Abstract
Vasopressin analogs are used as a supplement to norepinephrine in septic shock. The isolated effects of vasopressin agonists on sepsis-induced vascular dysfunction, however, remain controversial. Because V2-receptor stimulation induces vasodilation and procoagulant effects, a higher V1a- versus V2-receptor selectivity might be advantageous. We therefore hypothesized that a sole, titrated infusion of the selective V1a-agonist Phe2-Orn8-Vasotocin (POV) is more effective than the mixed V1a-/V2-agonist AVP for the treatment of vascular and cardiopulmonary dysfunction in methicillin resistant staphylococcus aureus pneumonia-induced, ovine sepsis. After the onset of hemodynamic instability, awake, chronically instrumented, mechanically ventilated, and fluid resuscitated sheep were randomly assigned to receive continuous infusions of either POV, AVP, or saline solution (control; each n = 6). AVP and POV were titrated to maintain mean arterial pressure above baseline − 10 mmHg. When compared with that of control animals, AVP and POV reduced neutrophil migration (myeloperoxidase activity, alveolar neutrophils) and plasma levels of nitric oxide, resulting in higher mean arterial pressures and a reduced vascular leakage (net fluid balance, chest and abdominal fluid, pulmonary bloodless wet-to-dry-weight ratio, alveolar and septal edema). Notably, POV stabilized hemodynamics at lower doses than AVP. In addition, POV, but not AVP, reduced myocardial and pulmonary tissue concentrations of 3-nitrotyrosine, VEGF, and angiopoietin-2, thereby leading to an abolishment of cumulative fluid accumulation (POV, 9 ± 15 ml/kg vs. AVP, 110 ± 13 ml/kg vs. control, 213 ± 16 ml/kg; P < 0.001 each) and an attenuated cardiopulmonary dysfunction (left ventricular stroke work index, PaO2-to-FiO2 ratio) versus control animals. Highly selective V1a-agonism appears to be superior to unselective vasopressin analogs for the treatment of sepsis-induced vascular dysfunction.
Keywords: angiopoietin-2, arginine vasopressin, vascular leakage, vascular endothelial growth factor
catecholamine-refractory arterial hypotension and vascular dysfunction are hallmarks of septic shock (6) and play a pivotal role in the pathogenesis of multiple organ failure and death (42, 51). Key mechanisms of vascular dysfunction include the activation of neutrophils and the subsequent interaction with the endothelium (diapedesis, “rolling and sticking”) (20) as well as the increased production of nitric oxide via stimulation of the inducible nitric oxide synthase and reactive nitrogen species, such as peroxynitrite (22). In addition, mediators released by the endothelial cells, like VEGF and angiopoietin-2, contribute to the development of vascular leakage (25, 32).
The Surviving Sepsis Campaign currently suggests that the mixed V1a/V2-receptor agonist AVP “may be subsequently added to norepinephrine” in volume- and catecholamine-refractory septic shock (8). However, despite numerous experimental (38, 40, 43, 45) and clinical studies (9, 26, 29, 31, 41) using vasopressin agonists as a supplement to norepinephrine in septic shock, their potential benefit and the individual role of the different vasopressin analogs are still subjects of controversial debates (36, 50).
Whereas vasoconstriction is mediated by vascular V1a-receptors (1, 34), stimulation of endothelial V2-receptors causes vasodilation (1, 17), leukocyte rolling (16), and secretion of procoagulant factors like von Willebrand factor (37). Therefore, a higher selectivity for the V1a- versus the V2-receptor could be advantageous in sepsis. However, the isolated effects of vasopressin analogs with different selectivity for the V1a-receptor on vascular dysfunction and fluid accumulation have not yet been investigated in a clinically relevant animal model.
We hypothesized that vasopressin agonists reduce sepsis-induced vascular dysfunction and that a sole, titrated infusion of the highly selective V1a-agonist Phe2-Orn8-Vasotocin (POV) is more effective than a titrated AVP infusion in attenuating vascular dysfunction in severe sepsis. Therefore, the present study was designed as a prospective, randomized, controlled laboratory experiment to elucidate the effects of these treatment strategies on neutrophil activation, nitrosative stress, and markers of vascular dysfunction, namely angiopoietin-2 and VEGF, as well as on cardiovascular and pulmonary function. The study hypothesis was tested in an established ovine two-hit model of severe sepsis resulting from methicillin resistant staphyloccocus aureus-induced pneumonia after smoke inhalation injury (11, 15).
MATERIALS AND METHODS
The study was approved by the Animal Care and Use Committee of the University of Texas Medical Branch at Galveston and conducted in compliance with the guidelines of the Public Health Service Policy on Humane Care and Use of Laboratory Animals and the American Physiological Society.
Instrumentation and surgical procedures.
The ovine model of methicillin resistant staphylococcus aureus pneumonia-induced sepsis has been described in detail by Enkhbaatar and colleagues (11). In brief, 24 female sheep were fasted for 24 h before the experiment began, but had free access to water. After induction of anesthesia with i.m. injection of ketamine (KetaVedTM; Phoenix Scientific, St. Joseph, MO), the animals were weighed and instrumented for chronic hemodynamic monitoring under deep anesthesia (isoflurane; Abbott Laboratories, Abbott Park, IL) with a right femoral artery catheter (18-GA, 36 inches; Parke-Davis, Sandy, UT) and a Swan-Ganz thermal dilution catheter (REF 131F7; Edwards Lifesciences, Irvine, CA). In addition, the left atrium was cannulated (Silastic catheter; Dow Corning, Midland, MI) through a thoracotomy incision in the sixth intercostal space. All intravascular catheters were connected to pressure transducers with continuous flushing devices (model PX3X3; Baxter-Edwards Critical Care, Irvine, CA). The animals were then allowed to recover for 5 days with free access to water and food to recover from the surgical trauma. Buprenorphine (0.3 mg) on a regular basis and whenever needed was used for analgesic therapy.
A Shiley tube (inner diameter 10 mm; Tyco Healthcare Group, Pleasanton, CA) was used for tracheostomy and a Dover catheter (12–14 Fr; Sherwood Medical, St Louis, MO) for urinary bladder catheterization.
Experimental protocol.
After 5 days of recovery, baseline (BL) measurements were performed in awake animals. After tracheostomy and placement of an urinary bladder catheter, sheep were subjected to 48 breaths of cotton smoke (<40°C) and methicillin resistant staphyloccocus aureus (4 × 1011 colony forming units) was placed into both lungs via bronchoscopy under deep anesthesia. After a 10-mmHg drop in mean arterial pressure (MAP) from BL, the awake animals were randomly assigned to receive an intravenous infusion of either POV, AVP (both provided by Ferring Research Institute, San Diego, CA), or the vehicle (0.9% saline solution; Baxter Healthcare, Deerfield, IL; n = 6 each). During the experiment the investigators were unaware of the animal's group assignment. Six sham-operated animals were not injured and used to obtain reference values for histological and immunohistochemical analyses.
POV and AVP were started at a dose of 30 pMol/min (∼0.01 units/min AVP) and, if necessary, increased every hour by 30 pMol/min to keep MAP above BL 10 mmHg. During the 24-h study period, all sheep were awake and mechanically ventilated according to an established protocol recognizing the specific physiology of the ovine airways (23). To ensure normovolemia, Ringer's lactate (Baxter Healthcare) was continuously infused. It was started at 2 ml·kg−1·h−1 and titrated to maintain hematocrit at BL ± 3%. At the end of the 24-h study period, sheep were deeply anesthetized with ketamine (15 mg/kg) and euthanized by injection of 60 ml of saturated potassium chloride.
Mechanical ventilation.
All animals were mechanically ventilated (Servo Ventilator 900C; Siemens-Elema, Sweden) with a tidal volume of 15 ml/kg, a positive end-expiratory pressure of 5 cmH2O and an I-to-E ratio of 1:2 throughout the entire experiment. In this context, it is important to consider that the pulmonary dead space in sheep is higher than in humans (23). Therefore, higher tidal volumes than in humans are required to guarantee adequate ventilation (23). The inspiratory oxygen fraction was set at 100% for the first 3 h postinjury and was then adjusted to maintain sufficient oxygenation (arterial oxygen saturation, >95%; partial pressure of oxygen, >90 mmHg), whenever possible. The respiratory rate was initially set at 20 breaths/min and was then adjusted according to individual blood gas analyses.
Hemodynamic monitoring.
Hemodynamic measurements were performed at BL and 3, 6, 9, 12, 15, 18, 21, and 24 h. Mean arterial pressure, central venous pressure, left atrial pressure, mean pulmonary artery pressure, and heart rate were continuously recorded on a hemodynamic monitor (model 7830A; Hewlett Packard, Santa Clara, CA). Cardiac output was measured in triplicate with the thermodilution technique using 10 ml of ice-cold normal saline as indicator. Cardiac index, left and right ventricular stroke work index, as well as pulmonary shunt fraction and PaO2-to-FiO2 ratio were calculated using standard equations (53). Because sheep do not perspire and the inhaled gases were humidified, fluid balance was calculated as the difference of fluid input and urinary output (52). During the experiment, the animals had free access to food but not to water to accurately determine fluid intake.
Laboratory variables.
