Abstract
The mechanisms underlying slow-transit constipation (STC) are unclear. In 50% of patients with STC, some form of outlet obstruction has been reported; also an elongated colon has been linked to patients with STC. Our aims were 1) to develop a murine model of STC induced by partial outlet obstruction and 2) to determine whether this leads to colonic elongation and, consequently, activation of the inhibitory “occult reflex,” which may contribute to STC in humans. Using a purse-string suture, we physically reduced the maximal anal sphincter opening in C57BL/6 mice. After 4 days, the mice were euthanized (acutely obstructed), the suture was removed (relieved), or the suture was removed and replaced repeatedly (chronically obstructed, over 24–31 days). In partially obstructed mice, we observed increased cyclooxygenase (COX)-2 levels in muscularis and mucosa, an elongated impacted large bowel, slowed transit, nonpropagating colonic migrating motor complexes (CMMCs), a lack of mucosal reflexes, a depolarized circular muscle with slow-wave activity due to a lack of spontaneous inhibitory junction potentials, muscle hypertrophy, and CMMCs in mucosa-free preparations. Elongation of the empty obstructed colon produced a pronounced occult reflex. Removal of the obstruction or addition of a COX-2 antagonist (in vitro and in vivo) restored membrane potential, spontaneous inhibitory junction potentials, CMMC propagation, and mucosal reflexes. We conclude that partial outlet obstruction increases COX-2 leading to a hyperexcitable colon. This hyperexcitability is largely due to suppression of only descending inhibitory nerve pathways by prostaglandins. The upregulation of motility is suppressed by the occult reflex activated by colonic elongation.
Keywords: colonic migrating motor complex, cyclooxygenase-2, elongation, inhibitory junction potentials, nitric oxide, occult reflex, prostaglandin E2, serotonin
chronic slow-transit constipation (STC) remains a challenge for today's gastroenterologists. Traditional treatments include increased fiber intake, osmotic laxatives, stimulant laxatives, prokinetic agents, biofeedback training, and surgery. These often are tried sequentially and episodically, with little evidence of long-term efficacy (29). Most commonly, the symptoms of STC are not alleviated; rather, STC is only managed throughout the patient's life (17). The mechanisms underlying STC are unclear, since hypo- and hypercontractility have been observed (8). Constipation is a common and costly complaint, affecting ∼15% of the US population. According to the Rome III criteria, patients are generally considered to have STC if they exhibit slow fecal transit and more than two of the following symptoms: straining during defecation, hard or protuberated stool, incomplete evacuation, sensation of blockage, manual mechanical evacuation, and less than three defecations per week (14, 15).
Chronic constipation is known to include outlet obstruction, colonic inertia, or both; in fact, most patients with colonic inertia have associated outlet obstruction (27, 39). It has been reported that >50% of all patients with STC have some form of outlet obstruction, which can be due to a variety of causes, including defective rectal filling sensation, idiopathic megarectum with or without megacolon, rectal hyposensitivity (blunted rectum), functional outlet obstruction, or inefficient inhibition of the internal anal sphincter (9, 28). Surgical intervention is considered a therapeutic option for patients with STC who do not respond to aggressive medical therapy (38). “Failure to address the outlet obstruction might explain the high rate of re-operation reported in most series of patients undergoing colectomy for colonic inertia” (27).
Luminal distension or mucosal stimulation of the colon evokes the peristaltic reflex, which consists of a contraction and relaxation above and below the stimulated region, respectively, due to activation of intrinsic ascending excitatory and descending inhibitory neural reflex pathways, respectively (4, 5, 11, 30, 36). Recent studies have characterized a conserved inhibitory reflex in guinea pig (10, 11, 36), mouse (19), and monkey (Dickson and Smith, unpublished observations) large intestine that is triggered by colonic elongation, rather than distension. Referred to as the “occult” (i.e., hidden from view) reflex, this phenomenon is responsible for depressing intrinsic nerve pathways underlying the peristaltic reflex and colonic migrating motor complexes (CMMCs), rather than a direct muscle response. When stretched longitudinally, mechanosensitive descending neuronal nitric oxide (NO) synthase (nNOS)-positive interneurons release NO to depress the intrinsic neural circuitry [interneurons and afterhyperpolarizing (AH) neurons] (10, 11, 19). Normally, the occult reflex allows the colon to accumulate and store fecal matter. Typically, the colon will elongate to accommodate contents, while only slight circumferential dilations occur (10, 11, 17).
