Abstract
The influence on mucosal healing of luminal nutrient flow and the forces it creates are poorly understood. We hypothesized that altered deformation and extracellular pressure mediate, in part, the effects of defunctionalization on mucosal healing. We created patent or partially obstructing defunctionalizing jejunal Roux-en-Y anastomoses in rats to investigate mucosal healing in the absence or presence of luminal nutrient flow and measured luminal pressures to document partial obstruction. We used serosal acetic acid to induce ulcers in the proximal, distal, and defunctionalized intestinal segments. After 3 days, we assessed ulcer area, proliferation, and phosphorylated ERK. In vitro, we measured proliferation and migration in Caco-2 and IEC-6 intestinal epithelial cells subjected to cyclic strain, increased extracellular pressure, or strain and pressure together. Defunctionalization of intestine without obstruction reduced phosphorylated ERK, slowed ulcer healing, and inhibited mucosal proliferation. This outcome was blocked by PD-98059. Partial obstruction delayed ulcer healing but stimulated proliferation independently of ERK. In vitro, strain increased Caco-2 and IEC-6 proliferation and reduced migration across collagen but reduced proliferation and increased migration across fibronectin. In contrast, increased pressure and the combination of pressure and strain increased proliferation and reduced migration independently of substrate. PD-98059 reduced basal migration but increased migration under pressure. These results suggest that loss of the repetitive distension may decrease mucosal healing in defunctionalized bowel, while increased luminal pressure above anastomoses or in spastic bowel disease could further inhibit mucosal healing, despite peristaltic repetitive strain. ERK may mediate the effects of repetitive deformation but not the effects of pressure.
Keywords: anastomosis, pressure, enterocyte, proliferation, ERK
the intestinal mucosa is constantly subjected to injuries that it must heal to maintain normal function and provide efficient nutrient supply. Thus wound healing is constantly active in the gut mucosa during normal function (3, 31). This healing of damaged mucosa requires epithelial cell migration and proliferation.
The small intestine displays various patterns of motility and contractility during normal physiological function and in disease. The intestine can stretch up to 200% (41) while its mucosa undergoes constant repetitive contraction and movements (43). Mucosal healing in vivo is affected by many pathophysiological states that are associated with alterations in intestinal mucosal deformation and pressure. Sepsis, postsurgical ileus, and prolonged fasting may be associated with altered contractile rhythms, altered villous motility, and decreased mucosal deformation from luminal contents (4). Excessive pressure is commonly observed in patients undergoing surgical intervention in the small intestine (20). Ileus after major surgery or trauma changes the physiology of the intestine by reducing and slowing contractility and alters small intestine motor patterns and pressure (38). However, the effects of changed contractility on intestinal physiology and reparative capabilities remain undefined.
Previous studies demonstrated that repetitive deformation of intestinal epithelial monolayers can modulate intestinal epithelial migration and proliferation in vitro (40, 47). In vivo, cyclic deformation initiates tyrosine kinase signaling within the intestinal mucosa (2), and obstructed mouse intestine seems to heal more slowly (16). However, how pressure and deformation affect the small intestinal mucosa in vivo, an issue important for normal bowel function and the healing of damaged small intestinal mucosa in disease states with altered patterns of luminal pressure and mucosal deformation (18), is otherwise poorly understood. Extrapolating from in vitro observations, we hypothesized that pressure and cyclic deformation initiate intracellular signals within the small intestinal epithelium that can modulate mucosal wound healing in vivo.
Using a Roux-en-Y anastomosis in rat jejunum, we created defunctionalized segments of jejunum in enterally nourished rats. The defunctionalized jejunum exhibited mucosal atrophy, reduced cell proliferation, and decreased ERK activation (24). Furthermore, mucosal ulcers generated by acetic acid in this Roux-en-Y model healed more slowly in the defunctionalized bowel than in the undiverted jejunum proximal or distal to the anastomosis. ERK suppression by PD-98059 administration via an osmotic minipump reduced healing in the intact bowel and eliminated the differences between ulcer healing in the defunctionalized bowel and its proximal or distal undiverted counterparts. Seeking to reconcile these observations with a previous preliminary study in mice that yielded different results (16), we noted that similar anastomoses in mice resulted in partial obstruction. We therefore performed parallel studies in which the rat anastomosis was deliberately narrowed to create a partial obstruction and demonstrated that, as previously observed in mice, partial obstruction slowed ulcer healing and stimulated enterocytic proliferation in the defunctionalized and the undiverted proximal bowel. ERK inhibition did not eliminate these differences. Finally, we hypothesized that these two models differed as follows: in the unobstructed case, we were observing the effects of decreased repetitive deformation from the defunctionalization, whereas the obstructed case was also characterized by an increased luminal pressure. We confirmed this hypothesis by directly measuring pressure in each segment of the anastomosis in vivo in parallel studies. We then confirmed in vitro in human colon cancer Caco-2 and rat normal IEC-6 intestinal epithelial cells that cyclic strain can improve monolayer wound healing, while increased extracellular pressure inhibits it, and that when constant pressure and cyclic strain are combined, the effects of pressure on cell proliferation and migration predominate. Taken together, these results suggest that repetitive deformation may support the healing of intestinal mucosa via ERK signaling, while increases in luminal pressure may inhibit mucosal healing by an ERK-independent mechanism.
MATERIALS AND METHODS
Animals and surgical procedures.
All experiments were approved by the Michigan State University Institutional Care and Use Committee. Female Wistar rats were purchased from Charles River (Wilmington, MA) at 8 wk of age and housed with a 12:12-h light-dark cycle. Chow diets and water were provided ad libitum.
Roux-en-Y anastomosis.
Rats were placed on a liquid diet (Jevity 1 cal, Abbott Nutrition, Columbus, OH) 2 days before surgery and for 3 days after surgery. Anesthesia was induced by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). To create a defunctionalized segment of jejunum in continuity with the remainder of the bowel, we performed a defunctionalizing Roux-en-Y anastomosis in rats through a midline laparotomy. Silk sutures (Ethicon, Somerville, NJ) were used to divide the jejunum 1 cm from the ligament of Treitz. For anastomosis of the proximal jejunum to the distal jejunum 3 cm distal to the original transection, we used running 7-0 Vicryl sutures to create a side-to-side anastomosis. The abdominal cavity was closed using a running 5-0 Vicryl suture. In some studies, we made a side-to-side anastomosis cross section 50% smaller in diameter to create a partially obstructed anastomosis. Sham surgeries consisting of a midline laparotomy followed by manipulations mimicking Roux-en-Y surgery were carried out in control animals.
Pressure measurements in vivo.
During Roux-en-Y surgery, radiometric devices (TA11PAC40, DSI, St. Paul, MN) were implanted intra-abdominally and attached to the abdominal wall. Flexible pressure-sensing tips were introduced into the lumen of the limb of interest of the Roux-en-Y anastomosis 3 cm from the surgical site or into the small intestine of sham-operated rats at the same location. Pressure was then recorded throughout the study at a sampling rate of 10 s each 2-min interval (Dataquest A.R.T. 4.1, DSI). Changes in strain were characterized by a computer-based analysis of serial manometric recordings, as previously described by Andrioli and colleagues (1). The threshold for contraction was measured at each time point. Over 30 pressure measurements, maximum and minimum pressure values were recorded after that threshold (T) was identified: T = minimum + [(maximum − minimum)/3]. Readings above threshold of >4-min duration (2 consecutive readings) were considered phasic contraction, and the average value of each such phasic contraction was defined as the amplitude of the contraction (1).
To quantitate the association of partial obstruction with an increased incidence of high intraluminal pressure, the 99th percentile of the normal intraluminal pressure in the small intestine of normal animals was selected as a threshold to define high intraluminal pressure. We chose this threshold on the basis of the technique previously used by Hansen and colleagues (21) to evaluate high blood pressure. Similar results were obtained using other thresholds, such as average of the normal group + 3SD (18.44 mmHg) and average of all groups + 3SD (21.88 mmHg), which are not shown here for brevity.
