Abstract
Epidemiological studies have consistently linked inhalation of particulate matter (PM) to increased cardiac morbidity and mortality, especially in at risk populations. However, few studies have examined the effect of PM on baseline cardiac function in otherwise healthy individuals. In addition, airborne PM contain environmentally persistent free radicals (EPFR) capable of redox cycling in biological systems. The purpose of this study was to determine whether nose-only inhalation of EPFRs (20 min/day for 7 days) could decrease baseline left ventricular function in healthy male Sprague-Dawley rats. The model EPFR tested was 1,2-dichlorobenzene chemisorbed to 0.2-μm-diameter silica/CuO particles at 230°C (DCB230). Inhalation of vehicle or silica particles served as controls. Twenty-four hours after the last exposure, rats were anesthetized (isoflurane) and ventilated (3 l/min), and left ventricular function was assessed using pressure-volume catheters. Compared with controls, inhalation of DCB230 significantly decreased baseline stroke volume, cardiac output, and stroke work. End-diastolic volume and end-diastolic pressure were also significantly reduced; however, ventricular contractility and relaxation were not changed. DCB230 also significantly increased pulmonary arterial pressure and produced hyperplasia in small pulmonary arteries. Plasma levels of C-reactive protein were significantly increased by exposure to DCB230, as were levels of heme oxygenase-1 and SOD2 in the left ventricle. Together, these data show that inhalation of EPFRs, but not silica particles, decreases baseline cardiac function in healthy rats by decreasing cardiac filling, secondary to increased pulmonary resistance. These EPFRs also produced systemic inflammation and increased oxidative stress markers in the left ventricle.
Keywords: pressure-volume, particulates, inflammation, oxidative stress, air pollution
epidemiological studies have consistently linked exposure to airborne fine and ultrafine particulate matter (PM) to significant increases in cardiac morbidity and mortality (9, 35, 41). In general, these studies have indicated that for every 10-μg/m3 increase in the long-term average concentration of fine particulates, there is a significant increase in overall mortality as well as mortality attributable to cardiopulmonary and cardiovascular disease (9, 35). Exposure to PM increases heart rate, mean arterial pressure (MAP), and the incidence of cardiac arrhythmias. It also exacerbates atherosclerosis and coronary and peripheral artery disease, leading to increases in the incidence of angina, myocardial ischemia, stroke, heart failure, and sudden cardiac death (9, 28). Therefore, despite not being the primary site of exposure, metabolism, or detoxification, the cardiovascular system is still significantly affected by inhalational exposure to PM. While the mechanisms underlying PM-mediated cardiovascular toxicity are poorly understood, systemic inflammation and oxidative stress are thought to play a major role (7, 9, 35). Although controversial, there is mounting evidence that fine and ultrafine PM can enter the circulation to produce localized effects (7, 30, 32, 39).
The majority of fine and ultrafine PM arises from the combustion of fossil fuels or hazardous waste (8, 14, 35). Evidence from our group and others has indicated that a significant component of combustion-generated PM is composed of environmentally persistent free radicals (EPFRs) (13, 25, 26). EPFRs are formed in the postflame cool zone of combustion reactions when halogenated hydrocarbons chemisorb to the surface of metal oxide-containing particles, reduce the metal, and form an organic free radical. These EPFRs persist in the air for days or weeks and, upon redox cycling, produce a sustained release of ROS (26). While the biological actions of EPFRs are only starting to be studied, evidence suggests that PM with intrinsic redox potential may have enhanced biological activity beyond that of the particle itself (4, 5, 16, 27). Inhalation of EPFRs has been shown to produce pulmonary toxicity in neonatal rats (5, 27). Diesel exhaust particles (DEPs) containing redox active species also produce endothelial damage and exacerbate atherosclerotic disease in mice (1).
Despite the strong association between increases in particulate exposure and increased morbidity and mortality from cardiac events in humans, few experimental studies have directly examined the effects of particulates on baseline cardiac function. The majority of previous studies that have been conducted examined the effects of particle exposure in animals with a preexisting cardiovascular disease (e.g., hypertension) or in animals subjected to a subsequent cardiovascular insult. Cozzi and colleagues (12) showed that compared with controls, prior intratracheal instillation (24 h) of DEP significantly increases infarct size and inflammation in mice. In senescent mice, exposure to fine carbon black particles reduces cardiac fractional shortening and ejection fraction (40). Moreover, ventricular function was decreased in isolated hearts from spontaneously hypertensive rats (SHRs) previously exposed to DEP in vivo compared with normotensive control rats (3). In these hearts, the functional deficits produced by global ischemia were greater and the recovery from ischemia was slower than in control hearts. In separate studies, left ventricular (LV) function was also significantly decreased when isolated hearts from SHRs and normotensive rats were directly perfused with DEP (43, 44). Our group (27) showed that a large, single intratracheal instillation of EPFRs decreased LV function and exacerbated the decreases in LV function produced in response to a brief period of ischemia and reperfusion. While particle administration can affect cardiac function in “at risk” animals or exacerbate the functional deficits and damage produced by cardiac ischemia, the question of whether exposure to airborne PM, in particular EPFRs, can alter baseline ventricular function in otherwise healthy rats has not been studied. Therefore, this study tested the hypothesis that inhalation of EPFRs would decrease baseline LV function in healthy rats.
METHODS
Synthesis of ultrafine 1,2-dichlorobenzene chemisorbed to 0.2-μm-diameter silica/CuO particles at 230°C.
The model EPFR used in this study was 1,2-dichlorobenzene chemisorbed to 0.2-μm-diameter silica/CuO particles at 230°C (DCB230), an environmentally relevant ortho-chlorophenoxyl EPFR synthesized using established protocols (26). Briefly, DCB230 is formed during the thermal reaction (230°C) of 1,2-dichlorobenzene with silica oxide particles (∼0.2 μm diameter) dosed with 5% CuO. The reaction temperature mimics the combustion conditions of the postflame cooldown zone where the transition metal, in this case copper, and the organic pollutant chemisorb to form a stable oxygen-centered radical (26). Silica particles (∼0.08 μm diameter) served as controls.
Animals.
Experiments were performed using male Sprague-Dawley rats (225–275 g, Harlan, Indianapolis, IN). All procedures were performed in accordance with National Institutes of Health Guidelines for the Care and Use of Experimental Animals and were approved by the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center. Rats were housed in a temperature- and humidity-controlled room with a 12:12-h light-dark cycle. Standard rat chow and tap water were available ad libitum.
DCB230 exposure.
