Abstract
Monocarboxylate transporter isoform-1 (MCT1) plays an important role in the absorption of short-chain fatty acids (SCFAs) in the colon. Butyrate, a major SCFA, serves as the primary energy source for the colonic mucosa, maintains epithelial integrity, and ameliorates intestinal inflammation. Previous studies have shown substrate (butyrate)-induced upregulation of MCT1 expression and function via transcriptional mechanisms. The present studies provide evidence that short-term MCT1 regulation by substrates could be mediated via a novel nutrient sensing mechanism. Short-term regulation of MCT1 by butyrate was examined in vitro in human intestinal C2BBe1 and rat intestinal IEC-6 cells and ex vivo in rat intestinal mucosa. Effects of pectin feeding on MCT1, in vivo, were determined in rat model. Butyrate treatment (30–120 min) of C2BBe1 cells increased MCT1 function {p-(chloromercuri) benzene sulfonate (PCMBS)-sensitive [14C]butyrate uptake} in a pertussis toxin-sensitive manner. The effects were associated with decreased intracellular cAMP levels, increased Vmax of butyrate uptake, and GPR109A-dependent increase in apical membrane MCT1 level. Nicotinic acid, an agonist for the SCFA receptor GPR109A, also increased MCT1 function and decreased intracellular cAMP. Pectin feeding increased apical membrane MCT1 levels and nicotinate-induced transepithelial butyrate flux in rat colon. Our data provide strong evidence for substrate-induced enhancement of MCT1 surface expression and function via a novel nutrient sensing mechanism involving GPR109A as a SCFA sensor.
Keywords: SCFA absorption, cyclic AMP, GPR109A
short-chain fatty acids (SCFAs) acetate, propionate, and butyrate are produced in the colonic lumen by bacterial fermentation of dietary fiber. Of these SCFAs, butyrate plays a prominent role to serve as primary fuel for the colonocytes, and its oxidation is critical to various important metabolic processes in the colon (7). It is believed that a major consequence of reduction in intracellular SCFA oxidation results in metabolic starvation and mucosal atrophy (7). Besides its role as energy nutrient, butyrate is also vital in maintaining health and integrity of colonic mucosa by virtue of its multiple beneficial effects (4, 12, 22), such as its ability to improve barrier function and ameliorate intestinal inflammation. However, the ability of butyrate to exert many of these cellular effects is concentration dependent and the absorption of luminal butyrate is critical for the health benefits it confers to the intestinal mucosa (33, 34).
We and others have shown that monocarboxylate transporter-1 (MCT1) plays an important role in the absorption of luminal SCFAs by the colonocytes (11, 28). Despite controversies regarding apical versus basolateral localization of MCT1 in various species (9, 14), recent studies utilizing specific inhibitors of MCT1 have established its role in luminal SCFA absorption. Its importance in luminal butyrate absorption has also been emphasized in recent studies demonstrating downregulation of MCT1 in diseases involving decreased SCFA absorption and metabolism (33, 34).
Besides the intracellular effects, recent discovery of G protein-coupled receptors for SCFAs suggested that multiple effects of SCFAs in various tissues might also be mediated extracellularly via activation of the receptors without entering into the cells (3, 18). Sensing of SCFAs in different tissues may be mediated by GPCRs, GPR41, and GPR43, which have been identified as SCFA receptors by the agonist's selectivity in gene-transfected mammalian cells (3, 18). GPR41 is abundantly expressed in adipose tissues, whereas GPR43 is predominantly expressed in immune cells (3, 18). Recently, the localization of both GPR43 and GPR41 on L-type enteroendocrine cells in rat colon has been demonstrated (31). However, whether SCFA effects on various colonic epithelial functions are mediated by the GPCR activation remains to be elucidated. On the other hand, recent studies provided evidence that butyrate may elicit effects on colonocyte functions extracellularly by serving as a ligand for GPR109A (also known as PUMA-G in mice and HM74A in humans), a receptor for nicotinate (niacin) (20, 26), which was recently shown to be activated by SCFAs (32). GPR109A is abundantly expressed in the lumen-facing apical membrane of colonocytes, supporting its potential to serve as a luminal SCFA sensor (32).
Functional activity of intestinal transporters may undergo modulation via specific sensing of luminal nutrients. This has been best described for the intestinal sugar transporter Glut2 in rat intestine that has basolateral localization but is recruited to apical membrane in response to higher levels of luminal sugar following a meal (15, 16, 21). However, despite the fact that colonic luminal SCFA levels vary significantly depending upon diet, gut microbial composition, and disease state, it is not known whether MCT1 may be regulated via luminal SCFA sensing. In this report, we provide evidence that membrane polarity of MCT1 and its functionality to transport luminal SCFAs could be modulated via nutrient-sensing mechanisms causing potential translocation of MCT1 to the apical membrane domains in the presence of higher luminal SCFA levels. Furthermore, our results also led us to speculate GPR109A as the candidate SCFA sensor mediating the effects of SCFA substrates on membrane localization and function of MCT1.
MATERIALS AND METHODS
Cell lines and cell culture.
C2BBe1 cells, a subclone of the Caco-2 cell line, were grown at 37°C in an atmosphere of 5% CO2, and maintained in DMEM supplemented with 10% FCS, 50 U/ml penicillin, 5 μg/ml streptomycin, 10 μg/ml transferrin, and 2 mM glutamine. IEC-6 cells, a nontransformed rat intestinal cell line, were maintained in DMEM with 4.5 g/l glucose, 50 U/ml penicillin, 5 g/ml streptomycin, 2 g/ml gentamicin, and 10% FBS. MCT1 function ([14C]butyrate uptake) was measured in fully differentiated C2BBe1 (14-day postplating) or IEC-6 (10-day postplating) cells grown on 24-well plastic supports or 12-well transwell inserts.