The arterial carboxyhemoglobin level was determined immediately after smoke inhalation to quantify the degree of injury. Arterial and mixed venous blood gas samples were analyzed for gas tensions, oxygen saturation, pH, base excess, and lactate concentration using a blood gas analyzer (GEM Premier 3000; Instrumental Laboratory, Lexington, MA) at BL and 3, 6, 9, 12, 15, 18, 21, and 24 h; all measurements were corrected for core body temperature. Neutrophil and platelet counts in the peripheral blood were immediately determined with a Hemavet 850 (Drew Scientific, Oxford, CT) adjusted for sheep at BL and 12 and 24 h. Hematocrit was measured by blood centrifugation in heparinized microhematocrit capillary tubes (Fisher Brand, PA). Plasma protein concentration was measured with a refractometer (National Instrument, Baltimore, MD). Oncotic pressure was determined through a semipermeable membrane in a colloid osmometer (model 4420; Wescor, Logan, UT). Citrated blood samples were centrifuged, and plasma samples frozen at −80°C for determination of nitric oxide concentration by measuring the intermediate and end products according to the manufacturer's protocol (nitrates/nitrites, Colorimetric Assay Kit; Cayman Chemicals, Ann Arbor, MI) at a later time point.
Immunhistochemical analyses.
After death, representative samples from the right middle lobe and from transmural tissue of the heart were snap frozen in liquid nitrogen, stored at −80°C, and used for immunhistochemical analyses at a later time point. VEGF protein expression and 3-nitrotyrosine in lung tissue were determined using anti-3-nitrotyrosine monoclonal antibody (Cayman Chemical, Ann Arbor, MI) and anti-VEGF (A-20) (Catalog No. sc-152-G; Santa Cruz, Santa Cruz, CA). The primary antibodies were used with biotinylated secondary antibody and donkey anti-goat IgG horseradish peroxidase secondary antibody (Catalog No. HAF109; R&D Systems, Minneapolis, MN), respectively, as described previously (14). Blots were completed using 20 g of protein and were quantified by the National Institutes of Health ImageJ (Image and Processing and Analysis in Java) scanning densitometry (National Institutes of Health, Bethesda, MD) and normalized to total actin (I-19) (Catalog No. sc-1616; Santa Cruz, Santa Cruz) expression.
Quantifications of myeloperoxidase activity and angiopoietin-2 concentration were performed according to the manufacturer's protocols using the NWLSS Myeloperoxidase Activity Assay (Northwest Life Science Specialties, Vancouver, WA) and the Human Angiopoietin-2 Immunoassay Kit (Invitrogen, Camarillo, CA), respectively.
Histological analyses.
At the beginning of autopsy, the thoracic and the peritoneal cavity were carefully opened and the effusions were collected with a suctioning catheter to determine the amounts of thoracic and peritoneal fluids. For assessment of the bloodless lung wet-to-dry weight ratio, the right lower lobe of each animal was excised at necropsy and blood samples were drawn. Bloodless lung wet-to-dry weight ratio was calculated as an index of lung water content (33). Samples of the right lung (middle and upper lobe) of each animal were excised, injected, and immersed with 10% phosphate-buffered formalin. Fixed samples were then embedded in paraffin, sectioned into 6-μm slices, and stained with hematoxylin-eosin. A pathologist unaware of the study protocol evaluated the degree of bronchiolar obstruction, alveolar and septal edema, alveolar neutrophils, and bacteria as well as hyaline membranes according to a standardized protocol as described in detail by Cox and colleagues (7).
Statistical analyses.
Sigma Stat 3.1 software (Systat Software, San Jose, CA) was used for statistical analyses. After normal distribution (Kolmogorov-Smirnov test) was confirmed, analysis of variance methodologies appropriate for two factor experiments with repeated measures across time for each animal were used. Each variable was analyzed separately for differences among groups, differences across time, and for group by time interaction. Differences between groups in variables with single measures were analyzed using the one-way ANOVA. After the significance of different group effects over time was confirmed, post hoc pair-wise comparisons among groups were performed using the Student-Newman-Keuls procedure to adjust for the elevated false-positive rate found otherwise in multiple testing. Data are expressed as means ± SE. Differences were considered statistically significant, when P was less than 0.05.
RESULTS
BL characteristics.
There were no differences among study groups in any of the investigated variables at BL. Mean body weight (31 ± 1 kg) and arterial carboxyhemoglobin values measured immediately after smoke inhalation injury (65 ± 3%) did not differ among groups. The average time to treatment initiation was similar in the AVP (8 ± 1 h) and the selective V1a-agonist group (7 ± 1 h; P = 0.645).
Cardiopulmonary hemodynamics and function.
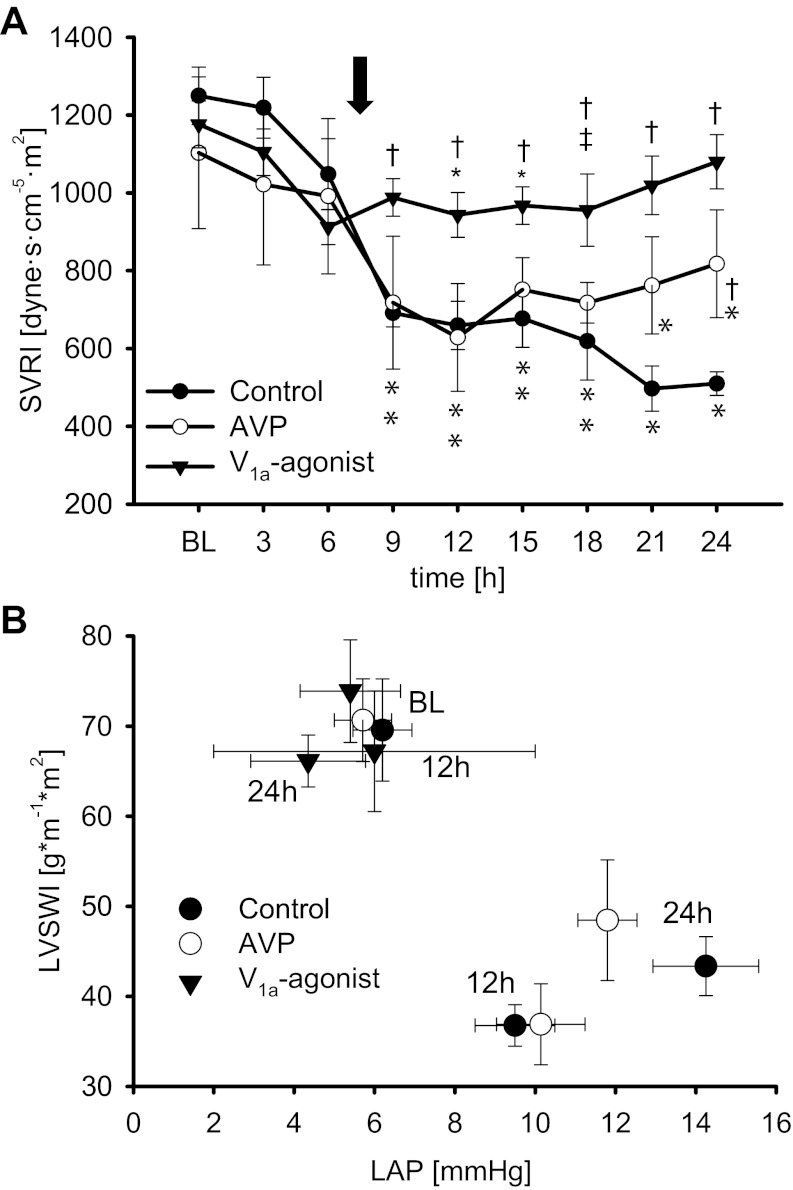
Changes in cardiopulmonary hemodynamics and function are presented in Figs. 1 and 2 and Tables 1 and 2. Sepsis-induced hemodynamic instability was characterized by decreases in MAP (P ≤ 0.01 vs. BL each) and systemic vascular resistance index (SVRI; P < 0.05 vs. BL each) as well as an increase in heart rate (P < 0.001 vs. BL each) in all injured groups at 6 h. In addition, cardiac index increased in all three injured groups as compared with BL (12 h: P < 0.05 each). Whereas these changes progressed in control animals over the 24-h study period, the selective V1a-agonist POV stabilized SVRI (9–24 h: P < 0.05 vs. control each) and kept MAP within the target range after treatment initiation (12–24 h: P ≤ 0.01 vs. control each). Contrary, AVP was only able to increase SVRI and MAP as compared with control animals at 24 h (Fig. 1A, Table 1).
Fig. 1.
Systemic vascular resistance (A) and left ventricular contractility, demonstrating the left ventricular stroke work index (LVSWI) values in relation to the individual left atrial pressure (LAP) values at this time for each group (B). *P < 0.05 vs. baseline (BL); †P < 0.05 vs. control; ‡P < 0.05 vs. arginine vasopressin (AVP). Arrow, average time point of treatment initiation in the 3 study groups. Data are represented as means ± SE; n = 6 each. SVRI, systemic vascular resistance index.
