Abstract
Acute pancreatitis is an inflammatory disease of the exocrine pancreas that carries considerable morbidity and mortality; its pathophysiology remains poorly understood. Recent findings from experimental models and genetically altered mice summarized in this review reveal that autophagy, the principal cellular degradative pathway, is impaired in pancreatitis and that one cause of autophagy impairment is defective function of lysosomes. We propose that the lysosomal/autophagic dysfunction is a key initiating event in pancreatitis and a converging point of multiple deranged pathways. There is strong evidence supporting this hypothesis. Investigation of autophagy in pancreatitis has just started, and many questions about the “upstream” mechanisms mediating the lysosomal/autophagic dysfunction and the “downstream” links to pancreatitis pathologies need to be explored. Answers to these questions should provide insight into novel molecular targets and therapeutic strategies for treatment of pancreatitis.
Keywords: macroautophagy, lysosome, cathepsin, lysosome-associated membrane protein, pancreatic acinar cell, trypsin
autophagy encompasses several intracellular pathways of lysosome-driven degradation and recycling of organelles and long-lived proteins. Recent studies have begun to elucidate the role of autophagy in the normal function of the exocrine pancreas and in pancreatitis, the most common disease of this organ. The purpose of this review is threefold: 1) to provide basic information on the process of autophagy for pancreatologists entering the field, 2) to discuss the recent findings and their implications, and 3) to delineate perspective directions for research. Thus we only give a brief background on autophagy, necessary for the discussion of its role in pancreatitis. A number of recent reviews (4, 20, 29, 50, 54, 59, 62, 68, 70, 71, 73, 83, 101, 106, 113), especially on the role of autophagy in the liver and pancreas (12), describe in detail the function and mechanisms of autophagy, as well as methods used in autophagy research.
The Process of Autophagy: A Brief Introduction
Autophagic pathways.
Living cells undergo continuous renewal during which old components are recycled and replaced with new ones. The degradation of macromolecules and organelles to generate new “building blocks” is necessary to maintain cellular homeostasis. Two major systems in eukaryotic cells degrade cellular components: the ubiquitin-proteasome system and autophagy. The former mainly degrades short-lived proteins, which are tagged by ubiquitin to be recognized and degraded by the proteasome (30). By contrast, autophagy degrades long-lived proteins, lipids, and cytoplasmic organelles through a lysosome-driven process (12, 20, 29, 71, 101). Autophagy occurs at a basal rate in most cells, where it acts as a quality control mechanism to eliminate protein aggregates and damaged or unneeded organelles. Equally important, autophagy constitutes a major protective mechanism that allows cells to survive and adapt to fluctuations in external conditions. In particular, nutrient deprivation is one of the strongest inducers of physiological autophagy; it generates/recycles components (e.g., amino acids) vital for cell survival.
Three major autophagic pathways, which differ in their routes to the lysosome, have been described: chaperone-mediated autophagy, microautophagy, and macroautophagy (29, 71). In chaperone-mediated autophagy, cytosolic proteins bearing a specific sequence motif bind to a chaperone (i.e., the heat-shock protein Hsc70) and are thus targeted to a receptor [lysosome-associated membrane protein (LAMP)-2a] on the lysosomal membrane, resulting in their translocation into the lysosome (11, 50). In microautophagy, the lysosome itself engulfs small parts of the cytoplasm by inward invagination of the lysosomal membrane (2, 70). A related process is crinophagy (28), in which lysosomes directly fuse with secretory granules, resulting in their degradation [e.g., insulin-containing granules in pancreatic β-cells (66)]. The molecular signals and mechanisms mediating crinophagy are not known.
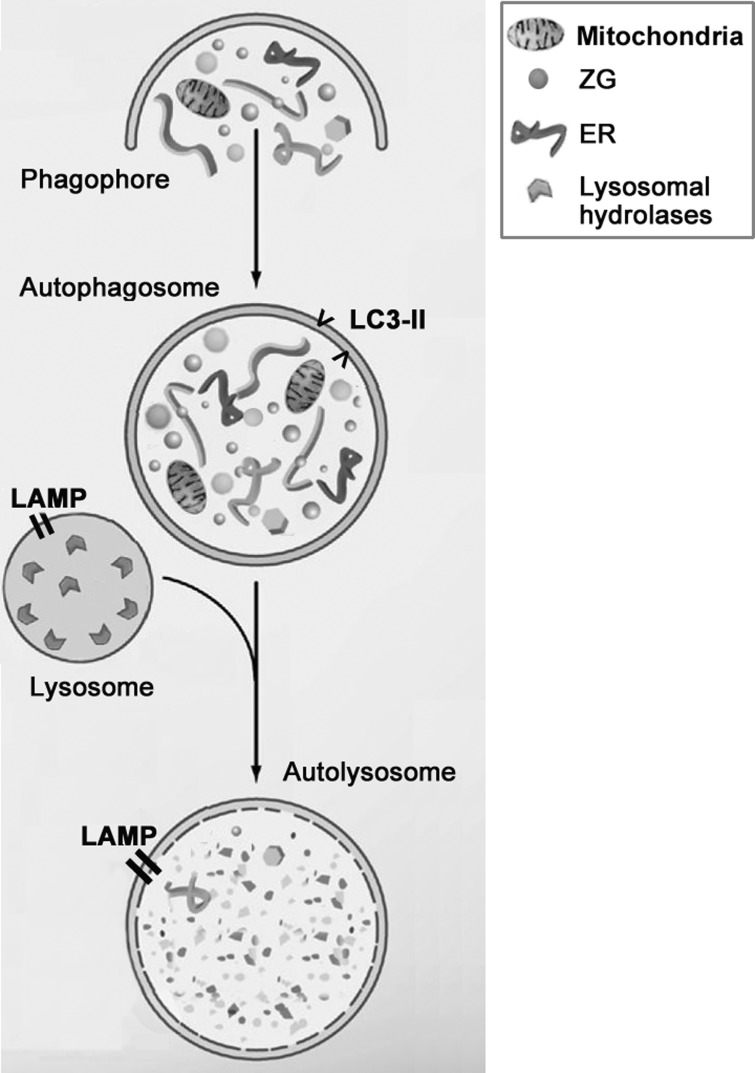
The major form of autophagy is macroautophagy. It has been studied more extensively than the other pathways and herein is referred to as “autophagy.” Macroautophagy (Fig. 1) is a multistep process (4, 20, 29, 68, 71, 101) that starts with the formation of an autophagosome, a unique double-membrane vacuole that sequesters organelles and proteins destined for degradation. Autophagosomes fuse with endosomes and then with lysosomes, generating single-membrane autolysosomes. Finally, the sequestered material is degraded by lysosomal hydrolases, and the degradation products, such as amino acids, are recycled back to the cytoplasm. A key, and sometimes underappreciated, aspect of autophagy is its dynamic character (54, 71, 73). Changes in the above-described steps affect the progression and function of autophagy; it is thus critical to assess the flux through this pathway (i.e., the turnover rate of autophagic vacuoles) and how it is affected by various stresses and in disease (54, 62, 83).
Fig. 1.
Schematic of autophagy progression. Autophagy starts with formation of membrane structures that sequester cytoplasmic organelles [e.g., mitochondria or zymogen granules (ZGs)], forming the double-membrane autophagosomes. Autophagosomes then fuse with lysosomes, thus becoming autolysosomes. LC3-II protein, a marker of autophagosomes; LAMP, integral lysosome-associated membrane protein; ER, endoplasmic reticulum.
Autophagy induction: formation and origin of autophagosomes; nonselective and selective autophagy.
Autophagosome formation is a complex process (29, 71, 74, 101); it begins with formation of a so-called isolation membrane, or phagophore, that elongates to engulf parts of the cytoplasm (including organelles, e.g., mitochondria) and, finally, closes to form the mature autophagosome, a globular double-membrane organelle. This process is controlled by a series of evolutionary conserved Atg genes, as well as lipid kinases such as the class III phosphatidylinositol 3-kinase Vps34 (29, 71, 74, 101). There are at least four complexes of Atg proteins (also involving other proteins, i.e., Vps34) that control individual steps of autophagosome formation. For example, ULK1/Atg1 is necessary for nucleation and Beclin1/Atg6-Vps34 and Atg5-Atg12-Atg16 complexes are required for assembly of the isolation membrane. A product of the Atg8 gene, LC3 protein, is necessary for phagophore closure; during this process, its cytosolic LC3-I form is modified (lipidated) to become LC3-II, which specifically translocates to the autophagosomal membrane. The intracellular origin of the phagophore is a matter of intense research; the current point of view is that it can be generated from multiple sources, including the endoplasmic reticulum (ER), the Golgi, the outer mitochondrial membrane, and the plasma membrane (38, 42, 78, 101).
Autophagy was long thought to be nonselective, with the best-studied example being autophagy induced by nutrient deprivation. More recently, several cargo-specific autophagy pathways have been characterized; these function under nutrient-normal conditions to remove damaged organelles and protein aggregates, the accumulation of which could be toxic for the cell (29, 101). Perhaps the best-understood type of selective autophagy is “mitophagy,” which removes damaged (e.g., uncoupled) mitochondria (106, 113). Recent studies have identified the sensors/mediators that control autophagic recognition of damaged mitochondria, such as the ubiquitin ligase Parkin and the mitochondria-residing kinase PINK1 (phosphatase and tensin homolog-induced putative kinase 1) (113).
