Abstract
The chemomechanosensory function of the gut enterochromaffin (EC) cell enables it to respond to dietary agents and mechanical stretch. We hypothesized that the EC cell, which also sensed alterations in luminal or mucosal oxygen level, was physiologically sensitive to fluctuations in O2. Given that low oxygen levels induce 5-HT production and secretion through a hypoxia inducible factor 1α (HIF-1α)-dependent pathway, we also hypothesized that increasing O2 would reduce 5-HT production and secretion. Isolated normal EC cells as well as the well-characterized EC cell model KRJ-I were used to examine HIF signaling (luciferase-assays), hypoxia transcriptional response element (HRE)-mediated transcription (PCR), signaling pathways (Western blot), and 5-HT release (ELISA) during exposure to different oxygen levels. Normal EC cells and KRJ-I cells express HIF-1α, and transient transfection with Renilla luciferase under HRE control identified a hypoxia-mediated pathway in these cells. PCR confirmed activation of HIF-downstream targets, GLUT1, IGF2, and VEGF under reduced O2 levels (0.5%). Reducing O2 also elevated 5-HT secretion (2–3.2-fold) as well as protein levels of HIF-1α (1.7–3-fold). Increasing O2 to 100% inhibited HRE-mediated signaling, transcription, reduced 5-HT secretion, and significantly lowered HIF-1α levels (∼75% of control). NF-κB signaling was also elevated during hypoxia (1.2–1.6-fold), but no significant changes were noted in PKA/cAMP. We concluded that gut EC cells are oxygen responsive, and alterations in O2 levels differentially activate HIF-1α and tryptophan hydroxylase 1, as well as NF-κB signaling. This results in alterations in 5-HT production and secretion and identifies that the chemomechanosensory role of EC cells extends to oxygen sensing.
Keywords: hypoxia, neuroendocrine, serotonin
neuroepithelial bodies (NEBs) are bronchopulmonary neuroendocrine cells that serve as airway chemoreceptor cells responsive to hypoxia. When exposed to low O2 levels, NEBs release serotonin (5-HT), which activates vagal afferents, thereby participating in the regulation of breathing. The gut counterpart of NEBs, the neuroendocrine enterochromaffin (EC) cells, are located in the base of the mucosal crypts. Similar to NEBs, they have a chemomechanosensory function and respond to alterations in the luminal milieu, including dietary agents and mechanical stretch via cAMP/ERK activation and 5-HT secretion (6, 28).
The mucosa in the gastrointestinal (GI) tract is, during multiple times a day, subject to large variations in blood perfusion, and therefore Po2. During fasting, the blood flow is low (5% of cardiac output), which then rises significantly to 30% after a meal. In addition, the mucosa has a steep oxygen gradient from the epithelial cells adjacent to the nearly anoxic lumen to the cells near the highly perfused submucosa (10, 47).
In inflammatory bowel disease (IBD), the GI mucosa is subjected to chronic inflammation. The subsequent increase in leukocyte numbers, increased metabolism, and vasculitis leaves the tissue in a chronic hypoxic state (22, 47). Hypoxia-inducible factor-1 α (HIF-1α) functions as a master regulator of cellular and systemic homeostatic response to hypoxia by activating transcription of many genes whose protein products increase oxygen delivery or facilitate metabolic adaptation to hypoxia. In a preliminary study by our group, we demonstrated that EC cells are hypoxia responsive and that the production and secretion of 5-HT is upregulated during hypoxia via a HIF-1α-related mechanism accompanied by NF-κB pathway activation (3).
Alterations in 5-HT release are critically relevant to the regulation of normal gut function. EC cells mediate gut secretion, peristalsis, and motility by the secretion of 5-HT, as well as of melatonin, substance P, and guanylin (27). 5-HT release is altered in pathological states such as IBD, irritable bowel syndrome (IBS), and Celiac disease. Increased 5-HT secretion (2, 17) and decreased levels of 5-HT transporters (SERT) (8) have been noted in IBD, the latter, a reuptake mechanism facilitating degradation of 5-HT. In diarrhea-dominated IBS (D-IBS), the EC cell number is also increased (31), which is accompanied by elevated postprandial plasma 5-HT (1). In addition, SERT is downregulated in D-IBS (8). In Celiac disease, the EC cell number is increased (51), and 5-HT is elevated in both the blood and mucosa (9). Multiple experimental studies indicate an important role for 5-HT in immune activation (20, 32), and 5-HT receptors have been identified on a wide array of immune cells, including lymphocytes, monocytes, macrophages, and dendritic cells (7). In the gut, this may be of particular interest in conditions like IBD and intestinal hypoxia with increased 5-HT secretion and concomitant inflammation.
We hypothesized that EC cells, based on their chemomechanoresponsive phenotype, would, similar to bronchopulmonary NEBs, be able to respond to different levels of oxygen. Furthermore, under conditions of elevated O2, we predicted that 5-HT production and secretion would be reduced compared with decreased O2 levels because tryptophan hydroxylase (TPH)1 transcription is positively regulated by HIF-1α (38). To test our hypothesis, we used isolated normal EC cells as well as the well-characterized EC cell model, KRJ-I, and examined the effects of an O2 gradient (0.5% O2→100% O2) on 5-HT production and secretion, focusing on signal pathway activation (HIF) in this process.
MATERIALS AND METHODS
Experimental approach.
Studies were performed on the continuously growing EC cell tumor-derived KRJ-I cells, a model for normal EC cells (6, 28), as well as isolated, short-term cultured human ileal EC cells isolated as described (27). HIF signaling pathway activation was measured using transient transfection with Renilla luciferase- hypoxia transcriptional response element (HRE) constructs in cells exposed to hypoxia, defined as 0.5% O2 (maximally responsive effective concentration; Ref. 24) and hyperoxia defined as 100% O2 for 30 and 120 min and compared with normoxia (defined as 20% O2 at the same time points). RT-PCR was performed to determine the HIF-1α downstream targets Glut1, IGF2, and VEGF. 5-HT secretion was measured under these conditions (ELISA), whereas signal pathway activation was assessed using Western blot protocols. These were performed to evaluate HIF-1α, OH-HIF, pTPH, pNF-κB/NF-κB, pERK/ERK, pAKT/AKT, catalytic PKA, pCREB, vesicle monoamine transporter type 1 (VMAT1), and chromogranin A (CgA).
Antibodies.
The following antibodies were used in the studies. pNF-κB (3033S), tot-NF-κB (4764S), pAkt (4058S), tot-Akt (4691S), PKA C-α (5842S), MAPK (4695S), pMAPK (4370S), pCREB (9198S), HIF-1α (3716), OH-HIF (3434), Rb IgG (7074S), Mouse IgG (7076S) were from Cell Signaling Technology. VMAT-1(58170) and pTPH-1(30574) were from Abcam. CgA1/2 (M0869) was from Dako. β-Actin (011M4793) was obtained from Sigma-Aldrich.