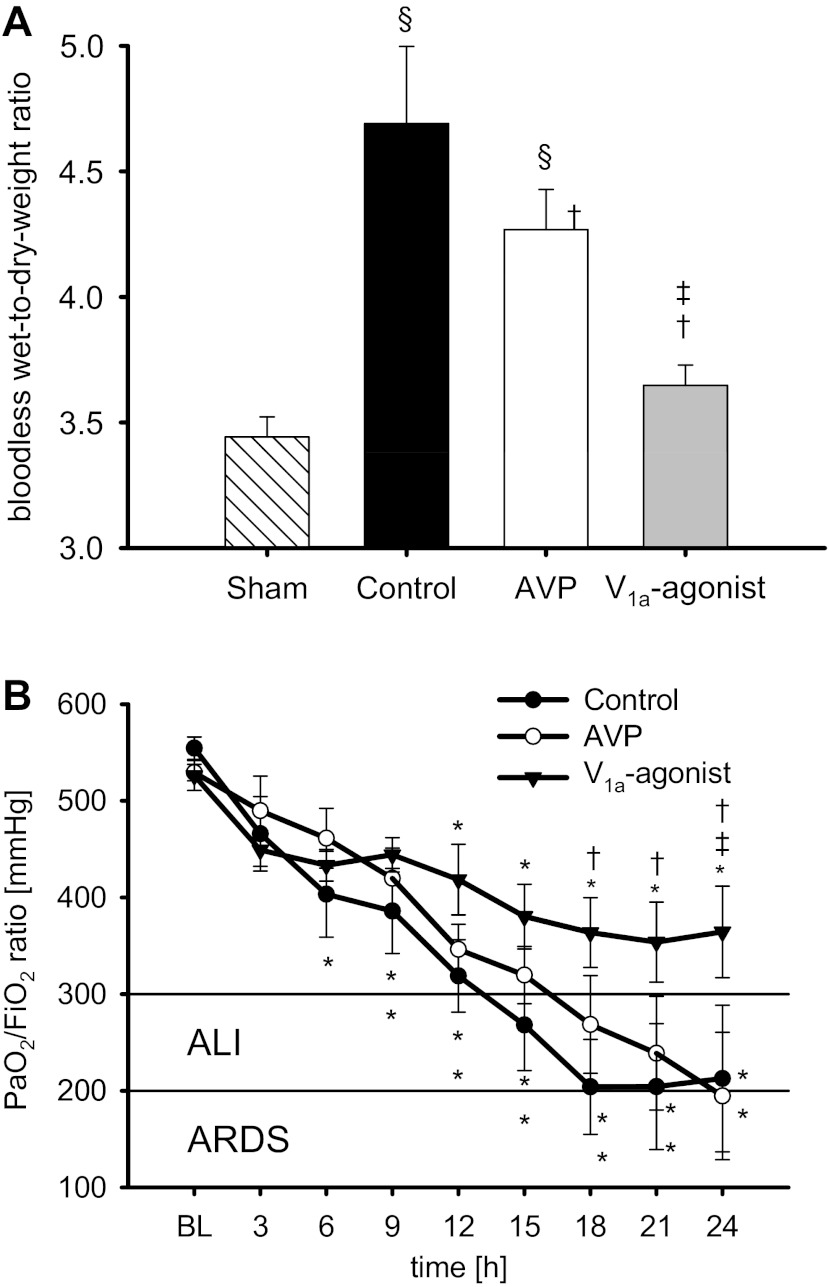
Fig. 2.
Bloodless wet-to-dry-weight ratio (A) and arterial partial pressure of oxygen (PaO2)-to-inspiratory fraction of oxygen (FiO2) ratio (B). *P < 0.05 vs. BL; †P < 0.05 vs. control; ‡P < 0.05 vs. AVP; §P < 0.05 vs. sham. Data are represented as means ± SE; n = 6 each. ALI, acute lung injury; ARDS, acute respiratory distress syndrome.
Table 1.
Cardiovascular hemodynamics
| Variable and Group | BL | 6 h | 12 h | 18 h | 24 h |
|---|---|---|---|---|---|
| Heart rate, beats/min | |||||
| Control | 85 ± 5 | 127 ± 9* | 137 ± 7* | 124 ± 3* | 122 ± 2* |
| AVP | 89 ± 8 | 143 ± 13* | 143 ± 8* | 126 ± 9* | 127 ± 4* |
| V1a agonist | 90 ± 4 | 141 ± 14* | 105 ± 4†‡ | 104 ± 7 | 102 ± 8 |
| Mean arterial pressure, mmHg | |||||
| Control | 92 ± 3 | 79 ± 2* | 65 ± 3* | 66 ± 5* | 66 ± 6* |
| AVP | 87 ± 2 | 74 ± 6* | 72 ± 6* | 78 ± 8* | 89 ± 4† |
| V1a agonist | 93 ± 4 | 80 ± 3* | 92 ± 4†‡ | 87 ± 4† | 91 ± 2† |
| Central venous pressure, mmHg | |||||
| Control | 4 ± 1 | 6 ± 1 | 8 ± 1 | 9 ± 1* | 12 ± 1* |
| AVP | 3 ± 0 | 5 ± 1 | 8 ± 2* | 7 ± 2* | 9 ± 2* |
| V1a agonist | 3 ± 0 | 5 ± 1 | 6 ± 1* | 5 ± 1 | 5 ± 1† |
| Left arterial pressure, mmHg | |||||
| Control | 7 ± 1 | 6 ± 2 | 10 ± 1* | 11 ± 1* | 14 ± 1* |
| AVP | 6 ± 1 | 7 ± 2 | 10 ± 2* | 11 ± 1* | 11 ± 1* |
| V1a agonist | 5 ± 2 | 5 ± 2 | 6 ± 4 | 5 ± 1†‡ | 4 ± 1†‡ |
| Cardiac index, l·min−1·m−2 | |||||
| Control | 5.7 ± 0.5 | 5.4 ± 0.7 | 7.1 ± 0.5* | 7.2 ± 0.8 | 7.6 ± 0.4* |
| AVP | 5.6 ± 0.3 | 5.8 ± 0.2 | 6.7 ± 0.3* | 7.8 ± 0.2* | 7.3 ± 0.4* |
| V1a agonist | 5.8 ± 0.3 | 6.0 ± 0.2 | 7.4 ± 0.4* | 6.7 ± 0.8 | 6.2 ± 0.4 |
| Left ventricular stroke work index, g·m−1·m2 | |||||
| Control | 70 ± 6 | 39 ± 2* | 37 ± 2* | 37 ± 4* | 43 ± 3* |
| AVP | 71 ± 5 | 38 ± 4* | 37 ± 5* | 51 ± 5*† | 48 ± 7* |
| V1a agonist | 73 ± 6 | 46 ± 2* | 67 ± 7†‡ | 62 ± 5† | 66 ± 3† |
| Right ventricular stroke work index, g·m−1·m2 | |||||
| Control | 13 ± 1 | 10 ± 1 | 14 ± 2 | 13 ± 1 | 15 ± 1 |
| AVP | 12 ± 1 | 9 ± 1 | 10 ± 1 | 15 ± 1 | 13 ± 2 |
| V1a agonist | 13 ± 1 | 11 ± 1 | 15 ± 2 | 14 ± 1 | 14 ± 2 |
Values are means ± SE; n = 6 for each group.
BL, baseline.
P < 0.05 vs. BL;
P < 0.05 vs. control;
P < 0.05 vs. AVP.
Table 2.
Acid-base balance, pulmonary hemodynamics, gas exchange, and ventilarory pressures
| Variable and Group | BL | 6 h | 12 h | 18 h | 24 h |
|---|---|---|---|---|---|
| Arterial potential of hydrogen | |||||
| Control | 7.50 ± 0.01 | 7.60 ± 0.02* | 7.55 ± 0.02 | 7.53 ± 0.02 | 7.54 ± 0.03 |
| AVP | 7.51 ± 0.01 | 7.58 ± 0.03* | 7.56 ± 0.02 | 7.51 ± 0.02 | 7.49 ± 0.04 |
| V1a agonist | 7.52 ± 0.01 | 7.61 ± 0.01* | 7.56 ± 0.01 | 7.54 ± 0.02 | 7.54 ± 0.01 |
| Base excess, mmol/l | |||||
| Control | 6.9 ± 1.1 | 3.9 ± 1.0* | 0.9 ± 1.0* | −0.5 ± 1.6* | 0.3 ± 0.9* |
| AVP | 6.6 ± 0.6 | 3.8 ± 1.3* | 2.1 ± 1.3* | −0.4 ± 1.1* | −1.1 ± 0.5* |
| V1a agonist | 8.0 ± 0.2 | 4.4 ± 0.9* | 1.0 ± 0.6* | −0.4 ± 0.4* | 0.2 ± 0.8* |
| Lactate, mmol/l | |||||
| Control | 0.5 ± 0.1 | 3.2 ± 0.4* | 4.1 ± 0.6* | 3.8 ± 0.9* | 4.3 ± 1.2* |
| AVP | 0.3 ± 0.1 | 3.7 ± 0.4* | 3.6 ± 0.3* | 2.8 ± 0.4* | 1.5 ± 0.5*† |
| V1a agonist | 0.4 ± 0.1 | 2.8 ± 0.2* | 2.7 ± 0.2* | 2.1 ± 0.3* | 2.0 ± 0.4*† |
| Mean pulmonary artery pressure, mmHg | |||||
| Control | 18 ± 1 | 23 ± 1* | 26 ± 1* | 29 ± 2* | 29 ± 2* |
| AVP | 17 ± 1 | 22 ± 1* | 24 ± 1* | 27 ± 1* | 28 ± 2* |
| V1a agonist | 17 ± 1 | 23 ± 1* | 23 ± 1* | 21 ± 1†‡ | 22 ± 2†‡ |
| Pulmonary shunt fraction, % | |||||
| Control | 19 ± 1 | 26 ± 2* | 33 ± 4* | 41 ± 5* | 43 ± 9* |
| AVP | 19 ± 1 | 22 ± 1 | 34 ± 3* | 36 ± 5* | 39 ± 5* |
| V1a agonist | 20 ± 1 | 23 ± 1 | 24 ± 1†‡ | 24 ± 2†‡ | 26 ± 2†‡ |
| Mixed venous oxygen saturation, % | |||||
| Control | 60 ± 2 | 63 ± 3 | 60 ± 3 | 52 ± 5 | 59 ± 4 |
| AVP | 59 ± 1 | 60 ± 2 | 64 ± 2 | 60 ± 1 | 62 ± 3 |
| V1a agonist | 61 ± 3 | 66 ± 2 | 59 ± 2 | 61 ± 2 | 61 ± 3 |
| Peak airway pressure, cmH2O | |||||
| Control | 18 ± 0 | 21 ± 1 | 26 ± 2* | 32 ± 2* | 28 ± 3* |
| AVP | 19 ± 1 | 19 ± 1 | 21 ± 1† | 24 ± 3*† | 26 ± 3* |
| V1a agonist | 19 ± 1 | 20 ± 1 | 20 ± 1† | 22 ± 1† | 21 ± 1† |
| Pause airway pressure, cmH2O | |||||
| Control | 16 ± 0 | 18 ± 1 | 22 ± 1* | 29 ± 3* | 26 ± 4* |
| AVP | 16 ± 1 | 17 ± 1 | 18 ± 1 | 21 ± 2*† | 24 ± 4* |
| V1a agonist | 17 ± 1 | 18 ± 1 | 18 ± 1 | 19 ± 1† | 18 ± 1†‡ |
Values are means ± SE; n = 6 for each group.
