Hospital Intensive Care Group Crit Care Med 26: 1164–1172, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Bai Y, Sun L, Yang T, Sun K, Chen J, Hui R. Increase in fasting vascular endothelial function after short-term oral l-arginine is effective when baseline flow-mediated dilation is low: a meta-analysis of randomized controlled trials. Am J Clin Nutr 89: 77–84, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Ban H, Shigemitsu K, Yamatsuji T, Haisa M, Nakajo T, Takaoka M, Nobuhisa T, Gunduz M, Tanaka N, Naomoto Y. Arginine and Leucine regulate p70 S6 kinase and 4E-BP1 in intestinal epithelial cells. Int J Mol Med 13: 537–543, 2004 [PubMed] [Google Scholar]
- 10. Bansal V, Ochoa JB. Arginine availability, arginase, and the immune response. Curr Opin Clin Nutr Metab Care 6: 223–228, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Barbul A. Arginine: biochemistry, physiology, and therapeutic implications. J Parenter Enteral Nutr 10: 227–238, 1986 [DOI] [PubMed] [Google Scholar]
- 12. Barbul A, Lazarou SA, Efron DT, Wasserkrug HL, Efron G. Arginine enhances wound healing and lymphocyte immune responses in humans. Surgery 108: 331–336; discussion 336–337, 1990 [PubMed] [Google Scholar]
- 13. Barr FE, Macrae D. Inhaled nitric oxide and related therapies. Pediatr Crit Care Med 11: S30–S36, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bauchart-Thevret C, Cui L, Wu G, Burrin DG. Arginine-induced stimulation of protein synthesis and survival in IPEC-J2 cells is mediated by mTOR but not nitric oxide. Am J Physiol Endocrinol Metab 299: E899–E909, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Beach PK, Spain DA, Kawabe T, Harris PD, Garrison RN. Sepsis increases NOS-2 activity and decreases non-NOS-mediated acetylcholine-induced dilation in rat aorta. J Surg Res 96: 17–22, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Benjamin N, O'Driscoll F, Dougall H, Duncan C, Smith L, Golden M, McKenzie H. Stomach NO synthesis. Nature 368: 502, 1994 [DOI] [PubMed] [Google Scholar]
- 17. Bertolini G, Iapichino G, Radrizzani D, Facchini R, Simini B, Bruzzone P, Zanforlin G, Tognoni G. Early enteral immunonutrition in patients with severe sepsis: results of an interim analysis of a randomized multicentre clinical trial. Intensive Care Med 29: 834–840, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Blouet C, Mariotti F, Mathe V, Tome D, Huneau JF. Nitric oxide bioavailability and not production is first altered during the onset of insulin resistance in sucrose-fed rats. Exp Biol Med (Maywood) 232: 1458–1464, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Boelens PG, Melis GC, van Leeuwen PA, ten Have GA, Deutz NE. Route of administration (enteral or parenteral) affects the contribution of l-glutamine to de novo l-arginine synthesis in mice: a stable-isotope study. Am J Physiol Endocrinol Metab 291: E683–E690, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Boelens PG, van Leeuwen PA, Dejong CH, Deutz NE. Intestinal renal metabolism of l-citrulline and l-arginine following enteral or parenteral infusion of l-alanyl-l-[2,15N]glutamine or l-[2,15N]glutamine in mice. Am J Physiol Gastrointest Liver Physiol 289: G679–G685, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Boger RH. The pharmacodynamics of l-arginine. J Nutr 137: 1650S–1655S, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Boger RH, Bode-Boger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, Blaschke TF, Cooke JP. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation 98: 1842–1847, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Boger RH, Endres HG, Schwedhelm E, Darius H, Atzler D, Luneburg N, von Stritzky B, Maas R, Thiem U, Benndorf RA, Diehm C. Asymmetric dimethylarginine as an independent risk marker for mortality in ambulatory patients with peripheral arterial disease. J Intern Med 269: 349–361, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Bouby N, Coutaud C, Bankir L. Arginine synthesis by the proximal convoluted tubule in rats with chronic renal failure. Miner Electrolyte Metab 18: 101–103, 1992 [PubMed] [Google Scholar]
- 25. Bouby N, Hassler C, Parvy P, Bankir L. Renal synthesis of arginine in chronic renal failure: in vivo and in vitro studies in rats with 5/6 nephrectomy. Kidney Int 44: 676–683, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Bower RH, Cerra FB, Bershadsky B, Licari JJ, Hoyt DB, Jensen GL, Van Buren CT, Rothkopf MM, Daly JM, Adelsberg BR. Early enteral administration of a formula (Impact) supplemented with arginine, nucleotides, and fish oil in intensive care unit patients: results of a multicenter, prospective, randomized, clinical trial. Crit Care Med 23: 436–449, 1995 [DOI] [PubMed] [Google Scholar]
