Abstract
Secondary lymphedema in humans is a common consequence of axillary lymph node dissection (ALND) to treat breast cancer. Remarkably, secondary lymphedema generally first appears following a delay of over a year and can be triggered suddenly by an inflammatory insult. However, it remains unclear why the apparently functional lymphatic system is unable to accommodate an inflammatory trigger. To provide mechanistic insight into the delayed and rapid secondary lymphedema initiation, we compared the ability of the ALND-recovered rat foreleg lymphatic system to prevent edema during an inflammatory challenge with that of the uninjured lymphatic system. At 73 days postsurgery, the forelegs of ALND−- and ALND+-sensitized rats were exposed to the proinflammatory agent oxazolone, which was found to reduce fluid drainage and increase skin thickness in both ALND− and ALND+ forelegs (P < 0.05). However, drainage in the ALND-recovered forelegs was more severely impaired than ALND− forelegs, as visualized by indocyanine green lymphography and quantified by interstitial transport of fluid marker (P < 0.05). Although both ALND+ and ALND− forelegs experienced significant inflammation-induced edema with the oxazolone exposure (P < 0.05), the peak tissue swelling in the ALND+ group was significantly greater than that of the ALND− forelegs (arm area peaked at ∼13.4 vs. ∼5.7% swelling, respectively, P < 0.005; wrist diameter peaked at 9.7 vs. 2.2% swelling, respectively, P < 0.005). The findings demonstrate that outward recovery from ALND in the rat foreleg masks an ensuing chronic and latent lymphatic insufficiency, which reduces the ability of the foreleg lymphatic system to prevent edema during an acute inflammatory process.
Keywords: lymphangiogenesis, lymphatic drainage, axillary lymph node dissection, secondary lymphedema, inflammation, oxazolone
secondary lymphedema in humans is a common consequence of axillary lymph node dissection (ALND) to treat breast cancer, which disconnects lymphatic vessels and excises extracellular matrix from the axilla (6). The intervening surgical space forms into scar tissue, a matrix material that has been demonstrated to hinder lymphatic regeneration and interstitial flows (2, 26, 35, 41, 42). An important characteristic of secondary lymphedema is that 25% of patients experience chronic life-long swelling, which generally appears within several years after the initial lymphatic-related injury has healed, although there may be a period of acute transient swelling that appears immediately following the surgery (4, 6, 16, 20, 21).
Because most studies of lymphedema are conducted on humans with the chronic swelling, relatively little is known about lymphatic function before edema appears. A lack of lymphatic drainage impairment from the operated limb was reported in women assessed pre- and 3 mo post-ALND (27). A 7-mo post-ALND study showed no deterioration in the muscle and subcutis drainage in the arms of women who ultimately incurred lymphedema relative to women who did not incur lymphedema (33). We have found in the mouse that lymph drainage recovers within several weeks following ALND (19). How lymphatic drainage is maintained in the post-ALND limb despite an inability to achieve sufficient physiological lymphangiogenesis across the wound site has been an open question. In the absence of adequate lymphatic regeneration across the wound site, there is evidence that accumulating lymph may experience compensatory drainage at the expense of higher outflow resistance through a reduced number of surviving local lymphatics, rerouted lymphatics that bypass the obstructive tissue, or lymphovenous communications (20, 34). Each of these may help resolve any acute lymphedema that develops and restore local lymph drainage, yet fail to prevent eventual lymphatic pump failure and chronic lymphedema (34, 37).
Although there is evidence that an inability of the lymphatic system to adequately regenerate during normal wound repair may predispose the tissue to swell during secondary lymphedema (26, 41), the series of events that causes the initiation of secondary lymphedema in the human has been relatively unexplored. Inflammation has been shown to play an important role in determining the amount of swelling in lymphedema (22, 45, 46) and may be an important stimulus promoting the appearance and perpetuation of the swelling (8). Indeed, the initial appearance of the chronic form of lymphedema in the human can occur rapidly (within a few hours to days) following an acute inflammatory insult (4), although subsequently it may slowly worsen. It is presently unclear why the apparently functional lymphatic system is unable to accommodate an acute inflammatory stimulus. We recently found that the functional recovery of fluid drainage and the resolution of acute lymphedema produced by ALND in the mouse foreleg are not hindered by vascular endothelial growth factor receptor-3 neutralization (which completely prevents any lymphatic regrowth) (19), demonstrating that recovery of fluid drainage and resolution of tissue swelling in acute lymphedema are lymphangiogenesis-independent. This finding raises the possibility that the lymphatic system may remain chronically impaired even though the lymphatic system continues to effectively drain capillary filtrate and prevent edema.
We hypothesized that a chronic lymphatic impairment may be a natural consequence of the poor physiological lymphatic regeneration that follows ALND. This chronic impairment may create a latent condition of reduced lymphatic reserve capacity (the difference between the normal and maximum lymph flow rate), which may reduce the ability of the tissue to successfully resolve an inflammatory response to foreign antigen. This lymphatic deficiency may be undetectable under normal conditions post-ALND yet predispose the tissue to chronic swelling following an inflammatory stimulus. To test our hypothesis, we compared the ability of the lymphatic system that regenerates in the rat foreleg following ALND to prevent edema during an acute inflammatory challenge with that of the normal foreleg lymphatic system. Such a comparison is necessary to reveal the presence of any latent lymphatic functional deficiency that may follow the physiological lymphatic regeneration process and to clarify the mechanism of secondary lymphedema initiation in the human, which may follow ALND and a coincidental acute inflammatory stimulus.
METHODS
Foreleg rat lymphedema model.