Another recent development is the finding of “alternative” autophagy, which does not involve the “canonical” Atg5 and Atg7 proteins (78). This pathway does not require the LC3-I to LC3-II transition.
Autophagic flux: the role of lysosomes.
The final steps of autophagy (Fig. 1) following autophagosome formation (20, 21, 29, 71, 89) are controlled by the lysosome, the principal cellular degradative organelle that contains acid hydrolases, the enzymes capable of breaking down all kinds of biological material (57, 64, 89). Two classes of proteins are critical for lysosomal function: the soluble acid hydrolases and lysosomal membrane proteins. The lysosome contains ∼50 hydrolases targeting specific substrates for degradation. These include proteases, lipases, nucleases, glycosidases, phospholipases, phosphatases, and sulfatases, which usually exert maximal enzymatic activity at low pH. This acidic (pH ≤5) milieu of lysosomes is maintained by a vacuolar ATPase (vATPase) that pumps protons from the cytosol into the lysosomal lumen.
The delivery of hydrolases to the lysosome is a multistep process controlled by the Golgi and the endosomal system (8, 27, 47, 57). Lysosomal hydrolases are synthesized in the ER as inactive proforms and then transported to the Golgi, where mannose 6-phosphate (M6P) moieties are added onto the hydrolases. These moieties form strong complexes with two types of M6P receptors that mediate endosomal trafficking of hydrolases, such as cathepsins, to the lysosome. Cathepsins comprise a family of serine, aspartic [e.g., cathepsin D (CatD)], and mainly cysteine (e.g., CatB and CatL) proteases, which are important for lysosomal, autophagic, and other functions (84). During trafficking, cathepsins undergo proteolytic processing (maturation) to become active enzymes: first, in endosomes, this process generates an intermediate, “single-chain” form of cathepsins and then, mainly in the lysosome, a fully mature “double-chain” active form (18, 47, 88).
Lysosomal integrity and degradative capacity critically depend on LAMP-1 and -2. LAMPs are heavily glycosylated transmembrane proteins comprising >70% of all lysosomal membrane proteins; they play diverse and crucial roles in the function of lysosomes (21, 89). LAMPs are necessary for protection of the cytoplasm (and the limiting lysosomal membrane itself) from the action of acid hydrolases. They regulate fusion of lysosomes with other organelles, in particular autophagosomes, lysosomal proteolytic activity, and endocytosis. As mentioned above, LAMP-2a acts as a specific translocation receptor in chaperone-mediated autophagy.
Thus the efficiency of autophagic flux depends primarily on the rates of formation and degradative activity of autolysosomes, the latter being controlled by the levels and proteolytic activities of lysosomal hydrolases, the LAMPs, intralysosomal pH, and other factors. Because the properties and functions of autophagosomes and autolysosomes are so different, it is important to discriminate between these two types of autophagic organelles (Table 1).
Table 1.
Properties of autophagosomes vs. autolysosomes
| Autophagosome | Autolysosome |
|---|---|
| Has double (or multiple) membrane(s) | Has a single membrane |
| Has neutral luminal pH | Has acidic luminal pH |
| Does not express LAMPs on the surface | Expresses LAMPs on the surface |
| All autophagosomes express LC3-II | Only early autolysosomes express LC3-II |
| Does not contain hydrolases | Contains various acid hydrolases |
| Does not degrade cargo | Degrades cargo |
LAMP, lysosome-associated membrane protein.
Autophagy impairment.
It has become clear that a wide variety of diseases are associated with autophagy impairment (20, 62, 83, 89). Dysfunction could occur at various steps of autophagy, including decreased or defective formation of autophagosomes, their impaired fusion with lysosomes, or inefficient lysosomal proteolytic activity. For example, mutations in autophagy proteins, such as ATG16L1, cause defects in autophagosome formation in Crohn's disease, leading to inhibition of autophagy and, hence, persistent inflammation (97). In Danon disease (X-linked cardiomyopathy), mutations in LAMP-2 hamper the fusion of autophagosomes and lysosomes, resulting in vacuole accumulation and cardiomyopathy (100). Mucolipidosis type II (“I-cell disease”) is a rapidly progressive and fatal lysosomal storage disorder characterized by impaired delivery of acid hydrolases to the lysosome, their extrusion from the cell, and the appearance of phase-dense cytoplasmic inclusions in a number of cell types, especially mesenchymal fibroblasts (56, 61). The disease is caused by mutations in a critical enzyme, N-acetylglucosamine-1-(GlcNAc-1)-phosphotransferase (see Defective processing and activities of lysosomal hydrolases), leading to deficient lysosomal hydrolytic activity and, thus, accumulation of undigested cargo [as shown in mice with genetic ablation of this enzyme (6)].
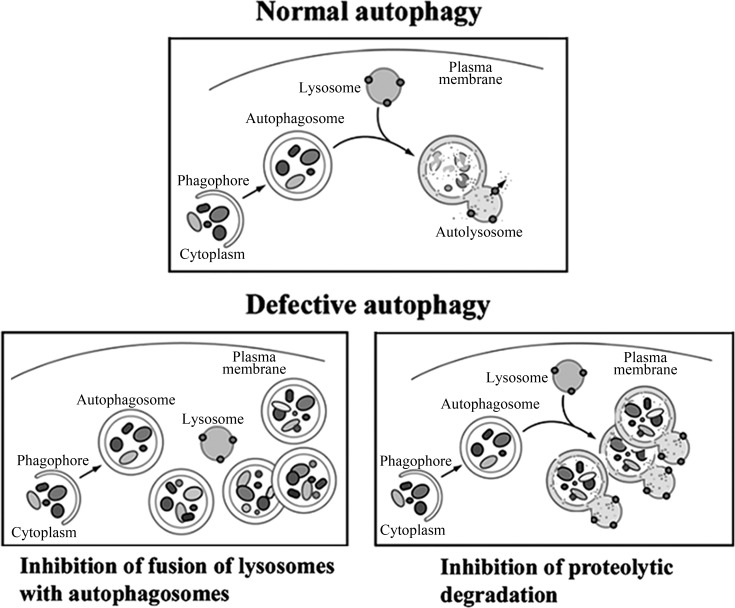
Impairment of the early and late steps of autophagy can have opposite effects on the number of autophagy-related vacuoles in a cell (54, 62, 73). Inhibition of autophagosome formation results in a decreased number of autophagic vacuoles, whereas blockade of the later stages of autophagy increases cell vacuolation. Blockade of the fusion of autophagosomes with lysosomes causes accumulation of autophagosomes, while deficient lysosomal proteolytic activity results in accumulation of autolysosomes with partially degraded cargo (Fig. 2). A hallmark of impaired autophagic flux is accumulation of abnormally large autophagic vacuoles. When autophagy is efficient, the turnover of autophagic vacuoles is high [the normal autophagosome half-life in mammalian cells is ∼10 min (95)], and they are small. Impaired autophagic flux prolongs the half-life of autophagic vacuoles; they fuse with each other, forming vacuoles that can be larger than the nucleus and occupy a large part of the cytoplasm (65).
Fig. 2.
Schematic illustrating 2 major mechanisms of defective autophagic flux. [Adapted from Klionsky et al. (54).]
During the past several years, significant progress has been achieved in understanding the mechanisms through which impaired autophagy leads to cell pathology. For example, a major consequence of impaired autophagy is accumulation of damaged (e.g., uncoupled) mitochondria (26, 113). In particular, mitochondrial membrane permeabilization caused by various stressors results in ATP decrease, overproduction of reactive oxygen species (ROS), and, ultimately, apoptotic or necrotic cell death. Removal of damaged mitochondria via mitophagy can rescue the cell from these pathological effects. Excessive mitochondrial ROS generation causes activation of the inflammasome, leading to the inflammatory response (33, 59). Another consequence of impaired autophagy is accumulation of p62/SQSTM1, a protein that is required for sequestration of ubiquitinated protein aggregates in autophagosomes and, on the other hand, is specifically degraded through autophagy (46, 49, 76). Impaired autophagy causes accumulation of aggregates that contain p62 and ubiquitin, particularly in neurodegenerative (e.g., Alzheimer's) and liver (e.g., alcoholic hepatitis and steatohepatitis) diseases (46, 49). p62 also functions as a signaling hub for oxidative stress and NF-κB pathways (49, 76), the derangement of which may lead to inflammation and cell death.
Tools to study autophagy.
There are several recent comprehensive reviews on the methods to study autophagy (4, 54, 73). A gold standard of autophagy measurements is transmission electron microscopy (TEM). However, quantitative TEM is expensive and time-consuming and requires specialized expertise (19); thus TEM is used largely for illustrative purposes. The most widely used methods to monitor autophagy utilize changes in the LC3 protein, a specific marker of autophagic vacuoles. As stated above, its LC3-II (lipidated) form, generated upon autophagy induction, localizes almost exclusively to autophagosomal membranes (29, 68, 71, 74, 101). Therefore, measurements of LC3-II level (by immunoblot) or LC3-positive vesicular structures [“puncta,” by immunofluorescence or green fluorescent protein (GFP)-LC3 fluorescence] are commonly applied to assess changes in the number of autophagic vacuoles (4, 54, 72, 73).