- 27. Brosnan JT, Brosnan ME. Creatine metabolism and the urea cycle. Mol Genet Metab 100, Suppl 1: S49–S52, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Bryk J, Ochoa JB, Correia MI, Munera-Seeley V, Popovic PJ. Effect of citrulline and glutamine on nitric oxide production in RAW 264.7 cells in an arginine-depleted environment. J Parenter Enteral Nutr 32: 377–383, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Buijs N, van Bokhorst-de van der Schueren MA, Langius JA, Leemans CR, Kuik DJ, Vermeulen MA, van Leeuwen PA. Perioperative arginine-supplemented nutrition in malnourished patients with head and neck cancer improves long-term survival. Am J Clin Nutr 92: 1151–1156, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, Tsai AL, Berka V, Zweier JL. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J Biol Chem 282: 879–887, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Castillo L, Beaumier L, Ajami AM, Young VR. Whole body nitric oxide synthesis in healthy men determined from [15N]arginine-to-[15N]citrulline labeling. Proc Natl Acad Sci USA 93: 11460–11465, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Castillo L, Chapman TE, Sanchez M, Yu YM, Burke JF, Ajami AM, Vogt J, Young VR. Plasma arginine and citrulline kinetics in adults given adequate and arginine-free diets. Proc Natl Acad Sci USA 90: 7749–7753, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Castillo L, Chapman TE, Yu YM, Ajami A, Burke JF, Young VR. Dietary arginine uptake by the splanchnic region in adult humans. Am J Physiol Endocrinol Metab 265: E532–E539, 1993 [DOI] [PubMed] [Google Scholar]
- 34. Castillo L, Sanchez M, Chapman TE, Ajami A, Burke JF, Young VR. The plasma flux and oxidation rate of ornithine adaptively decline with restricted arginine intake. Proc Natl Acad Sci USA 91: 6393–6397, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Castillo L, Sanchez M, Vogt J, Chapman TE, DeRojas-Walker TC, Tannenbaum SR, Ajami AM, Young VR. Plasma arginine, citrulline, and ornithine kinetics in adults, with observations on nitric oxide synthesis. Am J Physiol Endocrinol Metab 268: E360–E367, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Ceballos I, Chauveau P, Guerin V, Bardet J, Parvy P, Kamoun P, Jungers P. Early alterations of plasma free amino acids in chronic renal failure. Clin Chim Acta 188: 101–108, 1990 [DOI] [PubMed] [Google Scholar]
- 37. Cheang WS, Wong WT, Tian XY, Yang Q, Lee HK, He GW, Yao X, Huang Y. Endothelial nitric oxide synthase enhancer reduces oxidative stress and restores endothelial function in db/db mice. Cardiovasc Res 92: 267–275, 2011 [DOI] [PubMed] [Google Scholar]
- 38. Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 468: 1115–1118, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen GF, Baylis C. In vivo renal arginine release is impaired throughout development of chronic kidney disease. Am J Physiol Renal Physiol 298: F95–F102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen K, Pittman RN, Popel AS. Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal 10: 1185–1198, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cheriyan J, Webb AJ, Sarov-Blat L, Elkhawad M, Wallace SM, Maki-Petaja KM, Collier DJ, Morgan J, Fang Z, Willette RN, Lepore JJ, Cockcroft JR, Sprecher DL, Wilkinson IB. Inhibition of p38 mitogen-activated protein kinase improves nitric oxide-mediated vasodilatation and reduces inflammation in hypercholesterolemia. Circulation 123: 515–523, 2011 [DOI] [PubMed] [Google Scholar]
- 42. Choi S, Park C, Ahn M, Lee JH, Shin T. Immunohistochemical study of arginase 1 and 2 in various tissues of rats. Acta Histochem 114: 487–494, 2012 [DOI] [PubMed] [Google Scholar]
- 43. Crabtree MJ, Channon KM. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide 25: 81–88, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol 299: H1633–H1641, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Crenn P, Coudray-Lucas C, Thuillier F, Cynober L, Messing B. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology 119: 1496–1505, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr 27: 328–339, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Benazeth S, Cynober L. Almost all about citrulline in mammals. Amino Acids 29: 177–205, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Cynober L. Pharmacokinetics of arginine and related amino acids. J Nutr 137: 1646S–1649S, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Cynober L, Le Boucher J, Vasson MP. Arginine metabolism in mammals. Nutr Biochem 6: 402–413, 1995 [Google Scholar]