Measurement of [14C]butyrate uptake.
Apical uptake of [14C]butyrate was measured as described previously (1), in the presence or absence of p-chloromercuri-benzene sulfonate (pCMBS), a specific inhibitor of MCT1 (17). Uptake values were calculated as nanomoles of [14C]butyrate per milligram protein per 5 min, and results were expressed as percent of control.
Cell surface biotinylation and immunoblotting.
Cell surface biotinylation was performed using sulfo-NHS-SS-biotin (Thermo Scientific, Rockford, IL) (0.5 g/l) in borate buffer (in mmol/l: 154 NaCl, 7.2 KCl, 1.8 CaCl2, 10 H3BO3, pH 9.0) as previously described (8). Labeling was allowed to proceed for 60 min at 4°C to prevent endocytosis and internalization of antigens. After immunoprecipitation of biotinylated antigens with streptavidin agarose, biotinylated proteins were released by boiling in Laemmli buffer containing dithiothreitol, subjected to SDS-PAGE, and then probed with anti-MCT1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). The surface MCT1 was compared with total cellular MCT1 as determined by immunoblotting of the solubilized cell extract.
siRNA silencing of GPR109A.
siRNA for human GPR109A (cat. no. SI03060085) and scrambled siRNA were obtained commercially from Qiagen Sciences (Germantown, MD), and transfection of cells (1 × 105 cells per well in 6-well plates) was performed following the manufacturer's protocol. Forty-eight hours after transfection, cells were treated with butyrate for 1–2 h, and cell surface MCT1 levels were assessed by cell surface biotinylation. The extent of GPR109A silencing was assessed by measuring GPR109A levels in the cell lysates by immunoblotting with anti-GPR109A antibody (a kind gift from Dr. V. Ganapathy from the Medical College of Georgia, Augusta, GA).
Immunofluorescence staining in C2BBe1 cells.
C2BBe1 cells were transiently transfected with the MCT1-green fluorescent protein (GFP) fusion construct in pEGFPN (Invitrogen, Carlsbad, CA) and were plated on transwell inserts at a density of 1 × 104. Forty-eight hours posttransfection, cells were treated with butyrate (10 mM) for 1 h from the apical side. During the last 10 min of incubation with butyrate, cells were labeled with 1 mg/ml fluorescent wheat germ agglutinin-Alexa Fluor 594 conjugate in standard buffer (PBS), washed, and fixed with 2% paraformaldehyde for 10 min. After being mounted under coverslip, the cells were visualized utilizing Carl Zeiss LSM 510 laser scanning confocal microscope equipped with a ×63 water-immersion objective. Green and red fluorescence emissions were detected through LP485 and 585 filters, respectively. The two different fluorochromes were scanned sequentially by using the multitracking function to avoid any bleed through among these fluorescent dyes.
Rats and pectin feeding experiments.
All animal studies were conducted with prior approval of the protocols by the Institutional Animal Care and Use Committee of the University of Illinois and Jesse Brown VA Medical Center, Chicago. Male Sprague-Dawley rats (4–6 wk old) procured from Jackson Laboratory were divided into a control group (n = 10) and a pectin-fed group (n = 10). The two groups were fed a fiber-free diet or pectin (6%) diet, respectively, as described previously (24). Rats were killed at the 7th and the 14th day. Colonic mucosa stripped off the muscle layer was used immediately to measure transepithelial butyrate flux in the Ussing chamber. Colonic tissues from pectin or fiber-free diet fed rats were also frozen in OCT medium (Sakura Finetek, Torrance, CA) and 5-μM sections were cut using a cryostat to be utilized for immunofluorescence studies.
Measurement of transepithelial flux of butyrate.
MCT1 function in rat colonic mucosa ex vivo was determined by measuring transepithelial flux of [14C]butyrate in the Ussing chamber under open-circuit conditions without clamping the voltage and in the presence or absence of pCMBS (1 mM). The intestine was stripped off the muscle layer, mounted in the Ussing chamber so that the mucosal sheets are exposed on each side to the Kreb's solution (in mmol/l: 0.4 KH2PO4, 2.4 K2HPO4, 115 NaCl, 25 NaHCO3, 1.2 CaCl2, 1.2 MgCl2, 10 mannitol). Solutions were continuously gassed throughout the experiment with 95% O2/5% CO2. After incubating with [14C]butyrate for 30 min, aliquots were collected from the serosal reservoir to measure the radioactivity in a liquid scintillation counter and to calculate mucosal to serosal (Jm-s) flux rates. Inhibitor (pCMBS)-sensitive flux was considered to represent MCT1-mediated butyrate uptake.
Immunofluorescence staining in rat colonic tissues.
Sections of colonic tissues from rats fed pectin or fiber-free diet were snap frozen in optimal cutting temperature embedding medium (Tissue-Tek OCT compound; Sakura). For immunostaining, 5-μM frozen sections were fixed with 1% paraformaldehyde in PBS for 10 min at room temperature. Fixed sections were washed in PBS, permeabilized with 5% NP-40 for 5 min, and blocked with 5% normal goat serum (NGS) for 30 min. Tissues were incubated with MCT1 antibody (1:1,500 AB1286; Chemicon, Temecula, CA) in PBS with 1% NGS for 90 min at room temperature. After washing, sections were incubated with Alexa Fluor 594-conjugated goat anti-rabbit IgG, Alexa Fluor 488-conjugated phalloidin (5 U/ml; Invitrogen) and Hoechst 33342 (Invitrogen) for 60 min. Sections were then washed and mounted under coverslips using ProLong Gold antifade reagent (Invitrogen). Sections were imaged using Carl Zeiss LSM 510 laser scanning confocal microscope equipped with ×20 water immersion objective.