EC cell isolation and culture conditions.
Macroscopically “normal” tissue obtained from patients undergoing surgery for diverticulitis (n = 3) or colon cancer (colon: n = 4) (all tissue collected between 2009 and 2011 at Yale University, Department of Surgery and designated by the IRB as nonhuman subjects research). EC cells (>98% purity) were isolated by mucosal stripping, enzymatic digestion, and a combination of Nycodenz gradient fractionation and fluorescence-activated cell sorting as described (27). Approximately 1 × 106 cells were obtained per mucosal sample, a quantity sufficient for real-time PCR, short-term culture, and Western blots. For short-term culture, cells were maintained for <12 h (after isolation) in Quantum 263 complete tumor growth medium supplemented with penicillin (100 IU/ml) and streptomycin (100 μg/ml).
The EC cell tumor-derived cell line KRJ-I (33, 45) was maintained as floating cell aggregates in the same media as for normal cells. All experiments were performed without antibiotics; the tumor cell line was demonstrated to be mycoplasma free.
Hypoxic/hyperoxic treatment.
Hypoxic/hyperoxic conditions were induced using a modular incubator chamber (MIC-101; Billups-Rothenberg). Briefly, cultured KRJ-I cells (48 h) were transferred to the humidified hyperoxic chamber; the chamber was flushed with CO2 (0.5% O2) or 100% O2 for 4 min to maintain hypoxic/hyperoxic conditions. Cells were exposed to hypoxia/hyperoxia for 30 and 120 min, respectively.
RLU studies.
The Cignal HIF Pathway Reporter Assay Kit (LUC) (CCS-007L) was used to evaluate HIF signaling in isolated normal EC cells and in KRJ-I cells. Briefly, the basis of this protocol is transient transfection with a HIF-responsive luciferase construct that encodes the firefly luciferase reporter gene under the control of a minimal (m)CMV promoter and tandem repeats of HRE. This is designed to monitor the activity of HIF-regulated signal transduction pathways in cultured cells. Each reporter is premixed with constitutively expressing Renilla luciferase, which serves as an internal control for normalizing transfection efficiencies and monitoring cell viability. Short-term cultured EC cells (10,000/well) or KRJ-I cells (10,000/well) were transfected per protocol and exposed to hypoxia or hyperoxia for 30–120 min. O2-activated Renilla luciferase was measured using the dual luciferase assay (Glomax). The average maximum response per kit is four relative light units (RLU); in these experiments two RLU were identified. Exposure to normoxia was used as a control.
Knockdown studies.
The Ambion Silencer Select Validated siRNA approach (gene number 42840, Ambion) was used to evaluate HIF signaling in KRJ-I cells [200,000 cells/well in 6-well plates (Falcon, BD Biosciences)] (21). HIF-1α was silenced using the reverse transfection approach (12.5 pmol) and Lipofectamine RNAiMAX (Invitrogen). Knockdown was confirmed using PCR and Western blot after 24 and 48 h of incubation. Transfected cells (48 h) were exposed to hypoxia for 30 min, and serotonin release was measured. The average knockdown was 50% after 48 h. Exposure to normoxia was used as a control.
RT-PCR analyses.
RNA was extracted from isolated, short-term cultured EC cells (1 × 105, n = 4) or KRJ-I cells (1 × 106, n = 6) using TRIZOL (Invitrogen) and cleaned (Qiagen, RNeasy kit). After conversion to cDNA (High Capacity cDNA Archive Kit, Applied Biosystems) (27), quantitative RT-PCR analysis was performed using Assays-on Demand and the ABI 7900 Sequence Detection System (25, 26). Primer sets were all obtained from Applied Biosystems, and PCR mix on gels were performed to confirm presence of single bands for each primer set. PCR data were normalized using the ΔΔCT method; ALG9 was used as a housekeeping gene (29).
5-HT secretion analysis.
5-HT levels were analyzed using commercially available ELISA assays (5-HT: BA 10–0900; Rocky Mountain Diagnostics) as previously described (26, 33). Briefly, cells were seeded into six-well plates (Falcon, BD Biosciences) and stimulated with hypoxia/hyperoxia (30 and 120 min). Supernatant was immediately collected, and 5-HT concentration was evaluated according to the manufacturer's instructions.
Protein extraction.
KRJ-I cells (4 × 105 cells/ml) were seeded in six-well plates (Falcon, BD Biosciences) and stimulated with hypoxia/hyperoxia for 30 and 120 min. After cells were harvested, whole cell lysates were prepared by adding 200 μl of ice-cold cell lysis buffer [10 × RIPA lysis buffer (Millipore), complete protease inhibitor (Roche), phosphatase inhibitor set 1&2 (Calbiochem), 100 mM PMSF (Roche), 200 mM Na3VO4 (Acros Organics), 12.5 mg/ml SDS (American Bioanalytical)]. Tubes were centrifuged at 12,000 g for 20 min, and protein amount in the supernatant was quantified using the BCA protein assay kit (Thermo Fisher Scientific) (46).
Western blot analysis.
Total protein lysates (20 μg) were denaturated in SDS sample buffer, separated on a Tris-Glycine gel (10%) and transferred to an Immobilon P (PVDF) membrane (Millipore). After blocking (5% BSA for 60 min at room temperature), the membrane was incubated with primary antibodies in 5% BSA/PBS/Tween20 overnight at 4°C. The membranes were incubated with the horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology) for 60 min at room temperature and immunodetection was performed using the Western Lightning Plus-ECL (PerkinElmer). Blots were exposed on X-OMAT-AR films. The optical density of the appropriately sized bands was measured using ImageJ software (NIH). The ratio between phospho-protein and total protein was calculated, and total protein expression was reported relative to that of β-actin (Sigma-Aldrich) (46).
Statistics.
Results were expressed as means ± SE. All statistical analyses were performed using Prism 5 (GraphPad Software). Results were compared between control and stimulated cells using the Mann-Whitney test.
RESULTS
Assessment of HIF-1α and differential oxygen activation in EC cells.