- 50. Cynober L, Moinard C, De Bandt JP. The 2009 ESPEN Sir David Cuthbertson. Citrulline: a new major signaling molecule or just another player in the pharmaconutrition game? Clin Nutr 29: 545–551, 2010 [DOI] [PubMed] [Google Scholar]
- 51. Daff S. NO synthase: structures and mechanisms. Nitric Oxide 23: 1–11, 2010 [DOI] [PubMed] [Google Scholar]
- 52. Daly JM, Reynolds J, Sigal RK, Shou J, Liberman MD. Effect of dietary protein and amino acids on immune function. Crit Care Med 18: S86–S93, 1990 [PubMed] [Google Scholar]
- 53. Dejong CH, Welters CF, Deutz NE, Heineman E, Soeters PB. Renal arginine metabolism in fasted rats with subacute short bowel syndrome. Clin Sci (Colch) 95: 409–418, 1998 [PubMed] [Google Scholar]
- 54. Dhanakoti SN, Brosnan JT, Herzberg GR, Brosnan ME. Renal arginine synthesis: studies in vitro and in vivo. Am J Physiol Endocrinol Metab 259: E437–E442, 1990 [DOI] [PubMed] [Google Scholar]
- 55. Dioguardi FS. To give or not to give? Lessons from the arginine paradox. J Nutrigenet Nutrigenomics 4: 90–98, 2011 [DOI] [PubMed] [Google Scholar]
- 56. Domenico R. Pharmacology of nitric oxide: molecular mechanisms and therapeutic strategies. Curr Pharm Des 10: 1667–1676, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Featherston WR, Rogers QR, Freedland RA. Relative importance of kidney and liver in synthesis of arginine by the rat. Am J Physiol 224: 127–129, 1973 [DOI] [PubMed] [Google Scholar]
- 58. Feun L, You M, Wu CJ, Kuo MT, Wangpaichitr M, Spector S, Savaraj N. Arginine deprivation as a targeted therapy for cancer. Curr Pharm Des 14: 1049–1057, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fink MP. Cytopathic hypoxia. Mitochondrial dysfunction as mechanism contributing to organ dysfunction in sepsis. Crit Care Clin 17: 219–237, 2001 [DOI] [PubMed] [Google Scholar]
- 60. Flam BR, Eichler DC, Solomonson LP. Endothelial nitric oxide production is tightly coupled to the citrulline-NO cycle. Nitric Oxide 17: 115–121, 2007 [DOI] [PubMed] [Google Scholar]
- 61. Forstermann U, Li H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br J Pharmacol 164: 213–223, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Freund H, Atamian S, Holroyde J, Fischer JE. Plasma amino acids as predictors of the severity and outcome of sepsis. Ann Surg 190: 571–576, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Galban C, Montejo JC, Mesejo A, Marco P, Celaya S, Sanchez-Segura JM, Farre M, Bryg DJ. An immune-enhancing enteral diet reduces mortality rate and episodes of bacteremia in septic intensive care unit patients. Crit Care Med 28: 643–648, 2000 [DOI] [PubMed] [Google Scholar]
- 64. Gardiner KR, Gardiner RE, Barbul A. Reduced intestinal absorption of arginine during sepsis. Crit Care Med 23: 1227–1232, 1995 [DOI] [PubMed] [Google Scholar]
- 65. Georgieff M, Tugtekin IF. Positive role of immune nutrition on metabolism in sepsis and multi-organ failure. Kidney Int Suppl 64: S80–S83, 1998 [PubMed] [Google Scholar]
- 66. Gielis JF, Lin JY, Wingler K, Van Schil PE, Schmidt HH, Moens AL. Pathogenetic role of eNOS uncoupling in cardiopulmonary disorders. Free Radic Biol Med 50: 765–776, 2011 [DOI] [PubMed] [Google Scholar]
- 67. Grimble GK. Adverse gastrointestinal effects of arginine and related amino acids. J Nutr 137: 1693S–1701S, 2007 [DOI] [PubMed] [Google Scholar]
- 68. Groeneveld PH, Kwappenberg KM, Langermans JA, Nibbering PH, Curtis L. Relation between pro- and anti-inflammatory cytokines and the production of nitric oxide (NO) in severe sepsis. Cytokine 9: 138–142, 1997 [DOI] [PubMed] [Google Scholar]
- 69. Gronros J, Jung C, Lundberg JO, Cerrato R, Ostenson CG, Pernow J. Arginase inhibition restores in vivo coronary microvascular function in type 2 diabetic rats. Am J Physiol Heart Circ Physiol 300: H1174–H1181, 2011 [DOI] [PubMed] [Google Scholar]
- 70. Gurbuz AT, Kunzelman J, Ratzer EE. Supplemental dietary arginine accelerates intestinal mucosal regeneration and enhances bacterial clearance following radiation enteritis in rats. J Surg Res 74: 149–154, 1998 [DOI] [PubMed] [Google Scholar]