Recently, the occult reflex was suggested to contribute to STC in humans, because “Interestingly we have observed that many patients with slow transit constipation have an elongated transverse colon” (32, 33). Historically, an elongated colon was believed to be associated with STC (reviewed in Refs. 14 and 19): “The most common related cause of constipation after ARM (anorectal myectomy) was a redundant colon” (39). Barium enema X-ray studies confirmed an elongated colon in patients with STC (26, 32, 33). Also called a redundant or dolichocolon (Dolichos, a Greek long-distance runner), elongated colons were at times surgically shortened; however, this method of treatment was not successful.
To further investigate the role of the occult reflex on slow-transit intestinal disorders, we have used partial outlet obstruction to develop a simple mouse model of STC that displays the phenotype of an elongated colon. Few animal models of constipation/obstruction exist; most have problematic methods of implementation, since they require significant invasive surgical intervention or use opiate drugs or serotonin receptor (5-HT3) antagonists, which impede neurotransmission (14). A suitable animal model of STC is needed to begin to understand its mechanisms, so that new and more effective therapies can be developed for this chronic disorder. The aim of this study was to develop a drug-free, easily inducible murine model of STC that parallels some of the changes observed in the human condition.
METHODS
Male C57BL/6 mice (28–42 days old) were euthanized by inhalation of a 5% concentration of isoflurane, followed by cervical dislocation. These studies were performed in accordance with National Institutes of Health guidelines for the use and care of laboratory animals, and all procedures were approved by the Animal Ethics Committee and the Institutional Animal Care and Use Committee at the University of Nevada, Reno.
Partial Reduction of the External Anal Sphincter Opening
A simple local procedure that does not require a laparotomy was used to cause partial outlet obstruction in mice to reduce daily fecal output. Anesthesia was induced with 3–4% isoflurane and maintained at 1–3%. The perianal area of the animal was prepared with a Betadine scrub prior to lidocaine injection. Lidocaine (2%, up to 0.25 ml as needed) was injected into the skin surrounding the perianal area. A smooth 2.5-mm-diameter glass rod was inserted into the anus. A purse-string suture (size 6-0, Prolene, Ethicon, San Lorenzo, Puerto Rico) was placed in the external perianal region to close the lumen of the anus around the glass rod; then the rod was removed. The mouse was revived and observed for 4 days until it was euthanized or until the suture was removed. In animals that required suture removal, anesthesia was induced as described above, the suture was removed, and the animal was revived. In acute partially obstructed animals, the suture remained for 4 days, and the animals were euthanized. In chronic partially obstructed animals, the suture remained for 3–4 days and was removed for 4–5 days, and the process was repeated three additional times (over 24–31 days). In relieved animals, the suture was removed for 7 days following partial obstruction. During model development, animals obstructed for >5 days exhibited signs of stress (arched back and a lack of foraging) and occasionally a twisted necrotic small intestine (volvulus; 4 of 40 mice).
Preparations
A ventral midline incision was made in the animal, the gastrointestinal tract (stomach to external anal sphincter) was carefully excised, and several preparations were derived.
Artificial pellet propulsion.
An isolated segment of colonic tissue was pinned taut in a Sylgard (WPI, Sarosota, FL)-lined organ bath continuously perfused with oxygenated Krebs-Ringer bicarbonate solution (see Drugs and Solutions) at 36.0 ± 0.5°C. An artificial pellet was inserted into the oral end, and the movement was recorded with a video camera (model WV-BP330, Panasonic CCTV) directly to a computer (iMac, Apple, Cupertino, CA) and analyzed using previously described methods (13, 19).