For exposure, samples of DCB230 and silica were suspended in vehicle (isotonic saline containing 0.02% Tween 80). To avoid particle aggregation, all samples were prepared fresh before exposure and sonicated on ice in 30-s intervals for 4 min at an output wattage of 10–14 W. Rats were exposed, in groups of four rats/group, to vehicle, silica, or DCB230 (1.4, 2.14, or 2.86 mg/ml) in a total volume of 7 ml using an inExpose, nose-only inhalation system (SciReq Scientific Respiratory Equipment). Each 7-ml sample of particles or vehicle was aerosolized using a VixOne nebulizer in conjunction with a Vios air pump. The particle suspension was passed through a silica gel desiccant column at ∼5.5 l/min to reduce moisture before the suspension reached the animals. The air output from the desiccant column was then combined with an additional 2 l/min dried air and channeled through the inExpose rat inhalation exposure system. Exposures lasted ∼20 min. Rats received 1 exposure/day for 7 consecutive days. All functional and biochemical experiments were performed 24 h after the final exposure. An epidemiological study (9) has reported that hospital admissions for cardiovascular events peak 24–48 h after increases in the levels of airborne PM.
To determine the actual particle dose, 7 ml of the DCB230 solution (1.4, 2.14, or 2.86 mg/ml, n = 3 each) was nebulized and delivered through the inExpose rat inhalation exposure system as described above. The accessory holes were sealed to accurately simulate the exposure condition. The nebulized DCB230 particles exiting the base of the system were gently bubbled into 5 ml of milli-Q water for capture and gravimetric analysis. At the end of the exposure, particulates captured in the aqueous solution were transferred into preweighed tubes, desalted by three cycles of milli-Q water wash, and then centrifuged at 16,000 relative centrifugal force (RCF) for 5 min, and the particle-free supernatant was discarded. Finally, the wet DCB230 particles were dried at 90°C overnight, and their mass was determined using a Mettler-Toledo MT-5 microbalance. It was determined that nebulization of the 1.4 mg/ml solution of DCB230 exposed each rat to 112 μg/day. Similarly, nebulization of the 2.14 and 2.86 mg/ml solution produced exposures of 171 and 230 μg/day, respectively. Taking into account the flow rate through our system and the mass of particles delivered, the concentrations of DCB230 to which our rats were exposed ranged between 2 and 6 mg/m3. This is comparable to the PM concentrations used in inhalational studies by several laboratories (19, 21, 42). However, it should be noted that in the previous studies, rats were exposed for 4–6 h/day over 4–5 days.
Measurement of LV function.
In vivo LV performance was assessed in vehicle-, silica-, and DCB230-treated rats 24 h after the final exposure using Millar pressure-volume (P-V) conductance catheters (model SPR-838). Treated rats were anesthetized (5% isoflurane), intubated (14-gauge catheter), and ventilated (2–3% isoflurane with 3 l/min O2). Tidal volume (3–5 ml) and respiratory rate (70–80 breaths/min) were adjusted as required. Body temperature was maintained at 37 ± 1°C using a heating pad. The right jugular vein was cannulated (polyethylene-50, 0.038-in outer diameter) to allow venous access for saline administration. The P-V catheter was introduced into the right carotid artery, and MAP signals were recorded (sampling rate: 1,000 Hz, MPVS-400, Millar Instruments) in the artery at the level of the aortic arch. The catheter was then advanced in a retrograde fashion into the LV. After a 15-min equilibration period, pressure and volume signals were continuously recorded. To determine load-independent parameters of cardiac function, preload was gradually reduced by slowly occluding the vena cava at the level of the diaphragm using a cotton-tipped applicator. Volume calibration of the P-V catheter was performed as previously described (27).
MAP, heart rate, and baseline LV measures of end-systolic pressure (ESP), end-diastolic pressure (EDP), and end-diastolic volume (EDV) were recorded using the P-V catheter. The maximal slope of the systolic pressure increment (dP/dtmax), maximal slope of the diastolic pressure increment (−dP/dtmin), load independent measure of relaxation (τ), stroke volume (SV), cardiac output (CO), and stroke work (SW) were computed from the recorded pressure and volume signals using AD Instruments LabChart Pro analysis software (27). dP/dtmax/EDV was calculated to assess contractility after accounting for changes in EDV. Preload- and afterload-independent measures of LV function, such as the end-systolic pressure-volume relationship (ESPVR) and end-diastolic pressure-volume relationship (EDPVR), were assessed using data obtained during gradual occlusion of the inferior vena cava. For analyses of all preload- and afterload-independent measures of cardiac function, a minimum of three consecutive P-V loops were used.
After the ventricular function experiments had been completed, the lungs and hearts were removed, rinsed in cold PBS, flash frozen in liquid nitrogen, and stored at −80°C. In addition, 3–5 ml of blood were drawn from the LV. Blood was collected into vacuum K2EDTA BD Vacutainer tubes and centrifuged at 14,000 RCF for 10 min at 4°C. The plasma was removed and stored at −80°C for future analysis.
ELISA for C-reactive protein.
Concentrations of C-reactive protein (CRP) in plasma samples from vehicle-treated (n = 3 rats/group), silica-treated (n = 4 rats/group), and DCB230-treated (1.4, 2.14, or 2.86 mg/ml, n = 4 rats/group) rats were measured using the BD Biosciences Rat CRP ELISA kit per the manufacturer's protocol. Briefly, the sandwich ELISA used an antibody specific for rat CRP coated on a 96-well plate. Standards and serum samples were added to the appropriate wells and incubated to allow the binding of protein to the immobilized antibody. Wells were washed, and horseradish peroxidase-conjugated anti-rat CRP antibody was added to the wells and allowed to incubate. The plate was again washed, tetramethylbenzidine was added to wells, and, after a 5-min incubation period, phosphoric acid was added to each well to stop the color development. Absorbance was measured at a wavelength of 450 nm on a spectrophotometer and plotted on a log graph to determine relative concentrations.
Pulmonary artery pressure.
Pulmonary artery pressure was measured using methods previously described by Pankey and colleagues (33). Briefly, separate groups of rats were treated with DCB230 (2.86 mg/ml, n = 8) or vehicle (n = 6) as previously described. Twenty-four hours after the final exposure, rats were anesthetized and ventilated. After baseline LV function had been measured, the P-V catheter was withdrawn, and a specially designed 3-Fr single-lumen catheter, having both a curved tip and radioopaque marker, was advanced into the pulmonary artery via the right jugular vein using fluoroscopic guidance (18). Mean pulmonary arterial pressure was measured using a Boston Scientific Namic Preceptor DT transducer and digitized by a Biopac MP100 data-acquisition system connected to a personal computer.
Pulmonary histology.