- 71. Hallemeesch MM, Janssen BJA, De Jonge WJ, Soeters PB, Lamers WH, Deutz NEP. NO production by cNOS and iNOS reflects blood pressure changes in LPS-challenged mice. Am J Physiol Endocrinol Metab 285: E871–E875, 2003 [DOI] [PubMed] [Google Scholar]
- 72. Haussinger D. Liver regulation of acid-base balance. Miner Electrolyte Metab 23: 249–252, 1997 [PubMed] [Google Scholar]
- 73. Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. J Parenter Enteral Nutr 27: 355–373, 2003 [DOI] [PubMed] [Google Scholar]
- 74. Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA 286: 944–953, 2001 [DOI] [PubMed] [Google Scholar]
- 75. Heyland DK, Samis A. Does immunonutrition in patients with sepsis do more harm than good? Intensive Care Med 29: 669–671, 2003 [DOI] [PubMed] [Google Scholar]
- 76. Heys SD, Gardner E. Nutrients and the surgical patient: current and potential therapeutic applications to clinical practice. J R Coll Surg Edinb 44: 283–293, 1999 [PubMed] [Google Scholar]
- 77. Hibbs JB, Jr, Westenfelder C, Taintor R, Vavrin Z, Kablitz C, Baranowski RL, Ward JH, Menlove RL, McMurry MP, Kushner JP. Evidence for cytokine-inducible nitric oxide synthesis from l-arginine in patients receiving interleukin-2 therapy. J Clin Invest 89: 867–877, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Hara K, Jitsuiki D, Goto C, Oshima T, Chayama K, Yoshizumi M. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis 186: 390–395, 2006 [DOI] [PubMed] [Google Scholar]
- 79. Ince C, Sinaasappel M. Microcirculatory oxygenation and shunting in sepsis and shock. Crit Care Med 27: 1369–1377, 1999 [DOI] [PubMed] [Google Scholar]
- 80. Jalan R, Olde Damink SW, Ter Steege JC, Redhead DN, Lee A, Hayes PC, Deutz NE. Acute endotoxemia following transjugular intrahepatic stent-shunt insertion is associated with systemic and cerebral vasodilatation with increased whole body nitric oxide production in critically ill cirrhotic patients. J Hepatol 54: 265–271, 2011 [DOI] [PubMed] [Google Scholar]
- 81. Jenkinson CP, Grody WW, Cederbaum SD. Comparative properties of arginases. Comp Biochem Physiol B Biochem Mol Biol 114: 107–132, 1996 [DOI] [PubMed] [Google Scholar]
- 82. Jung C, Gonon AT, Sjoquist PO, Lundberg JO, Pernow J. Arginase inhibition mediates cardioprotection during ischaemia-reperfusion. Cardiovasc Res 85: 147–154, 2010 [DOI] [PubMed] [Google Scholar]
- 83. Kao C, Hsu J, Bandi V, Jahoor F. Impaired synthesis of citrulline from glutamine in sepsis. Am J Respir Crit Care Med 185: A1137, 2012 [Google Scholar]
- 84. Kao CC, Bandi V, Guntupalli KK, Wu M, Castillo L, Jahoor F. Arginine, citrulline and nitric oxide metabolism in sepsis. Clin Sci (Lond) 117: 23–30, 2009 [DOI] [PubMed] [Google Scholar]
- 85. Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J 298: 249–258, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Koretz RL. The impact of immunonutrition. Gastroenterology 109: 1713–1714, 1995 [DOI] [PubMed] [Google Scholar]
- 87. Lacza Z, Pankotai E, Csordas A, Gero D, Kiss L, Horvath EM, Kollai M, Busija DW, Szabo C. Mitochondrial NO and reactive nitrogen species production: does mtNOS exist? Nitric Oxide 14: 162–168, 2006 [DOI] [PubMed] [Google Scholar]
- 88. Lam TL, Wong GK, Chow HY, Chong HC, Chow TL, Kwok SY, Cheng PN, Wheatley DN, Lo WH, Leung YC. Recombinant human arginase inhibits the in vitro and in vivo proliferation of human melanoma by inducing cell cycle arrest and apoptosis. Pigment Cell Melanoma Res 24: 366–376, 2011 [DOI] [PubMed] [Google Scholar]
- 89. Lee CH, Hsiao CC, Hung CY, Chang YJ, Lo HC. Long-term enteral arginine supplementation in rats with intestinal ischemia and reperfusion. J Surg Res 175: 67–75, 2011 [DOI] [PubMed] [Google Scholar]
- 90. Lehr HA, Bittinger F, Kirkpatrick CJ. Microcirculatory dysfunction in sepsis: a pathogenetic basis for therapy? J Pathol 190: 373–386, 2000 [DOI] [PubMed] [Google Scholar]
- 91. Leiper J, Nandi M. The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nat Rev Drug Discov 10: 277–291, 2011 [DOI] [PubMed] [Google Scholar]
- 92. Leiper J, Vallance P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res 43: 542–548, 1999 [DOI] [PubMed] [Google Scholar]