In vivo ERK blockade.
In vivo, ERK activation was blocked using the MEK inhibitor PD-98059 (Calbiochem, La Jolla, CA) diluted to 20 mmol/l in sterile saline containing 0.1% DMSO and loaded into an osmotic pump (model 1003D, Alzet, Cupertino, CA). Filled pumps placed subcutaneously in the rats after Roux-en-Y surgery delivered PD-98059 at 26.75 μg·kg−1·day−1. Control rats were implanted with pumps filled with the 0.1% DMSO vehicle control in the same manner and location.
Mucosal ulcers.
A circumscribed ischemic mucosal ulcer was created by application of tissue paper disks (3.4 mm2) soaked with a 75% acetic acid solution to the small bowel serosa of each limb of the Roux-en-Y anastomosis or appropriate areas of intestine in control animals for 15 s according to a modification of a method that was originally described to create gastric ulcers (37) and was previously extended to jejunal ulcers in mice (16). All rats were euthanized 3 days after ulcer induction. Ulcerated segments were excised, and the mucosal ulcer was photographed through a dissecting microscope equipped with a digital camera (Q-color5, Olympus, Tokyo, Japan). Images were analyzed on a Kodak Image Station (Perkin Elmer, Boston, MA).
Immunohistochemical studies of proliferating cell nuclear antigen immunoreactivity.
Samples of intestinal segments from the proximal, distal, and Roux limbs were fixed in 10% formalin for 24 h and embedded in paraffin. Step sections (4.0 μm thick) were prepared from all the blocks and stained with hematoxylin and eosin. A PCNA kit (Zymed) was used for proliferating cell nuclear antigen (PCNA) immunohistochemical analysis of unstained, formalin-fixed paraffin sections. Sections were counterstained with hematoxylin for histological orientation and visualized and photographed (Microphot-FXA, Nikon, Tokyo, Japan).
Cell culture.
Caco-2BBE intestinal epithelial cells (CRL-2102, American Type Culture Collection), a common model of intestinal epithelial biology able to differentiate in culture (6), were maintained at 37°C with 8% CO2 in DMEM with 25 mM d-glucose, 4 mM glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 g/ml transferrin, 10 mM HEPES (pH 7.4), and 3.7 g/l NaHCO3 supplemented with 10% heat-inactivated fetal bovine serum. IEC-6 nonmalignant rat intestinal epithelial cells (CRL-1592, American Type Culture Collection) were maintained in 5% CO2 in high-glucose (4.5 g/l) DMEM supplemented with 10% fetal bovine serum and 0.1 U/ml bovine insulin.
Matrix and medium modulation.
For migration and proliferation studies, six-well petri dishes were precoated with 12.5 g/ml collagen I (Sigma Chemical, St. Louis, MO) or tissue fibronectin (Chifyx, Chicago, IL), and ERK activation was blocked by 30 min of pretreatment with PD-98059 (20 mmol/l). Control cells were treated with the 0.1% DMSO vehicle.
Motility measurement.
IEC-6 and Caco-2 cells cultured to confluence on sterile six-well plates precoated with tissue fibronectin or collagen I were subjected to 40 mmHg increased pressure and/or repetitive deformation over ambient pressure and/or repetitive deformation for 12 or 24 h, respectively, while control monolayers were maintained in the same incubator under ambient pressure. Small uniform circular wounds in the cell monolayer were created as previously described (7). A SPOT Advanced digital camera (version 3.0.6) attached to a microscope (Eclipse TE 300, Nikon) was used to photograph each hole at time 0 and 12 or 24 h after the initiation of strain, and wound areas were calculated using Kodak 1D software (version 3.6) on a Kodak Image Station. To account for variability in the size of the initial holes, the remaining wound area in each wound at 12 or 24 h was expressed as a ratio to the initial size of each individual wound. Migration was then expressed as the percentage of wound closure in each wound and calculated as 100 × (initial wound − final wound)/initial wound and compared with wound closure at ambient pressure.
Pressure regulation in vitro.
Pressure was controlled using an airtight box with an inlet valve for gas application and an outlet valve connected to a manometer (9). The box was prewarmed to 37°C. Temperature was maintained within ±2°C and pressure within ±1.5 mmHg.
Strain application.
For studies of repetitive deformation, cells were grown on Flexwell plates (Flexcell, McKeesport, PA) precoated with saturating concentrations of tissue fibronectin or type I collagen, as previously described (47). After induction of small uniform circular wounds, Flexwell plate membranes with monolayers of Caco-2 or IEC-6 cells were repetitively deformed for 24 and 12 h, respectively, utilizing a computer-controlled vacuum manifold (model FX4000, Flexcell) by 20-kPa vacuum at 10 cycles/min, producing an average 10% strain on the adherent cells during periods of deformation. For constant-strain experiments, cells were subjected to 10% constant stretch/deformation (Caco-2 for 24 h and IEC-6 for 12 h) after wound induction. Plates were maintained at 37°C in a humidified incubator with 5% CO2 during repetitive and constant deformation. Control plates were placed in the same incubator but were not attached to the Flexcell unit.
Proliferation.
IEC-6 and Caco-2 cells were seeded at 50,000 cells/well on six-well culture plates precoated with type I collagen or fibronectin for 12 or 24 h, respectively. Subconfluent (30–40%) cells were serum-starved for 24 h. A single six-well plate was reserved for a time 0 measurement, and the remaining serum-starved cells were incubated in normal growth medium under static or pressure conditions for 12 or 24 h before cells were counted using a crystal violet absorption assay within its linear range, as previously described. Cell number and cell area were determined in each of the six wells independently with an automated cell counter (Countess, Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Data from each experiment were analyzed with six observations in each group. Data represent one of at least three individual experiments with significant and similar results.
Protein isolation and Western blot analysis.
Mucosal scrapings from target intestinal segments after harvest were immediately immersed in ice-cold lysis buffer (50 mM Tris·HCl, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 1% deoxycholic acid, 1% glycerol, 10 mM sodium pyrophosphate, and 50 mM NaF). Tissue was homogenized using a Bullet Blender (Next Advance, Averill Park, NY) and then centrifuged at 15,000 g for 10 min at 4°C. Caco-2 and IEC-6 cells were lysed in lysis buffer, centrifuged at 15,000 g for 10 min at 4°C, and resolved by SDS-PAGE, as previously described (47). Membranes were blotted for phosphorylated ERK1/2 (pERK1 at Thr202/Tyr204 and pERK2 at Thr185/Tyr187) or total ERK (Cell Signaling Technology, Danvers, MA), as previously described (10). Membranes were then reprobed with antibodies specific for β-actin and appropriate fluorophore-conjugated secondary antibody as a loading control. Bands were visualized using the Odyssey imaging system (LI-COR, Lincoln, NE) and analyzed with the Kodak Image Station (model 440CF). All exposures of probed membranes used for densitometric analysis were within the linear range.
Data analysis.
Values are group means ± SE of the nontransformed data. Treatment effects were compared by Student's t-test or one- or two-way ANOVA using the SAS statistical software package (version 9.1.2, SAS, Cary, NC) followed by pair-wise comparisons using Fisher's protected least significance difference. Prior to analysis, we used the plot of predicted values vs. residuals, as well as the Shapiro-Wilk and Kolmogorov-Smirnov tests for normality, to ensure that all data fit a normal distribution. Two-tailed Student's t-test was used when appropriate. Skewed or nonnormally distributed data were logarithmically transformed prior to analysis, and the correction to a normal distribution was confirmed using the tests described above. Differences between means were considered significant at P < 0.05.
RESULTS
Defunctionalizing Roux-en-Y surgery alters contraction and reduces ulcer healing rate and proliferation in the defunctionalized Roux limb.