To assess evidence of pulmonary inflammation and increased pulmonary vascular resistance, histology and analysis were performed on the left lobe of the lung from rats treated with vehicle, silica, or DCB230 (n = 4 rats/group). Lungs were gravity perfused with 4% buffered zinc formalin and then paraffin imbedded. The left lobe was then sectioned (5 μm) and stained with hematoxylin-eosin. Small pulmonary arteries were analyzed in two random sections from each lung (8 sections from each treatment group) using ×40 magnification. From the resulting digitized images, vessel wall and lumenal area were calculated for 13–17 small pulmonary arteries/treatment group using Axio Vision 4.6.3 software. The wall index for each vessel was calculated as follows: wall index = wall area/(wall area + lumen area) (6).
Western blot analysis.
LV tissue from vehicle-, silica-, and DCB230-exposed rats was homogenized using tissue lysis buffer [0.1% Triton X-100, 0.1% deoxycholate, 25 mM HEPES (pH 7.4), 50 mM NaCl, 1 mM MgCl2, 2 mM EGTA, 10 mM pyrophosphate, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 0.5 mM PMSF, and 500 μM Na3VO4]. Samples were then sonicated for 10 s and centrifuged at 14,000 RCF for 10 min at 4°C. Protein concentrations were assessed using the Pierce BCA protein assay.
Lysates from cardiac tissue were analyzed by Western blot by running equal amounts of protein on SDS-PAGE gels for separation. Protein was then transferred to polyvinylidene difluoride membranes. Membranes were blocked for 1 h in 3% BSA in Tris-buffered saline-Tween 20 (TBST) and then incubated with the following primary antibodies: rabbit polyclonal anti-SOD2 (Abcam no. ab13533) or rabbit polyclonal anti-heme oxygenase (HO)-1 (Enzo no. ADI-SPA-895) in TBST with 3% BSA for an additional 2 h at room temperature. Membranes were rinsed with TBST and then incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibodies (1:10,000) for 1 h at room temperature. Immunoblots were visualized by chemiluminescence using Amersham ECL reagents according to the manufacturer's protocol. Images were captured using a GE ImageQuant LAS4000 imager and quantified with GE ImageQuant software.
Statistical analysis.
All data are expressed as means ± SE. Parameters of LV function, histology, CRP levels, and immunoblot analysis were analyzed by one-way ANOVA, and differences between means were determined by Dunnet's post hoc test (GraphPad Prism). Changes in pulmonary artery pressures were compared using an unpaired Student's t-test. P values of <0.05 were considered as statistically significant.
RESULTS
Ventricular function.
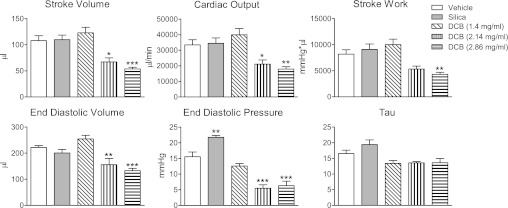
To examine the effects of DCB230 inhalation on baseline LV function, groups of rats were exposed to vehicle (n = 9), silica (n = 6), or DCB230 (1.4 mg/ml, n = 10; 2.14 mg/ml, n = 6; and 2.86 mg/ml, n = 8) by nose-only inhalation for 7 days. Twenty-four hours after the final exposure, rats were anesthetized, and P-V curves were generated with the chest closed. After exposure, baseline MAP and heart rate were not significantly different between any of the treatment groups (Table 1). Compared with vehicle- and silica-exposed rats, SV, CO, and SW were significantly reduced in rats exposed to the two highest does of DCB230 (Fig. 1). In addition, EDV and EDP were also significantly decreased in rats exposed to the highest concentrations of DCB230 (Fig. 1). Exposure to silica significantly increased EDP (Fig. 1). τ was not significantly different between any of the groups (Fig. 1). Likewise, −dP/dtmin was not different between any of the groups (data not shown).
Table 1.
DCB230 did not alter HR or MAP
| DCB230 Treatment |
|||||
|---|---|---|---|---|---|
| Vehicle Treatment | Silica Treatment | 1.4 mg/ml | 2.14 mg/ml | 2.86 mg/ml | |
| Number of rats/group | 9 | 6 | 10 | 6 | 8 |
| HR, beats/min | 309 ± 11 | 311 ± 11 | 326 ± 15 | 312 ± 10 | 325 ± 14 |
| MAP, mmHg | 88 ± 4 | 98 ± 5 | 93 ± 5 | 89 ± 6 | 86 ± 2 |
Values are means ± SE. Twenty-four hours after the final exposure, baseline heart rate (HR) and mean arterial pressure (MAP) were not significantly different between treatment groups. HR and MAP were recorded in the aortic arch of anesthetized and ventilated (2-3% isoflurane and 3 l/min oxygen) rats before the analysis of ventricular function. DCB230, 1,2-dichlorobenzene chemisorbed to 0.2-μm-diameter silica/CuO particles at 230°C.
Fig. 1.
Effect of exposure to vehicle, silica particles, or 1.4–2.86 mg/ml 1,2-dichlorobenzene chemisorbed to 0.2-μm-diameter silica/CuO particles at 230°C [DCB230 (DCB)] on ventricular performance, diastolic filling, and ventricular relaxation in adult rats. Inhalational exposure to DCB230 (20 min/day for 7 days) caused significant dose-related decreases in stroke volume, cardiac output, stroke work, end-diastolic volume (EDV), and end-diastolic pressure (EDP). A preload-independent measure of isovolumic relaxation (τ) was not significantly altered by DCB230 exposure. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. vehicle.
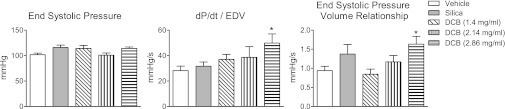
With respect to systolic function, neither exposure to silica nor any concentration of DCB230 significantly affected ESP (Fig. 2). To account for treatment-mediated changes in EDV and to compare contractility between the groups, dP/dt was indexed by dividing it by EDV. DCB230 exposure tended to elicit dose-related increases in dP/dtmax/EDV that were significantly different from vehicle at the highest dose of DCB230 (Fig. 2). ESPVR was also significantly greater than vehicle after exposure to the highest dose of DCB230 (Fig. 2).
Fig. 2.
Effect of exposure to vehicle, silica, or DCB230 (1.4–2.86 mg/ml) on end-systolic pressure (ESP), ventricular dP/dtmax adjusted for EDV (dP/dtmax/EDV), and the end-systolic pressure-volume relationship (ESPVR). ESP was not significantly altered by exposure to DCB230; however, the highest dose of DCB230 significantly increased dP/dtmax/EDV and ESPVR. *P < 0.05 relative to vehicle.
Pulmonary artery pressure.