Female Sprague Dawley rats at 250–300 grams were used for all experiments. Rats were anesthetized with 2.5% isoflurane mixed with oxygen gas for surgical procedures and were killed at experimental endpoints by CO2 asphyxiation. All protocols were approved by the Animal Care and Use Committee of Michigan Technological University. Axillary lymph nodes were removed from female rats similar to what has been described previously for mice (39). Briefly, a 10-mm-long surgical incision through the dermis was placed across the axilla on the right side, and the axillary lymph nodes were identified and excised along with visible portions of pre- and postnodal collecting vessels under an Olympus SZX7 stereomicroscope. The dermis was sutured with 6–0 suture thread (Harvard Apparatus, Holliston, MA).
Oxazolone exposure.
Oxazolone (catalog no. E0753; Sigma) is a potent proinflammatory agent. Oxazolone induces contact hypersensitivity, an immunological response against the foreign chemical. During the sensitization phase, cells of the innate immune system take up the oxazolone and promote the formation of antigen-specific memory T cells (15). Subsequent contact with the oxazolone activates the memory T cells and causes the recruitment of inflammatory cells to the exposure site, which becomes outwardly manifest as tissue inflammation (14, 28). We allowed rats to fully recover from ALND. At 66 days post-ALND surgery, at which time there was no edema (data not shown), the rats in the ALND− oxazolone+ and ALND+ oxazolone+ groups were sensitized by applying 300 μl of 2% oxazolone in ethanol to the abdomen. A 1 × 1″ area was shaved on the rat abdomen, and 300 μl of the oxazolone solution was evenly applied throughout the shaved area using a micropipette. The rat was kept anesthetized until the applied oxazolone had dried. To initiate dermatitis, 300 μl of a 1.6% oxazolone solution of acetone and olive oil (4:1) was applied to the forelegs in the same groups as before starting at day 7 postsensitization (day 73 post-ALND surgery) and proceeding every three days thereafter until day 82 postsurgery, for a total of four applications. For these applications, rats were anesthetized and had their foreleg shaved. Next, 300 μl of the 1.6% oxazolone solution were evenly applied to the entire foreleg up to the shoulder using a micropipette and allowed to dry before the rat was allowed to regain consciousness. Optical images of the rat forelegs were collected before each application of oxazolone. Finally, rats were imaged via indocyanine green (ICG) lymphography, injected with lymphatic fluid tracer, and killed for cryosectioning of the foreleg on day 85 post-ALND.
Quantifying interstitial transport and lymphatic function.
Tetramethylrhodamine-conjugated lysine fixable dextran 70,000 mol wt (catalog no. D1818; Invitrogen, Carlsbad, CA) at 1 mg/ml in PBS was used as a fluorescent lymph tracer to quantify fluid drainage in the rat foreleg. At the specified day postsurgery, 15 μl of fluorescent tracer solution were injected intradermally into the posterior of both foreleg paws. We allowed all rats to recover for 6 h following dextran injections to quantify lymph drainage postsurgery. Because the presence and distribution of the tracer across the foreleg depends upon lymphatic drainage, the coverage of fluorescent tracer that is measured later in foreleg cross sections can serve to quantify interstitial transport and lymphatic function across the foreleg. The extent of fluid marker coverage in cross sections made at the wrist is a direct measurement of the ability of fluid marker to spread interstitially from the paw injection site toward the lymphatics proximal to the wrist. The extent of fluid marker coverage at the middle and upper locations of the foreleg is indicative of fluid accumulation. At all locations, the absence of fluid marker can be an indication of either highly efficient or highly dysfunctional lymphatics. Collected forelegs were cryosectioned to produce 100-μm cross sections at the elbow joint (designated as the upper location), midway between the elbow and wrist (middle location), and near the wrist (lower location). Sections were counterstained for cell nuclei with 4′,6-diamino-2-phenylindole (DAPI, catalog no. H-1200; Vector Laboratories, Burlingame, CA) and imaged under an Olympus BX51 Fluorescent Microscope. Fluorescent tracer area of coverage was quantified using Metamorph Offline 6.3r7 software and expressed as a percentage of total cross-sectional area of the foreleg tissue section.
Fluorescence imaging.
Fluorescent imaging was conducted on foreleg specimens cut into 100-μm cross sections. The path taken by lymph through the foreleg following the injection of 70,000 mol wt tetramethylrhodamine-conjugated dextran was identified in the cross sections by immobilizing the lysine-fixable fluid tracer. Cell nuclei were counterstained with DAPI. Fluorescent images were captured with a Zeiss MRm camera on a Zeiss Axiovert 200M fluorescence microscope with the Apotome system. This system collects a stack of two-dimensional images that are then compressed into a single image.
Physiological measurements.
Foreleg wrist thickness was measured in Metamorph from digital images of the rat foreleg. Foreleg area was measured in Metamorph from digital images of the right rat foreleg by outlining the paw, wrist, and leg 2.5 cm proximally from the wrist. Foreleg wrist thickness and foreleg area measurements for the oxazolone+ groups were normalized to the oxazolone− condition. Skin thickness of the swollen and nonswollen ALND− oxazolone− arm of each rat was measured with MetaMorph Imaging software (Molecular Devices) from sections obtained 4 mm distal to the elbow of each arm. Skin thickness of the edematous oxazolone+ groups was normalized to the oxazolone− condition.
Imaging of functional lymphatic vessels via ICG fluorescence lymphography.