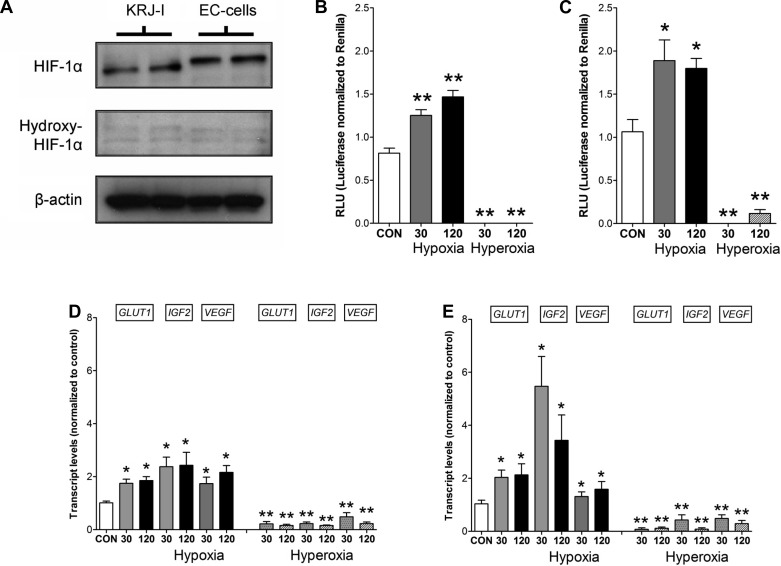
We initially examined normal EC cells and the EC cell tumor-derived cell line KRJ-I to determine baseline HIF-1α and hydroxy-HIF-1α expression and evaluate whether these cells express HIF-mediated oxygen-signaling pathways. Protein expression of HIF-1α (Fig. 1A) was identified in normal and KRJ-I cells. HIF-1α that can be activated was identified in both cell types, whereas the degradable, hydroxylated form hydroxy-HIF-1α was not expressed. An inducible HIF pathway was designed using transient transfection of normal and KRJ-I cells with a HIF-responsive firefly luciferase construct under HRE-transcription control. In normal EC cells (Fig. 1B), hypoxia (0.5% O2) resulted in significant activation (∼2-fold RLU) at both 30-min and 120-min exposure. KRJ-I cells responded in a similar fashion, (∼1.5-fold RLU) at both time points (Fig. 1C). Hyperoxia (100% O2) had no effect on luciferase production in either cell type. These results demonstrate intact HIF signaling pathways in both cell types and that activation of HIF signaling can be completely blocked by high levels of O2. An analysis of the HIF-1α downstream targets Glut1 (∼2-fold, P < 0.05), IGF2 (∼2–4-fold, P < 0.05), and VEGF (∼2-fold, P < 0.05) identified that these were significantly elevated in both normal EC cells (Fig. 1D) and in KRJ-I cells during hypoxia (Fig. 1E).
Fig. 1.
Protein levels of hydroxy-hypoxia-inducible factor (HIF)-1α, HIF-1α, and β-actin in KRJ-I and enterochromaffin (EC) cells identify an intact HIF-1α in these cells (A). Transient transfection with Renilla luciferase-encoding constructs identified activation of luciferase (RLU) in normal EC cells (B) and KRJ-I cells (C) under hypoxic conditions. Oxygen inhibited RLU formation. Downstream HRE targets including Glut1, IGF2, and VEGF were elevated by hypoxia and significantly decreased by hyperoxia at 30 and 120 min in normal (D) and KRJ-I cells (E). CON, cells under normoxic conditions. Means ± SE, n = 4–6, *P < 0.01, **P < 0.05.
Differential oxygen exposure and HIF-1α/OH-HIF.
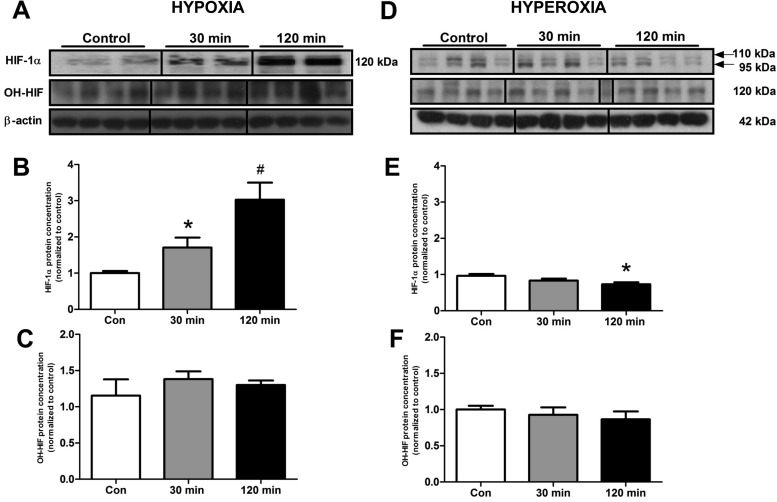
After demonstrating an intact HIF signaling pathway using luciferase constructs, we next evaluated whether variations in O2 altered protein levels of HIF. Hypoxia was associated with an increase in HIF-1α expression [30 min: 1.7 ± 0.3, P < 0.001; 120 min: 3.0 ± 0.47, P < 0.0001 (fold expression compared with normoxia)] (Fig. 2, A–B). In contrast, cells subjected to elevated O2 levels exhibited a different HIF-1α phenotype. Instead of one band at 120 kDa, there were two bands identified, one at 110 kDa, the second at 95 kDa (Fig. 2D). When these were quantitated, a significant decrease in expression was noted compared with normoxia at 120 min (0.73 ± 0.05, P < 0.01). As anticipated, levels of HIF-1α were significantly elevated at 30 min (P < 0.005) and 120 min (P < 0.0001) by hypoxia compared with hyperoxia. Interestingly, no significant alterations were noted in the expression of the hydroxylated form of HIF (OH-HIF) (Fig. 2, C and F) in response to differential O2 exposure.
Fig. 2.
A–C: hypoxia. D–F: hyperoxia. Protein levels of HIF-1α and OH-HIF in KRJ-I cells, in response to different O2 levels. There was a significant increase of HIF-1α protein after decreasing O2 saturation (30 min, 120 min). After increasing O2 saturation, there was a significant decrease of HIF-1α at 120 min of hyperoxia. No changes in OH-HIF levels were identified. Means ± SE, n = 4–18, *P < 0.006, #P < 0.0001.
Differential oxygen exposure, phosphorylated (activated) TPH1, and 5-HT release.