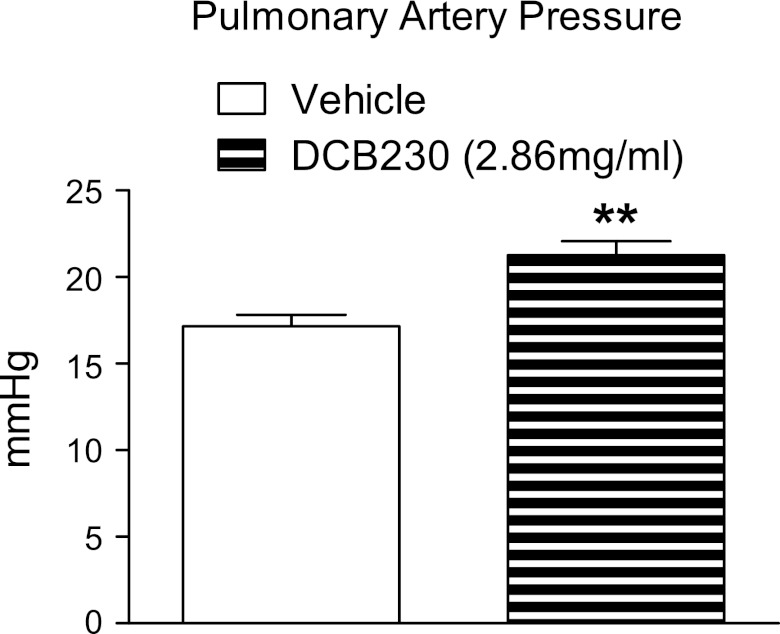
To determine whether the significant decreases in EDV and EDP in DCB230-exposed rats resulted from an increase in pulmonary vascular resistance, pulmonary artery pressures were measured in a separate cohort of vehicle-exposed (n = 6) and DCB230-exposed (2.86 mg/ml, n = 8) rats. Twenty-four hours after the final exposure, pulmonary artery pressure was significantly greater in rats exposed to DCB230 than in those exposed to vehicle (Fig. 3). P-V analysis showed that, as before, EDV (224 ± 8.7 vs. 154 ± 17.5 μl), EDP (14 ± 1.1 vs. 6 ± 0.8 mmHg), and CO (34,898 ± 4,442 vs. 16,840 ± 1,666 μl/min) were significantly lower in rats exposed to DCB230 than in rats exposed to vehicle.
Fig. 3.
Inhalation of DCB230 increases pulmonary artery pressure. Twenty-four hours after the final exposure, arterial pressure in DCB230-treated rats (2.86 mg/ml) was significantly greater than in vehicle-exposed rats. **P < 0.01 vs. vehicle.
Pulmonary histology.
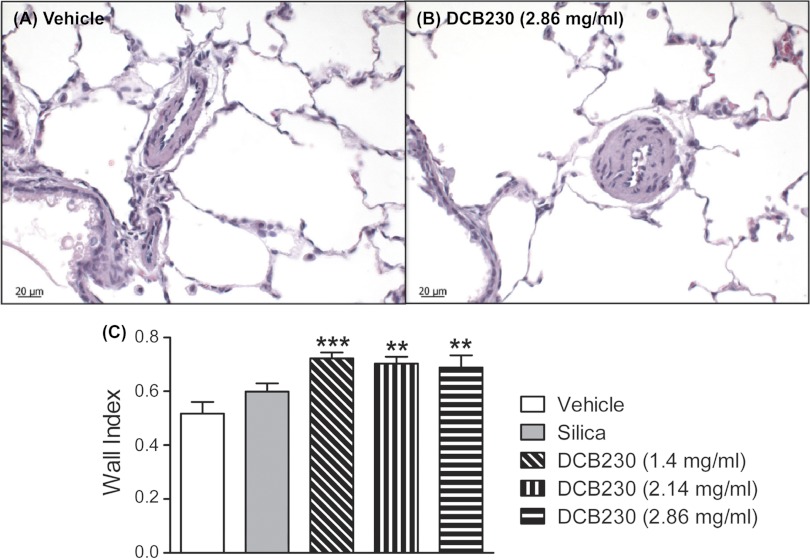
Figure 4, A and B, shows representative histological sections of the lung from rats exposed to vehicle and DCB230 (2.86 mg/ml). There were no obvious signs of active inflammation (e.g., mucus or inflammatory infiltration) in DCB230-treated lungs 24 h after the last exposure. Exposure to DCB230 (1.4, 2.14, and 2.86 mg/ml) induced hyperplasia in the walls of small pulmonary arteries (Fig. 4, A and B). Calculation of the wall index of small arteries confirmed that compared with inhalation of vehicle or silica, inhalational exposure to all concentrations of DCB230 significantly increased the wall index (i.e., wall thickness) in small pulmonary arteries (Fig. 4).
Fig. 4.
DCB230 exposure was associated with hypertrophy of small pulmonary arteries. A and B: representative histological sections from the left lobe of the lung from rats exposed to vehicle (A) or DCB230 (2.86 mg/ml; B). C: summary of the wall index of small pulmonary arteries in rats exposed to vehicle, silica, or the three concentrations of DCB230. Relative to vehicle, exposure to all doses of DCB230 significantly increased the small pulmonary artery wall index. Lung sections from 4 rats/treatment group were analyzed, with 12–17 small arteries/group measured. **P < 0.01 and ***P < 0.001 relative to vehicle treatment.
CRP.
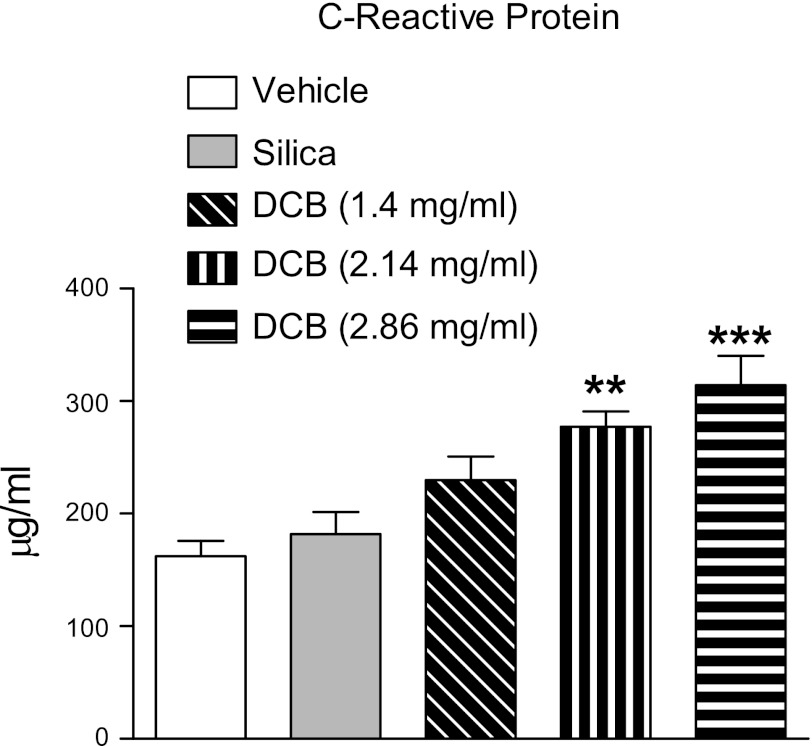
CRP, a marker of systemic inflammation and oxidant injury, was measured to determine whether inhalation of DCB230 produced systemic inflammation in rats. Compared with vehicle- or silica-exposed rats, exposure to DCB230 caused dose-related increases in CRP in the plasma (Fig. 5). The increases in CRP caused by exposure to the two highest doses of DCB230 were significantly greater than those produced by vehicle or silica.