We employed ICG fluorescence lymphography to identify functional lymphatic vessels following ALND and oxazolone exposure (n = 5/group). An imaging system recently developed by Drs. N. Unno, F. Ogata, and Eva M. Sevick-Muraca (23, 24, 31, 32, 40) was used to detect functional lymphatic vessels in the rat foreleg. Seven point five microliters of a 5 mg/ml solution of the fluorescent near-infrared dye ICG (catalog no. 17478–701-02; Akorn) were injected into the rat paw. Detection of ICG was performed with an electron multiplying charge-coupled device (C9100–13; Hamamatsu) using Hamamatsu recommendations as described previously (40). ICG was illuminated with an array of 760-nm light-emitting diodes (Epitex) placed before a 760-nm band pass filter (model 760FS10–50; Andover) to provide the excitation light for activating ICG. A 785- and 763-nm custom made holographic notch band rejection filter (models HNPF-785.0–2.0 and HNPF-763.0–2.0; Kaiser Optical Systems) and a 830-nm image quality bandpass filter (model 830FS20–25; Andover) were placed before the camera lens to selectively reject the excitation light and pass the emitted 830-nm wavelength. Following microdose paw injections of ICG dye, images were collected at 10 min to visualize the path of ICG drainage through the rat foreleg. Careful attention was made to inject the ICG dye at the proper depth in the paw dermis. For this, the needle insertion was made parallel to the plane of the paw so that the needle tip was fully covered yet within the dermis.
Statistical methods.
Five animals were used for each experimental group. Data are presented as means with SDs. P values were calculated using ANOVA or Mann-Whitney U-test.
RESULTS
Increased inflammation-induced edema post-ALND.
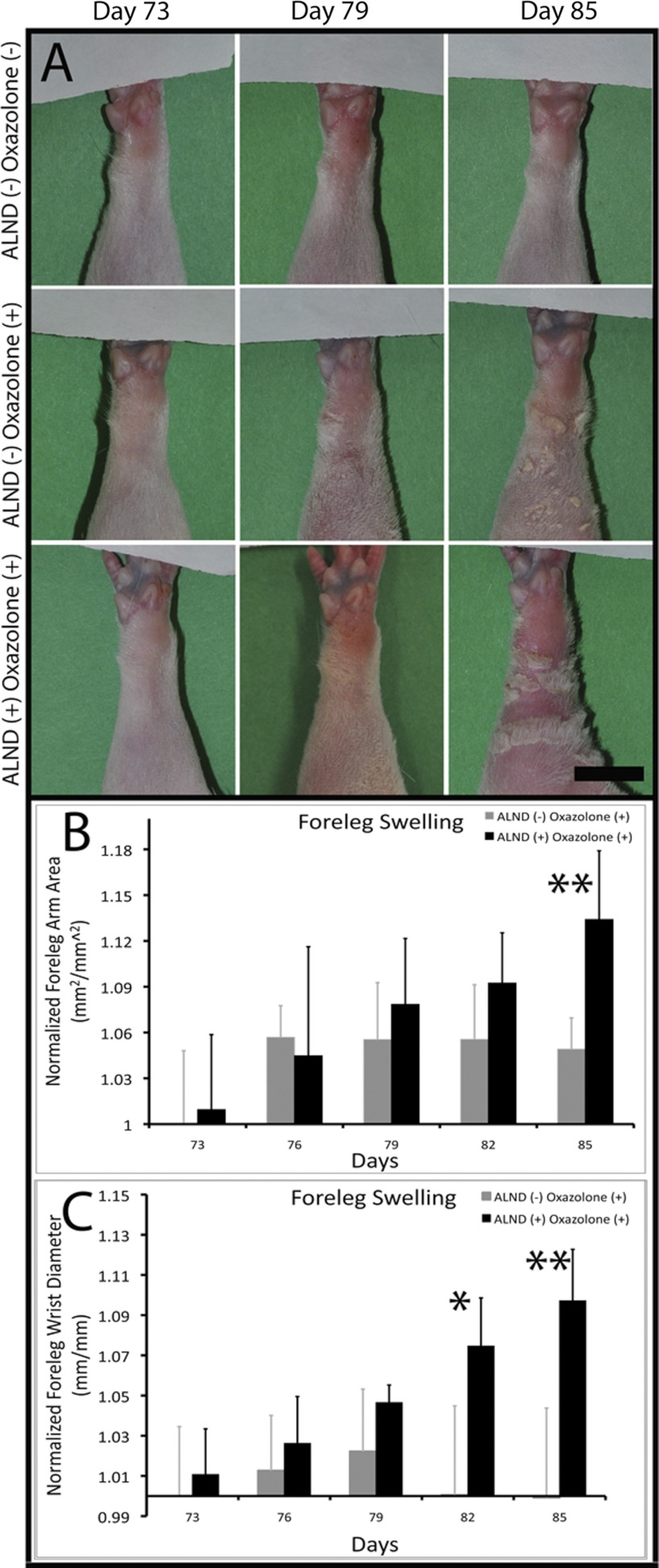
To reveal the presence of a chronic latent lymphatic deficiency, we compared the ability of the lymphatic system that had regenerated in the rat foreleg following ALND to prevent edema during an acute inflammatory challenge with that of the uninjured foreleg lymphatic system. Following the ALND surgery and at all other times up to day 73 (measurements made every 5 days), there was no detectable swelling in any of the forelegs (data not shown). Beginning at 73 days post-ALND, long after quiescence of the acute wound healing phase, the forelegs of sensitized rats were exposed to the proinflammatory agent oxazolone to challenge the foreleg lymphatic system. Such an approach was intended to increase lymphatic load to failure to reveal the presence of latent lymphatic deficiencies in the ALND-regenerated foreleg. We detected significant swelling in the ALND+ oxazolone+ foreleg relative to ALND− oxazolone+ forelegs by day 82, which was further increased at day 85 (Fig. 1), at which time the rats were killed for histological analyses. At day 82, the foreleg wrist swelling was significantly increased in ALND+ oxazolone+ forelegs relative to ALND− oxazolone+ (7.46 ± 2.39 vs. 0.06 ± 4.43%, respectively, P < 0.05 by ANOVA). At day 85, the increase in wrist and foreleg area swelling was both highly significant in the ALND+ oxazolone+ forelegs relative to ALND− oxazolone+ (9.71 ± 2.55% for ALND+ wrist swelling vs. −0.08 ± 4.46% for ALND−, P < 0.005 by ANOVA; 13.4 ± 4.5% for ALND+ foreleg area swelling vs. 4.91 ± 2.04% for ALND−, P < 0.005 by ANOVA). ALND− oxazolone+ mean foreleg area was found to have peaked at ∼5.7% swelling on day 76, whereas ALND+ oxazolone+ mean foreleg area was highest at ∼13.4% swelling on day 85. ALND− oxazolone+ mean wrist diameter peaked at ∼2.2% swelling on day 79, whereas ALND+ oxazolone+ mean wrist diameter peaked at ∼9.7% swelling at day 85. The peak trends suggest that edema in the ALND− oxazolone+ foreleg had stabilized by as early as days 76–79, whereas edema in the ALND-recovered foreleg had not yet stabilized even at the time of death (day 85), indicating that swelling in the ALND-recovered forelegs may have continued to increase over time. The data demonstrate a significantly reduced capacity of the ALND-regenerated lymphatic system to prevent edema during the oxazolone-induced acute inflammation.