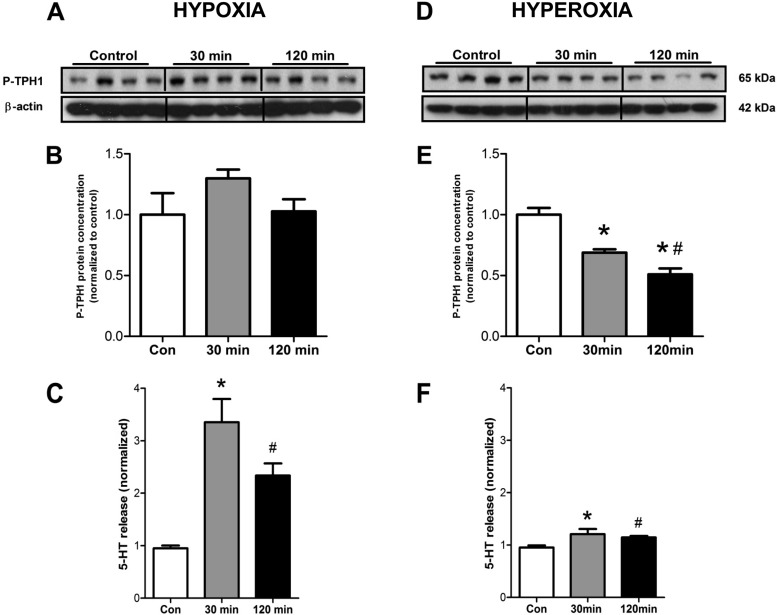
We next evaluated whether the rate-limiting enzyme in 5-HT synthesis, TPH1, was activated by alterations in O2. Hypoxia tended to induce phosphorylation and activation of TPH1 (Fig. 3, A–B), whereas increasing O2 significantly reduced phosphorylation of TPH1 compared with normoxia (30 min: 0.7 ± 0.03, P < 0.0005; 120 min: 0.5 ± 0.05, P < 0.0005) (Fig. 3, D–E). In addition, pTPH was further decreased at 120 min compared with 30 min (P < 0.01). Comparing the two groups confirmed that hyperoxia was associated with decreased pTPH at both 30 min (P < 0.005) and 120 min (P < 0.005). This indicates that EC cells respond to low O2 levels with activation of TPH1, a critical enzyme for 5-HT synthesis. Decreasing O2 to 0.5% elevated levels of 5-HT after 30 min (3.2 ± 0.4, P < 0.005), as well as at 120 min (2.2 ± 0.2, P < 0.01) compared with normoxic-mediated secretion (Fig. 3C). Increasing O2 to 100% also elevated levels of 5-HT after 30 min (1.2 ± 0.07, P < 0.001), as well as after 120 min (1.1 ± 0.06, P < 0.01), but these levels were not as marked as in response to hypoxia (Fig. 3F). Comparing each time point identified that release was significantly lower at both 30 min (P < 0.0001) and 120 min hyperoxia (P < 0.05) compared with hypoxic conditions, suggesting that EC cell 5-HT secretion is most sensitive to low O2 concentrations.
Fig. 3.
Protein levels of phosphorylated tryptophan hydroxylase 1 (TPH1) and total TPH1 in KRJ-I cells and serotonin (5-HT) secretion, in response to different O2 levels. Hypoxia was associated with a trend to an increase in P-TPH1 (A and B) and an elevation in secreted 5-HT at both 30 and 120 min (C). Elevating O2, in contrast, resulted in a significant decrease in P-TPH1 (30 min, 120 min) (#D and E). 5-HT secretion, however, did still occur at levels 20% above control during hyperoxia (F). Means ± SE, n = 4–8, *P < 0.003, #P = 0.05.
Effect of HIF-1α knockdown on normoxic and hypoxia-mediated 5-HT release.
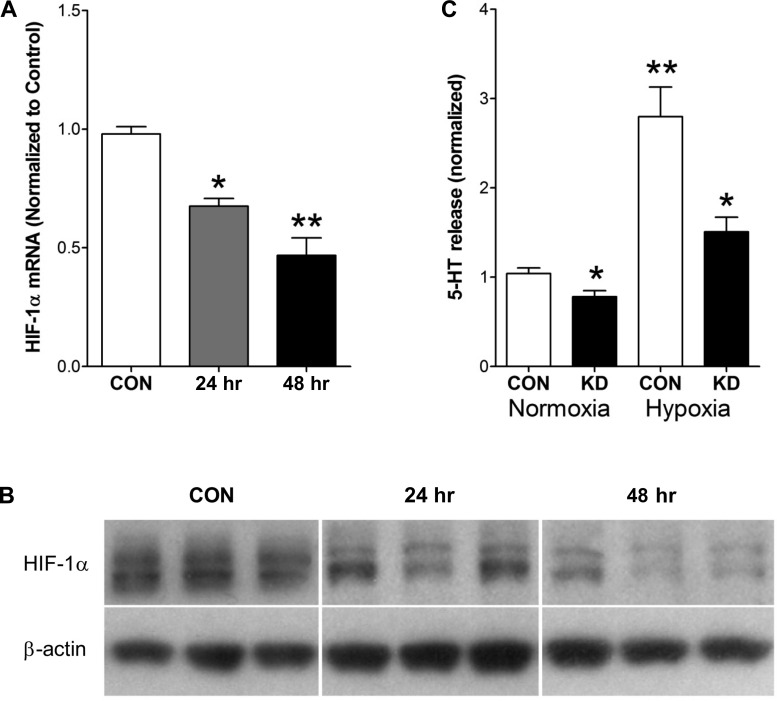
To examine the relevance of HIF-1α to serotonin release, we evaluated whether knocking down gene expression affected serotonin release under hypoxic conditions. The Ambion approach effectively reduced HIF-1α mRNA (Fig. 4A) and protein (Fig. 4B) at 24 and particularly 48 h. Examination of 5-HT release identified that basal release was significantly lower (∼75%, P < 0.05) under normoxic conditions, whereas the normal secretory response to 0.5% hypoxia was reduced approximately twofold (P < 0.01) by knockdown (Fig. 4C).
Fig. 4.
Transcripts, protein, and 5-HT release in response to HIF-1α knockdown. Knockdown (siRNA silencing) effectively reduced HIF-1α mRNA (A) and protein (B) at both 24 and 48 h. Basal 5-HT release was significantly lower during normoxia. Under hypoxic conditions, 5-HT secretion was reduced ∼2-fold (C). Means ± SE, n = 3–5, *P < 0.05 vs. control. **P < 0.01 vs. normoxia. KD, knockdown.
Differential oxygen sensing, 5-HT vesicular accumulation, and granule formation.
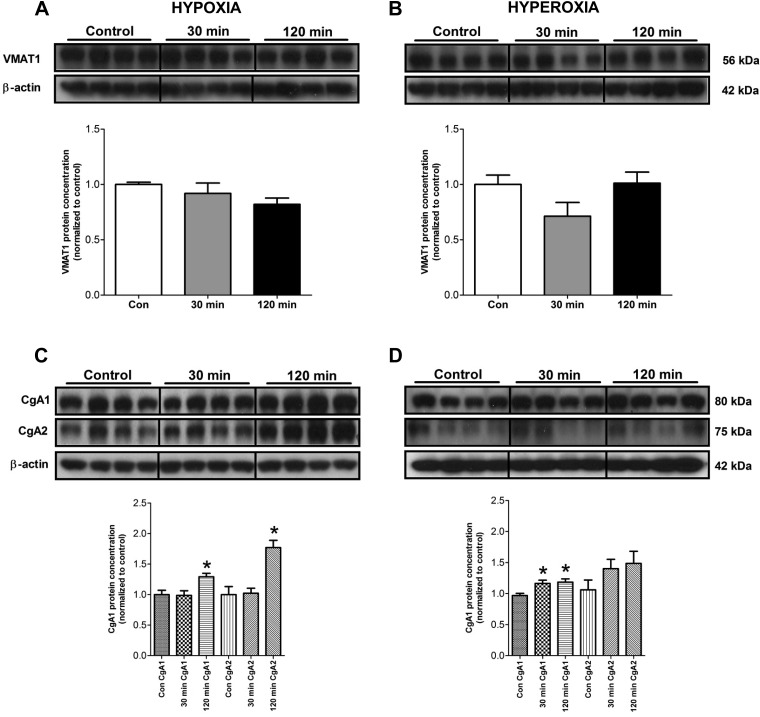
We next investigated the effects of altering O2 levels on VMAT1 and CgA expression. VMAT-1 functions to accumulate cytosolic monoamines, like 5-HT, into secretory vesicles (16), whereas CgA is important for granulogenesis and secretion of amines and peptides in neuroendocrine cells (33). Neither hypoxia nor hyperoxia had any effect on VMAT1 (Fig. 5, A–B). In contrast, significant alterations were noted in CgA. At 120 min, hypoxia was associated with significant increases in the two major CgA band sizes (75–80 kDa; 1.3 ± 0.06, P < 0.05 and 1.8 ± 0.12, P < 0.05, respectively) (Fig. 5C). High O2 levels were associated with increased expression of the major 75-kDa band (30 min: 1.17 ± 0.05, P < 0.05; 120 min: 1.19 ± 0.05, P = 0.01) (Fig. 5D). This indicates that EC cell O2 sensing is not associated with alterations in 5-HT vesicular accumulation but with translation and processing of CgA, an important component of the neuroendocrine secretory pathway.