Genetic, molecular [e.g., small interfering RNA (siRNA)], and pharmacological approaches are used to manipulate autophagy in vitro and in vivo. A number of mouse strains deficient in key autophagy proteins, such as Atg5, Beclin1, and Atg7, have been developed as general and tissue-specific knockouts (46). Widely used [with some caveats (54, 73, 112)] pharmacological inhibitors of autophagy include 3-methyladenine (3-MA), a phosphatidylinositol 3-kinase inhibitor that blocks autophagosome formation; chloroquine, a weak base amine, which increases intralysosomal pH and thus blocks lysosomal hydrolytic activity; and bafilomycin A1, an inhibitor of vATPase.
The number of autophagosomes observed at any time is a function of the balance between their rates of generation and degradation during the subsequent stages of autophagy. Furthermore, LC3-II is ultimately degraded in the autolysosome. Therefore, an increase in LC3-II, indicating an increased number of autophagosomes (or, in general, autophagic vacuoles) does not necessarily imply that autophagy is activated. Such an increase could result from two opposite scenarios: autophagy induction or suppression of steps downstream of autophagosome formation, i.e., impaired autophagic flux.
Assessing the efficiency of the autophagic flux is thus necessary to distinguish between autophagy activation and its inhibition. Autophagic flux could be impaired as a result of defective fusion between autophagosomes and lysosomes and/or deficient lysosomal proteolytic activity (Fig. 2). A direct method to measure the efficiency of autophagic flux in cells is degradation of long-lived proteins by pulse-chase assay (4, 54, 65, 73, 100). To discriminate between autophagic and nonautophagic degradation, the measurements are done in parallel samples, for example, with and without 3-MA. Flux through the autophagy pathway in vitro can be also assessed by evaluating LC3-II turnover with immunoblot in conditions of intact vs. blocked (e.g., using bafilomycin A1) lysosomal degradation (4, 54, 73). If flux is efficient, blockade of lysosomal degradation will result in an increased amount of LC3-II (compared with the absence of the inhibitor).
Assessing the efficiency of autophagic flux in vivo is a challenge; one method suitable for measurements in tissue is to determine in parallel the changes in LC3-II and p62 levels, as discussed in detail elsewhere (4, 49, 54, 73). Because p62 is specifically degraded via autophagy, a decrease in its level associated with increased LC3-II indicates that autophagy is activated and efficient. In contrast, an increase in both p62 and LC3-II indicates inefficient, retarded autophagic flux.
Autophagy in Pancreatitis
Physiological autophagy in the exocrine pancreas.
On the basis of the abundance of GFP-LC3 puncta, the basal autophagy level is higher in mouse exocrine pancreas than in liver, kidney, heart, or endocrine pancreas; furthermore, the number of GFP-LC3 puncta after 24 h of starvation is greater in the exocrine pancreas than in the other organs (72). Our in vitro data, using the comparative analysis of LC3-II level in conditions of intact vs. blocked lysosomal degradation, also indicate efficient basal autophagy in mouse pancreatic acinar cells (Jia et al., unpublished observations). One could speculate that because the exocrine pancreas has a very high rate of protein synthesis, it might have a greater need to remove defective (or excessive) proteins. Indeed, the quintessential function of the pancreatic acinar cell is to secrete digestive enzymes, many of which are produced as inactive zymogens prepacked and stored in zymogen granules (ZGs) and normally become activated after they are secreted and reach the intestine (82). Thus an attractive idea is that macroautophagy may have a role in regulating the number of ZGs, to adjust to the needs of the acinar cell and the whole organism. However, the role of autophagy in regulating the level and secretion of digestive enzymes has not been defined, and in general, the interrelations between the synthesis/secretion of secretory granules (in particular, ZGs) and their degradation are largely unknown.
The basal and starvation-induced autophagy in the pancreatic acinar cell is nonselective, as autophagosomes contain various organelles: mitochondria, ER, and ZGs (65, 72). Starvation greatly stimulates autophagy in the pancreas (65, 72), resulting in a dramatic decrease in the number of ZGs in acinar cells (72). This is in agreement with studies from decades ago (5, 53, 75) that showed a decreased number of ZGs in starved rodents. However, stimulation of crinophagy [direct fusion of secretory granules with lysosomes (28, 66)], rather than autophagy, was thought to mediate ZG degradation during starvation (53). It would be worthwhile to revisit this issue using modern methods.
Acute pancreatitis.
Acute pancreatitis is a potentially fatal disease with considerable morbidity and mortality; its pathogenesis of remains obscure, and no specific or effective treatment has been developed (22, 82). The disease is believed to initiate in the acinar cell (82, 98). Hallmark responses of pancreatitis include hyperamylasemia, the inappropriate, intra-acinar activation of digestive enzymes (e.g., conversion of trypsinogen to trypsin), accumulation of large vacuoles in acinar cells, induction of proinflammatory mediators (e.g., the key transcription factor NF-κB) resulting in inflammatory cell infiltration in the pancreas and systemic inflammatory response, and acinar cell death through apoptosis and necrosis.
There are several widely used rodent models of nonalcoholic acute pancreatitis, such as pancreatitis induced in rats or mice by administration of high-dose caerulein (an analog of CCK-8), l-arginine, or bile acids and, in young mice, by feeding a choline-deficient, ethionine-supplemented diet (60, 99). Models of alcoholic pancreatitis combine ethanol feeding with another stressor [low-dose caerulein (81) or LPS (24)], because alcohol alone does not cause pronounced pancreatic damage in rodents. Also widely used are the ex vivo models, i.e., isolated acinar cells stimulated with caerulein or bile acids, which reproduce many pathological responses of acute pancreatitis, such as vacuole accumulation and trypsinogen and NF-κB activation.
Autophagic flux is impaired in pancreatitis.
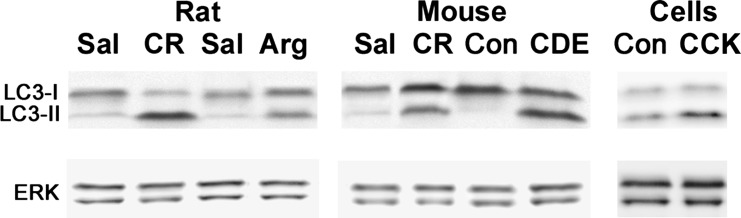
Accumulation of large vacuoles in acinar cells (Fig. 3) is a long-noted and prominent feature of experimental and human pancreatitis (1, 3, 7, 43, 55, 77, 108, 111); however, the mechanism of their formation and relation to other pathological responses of pancreatitis remained unclear. In a recent study (35, 65), we showed that most of these vacuoles are autophagic. Many more vacuoles are induced in acinar cells by pancreatitis than by starvation, and these vacuoles are also strikingly larger; furthermore, the autophagic vacuoles in pancreatitis are predominantly autolysosomes. The accumulation of large vacuoles is accompanied by increased pancreatic levels of LC3-II, observed in all experimental models (Fig. 4 and Ref. 65). Importantly, and in contrast to starvation, pancreatitis greatly decreases autophagic efficiency, which is manifest by a decreased rate of long-lived protein degradation and an increased level of p62 (37, 65).
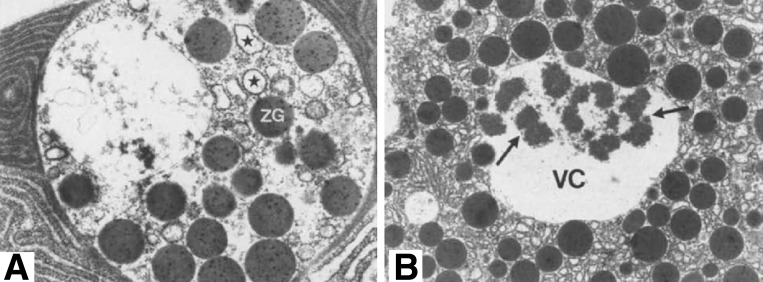
Fig. 3.
Electron micrographs of pancreatic tissue from patients with acute pancreatitis. Note large autophagic vacuoles (VC) containing ZGs and fragments of rough endoplasmic reticulum (*, A) and zymogen content (arrows) immunoreactive for trypsinogen (B). [From Willemer et al. (111), with kind permission from Springer Science and Business Media.]
Fig. 4.
LC3-I to LC3-II conversion in rat and mouse models of acute pancreatitis and the ex vivo model of mouse acinar cells hyperstimulated with CCK-8. Arg, l-arginine; CDE, choline-deficient, ethionine-supplemented diet; Con, control; CR, caerulein; Sal, saline control.
Taken together, the vacuole accumulation, decreased efficiency of autophagic degradation, and increased levels of LC3-II and p62 reveal that autophagic flux is impaired in pancreatitis. Furthermore, the predominant accumulation of large autolysosomes with partially digested cargo indicates that lysosomal hydrolytic activity is compromised. Pancreatitis does not block the fusion of autophagosomes with lysosomes, which is evident from accumulation of autolysosomes in acinar cells and increased colocalization of lysosomal markers with LC3-II (65). It is possible, however, that the fusion is somehow compromised in pancreatitis.
Effect of pancreatitis on autophagosome formation.
Pancreatitis does not block autophagosome formation; on the contrary, our results (37, 65) and the data on the autophagy-mediating protein vacuole membrane protein 1 (VMP1; see below) indicate that autophagosome formation is stimulated in pancreatitis. TEM and immunogold-TEM show autophagic vacuoles, with all the characteristics of autophagosomes, i.e., double membrane, LC3-II, and intact cargo, in acinar cells (65). Genetic, molecular, and pharmacological manipulations indicate that Atg5 is involved in autophagosome formation in acinar cells (41, 65), suggesting that it proceeds through the canonical pathway. Data also suggest involvement of the ER in autophagosome formation in acinar cells, as heterozygous deletion of X-box binding protein 1, a key ER mediator of the adaptive “Unfolded Protein Response,” stimulates pancreatic autophagy in ethanol-fed mice (63).