Statistical analysis.
All experiments were performed in triplicate on 4–5 separate sets. Experiments with animals were replicated using tissues from different animals. Data were analyzed by ANOVA using GraphPad Prism software. Results shown are means ± SE, P < 0.05 was considered statistically significant.
RESULTS
Short-term butyrate treatment enhances MCT1 function in C2BBe1 and IEC-6 cells.
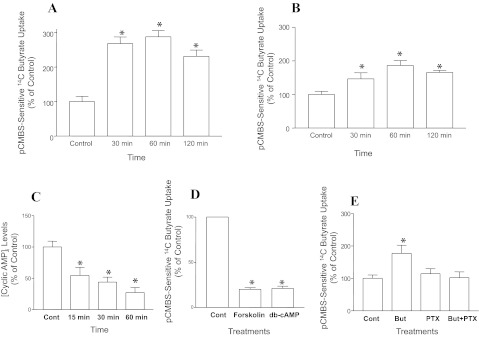
Mechanisms underlying upregulation of MCT1 expression and function in vitro in response to long-term treatments with butyrate have been shown in earlier studies (1, 2). Our present set of initial studies examined the short-term effects of butyrate treatment on MCT1 function, as measured by pCMBS-sensitive [14C]butyrate uptake in human intestinal C2BBe1 and rat intestinal IEC-6 cells. Results showed that short-term (30–120 min) incubation with butyrate (10 mM) significantly enhanced MCT1 function in C2BBe1 (Fig. 1A) and IEC-6 (Fig. 1B) cells.
Fig. 1.
Butyrate (But)-induced enhancement of monocarboxylate transporter-1 (MCT1) function is pertussis toxin (PTX)-sensitive and associated with decreased intracellular cAMP levels. {MCT1 function (pCMBS-sensitive [14C]butyrate uptake)} was calculated as nanomols butyrate per milligram protein per 5 min and results are expressed as % of control]. Time-course of butyrate (10 mM) effects on MCT1 function in C2BBe1 cells (14-day postplating) (n = 5, P < 0.05 vs. control) (A) and in IEC-6 cells (10-day postplating) (n = 3, *P < 0.05) (B). Time-course of butyrate (10 mM) effects on intracellular cAMP levels (n = 3, *P < 0.05) (C); forskolin (10 μM) and dibutyryl cAMP (50 μM) effects on MCT1 function (n = 3, *P < 0.001) (D); PTX (1 μg/ml)-sensitivity of butyrate (10 mM) induction of MCT1 function (n = 3, *P < 0.05) (E). pCMBS, p-chloromercuribenzene sulfonate.
Butyrate enhancement of MCT1 function is associated with decreased cAMP and is pertussis toxin sensitive.
Recent reports showed that SCFAs might exert their effects acting extracellularly via GPCR activation (3, 18). To examine whether short-term effects of butyrate on MCT1 function are GPCR mediated, we sought to investigate the role of cAMP signaling in mediating these effects. Previous studies also reported altered cAMP signaling in mediating short-term effects of butyrate on intestinal secretory functions (27). Initially, we measured intracellular cAMP levels in response to 10 mM butyrate that enhanced MCT1 function in C2BBe1 cells (Fig. 1A). As shown in Fig. 1C, there was a time-dependent decrease in intracellular cAMP in C2BBe1 cells in response to treatments with 10 mM butyrate (15–60 min). These results suggest that butyrate enhancement of MCT1 function could involve modulation of cAMP pathway. We next measured the effects of cAMP agonists on MCT1 function. As shown in Fig. 1D, treatment with 10 μM forskolin or 50 μM dibutyryl cAMP for 15 min significantly inhibited butyrate uptake. Furthermore, in cells preloaded with 10 μM forskolin (to increase cAMP) for 30 min and subsequently coincubated with 10 mM butyrate for 60 min, forskolin inhibited the increase in MCT1 function by butyrate by ∼60% (data not shown). Our data showing involvement of altered cAMP signaling in short-term regulation of MCT1 function by butyrate suggests that these effects could be receptor mediated. On the other hand, decreased intracellular cAMP associated with butyrate enhancement of MCT1 function suggests that butyrate may act through activation of G protein coupled receptor(s) that leads to reduction of intracellular cAMP. Therefore, we examined the effects of pertussis toxin (PTX), an inhibitor of Gαi type of G-proteins (19), on butyrate-induced enhancement of MCT1 function. Results showed that PTX (1 μM) blocked butyrate enhancement of pCMBS-sensitive [14C]butyrate uptake (MCT1 function) (Fig. 1E), suggesting the role of Gαi-coupled GPCR as a potential SCFA sensor in mediating the effects of butyrate on MCT1 function.
Nicotinate, a GPR109A agonist, mimics butyrate effects on MCT1 function and intracellular cAMP.