Fig. 1.
Inflammation-induced edema is worsened in the post-axillary lymph node dissection (ALND) rat foreleg. Shown are representative images of ALND− oxazolone− (A, row on top row), ALND− oxazolone+ (A, row in middle), and ALND+ oxazolone+ (A, row on bottom) rat forelegs at days 73, 79, and 85 postsurgery. Black scale bar in A, bottom right = 10 mm. Foreleg arm area (B) and wrist diameter (C) were measured for all forelegs and graphed with means and SDs. *Statistical significance. **High statistical significance relative to the control condition. n = 5 Forelegs/group.
Appearance of edematous skin during acute inflammation.
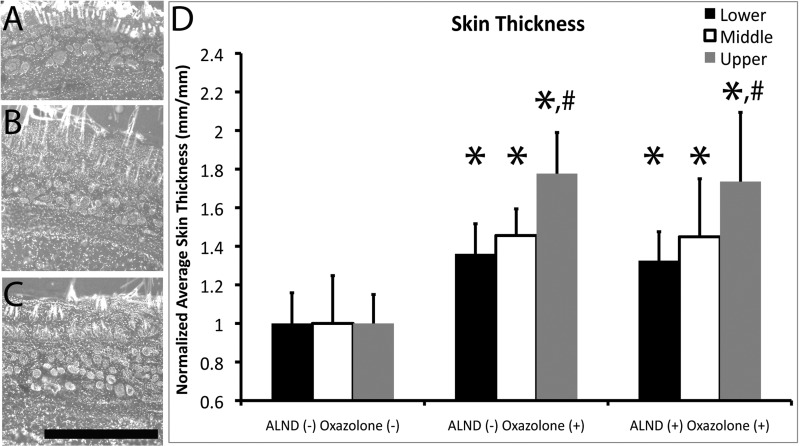
To identify histological changes associated with the foreleg swelling differences that were measured in Fig. 1, we measured skin thickness in histological foreleg cross sections. Skin thickness was found to have significantly increased in both oxazolone-treated foreleg groups relative to the ALND− oxazolone− foreleg by ∼35% at the lower location, 45% at the middle location, and 75% at the upper location (P < 0.05, by ANOVA; Fig. 2D), suggesting that the oxazolone treatment induced long-term cellular and structural changes in the skin irrespective of the presence of a healed lymphatic injury. The increase in skin thickness was predominantly seen in the dermal and epidermal layers, with less expansion seen in the hypodermis. However, there were no significant skin thickness differences between the ALND-recovered and ALND− oxazolone+ forelegs (P > 0.05). There was a significant increase for both oxazolone-treated foreleg groups in the upper foreleg location relative to the lower location (P < 0.05, by ANOVA; Fig. 2D). This may have occurred because there are more soft tissues in upper foreleg locations, so the ability to accumulate fluid in this area is greater.
Fig. 2.
Foreleg skin thickness increases during acute inflammation. Shown are fluorescent images of rat foreleg skin cryosections stained with 4′,6-diamino-2-phenylindole (DAPI) to identify cell nuclei (blue) at 85 days postsurgery and at the middle foreleg location for ALND− oxazolone− (A), ALND− oxazolone+ (B), and ALND+ oxazolone+ (C) skin. Black scale bar in C = 1 mm. Skin thickness was measured and graphed for all groups at all foreleg locations (D). *Statistical significance relative to the normal condition. #Statistical significance relative to the lower location within a particular condition. n = 5 Forelegs/group.
Reduced lymphatic drainage during post-ALND inflammation.