Fig. 5.
Protein levels of vesicle monoamine transporter type 1 (VMAT1) and chromogranin A (CgA) (both major bands, CgA1 and CgA2) in KRJ-I cells, in response to different O2 levels. VMAT1 protein expression remained unchanged during both hypoxia and hyperoxia (A and B). In contrast, there was a significant increase in CgA1 and CgA2 protein levels after 120 min of hypoxia (C). Hyperoxia induced a small but significant increase in CgA1, whereas CgA2 did not change significantly (D). Means ± SE, n = 4–8, *P < 0.04 compared with control.
Identification of O2-dependent activation of non-HIF-signaling pathways.
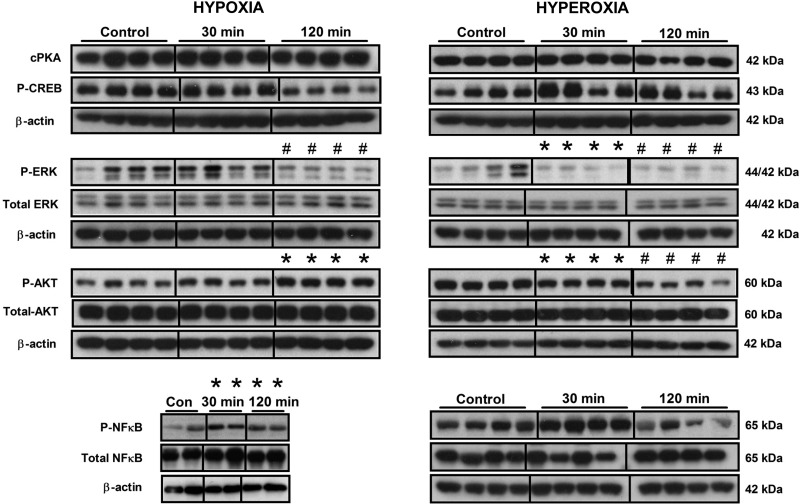
Having demonstrated an intact HIF signaling pathway in EC cells that was associated with 5-HT, we next analyzed other signaling pathways to determine whether O2 sensing activated other pathways associated with 5-HT secretion. These include CREB, MAPK, AKT, and NF-κB. PKA activation is associated with pCREB signaling, a pathway activated in EC cell neoplasia (15). Exposure to alterations in O2 levels had no significant effects on PKA (Fig. 6). In contrast, hyperoxia was associated with a decrease in phosphorylated CREB at 120 min (0.53 ± 0.04, P < 0.03). These data indicate that a cAMP-regulated PKA/pCREB pathway is not activated by decreases in O2. MAPK (ERK) plays a critical role in regulating 5-HT secretion (6, 28). Hypoxia did not affect ERK phosphorylation at 30 min but significantly reduced it at 120 min (0.6 ± 0.06, P < 0.001) compared with normoxia (Fig. 6). Hyperoxia, in contrast, significantly reduced phosphorylated ERK at both time points (30 min: 0.17 ± 0.07, P = 0.001; 120 min: 0.20 ± 0.08, P < 0.005). In addition, ERK activation was significantly decreased by hyperoxia at 30 min (P < 0.001) and 120 min (P = 0.005) compared with hypoxia. No significant alterations were noted for total ERK. This indicates that EC cells respond to high O2 levels with inhibition of ERK signaling. Activation of phosphatidylinositol 3-kinase/AKT signaling is also associated with hypoxia (5). In EC cells, hypoxia was associated with significant AKT phosphorylation at 120 min (1.6 ± 0.10, P < 0.005) compared with normoxia (Fig. 6). Increased O2 levels were associated conversely with a decrease in phosphorylated AKT levels (30 min: 0.76 ± 0.04, P < 0.0001; 120 min: 0.55 ± 0.05, P < 0.0001). In addition, there was a significant decrease between 30 min and 120 min (P < 0.05) during hyperoxia. Comparing the two conditions (low vs. high O2) confirmed that hyperoxia was associated with decreased pAKT at all time points. No significant alterations were noted for total AKT. This indicates that this pathway is activated in EC cells by alterations in O2; a response similar to chemoresistant tumor cell lines (5). Finally, we focused on the NF-κB, a pathway associated with hypoxia-mediated 5-HT synthesis (3). Hypoxia, as expected, was associated with an elevation in phosphorylated NF-κB levels compared with normoxia (30 min: 1.24 ± 0.07, P = 0.001; 120 min: 1.59 ± 0.24, P < 0.05) (Fig. 6). Hyperoxia was not associated with any sustained alterations. Comparing the two groups identified that pNF-κB was significantly lowered by 120 min of hyperoxia (P < 0.05). No significant alterations were noted for total NF-κB for any of the conditions. These data indicate that EC cells respond to alterations in O2 levels with NF-κB signaling, an effect most noticeable at low O2 levels.
Fig. 6.
Protein levels of alternate signaling pathways in response to different O2 exposure. No significant differences in catalytic (c)PKA were identified in cells subject to hypoxic or hyperoxic exposure (30 min, 120 min). A significant decrease in P-CREB protein levels was identified after 120 min of decreased O2 saturation. Decreasing the O2 resulted in a decrease in P-ERK at 120 min, but increasing O2 significantly decreased pERK at both time points. No changes in total ERK levels were identified. Reducing O2 significantly increased pAKT (120 min), but an elevated O2 was associated with a significant decrease in pAKT (30 min, 120 min). No changes in total AKT levels were identified. There was a significant increase in P-NF-κB after decreasing O2 (30 min, 120 min). No changes in total NF-κB levels were identified. Means ± SE, n = 4–16, *P < 0.01, #P < 0.05.