Tension recordings of CMMCs.
Three isometric tension transducers (model TST125C, Biopac Systems, Santa Barbara, CA) were attached by suture silk at regular intervals [proximal colon (To), at the colonic flexure (Tm), and 10–15 mm from the anal end (Ta)]; the silk was glued to the colon by a bead of Gluture (WPI).
The whole colon was stretched and then repinned, as described previously (19), to achieve elongation (longitudinal stretch). Mucosal reflexes were evoked by gentle stroking (2–5 strokes) of the mucosa with an artist brush (size 0) (1–3, 18, 36).
The tube was opened into a flat sheet along the mesenteric border to produce mucosa-free preparations. The mucosa was carefully sharp-dissected away. Pins were used to secure one mesenteric border to the base of the organ bath, and tension transducers were attached to the opposite side of the mesenteric border.
Pellet weighing.
Pellets were weighed immediately following harvest and also after they were allowed to dry for 48 h.
Electrophysiological recordings.
Microelectrodes were advanced into the circular muscle, as previously described (10, 11–13, 36), for electrophysiological recordings.
Histological staining.
Cross sections from colonic segments were cut to 5 μm thickness with a cryostat (model CM 3050S, Leica, Wetzlar, Germany) and fixed in 10% (vol/vol) neutral buffered formalin solution. Samples were then embedded in paraffin wax and stained with hematoxylin and eosin solutions.
Immunohistochemistry.
After electrophysiological experiments, tissues were removed from the bath and repinned in a Sylgard-lined fixation dish. Tissues were fixed with 4°C paraformaldehyde for 60 min, washed overnight in 0.01 M PBS (pH 7.2), and treated with BSA (1%) for 60 min. Dual staining was performed sequentially: the first primary incubation was carried out for 48 h at 4°C, and tissues were washed in PBS for 4 h at 20°C and then incubated with the second primary antibody. To identify nitrergic enteric neurons, nNOS (NOS1; a gift from P. C. Empson) was used (1). Cyclooxygenase (COX)-2-containing cells were labeled using an antibody against prostaglandin-endoperoxide synthase II (PTGS2, COX II; Cayman Chemical, Ann Arbor, MI). After incubation with primary antibodies, tissues were washed in PBS for 24 h and incubated with secondary antibodies [Alexa Fluor 488 donkey anti-rabbit IgG (H+L), 1:1,000 dilution, and Alexa Fluor 594 donkey anti-sheep IgG (H+L), 1:1,000 dilution; Molecular Probes/Invitrogen], as described elsewhere (25). Whole-mount preparations were viewed and recorded using a confocal scanning microscope (model LSM 510 META, Carl Zeiss Microimaging, Thornwood, NY) with ×10–100 lenses.
Western blots.
Total protein was extracted from control, obstructed, and relieved colons. Protein concentration of isolated muscle extracts was determined by the Bradford assay (Bio-Rad, Richmond, CA). A total of 30 μg of protein from each lysate was used for the blot. Proteins were subjected to 10% SDS-PAGE and probed with antibodies against IL-6 (Abcam), PTGS2 (COX II; Cayman Chemical), NOS2 (inducible NOS, Transduction Labs), and GAPDH (Santa Cruz Biotechnology). The blot membrane was washed and then incubated with alkaline phosphatase-conjugated anti-rabbit or anti-mouse immunoglobulin G antibody (Santa Cruz Biotechnology). After another wash, the color development was stopped. ImageJ software (National Institutes of Health) was used to calculate the relative densities of protein compared with the housekeeping gene GAPDH. Control experiments were performed in the absence of primary or secondary antibodies.
Intraperitoneal injections of COX-2 inhibitor.
Valdecoxib (1 mg/kg) was injected into the abdominal cavity once each day over the 4 days of partial outlet obstruction. Vehicle (saline solution) was injected in controls.