P < 0.05 vs. BL;
P < 0.05 vs. control;
P < 0.05 vs. AVP.
Impairment of myocardial function was evidenced by a decrease in left ventricular stroke work index (P < 0.001 vs. BL each) in all injured groups (Table 1). At 24 h, left ventricular contractility plotted as a Starling-based relation between left ventricular stroke work index and preload, namely the left atrial pressure, was severely impaired in the control and the AVP group (Fig. 1B). Central venous and left atrial pressures, as surrogate variables of right and left ventricular filling pressures, increased over time in control and AVP-treated animals (24 h: P < 0.001 vs. BL each). Notably, treatment with POV increased left ventricular stroke work index (12–24 h: P ≤ 0.01 vs. control each) and left ventricular contractility almost back to BL level (Table 1, Fig. 1B). In addition, left atrial pressures were significantly lower in POV-treated animals than in both other injured groups (18–24 h: P < 0.05 each).
Pulmonary hemodynamics were characterized by progressive increases in mean pulmonary artery pressures and pulmonary shunt fraction in control and AVP-treated animals (Table 2). POV significantly attenuated these changes (18–24 h: P < 0.01 vs. control each; P < 0.01 vs. AVP each). Furthermore, ventilatory pressures were lower in animals treated with vasopressin agonists than in the control group (18 h: P < 0.001 vs. control each for AVP and POV, respectively).
Acute lung injury, defined as a PaO2-to-FiO2 ratio <300 mmHg (3), occurred after 15 h in control animals and after 18 h in the AVP group. Treatment with POV led to higher PaO2-to-FiO2 ratios as compared with the other groups at 24 h (P < 0.05 each; Fig. 2B).
Vascular leakage and fluid accumulation.
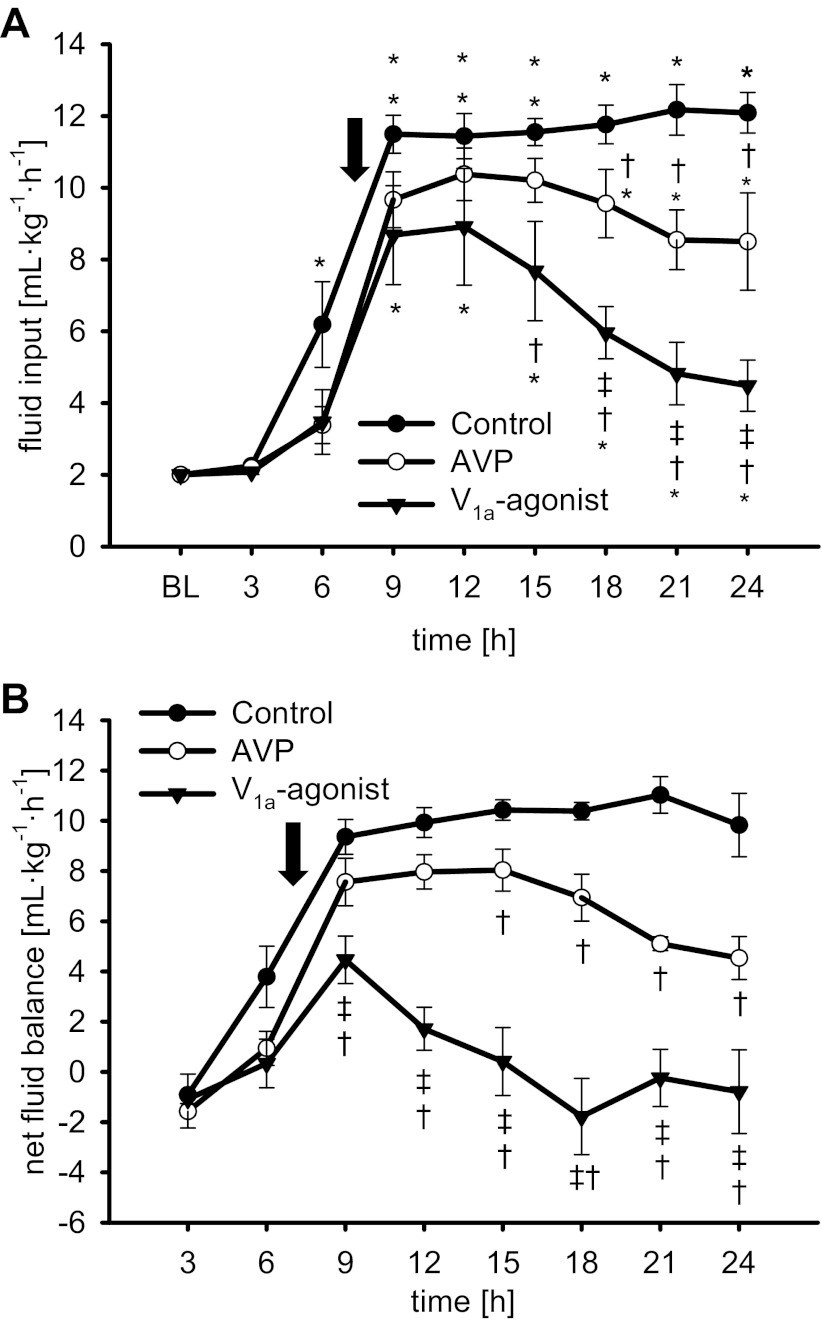
After an initial increase in hematocrit (6 h: P < 0.001 vs. BL each), aggressive fluid resuscitation kept hematocrit within 3% of BL values in all study groups (Table 3). After treatment initiation, the amounts of fluid, however, were lower in treated versus control animals from 18 to 24 h (AVP, P < 0.05 each; POV, P < 0.01 each; Fig. 3A). In addition, POV reduced fluid rates as compared with AVP (18–24 h: P < 0.05 each). At the same time, protein concentrations as well as plasma oncotic pressures significantly fell as compared with BL (24 h: P < 0.001 each). This decrease was attenuated by POV (24 h: P < 0.01 vs. control each, P < 0.05 vs. AVP each; Table 3).
Table 3.
Indexes of fluid balance, renal function, and counts of neutrophils and platelets
| Variable and Group | BL | 6 h | 12 h | 18 h | 24 h |
|---|---|---|---|---|---|
| Hematocrit, % | |||||
| Control | 24 ± 1 | 30 ± 1* | 27 ± 1* | 28 ± 1* | 27 ± 1 |
| AVP | 25 ± 1 | 30 ± 2* | 26 ± 1 | 26 ± 0 | 27 ± 1 |
| V1a agonist | 25 ± 1 | 30 ± 2* | 26 ± 2 | 26 ± 2 | 26 ± 2 |
| Proteinplasma, g·dL−2 | |||||
| Control | 6.1 ± 0.1 | 5.7 ± 0.1* | 4.2 ± 0.2* | 3.3 ± 0.2* | 3.1 ± 0.3* |
| AVP | 6.2 ± 0.1 | 5.7 ± 0.2* | 4.3 ± 0.2* | 3.7 ± 0.2* | 3.9 ± 0.2* |
| V1a agonist | 5.9 ± 0.2 | 5.4 ± 0.2* | 4.7 ± 0.2* | 4.6 ± 0.3*†‡ | 4.5 ± 0.3*†‡ |
| Oncotic pressure, cmH2O | |||||
| Control | 19 ± 1 | 16 ± 1* | 11 ± 0* | 9 ± 0* | 8 ± 1* |
| AVP | 19 ± 1 | 16 ± 1* | 11 ± 1* | 11 ± 1* | 11 ± 1* |
| V1a agonist | 19 ± 1 | 17 ± 1 | 14 ± 1* | 13 ± 1*†‡ | 15 ± 1*†‡ |
| Urine output, ml·kg−1·h−1 | |||||
| Control | / | 2.7 ± 0.4 | 1.4 ± 0.3 | 0.5 ± 0.1 | 0.4 ± 0.1 |
| AVP | / | 2.4 ± 0.5 | 2.4 ± 0.6 | 1.5 ± 0.5 | 2.0 ± 0.5† |
| V1a agonist | / | 2.8 ± 0.4 | 5.8 ± 0.9†‡ | 4.0 ± 0.6†‡ | 2.0 ± 0.3† |
| Creatinine clearance, ml/min | |||||
| Control | / | 84 ± 16 | 72 ± 8 | / | 75 ± 5 |
| AVP | / | 80 ± 10 | 127 ± 26† | / | 136 ± 22† |
| V1a agonist | / | 82 ± 17 | 125 ± 22† | / | 132 ± 32† |
| Neutrophils, % of BL | |||||
| Control | 100 ± 0 | / | 35 ± 6* | / | 39 ± 5* |
| AVP | 100 ± 0 | / | 40 ± 4* | / | 39 ± 5* |
| V1a agonist | 100 ± 0 | / | 83 ± 15†‡ | / | 74 ± 14†‡ |
| Platelets, % of BL | |||||
| Control | 100 ± 0 | / | 67 ± 13* | / | 65 ± 16* |
| AVP | 100 ± 0 | / | 78 ± 7* | / | 61 ± 5* |
| V1a agonist | 100 ± 0 | / | 82 ± 18 | / | 118 ± 8†‡ |
Values are means ± SE; n = 6 for each group.