DISCUSSION
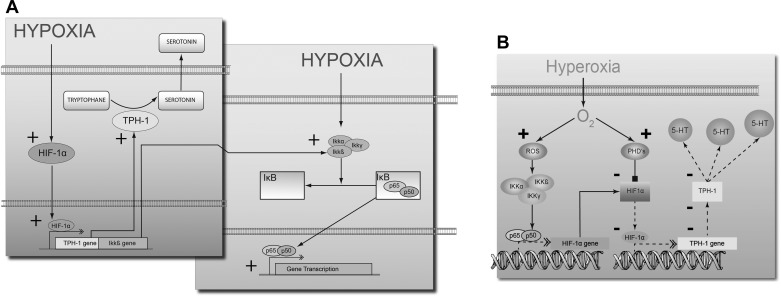
In this study, we demonstrate that the neuroendocrine EC cell, a cell type known for its chemomechanosensory role in gut function, is also, similar to pulmonary neuroepithelial 5-HT-secreting cells (13), an O2 sensor that can respond with increased hypoxia-related intracellular signaling and 5-HT secretion during exposure to hypoxia. These responses included a direct HRE-mediated response and transcription of known downstream targets (GLUT1, IGF2, and VEGF), activation of pNF-κB, and pAKT, as well as increased production of CgA. Increasing O2 to 100% inhibited HRE-mediated signaling, transcription, reduced 5-HT secretion (compared to hypoxia) and significantly lowered HIF-1α levels (∼75% of control). These data indicate that the gut EC cell can metabolically adapt to changes in the oxygen environment (Fig. 7).
Fig. 7.
Cartoon describing differential O2 sensing in EC cells and signal pathway/5-HT secretion. Under hypoxic conditions (A), a significant increase in 5-HT secretion and production occurs via a direct HIF-1α-mediated mechanism. Activation of NF-κB may also occur via cross-activation of this pathway. Hyperoxic conditions resulted in an O2 dependent prolyl hydroxylase (PHD)-mediated degradation of HIF-1α, which lead to a decrease in TPH1 transcription and translation (B). With less TPH1, the rate-limiting enzyme in 5-HT production and 5-HT synthesis will decrease. Solid lines and + reflect activation. Dotted lines reflect a decreased effect and -, inhibition. ROS, reactive oxygen species.
Under normal conditions, the intestinal mucosa is subject to a “physiological hypoxia” (45), and mucosal cells adapt to a steadily changing O2 supply, e.g., increased blood supply during digestion. The canonical regulator of hypoxic responses is HIF-1α (43), a heterodimer consisting of a constantly expressed β-subunit, and an oxygen-regulated α-subunit. Under normoxic conditions, the α-subunit of HIF-1 is marked for degradation by O2-dependent prolyl hydroxylases (PHDs) (11), whereas, in hypoxia, HIF-1α escapes degradation (42). In the present study, HIF-1α was significantly increased under hypoxic conditions. Interestingly, we noted two separate bands (∼95 and 110 kDa) in cells exposed to hyperoxia in contrast to a single, larger band in hypoxia. Wenger et al. (50) demonstrated that mouse HIF-1α can exist as two different mRNA isoforms, one being ubiquitously expressed, the other being tissue specific (50); different isoforms have also been identified in humans (14). It is noteworthy that the different isoforms are regulated in distinct manners and that effects on downstream genes can differ (41). Our data indicate that HIF-1α isoforms are present in gut EC cells and that oxygen may regulate expression.
To evaluate whether O2 exposure regulated HIF signaling, we examined HRE-mediated luciferase production in transiently infected EC cells. Hypoxia was associated with luciferase production; hyperoxia inhibited expression. This suggests that hypoxia is a physiological process in EC cells and that reducing O2 exposure stimulates this pathway. Conversely, elevated O2 exposure completely abrogates HRE-mediated signaling presumably via a PHD-mediated degradation. To further evaluate HRE signaling, we examined expression of a subset of the 40 genes that have been identified with HREs in their promoter regions. The known HIF-1α downstream target genes Glut1, IGF2, and VEGF were all upregulated by hypoxia in EC cells, confirming that O2 sensing results in HRE-mediated transcription. Interestingly, hyperoxia was associated with a decreased transcription of these targets, consistent with a loss of HRE-mediated signaling.
An HRE has also been identified in the promoter region for TPH1 (38, 40), and under hypoxic conditions (1% O2) TPH1 is strongly upregulated (38), suggesting that HIF-1α may directly drive 5-HT production in cells subject to hypoxia (38). Our measurements of 5-HT secretion confirmed that hypoxia was associated with 5-HT secretion. Given that there was no significant reduction in cell viability (MTT uptake/GLOMAX live-dead assay, data not shown) following exposure to different O2 levels, 5-HT release appears to be a physiological consequence of O2 sensing, particularly under conditions of hypoxia. The direct relevance of HIF-1α to 5-HT release was confirmed by the silencing studies. Knockdown reduced both basal secretion (by ∼25%), whereas the hypoxic response was reduced twofold.
Interestingly, we noted an increase in 5-HT secretion in cells exposed to hyperoxia; levels were, however, substantially lower than when cells were exposed to anoxic conditions and could reflect metabolic function. These findings were supported by pTPH, the rate-limiting enzyme in 5-HT production; levels were significantly lower at 30 as well as 120 min in cells exposed to high O2 levels compared with hypoxic conditions. In colorectal surgery, use of intraoperative oxygen reduces 5-HT levels in plasma and platelets, with a reduction in postoperative GI symptoms (35). We postulate that this effect may be indirectly mediated by the EC cell.
Additional analysis of the EC cell secretory machinery identified no significant alteration in VMAT1 (a 5-HT transporter involved in 5-HT uptake into secretory vesicles, Ref. 16), but CgA was significantly increased, particularly by hypoxia. CgA plays an important role in granulogenesis and secretion in neuroendocrine cells (33), and activation presumably identifies de novo regulation of the neuroendocrine secretory pathway, further confirming a role for hypoxia in 5-HT secretion.
Although HIF-1α represents the predominant cellular response to hypoxia, we examined other potential signaling pathways to evaluate whether they played a role in oxygen sensing and 5-HT release. In the present studies, PKA and pCREB were not significantly associated with 5-HT synthesis and release. In contrast, signaling via MAPK and AKT were differentially altered by O2 levels. Phosphorylation of ERK was significantly reduced by both low and high O2, an effect most noted at hyperoxia. In lung epithelial cells subjected to hyperoxia, several investigators have identified ERK activation as well as the fact that this activation protects against hyperoxia-induced cell death (4, 36, 49). We identified a decrease in pERK, indicating that oxygen sensing in EC cells is not associated with the ERK pathway. In contrast, AKT was differentially regulated, increased at low O2 levels, and decreased at high levels. In a study by Truong et al. (44), low O2 levels were associated with an increase in pAKT, whereas increased O2 resulted in a decrease in pAKT, responses similar to the gut EC cells. AKT signaling therefore may be a measure of O2 sensing in these cells although the multifactorial role of AKT in HIF-1 stabilization, i.e., both oxygen dependent and independent, should be recognized (44). The AKT response may also reflect a cellular response to oxygen.