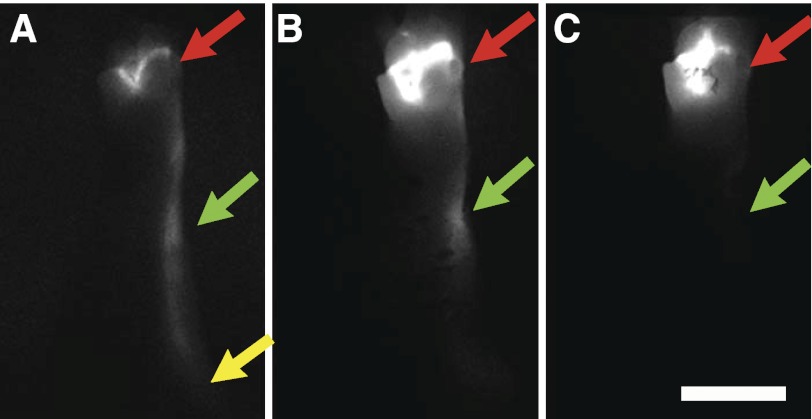
Because it was evident that oxazolone application had resulted in greater edema in the ALND+ oxazolone+ forelegs relative to ALND− oxazolone+ forelegs (Fig. 1), we asked whether the increased swelling was a consequence of deficient lymphatic drainage. We therefore visualized lymphatic drainage with ICG fluorescent lymphography at day 85, before being killed. ICG dye in ALND− oxazolone− forelegs was seen to drain from the injection site by large lymphatic vessels leading to the axillary lymph nodes (Fig. 3A), indicative of normally functioning lymphatic vessels in the foreleg. ALND− oxazolone+ forelegs were found to have incurred deficient lymph drainage, since the small ICG dye was seen mainly to spread interstitially throughout the paw and leg, reaching up to the elbow of the foreleg (Fig. 3B). However, an even more dramatically impaired lymph drainage was seen in the ALND-recovered forelegs treated with oxazolone. ICG dye in these forelegs was mainly restricted to the paw, near the injection site (Fig. 3C), indicative of a more serious lymphatic transport impairment. Thus, whereas reduced lymphatic drainage was apparent in the ALND− oxazolone+ forelegs due to the oxazolone-induced inflammation, a greater lymphatic drainage impairment was seen in the ALND-recovered forelegs. The ICG lymphography images correspond with the swelling data (Fig. 1) and confirm lymphatic drainage deficiency as a potential contributing source of the increased inflammation-induced edema in the ALND-recovered forelegs.
Fig. 3.
Acute inflammation impairs post-ALND lymph drainage. Shown are indocyanine green (ICG) fluorescence lymphography images captured from ALND− oxazolone− (A), ALND− oxazolone+ (B), and ALND+ oxazolone+ (C) forelegs at 85 days postsurgery. Images were captured at 10 min following the ICG paw injection. Red arrows identify the paw injection site. Green arrows identify the elbow. Yellow arrows identify the axillary region. White scale bar in C = 10 mm. n = 5 Forelegs/group.
Reduced interstitial transport and lymphatic function during acute inflammation.
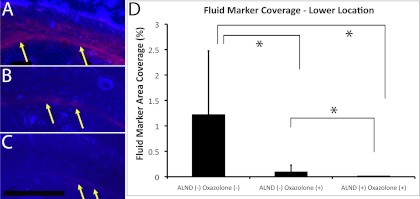
A second method was employed to assess the degree of lymph drainage impairment in the rat forelegs. Fluorescent rhodamine-dextran was injected in rat paws to trace the path of lymph drainage through the foreleg and quantitatively assess the ability of the lymphatic system to drain macromolecules from the paw by measuring fluorescent fluid tracer area coverage in histological foreleg cross sections at the elbow (upper location), midway between the elbow and wrist (middle location), and at the wrist (lower location). Fluid marker was found to be strongly present interstitially and subcutaneously in the lower location, near the injection site of ALND− oxazolone− forelegs (Fig. 4A). Fluid marker was more weakly present but was also seen spreading interstitially and subcutaneously in the lower location of ALND+/− oxazolone+ forelegs (Fig. 4, B and C). Fluid marker coverage in the lower location of ALND+/− oxazolone+ forelegs was significantly lower than in the control foreleg (P < 0.05, by Mann-Whitney U-test; Fig. 4D). The data may indicate a reduced interstitial transport of fluid marker from the injection site in the paw to more proximal lymphatic vessels due to the oxazolone treatment. However, the data may also indicate an enormously increased interstitial transport due to a dramatically increased blood capillary permeability, which has been shown to occur from the oxazolone (9). Importantly, we also found that the ALND+ oxazolone+ fluid marker area coverage was significantly lower relative to the ALND− oxazolone+ condition, significantly reducing by ∼80% (P < 0.05 by Mann-Whitney U-test). Because the significantly decreased fluid marker coverage was associated with the lymphatic injury, this suggests that a reduction in fluid marker coverage indicates a reduced lymphatic function. Indeed, a reduced lymphatic flow rate has been recently shown by direct lymphatic flow measurement conducted in a model of chronic skin inflammation induced by oxazolone (11). Fluid marker was scarcely detectable in the cross sections of all three groups at the middle and upper locations (data not shown). We have found in a recent study in the mouse foreleg that fluid marker signal increases at the middle and upper locations of mildly edematous tissues, indicative of fluid accumulation (19). We also found that signal is near zero at the middle and upper locations of healthy tissues, since the lymphatic system is highly effective in clearing fluid marker from the limb once the marker has entered the lymphatic system (19). We were surprised in the present study to find a near-zero coverage of fluid marker in the edematous limb at the middle and upper locations. When taken together with the presence of edema in the oxazolone-treated limb, a worsened edema in the post-ALND foreleg relative to the ALND− oxazolone+ foreleg, the dramatic lymph drainage impairment that was apparent with the ICG imaging, and the drastically reduced interstitial drainage that we measured from the reduced marker coverage in the wrist cross sections (including the significant 80% reduction in ALND+ oxazolone+ signal coverage relative to the ALND− oxazolone+ condition), we conclude that lymph drainage is severely impaired in the oxazolone-treated forelegs and is more impaired in the ALND+ oxazolone+ foreleg relative to the ALND− oxazolone+ foreleg.
Fig. 4.