Fig. 5.
Effect of exposure to DCB230 on plasma levels of C-reactive protein. Compared with vehicle and silica treatment, inhalation of DCB230 elicited dose-related increases in C-reactive protein. Serum samples were collected 24 h after the final exposure. **P < 0.01 and ***P < 0.001 relative to vehicle treatment.
Western blot analysis.
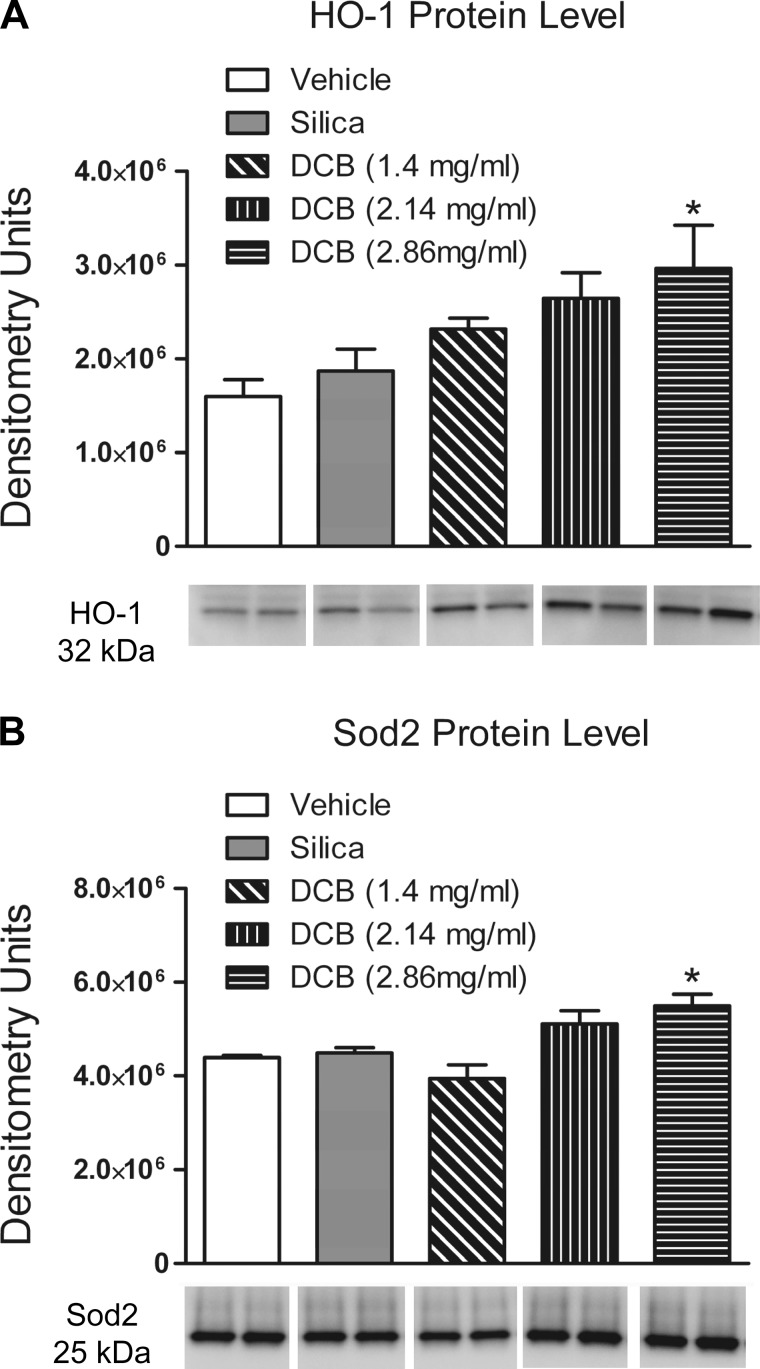
Since an increase in CRP was detected in the plasma of DCB230-exposed rats, the levels of the antioxidant enzymes HO-1 and SOD2 were determined in the LV using immunoblots. Exposure to DCB230 (2.86 mg/ml) significantly increased HO-1 protein levels in the myocardium (Fig. 6B). HO-1 induction was also observed at lower doses of DCB230, although the increases were not significantly greater than the vehicle. Exposure to DCB230 (2.86 mg/ml) also increased levels of SOD2 in the LV (Fig. 6A).
Fig. 6.
Heme oxygenase (HO)-1 and SOD2 protein levels in the left ventricle after inhalational exposure to vehicle, silica, or 1.4–2.86 mg/ml DCB230. Protein levels were measured by Western blot analysis 24 h after the last exposure. A: representative Western blot demonstrating dose-related increases in HO-1 protein levels and densitometric quantification of HO-1 protein levels. B: representative Western blots of SOD2 protein levels and densitometric quantification of increases in SOD2 protein levels. *P < 0.05 relative to vehicle treatment.