We also examined the NF-κB signaling pathway, as this is activated under changing O2 conditions (11, 12). NF-κB is also thought to be a master regulator in inflammatory responses, including in the gut (37). NF-κB is activated by reactive oxygen species (ROS) (30) and is inhibited, interestingly, in the same manner as HIF-1α, by hydroxylation of PHDs (11). In the present study, we noted a significant increase in pNF-κB levels under hypoxic conditions, indicating that the inhibitory effect of PHDs on NF-κB activation does not occur in the EC cell. However, we noted a significant increase of pNF-κB also after 30 min of hyperoxia with no difference at 120 min. We interpret this as dynamic responses to increased ROS levels, which are known to be powerful NF-κB activators. In lung epithelia, NF-κB protects against hyperoxic cell death. Whereas ROS is known to induce cell death via apoptosis, NF-κB could protect cells via glutathione (39) and manganese superoxide dismutase (19), which both have antioxidant effects and directly protect against ROS.
In conclusion, we have demonstrated that gut EC cells are oxygen responsive. Alterations in O2 levels differentially activate HIF-1α and NF-κB signaling as well as TPH1. This results in alterations in 5-HT production and secretion. Localized hypoxia occurs as a result of chronic inflammation in the gut during pathophysiological processes including IBD (48). Given the relevance of 5-HT in the etiopathology of this complex disease (TPH-1 knockout mice respond to chemically-induced colitic agents with a less severe phenotype and delayed onset of disease compared with wild-type mice treated in the same protocol, Ref. 20), it appears likely that hypoxic sensing via HIF-1α plays a role in EC cell responses. This is strengthened by observations that the majority (∼90%) of EC cells in hypoxic mucosa (e.g., Crohn's disease) exhibit HIF-1α protein (unpublished observations). In addition, as 5-HT is a potent modulator of the immune system, e.g., innate-sensing dendritic cells express 5-HTR3,4,7, the activation of which regulates IL16 (34) and IL8 secretion (23), EC cell-mediated signaling likely plays a significant role as an inducer of inflammation during intestinal hypoxia. As hypoxia is associated with increased adenosine release (18) as well as stabilization of HIF-1α and ATP-mediated responsiveness (42), and EC cell 5-HT release is regulated in this milieu (6), it is likely that this cell type may be a canonical sensing system during gut dysfunction.
GRANTS
These studies were funded by NIH R01DK080871 and Kontaktutvalget at St Olavs University Hospital and Faculty of Medicine, NTNU, Trondheim, Norway.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.H., R.D., B.S., B.I.G., and M.K. conception and design of research; M.H., R.D., B.S., and M.K. performed experiments; M.H., R.D., B.S., B.I.G., and M.K. analyzed data; M.H., R.D., B.S., B.I.G., R.P., I.M.M., and M.K. interpreted results of experiments; M.H., R.D., B.S., and M.K. prepared figures; M.H., R.D., B.I.G., R.P., and M.K. drafted manuscript; M.H., R.D., B.S., B.I.G., R.P., I.M.M., and M.K. edited and revised manuscript; M.H., R.D., B.S., B.I.G., R.P., I.M.M., and M.K. approved final version of manuscript.
REFERENCES
- 1. Bearcroft CP, Perrett D, Farthing MJ. Postprandial plasma 5-hydroxytryptamine in diarrhoea predominant irritable bowel syndrome: a pilot study. Gut 42: 42–46, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bishop AE, Pietroletti R, Taat CW, Brummelkamp WH, Polak JM. Increased populations of endocrine cells in Crohn's ileitis. Virchows Arch A Pathol Anat Histopathol 410: 391–396, 1987 [DOI] [PubMed] [Google Scholar]
- 3. Brønstad M, Pfragner R, Gustafsson B, Kidd M, Solligard E. Hypoxia and gut neuroendocrine cells: the role of HIF-1α, NfκB and serotonin production from enterochromaffin cells in intestinal ischemia. Gastroenterology 140: S698; Mo2010, 2011 [Google Scholar]
- 4. Buckley S, Driscoll B, Barsky L, Weinberg K, Anderson K, Warburton D. ERK activation protects against DNA damage and apoptosis in hyperoxic rat AEC2. Am J Physiol Lung Cell Mol Physiol 277: L159–L166, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Bussink J, van der Kogel AJ, Kaanders JH. Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol 9: 288–296, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Chin ASB, Gustafsson BI, Granlund AvB, Sandvik AK, Timberlake A, Sumpio B, Pfragner R, Modlin IM, Kidd M. The role of mechanical forces and adenosine in the regulation of intestinal enterochromaffin cell serotonin secretion. Am J Physiol Gastrointest Liver Physiol 302: G397–G405, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cloez-Tayarani I, Changeux JP. Nicotine and serotonin in immune regulation and inflammatory processes: a perspective. J Leukoc Biol 81: 599–606, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 126: 1657–1664, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Coleman NS, Foley S, Dunlop SP, Wheatcroft J, Blackshaw E, Perkins AC, Singh G, Marsden CA, Holmes GK, Spiller RC. Abnormalities of serotonin metabolism and their relation to symptoms in untreated celiac disease. Clin Gastroenterol Hepatol 4: 874–881, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol 7: 281–287, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci USA 103: 18154–18159, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cummins EP, Taylor CT. Hypoxia-responsive transcription factors. Pflügers Arch 450: 363–371, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Cutz E, Yeger H, Pan J. Pulmonary neuroendocrine cell system in pediatric lung disease-recent advances. Pediatr Dev Pathol 10: 419–435, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Depping R, Hagele S, Wagner KF, Wiesner RJ, Camenisch G, Wenger RH, Katschinski DM. A dominant-negative isoform of hypoxia-inducible factor-1 alpha specifically expressed in human testis. Biol Reprod 71: 331–339, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Drozdov I, Svejda B, Gustafsson B, Mane S, Pfragner R, Kidd M, Modlin I. Gene network inference and biochemical assessment delineates GPCR pathways and CREB targets in small intestinal neuroendocrine neoplasia. Plos One 6: e22457, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eiden LE, Schafer MK, Weihe E, Schutz B. The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pflügers Arch 447: 636–640, 2004 [DOI] [PubMed] [Google Scholar]