Analysis of Data
Video imaging, tension recordings, and microelectrode recordings were analyzed as described previously (10, 18, 19). Tension was recorded (MP100 acquisition system, Biopac, Santa Barbara, CA) and stored on a personal computer. Frequency, propagation, and amplitude of contractile complexes were measured using Acqknowledge 3.2.6 (Biopac Systems, Goleta, CA), and tests for statistical significance were made using SigmaPlot 5.0 (Jandel Scientific, San Rafael, CA). The delay between the 50% amplitude points of CMMCs was calculated to determine propagation velocity. Microelectrode electrophysiology was analyzed using algorithms written in-house, as described previously (10, 12, 18, 19, 37).
Statistical Methods
Statistical comparisons of data were performed using Student's (paired or unpaired) t-tests or ANOVA, where P < 0.05 was considered statistically significant; n refers to the number of animals from which colons were taken. Values are means ± SE.
Drugs and Solutions
Atropine, chremophor, indomethacin, hexamethonium bromide, lidocaine, N′-nitro-l-arginine, and PGE2 were purchased from Sigma-Aldrich (St. Louis, MO). Valdecoxib was purchased from Tocris (Bristol, UK). Krebs-Ringer bicarbonate solution (in mM: 120.35 NaCl, 5.9 KCl, 15.5 NaHCO3, 1.2 NaH2PO4, 1.2 MgCl2, 2.5 CaCl2, and 11.5 glucose, pH 7.3–7.4) was continuously gassed with 3% CO2-97% O2.
RESULTS
Effect of Manipulation of External Anal Sphincter Diameter
Restriction of the opening diameter of the external anal sphincter with a purse-string suture reduced daily pellet output from 29.7 ± 1.2 to 8.2 ± 1.5 pellets/day (n = 15, P = 0.005) by day 4, a reduction of ∼73%. At 3–4 days following removal of the suture, average defecation returned to 28.8 ± 2.3 pellets/day (n = 8; Fig. 1, A and D).
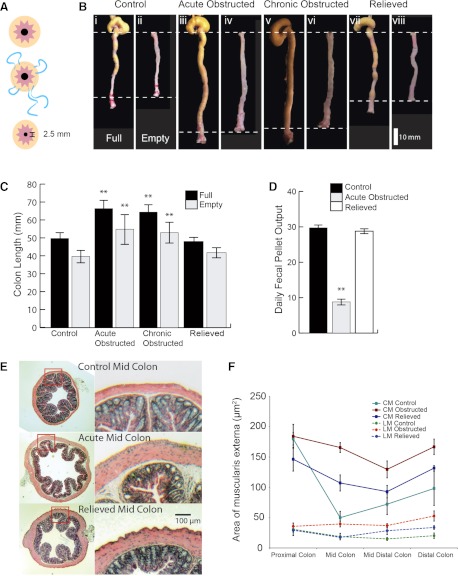
Fig. 1.
Anatomic consequences of partial outlet obstruction. A: purse-string suture applied to perianal region restricts sphincter outlet to 2.5 mm. B: length changes in full and empty control (i and ii), acutely obstructed (iii and iv), chronically obstructed (v and vi), and relieved (vii and viii) colons. C: changes in colon length. D: daily fecal pellet output in control and acutely obstructed colons. **P < 0.001. E: hematoxylin-eosin-stained sections of acutely obstructed midproximal region of colon. F: area of longitudinal muscle (LM) and circular muscle (CM) along the length of acutely obstructed colon.
Fecal pellets in the middle and distal colon of obstructed mice did not properly form; shape, compaction, and desiccation did not occur normally. Fecal pellets were of variable length and often longer in obstructed mice. Wet weight and dry weight of fecal pellets was significantly higher for obstructed than normal mice: 0.03 ± 0.01 g wet wt and 0.01 ± 0.00 g dry wt for normal mice and 0.2 ± 0.03 g wet wt (P < 0.001) and 0.05 ± 0.01 g dry wt (P < 0.005) for obstructed mice (n = 10). Pellets from the obstructed mice were often surrounded by dry powdery fecal matter, which may have prevented water absorption. The pellets crumbled when handled; therefore, dimensional analysis was difficult.