P < 0.05 vs. BL;
P < 0.05 vs. control;
P < 0.05 vs. AVP.
Fig. 3.
Fluid input (A) and hourly calculated net fluid balance (B). *P < 0.05 vs. BL; †P < 0.05 vs. control; ‡P < 0.05 vs. AVP. Arrow, average time point of treatment initiation in the 3 study groups. Data are represented as means ± SE; n = 6 each.
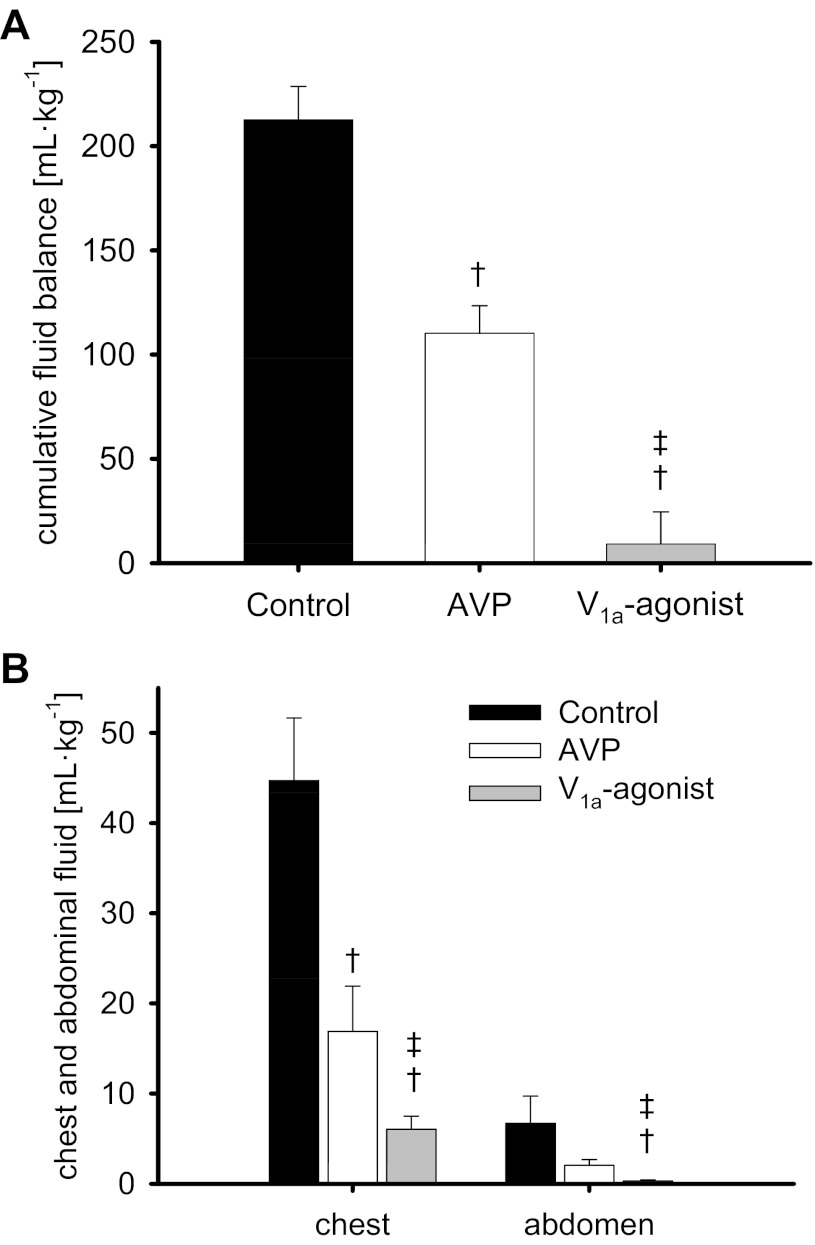
Sepsis-induced fluid accumulation was evidenced by a net fluid balance of about 10 ml·kg−1·h−1 from 9 to 24 h in control animals (Fig. 3B) resulting in a cumulative net fluid balance of 213 ± 16 ml/kg (Fig. 4A) and 45 ± 17 ml/kg fluid in the chest and 7 ± 2 ml/kg in the peritoneal cavity over the 24-h study period (Fig. 4B). Whereas both vasopressin agonists significantly reduced all these variables of fluid accumulation, the selective V1a-agonist POV was superior to AVP and led to a cumulative net fluid balance of only 9 ± 15 ml/kg (P <0.001 vs. control and AVP).
Fig. 4.
Cumulative fluid balance (A) and chest and abdominal fluid volume (B). †P < 0.05 vs. control; ‡P < 0.05 vs. AVP. Data are represented as means ± SE; n = 6 each.
Mechanisms and mediators of vascular dysfunction.
Variables characterizing neutrophil migration, nitrosative stress, as well as concentrations of VEGF and angiopoietin-2 are presented in Fig. 5 and Tables 3 and 4. Transvascular migration of neutrophils in control animals was represented by increases in pulmonary myeloperoxidase activity (P = 0.026 vs. sham) and polymorphonuclear cells in the lungs (P < 0.001 vs. sham), as well as neutrophil reduction in the peripheral blood (24 h: P < 0.001 vs. BL). Both AVP and POV reduced pulmonary myeloperoxidase activity (P < 0.001 vs. control; Table 4). However, only the selective V1a-agonist POV attenuated the decrease in neutrophil count (24 h: P < 0.05 vs. AVP and control; Table 3). There were no statistical significant differences in myeloperoxidase activity in cardiac tissues among study groups.
Fig. 5.
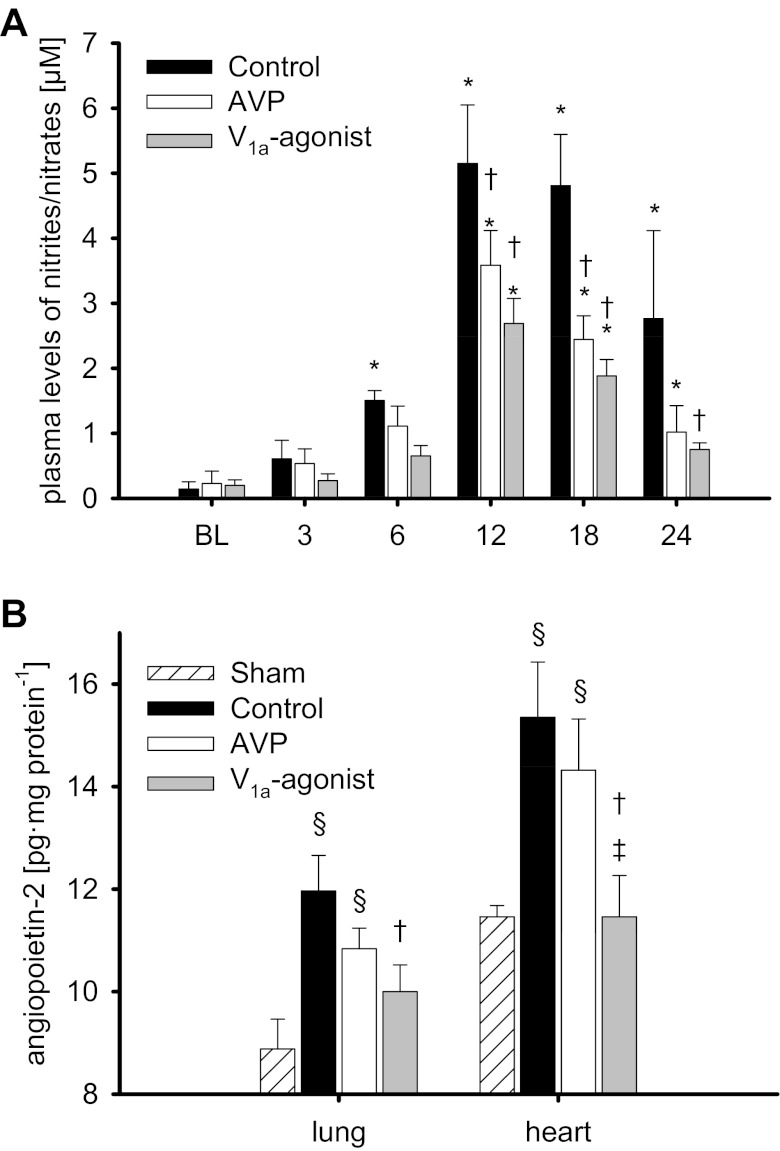
Plasma levels of nitrites/nitrates (A) and tissue concentration of angiopoietin-2 (B). *P < 0.05 vs. BL; †P < 0.05 vs. control; ‡P < 0.05 vs. AVP; §P < 0.05 vs. sham. Data are represented as means ± SE; n = 6 each.