Studies from Vaccaro's group (32, 87, 103) show that VMP1 is involved in autophagy induction in acinar cells. VMP1, which primarily resides in the ER, was cloned (17) as one of the proteins upregulated in acute pancreatitis (although it is expressed in many tissues in normal conditions). It interacts with Beclin1, colocalizes with LC3-II, and is an important mediator of autophagosome formation. There are several interesting implications from these studies. 1) The fact that there was no sign of pancreatitis in mice with acinar cell-specific overexpression of VMP1 indicates that, by itself, increased autophagosome formation is not harmful to acinar cells. 2) The results with VMP1 overexpression and siRNA knockdown suggest a protective role for VMP1 in caerulein-induced pancreatitis. However, VMP1 upregulation was reported 3 h after initiation of caerulein treatment (32), whereas vacuole accumulation and increased trypsin activity occur within 30 min in this model. These data suggest that autophagy impairment precedes VMP1-mediated effects. 3) VMP1 overexpression might also facilitate autophagic efficiency, as the p62 level time dependently decreased in the pancreas of VMP1-overexpressing mice with caerulein-induced pancreatitis, while there was no such p62 decrease in wild-type mice (32).
VMP1 was proposed to mediate “zymophagy,” a novel autophagic pathway to selectively remove ZGs in pancreatitis, on the basis of the finding that, in VMP1-overexpressing mice with caerulein-induced pancreatitis, autophagosomes selectively sequester ZGs, but not other organelles, such as mitochondria or ER (32). These results were obtained by immunoisolation of autophagic vacuoles, and it would be important to confirm the alterations in cargo composition with quantitative TEM (19). The existence of a selective autophagic pathway for ZG removal, and its induction by pancreatitis, is an intriguing idea. However, in wild-type mice with caerulein-induced pancreatitis, as well as in other experimental models (65) and in human disease (Fig. 3A), autophagosomes sequester all types of organelles, including mitochondria, ER, and ZGs. Also, zymophagy is not induced by VMP1 overexpression per se, that is, with stimulation of autophagosome formation, but is only proposed to occur after caerulein-induced pancreatitis in VMP1-overexpressing mice (32). This implies that, for zymophagy to occur, ZGs in pancreatitis must acquire some pathological signal that triggers their selective sequestration; such signals have yet to be identified. Finally, the effects of VMP1 overexpression may be more complex, as it causes cell death in (nonpancreatic) cell lines (17), and VMP1 may also have functions other than autophagy, as shown in Dictyostelium discoideum (9).
In summary, the available data indicate that autophagosome formation is stimulated in acute pancreatitis. Whether this occurs in all models of pancreatitis and the extent and kinetics of autophagy induction remain to be characterized. Other important questions are What are the exact pathways of autophagosome formation in acinar cells? What is the source of the autophagosomal membrane? Is autophagy in pancreatitis selective (the idea of zymophagy)?
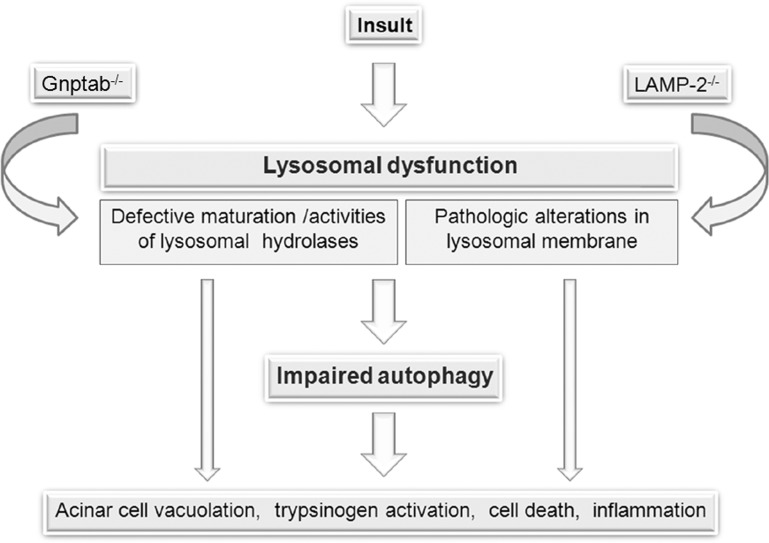
Lysosomal Dysfunction Mediates Autophagy Impairment in Pancreatitis
Recent findings (see below) reveal that lysosomal function is impaired in pancreatitis through at least two mechanisms, deficient lysosomal proteolytic activity and pathological alterations in lysosomal membrane proteins such as LAMPs, both of which cause retarded autophagic flux.
Defective processing and activities of lysosomal hydrolases.
More than two decades ago, Steer and Saluja and colleagues (91–93, 98) showed that experimental acute pancreatitis caused a dramatic decrease in the activity of CatB, a major acid hydrolase, in lysosome-enriched pancreatic tissue subcellular fractions. Furthermore, our data (Table 2 and Ref. 65) and data from the literature (93, 104) show that such a decrease is not limited to CatB but occurs with a number of lysosomal hydrolases, including CatL, CatD, asparaginyl endopeptidase (or legumain), aryl-sulfatase, and others, indicating a general defect. In search for the underlying mechanism, we analyzed the effect of pancreatitis on the processing of CatB and CatL (35, 37, 65). As stated above, cathepsins undergo proteolytic processing/maturation during their transit from Golgi to lysosomes, culminating in the formation of the fully mature and stable “double-chain” active form. We found (65; unpublished data) that cathepsin processing is impaired in pancreatitis, resulting in a decrease of the mature form and concomitant accumulation of intermediate and proforms. The impaired maturation of lysosomal hydrolases implies their defective activation, thus providing an explanation for their decreased activity in pancreatic tissue lysosomal fractions.
Table 2.
Caerulein-induced pancreatitis decreases hydrolase activities in lysosome-enriched pancreatic tissue fractions
| Lysosomal Hydrolase | Activity vs. Saline Control |
|---|---|
| CatB | 0.43 ± 0.07 |
| CatL | 0.27 ± 0.11 |
| Asparaginyl endopeptidase (or legumain) | 0.36 ± 0.10 |
| CatD | 0.49 ± 0.05 |
Values are means ± range from 2 independent experiments. Activity was measured in rat pancreatic tissue fractions obtained as described by Mareninova et al. (65). CatB, CatL, and CatD, cathepsins B, L, and D.
As discussed above (see Autophagy impairment), deficient lysosomal hydrolytic activity causes reduced autophagic flux, manifest by accumulation of autolysosomes containing partially digested cargo. We showed that specific inhibitors of CatB and CatL as well as the general inhibitor of cysteine proteases E-64d all cause accumulation of large autolysosomes in pancreatic acinar cells (65). A similar effect was observed with leupeptin, another broad-spectrum inhibitor of cysteine proteases (102), and in embryonic fibroblasts derived from CatL knockout mice (15).
An important (and different) line of evidence that dysfunctional lysosomal hydrolases, in particular cathepsins, cause autophagy impairment in acinar cells comes from studies of mice deficient in GlcNAc-1-phosphotransferase (6, 56, 105). This enzyme, subunits of which are coded by the Gnptab and Gnptg genes, mediates the addition of M6P moieties onto acid hydrolases, which serve as a recognition signal for specific targeting of hydrolases to the lysosome. Gnptab genetic ablation blocks cathepsins' transport from Golgi to lysosomes and creates a mouse model of human mucolipidosis type II, reproducing many (albeit not all) features of this disease (6). Gnptab−/− mice show a dramatic decrease in lysosomal degradative capacity of the exocrine pancreas resulting in impaired autophagy in acinar cells (Fig. 5A), manifest by numerous enlarged autolysosomes containing undigested cargo (6, 105). Of note, the fusion of autophagosomes with lysosomes is not blocked in these mice (6).
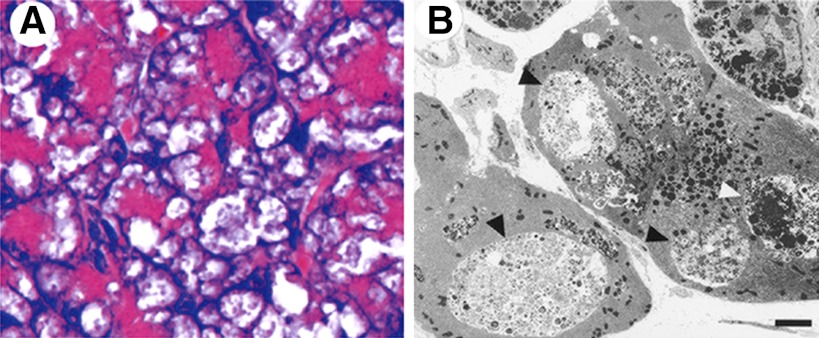
Fig. 5.
Pancreatitis-like injury resulting from lysosomal/autophagic dysfunction in genetic models. A: hematoxylin-eosin-stained section of pancreas of N-acetylglucosamine-1-phosphotransferase-deficient (Gnptab−/−) mouse. Note large cytoplasmic vacuoles in acinar cells containing partially degraded cargo. [From Vogel et al. (105), by permission from Sage Publications.] B: electron micrograph showing accumulation of large autophagic vacuoles (arrowheads) in pancreas of LAMP-2-deficient mice. [From Tanaka et al. (100), by permission from Macmillan Publishers Ltd.]