Similar to the anatomic changes we previously described in a preliminary characterization (24), the diameter of the defunctionalized bowel at euthanization (4.8 ± 0.22 mm) was 14.7 ± 2.01% and 11.56 ± 3.9% smaller than the diameter of the proximal or distal limbs of the anastomosis (5.68 ± 0.12 and 5.50 ± 0.1 mm, respectively, n = 6, P < 0.05; data not shown). The amplitude of contractions in the defunctionalized bowel was significantly reduced by 50.1 ± 8.5% compared with the amplitude of contractions in the same bowel segment in sham-operated animals and 51.2 ± 8.2% or 32 ± 12.9% lower than the amplitude of contractions in the proximal or distal limbs of the anastomosis (Fig. 1A; n = 4, P < 0.05).
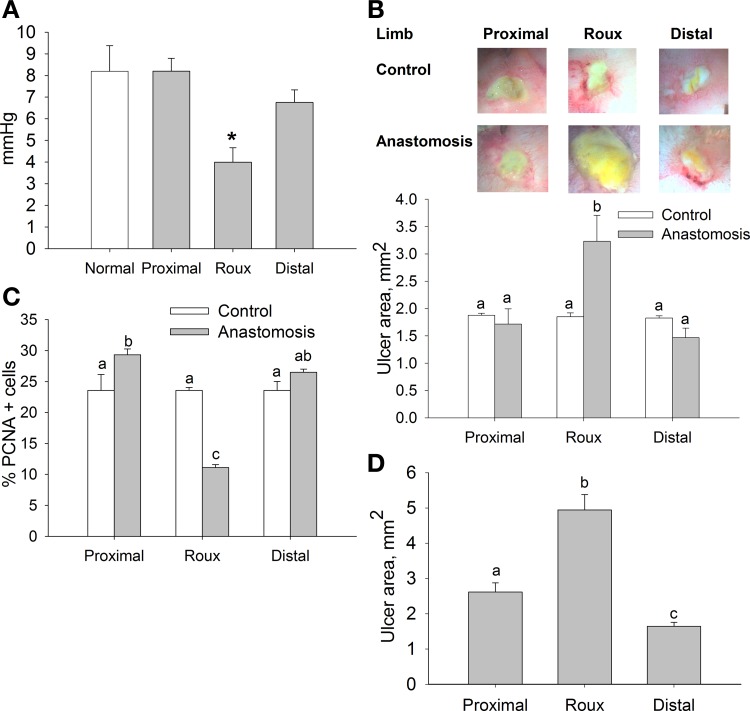
Fig. 1.
Impaired ulcer healing in defunctionalized rat intestinal limb. A: contraction amplitude in intact intestine of control animals and proximal, defunctionalized (Roux), and distal limbs of the anastomosis group (n = 4). *P < 0.05. B: representative images of 3-day ulcers in corresponding areas of control animals and animals subjected to Roux-en-Y anastomosis. Ulcer size was significantly greater in the Roux limb. C: proliferation in intestinal mucosa, assessed as percentage of proliferating cell nuclear antigen (PCNA)-positive cells, was lowest in the Roux limb (n = 5). D: healing was also impaired in the Roux limb in ulcers induced 30 days after Roux-en-Y anastomosis (n = 12). Bars without a common letter are significantly different at P < 0.05.
Mucosal ulcers were induced at surgery in the proximal, Roux, and distal limbs of the anastomosis to investigate the effects of defunctionalization on mucosal healing. At 24 h after surgery, ulcer area averaged 3.84 ± 0.11 mm2 and was similar in all three segments of the intestine (data not shown), confirming the reproducibility of the model. In intact animals, ulcer area was also not different 3 days after ulcer induction among the segments of the jejunum that were also studied in the experimental anastomotic preparation (Fig. 1B). In contrast, in rats in which a defunctionalizing anastomosis was constructed, ulcer diameter in the defunctionalized limb was 88.3 ± 27.9% and 102.8 ± 23.8% larger than in the proximal and distal limbs of the anastomosis 3 days after surgery (n = 9, P < 0.05). The defunctionalized limb had the lowest PCNA immunoreactivity: 52.4 ± 5.8% and 44.2 ± 2.8% lower than the proximal and distal limbs in control animals (Fig. 1C; n = 5, P < 0.05). In parallel studies, we confirmed the sustained effect of the loss of function on mucosal wound healing. In these studies, anastomoses were created and ulcers were induced 30 days after the anastomosis at a second celiotomy. Results were similar to those in the rats in which anastomoses and ulcers were created simultaneously. At 30 days after anastomotic surgery and 3 days after ulcer creation, ulcers were 98.8 ± 8.3% and 139.2 ± 15.2% larger in the defunctionalized bowel than in the simultaneously created proximal or distal limbs of the anastomosis, respectively (Fig. 1D; n = 12, P < 0.05). Interestingly, ulcers in the distal limb of the anastomosis in this group were 42.5 ± 12.4% smaller than in proximal limb (Fig. 1D).
Defunctionalizing Roux-en-Y surgery with partial obstruction alters anatomic characteristics and intraluminal pressure in partially obstructed intestine.
Since patterns of variation in ulcer healing in these rat anastomoses were different from those previously observed in partially obstructed mouse intestine (16), we constructed partially obstructed anastomoses in the rats to determine whether the difference between the above-described results and those reported previously reflects a species difference or the effects of partial obstruction. Rats with partial obstruction tolerated feeding, and the Roux-en-Y anastomosis was probe-patent at the time of harvest, with luminal chyme present in the distal bowel.
The diameter of the proximal and defunctionalized limbs was 26.88 ± 8.7% and 23.61 ± 9.09% larger in rats with partially obstructed anastomosis than in control animals (Fig. 2A; n = 6, P < 0.05). The 99th percentile of the normal pressure of sham-operated animals was selected as a threshold for high intraluminal pressure (21). Compared with sham-operated animals, partial obstruction significantly increased the incidence of high pressure by 13.6-fold in the proximal limb and 2.6-fold in the defunctionalized limb (Fig. 2B; n = 4, P < 0.05). In unobstructed intestinal anastomoses, the incidence of high pressure was not significantly different among any of the intestinal anastomotic segments or normal small intestine (1.3 ± 0.61%, n = 4; data not shown). Similarly, the amplitude of the contractions in the proximal limb in partially obstructed anastomosis was 15.32 ± 1.27 mmHg, significantly higher than in normal animals (8.19 ± 1.18 mmHg), while the amplitude of the contractions in the defunctionalized and distal limb of partially obstructed anastomoses did not differ from control (9 ± 1.4 and 6.4 ± 0.51 mmHg, respectively; data not shown).
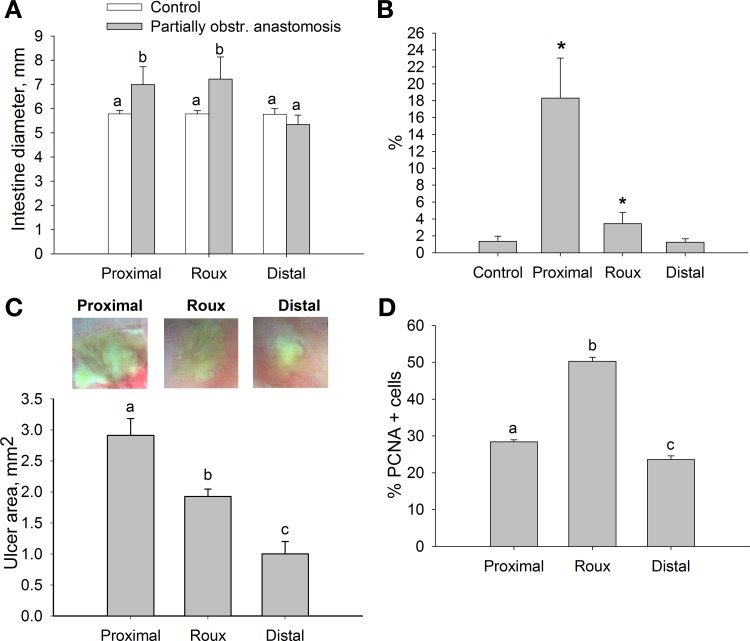
Fig. 2.