- 93. Li H, Cui H, Kundu TK, Alzawahra W, Zweier JL. Nitric oxide production from nitrite occurs primarily in tissues not in the blood: critical role of xanthine oxidase and aldehyde oxidase. J Biol Chem 283: 17855–17863, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li H, Meininger CJ, Hawker JR, Jr, Haynes TE, Kepka-Lenhart D, Mistry SK, Morris SM, Jr, Wu G. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab 280: E75–E82, 2001 [DOI] [PubMed] [Google Scholar]
- 95. Li P, Yin YL, Li D, Kim SW, Wu G. Amino acids and immune function. Br J Nutr 98: 237–252, 2007 [DOI] [PubMed] [Google Scholar]
- 96. Ligthart-Melis GC, van de Poll MC, Boelens PG, Dejong CH, Deutz NE, van Leeuwen PA. Glutamine is an important precursor for de novo synthesis of arginine in humans. Am J Clin Nutr 87: 1282–1289, 2008 [DOI] [PubMed] [Google Scholar]
- 97. Ligthart-Melis GC, van de Poll MC, Dejong CH, Boelens PG, Deutz NE, van Leeuwen PA. The route of administration (enteral or parenteral) affects the conversion of isotopically labeled l-[2–15N]glutamine into citrulline and arginine in humans. J Parenter Enteral Nutr 31: 343–348; discussion 349–350, 2007 [DOI] [PubMed] [Google Scholar]
- 98. Liu H, Lee SS. Acute-on-chronic liver failure: the heart and systemic hemodynamics. Curr Opin Crit Care 17: 190–194, 2011 [DOI] [PubMed] [Google Scholar]
- 99. Liu P, Jing Y, Zhang H. Age-related changes in arginine and its metabolites in memory-associated brain structures. Neuroscience 164: 611–628, 2009 [DOI] [PubMed] [Google Scholar]
- 100. Luiking YC, Deutz NE. Biomarkers of arginine and lysine excess. J Nutr 137: 1662S–1668S, 2007 [DOI] [PubMed] [Google Scholar]
- 101. Luiking YC, Deutz NE. Isotopic investigation of nitric oxide metabolism in disease. Curr Opin Clin Nutr Metab Care 6: 103–108, 2003 [DOI] [PubMed] [Google Scholar]
- 102. Luiking YC, Hallemeesch MM, Vissers YL, Lamers WH, Deutz NE. In vivo whole body and organ arginine metabolism during endotoxemia (sepsis) is dependent on mouse strain and gender. J Nutr 134: 2768S–2774S, 2004 [DOI] [PubMed] [Google Scholar]
- 103. Luiking YC, Poeze M, Ramsay G, Deutz NE. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am J Clin Nutr 89: 142–152, 2009 [DOI] [PubMed] [Google Scholar]
- 104. Luneburg N, Xanthakis V, Schwedhelm E, Sullivan LM, Maas R, Anderssohn M, Riederer U, Glazer NL, Vasan RS, Boger RH. Reference intervals for plasma l-arginine and the l-arginine:asymmetric dimethylarginine ratio in the Framingham Offspring Cohort. J Nutr 141: 2186–2190, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Marini JC, Didelija IC, Castillo L, Lee B. Glutamine: precursor or nitrogen donor for citrulline synthesis? Am J Physiol Endocrinol Metab 299: E69–E79, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Marini JC, Erez A, Castillo L, Lee B. Interaction between murine spf-ash mutation and genetic background yields different metabolic phenotypes. Am J Physiol Endocrinol Metab 293: E1764–E1771, 2007 [DOI] [PubMed] [Google Scholar]
- 107. Marini JC, Keller B, Didelija IC, Castillo L, Lee B. Enteral arginase II provides ornithine for citrulline synthesis. Am J Physiol Endocrinol Metab 300: E188–E194, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. McCowen KC, Bistrian BR. Immunonutrition: problematic or problem solving? Am J Clin Nutr 77: 764–770, 2003 [DOI] [PubMed] [Google Scholar]
- 109. McDonald KK, Zharikov S, Block ER, Kilberg MS. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the “arginine paradox”. J Biol Chem 272: 31213–31216, 1997 [DOI] [PubMed] [Google Scholar]
- 110. Milewski PJ, Threlfall CJ, Heath DF, Holbrook IB, Wilford K, Irving MH. Intracellular free amino acids in undernourished patients with or without sepsis. Clin Sci (Lond) 62: 83–91, 1982 [DOI] [PubMed] [Google Scholar]
- 111. Moens AL, Kietadisorn R, Lin JY, Kass D. Targeting endothelial and myocardial dysfunction with tetrahydrobiopterin. J Mol Cell Cardiol 51: 559–563, 2011 [DOI] [PubMed] [Google Scholar]
- 112. Moinard C, Cynober L. Citrulline: a new player in the control of nitrogen homeostasis. J Nutr 137: 1621S–1625S, 2007 [DOI] [PubMed] [Google Scholar]
- 113. Moinard C, Nicolis I, Neveux N, Darquy S, Benazeth S, Cynober L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: the Citrudose pharmacokinetic study. Br J Nutr 99: 855–862, 2008 [DOI] [PubMed] [Google Scholar]
- 114. Moncada S, Higgs A. The l-arginine-nitric oxide pathway. N Engl J Med 329: 2002–2012, 1993 [DOI] [PubMed] [Google Scholar]
- 115. Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol 147, Suppl 1: S193–S201, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Moncada S, Radomski MW, Palmer RM. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem Pharmacol 37: 2495–2501, 1988 [DOI] [PubMed] [Google Scholar]