In accord with the data from Gnptab−/− mice, genetic ablation of one of the two M6P receptors mediating delivery of acid hydrolases to lysosomes was found to cause acinar cell vacuolation (69).
LAMP degradation.
In a collaborative study with Lerch's group, we showed that pancreatitis markedly decreases pancreatic levels of LAMP-1 and -2, major integral components of the lysosomal membrane (Mareninova et al., unpublished observations). We found LAMP degradation in four dissimilar rat and mouse models of nonalcoholic pancreatitis, in the ethanol + caerulein model of alcoholic pancreatitis, and in the ex vivo model of caerulein-hyperstimulated acinar cells. LAMP decrease was also observed in the ethanol + LPS model (24) and in human pancreatitis (24; Mareninova et al., unpublished observations).
Our results indicate that LAMP degradation in pancreatitis occurs in lysosomes and is mediated by cathepsins. The data further indicate that LAMP degradation occurs through a CatB-mediated pathway, suggesting that it might result from impaired cathepsin maturation. Acid hydrolases are normally present in the lysosome as large multiprotein complexes spatially separated from LAMPs (58, 64). One may speculate that, because of abnormal maturation of cathepsins, their interactions and localization within the lysosome are altered in pancreatitis, making LAMPs accessible to proteolytic cleavage by cathepsins.
Importantly, LAMP-2 genetic ablation causes autophagy impairment in the pancreas (100), with accumulation of large autolysosomes containing poorly degraded material (Fig. 5B). The exact mechanisms whereby LAMP deficiency causes impaired autophagy in the exocrine pancreas remain to be determined (21, 89, 100). To assess the role of LAMP decrease in pancreas damage, our study examined in detail the effects of LAMP-2 deficiency on the exocrine pancreas (Mareninova et al., unpublished observations). The accumulation of autophagic vacuoles was prominent in the pancreas of LAMP-2-null mice as early as 1 mo of age and was associated with progressive acinar cell damage.
Role of Lysosomal and Autophagic Dysfunction in Pancreatitis
Hypothesis: lysosomal/autophagic dysfunction is a key initiating event in pancreatitis.
On the basis of the findings described above, we propose that lysosomal/autophagic dysfunction is a key initiating event in pancreatitis (Fig. 6). Indeed, features of impaired autophagy are evident in human disease (3, 43, 111) and in all experimental models of pancreatitis (1, 7, 55, 65, 77, 108), including those induced by ethanol + LPS (24) or coxsackievirus infection (51). The lysosomal/autophagic disordering is an early event in development of the disease. For example, inhibition of lysosomal activity of cathepsins, decrease in LAMPs, and impairment of autophagy are evident as early as 30 min after the induction of caerulein-induced pancreatitis (37, 65; Mareninova et al., unpublished observations), that is, concurrent with other early pathological responses of pancreatitis, such as trypsinogen and NF-κB activation.
Fig. 6.
Schematic illustrating the hypothesis on a key initiating role of lysosomal/autophagic dysfunction in pancreatitis.
Importantly, genetic alterations that specifically target the lysosomal or autophagic function induce pancreatitis-like injury. The lysosomal/autophagic dysfunction in LAMP-2-deficient mice causes spontaneous pancreatitis manifest by acinar cell vacuolation, progressive inflammatory infiltration in the pancreas, and acinar cell necrosis (Mareninova et al., unpublished observations). The blockade of cathepsins' delivery to lysosomes in Gnptab−/− mice, resulting in impaired autophagic flux (see Defective processing and activities of lysosomal hydrolases), causes pancreatitis responses such as acinar cell vacuolation, inflammation, and disorganization of pancreas tissue architecture (6, 105). Knockout of one of the two M6P receptors potentiates trypsinogen activation in caerulein-induced pancreatitis (69).
Moreover, a number of studies reveal that lysosomal/autophagic dysfunction is involved in pancreatitis induced by genetic or molecular manipulations of other, seemingly unrelated, pathways. Genetic ablation of Spink-3 (mouse ortholog of SPINK1, the main endogenous trypsin inhibitor in humans) causes autophagy impairment, acinar cell vacuolation, and, ultimately, pancreas degeneration (79, 86). Mice with defective exocytosis caused by inability of ZGs to fuse with the apical membrane (knockout of interferon regulatory factor 2) develop massive accumulation of autolysosomes in acinar cells and other manifestations of pancreatitis (67). Overexpression (or administration) of IL-22, a cytokine that belongs to the IL-10 family, ameliorates mouse caerulein-induced pancreatitis through autophagy (23). A study from Karin's laboratory (Li et al., unpublished observations) found that pancreas-specific deletion of IKKα, a component of the IKK kinase complex responsible for NF-κB activation, results in acinar cell damage progressing from vacuole accumulation to severe chronic pancreatitis, with sustained inflammation and fibrosis. Importantly, this novel role of IKKα in pancreas homeostasis is independent of its kinase activity and is unrelated to NF-κB; rather, the IKKα deficiency causes defective autophagy completion in acinar cells (Li et al., unpublished observations).
Autophagy and the intra-acinar trypsinogen activation.
Our data and data from the literature (41, 65) show that, in addition to acinar cell vacuolation, the lysosomal/autophagic dysfunction mediates another hallmark response of pancreatitis, the accumulation of active trypsin in acinar cells. The mechanism and the role of the pathological, intra-acinar trypsinogen activation in pancreatitis is a subject of extensive research and much debate; here we only reflect on the role of autophagy in this process. The current hypothesis for the mechanism, proposed by Steer and Saluja and colleagues (91, 92, 98, 104), is that the intra-acinar trypsinogen activation results from redistribution (“missorting”) of CatB into ZG-containing compartment(s) and, thus, colocalization of CatB with trypsinogen. The colocalization compartment remains unidentified, but evidence indicates that it is not the ZGs themselves (31, 80). The colocalization hypothesis has been criticized (39), in part because a significant CatB activity in the ZG-enriched fraction is observed in the normal pancreas (91, 104).
Our study (65) compared the autophagic response in models of pancreatitis with the physiological autophagy induced by starvation. It showed that colocalization of digestive enzymes with cathepsins occurs in autolysosomes during autophagic degradation of ZGs in normal conditions. Starvation stimulates autophagic degradation in acinar cells, as manifest, in particular, by an increased rate of long-lived protein degradation (65). This results in loss of ZGs (72) but does not cause manifestations of pancreatitis. Hence, colocalization of cathepsins with digestive enzymes is not pathological in itself. Alternatively, we propose that it is the defective lysosomal proteolytic activity that leads to inefficient autophagic degradation of ZGs and the intra-acinar accumulation of trypsin. CatB and CatL are involved in this process, but their roles are exactly opposite: whereas CatB converts trypsinogen to trypsin (40, 94, 98, 104), CatL does not activate trypsinogen but, on the contrary, degrades both trypsin and trypsinogen (65, 107). Our data indicate that lysosomal dysfunction in pancreatitis results in an imbalance between CatB and CatL, such that CatL activity is decreased relative to CatB and is not sufficient to degrade trypsin, resulting in its accumulation in autophagic vacuoles (65). In this context, the significance of the colocalization hypothesis is that it was the first to draw attention to a possible role for lysosomal hydrolases in the initiation of pancreatitis.
Until very recently (13, 25, 48, 90), intra-acinar trypsinogen activation has been the prevailing paradigm for the mechanism initiating pancreatitis, providing a molecular basis for the >100-year-old idea of pancreas autodigestion (48, 82, 90, 94, 98, 110). This “trypsin central” paradigm has been challenged by recent results from Logsdon's and Saluja's groups. The study from Logsdon's group (25; commentary in Ref. 90) showed that high levels of intra-acinar spontaneous activation of genetically engineered trypsinogen are sufficient to cause severe acute pancreatitis; this, however, did not result in sustained acinar cell injury or progression to chronic pancreatitis. Saluja's group generated mice deficient in the gene for trypsinogen-7, the mouse homolog of human cationic trypsinogen (13; commentary in Ref. 48). In these mice, there was no pathological trypsinogen activation, and acinar cell necrosis in caerulein-induced pancreatitis was 50% less, but the extent of local and systemic inflammation was the same as in the wild-type mice. The authors conclude that trypsinogen activation mediates early acinar cell injury but not the progression of pancreatitis.
These studies indicate that intra-acinar accumulation of trypsin is important but may play a more restricted role in pancreatitis pathogenesis. Reflecting the recent findings, a “multifaceted” mechanism for initiation of pancreatitis has been proposed (48). We posit that the lysosomal/autophagic dysfunction is a critical “facet” of the pathogenic mechanism of pancreatitis; furthermore, it represents a converging point through which various stressors trigger acinar cell injury. As illustrated above (see Hypothesis: lysosomal/autophagic dysfunction is a key initiating event in pancreatitis), lysosomal dysfunction resulting in impaired autophagy can lead not only to the accumulation of vacuoles and active trypsin in acinar cells, but also to inflammation and cell death, all key responses of pancreatitis. The first indication of the link between autophagy and pancreatitis was the study from Yamamura's group (41) which showed that caerulein-induced accumulation of vacuoles and active trypsin was markedly inhibited in Atg5-deficient mice. The authors concluded that enhanced autophagy mediates pancreatitis responses. In contrast, we emphasize that it is the impaired, inefficient autophagy, and not the induction of “normal,” efficient autophagy (41), that is critical for the development of pancreatitis. Indeed, starvation, which potently stimulates the physiological, efficient autophagy, does not cause pancreatitis. Moreover, one may speculate that, in the injured acinar cells, there is an increased demand for removal of damaged organelles, and the inability of impaired autophagy to cope with this demand causes or exacerbates pancreatitis pathologies.