Acute inflammation impairs interstitial drainage. Dextran fluid marker was imaged in cross sections from the ALND− oxazolone− (A), ALND− oxazolone+ (B), and ALND+ oxazolone+ (C) forelegs at the lower location. Red color in each image is fluorescent dextran lymph fluid tracer. Blue color in each image is DAPI-labeled cell nuclei. Yellow arrows identify fluid marker. Black scale bar in C = 1 mm. Fluid marker coverage and peak intensity are graphically depicted (D). *Statistical difference. n = 5 Forelegs/group.
DISCUSSION
Although secondary lymphedema is a common consequence of surgery to treat breast cancer, it remains unclear why the apparently functional lymphatic system in the human arm is unable to accommodate an acute inflammatory stimulus, which can induce chronic lymphedema. Here, we are the first to demonstrate that the lymphatic-independent recovery of acute lymphedema in the foreleg masks an ensuing chronic and latent lymphatic insufficiency, which reduces the ability of the foreleg lymphatic system to prevent edema during acute inflammation. The present study represents, to our knowledge, the first experimental evidence in support of the largely unexplored clinical observation that secondary lymphedema is often preceded by an acute inflammatory stimulus (18, 21, 29). Indeed, we showed that an acute inflammatory challenge significantly reduced lymphatic drainage, even in the lymphatic-intact foreleg, leading to an increased post-ALND foreleg edema. Our findings therefore demonstrate a clear link between inflammatory stimulation and increased post-ALND fluid accumulation and demonstrate that insufficient lymphatic drainage may be a potential contributing mechanism.
The similar lymphatic injury, period of latency, and disease onset between the post-ALND rat foreleg and the human arm suggest that the post-ALND rat foreleg may be a useful model for clarifying the lymphatic deficiency that preexists human secondary lymphedema and its response to acute inflammation. Although lymphatic function may be chronically impaired by the ALND surgery, there was no measured edema or deficient lymphatic drainage before the inflammatory stimulus. The appearance of an increased inflammation-induced post-ALND foreleg edema supports the hypothesis that the ALND surgery produces a chronic latent lymphatic functional deficiency that is not adequately restored by the natural wound healing process. We show here that the chronic latent deficiency becomes outwardly manifest during periods of acute inflammation. This chronic latent lymphatic functional deficiency may consist, at least in part, of a reduced maximum lymph flow rate caused by an insufficient wound healing lymphangiogenesis. Indeed, we and others have reported deficient physiological wound healing lymphangiogenesis in the mouse (2, 3, 41). Because the onset of secondary lymphedema in the human arm also follows a time delay (25) during which time lymph continues to drain normally (27, 33, 34), a similar latent lymphatic deficiency may be present in the post-ALND human arm, which predisposes the arm to chronically swell following an acute inflammatory stimulus. A straightforward explanation for the increased post-ALND edema is that lymphatic obstruction in the rat foreleg reduces the maximum lymphatic flow rate (i.e., lymphatic reserve) without altering lymph outflow under normal conditions. In conditions of acute inflammation, the reduced lymphatic reserve results in a greater lymphatic flow reduction and an increased fluid accumulation relative to what occurs in a healthy lymphatic system.
It has recently been demonstrated that oxazolone-mediated inflammatory responses increase capillary permeability (9), spur inducible nitric oxide (NO) release (17), and reduce lymphatic flow (11). NO is a key molecular regulator of lymphatic contractility (1) that is known to slow lymphatic drainage by reducing lymphatic contraction (30, 43). Reduced lymphatic pumping activity due to increased NO production may explain the appearance of edema in the ALND− oxazolone+ forelegs. A reduced maximum lymphatic flow rate in the post-ALND limb due to an increased lymphatic outflow resistance may explain the further increase in edema that we saw in the ALND+ oxazolone+ forelegs. Thus, we hypothesize that the increased intralymphatic pressure and weakening of lymphatic contractility and pump function caused by the increased regional outflow lymphatic network resistance after ALND may be worsened by acute inflammation due to an accelerated pump failure.
It has long been hypothesized that an increased lymphangiogenesis may be beneficial for increasing fluid drainage in humans with secondary lymphedema. A number of groups have reported that lymphatic growth-promoting molecular therapies hasten the natural resolution of experimental acute lymphedema (5, 13, 38, 44), thereby raising the prospect that similar approaches may be useful for resolving chronic lymphedema in the human. However, we have found that acute lymphedema in experimental models resolves via a lymphangiogenesis-independent mechanism (19, 26). Therefore, conclusions regarding the therapeutic effectiveness of stimulated lymphangiogenesis drawn from acute lymphedema models may not necessarily apply to chronic lymphedema patients.
In contrast to acute lymphedema, the inflammation-induced swelling and lymphatic deficiency that we have found in the post-ALND rat foreleg is independent of an ongoing wound repair process and is entirely dependent on the functional status of the foreleg lymphatic system as it had regenerated following the surgery to remove the axillary lymph nodes. The present experimental model more closely parallels the human disease etiology relative to previously employed acute lymphedema animal models in that there is a delay between the surgery and the appearance of swelling as opposed to an immediate edema onset, an inflammatory stimulus that initiates the swelling as opposed to a wound obstruction, the lack of an ongoing wound repair process with active remodeling of multiple interrelated systems, and an edema caused by a chronically (as opposed to acutely) impaired lymphatic system. We have used this more realistic animal model to provide novel insights into the latent lymphatic deficiency that precedes secondary lymphedema.