Colonic Elongation and Hypertrophy
As a result of the increase in stool accommodation in the large bowel, colon length markedly increased. In the full state, colon length was 49.7 ± 0.8 mm (n = 10), 66.3 ± 1.6 mm (25% increase, P < 0.005, n = 10), 70.1 ± 1.9 mm (30% increase, P < 0.005, n = 10), and 48.0 ± 1.5 mm (n = 10) in control, acutely obstructed, chronically obstructed, and relieved animals, respectively. In the empty state, colon length was 39.6 ± 1.1 mm (n = 10), 43.5 ± 2.4 mm (9% increase, n = 10), 41.3 ± 1.8 mm (4% increase, n = 10), and 41.8 ± 0.7 mm (n = 10) in control, acutely obstructed, chronically obstructed, and relieved animals, respectively (Fig. 1C).
Hypertrophy of the longitudinal and circular muscle layers was observed along the colon in acutely obstructed mice. The proximal (farthest from obstruction) region appeared to be less affected than regions nearer the obstruction (Fig. 1, E and F; n = 5).
Furthermore, mast cells and macrophages were not observed in preparations from obstructed mice. Also the mucosa appeared to be unaffected and intact in obstructed mice.
Muscle Tension Recordings From the Full Partially Obstructed Colon
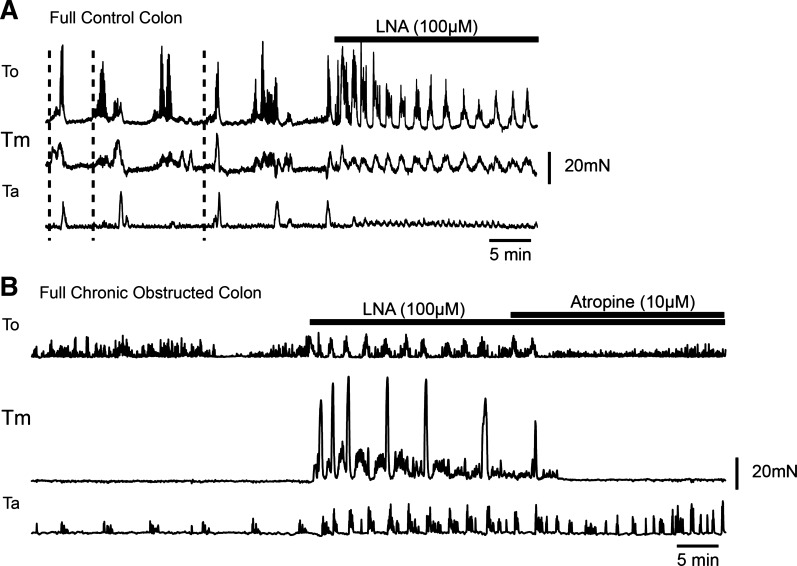
Contractile activity in the isolated, elongated and impacted colons from control mice consisted of CMMCs that often propagated in an oral-to-anal direction (Fig. 2A). In contrast, contractile activity in isolated, elongated and impacted colons from acutely and chronically partially obstructed mice ranged from relative quiescence to fairly rhythic low-amplitude contractions (Fig. 2B). Bath application of N′-nitro-l-arginine (100 μM, n = 5) to control (19) and partially obstructed full colons increased amplitude and frequency of contractile activity (Fig. 2, A and B). Subsequent addition of atropine (10 μM) depressed activity (n = 3; Fig. 2, A and B).
Fig. 2.
Contractile activity in obstructed mice. A: tension recordings from a full normal colon along 3 points [oral (To), middle (Tm), and anal (Ta)]. N′-nitro-l-arginine (l-NA) was added to block nitric oxide (NO). B: tension recordings from a full chronically obstructed colon at To, Tm, and Ta. l-NA increased amplitude and frequency of contractile activity, and atropine depressed contractile activity.