DISCUSSION
This study showed, for the first time, that short-term inhalational exposure to EPFRs, specifically DCB230, decreased baseline LV function in rats, as revealed by significant decreases in SV, CO, and SW. Surprisingly, despite these functional deficits, ESP was not significantly reduced, indicating that contractile function had not been affected by inhalation of this EPFR. In fact, ESPVR and dP/dtmax/EDV (load- and volume-independent measures of contractile function, respectively) were significantly increased after exposure to the highest concentration of DCB230, indicating that contractility was actually increased, most likely in an attempt to compensate for the decreased CO. Although contractility was not decreased after inhalation of DCB230, the decreases in CO, SV, and SW were accompanied by significant decreases in both EDV and EDP, indicating a significant reduction in ventricular filling. τ, a volume-independent measure of ventricular relaxation, and −dP/dtmin were not significantly reduced by exposure to DCB230, demonstrating that impaired relaxation was not responsible for the observed decrease in ventricular filling. Taken together, our data show that the DCB230-mediated reductions in SV, CO, and SW were caused by decreased ventricular filling rather than by deficits in ventricular relaxation or contractility. Therefore, we hypothesized that the observed decreases in EDV and EDP were the result of increased pulmonary vascular resistance, secondary to particle-mediated pulmonary inflammation. To determine whether DCB230 could increase pulmonary vascular resistance, pulmonary arterial pressure was measured in rats 24 h after exposure to either vehicle or the highest concentration of DCB230. Pulmonary artery pressure in DCB230-exposed rats was significantly higher relative to that in vehicle-exposed animals and was accompanied by significant decreases in ventricular filling and CO. Tankersley and colleagues (40) also demonstrated that inhalation of carbon black in 28-mo-old mice (3 h/day for 4 days over 2 wk) significantly increased right ventricular and pulmonary vascular pressures accompanied by a decrease in ejection fraction relative to age-matched controls exposed to filtered air. Pulmonary pressures were not increased in middle-aged (18 mo old) mice exposed to the same particles. In patients with moderate to severe heart failure, it has been shown that 11.62-μg/m3 increases in same-day mean fine particulate concentration are associated with significant increases in estimated pulmonary artery diastolic pressure (36). Increases in pulmonary arterial pressure were also noted by Doppler echocardiography in children chronically exposed to levels of PM and ozone that exceed United States air quality standards (10).
Although the exact mechanism mediating the increase in pulmonary arterial pressure is not known, we found that exposure to DCB230 was associated with hypertrophy of the vessel walls in small pulmonary arteries. At all levels of exposure, the remodeling of the small pulmonary arteries in DCB230-treated rats was characterized by significant increases in the wall index of small pulmonary arteries compared with small arteries in vehicle-treated or silica-treated rats. To our knowledge, ours is the first study to show PM-mediated vascular hypertrophy in the lung. Increases in the intimal thickness of the common carotid artery have been reported in response to 10-μg/m3 elevations in daily fine particulates (22) as well as in response to long-term elevations in ambient PM concentrations (15). Whether the remodeling of the small arteries in the lungs of our rats were responsible for the observed increases in pulmonary arterial pressure remains to be determined. We were not able to measure pulmonary wedge pressure in our experiments, so an exact determination of pulmonary vascular resistance could not be made.
The increases in pulmonary arterial pressure may also reflect changes in the responsiveness of blood vessels to vasoactive factors, such as endothelin (10), or changes in cardiopulmonary reflex function (17). Several studies (11, 24, 31) have reported that exposure to PM consistently increases the circulating levels of endothelin, impairs endothelium-dependent vasodilation, and augments responses to adrenergic vasoconstrictors. Alternatively, constriction of small pulmonary arteries and hence increases in pressure could occur through stimulation of bronchopulmonary C-fiber afferents that mediate defensive cardiopulmonary chemoreflexes directed at reducing the amount of inhaled pollutants transported into the bloodstream (2, 23).
What role pulmonary inflammation and oxidative stress may play in mediating the increases in pulmonary arterial pressure and the remodeling of small pulmonary arteries is currently unknown. Although pulmonary inflammation was not specifically addressed in our study, there is a large body of clinical and experimental literature demonstrating that many types of PM produce pulmonary inflammation and increase oxidative stress (20, 38). More important with respect to our data are reports showing that exposure to EPFRs produced pulmonary inflammation and toxicity in vitro and in vivo. Exposure of cultured human bronchial epithelial cells to an EPFR formed from the thermal reaction (230°C) of CuO and monochlorophenol at 230°C resulted in increased levels of ROS and decreased levels of the cellular antioxidants glutathione, glutathione peroxidase, and SOD (4). Treatment with the antioxidant resveratrol prevented the decline in cellular antioxidant levels (4). In neonatal rats, exposure (20 min/day for 7 days) to DCB230 produced pulmonary oxidative stress, as demonstrated by decreases in the oxidized-to-reduced glutathione ratio and increases in 8-isoprostanes in the bronchoalveolar lavage fluid (BALF) and lung tissue 24 h after the final exposure (5). The oxidative stress in the lungs was associated with increased numbers of lymphocytes and neutrophils and decreased numbers of macrophages in the BALF (5). There were also increases in levels of the proinflammatory cytokines IL-6, TNF-α, and interferon (IFN)-γ in the BALF. In adult rats, a single intratracheal instillation of DCB230 increased the levels of monocyte chemoattractant protein-1, macrophage inflammatory protein-1α, IL-1β/6/18, IFN-γ, and TNF-α in the BALF from adult rats (27).
Reports describing the effects of airborne particulates on baseline cardiac function in vivo are limited. Our data showing that inhalation of the EPFR DCB230 produced dose-related decreases in SV and CO are consistent with a report by Yan and colleagues (44), who showed using echocardiography that intratracheal instillation of DEP decreases ejection fraction in anesthetized rats. Our laboratory (27) has previously demonstrated that a single intratracheal instillation of DCB230 (8 mg/kg) significantly decreases baseline EDV but not EDP. In addition, the intratracheal instillation of these particles also produced diastolic dysfunction, as indicated by significant reductions in τ and −dP/dtmin. Reduced ventricular relaxation is most likely the reason why EDP was not significantly reduced in these rats, despite the significant reduction in EDV. The intratracheal instillation of DCB230 also significantly reduced baseline CO and SW but not SV. Unlike the inhalation of DCB230, the intratracheal instillation of these particles also produced contractile dysfunction (e.g., decreased dP/dtmax and ESP), indicating that the decrease in CO was the result of decreased filling and contractility. From these data, it would appear that the intratracheal administration of DCB230 produced greater cardiac toxicity (decreased filling and diastolic and systolic dysfunction) at baseline than did inhalational exposure. The reason for the differential effects of DCB230 on baseline function between these two studies probably reflects differences in the dose and route of administration. In our previous study, rats received a single intratracheal dose of DCB230 (∼2.5 mg) suspended in 0.3 ml of vehicle. In contrast, in the inhalational study, the maximum amount of particles that a rat could be exposed to was 230 μg, of which only of fraction was actually inhaled. Therefore, it is likely that the intratracheal administration of DCB230 produced a greater degree of pulmonary and systemic inflammation and markedly increased the number of particles available to translate from the lung to the systemic circulation. In contrast, inhalational exposure produced a milder, more prolonged inflammatory response and reduced particle availability. Impaired LV contractility was also demonstrated ex vivo in isolated perfused hearts from normotensive rats and SHRs 4 h after the intratracheal instillation of combustion-generated fine particles or when DEPs were directly added to the perfusion buffer (3, 43). Inhalational exposure to carbon black particles (≤0.1 μm) significantly decreased baseline systolic and diastolic function in senescent (28 mo old) mice; however, cardiac function was not altered when middle-aged (18 mo old) healthy mice were similarly exposed to the same particles (40).