- 17. El-Salhy M, Danielsson A, Stenling R, Grimelius L. Colonic endocrine cells in inflammatory bowel disease. J Intern Med 242: 413–419, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Eltzschig HK. Adenosine: an old drug newly discovered. Anesthesiology 111: 904–915, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Franek WR, Morrow DM, Zhu H, Vancurova I, Miskolci V, Darley-Usmar K, Simms HH, Mantell LL. NF-kappaB protects lung epithelium against hyperoxia-induced nonapoptotic cell death-oncosis. Free Radic Biol Med 37: 1670–1679, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Ghia JE, Li N, Wang H, Collins M, Deng Y, El-Sharkawy RT, Cote F, Mallet J, Khan WI. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 137: 1649–1660, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Guadall A, Orriols M, Rodriguez-Calvo R, Calvayrac O, Crespo J, Aledo R, Martinez-Gonzalez J, Rodriguez C. Fibulin-5 is up-regulated by hypoxia in endothelial cells through a hypoxia-inducible factor-1 (HIF-1alpha)-dependent mechanism. J Biol Chem 286: 7093–7103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hatoum OA, Binion DG, Gutterman DD. Paradox of simultaneous intestinal ischaemia and hyperaemia in inflammatory bowel disease. Eur J Clin Invest 35: 599–609, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Idzko M, Panther E, Stratz C, Muller T, Bayer H, Zissel G, Durk T, Sorichter S, Di Virgilio F, Geissler M, Fiebich B, Herouy Y, Elsner P, Norgauer J, Ferrari D. The serotoninergic receptors of human dendritic cells: identification and coupling to cytokine release. J Immunol 172: 6011–6019, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol Cell Physiol 271: C1172–C1180, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Kidd M, Eick G, Shapiro MD, Camp RL, Mane SM, Modlin IM. Microsatellite instability and gene mutations in transforming growth factor-beta type II receptor are absent in small bowel carcinoid tumors. Cancer 103: 229–236, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Kidd M, Eick GN, Modlin IM, Pfragner R, Champaneria MC, Murren J. Further delineation of the continuous human neoplastic enterochromaffin cell line, KRJ-I, and the inhibitory effects of lanreotide and rapamycin. J Mol Endocrinol 38: 181–192, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Kidd M, Gustafsson BI, Drozdov I, Modlin IM. IL1beta- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn's disease. Neurogastroenterol Motil 21: 439–450, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kidd M, Modlin IM, Gustafsson BI, Drozdov I, Hauso O, Pfragner R. Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am J Physiol Gastrointest Liver Physiol 295: G260–G272, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Kidd M, Nadler B, Mane S, Eick G, Malfertheiner M, Champaneria M, Pfragner R, Modlin I. GeneChip, geNorm, and gastrointestinal tumors: novel reference genes for real-time PCR. Physiol Genomics 30: 363–370, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Kretz-Remy C, Mehlen P, Mirault ME, Arrigo AP. Inhibition of I kappa B-alpha phosphorylation and degradation and subsequent NF-kappa B activation by glutathione peroxidase overexpression. J Cell Biol 133: 1083–1093, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kyosola K, Penttila O, Salaspuro M. Rectal mucosal adrenergic innervation and enterochromaffin cells in ulcerative colitis and irritable colon. Scand J Gastroenterol 12: 363–367, 1977 [DOI] [PubMed] [Google Scholar]
- 32. Leon-Ponte M, Ahern GP, O'Connell PJ. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood 109: 3139–3146, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A–biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol 17: 2427–2443, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Muller T, Durk T, Blumenthal B, Grimm M, Cicko S, Panther E, Sorichter S, Herouy Y, Di Virgilio F, Ferrari D, Norgauer J, Idzko M. 5-hydroxytryptamine modulates migration, cytokine and chemokine release and T-cell priming capacity of dendritic cells in vitro and in vivo. PLoS One 4: e6453, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ochmann C, Tuschy B, Beschmann R, Hamm F, Rohm KD, Piper SN. Supplemental oxygen reduces serotonin levels in plasma and platelets during colorectal surgery and reduces postoperative nausea and vomiting Eur J Anaesthesiol 27: 1036–1043, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Parinandi NL, Kleinberg MA, Usatyuk PV, Cummings RJ, Pennathur A, Cardounel AJ, Zweier JL, Garcia JG, Natarajan V. Hyperoxia-induced NAD(P)H oxidase activation and regulation by MAP kinases in human lung endothelial cells. Am J Physiol Lung Cell Mol Physiol 284: L26–L38, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Pasparakis M. IKK/NF-kappaB signaling in intestinal epithelial cells controls immune homeostasis in the gut. Mucosal Immunol 1, Suppl 1: S54–S57, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Pocock R, Hobert O. Hypoxia activates a latent circuit for processing gustatory information in C. elegans. Nat Neurosci 13: 610–614, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rahman I, Mulier B, Gilmour PS, Watchorn T, Donaldson K, Jeffery PK, MacNee W. Oxidant-mediated lung epithelial cell tolerance: the role of intracellular glutathione and nuclear factor-kappaB. Biochem Pharmacol 62: 787–794, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Rahman MS, Thomas P. Molecular cloning, characterization and expression of two tryptophan hydroxylase (TPH-1 and TPH-2) genes in the hypothalamus of Atlantic croaker: down-regulation after chronic exposure to hypoxia. Neuroscience 158: 751–765, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Ramanathan M, Luo W, Csoka B, Hasko G, Lukashev D, Sitkovsky MV, Leibovich SJ. Differential regulation of HIF-1alpha isoforms in murine macrophages by TLR4 and adenosine A(2A) receptor agonists. J Leukoc Biol 86: 681–689, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Semenza GL. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 107: 1–3, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Semenza GL. Life with oxygen. Science 318: 62–64, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci 5: 437–448, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Shepherd AP. Metabolic control of intestinal oxygenation and blood flow. Fed Proc 41: 2084–2089, 1982 [PubMed] [Google Scholar]
- 46. Svejda B, Kidd M, Kazberouk A, Lawrence B, Pfragner R, Modlin IM. Limitations in small intestinal neuroendocrine tumor therapy by mTor kinase inhibition reflect growth factor-mediated PI3K feedback loop activation via ERK1/2 and AKT. Cancer 17: 4141–4154, 2011 [DOI] [PubMed] [Google Scholar]
- 47. Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med 85: 1295–1300, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med 85: 1295–1300, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Truong SV, Monick MM, Yarovinsky TO, Powers LS, Nyunoya T, Hunninghake GW. Extracellular signal-regulated kinase activation delays hyperoxia-induced epithelial cell death in conditions of Akt downregulation. Am J Respir Cell Mol Biol 31: 611–618, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Wenger RH, Rolfs A, Spielmann P, Zimmermann DR, Gassmann M. Mouse hypoxia-inducible factor-1alpha is encoded by two different mRNA isoforms: expression from a tissue-specific and a housekeeping-type promoter. Blood 91: 3471–3480, 1998 [PubMed] [Google Scholar]
- 51. Wheeler EE, Challacombe DN. Quantification of enterochromaffin cells with serotonin immunoreactivity in the duodenal mucosa in coeliac disease. Arch Dis Child 59: 523–527, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]