Isolated impacted colons were allowed to spontaneously empty their fecal pellets (11, 19). Control full colons emptied (5–7 pellets) in ∼30 min (11, 19); in obstructed colons, only one to four pellets emptied within 4.5 ± 0.5 h (n = 4). As each pellet emptied from the impacted colon, it shortened, together with an increase in contractile activity, as observed in control colons (11, 19).
Fecal Pellet Transit in the Colon
Epoxy-coated fecal pellets were allowed to spontaneously migrate along isolated segments of colon. Speed of pellet transit was 0.8 ± 0.1, 0.3 ± 0.1, 0.3 ± 0.1, and 0.5 ± 0.1 mm/s in control, acutely obstructed, chronically obstructed, and relieved animals, respectively (n = 6 in each group; Fig. 3).
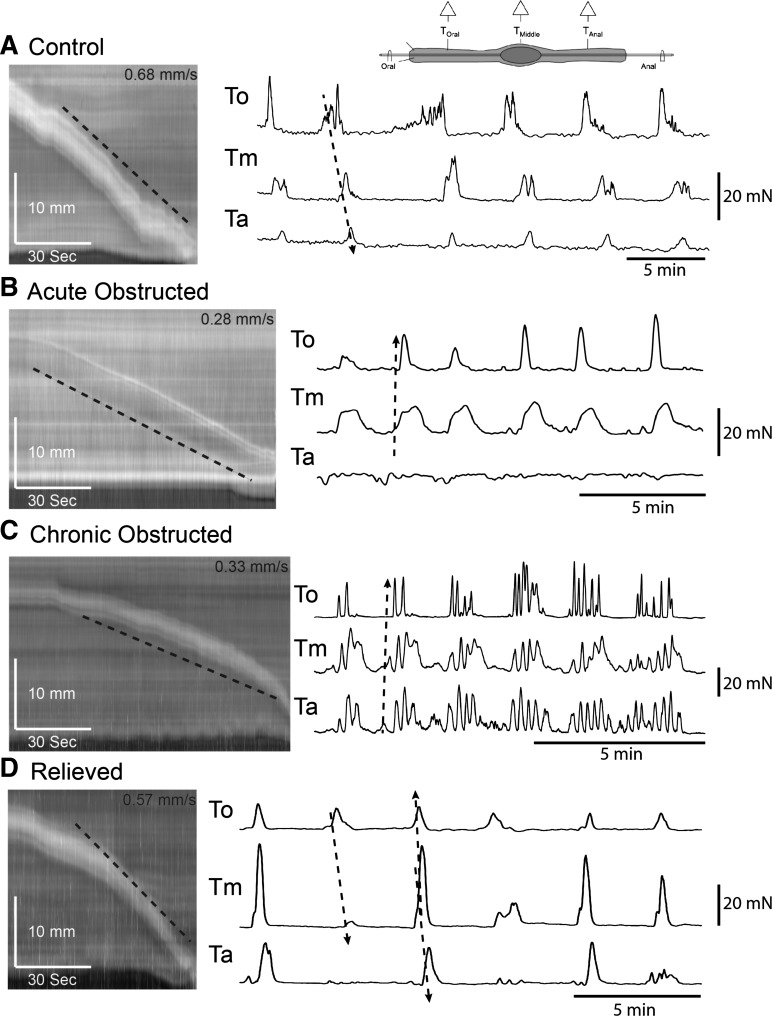
Fig. 3.
Pellet propulsion and colonic migrating motor complexes (CMMCs) in an empty obstructed colon. Left: spatiotemporal maps of artificial pellet propagation in control, acutely obstructed, chronically obstructed, and relieved colons. Right: tension recordings from a colon that contained a fixed fecal pellet along points To, Tm, and Ta. Oral-to-anal propagation is less likely in B and C than in A and D.