Presently, therapies for treating secondary lymphedema have largely been directed at increasing lymphatic drainage to clear excess fluid after disease onset. Interestingly, it has recently been reported in the mouse ear that an increased lymph flow may help resolve acute inflammation and reduce inflammation-induced edema (10), whereas decreased lymphangiogenesis or lymphatic function may have the opposite effect (7, 36). It may therefore be beneficial to apply prolymphangiogenic therapy prophylactically to increase lymphatic vasculogensis to reduce susceptibility for developing lymphedema during periods of acute inflammation, since capillary lymphatics by themselves will not be able to conduct all upper limb tissue fluid/lymph. However, it should be noted that lymphatic vasculogensis has not been shown to occur in humans, although it has been shown to occur in the mouse (12, 19). The present experimental model may be more ideal than existing models for evaluating experimental lymphatic growth-promoting therapies that could be applied to the post-ALND foreleg either during the wound healing phase or during the subsequent period of latency and then tested at a later time by challenging the lymphatic system with an acute inflammatory stimulus, as we have done here.
GRANTS
This work was funded by National Institutes of Health Grants R21-AR-053094, R21-HL-093568, and R15-HL-093705.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Akl TJ, Nagai T, Cote GL, Gashev AA. Mesenteric lymph flow in adult and aged rats. Am J Physiol Heart Circ Physiol 301: H1828–H1840, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avraham T, Clavin NW, Daluvoy SV, Fernandes J, Soares MS, Corderio AP, Mehrara BJ. fibrosis is a key inhibitor of lymphatic regneration. Plastic Reconstr Surg 124: 438–450, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Avraham T, Daluvoy S, Zampell J, Yan A, Haviv YS, Rockson SG, Mehrara BJ. Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair. Am J Pathol 177: 3202–3214, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates DO. An interstitial hypothesis for breast cancer related lymphoedema. Pathophysiology 17: 289–294, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung L, Han J, Beilhack A, Joshi S, Wilburn P, Dua A, An A, Rockson SG. An experimental model for the study of lymphedema and its response to therapeutic lymphangiogenesis. BioDrugs 20: 363–370, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Clarke D, Martinez A, Cox RS. Analysis of cosmetic results and complications in patients with stage-I and stage-II breast-cancer treated by biopsy and irradiation. Int J Radiat Oncol 9: 1807–1813, 1983 [DOI] [PubMed] [Google Scholar]
- 7.Guo RL, Zhou Q, Proulx ST, Wood R, Ji RC, Ritchlin CT, Pytowski B, Zhu ZP, Wang YJ, Schwarz EM, Xing LP. Inhibition of lymphangiogenesis and lymphatic drainage via vascular endothelial growth factor receptor 3 blockade increases the severity of inflammation in a mouse model of chronic inflammatory arthritis. Arthritis Rheum 60: 2666–2676, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halsted WS. The swelling of the arm after operation for cancer of the breast, elephantiasis chirurgica, its causes and prevention. Bull Johns Hopkins Hosp 32: 309–313, 1921 [Google Scholar]
- 9.He CF, Young AJ, West CA, Su M, Konerding MA, Mentzer SJ. Stimulation of regional lymphatic and blood flow by epicutaneous oxazolone. J Appl Physiol 93: 966–973, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Huggenberger R, Siddiqui SS, Brander D, Ullmann S, Zimmermann K, Antsiferova M, Werner S, Alitalo K, Detmar M. An important role of lymphatic vessel activation in limiting acute inflammation. Blood 117: 4667–4678, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huggenberger R, Ullmann S, Proulx ST, Pytowski B, Alitalo K, Detmar M. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J Exp Med 207: 2255–2269, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikomi F, Kawai Y, Nakayama J, Ogiwara N, Sasaki K, Mizuno R, Ohhashi T. Critical roles of VEGF-C-VEGF receptor 3 in reconnection of the collecting lymph vessels in mice. Microcirculation 15: 591–603, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Jin da P, An A, Liu J, Nakamura K, Rockson SG. Therapeutic responses to exogenous VEGF-C administration in experimental lymphedema: immunohistochemical and molecular characterization. Lymphat Res Biol 7: 47–57, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Kaplan DH, Igyarto BZ, Gaspari AA. Early immune events in the induction of allergic contact dermatitis. Nat Rev Immunol 12: 114–124, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol 38: 2369–2376, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Kissin MW, Querci della Rovere G, Easton D, Westbury G. Risk of lymphoedema following the treatment of breast cancer. Br J Surg 73: 580–584, 1986 [DOI] [PubMed] [Google Scholar]
- 17.Liao S, Cheng G, Conner DA, Huang YH, Kucherlapati RS, Munn LL, Ruddle NH, Jain RK, Fukumura D, Padera TP. Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci USA 108: 18784–18789, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mak SS, Yeo W, Lee YM, Tse SM, Ho FP, Zee B, Chan E. Risk factors for the initiation and aggravation of lymphoedema after axillary lymph node dissection for breast cancer. Hong Kong Med J 15: 8–12, 2009 [PubMed] [Google Scholar]
- 19.Mendez U, Brown EM, Ongstad EL, Slis JR, Goldman J. Functional recovery of fluid drainage precedes lymphangiogenesis in acute murine foreleg lymphedema. Am J Physiol Heart Circ Physiol 302: H2250–H2256, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mortimer PS. The pathophysiology of lymphedema. Cancer 83: 2798–2802, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Mortimer PS, Bates DO, Brassington HD, Stanton AWB, Strachan DP, Levick JR. The prevalence of arm oedema following treatment for breast cancer. Qjm-Mon J Assoc Phys 89: 377–380, 1996 [Google Scholar]