In addition to producing pulmonary effects, inhalation of DCB230 also produced systemic inflammation, as demonstrated by significant increases in plasma CRP. CRP is an acute-phase protein that is increased in response to inflammation, specifically increases in the inflammatory cytokine IL-6. CRP levels are also increased in healthy men after elevations in daily fine PM concentrations (34, 37). The systemic inflammatory response observed after DCB230 exposure is consistent with the widely held theory that pulmonary-derived systemic inflammation and oxidative stress play major causative roles in the pathogenesis of PM-mediated cardiovascular and cardiac toxicity (9, 28, 35). There is also a growing body of evidence demonstrating that fine and ultrafine PMs directly elicit inflammatory and oxidative stress responses in the circulation and organs after transiting the lung to the circulation (7, 29). Whether EPFRs, by virtue of their ability to undergo redox cycling, would produce enhanced systemic or localized organ toxicity is currently being studied.
In addition to systemic inflammation, we also found significant increases in the protein levels of the antioxidants HO-1 and SOD2 in the ventricles of our DCB230-treated rats, suggesting that although there was no discernable dysfunction of the myocardium itself after exposure to the EPFRs, the heart was being exposed to increased ROS. The question of whether ROS levels would continue to rise and would be associated with systolic and/or diastolic dysfunction after more prolonged exposure to DCB230 is currently being studied. Another important question is whether the increased ROS in ventricles arose from circulating pulmonary-derived inflammatory factors and or from contact with PM that translocated from the lung.
This study demonstrated that short-term (7 days) inhalational exposure to DCB230 can significantly reduce baseline cardiac function in otherwise healthy rats as a consequence of EPFR-mediated increases in pulmonary arterial pressure. Although our rats did not show overt contractile diastolic or systolic dysfunction at baseline, the ventricles did show signs of oxidative stress and inflammation, which could reflect early stages of cardiac disease. It is reasonable to assume that the severity of cardiac disease would increase with more prolonged exposures. Nonetheless, the decrease in ventricular filling and CO could have important clinical implications. The increases in heart rate and blood pressure noted with PM exposures could, at least in part, result from increased sympathetic drive to maintain adequate CO. A reduction in ventricular filling, and thus CO, could increase the incidence of cardiac ischemia, especially in individuals with coronary artery diseases due to a decrease in coronary reserve and oxygen supply. Similarly, the decreases in CO and coronary flow and increases in ROS in the ventricles (reducing antioxidant defenses) could exacerbate myocardial injury produced by cardiac ischemia and may help explain the increase in cardiac morbidity and mortality associated with PM exposure. Moreover, our observation that the functional deficits and toxicities elicited by DCB230 were significantly greater than those elicited by silica particles alone suggests that EPFRs can produced a level of biological activity/toxicity that is greater than that of the particle alone. This suggests new concerns regarding the health effects of combustion-generated particles and points to the continued need to further examine the toxic potential of these specific environmental pollutants.
GRANTS
This work was supported by National Institutes of Health Grants P42-ES-0137648 (subaward 61365) and P20-RR-018766.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.M., G.C.C., E.A.P., and L.K. performed experiments; S.M., G.C.C., E.A.P., and P.J.K. analyzed data; S.M. and K.J.V. interpreted results of experiments; S.M. prepared figures; S.M. drafted manuscript; S.M., G.C.C., E.A.P., L.K., P.J.K., and K.J.V. edited and revised manuscript; B.D. and K.J.V. conception and design of research; K.J.V. approved final version of manuscript.
REFERENCES
- 1. Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res 102: 589–596, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aviado DM, Aviado DG. The Bezold-Jarisch reflex. Ann NY Acad Sci 940: 48–58, 2001 [PubMed] [Google Scholar]
- 3. Bagate K, Meiring JJ, Gerlofs-Nijland ME, Cassee FR, Wiegand H, Osornio-Vargas A, Borm PJ. Ambient particulate matter affects cardiac recovery in a Langendorff ischemia model. Inhal Toxicol 18: 633–643, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Balakrishna S, Lomnicki S, McAvey KM, Cole RB, Dellinger B, Cormier SA. Environmentally persistent free radicals amplify ultrafine particle mediated cellular oxidative stress and cytotoxicity. Part Fibre Toxicol 6: 11, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balakrishna S, Saravia J, Thevenot P, Ahlert T, Lominiki S, Dellinger B, Cormier SA. Environmentally persistent free radicals induce airway hyperresponsiveness in neonatal rat lungs. Part Fibre Toxicol 8: 11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bianda N, Di Valentino M, Periat D, Segatto JM, Oberson M, Moccetti M, Sudano I, Santini P, Limoni C, Froio A, Stuber M, Corti R, Gallino A, Wyttenbach R. Progression of human carotid and femoral atherosclerosis: a prospective follow-up study by magnetic resonance vessel wall imaging. Eur Heart J 33: 230–237, 2011 [DOI] [PubMed] [Google Scholar]
- 7. Brook RD. Cardiovascular effects of air pollution. Clin Sci (Lond) 115: 175–187, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 109: 2655–2671, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121: 2331–2378, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Calderon-Garciduenas L, Vincent R, Mora-Tiscareno A, Franco-Lira M, Henriquez-Roldan C, Barragan-Mejia G, Garrido-Garcia L, Camacho-Reyes L, Valencia-Salazar G, Paredes R, Romero L, Osnaya H, Villarreal-Calderon R, Torres-Jardon R, Hazucha MJ, Reed W. Elevated plasma endothelin-1 and pulmonary arterial pressure in children exposed to air pollution. Environ Health Perspect 115: 1248–1253, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cascio WE, Cozzi E, Hazarika S, Devlin RB, Henriksen RA, Lust RM, Van Scott MR, Wingard CJ. Cardiac and vascular changes in mice after exposure to ultrafine particulate matter. Inhal Toxicol 19, Suppl 1: 67–73, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Cozzi E, Hazarika S, Stallings HW, Cascio WE, Devlin RB, Lust RM, Wingard CJ, Van Scott MR. Ultrafine particulate matter exposure augments ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol 291: H894–H903, 2006 [DOI] [PubMed] [Google Scholar]