Table 4.
Histological analyses of the lung and immunohistochemical analyses of lung and heart samples
| Variable | Control | AVP | V1a agonist | Sham |
|---|---|---|---|---|
| Bronchiolar obstruction, score | 1.6 ± 0.3§ | 0.6 ± 0.3 | 0.5 ± 0.3† | 0 ± 0 |
| Septal edema, score | 1.1 ± 0.2§ | 0.4 ± 0.2† | 0.4 ± 0.2† | 0.1 ± 0.1 |
| Alveolar edema, score | 2.6 ± 0.4§ | 1.3 ± 0.6§ | 1.1 ± 0.4†§ | 0.1 ± 0.1 |
| Alveolar neutrophils, score | 2.4 ± 0.4§ | 1.2 ± 0.6§ | 1.2 ± 0.4§ | 0 ± 0 |
| Alveolar bacteria, score | 1.9 ± 0.2§ | 0.5 ± 0.2† | 0.5 ± 0.3† | 0 ± 0 |
| Hyaline membranes, score | 0.5 ± 0.3 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0 ± 0 |
| MPO activity lung, units/ml | 34.2 ± 2.3§ | 28.5 ± 1.0† | 27.6 ± 0.9† | 27.0 ± 1.5 |
| 3-NT lung, ng/μg protein | 246 ± 7§ | 234 ± 5§ | 169 ± 9†‡ | 140 ± 9 |
| VEGF lung, ng/μg protein | 62 ± 3§ | 51 ± 3§† | 40 ± 1§†‡ | 32 ± 2 |
| MPO activity heart, units/ml | 22.4 ± 1.9 | 18.9 ± 0.4 | 19.3 ± 0.3 | 19.6 ± 0.7 |
| 3-NT heart, ng/μg actin | 1.78 ± 0.16§ | 0.26 ± 0.02§† | 0.17 ± 0.03†‡ | 0.14 ± 0.02 |
| VEGF heart, ng/μg actin | 0.92 ± 0.07§ | 0.83 ± 0.07§ | 0.63 ± 0.04§†‡ | 0.46 ± 0.04 |
Values are means ± SE; n = 6 for each group.
MPO, myeloperoxydase; 3-NT, 3-nitrotyrosine.
P < 0.05 vs. control;
P < 0.05 vs. AVP;
P < 0.05 vs. sham.
Nitrosative stress was evidenced by a significant increase in plasma levels of nitrites/nitrates, stable metabolites of nitric oxide (12 h: P < 0.001 vs. BL each; Fig. 5A), in all injured groups as well as higher concentrations of 3-nitrotyrosine, a reliable in vivo biomarker of peroxynitrite formation, in the lung and the heart in control as compared with sham-operated animals (P < 0.001 each; Table 4). Treatment with vasopressin agonists reduced plasma levels of nitrites/nitrates (12–18 h: P < 0.01 vs. control each), as well as myocardial (P < 0.001 vs. control each) and pulmonary 3-nitrotyrosine concentrations (POV: P < 0.001 vs. control). Notably, POV was associated with lower 3-nitrotyrosine concentrations than AVP-treated animals (P < 0.05 each).
Severe sepsis led to higher myocardial and pulmonary tissue concentrations of VEGF in injured as compared with sham animals (P < 0.05 each; Table 4). Infusion of the selective V1a-agonist POV significantly attenuated this increase (P < 0.001 vs. control each, P < 0.05 vs. AVP each). Angiopoietin-2 concentrations in lung and heart tissues were increased in control and AVP-treated animals as compared with the sham group (P < 0.05 each; Fig. 5B). POV reduced angiopoietin-2 concentrations in the lung (P = 0.046 vs. control) and the heart (P = 0.016 vs. control, P = 0.045 vs. AVP).
Metabolic changes.
Septic pneumonia was associated with a decrease in base excess (P < 0.05 vs. BL each) and an increase in arterial lactate concentrations (P < 0.001 vs. BL each) in all groups. These metabolic changes continuously progressed in all animals during the observation period. However, the increase in arterial lactate concentration was attenuated by AVP and POV as compared with control animals (24 h: P < 0.05 each). There were no statistical differences in arterial pH between groups. The metabolic changes are summarized in Table 2.
Histological analyses.
Histological analyses of lung tissues (Table 4) revealed increased scores for septal and alveolar edema (P ≤ 0.001 each), as well as an increased pulmonary bloodless wet-to-dry-weight ratio (Fig. 2A; P = 0.003) in the control versus sham-operated group. Only the selective V1a-agonist POV, but not AVP, was associated with a significant reduction of septal and alveolar edema as well as pulmonary wet-to-dry-weight ratio (P < 0.05 vs. control each). In addition, histological scores for bronchiolar obstruction and alveolar bacteria were higher in control as compared with sham-operated animals (P < 0.001 each). Both vasopressin agonists reduced these scores (bronchiolar obstruction: P = 0.036 POV vs. control, alveolar bacteria: P < 0.01 each), but statistical significance was not achieved for AVP versus control in bronchiolar obstruction (P = 0.055). There were no statistical differences in hyaline membranes between groups.
Doses of AVP and POV.
AVP was titrated up to the predefined maximum dose of 180 pMol/min (∼0.06 units/min AVP) in four of the six animals at 14 h already. In contrast, only one animal in the POV group needed a dose of 180 pMol/min at all, and none of the other five was titrated above 120 pMol/min during the 24-h study period (Fig. 6).
Survival.
Survival rates over the 24-h study period were 50% in the control group, 83.3% in the AVP group, and 100% in the POV group (P = 0.15).
DISCUSSION
The major findings of the present study are that a sole, titrated infusion of the highly selective V1a-agonist POV 1) reduced vascular dysfunction by attenuating neutrophil migration, nitrosative stress, and concentrations of angiopoietin-2 and VEGF; 2) reversed vascular leakage; 3) stabilized MAP more effectively; and 4) improved myocardial and pulmonary function as compared with the mixed V1a/V2-agonist AVP and control animals in a clinically relevant two-hit model of ovine severe sepsis.
Sepsis-induced vascular dysfunction was suggested by a marked fall in SVRI and MAP as well as a severe vascular leakage as evidenced by a decrease in plasma protein concentration and oncotic pressure in parallel with an increase in hematocrit as early as 6 h. Early fluid resuscitation was performed in accordance with the recommendations of the current guidelines for the treatment of severe sepsis and septic shock (8). The observed reversal of hemoconcentration suggests a restoration of intravascular volume. SVRI and MAP, however, continued to decrease, thus necessitating the use of vasopressors (8). Contrary with previous studies using constant infusions of vasopressin analogs as a supplement to norepinephrine, sole AVP or the selective V1a-agonist POV were titrated up to 180 pMol/min (∼0.06 units/min AVP) to maintain MAP. The maximum dose was based on a study of Torgersen et al. (47) who reported the safety of AVP doses up to 0.067 units/min in patients with septic shock. Because POV has a similar potency (EC50) as AVP at the human V1a receptor (28, 54), infusion preparations had the same concentrations. However, the selectivity of POV for the V1a- versus the V2 receptor is more than 220 times higher than AVP, thus resulting in a lack of V2-receptor activity (2). This probably explains the more pronounced vasoconstriction in the POV group, as suggested by a higher SVRI and MAP as compared with AVP-treated animals.
Notably, POV stabilized MAP already at lower doses immediately after the start of the infusion, whereas AVP was only effective at higher doses. The present observation is in line with previous experimental (40) and clinical data (47), suggesting that higher doses of AVP than the currently recommended 0.033 units/min might be necessary to restore MAP in septic shock. In addition, the premature death of one AVP-treated animal probably contributes, at least in part, to the “increase” in mean MAP and SVRI in the AVP group at the end of the study period.
Vascular leakage, the second manifestation of vascular dysfunction, led to massive fluid accumulation in the control group as suggested by hourly calculated and cumulative net fluid balance, the amounts of fluids in the thoracic and abdominal cavity. Furthermore, control animals suffered from pulmonary edema as evidenced by increased bloodless wet-to-dry-weight ratios and higher histological scores for alveolar and septal edema in the lung. The titrated infusion of the mixed V1a/V2-agonist AVP attenuated fluid accumulation as compared with the control group (213 ± 16 ml/kg). However, after 24 h animals still had a cumulative fluid balance of 110 ± 13 ml/kg.