Open questions and future directions.
Investigation of autophagy in pancreatitis has only recently started, and there are many questions to answer (some of which we already discussed). It will be important to examine the roles of other forms of autophagy, chaperone-mediated autophagy, microautophagy, and crinophagy, in normal function of the exocrine pancreas and pancreatitis. [In our study on LAMP-2 degradation (Mareninova et al., unpublished observations), we observed a decrease in LAMP-2a in experimental pancreatitis, suggesting impairment of chaperone-mediated autophagy.] Equally important is investigation of the role of selective autophagy pathways, mitophagy and lipophagy (autophagy of lipids), as well as zymophagy. For example, mitophagy and lipophagy were shown to protect against acute ethanol-induced hepatotoxicity in mice (12, 16).
Another critical issue is the interrelation between lysosomal/autophagic dysfunction and major “established” players in pancreatitis, such as Ca2+ and NF-κB. Abnormal Ca2+ signaling is believed to mediate the development of pancreatitis (10); it is likely involved in the regulation of autophagy in acinar cells, as preventing excessive Ca2+ influx inhibits vacuole accumulation and other signs of impaired autophagy in pancreatitis (52). The interrelations between autophagy and Ca2+, in general, are complex and not well understood (14). The NF-κB pathway is another important player in the mechanism of pancreatitis, triggering the inflammatory response (13, 25, 82); the links between autophagy and the NF-κB pathway, in general, have not been elucidated (68).
There are many gaps in our understanding of the “upstream” mechanisms underlying the lysosomal/autophagic dysfunction in pancreatitis, such as What mediates the abnormal maturation of cathepsins? Are there other pathways leading to impaired lysosomal degradation, e.g., changes in the intralysosomal pH caused by pathological alterations in vATPase (109)? What are the pathological roles of cathepsins in pancreatitis; is there a role for other lysosomal hydrolases; and what is the mechanism for LAMP degradation in pancreatitis? Furthermore, the lysosomal/autophagic dysfunction may be a manifestation of a more general phenomenon, indicating disordering of endolysosomal traffic in pancreatitis. For example, trypsinogen activation has been reported (96) to occur in endocytic vacuoles in CCK-8-hyperstimulated acinar cells.
It is also critical to elucidate the “downstream” mechanisms linking the lysosomal/autophagic dysfunction to cell death and inflammatory responses of pancreatitis. In general, the role of autophagy in cell death is a subject of intense research and much debate. The predominant view is that efficient, physiological autophagy is prosurvival, whereas defective autophagy promotes cell death (59, 62). One mechanism whereby impaired autophagy may stimulate acinar cell death is through accumulation of damaged (e.g., uncoupled) mitochondria (33, 59), as the mitochondrial damage is a key regulator of cell death in pancreatitis (34, 36). Furthermore, it is possible that the accumulation of damaged mitochondria mediates the inflammatory response (e.g., through a ROS-dependent mechanism) (26, 33). Another candidate linking impaired autophagy to inflammation is p62, the pathological accumulation of which may promote inflammation and cell death (49, 76). Pathways linking acinar cell death and inflammation may involve the inflammasome, recently shown to mediate pancreatitis pathologies (45).
In addition to mitochondria, the ER is another organelle in the acinar cell that is likely to regulate autophagy in pancreatitis, as the processes of autophagy and ER stress are interrelated and the ER is often a source for the isolation membrane. It is also important to investigate the role of dysregulated secretion, which may help us understand why the pancreatic acinar cell is particularly sensitive to lysosomal/autophagic dysfunction. Indeed, Gnptab−/− mice show accumulation of autolysosomes in pancreatic and salivary gland acinar cells, but not in liver, brain, or muscle (6, 105). Similarly, LAMP-2 deficiency severely affects the exocrine pancreas, while many other organs do not show overt pathology (100).
Finally, although the focus of this review is on acute pancreatitis, impaired autophagy may be also important in the pathobiology of chronic pancreatitis, in particular its hallmark response, fibrosis. For example, fibrosis might be promoted if autophagic removal of ubiquitinated protein aggregates is impaired (due to defective clearance of p62), as was shown in a mouse model of the liver disease α1-antitrypsin deficiency (44). Autophagy may also regulate the activation of stellate cells in pancreatitis (85).
Elucidating the mechanisms underlying the lysosomal and autophagic dysfunctions will lead to insights into potential molecular targets to treat or mitigate the severity of pancreatitis. The challenge will be to “normalize” these pathways, rather than simply stimulate or block autophagy. Because of defective lysosomal degradation, stimulating autophagy in pancreatitis might even exacerbate the “traffic jam.” This may explain why inhibiting autophagy through Atg5 genetic ablation (or siRNA) or with 3-MA improves caerulein-induced pancreatitis (41, 65). However, prolonged autophagy inhibition would likely be detrimental, i.e., for the later recovery phase of pancreatitis.
GRANTS
Our research is supported by the Department of Veterans Affairs, National Institutes of Health Grants R01 DK-59936 and AA-19730 and, in part, by the Southern California Research Center for Alcoholic Liver and Pancreatic Diseases and Cirrhosis (National Institutes of Health Grant P50 AA-11999).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.S.G. and I.G. are responsible for conception and design of the research; analyzed the data; interpreted the results of the experiments; prepared the figures; drafted the manuscript; edited and revised the manuscript; approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank members of the University of California at Los Angeles/Veterans Affairs Greater Los Angeles Healthcare System Pancreatic Research Group involved in the studies discussed in this review, especially Drs. Olga Mareninova and Stephen Pandol.
REFERENCES
- 1. Adler G, Rohr G, Kern HF. Alteration of membrane fusion as a cause of acute pancreatitis in the rat. Dig Dis Sci 27: 993–1002, 1982 [DOI] [PubMed] [Google Scholar]
- 2. Ahlberg J, Marzella L, Glaumann H. Uptake and degradation of proteins by isolated rat liver lysosomes. Suggestion of a microautophagic pathway of proteolysis. Lab Invest 47: 523–532, 1982 [PubMed] [Google Scholar]
- 3. Aho HJ, Nevalainen TJ, Havia VT, Heinonen RJ, Aho AJ. Human acute pancreatitis: a light and electron microscopic study. Acta Pathol Microbiol Immunol Scand A 90: 367–373, 1982 [PubMed] [Google Scholar]
- 4. Barth S, Glick D, Macleod KF. Autophagy: assays and artifacts. J Pathol 221: 117–124, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bendayan M, Bruneau A, Morisset J. Morphometrical and immunocytochemical studies on rat pancreatic acinar cells under control and experimental conditions. Biol Cell 54: 227–234, 1985 [DOI] [PubMed] [Google Scholar]
- 6. Boonen M, van Meel E, Oorschot V, Klumperman J, Kornfeld S. Vacuolization of mucolipidosis type II mouse exocrine gland cells represents accumulation of autolysosomes. Mol Biol Cell 22: 1135–1147, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brackett KA, Crocket A, Joffe SN. Ultrastructure of early development of acute pancreatitis in the rat. Dig Dis Sci 28: 74–84, 1983 [DOI] [PubMed] [Google Scholar]
- 8. Braulke T, Bonifacino JS. Sorting of lysosomal proteins. Biochim Biophys Acta 1793: 605–614, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Calvo-Garrido J, Carilla-Latorre S, Escalante R. Vacuole membrane protein 1, autophagy and much more. Autophagy 4: 835–837, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Criddle DN, McLaughlin E, Murphy JA, Petersen OH, Sutton R. The pancreas misled: signals to pancreatitis. Pancreatology 7: 436–446, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science 273: 501–503, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Czaja MJ. Functions of autophagy in hepatic and pancreatic physiology and disease. Gastroenterology 140: 1895–1908, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dawra R, Sah RP, Dudeja V, Rishi L, Talukdar R, Garg P, Saluja AK. Intra-acinar trypsinogen activation mediates early stages of pancreatic injury but not inflammation in mice with acute pancreatitis. Gastroenterology 141: 2210–2217, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Decuypere JP, Bultynck G, Parys JB. A dual role for Ca2+ in autophagy regulation. Cell Calcium 50: 242–250, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Dennemarker J, Lohmuller T, Muller S, Aguilar SV, Tobin DJ, Peters C, Reinheckel T. Impaired turnover of autophagolysosomes in cathepsin L deficiency. Biol Chem 391: 913–922, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W, Lu B, Stolz DB, Clemens DL, Yin XM. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology 139: 1740–1752, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dusetti NJ, Jiang Y, Vaccaro MI, Tomasini R, Azizi Samir A, Calvo EL, Ropolo A, Fiedler F, Mallo GV, Dagorn JC, Iovanna JL. Cloning and expression of the rat vacuole membrane protein 1 (VMP1), a new gene activated in pancreas with acute pancreatitis, which promotes vacuole formation. Biochem Biophys Res Commun 290: 641–649, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Erickson AH. Biosynthesis of lysosomal endopeptidases. J Cell Biochem 40: 31–41, 1989 [DOI] [PubMed] [Google Scholar]