- 13. dela Cruz AL, Gehling W, Lomnicki S, Cook R, Dellinger B. Detection of environmentally persistent free radicals at a superfund wood treating site. Environ Sci Technol 45: 6356–6365, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dellinger B, Pryor WA, Cueto R, Squadrito GL, Hegde V, Deutsch WA. Role of free radicals in the toxicity of airborne fine particulate matter. Chem Res Toxicol 14: 1371–1377, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Diez Roux AV, Auchincloss AH, Franklin TG, Raghunathan T, Barr RG, Kaufman J, Astor B, Keeler J. Long-term exposure to ambient particulate matter and prevalence of subclinical atherosclerosis in the multi-ethnic study of atherosclerosis. Am J Epidemiol 167: 667–675, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Fahmy B, Ding L, You D, Lomnicki S, Dellinger B, Cormier SA. In vitro and in vivo assessment of pulmonary risk associated with exposure to combustion generated fine particles. Environ Toxicol Pharmacol 29: 173–182, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghelfi E, Rhoden CR, Wellenius GA, Lawrence J, Gonzalez-Flecha B. Cardiac oxidative stress and electrophysiological changes in rats exposed to concentrated ambient particles are mediated by TRP-dependent pulmonary reflexes. Toxicol Sci 102: 328–336, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Hyman AL, Hao Q, Tower A, Kadowitz PJ, Champion HC, Gumusel B, Lippton H. Novel catheterization technique for the in vivo measurement of pulmonary vascular responses in rats. Am J Physiol Heart Circ Physiol 274: H1218–H1229, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Ito T, Suzuki T, Tamura K, Nezu T, Honda K, Kobayashi T. Examination of mRNA expression in rat hearts and lungs for analysis of effects of exposure to concentrated ambient particles on cardiovascular function. Toxicology 243: 271–283, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Kelly FJ, Fussell JC. Air pollution and airway disease. Clin Exp Allergy 41: 1059–1071, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Kodavanti UP, Moyer CF, Ledbetter AD, Schladweiler MC, Costa DL, Hauser R, Christiani DC, Nyska A. Inhaled environmental combustion particles cause myocardial injury in the Wistar Kyoto rat. Toxicol Sci 71: 237–245, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Kunzli N, Jerrett M, Mack WJ, Beckerman B, LaBree L, Gilliland F, Thomas D, Peters J, Hodis HN. Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect 113: 201–206, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol 125: 47–65, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Li Z, Carter JD, Dailey LA, Huang YC. Pollutant particles produce vasoconstriction and enhance MAPK signaling via angiotensin type I receptor. Environ Health Perspect 113: 1009–1014, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lomnicki S, Dellinger B. A detailed mechanism of the surface-mediated formation of PCDD/F from the oxidation of 2-chlorophenol on a CuO/silica surface. J Phys Chem A 107: 4387–4395, 2003 [Google Scholar]
- 26. Lomnicki S, Truong H, Vejerano E, Dellinger B. Copper oxide-based model of persistent free radical formation on combustion-derived particulate matter. Environ Sci Technol 42: 4982–4988, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Lord K, Moll D, Lindsey JK, Mahne S, Raman G, Dugas T, Cormier S, Troxlair D, Lomnicki S, Dellinger B, Varner K. Environmentally persistent free radicals decrease cardiac function before and after ischemia/reperfusion injury in vivo. J Recept Signal Transduct Res 31: 157–167, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med 356: 447–458, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Nemmar A, Hoylaerts MF, Hoet PH, Nemery B. Possible mechanisms of the cardiovascular effects of inhaled particles: systemic translocation and prothrombotic effects. Toxicol Lett 149: 243–253, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PH, Verbruggen A, Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am J Respir Crit Care Med 164: 1665–1668, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Nurkiewicz TR, Porter DW, Barger M, Castranova V, Boegehold MA. Particulate matter exposure impairs systemic microvascular endothelium-dependent dilation. Environ Health Perspect 112: 1299–1306, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, Kreyling W, Cox C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A 65: 1531–1543, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Pankey EA, Bhartiya M, Badejo AM, Jr, Haider U, Stasch JP, Murthy SN, Nossaman BD, Kadowitz PJ. Pulmonary and systemic vasodilator responses to the soluble guanylyl cyclase activator, BAY 60–2770, are not dependent on endogenous nitric oxide or reduced heme. Am J Physiol Heart Circ Physiol 300: H792–H802, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peters A, Frohlich M, Doring A, Immervoll T, Wichmann HE, Hutchinson WL, Pepys MB, Koenig W. Particulate air pollution is associated with an acute phase response in men; results from the MONICA-Augsburg study. Eur Heart J 22: 1198–1204, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Pope CA, 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc 56: 709–742, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Rich DQ, Freudenberger RS, Ohman-Strickland P, Cho Y, Kipen HM. Right heart pressure increases after acute increases in ambient particulate concentration. Environ Health Perspect 116: 1167–1171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Riediker M. Cardiovascular effects of fine particulate matter components in highway patrol officers. Inhal Toxicol 19: 99–105, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Ristovski ZD, Miljevic B, Surawski NC, Morawska L, Fong KM, Goh F, Yang IA. Respiratory health effects of diesel particulate matter. Respirology 17: 201–212, 2012 [DOI] [PubMed] [Google Scholar]
- 39. Takenaka S, Karg E, Roth C, Schulz H, Ziesenis A, Heinzmann U, Schramel P, Heyder J. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ Health Perspect 109: 547–551, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tankersley CG, Champion HC, Takimoto E, Gabrielson K, Bedja D, Misra V, El-Haddad H, Rabold R, Mitzner W. Exposure to inhaled particulate matter impairs cardiac function in senescent mice. Am J Physiol Regul Integr Comp Physiol 295: R252–R263, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. United States Environmental Protection Agency Integrated Science Assessment for Particulate Matter (online). http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=216546 [5 September 2012]. [PubMed]
- 42. Wellenius GA, Coull BA, Godleski JJ, Koutrakis P, Okabe K, Savage ST, Lawrence JE, Murthy GG, Verrier RL. Inhalation of concentrated ambient air particles exacerbates myocardial ischemia in conscious dogs. Environ Health Perspect 111: 402–408, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wold LE, Simkhovich BZ, Kleinman MT, Nordlie MA, Dow JS, Sioutas C, Kloner RA. In vivo and in vitro models to test the hypothesis of particle-induced effects on cardiac function and arrhythmias. Cardiovasc Toxicol 6: 69–78, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Yan YH, Huang CH, Chen WJ, Wu MF, Cheng TJ. Effects of diesel exhaust particles on left ventricular function in isoproterenol-induced myocardial injury and healthy rats. Inhal Toxicol 20: 199–203, 2008 [DOI] [PubMed] [Google Scholar]