Notably, the selective V1a-agonist POV was not only more effective than AVP in reducing fluid accumulation, POV reversed the sepsis-induced vascular leakage resulting in an almost even cumulative fluid balance over the 24-h study period (9 ± 15 ml/kg). The relevance of this finding is emphasized by a post hoc analysis of the VASST study, demonstrating an association between a positive fluid balance and mortality in septic shock (4). The effect of POV becomes most obvious by the time course of the hourly calculated net fluid balance (Fig. 2B). After treatment initiation, POV immediately reduced net fluid balance and finally led to negative values after 15 h. In accordance, urine output was significantly higher and the amount of fluid input, necessary to maintain intravascular volume, significantly lower in the POV group as compared with the other groups after 12 h. In view of these data, it is most likely that POV, at least in part, restored endothelial function and stopped vascular leakage. Thus the amounts of fluid that were accumulated during the first hours before POV treatment were excreted at the end. This hypothesis is supported by an experimental study in rats demonstrating that platelet activating factor-induced vascular leak is reversed by a selective V1a agonist and only attenuated by AVP (24).
The performed analyses of neutrophil activation, nitrosative stress, and markers of vascular dysfunction provide further insights into the potential mechanism of action of selective V1a agonists. Whereas both AVP and POV reduced neutrophil activation (myeloperoxidase activity in the lung, alveolar neutrophils) and plasma levels of nitric oxide as compared with control animals, only the selective V1a-agonist POV reduced myocardial and pulmonary tissue concentrations of two important mediators of vascular dysfunction, namely VEGF and angiopoietin-2. VEGF, a potent stimulator of vascular leakage, is released from the endothelium, and plasma levels of VEGF correlate with the severity of sepsis and septic shock (35, 49, 55). The angiopoietin-Tie2 system represents another important regulator in angiogenesis and endothelial inflammation (25). Whereas binding of angiopoietin-1 to the Tie2-receptor closes the gaps between endothelial cells and prevents the leakage of protein and fluid from the vascular compartment to the interstitial space (19, 46), angiopoietin-2 acts as a functional antagonist and promotes vascular leakage by disrupting the protective Tie2 signaling (27). Multiple clinical studies showed a correlation between elevated angiopoietin-2 concentrations and the severity of illness (13, 21, 30). Notably, in endothelial Weibel-Palade bodies angiopoietin-2 and von Willebrand factor are stored and secreted together (12), and the release of von Willebrand factor is known to be mediated by V2-receptor activation (18). Recently, our group was able to demonstrate that the mixed V1a/V2-agonist AVP induces the release of von Willebrand factor in doses used to treat septic shock, whereas a selective V1a-agonist does not (37).
A more mechanistic explanation for the reduction of vascular leakage by POV is represented by a potentially greater vasoconstrictor effect on precapillary resistance vessels. Thereby, POV would cause a reduction of the post-to-precapillary resistance ratio and decrease the capillary hydrostatic pressure. This hypothesis, however, needs to be verified in future studies.
Based on the published evidence on this topic, the superior effect of POV versus AVP in treating vascular dysfunction might be caused by its lack of V2 activity. This assumption is supported by a study of Traber (48) who showed that vascular leakage is promoted by V2-receptor stimulation in an ovine model of pneumonia-induced sepsis. The relevance of V1a-receptor selectivity is further emphasized by an experimental trial reporting that a selective V2 antagonist reduced metabolic, liver, and renal dysfunction as compared with norepinephrine and AVP in an ovine model of peritonitis-induced septic shock (39).
In the present study impairment of myocardial function, as indicated by a low left ventricular stroke work index for a given left ventricular filling pressure (left atrial pressure), and pulmonary dysfunction, as suggested by PaO2-to-FiO2 ratios <300 mmHg, were attenuated in POV- compared with AVP-treated and control animals. In addition to the reduction in vascular leakage and subsequent pulmonary edema, these findings may be explained by the attenuated nitrosative stress, as evidenced by lower concentrations of 3-nitrotyrosine in the lung and the heart in POV-treated animals as compared with the other groups. This assumption is supported by numerous experimental studies reporting the pivotal role of nitrosative stress in the pathogenesis of septic cardiomyopathy (44) as well as acute lung injury (10).
This study has some limitations that we want to acknowledge. In a study with numerous outcome variables and time points the risk of false-positive results has to be taken into consideration. A second limitation is the estimation of left ventricular stroke work index by standard formulas and the use of a correlation of left ventricular stroke work index and left atrial pressure as an index of myocardial contractility, because inotropy should be assessed by load-independent measures. However, these are only available by echocardiography or calculations of end-systolic pressure-volume relationships (5). Furthermore, due to the two-hit model of smoke inhalation injury and methicillin resistant staphylococcus aureus pneumonia, pulmonary mechanics might be influenced by numerous pathophysiological factors. In addition, interpretation of the current data is limited by the use of previously healthy animals, whereas the majority of patients typically suffer from comorbidities. Another potential limitation is the lack of dose response studies in the present sepsis model. However, appropriate pharmacological studies with POV and AVP have been published recently (54).
In summary, the present study provides evidence that a sole, titrated infusion of vasopressin analogs attenuates vascular dysfunction in ovine severe sepsis. Notably, the highly selective V1a-agonist POV stabilized MAP more effectively and at lower doses than mixed V1a/V2-agonist AVP. In addition, only POV, but not AVP, reversed vascular leakage and improved myocardial and pulmonary function as compared with control animals. The present findings suggest that the use of highly selective V1a-receptor agonists may be superior to unselective vasopressin analogs for the treatment of severe sepsis and septic shock. The first phase II trial using a selective V1a-agonist (ClinicalTrials.gov: NCT01000649) has been completed in October 2011, and the results should be available shortly.
GRANTS
This work was supported by Shriners of North America (Grant No. 8954, 8450, 8520, 8630) and National Institute for General Medical Sciences (Grant No. GM066312, GM060688). The compounds arginine vasopressin and Phe2-Orn8-Vasotocin were kindly provided by the Ferring Research Institute, San Diego, CA.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.R., L.D.T., P.E., and D.L.T. conception and design of research; S.R., Y.Y., L.E.S., E.B., C.C.J., A.K.H., and R.A.C. performed experiments; S.R., L.D.T., M.W., P.E., and D.L.T. analyzed data; S.R., L.D.T., M.W., P.E., and D.L.T. interpreted results of experiments; S.R. prepared figures; S.R. drafted manuscript; S.R., L.D.T., M.W., P.E., and D.L.T. edited and revised manuscript; S.R., Y.Y., L.E.S., E.B., C.C.J., A.K.H., L.D.T., R.A.C., M.W., P.E., and D.L.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the technicians of the Investigational Intensive Care Unit for expert technical assistance during the study.
REFERENCES
- 1.Barrett LK, Singer M, Clapp LH. Vasopressin: mechanisms of action on the vasculature in health and in septic shock. Crit Care Med 35: 33–40, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Berde B, Boissonnas RA, Huguenin RL, Sturmer E. Vasopressin analogues with selective pressor activity. Experientia 20: 42–43, 1964 [DOI] [PubMed] [Google Scholar]
- 3.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149: 818–824, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 39: 259–265, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol 289: H501–H512, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Chappell D, Westphal M, Jacob M. The impact of the glycocalyx on microcirculatory oxygen distribution in critical illness. Curr Opin Anaesthesiol 22: 155–162, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Cox RA, Burke AS, Soejima K, Murakami K, Katahira J, Traber LD, Herndon DN, Schmalstieg FC, Traber DL, Hawkins HK. Airway obstruction in sheep with burn and smoke inhalation injuries. Am J Respir Cell Mol Biol 29: 295–302, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36: 296–327, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Dunser MW, Mayr AJ, Ulmer H, Knotzer H, Sumann G, Pajk W, Friesenecker B, Hasibeder WR. Arginine vasopressin in advanced vasodilatory shock: a prospective, randomized, controlled study. Circulation 107: 2313–2319, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Enkhbaatar P, Connelly R, Wang J, Nakano Y, Lange M, Hamahata A, Horvath E, Szabo C, Jaroch S, Holscher P, Hillmann M, Traber LD, Schmalstieg FC, Herndon DN, Traber DL. Inhibition of neuronal nitric oxide synthase in ovine model of acute lung injury. Crit Care Med 37: 208–214, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enkhbaatar P, Joncam C, Traber L, Nakano Y, Wang J, Lange M, Connelly R, Kulp G, Saunders F, Huda R, Cox R, Schmalstieg F, Herndon D, Traber D. Novel ovine model of methicillin-resistant Staphylococcus aureus-induced pneumonia and sepsis. Shock 29: 642–649, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 103: 4150–4156, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Ganter MT, Cohen MJ, Brohi K, Chesebro BB, Staudenmayer KL, Rahn P, Christiaans SC, Bir ND, Pittet JF. Angiopoietin-2, marker and mediator of endothelial activation with prognostic significance early after trauma? Ann Surg 247: 320–326, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Gillett AM, Wallace MJ, Gillespie MT, Hooper SB. Increased expansion of the lung stimulates calmodulin 2 expression in fetal sheep. Am J Physiol Lung Cell Mol Physiol 282: L440–L447, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Jonkam CC, Lange M, Traber DL, Maybauer DM, Maybauer MO, Bansal K, Hamahata A, Zhu Y, Esechie A, Traber LD, Sousse L, Rehberg S, Herndon DN, Enkhbaatar P. Cardiovascular collapse and vascular permeability changes in an ovine model of methicillin-resistant staphylococcus aureus sepsis. Shock 32: 621–625, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Kanwar S, Woodman RC, Poon MC, Murohara T, Lefer AM, Davenpeck KL, Kubes P. Desmopressin induces endothelial P-selectin expression and leukocyte rolling in postcapillary venules. Blood 86: 2760–2766, 1995 [PubMed] [Google Scholar]