- 117. Morris CR, Morris SM, Jr, Hagar W, Van Warmerdam J, Claster S, Kepka-Lenhart D, Machado L, Kuypers FA, Vichinsky EP. Arginine therapy: a new treatment for pulmonary hypertension in sickle cell disease? Am J Respir Crit Care Med 168: 63–69, 2003 [DOI] [PubMed] [Google Scholar]
- 118. Morris SM., Jr Arginine metabolism: boundaries of our knowledge. J Nutr 137: 1602S–1609S, 2007 [DOI] [PubMed] [Google Scholar]
- 119. Morris SM., Jr Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharmacol 157: 922–930, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Nakae H, Endo S, Kikuchi M, Yamada Y, Shibata M, Ishikura H, Tanaka T, Wakabayashi G, Kawamura T, Inada K, Sato S. Nitrite/nitrate (NOx) levels and hemodynamics during septic shock. Surg Today 30: 683–688, 2000 [DOI] [PubMed] [Google Scholar]
- 121. Nijveldt RJ, Teerlink T, Siroen MP, van Lambalgen AA, Rauwerda JA, van Leeuwen PA. The liver is an important organ in the metabolism of asymmetrical dimethylarginine (ADMA). Clin Nutr 22: 17–22, 2003 [DOI] [PubMed] [Google Scholar]
- 122. Nijveldt RJ, Teerlink T, Van Der Hoven B, Siroen MP, Kuik DJ, Rauwerda JA, van Leeuwen PA. Asymmetrical dimethylarginine (ADMA) in critically ill patients: high plasma ADMA concentration is an independent risk factor of ICU mortality. Clin Nutr 22: 23–30, 2003 [DOI] [PubMed] [Google Scholar]
- 123. Nijveldt RJ, Teerlink T, van Leeuwen PA. The asymmetrical dimethylarginine (ADMA)-multiple organ failure hypothesis. Clin Nutr 22: 99–104, 2003 [DOI] [PubMed] [Google Scholar]
- 124. Ochiai M, Hayashi T, Morita M, Ina K, Maeda M, Watanabe F, Morishita K. Short-term effects of l-citrulline supplementation on arterial stiffness in middle-aged men. Int J Cardiol Epub Nov 8: 2010 [DOI] [PubMed] [Google Scholar]
- 125. Osowska S, Moinard C, Neveux N, Loi C, Cynober L. Citrulline increases arginine pools and restores nitrogen balance after massive intestinal resection. Gut 53: 1781–1786, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol 293: H3227–H3245, 2007 [DOI] [PubMed] [Google Scholar]
- 127. Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from l-arginine. Nature 333: 664–666, 1988 [DOI] [PubMed] [Google Scholar]
- 128. Parrillo JE. Pathogenetic mechanisms of septic shock. N Engl J Med 328: 1471–1477, 1993 [DOI] [PubMed] [Google Scholar]
- 129. Piton G, Manzon C, Cypriani B, Carbonnel F, Capellier G. Acute intestinal failure in critically ill patients: is plasma citrulline the right marker? Intensive Care Med 37: 911–917, 2011 [DOI] [PubMed] [Google Scholar]
- 130. Poeze M, Bruins MJ, Kessels F, Luiking YC, Lamers WH, Deutz NE. Effects of l-arginine pretreatment on nitric oxide metabolism and hepatosplanchnic perfusion during porcine endotoxemia. Am J Clin Nutr 93: 1237–1247, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Poeze M, Bruins MJ, Luiking YC, Deutz NE. Reduced caloric intake during endotoxemia reduces arginine availability and metabolism. Am J Clin Nutr 91: 992–1001, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Preiser JC, De Backer D, Vincent JL. Nitroglycerin for septic shock. Lancet 361: 880; author reply 880, 2003. [DOI] [PubMed] [Google Scholar]
- 133. Prins HA, Houdijk AP, Wiezer MJ, Teerlink T, van Lambalgen AA, Thijs LG, van Leeuwen PA. The effect of mild endotoxemia during low arginine plasma levels on organ blood flow in rats. Crit Care Med 28: 1991–1997, 2000 [DOI] [PubMed] [Google Scholar]
- 134. Prins HA, Nijveldt RJ, Gasselt DV, van Kemenade F, Teerlink T, van Lambalgen AA, Rauwerda JA, van Leeuwen PA. The flux of arginine after ischemia-reperfusion in the rat kidney. Kidney Int 62: 86–93, 2002 [DOI] [PubMed] [Google Scholar]
- 135. Puiman PJ, Stoll B, van Goudoever JB, Burrin DG. Enteral arginine does not increase superior mesenteric arterial blood flow but induces mucosal growth in neonatal pigs. J Nutr 141: 63–70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Rajapakse NW, Mattson DL. Role of l-arginine in nitric oxide production in health and hypertension. Clin Exp Pharmacol Physiol 36: 249–255, 2009 [DOI] [PubMed] [Google Scholar]
- 137. Reade MC, Clark MF, Young JD, Boyd CA. Increased cationic amino acid flux through a newly expressed transporter in cells overproducing nitric oxide from patients with septic shock. Clin Sci (Lond) 102: 645–650, 2002 [DOI] [PubMed] [Google Scholar]