- 22.Nakamura K, Radhakrishnan K, Wong YM, Rockson SG. Anti-inflammatory pharmacotherapy with ketoprofen ameliorates experimental lymphatic vascular insufficiency in mice. Plos One 4: e8380, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogata F, Azuma R, Kikuchi M, Koshima I, Morimoto Y. Novel lymphography using indocyanine green dye for near-infrared fluorescence labeling. Ann Plast Surg 58: 652–655, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Ogata F, Narushima M, Mihara M, Azuma R, Morimoto Y, Koshima I. Intraoperative lymphography using indocyanine green dye for near-infrared fluorescence labeling in lymphedema. Ann Plast Surg 59: 180–184, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Olszewski W. On the pathomechanism of development of postsurgical lymphedema. Lymphology 6: 35–51, 1973 [PubMed] [Google Scholar]
- 26.Ongstad EL, Bouta EM, Roberts JE, Uzarski JS, Gibbs SE, Sabel MS, Cimmino VM, Roberts MA, Goldman J. Lymphangiogenesis-independent resolution of experimental edema. Am J Physiol Heart Circ Physiol 299: H46–H54, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pain SJ, Barber RW, Solanki CK, Ballinger JR, Britton TB, Mortimer PS, Purushotham AD, Peters AM. Short-term effects of axillary lymph node clearance surgery on lymphatic physiology of the arm in breast cancer. J Appl Physiol 99: 2345–2351, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Saint-Mezard P, Berard F, Dubois B, Kaiserlian D, Nicolas JF. The role of CD4+ and CD8+ T cells in contact hypersensitivity and allergic contact dermatitis. Eur J Dermatol 14: 131–138, 2004 [PubMed] [Google Scholar]
- 29.Sakorafas GH, Peros G, Cataliotti L, Vlastos G. Lymphedema following axillary lymph node dissection for breast cancer. Surg Oncol 15: 153–165, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Saul ME, Thomas PA, Dosen PJ, Isbister GK, O'Leary MA, Whyte IM, McFadden SA, van Helden DF. A pharmacological approach to first aid treatment for snakebite. Nat Med 17: 809–811, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Sevick-Muraca EM, Sharma R, Rasmussen JC, Marshall MV, Wendt JA, Pham HQ, Bonefas E, Houston JP, Sampath L, Adams KE, Blanchard DK, Fisher RE, Chiang SB, Elledge R, Mawad ME. Imaging of lymph flow in breast cancer patients after microdose administration of a near-infrared fluorophore: feasibility study. Radiology 246: 734–741, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma R, Wang W, Rasmussen JC, Joshi A, Houston JP, Adams KE, Cameron A, Ke S, Kwon S, Mawad ME, Sevick-Muraca EM. Quantitative imaging of lymph function. Am J Physiol Heart Circ Physiol 292: H3109–H3118, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Stanton AW, Modi S, Bennett Britton TM, Purushotham AD, Peters AM, Levick JR, Mortimer PS. Lymphatic drainage in the muscle and subcutis of the arm after breast cancer treatment. Breast Cancer Res Treat 117: 549–557, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Stanton AW, Modi S, Mellor RH, Levick JR, Mortimer PS. Recent advances in breast cancer-related lymphedema of the arm: lymphatic pump failure and predisposing factors. Lymphat Res Biol 7: 29–45, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Suami H, Pan WR, Taylor GI. The lymphatics of the skin filled by a dermal backflow: an observation in a scarred cadaver leg. Lymphology 40: 122–126, 2007 [PubMed] [Google Scholar]
- 36.Sugaya M, Kuwano Y, Suga H, Miyagaki T, Ohmatsu H, Kadono T, Okochi H, Blauvelt A, Tamaki K, Sato S. Lymphatic dysfunction impairs antigen-specific immunization, but augments tissue swelling following contact with allergens. J Invest Dermatol 132: 667–676, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Szuba A, Pyszel A, Jedrzejuk D, Janczak D, Andrzejak R. Presence of functional axillary lymph nodes and lymph drainage within arms in women with and without breast cancer-related lymphedema. Lymphology 40: 81–86, 2007 [PubMed] [Google Scholar]
- 38.Szuba A, Skobe M, Karkkainen MJ, Shin WS, Beynet DP, Rockson NB, Dakhil N, Spilman S, Goris ML, Strauss HW, Quertermous T, Alitalo K, Rockson SG. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J 16: 1985–1987, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Tammela T, Saaristo A, Holopainen T, Lyytikka J, Kotronen A, Pitkonen M, Abo-Ramadan U, Yla-Herttuala S, Petrova TV, Alitalo K. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med 13: 1458–1466, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Unno N, Inuzuka K, Suzuki M, Yamamoto N, Sagara D, Nishiyama M, Konno H. Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema. J Vasc Surg 45: 1016–1021, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Uzarski J, Drelles MB, Gibbs SE, Ongstad EL, Goral JC, McKeown KK, Raehl AM, Roberts MA, Pytowski B, Smith MR, Goldman J. The resolution of lymphedema by interstitial flow in the mouse tail skin. Am J Physiol Heart Circ Physiol 294: H1326–H1334, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Warren AG, Slavin SA. Scar lymphedema: fact or fiction? Ann Plas Surg 59: 41–45, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Weiler M, Kassis T, Dixon JB. Sensitivity analysis of near-infrared functional lymphatic imaging. J Biomed Opt 17: 066019, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoon YS, Murayama T, Gravereaux E, Tkebuchava T, Silver M, Curry C, Wecker A, Kirchmair R, Hu CS, Kearney M, Ashare A, Jackson DG, Kubo H, Isner JM, Losordo DW. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. J Clin Invest 111: 717–725, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zampell JC, Elhadad S, Avraham T, Weitman E, Aschen S, Yan A, Mehrara BJ. Toll-like receptor deficiency worsens inflammation and lymphedema after lymphatic injury. Am J Physiol Cell Physiol 302: C709–C719, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zampell JC, Yan A, Avraham T, Daluvoy S, Weitman ES, Mehrara BJ. HIF-1alpha coordinates lymphangiogenesis during wound healing and in response to inflammation. FASEB J 26: 1027–1039, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]