Partial obstruction of the Roux-en-Y anastomosis increases intraluminal pressures and impairs upstream ulcer healing, despite increased proliferation. A: intestinal diameter of proximal and Roux limbs was increased in partially obstructed anastomosis 3 days after surgery (n = 6). B: incidence of pressures exceeding 99% of the pressure of normal nonoperated animals was higher in proximal and Roux limbs of partially obstructed rats (n = 4). *P < 0.05. C: representative images of ulcers (top) and histogram showing ulcer size (bottom) in proximal, Roux, and distal limbs of partially obstructed animals. Ulcer size was greatest in the proximal limb experiencing the highest incidence of increased pressure (n = 9). D: proliferation was elevated in proximal and Roux limbs compared with distal limb in partially obstructed rats (n = 4). Bars without a common letter are significantly different at P < 0.05.
Defunctionalizing Roux-en-Y surgery with partial obstruction alters the healing rate and proliferation in partially obstructed intestine.
Ulcer size in response to the partially obstructing anastomosis was significantly increased in the proximal bowel compared with the defunctionalized and distal bowel by 30.1 ± 7.5% and 65.6 ± 6.5%, respectively (Fig. 2C; n = 9, P < 0.05). However, in rats with a partially obstructing anastomosis, the ulcer size was 35.5 ± 3.6% larger in the defunctionalized bowel than in the distal bowel (Fig. 2C; n = 9, P < 0.05). The partially obstructed anastomosis-defunctionalized limb contained the highest PCNA immunoreactivity independent of the similar pressure effect and dilations due to obstructions in the proximal limb in obstructed animals (Fig. 2D; n = 4).
Pressure reduces intestinal Caco-2 and IEC-6 cell migration on any substrate in an ERK-independent manner.
Although defunctionalization without or with partially obstructing anastomosis would be expected to change the forces to which the mucosa is subject, this procedure could also affect many other influences on the mucosa, including patterns of energy metabolism, blood flow, food absorption, and secretion. To determine whether isolated changes in pressure or cyclic deformation per se could alter intestinal epithelial migration or proliferation, we seeded Caco-2 cells on different substrates and exposed them to 40 mmHg increased extracellular pressure, cyclic strain, or the combination of both. We chose 40 mmHg pressure on the basis of our previous observation that pressure has similar antimotogenic effects between 20 and 40 mmHg (16). Proximal limbs of the partially obstructed anastomoses in vivo had significantly higher incidences of high pressures exceeding 18.33 mmHg (99th percentile of normal animal), which approximates 20 mmHg, while maximal pressure in the partially obstructed proximal bowel was 58.5 ± 5.1 mmHg. We assessed migration in each condition by measuring circular wound closure. Migration of human Caco-2 intestinal epithelial cells on collagen I was significantly reduced after exposure to pressure, strain, or the combination of both for 24 h vs. static controls (Fig. 3A; n = 15, P < 0.05). Studies in nonmalignant immortalized rat small intestinal IEC-6 cells yielded similar results (Fig. 3B; n = 15, P < 0.05).
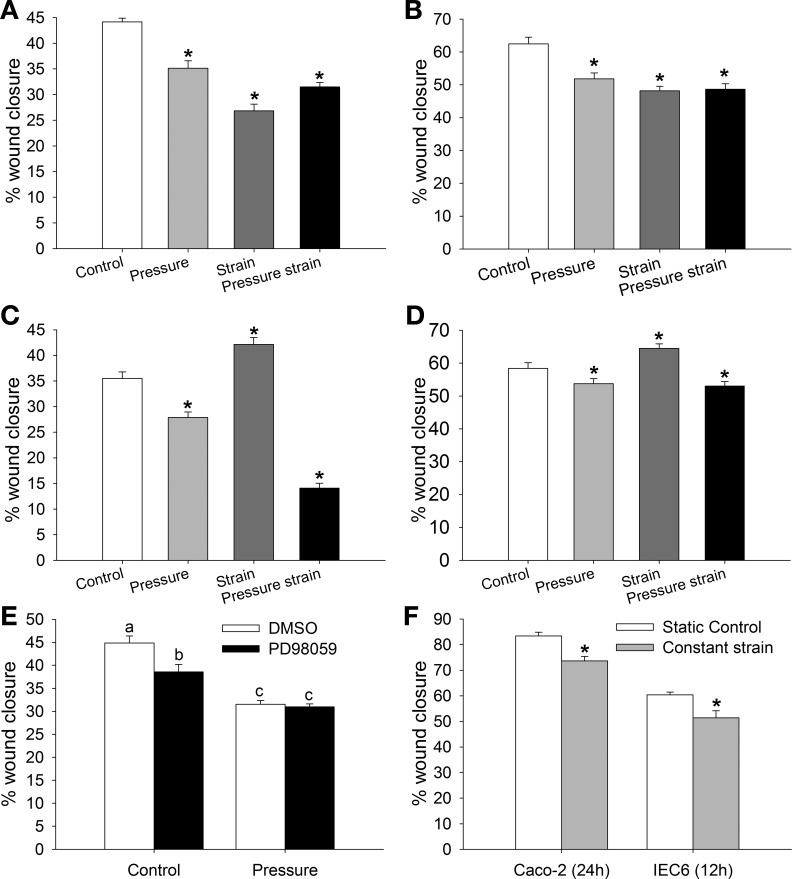
Fig. 3.
Effects of pressure, strain, and the combination of pressure and strain on migration of Caco-2 (A and C) and IEC-6 (B, D, and E) cells grown on a collagen I (A, B, and E) or tissue fibronectin (C and D) matrix. A and B: pressure, strain, or the combination of pressure and strain inhibits migration of Caco-2 (A) and IEC-6 (B) cells grown on collagen I (n = 15). C and D: strain enhances and pressure decreases migration of Caco-2 (C) and IEC-6 (D) cells grown on tissue fibronectin. Pressure effect predominates when cells are exposed to the combination of pressure and strain (n = 15). E: pretreatment with the MEK inhibitor PD-98059 lowers basal migration of IEC-6 cells grown on a collagen I matrix but has no effect in cells exposed to increased pressure for 12 h (n = 15). Bars without a common letter are significantly different at P < 0.05. F: migration of Caco-2 and IEC-6 cells on collagen I after exposure to constant strain for 24 and 12 h, respectively (n = 6). Constant strain decreases migration in each cell type. *P < 0.05 (by Student's t-test).
We previously showed that matrix can change the motogenic effects of strain (47). The response of Caco-2 and IEC-6 cells cultured on membranes covered with tissue fibronectin to repetitive deformation was different from that of cells grown on collagen I substrates. Pressure reduced migration of Caco-2 and IEC-6 cells on tissue fibronectin, whereas strain increased wound closure compared with control cells in the static condition (n = 15). However, as observed in cells grown on collagen I, combined pressure and strain significantly reduced wound closure in cells grown on fibronectin (Fig. 3, C and D; n = 15, P < 0.05 for each).
ERK signaling may change the motogenic properties of murine fibroblasts and Caco-2 cells (15, 46). In rat intestinal IEC-6 cells, the MEK inhibitor of ERK signaling PD-98059 significantly reduced wound closure in response to increased pressure compared with static control (P < 0.05), but the antimotogenic effects of PD-98059 were lost when the cells were exposed to 40 mmHg (Fig. 3E; n = 15).
Constant strain may mimic the modification of the intestinal wall under conditions of increased pressure. Migration of Caco-2 and IEC-6 intestinal epithelial cells was significantly reduced after exposure to 10% constant strain (Fig. 3F; n = 6, P < 0.05).
Pressure increases intestinal Caco-2 and IEC-6 cell proliferation on any substrate.