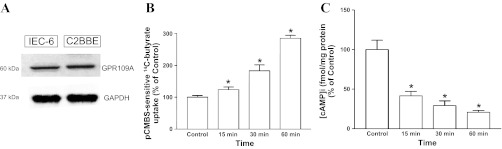
GPR109A, a Gαi-coupled GPCR for nicotinate, has recently been shown to be highly expressed on the apical surface of colonic mucosa and activated by SCFA (5, 32). We have shown the expression of GPR109A in C2BBe1 and IEC-6 cells (Fig. 2A). Since butyrate enhancement of MCT1 function is accompanied by decreased intracellular cAMP (Fig. 1C), we sought to examine whether GPR109A could act as a potential SCFA sensor and butyrate effects on MCT1 function is secondary to activation of GPR109A. Therefore, we examined the effects of the GPR109A agonist nicotinate, which has higher affinity for the receptor compared with butyrate, on MCT1 function and intracellular cAMP. Our results showed that, compared with butyrate (10 mM), 10-fold lower concentration (100 μM) of nicotinate enhanced MCT1 function (Fig. 2B) and decreased cAMP levels (Fig. 2C) in a time-dependent manner.
Fig. 2.
Nicotinate receptor GPR109A as potential mediator of butyrate effects on MCT1 function. GPR109A protein expression in IEC-6 and C2BBe1 cells (75 μg protein in cell lysate was loaded, probed with anti-human GPR109A antibody) (A); time-course of 100 μM nicotinate effects on MCT1 function (B); and time-course of nicotinate effects on intracellular cAMP (n = 3, *P < 0.05) (C).
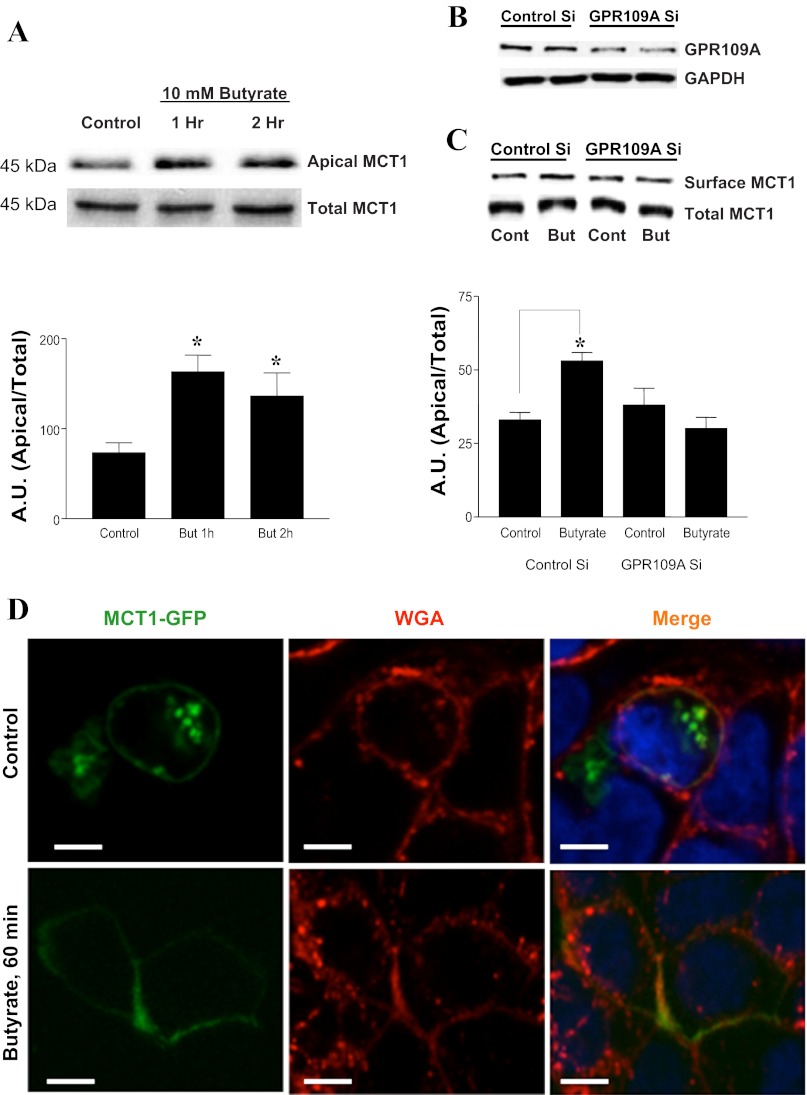
Butyrate increases Vmax of MCT1-mediated butyrate uptake and induces GPR109A-dependent increase in cell surface MCT1.
To examine the mechanisms of butyrate enhancement of MCT1 function, we performed kinetic analysis of the butyrate effects. Effects of 10 mM butyrate on pCMBS-sensitive [14C]butyrate uptake were measured at different substrate concentrations (0.5 mM-15 mM), and kinetic parameters Km and Vmax were calculated. As shown in Table 1, butyrate increases Vmax of MCT1-mediated butyrate uptake without altering the Km. Increased Vmax implies that butyrate treatment increases MCT1 levels at the cell surface. We next utilized cell surface biotinylation to measure apical membrane levels of MCT1 in response to butyrate treatment. Consistent with increased function, butyrate treatment (10 mM, 1 and 2 h) significantly increased apical membrane MCT1 levels (Fig. 3A). To examine the role of GPR109A in mediating these effects, we assessed butyrate-induced membrane targeting of MCT1 in cells following siRNA silencing of GPR109A. As shown in Fig. 3B, GPR109A protein was significantly reduced by 48 h after siRNA transfection. Furthermore, in GPR109A-silenced cells, butyrate treatment did not alter MCT1 abundance in the apical membrane (Fig. 3C). Butyrate-induced membrane targeting of MCT1 is further supported by our confocal immunofluorescence studies (Fig. 3D) showing increased intensity of GFP at the cell surface of MCT1-GFP overexpressing C2BBe1 cells treated with 10 mM butyrate for 1 h.