- 17.Kaufmann JE, Lezzi M, Vischer UM. Desmopressin (DDAVP) induces NO production in human endothelial cells via V2 receptor- and cAMP-mediated signaling. J Thromb Haemost 1: 821–828, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann JE, Vischer UM. Cellular mechanisms of the hemostatic effects of desmopressin (DDAVP). J Thromb Haemost 1: 682–689, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Kim I, Kim HG, So JN, Kim JH, Kwak HJ, Koh GY. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Circ Res 86: 24–29, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Kumar P, Shen Q, Pivetti CD, Lee ES, Wu MH, Yuan SY. Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev Mol Med 11: e19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumpers P, Lukasz A, David S, Horn R, Hafer C, Faulhaber-Walter R, Fliser D, Haller H, Kielstein JT. Excess circulating angiopoietin-2 is a strong predictor of mortality in critically ill medical patients. Crit Care 12: R147, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lange M, Connelly R, Traber DL, Hamahata A, Nakano Y, Esechie A, Jonkam C, von Borzyskowski S, Traber LD, Schmalstieg FC, Herndon DN, Enkhbaatar P. Time course of nitric oxide synthases, nitrosative stress, and poly(ADP ribosylation) in an ovine sepsis model. Crit Care 14: R129, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lange M, Traber DL, Enkhbaatar P. The authors reply. Crit Care Med 40: 356–357, 2011 [Google Scholar]
- 24.Laporte R, Wisniewska H, Callejas L, Russell J, Landry D, Rivière P. The selective V1a receptor agonist FE 202158 reverses platelet activating factor (PAF)-induced hypotension, vascular leak, impaired tissue perfusion, and mortality in rats (Abstract). Shock 1, Suppl 2: P21, 2008 [Google Scholar]
- 25.Lee WL, Liles WC. Endothelial activation, dysfunction and permeability during severe infections. Curr Opin Hematol 18: 191–196, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Luckner G, Dunser MW, Jochberger S, Mayr VD, Wenzel V, Ulmer H, Schmid S, Knotzer H, Pajk W, Hasibeder W, Mayr AJ, Friesenecker B. Arginine vasopressin in 316 patients with advanced vasodilatory shock. Crit Care Med 33: 2659–2666, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277: 55–60, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Mihara T, Tarumi T, Sugimoto Y, Chen Z, Kamei C. [Arg8]-vasopressin-induced increase in intracellular Ca2+ concentration in cultured rat hippocampal neurons. Brain Res Bull 49: 343–347, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Morelli A, Ertmer C, Rehberg S, Lange M, Orecchioni A, Cecchini V, Bachetoni A, D′Alessandro M, Van Aken H, Pietropaoli P, Westphal M. Continuous terlipressin versus vasopressin infusion in septic shock (TERLIVAP): a randomized, controlled pilot study. Crit Care 13: R130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orfanos SE, Kotanidou A, Glynos C, Athanasiou C, Tsigkos S, Dimopoulou I, Sotiropoulou C, Zakynthinos S, Armaganidis A, Papapetropoulos A, Roussos C. Angiopoietin-2 is increased in severe sepsis: correlation with inflammatory mediators. Crit Care Med 35: 199–206, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Patel BM, Chittock DR, Russell JA, Walley KR. Beneficial effects of short-term vasopressin infusion during severe septic shock. Anesthesiology 96: 576–582, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Paulus P, Jennewein C, Zacharowski K. Biomarkers of endothelial dysfunction: can they help us deciphering systemic inflammation and sepsis? Biomarkers 16, Suppl 1: S11–S21, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Pearce ML, Yamashita J, Beazell J. Measurement of Pulmonary Edema. Circ Res 16: 482–488, 1965 [DOI] [PubMed] [Google Scholar]
- 34.Petersen MB. The effect of vasopressin and related compounds at V1a and V2 receptors in animal models relevant to human disease. Basic Clin Pharmacol Toxicol 99: 96–103, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Pickkers P, Sprong T, Eijk L, Hoeven H, Smits P, Deuren M. Vascular endothelial growth factor is increased during the first 48 hours of human septic shock and correlates with vascular permeability. Shock 24: 508–512, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Polito A, Parisini E, Ricci Z, Picardo S, Annane D. Vasopressin for treatment of vasodilatory shock: an ESICM systematic review and meta-analysis. Intensive Care Med 38: 9–19, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Rehberg S, Enkhbaatar P, Rehberg J, La E, Ferdyan N, Qi S, Wisniewski K, Traber LD, Schteingart C, Rivière P, Laporte R, Traber DL. Unlike arginine vasopressin, the selective V1a receptor agonist FE 202158 does not cause procoagulant effects by releasing von Willebrand factor. Crit Care Med 40: 1957–1960, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rehberg S, Ertmer C, Kohler G, Spiegel HU, Morelli A, Lange M, Moll K, Schlack K, Van Aken H, Su F, Vincent JL, Westphal M. Role of arginine vasopressin and terlipressin as first-line vasopressor agents in fulminant ovine septic shock. Intensive Care Med 35: 1286–1296, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Rehberg S, Ertmer C, Lange M, Morelli A, Whorton EB, Dunser M, Strohhaecker AK, Lipke E, Kampmeier TG, Van Aken H, Traber DL, Westphal M. Role of selective V2-receptor antagonism in septic shock: a randomized, controlled, experimental study. Crit Care 14: R200, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rehberg S, Ertmer C, Vincent JL, Morelli A, Schneider M, Lange M, Van Aken H, Traber DL, Westphal M. Role of selective V1a receptor agonism in ovine septic shock. Crit Care Med 39: 119–125, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Russell J, Walley K, Singer J, Gordon A, Hébert P, Cooper D, Holmes C, Mehta S, Granton J, Storms M, Cook D, Presneill J, Ayers D. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 358: 877–887, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Russell JA. Management of sepsis. N Engl J Med 355: 1699–1713, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Simon F, Giudici R, Scheuerle A, Groger M, Asfar P, Vogt JA, Wachter U, Ploner F, Georgieff M, Moller P, Laporte R, Radermacher P, Calzia E, Hauser B. Comparison of cardiac, hepatic, and renal effects of arginine vasopressin and noradrenaline during porcine fecal peritonitis: a randomized controlled trial. Crit Care 13: R113, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soriano FG, Nogueira AC, Caldini EG, Lins MH, Teixeira AC, Cappi SB, Lotufo PA, Bernik MM, Zsengeller Z, Chen M, Szabo C. Potential role of poly(adenosine 5′-diphosphate-ribose) polymerase activation in the pathogenesis of myocardial contractile dysfunction associated with human septic shock. Crit Care Med 34: 1073–1079, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Sun Q, Dimopoulos G, Nguyen DN, Tu Z, Nagy N, Hoang AD, Rogiers P, De Backer D, Vincent JL. Low-dose vasopressin in the treatment of septic shock in sheep. Am J Respir Crit Care Med 168: 481–486, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med 6: 460–463, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Torgersen C, Dunser MW, Wenzel V, Jochberger S, Mayr V, Schmittinger CA, Lorenz I, Schmid S, Westphal M, Grander W, Luckner G. Comparing two different arginine vasopressin doses in advanced vasodilatory shock: a randomized, controlled, open-label trial. Intensive Care Med 36: 57–65, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Traber DL. Selective V1a receptor agonists in experimental septic shock (Abstract). Crit Care 11: P51, 2007 [Google Scholar]
- 49.van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, van Hinsbergh VW, Groeneveld AB. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax 63: 903–909, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Vincent JL. Vasopressin: useful or not? Curr Infect Dis Rep 10: 351–352, 2008 [PubMed] [Google Scholar]
- 51.Vincent JL, De Backer D. Microvascular dysfunction as a cause of organ dysfunction in severe sepsis. Crit Care 9, Suppl 4: S9–S12, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westphal M, Enkhbaatar P, Schmalstieg FC, Kulp GA, Traber LD, Morita N, Cox RA, Hawkins HK, Westphal-Varghese BB, Rudloff HE, Maybauer DM, Maybauer MO, Burke AS, Murakami K, Saunders F, Horvath EM, Szabo C, Traber DL. Neuronal nitric oxide synthase inhibition attenuates cardiopulmonary dysfunctions after combined burn and smoke inhalation injury in sheep. Crit Care Med 36: 1196–1204, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Westphal M, Sielenkamper AW, Van Aken H, Stubbe HD, Daudel F, Schepers R, Schulte S, Bone HG. Dopexamine reverses the vasopressin-associated impairment in tissue oxygen supply but decreases systemic blood pressure in ovine endotoxemia. Anesth Analg 99: 878–885, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Wisniewski K, Galyean R, Tariga H, Alagarsamy S, Croston G, Heitzmann J, Kohan A, Wisniewska H, Laporte R, Riviere PJ, Schteingart CD. New, potent, selective, and short-acting peptidic V1a receptor agonists. J Med Chem 54: 4388–4398, 2011 [DOI] [PubMed] [Google Scholar]
- 55.Yano K, Liaw PC, Mullington JM, Shih SC, Okada H, Bodyak N, Kang PM, Toltl L, Belikoff B, Buras J, Simms BT, Mizgerd JP, Carmeliet P, Karumanchi SA, Aird WC. Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J Exp Med 203: 1447–1458, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]