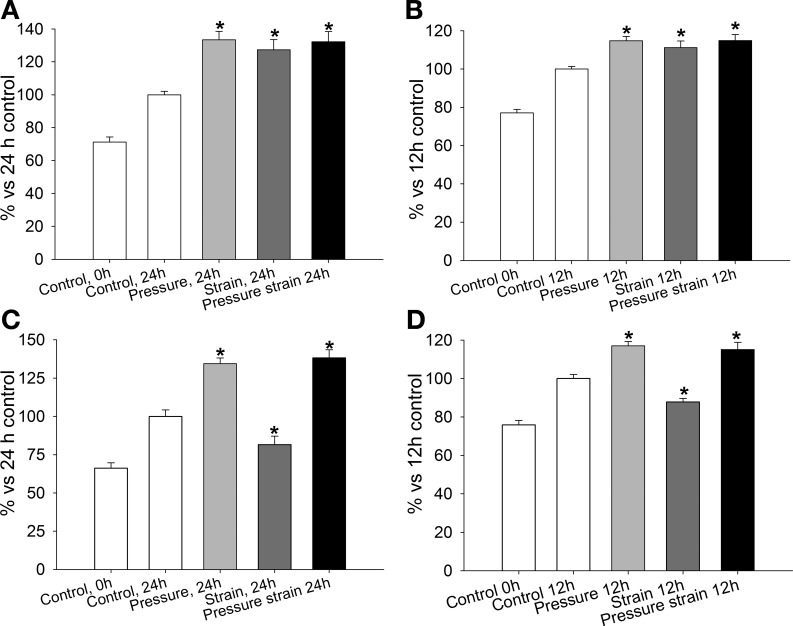
To confirm the proliferative changes in anastomosed intestinal limbs in normal and obstructed animals, we tested the effect of pressure, repetitive deformation, and their combination on proliferation of Caco-2 and IEC-6 cells grown on collagen I or tissue fibronectin substrates. Pressure and repetitive deformation stimulate Caco-2 cell proliferation on collagen I and tissue fibronectin (40, 46). However, the combined effect of pressure and strain has not been studied. On collagen I, proliferation was increased in Caco-2 (Fig. 4A; n = 12) and IEC-6 (Fig. 4B; n = 12) cells exposed to pressure, repetitive deformation, or the combination of both when compared with static cells under ambient pressure (P < 0.05). Similarly, proliferation of Caco-2 (Fig. 4C; n = 12) and IEC-6 (Fig. 4D; n = 12) cells grown under 40 mmHg pressure on tissue fibronectin was significantly increased vs. intact control. Conversely, repetitive deformation significantly reduced absolute cell number in Caco-2 and IEC-6 cell lines over 24 and 12 h, respectively (P < 0.05). However, exposure of the cells to the combination of pressure and repetitive deformation significantly increased cell number compared with 24-h control.
Fig. 4.
Proliferative effects of pressure, strain, and the combination of pressure and strain on Caco-2 (A and C) and IEC-6 (B and D) cells grown on collagen I or tissue fibronectin matrix. A and B: pressure, strain, and the combination of pressure and strain significantly enhance proliferation of Caco-2 (A) and IEC-6 (B) cells grown on collagen I (n = 12). C and D: pressure decreases and strain increases proliferation of Caco-2 (C) and IEC-6 (D) cells grown on tissue fibronectin. Proliferation is significantly higher in both cell types after exposure to pressure + strain (n = 12). *P < 0.05.
Suppression of ERK signaling changes healing rate in the Roux-en-Y model.
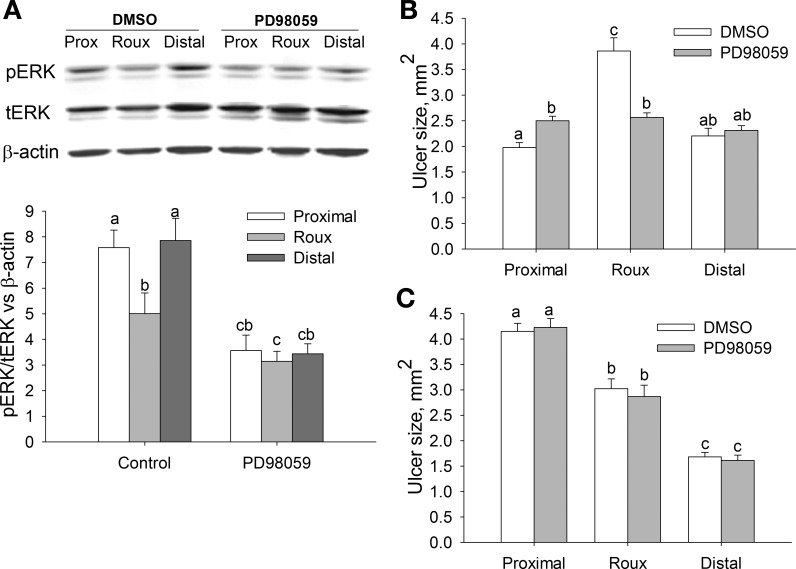
Next, we wanted to confirm our in vitro observation of the importance of ERK signaling to the motogenic effects of strain in human intestinal epithelial cells in vivo (47). The ratio of phosphorylated ERK (pERK) to total ERK (tERK) was significantly lower in the defunctionalized limb of the Roux-en-Y animals than in the proximal and distal limbs (Fig. 5A; n = 7, P < 0.05). PD-98059 lowered the pERK-to-tERK ratio in proximal, defunctionalized, and distal limbs of surgically modified, treated animals compared with the corresponding intestinal limbs of DMSO-treated animals (Fig. 5A; n = 6, P < 0.05).
Fig. 5.
ERK inhibition improves ulcer healing in the defunctionalized (Roux) limb but has no effect on ulcer healing in partially obstructed animals. A: representative Western blots (top) and densitometric analysis (bottom) of phosphorylated ERK (pERK), total ERK (tERK), and β-actin in proximal (Prox), Roux, and distal limbs of the anastomosis after treatment with vehicle (0.1% DMSO) or the MEK inhibitor PD-98059 (26.75 μg·kg−1·day−1) (n = 6–7). pERK was significantly decreased in the Roux limb of vehicle-treated animals; treatment was effective, as PD-98059 lowered pERK in all limbs. B: PD-98059 significantly improved ulcer healing in the Roux limb of nonobstructed animals (n = 10). C: ERK inhibition by PD-98059 had no effect on ulcer healing in partially obstructed animals (n = 10). Bars without a common letter are significantly different at P < 0.05.
The effect of PD-98059 on ulcer healing varied among the different limbs. Ulcer size in the proximal limb was significantly larger (26.6 ± 6.1%) in PD-98059- than DMSO-treated animals 3 days after surgery (Fig. 5B; n = 10, P < 0.05). Ulcer size in DMSO-treated animals was significantly larger (95.4 ± 10.7% and 83.9 ± 6.7%) in the Roux limb than in the proximal or distal limb (P < 0.05). However, ERK signal inhibition improved ulcer healing (65.6 ± 12.8% smaller size) with loss of strain in the defunctionalized limb (Fig. 5B; n = 10, P < 0.05).
The effect of defunctionalization or PD-98059 treatment on ulcer size was lost in animals with obstructed anastomosis. Ulcer size did not differ in each limb between DMSO- or PD-98059-treated animals. However, ulcer size in the DMSO- and PD-98059-treated animals was significantly greater (27.1 ± 1.7% and 59.4 ± 3.8% in DMSO and 32.8 ± 3.3% and 63.1 ± 4.7% in PD-98059) in the proximal limb than in the Roux or distal limb of corresponding animals (Fig. 5C; P < 0.05, n = 10).
Interaction of pressure, loss of function, and ERK signaling inhibition on proliferation rate in the defunctionalized limb.