Table 1.
Kinetics of butyrate (10 mM, 1 h) effects on MCT1 function (pCMBS-sensitive [14C]butyrate uptake)
| Control | Butyrate, 10 mM | |
|---|---|---|
| Vmax, nmol/mg protein/5 min | 2.2 ± 0.2 | 4.5 ± 0.4* |
| Km, mM | 3.8 ± 0.1 | 3.8 ± 0.3 |
Values are means ± SE; n = 3. Kinetic parameters were analyzed by regression analysis using GraphPad Prism software. MCT1, monocarboxylate transporter 1; pCMBS, p-chloromercuribenzene sulfonate.
P < 0.05.
Fig. 3.
Butyrate increases cell surface MCT1 levels in C2BBe1 cells in a GPR109A-dependent manner: A: cell monolayers (14-day postplating) were treated with 10 mM butyrate for the indicated time periods, and apical membrane MCT1 levels were measured by cell surface biotinylation; top: the band intensities of apical vs. total MCT1 in different groups; bottom: densitometric analysis of band intensities (AU, arbitrary units of band intensity). B: cells were transfected with control-scrambled siRNA or GPR109A siRNA as described in materials and methods. Forty-eight hours after transfection, cells were either used to measure GPR109 levels by immunoblotting with anti-GPR109A antibody (B) or to measure surface MCT1 by cell surface biotinylation (C). C, bottom: densitometric analysis of MCT1 band intensities. D: plasma membrane MCT1-green fluorescent protein (GFP) levels in response to butyrate treatments were measured in C2BBE cells transiently transfected with MCT-GFP fusion constructs and probed with anti-GFP antibodies in immunofluorescence studies. GFP, green fluorescent protein; WGA, wheat germ agglutinin. *P < 0.05.
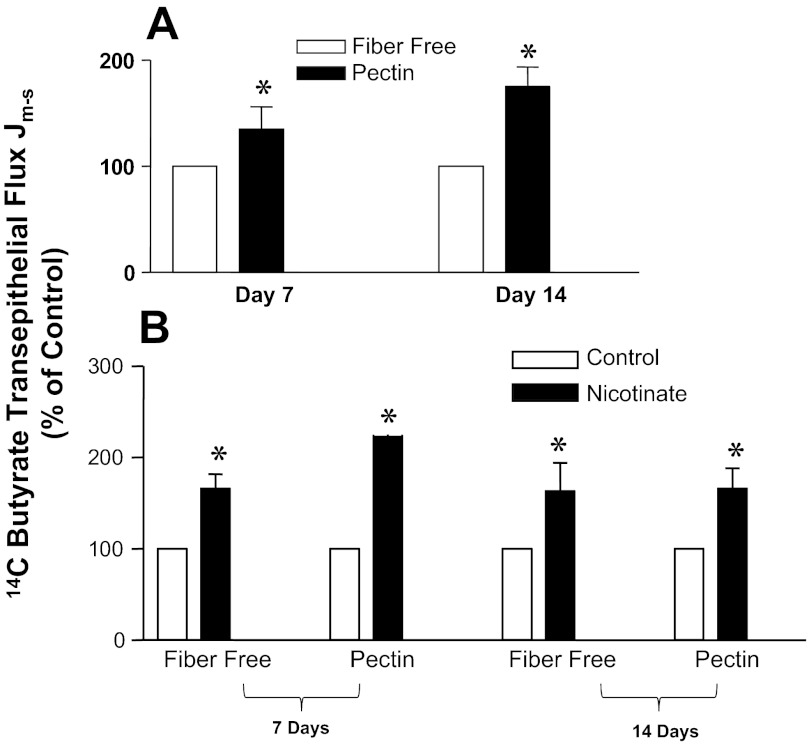
Nicotinate stimulates mucosal to serosal (Jm-s) flux of 14C butyrate in the colon of pectin-fed rats.
To assess the in vivo effects of SCFA substrates on MCT1 activity, we utilized a rat model of feeding pectin (a soluble fiber) or a fiber-free diet. Transepithelial flux of [14C]butyrate in colonic mucosa of rats fed pectin versus fiber-free diet for 7 or 14 days was measured in Ussing chambers. Additionally, to assess the role of GPR109A in mediating substrate-induced enhancement of MCT1 function, we measured transepithelial flux of [14C]butyrate in response to short-term treatment of the mucosa with nicotinate. Nicotinate, instead of butyrate, was used for two reasons: 1) to avoid the complicacy that might arise in measuring [14C]butyrate flux in the presence of high concentrations (10 mM) of cold butyrate and 2) for nicotinate being more potent agonist for GPR109A. As shown in Fig. 4A, mucosal-to-serosal (Jm-s) flux of [14C]butyrate was significantly higher in pectin-fed rats compared with those on fiber-free diet. The effect was more pronounced at 14th day, compared with 7th day of pectin feeding. On the other hand, the GPR109A agonist nicotinate treatment (100 μM, 1 h) increased butyrate flux in the colonic mucosa of both the fiber-free group and the pectin group, although the effect was more pronounced in the later (Fig. 4B).
Fig. 4.
Effects of pectin diet vs. fiber-free diet on MCT1 function (transepithelial butyrate flux) in native colonic mucosa of rats. A: pCMBS-sensitive Jm-s transepithelial flux of [14C]butyrate in the colonic mucosa of rats fed pectin or fiber-free diet for indicated periods. B: after mounting the colonic mucosa on the Ussing chamber, nicotinate (100 μM) was applied to the mucosal side of the compartment of for 60 min, followed by measurement of pCMBS-sensitive Jm-s transepithelial flux of [14C]butyrate. *P < 0.05.