- 138. Reckelhoff JF, Kellum JA, Jr, Racusen LC, Hildebrandt DA. Long-term dietary supplementation with l-arginine prevents age-related reduction in renal function. Am J Physiol Regul Integr Comp Physiol 272: R1768–R1774, 1997 [DOI] [PubMed] [Google Scholar]
- 139. Reynolds JV, Daly JM, Zhang S, Evantash E, Shou J, Sigal R, Ziegler MM. Immunomodulatory mechanisms of arginine. Surgery 104: 142–151, 1988 [PubMed] [Google Scholar]
- 140. Saetre T, Gundersen Y, Smiseth OA, Scholz T, Carlsen H, Nordsletten L, Lilleaasen P, Sautner T, Fugger R, Aasen AO. Hepatic oxygen metabolism in porcine endotoxemia: the effect of nitric oxide synthase inhibition. Am J Physiol Gastrointest Liver Physiol 275: G1377–G1385, 1998 [DOI] [PubMed] [Google Scholar]
- 141. Samlowski WE, Kondapaneni M, Tharkar S, McGregor JR, Laubach VE, Salvemini D. Endothelial nitric oxide synthase is a key mediator of interleukin-2-induced hypotension and vascular leak syndrome. J Immunother 34: 419–427, 2011 [DOI] [PubMed] [Google Scholar]
- 142. Santhanam L, Christianson DW, Nyhan D, Berkowitz DE. Arginase and vascular aging. J Appl Physiol 105: 1632–1642, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Schwartz D, Schwartz IF, Gnessin E, Wollman Y, Chernichovsky T, Blum M, Iaina A. Differential regulation of glomerular arginine transporters (CAT-1 and CAT-2) in lipopolysaccharide-treated rats. Am J Physiol Renal Physiol 284: F788–F795, 2003 [DOI] [PubMed] [Google Scholar]
- 144. Scott JA, Mehta S, Duggan M, Bihari A, McCormack DG. Functional inhibition of constitutive nitric oxide synthase in a rat model of sepsis. Am J Respir Crit Care Med 165: 1426–1432, 2002 [DOI] [PubMed] [Google Scholar]
- 145. Sharma V, Ten Have G, Ytrebo L, Sen S, Rose C, Dalton N, Turner C, Revhaug A, van Eijk H, Deutz N, Jalan R, Mookerjee R, Davies N. Nitric oxide and l-arginine metabolism in a devascularized porcine model of acute liver failure. Am J Physiol Gastrointest Liver Physiol 303: G435–G441, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Shi W, Meininger CJ, Haynes TE, Hatakeyama K, Wu G. Regulation of tetrahydrobiopterin synthesis and bioavailability in endothelial cells. Cell Biochem Biophys 41: 415–434, 2004 [DOI] [PubMed] [Google Scholar]
- 147. Shin S, Mohan S, Fung HL. Intracellular l-arginine concentration does not determine NO production in endothelial cells: implications on the “l-arginine paradox”. Biochem Biophys Res Commun 414: 660–663, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Sibal L, Agarwal SC, Home PD, Boger RH. The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr Cardiol Rev 6: 82–90, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol 587: 3885–3897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 10: 429–437, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Spronk PE, Ince C, Gardien MJ, Mathura KR, Oudemans-van Straaten HM, Zandstra DF. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet 360: 1395–1396, 2002 [DOI] [PubMed] [Google Scholar]
- 152. Suchner U, Heyland DK, Peter K. Immune-modulatory actions of arginine in the critically ill. Br J Nutr 87, Suppl 1: S121–S132, 2002 [DOI] [PubMed] [Google Scholar]
- 153. Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38: 274–279, 2001 [DOI] [PubMed] [Google Scholar]
- 154. Tang WH, Wang Z, Cho L, Brennan DM, Hazen SL. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J Am Coll Cardiol 53: 2061–2067, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Tharakan JF, Yu YM, Zurakowski D, Roth RM, Young VR, Castillo L. Adaptation to a long term (4 weeks) arginine- and precursor (glutamate, proline and aspartate)-free diet. Clin Nutr 27: 513–522, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S, Colton CA, Harris CC, Roberts DD, Wink DA. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med 45: 18–31, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Titheradge MA. Nitric oxide in septic shock. Biochim Biophys Acta 1411: 437–455, 1999 [DOI] [PubMed] [Google Scholar]
- 158. Urschel KL, Shoveller AK, Pencharz PB, Ball RO. Arginine synthesis does not occur during first-pass hepatic metabolism in the neonatal piglet. Am J Physiol Endocrinol Metab 288: E1244–E1251, 2005 [DOI] [PubMed] [Google Scholar]