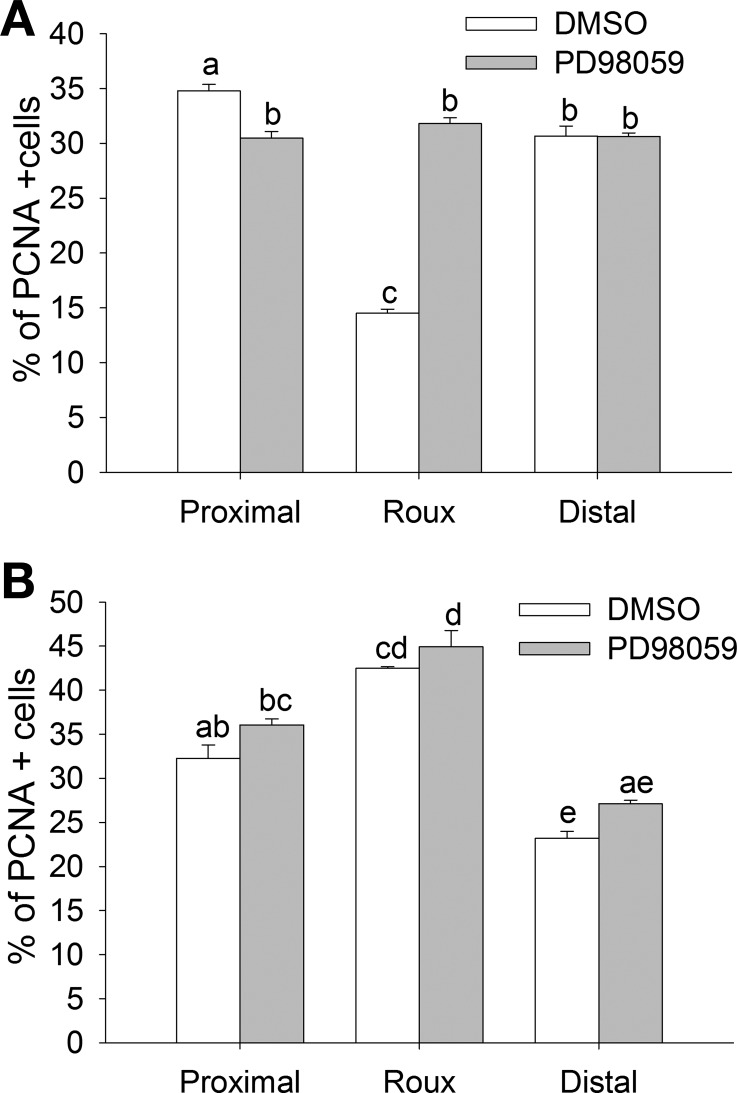
PCNA immunoreactivity was higher in the mucosa of the proximal limb of PD-98059-treated rats than the DMSO controls (Fig. 6A). Proliferation rate in the defunctionalized limb of DMSO-treated animals was reduced by 58.3 ± 5.4% and 46.4 ± 3.13% compared with the proximal and distal limb. This was restored to the level in the other limbs by PD-98059 treatment (Fig. 6A; n = 3, P < 0.05). Obstruction of the anastomosis resulted in a significant increase in proliferation rate in the defunctionalized limb of DMSO- or PD-98059-treated animals (Fig. 6B; n = 5, P < 0.05). In the obstructed animals, proliferation rate was higher in the proximal anastomotic limb of DMSO- and PD-98059-treated animals than in the distal limb (P < 0.05). Blocking ERK in vivo with PD-98059 did not have a significant effect on epithelial cell proliferation in animals with obstructed bowel compared with DMSO-treated animals.
Fig. 6.
Reduced proliferation in the defunctionalized Roux limb can be reversed by ERK inhibition or increased pressure as a result of partial obstruction. A: in nonobstructed animals, PD-98059 normalized proliferation rate in the defunctionalized Roux limb. B: PD-98059 had no further effect on the already high proliferation rate in proximal and Roux limbs of partially obstructed animals. Bars without a common letter are significantly different at P < 0.05.
DISCUSSION
The results of this study suggest that defunctionalized intestinal mucosa heals more slowly. We observed larger ulcer size and decreased proliferation in the defunctionalized bowel, but our in vitro data also suggest that cell migration is likely decreased as well and that the absence of repetitive deformation from in-continuity passage of the luminal contents through that segment of the gut could explain these phenomena. Partially obstructed intestine also healed more slowly, despite an increase in proliferative rate. ERK blockade eliminated differences in ulcer healing in the defunctionalized unobstructed bowel, but not the effects of partial obstruction.
Ulcer size at day 3 was reproducibly larger in the defunctionalized unobstructed bowel, although there were no differences in the size of the initial ulcer. Certainly, many factors could be altered in defunctionalized gut mucosa in this model, including the absence of luminal nutrients, differences in peristalsis frequency after disconnection from the proximal pacemaker, decreased villous motility because of the absence of luminal nutrients (44), changes in blood flow, and other factors. However, unlike total parenteral nutrition (TPN) models of mucosal atrophy, we would not expect differences in overall nutrition and its systemic sequelae, as each rat continued to eat normally and served as its own control. TPN initiates mucosal atrophy of the intestine associated with reduced cell proliferation and reduced cellular signaling (14). In patients with intestinal resection, proliferation rate was increased and morphological parameters were improved during the refeeding period after TPN (33). Additionally, atrophic processes may also change mucosal integrity, increasing intestinal epithelium permeability (22, 45). Moreover, consistent with previous in vitro studies (7, 47), our present in vitro study suggests that a decrease in or loss of the repetitive deformation to which villous motility or intermittent peristaltic contractility subject the enterocytes may be sufficient to explain the relative decrease in ulcer healing in the defunctionalized bowel. In vitro, cyclic strain promotes intestinal epithelial proliferation on most matrix substrates but suppresses intestinal epithelial migration across tissue fibronectin (47). Tissue fibronectin is strongly deposited into the extracellular matrix in and immediately around mucosal ulcers in this model and in vivo (25, 39). Thus, if decreased deformation in the defunctionalized bowel were important, one would predict decreased ulcer healing from decreased epithelial sheet migration at the fibronectin-rich edge of the ulcer, with decreased proliferation farther away on the relatively fibronectin-poor basement membrane, as we observed here. As most ulcer healing in the first 24–48 h is restitutive, one would predict that changes in rates of migration would dominate ulcer healing rates in this model, and, indeed, the finding that, in the partially obstructed model, we observed increased proliferation but still decreased ulcer healing in the defunctionalized limb would be consistent with this hypothesis. One possible concern would be that the defunctionalization and ulcerogenesis occurred simultaneously and that acute defunctionalization might have other effects. However, parallel studies in rats in which the defunctionalization was performed 30 days prior to ulcer induction at a second operation yielded similar results.
Phosphorylation of ERK1 at Thr202 or Tyr204, as well as ERK2 at Thr185 and Tyr187, leads to its increased kinase activity (34), which in turn leads to activation of ERK target proteins, such as ribosomal S6 kinase (36), cytosolic phospholipase A2 (28), and cytoskeletal proteins (23). Although we did not measure the activity of ERK1 or ERK2 kinase activity directly in this study, such ERK phosphorylation is commonly used as a surrogate for ERK activation in many settings, including the effects of physical forces on intestinal epithelial cells (46), cardiac myocytes (27), and pulmonary epithelial H441 cells (8). Furthermore, ERK phosphorylation at these sites correlates with ERK activation by in vitro kinase activity in response to repetitive deformation in human Caco-2 intestinal epithelial cells in vitro (26), as well as with the activation of downstream ERK targets such as Elk and ribosomal S6 kinase (19). We previously described a key role for ERK signaling in mediating the mitogenic effects of cyclic strain on collagen substrates and the motogenic effects of cyclic strain on fibronectin (47). Indeed, ERK activation was lower in the defunctionalized bowel in vivo, and ERK blockade appeared to eliminate differences in ulcer healing and mucosal PCNA staining index among the proximal, distal, and defunctionalized mucosa. This observation further supports the concept that differences in force-driven ERK signaling are important in the effects of defunctionalization on mucosal healing and suggests a proof-of-principle that modulation of deformation-driven signal pathways can modulate mucosal biology in some pathophysiological states in which forces are abnormal. In this work, we did not study the effects of ERK signaling changes on its potential downstream targets. ERK has numerous cytoplasmic and nucleic substrates, including myosin light chain kinase (23), kinase activity of nuclear Elk (19), and the expression of ERK target genes such as Fos and Jun (12). The potential role of these and other ERK downstream signals in mediating the effects of physical forces on intestinal epithelial cells in vivo awaits further study. These observations seemed at first to differ from previous preliminary studies from our laboratory in a similar model in mice (16). In those studies, we observed decreased ulcer healing in the defunctionalized bowel and the bowel proximal to the anastomosis compared with the distal gut, as well as a difference between the proximal bowel, which was in continuity, and the defunctionalized gut. In the second group of studies described here, we created deliberately narrowed anastomoses to determine whether the differences in our observations represented species differences or the effects of the partial obstruction. Indeed, partially obstructed rat anastomoses yielded the same effects as partially obstructed murine anastomoses.