Muscle Tension Recordings From the Empty Obstructed Colon
In empty acutely and chronically obstructed colons, CMMCs did not appear to migrate along the colon, despite the presence of a single fixed epoxy-coated fecal pellet in the center of the colon (18, 19), as measured by three transducers placed along the colon (Fig. 3, B and C): To (proximal colon), Tm (middle colon), and Ta (distal colon). CMMC amplitude in control animals was 24.6 ± 2.3, 18.1 ± 2.7, and 11.4 ± 1.9 mN at To, Tm, and Ta, respectively (Fig. 3A; n = 5). Amplitude was decreased in acutely obstructed animals: 12.3 ± 1.6, 13.0 ± 2.2, and 8.4 ± 1.8 mN at To, Tm, and Ta, respectively (n = 5). Amplitude was also decreased in chronically obstructed animals: 12.6 ± 1.3, 11.6 ± 1.5, and 14.2 ± 2.1 mN at To, Tm, and Ta, respectively (n = 5). In relieved animals, amplitude increased to near control measures: 24.5 ± 3.0, 20.6 ± 2.6, and 16.5 ± 5.0 mN at To, Tm, and Ta, respectively (n = 5).
Frequency of CMMC changed from 0.3 ± 0.04 min−1 in control animals (n = 5) to 0.45 ± 0.05, 0.6 ± 0.2, and 0.25 ± 0.05 min−1 in acutely obstructed, chronically obstructed, and relieved animals, respectively (n = 5 in each group; Fig. 3). CMMCs propagated from the oral to the anal direction in 97% of control animals, 32% of acutely obstructed animals, 36% of chronically obstructed animals, and 68% of relieved animals.
Occult Reflexes
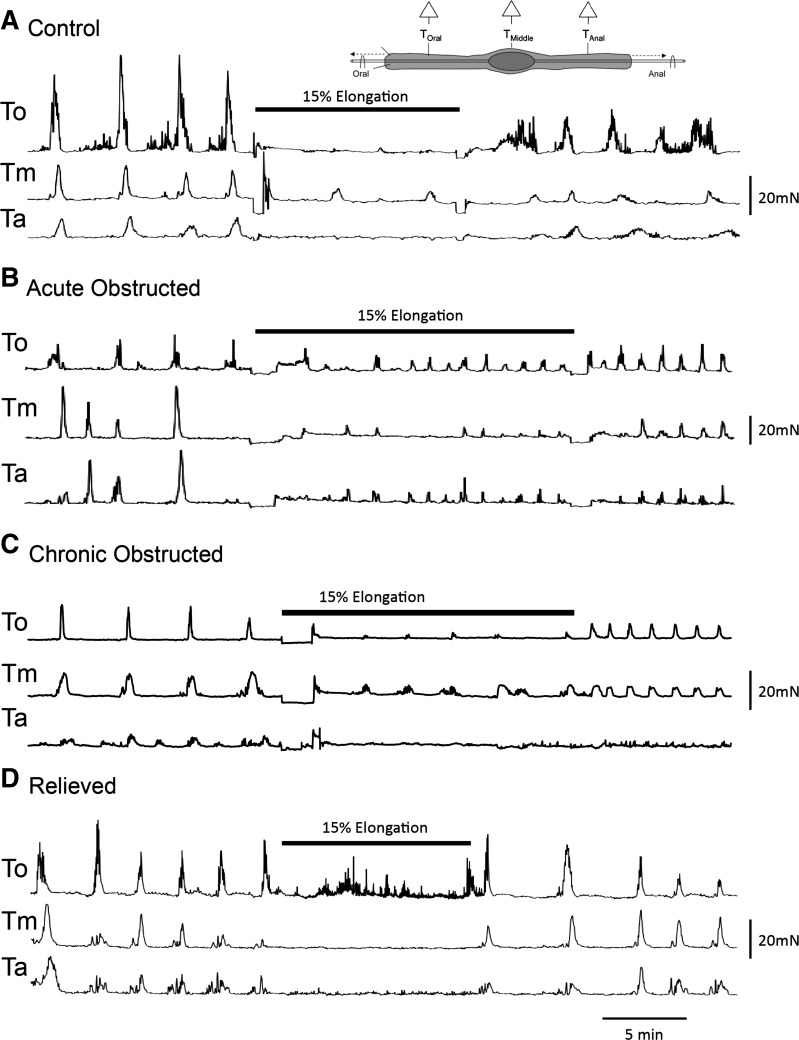
A 15% elongation of the colon (19) resulted in a similar depression of CMMCs in isolated colons from control, acutely obstructed, chronically obstructed, and relieved animals throughout the elongation period (n = 4; Fig. 4).