- 19. Eskelinen EL. To be or not to be? Examples of incorrect identification of autophagic compartments in conventional transmission electron microscopy of mammalian cells. Autophagy 4: 257–260, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Eskelinen EL, Saftig P. Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta 1793: 664–673, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Eskelinen EL, Tanaka Y, Saftig P. At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol 13: 137–145, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Everhart JE, Ruhl CE. Burden of digestive diseases in the United States. III. Liver, biliary tract, and pancreas. Gastroenterology 136: 1134–1144, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Feng D, Park O, Radaeva S, Wang H, Yin S, Kong X, Zheng M, Zakhari S, Kolls JK, Gao B. Interleukin-22 ameliorates cerulein-induced pancreatitis in mice by inhibiting the autophagic pathway. Int J Biol Sci 8: 249–257, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fortunato F, Burgers H, Bergmann F, Rieger P, Buchler MW, Kroemer G, Werner J. Impaired autolysosome formation correlates with Lamp-2 depletion: role of apoptosis, autophagy, and necrosis in pancreatitis. Gastroenterology 137: 350–360, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Gaiser S, Daniluk J, Liu Y, Tsou L, Chu J, Lee W, Longnecker DS, Logsdon CD, Ji B. Intracellular activation of trypsinogen in transgenic mice induces acute but not chronic pancreatitis. Gut 60: 1379–1388, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galluzzi L, Kepp O, Kroemer G. Mitochondrial dynamics: a strategy for avoiding autophagy. Curr Biol 21: R478–R480, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Ghosh P, Griffith J, Geuze HJ, Kornfeld S. Mammalian GGAs act together to sort mannose 6-phosphate receptors. J Cell Biol 163: 755–766, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glaumann H. Crinophagy as a means for degrading excess secretory proteins in rat liver. Revis Biol Celular 20: 97–110, 1989 [PubMed] [Google Scholar]
- 29. Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol 221: 3–12, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature 426: 895–899, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Grady T, Mah'Moud M, Otani T, Rhee S, Lerch MM, Gorelick FS. Zymogen proteolysis within the pancreatic acinar cell is associated with cellular injury. Am J Physiol Gastrointest Liver Physiol 275: G1010–G1017, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Grasso D, Ropolo A, Lo Re A, Boggio V, Molejon MI, Iovanna JL, Gonzalez CD, Urrutia R, Vaccaro MI. Zymophagy, a novel selective autophagy pathway mediated by VMP1-USP9x-p62, prevents pancreatic cell death. J Biol Chem 286: 8308–8324, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science 333: 1109–1112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gukovskaya AS, Gukovsky I. Which way to die: the regulation of acinar cell death in pancreatitis by mitochondria, calcium, and reactive oxygen species. Gastroenterology 140: 1876–1880, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gukovsky I, Gukovskaya AS. Impaired autophagy underlies key pathological responses of acute pancreatitis. Autophagy 6: 428–429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gukovsky I, Pandol SJ, Gukovskaya AS. Organellar dysfunction in the pathogenesis of pancreatitis. Antioxid Redox Signal 15: 2699–2710, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gukovsky I, Pandol SJ, Mareninova OA, Shalbueva N, Jia W, Gukovskaya AS. Impaired autophagy and organellar dysfunction in pancreatitis. J Gastroenterol Hepatol 27 Suppl 2: 27–32, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, Lippincott-Schwartz J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell 141: 656–667, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Halangk W, Lerch MM. Early events in acute pancreatitis. Clin Lab Med 25: 1–15, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Halangk W, Lerch MM, Brandt-Nedelev B, Roth W, Ruthenbuerger M, Reinheckel T, Domschke W, Lippert H, Peters C, Deussing J. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest 106: 773–781, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hashimoto D, Ohmuraya M, Hirota M, Yamamoto A, Suyama K, Ida S, Okumura Y, Takahashi E, Kido H, Araki K, Baba H, Mizushima N, Yamamura K. Involvement of autophagy in trypsinogen activation within the pancreatic acinar cells. J Cell Biol 181: 1065–1072, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hayashi-Nishino M, Fujita N, Noda T, Yamaguchi A, Yoshimori T, Yamamoto A. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat Cell Biol 11: 1433–1437, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Helin H, Mero M, Markkula H, Helin M. Pancreatic acinar ultrastructure in human acute pancreatitis. Virchows Arch A Pathol Anat Histol 387: 259–270, 1980 [DOI] [PubMed] [Google Scholar]
- 44. Hidvegi T, Ewing M, Hale P, Dippold C, Beckett C, Kemp C, Maurice N, Mukherjee A, Goldbach C, Watkins S, Michalopoulos G, Perlmutter DH. An autophagy-enhancing drug promotes degradation of mutant α1-antitrypsin Z and reduces hepatic fibrosis. Science 329: 229–232, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Hoque R, Sohail M, Malik A, Sarwar S, Luo Y, Shah A, Barrat F, Flavell R, Gorelick F, Husain S, Mehal W. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology 141: 358–369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ichimura Y, Komatsu M. Pathophysiological role of autophagy: lesson from autophagy-deficient mouse models. Exp Anim 60: 329–345, 2011 [DOI] [PubMed] [Google Scholar]
- 47. Ishidoh K, Kominami E. Processing and activation of lysosomal proteinases. Biol Chem 383: 1827–1831, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Ji B, Logsdon CD. Digesting new information about the role of trypsin in pancreatitis. Gastroenterology 141: 1972–1975, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy 7: 279–296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kaushik S, Bandyopadhyay U, Sridhar S, Kiffin R, Martinez-Vicente M, Kon M, Orenstein SJ, Wong E, Cuervo AM. Chaperone-mediated autophagy at a glance. J Cell Sci 124: 495–499, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kemball CC, Alirezaei M, Flynn CT, Wood MR, Harkins S, Kiosses WB, Whitton JL. Coxsackievirus infection induces autophagy-like vesicles and megaphagosomes in pancreatic acinar cells in vivo. J Virol 84: 12110–12124, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim MS, Lee KP, Yang D, Shin DM, Abramowitz J, Kiyonaka S, Birnbaumer L, Mori Y, Muallem S. Genetic and pharmacologic inhibition of the Ca2+ influx channel TRPC3 protects secretory epithelia from Ca2+-dependent toxicity. Gastroenterology 140: 2107–2115, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kitagawa T, Ono K. Ultrastructure of pancreatic exocrine cells of the rat during starvation. Histol Histopathol 1: 49–57, 1986 [PubMed] [Google Scholar]
- 54. Klionsky DJ, Abdalla FC, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8: 445–544, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Koike H, Steer ML, Meldolesi J. Pancreatic effects of ethionine: blockade of exocytosis and appearance of crinophagy and autophagy precede cellular necrosis. Am J Physiol Gastrointest Liver Physiol 242: G297–G307, 1982 [DOI] [PubMed] [Google Scholar]
- 56. Kollmann K, Pohl S, Marschner K, Encarnacao M, Sakwa I, Tiede S, Poorthuis BJ, Lubke T, Muller-Loennies S, Storch S, Braulke T. Mannose phosphorylation in health and disease. Eur J Cell Biol 89: 117–123, 2010 [DOI] [PubMed] [Google Scholar]
- 57. Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol 5: 483–525, 1989 [DOI] [PubMed] [Google Scholar]
- 58. Kreutzer R, Kreutzer M, Sewell AC, Techangamsuwan S, Leeb T, Baumgartner W. Impact of β-galactosidase mutations on the expression of the canine lysosomal multienzyme complex. Biochim Biophys Acta 1792: 982–987, 2009 [DOI] [PubMed] [Google Scholar]
- 59. Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell 40: 280–293, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lerch MM, Adler G. Experimental animal models of acute pancreatitis. Int J Pancreatol 15: 159–170, 1994 [PubMed] [Google Scholar]
- 61. Leroy JG, Ho MW, MacBrinn MC, Zielke K, Jacob J, O'Brien JS. I cell disease: biochemical studies. Pediatr Res 6: 752–757, 1972 [DOI] [PubMed] [Google Scholar]
- 62. Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell 132: 27–42, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lugea A, Tischler D, Nguyen J, Gong J, Gukovsky I, French SW, Gorelick FS, Pandol SJ. Adaptive unfolded protein response attenuates alcohol-induced pancreatic damage. Gastroenterology 140: 987–997, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol 8: 622–632, 2007 [DOI] [PubMed] [Google Scholar]