This prompted us to return to in vitro models to compare the effects of pressure and cyclic strain on intestinal epithelial monolayer wound closure and proliferation. While no in vitro model can ever reproduce the complex temporal and spatial pattern of different forces to which the intestinal mucosa is subject in vivo, the frequency and amplitude of repetitive deformation studied here are similar in magnitude and, if anything, more conservative than might be expected in vivo (29). Intra-abdominal pressures in bowel obstruction can exceed 30 mmHg above normal (42). Changes >20 mmHg in obstructed intestine may lead to impaired regional blood flow (13). Pressure was constant in our in vitro model, while strain was variable. However, very low-frequency strain has no effect on intestinal epithelial proliferation (32). We observed that constant strain mimicked the effect of pressure on any substrate, which may indicate that similar mechanisms may be involved. Moreover, different physical forces have very different effects in other cell types and systems. Vascular cells respond to the high frequency of 60–90 cycles/min that is observed in blood vessels (17), while human osteoblastic cells are responsive to 6 cycles/min (30). Diederichs et al. (11) reported that ERK signaling is activated by strain but is not necessary for strain-induced differentiation of human bone marrow stromal cells.
We confirmed that pressure induced proliferation and suppressed migration of Caco-2 and IEC-6 cells independent of matrix and cell line. Reduced healing of mucosal ulcers in the proximal limb is consistent with that of pressure-driven reduction of fibroblast migration on fibronectin (15) or Caco-2 cells on collagen (16). Conversely, healing in the Roux limb might be improved by a pressure-induced increase in proliferation, since such a stimulatory effect was previously observed in vivo in the biliary duct (35) or in SW620 and HCT 116 cells in vitro (40).
Strain stimulates intestinal epithelial proliferation on collagen and laminin substrates by a complex cytoskeletal-dependent pathway that involves PKC, focal adhesion kinase, Src, ERK, and several other signal molecules (5, 40, 46). Rhythmic distension increases mucosal tyrosine kinase activity in jejunum and colon in vivo (2). Increased pressure due to obstruction of the intestine impaired healing and increased ERK signaling in mice (16). In a fibronectin-rich environment, however, the signal network shifts and stimulates migration, while slightly inhibiting proliferation, in an ERK-independent manner (46). In contrast, increases in extracellular pressure stimulate intestinal epithelial proliferation in vitro but inhibit wound closure independently of ERK (40). Thus, while the decrease in deformation in the unobstructed defunctionalized bowel may be sufficient to explain decreased mucosal healing with decreased proliferation in this setting, the increase in luminal pressure proximal to a partially obstructing anastomosis might explain the decreased healing and increased proliferation in the partially obstructed mucosa. The inability of ERK blockade to ablate these differences in vivo is consistent with our in vitro observations that pressure effects are independent of ERK and that the antimotogenic effects of pressure can overcome the motogenic effects of cyclic strain.
In conclusion, loss of function in a rat Roux-en-Y model of anastomosis reduces mucosal ulcer healing. Factors that may contribute to delaying the healing process include pressure and reduced food flow due to impaired contractility. Mechanical forces in the intestine may influence mucosal wound healing via mechanisms involving ERK signaling. ERK inhibition can suppress recovery in normal intestine but improve it in defunctionalized limbs. Our work indicates that intraluminal pressure must also be monitored and should be reduced in patients with intestinal atrophy and ulcerations because of its overwhelming impact on the effects of other modulators. Further studies on ERK modulation during mucosal wound healing are required to provide additional insight into mechanisms capable of maintaining strain in the intestine to support adequate cell migration and proliferation.
GRANTS
This study was supported in part by a Veterans Affairs Merit Research Award (M. D. Basson) and National Institutes of Health Grants RO1 DK-067257 (M. D. Basson) and T32 GM-008420 (M. D. Basson and T. L. Flanigan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.L.K., T.L.F., and M.D.B. are responsible for conception and design of the research; P.L.K., T.L.F., and L.C. performed the experiments; P.L.K. and T.L.F. analyzed the data; P.L.K., T.L.F., and M.D.B. interpreted the results of the experiments; P.L.K., T.L.F., and L.C. prepared the figures; P.L.K. and T.L.F. drafted the manuscript; P.L.K. and M.D.B. edited and revised the manuscript; P.L.K., T.L.F., L.C., and M.D.B. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Hannah Garver and Dr. Fink for technical assistance with in vivo intraluminal pressure measurements, Drs. Cheri R. Owen and Christopher Gayer for technical assistance with the animal studies, Dr. Mary F. Walsh for reading and commenting on the manuscript, and Dr. Mark M. Meerschaert for assistance with statistical analysis of the data.
REFERENCES
- 1. Andrioli A, Wilmer A, Coremans G, Vandewalle J, Janssens J. Computer-supported analysis of continuous ambulatory manometric recordings in the human small bowel. Med Biol Eng Comput 34: 336–343, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Basson MD, Coppola CP. Repetitive deformation and pressure activate small bowel and colonic mucosal tyrosine kinase activity in vivo. Metabolism 51: 1525–1527, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Basson MD, Rashid Z, Turowski GA, West AB, Emenaker NJ, Sgambati SA, Hong F, Perdikis DM, Datta S, Madri JA. Restitution at the cellular level: regulation of the migrating phenotype. Yale J Biol Med 69: 119–129, 1996 [PMC free article] [PubMed] [Google Scholar]
- 4. Brodribb AJ, Condon RE, Cowles V, DeCosse JJ. Effect of dietary fiber on intraluminal pressure and myoelectrical activity of left colon in monkeys. Gastroenterology 77: 70–74, 1979 [PubMed] [Google Scholar]
- 5. Chaturvedi LS, Marsh HM, Basson MD. Src and focal adhesion kinase mediate mechanical strain-induced proliferation and ERK1/2 phosphorylation in human H441 pulmonary epithelial cells. Am J Physiol Cell Physiol 292: C1701–C1713, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Chaturvedi LS, Marsh HM, Shang X, Zheng Y, Basson MD. Repetitive deformation activates focal adhesion kinase and ERK mitogenic signals in human Caco-2 intestinal epithelial cells through Src and Rac1. J Biol Chem 282: 14–28, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Chaturvedi LS, Saad SA, Bakshi N, Marsh HM, Basson MD. Strain matrix-dependently dissociates gut epithelial spreading and motility. J Surg Res 156: 217–223, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chess PR, Toia L, Finkelstein JN. Mechanical strain-induced proliferation and signaling in pulmonary epithelial H441 cells. Am J Physiol Lung Cell Mol Physiol 279: L43–L51, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Craig DH, Owen CR, Conway WC, Walsh MF, Downey C, Basson MD. Colchicine inhibits pressure-induced tumor cell implantation within surgical wounds and enhances tumor-free survival in mice. J Clin Invest 118: 3170–3180, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Craig DH, Zhang J, Basson MD. Cytoskeletal signaling by way of α-actinin-1 mediates ERK1/2 activation by repetitive deformation in human Caco2 intestinal epithelial cells. Am J Surg 194: 618–622, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diederichs S, Freiberger F, van Griensven M. Effects of repetitive and short time strain in human bone marrow stromal cells. J Biomed Mater Res A 88: 907–915, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Ebisuya M, Kondoh K, Nishida E. The duration, magnitude and compartmentalization of ERK MAP kinase activity: mechanisms for providing signaling specificity. J Cell Sci 118: 2997–3002, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Enochsson L, Nylander G, Ohman U. Effects of intraluminal pressure on regional blood flow in obstructed and unobstructed small intestines in the rat. Am J Surg 144: 558–561, 1982 [DOI] [PubMed] [Google Scholar]