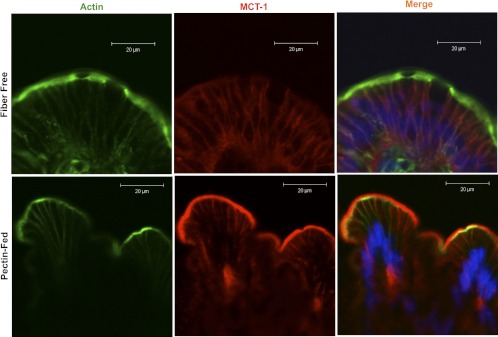
Pectin feeding increases MCT1 expression and apical membrane levels in rat colon.
We also compared the MCT1 abundance and its apical membrane levels in the colonic tissues of pectin diet or fiber-free diet fed rats, as evaluated by confocal microscopy. Consistent with an earlier report (17), pectin feeding in our studies also increased MCT1 (red) abundance as well as its apical membrane levels compared with fiber-free diet (Fig. 5).
Fig. 5.
Pectin feeding increases MCT1 protein expression as well as its apical membrane levels in the colonic mucosa compared with control rats fed fiber-free diet. MCT1 (red), actin (green), nuclei (blue); scale bar = 20 μm.
DISCUSSION
Our present findings implicate the role of a novel luminal nutrient sensing mechanism in modulating the activity of MCT1, and thereby absorption of SCFAs, by the colonocytes. SCFAs are produced in the colonic lumen via the action of specific gut microbiota on dietary fiber. Of the SCFAs, butyrate has a prominent role at the colonic level as it is the primary fuel for the colonocytes and its oxidation is involved in various important metabolic processes in the colon (7). Since MCT1 plays an important role in the absorption of butyrate anions produced in the colonic lumen (10, 11, 28), its regulation has major impact on colonic epithelial health. We and others have earlier demonstrated that long-term exposure of intestinal epithelial cells to SCFA substrates enhances MCT1 expression and function (2, 6) via NF-κB-dependent transcriptional mechanisms (2). Our present in vitro studies in human intestinal C2BBe1 and rat intestinal IEC-6 cells showed that butyrate enhances MCT1 function as early as 30 min, indicating the role of butyrate-induced rapid signaling events in mediating these effects. These results further implicate that butyrate elicits these effects extracellularly by activating sensors/receptors on the cell surface. Regulation of transporter function via specific sensing of luminal nutrient substrates has been described for the intestinal sugar transporter Glut2 (16, 21), the peptide transporter PEPT1 (23), and, recently, also for the intestinal iron transporters DMT1 and ferroporin 1 (25). However, the underlying mechanisms and pathways are not fully understood. Our present findings implicating regulation of the colonic SCFA transporter MCT1 via nutrient sensing are of considerable interest because, despite the fact that colonic luminal SCFA levels vary significantly depending upon dietary composition, gut microbiota, and disease, a SCFA-sensing mechanism regulating colonic luminal SCFA absorption has not been described previously.
In recent years, the importance of cell surface receptors in nutrient sensing has increasingly been recognized (21, 29). For example, recent discovery of the expression of T1R sweet taste receptor families on the apical membranes of enterocytes suggested that activities of these receptors might underlie the mechanisms of enhanced glucose absorption via increased apical membrane insertion of Glut2 after a meal (21). Recently, the GPCR deorphanizing strategy (13) has successfully identified GPR41 and GPR43 as receptors for SCFAs. GPR41 is mainly expressed in the adipocytes, whereas GPR43 expression has been localized primarily in the immune cells (3, 18). Although recent studies showed that GPR41/GPR43 are expressed in L-type enteroendocrine cells of the colon (31), their expression and role in absorptive colonic epithelial cells have not been demonstrated. Therefore, GPR41 and GPR43 are less likely to play the role of sensors for luminally produced SCFAs. On the other hand, GPR109A, the receptor for nicotinic acid, which is highly expressed and localized to the luminal membranes of colonocytes, has been shown to be activated by butyrate (32). Our results also demonstrated expression of GPR109A in C2BBe1 and IEC-6 cells. GPR109A is a GPCR linked to Gαi family proteins and upon activation leads to decreased intracellular cAMP levels. Our results also showed that butyrate-induced enhancement of MCT1 function is accompanied by a concomitant decrease in intracellular cAMP, whereas the cAMP agonists forskolin and dibutyryl cAMP inhibits MCT1 function. Nicotinate, the potent GPR109A agonist, stimulates the receptor at much lower concentrations compared with butyrate (32). Interestingly, our results showed that 100 μM nicotinate, compared with 10 mM butyrate, produced similar effects of enhancing MCT1 function and decreasing intracellular cAMP. These results further suggest that butyrate effects on MCT1 could be secondary to GPR109A activation. In adipocytes, nicotinate has been shown to stimulate adiponectin secretion via activation of GPR109A, and these effects were blocked when adipocytes were preexposed to PTX, an inhibitor of Gαi proteins (19). Consistently, we also found that butyrate enhancement of MCT1 function was blocked in the presence of PTX, presumably by inhibiting GPR109A activation-induced signaling events that stimulate MCT1 activity.