- 159. van de Poll MC, Ligthart-Melis GC, Boelens PG, Deutz NE, van Leeuwen PA, Dejong CH. Intestinal and hepatic metabolism of glutamine and citrulline in humans. J Physiol 581: 819–827, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. van de Poll MC, Siroen MP, van Leeuwen PA, Soeters PB, Melis GC, Boelens PG, Deutz NE, Dejong CH. Interorgan amino acid exchange in humans: consequences for arginine and citrulline metabolism. Am J Clin Nutr 85: 167–172, 2007 [DOI] [PubMed] [Google Scholar]
- 161. van de Poll MCG, Soeters PB, Deutz NEP, Fearon KCH, Dejong CHC. Renal metabolism of amino acids: its role in interorgan amino acid exchange. Am J Clin Nutr 79: 185–197, 2004 [DOI] [PubMed] [Google Scholar]
- 162. Van Eijk HM, Dejong CH, Deutz NE, Soeters PB. Influence of storage conditions on normal plasma amino-acid concentrations. Clin Nutr 13: 374–380, 1994 [DOI] [PubMed] [Google Scholar]
- 163. Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG, Jones AM. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol 299: R1121–R1131, 2010 [DOI] [PubMed] [Google Scholar]
- 164. Vente JP, von Meyenfeldt MF, van Eijk HM, van Berlo CL, Gouma DJ, van der Linden CJ, Soeters PB. Plasma-amino acid profiles in sepsis and stress. Ann Surg 209: 57–62, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Visek WJ. Arginine needs, physiological state and usual diets. A reevaluation. J Nutr 116: 36–46, 1986 [DOI] [PubMed] [Google Scholar]
- 166. Vissers YL, Dejong CH, Luiking YC, Fearon KC, von Meyenfeldt MF, Deutz NE. Plasma arginine concentrations are reduced in cancer patients: evidence for arginine deficiency? Am J Clin Nutr 81: 1142–1146, 2005 [DOI] [PubMed] [Google Scholar]
- 167. Vissers YL, von Meyenfeldt MF, Luiking YC, Dejong CH, Deutz NE. Interorgan synthesis of arginine is down-regulated in tumor-bearing mice undergoing surgical trauma. Metabolism 57: 896–902, 2008 [DOI] [PubMed] [Google Scholar]
- 168. Waugh WH, Daeschner CW, 3rd, Files BA, McConnell ME, Strandjord SE. Oral citrulline as arginine precursor may be beneficial in sickle cell disease: early phase two results. J Natl Med Assoc 93: 363–371, 2001 [PMC free article] [PubMed] [Google Scholar]
- 169. Weimann A, Bastian L, Bischoff WE, Grotz M, Hansel M, Lotz J, Trautwein C, Tusch G, Schlitt HJ, Regel G. Influence of arginine, omega-3 fatty acids and nucleotide-supplemented enteral support on systemic inflammatory response syndrome and multiple organ failure in patients after severe trauma. Nutrition 14: 165–172, 1998 [DOI] [PubMed] [Google Scholar]
- 170. Windmueller HG, Spaeth AE. Source and fate of circulating citrulline. Am J Physiol Endocrinol Metab 241: E473–E480, 1981 [DOI] [PubMed] [Google Scholar]
- 171. Wu G, Bazer FW, Davis TA, Kim SW, Li P, Marc Rhoads J, Carey Satterfield M, Smith SB, Spencer TE, Yin Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37: 153–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Wu G, Meininger CJ. Nitric oxide and vascular insulin resistance. Biofactors 35: 21–27, 2009 [DOI] [PubMed] [Google Scholar]
- 173. Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J 336: 1–17, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Yim CY, Bastian NR, Smith JC, Hibbs JB, Jr, Samlowski WE. Macrophage nitric oxide synthesis delays progression of ultraviolet light-induced murine skin cancers. Cancer Res 53: 5507–5511, 1993 [PubMed] [Google Scholar]
- 175. Yu H, Yoo PK, Aguirre CC, Tsoa RW, Kern RM, Grody WW, Cederbaum SD, Iyer RK. Widespread expression of arginase I in mouse tissues. Biochemical and physiological implications. J Histochem Cytochem 51: 1151–1160, 2003 [DOI] [PubMed] [Google Scholar]
- 176. Yu YM, Burke JF, Tompkins RG, Martin R, Young VR. Quantitative aspects of interorgan relationships among arginine and citrulline metabolism. Am J Physiol Endocrinol Metab 271: E1098–E1109, 1996 [DOI] [PubMed] [Google Scholar]
- 177. Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O'Neill A, Mier J, Ochoa AC. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res 65: 3044–3048, 2005 [DOI] [PubMed] [Google Scholar]
- 178. Zielinska M, Ruszkiewicz J, Hilgier W, Fresko I, Albrecht J. Hyperammonemia increases the expression and activity of the glutamine/arginine transporter y+ LAT2 in rat cerebral cortex: implications for the nitric oxide/cGMP pathway. Neurochem Int 58: 190–195, 2011 [DOI] [PubMed] [Google Scholar]
- 179. Zweier JL, Li H, Samouilov A, Liu X. Mechanisms of nitrite reduction to nitric oxide in the heart and vessel wall. Nitric Oxide 22: 83–90, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med 1: 804–809, 1995 [DOI] [PubMed] [Google Scholar]