- 65. Mareninova OA, Hermann K, French SW, O'Konski MS, Pandol SJ, Webster P, Erickson AH, Katunuma N, Gorelick FS, Gukovsky I, Gukovskaya AS. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Invest 119: 3340–3355, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Marsh BJ, Soden C, Alarcon C, Wicksteed BL, Yaekura K, Costin AJ, Morgan GP, Rhodes CJ. Regulated autophagy controls hormone content in secretory-deficient pancreatic endocrine beta-cells. Mol Endocrinol 21: 2255–2269, 2007 [DOI] [PubMed] [Google Scholar]
- 67. Mashima H, Sato T, Horie Y, Nakagawa Y, Kojima I, Ohteki T, Ohnishi H. Interferon regulatory factor-2 regulates exocytosis mechanisms mediated by SNAREs in pancreatic acinar cells. Gastroenterology 141: 1102–1113, 2011 [DOI] [PubMed] [Google Scholar]
- 68. Mehrpour M, Esclatine A, Beau I, Codogno P. Overview of macroautophagy regulation in mammalian cells. Cell Res 20: 748–762, 2010 [DOI] [PubMed] [Google Scholar]
- 69. Meister T, Niehues R, Hahn D, Domschke W, Sendler M, Lerch MM, Schnekenburger J. Missorting of cathepsin B into the secretory compartment of CI-MPR/IGFII-deficient mice does not induce spontaneous trypsinogen activation but leads to enhanced trypsin activity during experimental pancreatitis—without affecting disease severity. J Physiol Pharmacol 61: 565–575, 2010 [PubMed] [Google Scholar]
- 70. Mijaljica D, Prescott M, Devenish RJ. Microautophagy in mammalian cells: revisiting a 40-year-old conundrum. Autophagy 7: 673–682, 2011 [DOI] [PubMed] [Google Scholar]
- 71. Mizushima N. Autophagy: process and function. Genes Dev 21: 2861–2873, 2007 [DOI] [PubMed] [Google Scholar]
- 72. Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 15: 1101–1111, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell 140: 313–326, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 27: 107–132, 2011 [DOI] [PubMed] [Google Scholar]
- 75. Nevalainen TJ, Janigan DT. Degeneration of mouse pancreatic acinar cells during fasting. Virchows Arch B Cell Pathol 15: 107–118, 1974 [DOI] [PubMed] [Google Scholar]
- 76. Nezis IP, Stenmark H. p62 at the interface of autophagy, oxidative stress signaling, and cancer. Antioxid Redox Signal 17: 786–793, 2012 [DOI] [PubMed] [Google Scholar]
- 77. Niederau C, Grendell JH. Intracellular vacuoles in experimental acute pancreatitis in rats and mice are an acidified compartment. J Clin Invest 81: 229–236, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y, Shimizu S. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 461: 654–658, 2009 [DOI] [PubMed] [Google Scholar]
- 79. Ohmuraya M, Hirota M, Araki M, Mizushima N, Matsui M, Mizumoto T, Haruna K, Kume S, Takeya M, Ogawa M, Araki K, Yamamura K. Autophagic cell death of pancreatic acinar cells in serine protease inhibitor Kazal type 3-deficient mice. Gastroenterology 129: 696–705, 2005 [DOI] [PubMed] [Google Scholar]
- 80. Otani T, Chepilko SM, Grendell JH, Gorelick FS. Codistribution of TAP and the granule membrane protein GRAMP-92 in rat caerulein-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol 275: G999–G1009, 1998 [DOI] [PubMed] [Google Scholar]
- 81. Pandol SJ, Lugea A, Mareninova OA, Smoot D, Gorelick FS, Gukovskaya AS, Gukovsky I. Investigating the pathobiology of alcoholic pancreatitis. Alcohol Clin Exp Res 35: 830–837, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology 132: 1127–1151, 2007. Erratum: ibid, 133:1056 [DOI] [PubMed] [Google Scholar]
- 83. Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev 90: 1383–1435, 2010 [DOI] [PubMed] [Google Scholar]
- 84. Reiser J, Adair B, Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest 120: 3421–3431, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rickmann M, Vaquero EC, Malagelada JR, Molero X. Tocotrienols induce apoptosis and autophagy in rat pancreatic stellate cells through the mitochondrial death pathway. Gastroenterology 132: 2518–2532, 2007 [DOI] [PubMed] [Google Scholar]
- 86. Romac JM, Ohmuraya M, Bittner C, Majeed MF, Vigna SR, Que J, Fee BE, Wartmann T, Yamamura K, Liddle RA. Transgenic expression of pancreatic secretory trypsin inhibitor-1 rescues SPINK3-deficient mice and restores a normal pancreatic phenotype. Am J Physiol Gastrointest Liver Physiol 298: G518–G524, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ropolo A, Grasso D, Pardo R, Sacchetti ML, Archange C, Lo Re A, Seux M, Nowak J, Gonzalez CD, Iovanna JL, Vaccaro MI. The pancreatitis-induced vacuole membrane protein 1 triggers autophagy in mammalian cells. J Biol Chem 282: 37124–37133, 2007 [DOI] [PubMed] [Google Scholar]
- 88. Rowan AD, Mason P, Mach L, Mort JS. Rat procathepsin. Proteolytic processing to the mature form in vitro. J Biol Chem 267: 15993–15999, 1992 [PubMed] [Google Scholar]
- 89. Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol 10: 623–635, 2009 [DOI] [PubMed] [Google Scholar]
- 90. Sah RP, Saluja AK. Trypsinogen activation in acute and chronic pancreatitis: is it a prerequisite? Gut 60: 1305–1307, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Saluja A, Hashimoto S, Saluja M, Powers RE, Meldolesi J, Steer ML. Subcellular redistribution of lysosomal enzymes during caerulein-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol 253: G508–G516, 1987 [DOI] [PubMed] [Google Scholar]
- 92. Saluja A, Saito I, Saluja M, Houlihan MJ, Powers RE, Meldolesi J, Steer M. In vivo rat pancreatic acinar cell function during supramaximal stimulation with caerulein. Am J Physiol Gastrointest Liver Physiol 249: G702–G710, 1985 [DOI] [PubMed] [Google Scholar]
- 93. Saluja A, Saluja M, Villa A, Leli U, Rutledge P, Meldolesi J, Steer M. Pancreatic duct obstruction in rabbits causes digestive zymogen and lysosomal enzyme colocalization. J Clin Invest 84: 1260–1266, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Saluja AK, Lerch MM, Phillips PA, Dudeja V. Why does pancreatic overstimulation cause pancreatitis? Annu Rev Physiol 69: 249–269, 2007 [DOI] [PubMed] [Google Scholar]
- 95. Schworer CM, Shiffer KA, Mortimore GE. Quantitative relationship between autophagy and proteolysis during graded amino acid deprivation in perfused rat liver. J Biol Chem 256: 7652–7658, 1981 [PubMed] [Google Scholar]
- 96. Sherwood MW, Prior IA, Voronina SG, Barrow SL, Woodsmith JD, Gerasimenko OV, Petersen OH, Tepikin AV. Activation of trypsinogen in large endocytic vacuoles of pancreatic acinar cells. Proc Natl Acad Sci USA 104: 5674–5679, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Stappenbeck TS, Rioux JD, Mizoguchi A, Saitoh T, Huett A, Darfeuille-Michaud A, Wileman T, Mizushima N, Carding S, Akira S, Parkes M, Xavier RJ. Crohn disease: a current perspective on genetics, autophagy and immunity. Autophagy 7: 355–374, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Steer ML. Early events in acute pancreatitis. Baillieres Best Pract Res Clin Gastroenterol 13: 213–225, 1999 [DOI] [PubMed] [Google Scholar]
- 99. Su KH, Cuthbertson C, Christophi C. Review of experimental animal models of acute pancreatitis. HPB (Oxford) 8: 264–286, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lullmann-Rauch R, Janssen PM, Blanz J, von Figura K, Saftig P. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature 406: 902–906, 2000 [DOI] [PubMed] [Google Scholar]
- 101. Tanida I. Autophagosome formation and molecular mechanism of autophagy. Antioxid Redox Signal 14: 2201–2214, 2011 [DOI] [PubMed] [Google Scholar]
- 102. Telbisz A, Kovacs AL. Intracellular protein degradation and autophagy in isolated pancreatic acini of the rat. Cell Biochem Funct 18: 29–40, 2000 [DOI] [PubMed] [Google Scholar]
- 103. Vaccaro MI, Grasso D, Ropolo A, Iovanna JL, Cerquetti MC. VMP1 expression correlates with acinar cell cytoplasmic vacuolization in arginine-induced acute pancreatitis. Pancreatology 3: 69–74, 2003 [DOI] [PubMed] [Google Scholar]
- 104. Van Acker GJ, Weiss E, Steer ML, Perides G. Cause-effect relationships between zymogen activation and other early events in secretagogue-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 292: G1738–G1746, 2007 [DOI] [PubMed] [Google Scholar]
- 105. Vogel P, Payne BJ, Read R, Lee WS, Gelfman CM, Kornfeld S. Comparative pathology of murine mucolipidosis types II and IIIC. Vet Pathol 46: 313–324, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wang K, Klionsky DJ. Mitochondria removal by autophagy. Autophagy 7: 297–300, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wartmann T, Mayerle J, Kahne T, Sahin-Toth M, Ruthenburger M, Matthias R, Kruse A, Reinheckel T, Peters C, Weiss FU, Sendler M, Lippert H, Schulz HU, Aghdassi A, Dummer A, Teller S, Halangk W, Lerch MM. Cathepsin L inactivates human trypsinogen, whereas cathepsin L-deletion reduces the severity of pancreatitis in mice. Gastroenterology 138: 726–737, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Watanabe O, Baccino FM, Steer ML, Meldolesi J. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol 246: G457–G467, 1984 [DOI] [PubMed] [Google Scholar]
- 109. Waterford SD, Kolodecik TR, Thrower EC, Gorelick FS. Vacuolar ATPase regulates zymogen activation in pancreatic acini. J Biol Chem 280: 5430–5434, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Whitcomb DC. Genetic aspects of pancreatitis. Annu Rev Med 61: 413–424, 2010 [DOI] [PubMed] [Google Scholar]
- 111. Willemer S, Kloppel G, Kern HF, Adler G. Immunocytochemical and morphometric analysis of acinar zymogen granules in human acute pancreatitis. Virchows Arch A Pathol Anat Histopathol 415: 115–123, 1989 [DOI] [PubMed] [Google Scholar]
- 112. Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem 285: 10850–10861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12: 9–14, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]