The short-term regulation of the activity of a transporter protein may involve alterations of its membrane abundance or changes in some kinetic properties such as substrate affinity, interaction with other molecules, or ion dependence (23). Butyrate-induced activation of MCT1 function in polarized C2BBe1 cells was kinetically manifested by an increase in the Vmax, without altering the Km. Interestingly, earlier studies in rat brain endothelial cells showing cAMP agonist-induced inhibition of MCT1 function involved decrease in Vmax with no effects on Km (30). On the other hand, butyrate inhibition of cAMP agonist-induced chloride secretion in intestinal epithelial cells was associated with decreased cAMP (27). It appears that by modulating cAMP pathway, butyrate exerts opposite effects on ion absorptive and secretory processes, and both processes could involve activation of SCFA receptors. In our studies, increased Vmax suggests that butyrate-induced increase in the absorptive capacity of MCT1 was due to an increase in the apical membrane abundance of MCT1. In fact, our cell surface biotinylation and immmunofluorescence studies further supported that short-term treatment with butyrate increased apical membrane abundance of MCT1 with a resultant enhancement of its transport function, representing its regulation via nutrient-sensing as shown earlier for Glut2 (21). Interestingly, butyrate had no effect on MCT1 targeting to the apical membrane in GPR109A-deficient cells, further confirming the role of this SCFA receptor in mediating the substrate-induced membrane targeting of MCT1.
Controversies exist regarding apical versus basolateral membrane localization of MCT1 and its role in the absorption of luminal SCFAs in various mammalian species. We and others have shown predominantly apical membrane localization of MCT1 in the intestine of human (9, 28) and other mammalian species (14), whereas, few reports suggested basolateral localization in mice and human intestine (14). In this regard, various recent studies have shown that membrane polarity of transporters may be modulated by the substrates and membrane insertion of transporters could be a quick adaptive response to the availability of nutrients for absorption and represent a mechanism of short-term nutrient sensing (16).
A recent study demonstrated that pectin feeding increases the expression and function of MCT1 in rat small intestine and colon (17). Furthermore, MCT1 abundance in the apical membranes of enterocytes and colonocytes increased substantially in pectin-fed rats, while in control rats MCT1 was predominantly localized to the basolateral membranes. Similar to this published report, our present in vivo studies also demonstrated increased expression of MCT1 as well as its increased abundance in the apical surface of the colonic mucosal sections from pectin-fed rats compared with those from rats on fiber-free diet. There was a parallel increase in MCT1 function in the pectin group. Furthermore, our ex vivo studies showing increased transepithelial flux of [14C]butyrate in response to nicotinate, the GPR109A agonist, further support the role of GPR109A in mediating the effects of substrate-induced enhancement of MCT1 function in the native intestinal mucosa. Since GPR109A activation by nicotinate presumably causes a rapid mobilization of MCT1 to the apical membrane, we observed nicotinate-induced increase in butyrate flux in both the pectin group as well as the fiber-free group, although the effect was more pronounced in the former group. This could be due to a relatively greater rate of apical membrane insertion of MCT1 from a larger pool of total MCT1 expressed in pectin-fed rats. The observed increase in the translocation of MCT1 to the absorptive luminal surface of colonic mucosa appears to be an adaptive response to ensure efficient absorption of the bulk of SCFAs resulting from pectin feeding. This adaptive response to dietary changes may also underlie the differential membrane polarity of MCT1 reported by various investigators in the colonic epithelium of different species.
In summary, our results suggest that exposure of the absorptive luminal surfaces of intestinal epithelial cells to higher levels of SCFA substrates triggers short-term nutrient sensing mechanisms to mobilize more MCT1 molecules for apical membrane insertion to enhance its capacity to absorb more SCFAs. Our results also suggest that GPR109A is seemingly the potential SCFA-sensor, which, upon activation by SCFAs, triggers signaling pathways leading to decreased intracellular cAMP. The decreased cAMP levels could directly or indirectly influence the downstream events that underlie the mechanisms of increased targeting of MCT1 to the apical membranes. Since luminal concentrations of SCFAs are readily amenable to modulation by diet, detailed characterization of the players and pathways of luminal SCFA sensing in our future studies should help identify novel targets for intervention in diseases associated with impaired SCFA absorption.
GRANTS
These studies were supported by the Department of Veterans Affairs and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-54016 (to P. K. Dudeja), DK-81858 (to P. K. Dudeja), DK-92441 (to P. K. Dudeja), DK-71596 (W. A. Alrefai), DK-74458 (R. K. Gill) and the Program Project Grant DK-067887 (to P. K. Dudeja).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.B., R.K.G., W.A.A., and P.K.D. conception and design of research; A.B., S.P., A.K., and A.A.N. performed experiments; A.B., S.P., A.K., and A.A.N. analyzed data; A.B. interpreted results of experiments; A.B. prepared figures; A.B. drafted manuscript; A.B., R.K.G., W.A.A., and P.K.D. edited and revised manuscript; P.K.D. approved final version of manuscript.