- 14. Feng Y, McDunn JE, Teitelbaum DH. Decreased phospho-Akt signaling in a mouse model of total parenteral nutrition: a potential mechanism for the development of intestinal mucosal atrophy. Am J Physiol Gastrointest Liver Physiol 298: G833–G841, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flanigan TL, Craig DH, Gayer CP, Basson MD. The effects of increased extracellular deformation, pressure, and integrin phosphorylation on fibroblast migration. J Surg Res 156: 103–109, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flanigan TL, Owen CR, Gayer C, Basson MD. Supraphysiologic extracellular pressure inhibits intestinal epithelial wound healing independently of luminal nutrient flow. Am J Surg 196: 683–689, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frangos SG, Knox R, Yano Y, Chen E, Di Luozzo G, Chen AH, Sumpio BE. The integrin-mediated cyclic strain-induced signaling pathway in vascular endothelial cells. Endothelium 8: 1–10, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Gayer CP, Basson MD. The effects of mechanical forces on intestinal physiology and pathology. Cell Signal 21: 1237–1244, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gayer CP, Craig DH, Flanigan TL, Reed TD, Cress DE, Basson MD. ERK regulates strain-induced migration and proliferation from different subcellular locations. J Cell Biochem 109: 711–725, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Granger DN, Barrowman JA. Microcirculation of the alimentary tract. I. Physiology of transcapillary fluid and solute exchange. Gastroenterology 84: 846–868, 1983 [PubMed] [Google Scholar]
- 21. Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. JAMA 298: 874–879, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Hernandez G, Velasco N, Wainstein C, Castillo L, Bugedo G, Maiz A, Lopez F, Guzman S, Vargas C. Gut mucosal atrophy after a short enteral fasting period in critically ill patients. J Crit Care 14: 73–77, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol 137: 481–492, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kovalenko PL, Basson MD. Changes in morphology and function in small intestinal mucosa after Roux-en-Y surgery in a rat model. J Surg Res 177: 63–69, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levitan BN, Perova N, Astakhin AV, Trubnikov GA. [Plasma fibronectin in gastric and duodenal ulcer]. Klin Med (Mosk) 77: 31–33, 1999 [PubMed] [Google Scholar]
- 26. Li W, Duzgun A, Sumpio BE, Basson MD. Integrin and FAK-mediated MAPK activation is required for cyclic strain mitogenic effects in Caco-2 cells. Am J Physiol Gastrointest Liver Physiol 280: G75–G87, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Liang F, Lu S, Gardner DG. Endothelin-dependent and -independent components of strain-activated brain natriuretic peptide gene transcription require extracellular signal regulated kinase and p38 mitogen-activated protein kinase. Hypertension 35: 188–192, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell 72: 269–278, 1993 [DOI] [PubMed] [Google Scholar]
- 29. Madl C, Druml W. Gastrointestinal disorders of the critically ill. Systemic consequences of ileus. Best Pract Res Clin Gastroenterol 17: 445–456, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Matsuda N, Morita N, Matsuda K, Watanabe M. Proliferation and differentiation of human osteoblastic cells associated with differential activation of MAP kinases in response to epidermal growth factor, hypoxia, and mechanical stress in vitro. Biochem Biophys Res Commun 249: 350–354, 1998 [DOI] [PubMed] [Google Scholar]
- 31. McNeil PL, Ito S. Molecular traffic through plasma membrane disruptions of cells in vivo. J Cell Sci 96: 549–556, 1990 [DOI] [PubMed] [Google Scholar]
- 32. Osada T, Watanabe S, Tanaka H, Hirose M, Miyazaki A, Sato N. Effect of mechanical strain on gastric cellular migration and proliferation during mucosal healing: role of Rho dependent and Rac dependent cytoskeletal reorganisation. Gut 45: 508–515, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pironi L, Paganelli GM, Miglioli M, Biasco G, Santucci R, Ruggeri E, Di Febo G, Barbara L. Morphologic and cytoproliferative patterns of duodenal mucosa in two patients after long-term total parenteral nutrition: changes with oral refeeding and relation to intestinal resection. JPEN J Parenter Enteral Nutr 18: 351–354, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Robbins DJ, Zhen E, Owaki H, Vanderbilt CA, Ebert D, Geppert TD, Cobb MH. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J Biol Chem 268: 5097–5106, 1993 [PubMed] [Google Scholar]
- 35. Slott PA, Liu MH, Tavoloni N. Origin, pattern, and mechanism of bile duct proliferation following biliary obstruction in the rat. Gastroenterology 99: 466–477, 1990 [DOI] [PubMed] [Google Scholar]
- 36. Sturgill TW, Ray LB, Erikson E, Maller JL. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature 334: 715–718, 1988 [DOI] [PubMed] [Google Scholar]
- 37. Tarnawski A, Hollander D, Krause WJ, Dabros W, Stachura J, Gergely H. “Healed” experimental gastric ulcers remain histologically and ultrastructurally abnormal. J Clin Gastroenterol 12 Suppl 1: S139–S147, 1990 [DOI] [PubMed] [Google Scholar]
- 38. Toumadre JP, Barclay M, Fraser R, Dent J, Young R, Berce M, Jury P, Fergusson L, Burnett J. Small intestinal motor patterns in critically ill patients after major abdominal surgery. Am J Gastroenterol 96: 2418–2426, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Verspaget HW, Biemond I, Allaart CF, van Weede H, Weterman IT, Gooszen HG, Pena AS, Lamers CB. Assessment of plasma fibronectin in Crohn's disease. Hepatogastroenterology 38: 231–234, 1991 [PubMed] [Google Scholar]
- 40. Walsh MF, Woo RK, Gomez R, Basson MD. Extracellular pressure stimulates colon cancer cell proliferation via a mechanism requiring PKC and tyrosine kinase signals. Cell Prolif 37: 427–441, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Watters DA, Smith AN, Eastwood MA, Anderson KC, Elton RA. Mechanical properties of the rat colon: the effect of age, sex and different conditions of storage. Q J Exp Physiol 70: 151–162, 1985 [DOI] [PubMed] [Google Scholar]
- 42. Williams M, Simms HH. Abdominal compartment syndrome: case reports and implications for management in critically ill patients. Am Surg 63: 555–558, 1997 [PubMed] [Google Scholar]
- 43. Womack WA, Barrowman JA, Graham WH, Benoit JN, Kvietys PR, Granger DN. Quantitative assessment of villous motility. Am J Physiol Gastrointest Liver Physiol 252: G250–G256, 1987 [DOI] [PubMed] [Google Scholar]
- 44. Womack WA, Mailman D, Kvietys PR, Granger DN. Neurohumoral control of villous motility. Am J Physiol Gastrointest Liver Physiol 255: G162–G167, 1988 [DOI] [PubMed] [Google Scholar]
- 45. Yang H, Finaly R, Teitelbaum DH. Alteration in epithelial permeability and ion transport in a mouse model of total parenteral nutrition. Crit Care Med 31: 1118–1125, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Zhang J, Li W, Sanders MA, Sumpio BE, Panja A, Basson MD. Regulation of the intestinal epithelial response to cyclic strain by extracellular matrix proteins. FASEB J 17: 926–928, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Zhang J, Owen CR, Sanders MA, Turner JR, Basson MD. The motogenic effects of cyclic mechanical strain on intestinal epithelial monolayer wound closure are matrix dependent. Gastroenterology 131: 1179–1189, 2006 [DOI] [PubMed] [Google Scholar]