REFERENCES
- 1. Alrefai WA, Tyagi S, Gill R, Saksena S, Hadjiagapiou C, Mansour F, Ramaswamy K, Dudeja PK. Regulation of butyrate uptake in Caco-2 cells by phorbol 12-myristate 13-acetate. Am J Physiol Gastrointest Liver Physiol 286: G197– G203, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Borthakur A, Saksena S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Regulation of monocarboxylate transporter 1 (MCT1) promoter by butyrate in human intestinal epithelial cells: involvement of NF-κB pathway. J Cell Biochem 103: 1452– 1463, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278: 11312– 11319, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol 17: 1519– 1528, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cresci GA, Thangaraju M, Mellinger JD, Liu K, Ganapathy V. Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J Gastrointest Surg 14: 449– 461, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Cuff MA, Lambert DW, Shirazi-Beechey SP. Substrate-induced regulation of the human colonic monocarboxylate transporter, MCT1. J Physiol 539: 361– 371, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gassull MA. The intestinal lumen as a therapeutic target in inflammatory bowel disease. Aliment Pharmacol Ther 24, Suppl 3: 90–95, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G, Dudeja PK. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J Clin Invest 117: 428– 437, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gill RK, Saksena S, Alrefai WA, Sarwar Z, Goldstein JL, Carroll RE, Ramaswamy K, Dudeja PK. Expression and membrane localization of MCT isoforms along the length of the human intestine. Am J Physiol Cell Physiol 289: C846– C852, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Goncalves P, Araujo JR, Martel F. Characterization of butyrate uptake by nontransformed intestinal epithelial cell lines. J Membr Biol 240: 35– 46, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Hadjiagapiou C, Schmidt L, Dudeja PK, Layden TJ, Ramaswamy K. Mechanism(s) of butyrate transport in Caco-2 cells: role of monocarboxylate transporter 1. Am J Physiol Gastrointest Liver Physiol 279: G775– G780, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. The role of butyrate on colonic function. Aliment Pharmacol Ther 27: 104– 119, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Hirasawa A, Hara T, Katsuma S, Adachi T, Tsujimoto G. Free fatty acid receptors and drug discovery. Biol Pharm Bull 31: 1847– 1851, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Iwanaga T, Takebe K, Kato I, Karaki S, Kuwahara A. Cellular expression of monocarboxylate transporters (MCT) in the digestive tract of the mouse, rat, and humans, with special reference to slc5a8. Biomed Res (Tokyo) 27: 243– 254, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Kellett GL, Brot-Laroche E. Apical GLUT2: a major pathway of intestinal sugar absorption. Diabetes 54: 3056– 3062, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Kellett GL, Brot-Laroche E, Mace OJ, Leturque A. Sugar absorption in the intestine: the role of GLUT2. Annu Rev Nutr 28: 35– 54, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Kirat D, Kondo K, Shimada R, Kato S. Dietary pectin up-regulates monocaboxylate transporter 1 in the rat gastrointestinal tract. Exp Physiol 94: 422– 433, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, Parmentier M, Detheux M. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 278: 25481– 25489, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Li C, Dandridge KS, Di A, Marrs KL, Harris EL, Roy K, Jackson JS, Makarova NV, Fujiwara Y, Farrar PL, Nelson DJ, Tigyi GJ, Naren AP. Lysophosphatidic acid inhibits cholera toxin-induced secretory diarrhea through CFTR-dependent protein interactions. J Exp Med 202: 975– 986, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lukasova M, Malaval C, Gille A, Kero J, Offermanns S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J Clin Invest 121: 1163– 1173, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol 582: 379– 392, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Macfarlane GT, Macfarlane S. Fermentation in the human large intestine: its physiologic consequences and the potential contribution of prebiotics. J Clin Gastroenterol 45, Suppl: S120–S127, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Mertl M, Daniel H, Kottra G. Substrate-induced changes in the density of peptide transporter PEPT1 expressed in Xenopus oocytes. Am J Physiol Cell Physiol 295: C1332– C1343, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Musch MW, Bookstein C, Xie Y, Sellin JH, Chang EB. SCFA increase intestinal Na absorption by induction of NHE3 in rat colon and human intestinal C2/bbe cells. Am J Physiol Gastrointest Liver Physiol 280: G687– G693, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Nunez MT, Tapia V, Rojas A, Aguirre P, Gomez F, Nualart F. Iron supply determines apical/basolateral membrane distribution of intestinal iron transporters DMT1 and ferroportin 1. Am J Physiol Cell Physiol 298: C477– C485, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Plaisance EP, Lukasova M, Offermanns S, Zhang Y, Cao G, Judd RL. Niacin stimulates adiponectin secretion through the GPR109A receptor. Am J Physiol Endocrinol Metab 296: E549– E558, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Resta-Lenert S, Truong F, Barrett KE, Eckmann L. Inhibition of epithelial chloride secretion by butyrate: role of reduced adenylyl cyclase expression and activity. Am J Physiol Cell Physiol 281: C1837– C1849, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Ritzhaupt A, Wood IS, Ellis A, Hosie KB, Shirazi-Beechey SP. Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: its potential to transport L-lactate as well as butyrate. J Physiol 513: 719– 732, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shirazi-Beechey SP, Moran AW, Batchelor DJ, Daly K, Al-Rammahi M. Glucose sensing and signalling; regulation of intestinal glucose transport. Proc Nutr Soc 70: 185– 193, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Smith JP, Drewes LR. Modulation of monocarboxylic acid transporter-1 kinetic function by the cAMP signaling pathway in rat brain endothelial cells. J Biol Chem 281: 2053– 2060, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Tazoe H, Otomo Y, Kaji I, Tanaka R, Karaki SI, Kuwahara A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol 59, Suppl 2: 251–262, 2008 [PubMed] [Google Scholar]
- 32. Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, Mellinger JD, Smith SB, Digby GJ, Lambert NA, Prasad PD, Ganapathy V. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res 69: 2826– 2832, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thibault R, Blachier F, Darcy-Vrillon B, de Coppet P, Bourreille A, Segain JP. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm Bowel Dis 16: 684– 695, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Thibault R, De Coppet P, Daly K, Bourreille A, Cuff M, Bonnet C, Mosnier JF, Galmiche JP, Shirazi-Beechey S, Segain JP. Down-regulation of the monocarboxylate transporter 1 is involved in butyrate deficiency during intestinal inflammation. Gastroenterology 133: 1916– 1927, 2007 [DOI] [PubMed] [Google Scholar]