Fig. 4.
Occult reflexes in obstructed colon. A–D: tension recordings of occult reflex in response to elongation (15%) in control, acutely obstructed, chronically obstructed, and relieved empty colons.
Electrophysiological Recordings of Circular Muscle in the Colon
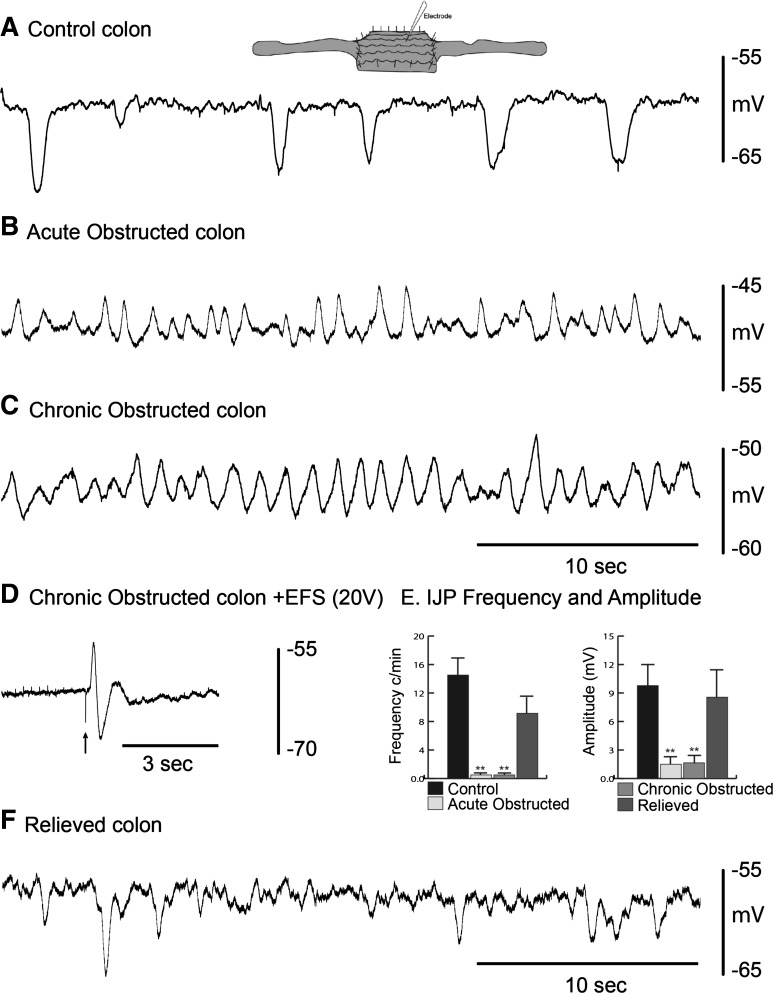
Resting membrane potential of circular muscle cells was −62 ± 3, −50 ± 6, −53 ± 2 mV, and −58 ± 5 mV in control, acutely obstructed, chronically obstructed, and relieved animals, respectively (n = 5 in each group; Fig. 5, A–C and F). Changes in resting membrane potential can be attributed to the loss of tonic inhibition (13, 34, 37), as observed in changes of inhibitory junction potential (IJP) frequency and amplitude and the unmasking of slow-wave activity in obstructed animals. In control animals, IJP frequency was 15 ± 3 min−1 and amplitude was 9.5 ± 2.6 mV; acutely obstructed mice rarely displayed spontaneous IJPs, and amplitude was 1.5 ± 0.8 mV; chronically obstructed mice also rarely (<5 h−) displayed spontaneous IJPs, and amplitude was 1.3 ± 0.6 mV; in relieved animals, frequency was 9 ± 3 min−1, and amplitude was 8.3 ± 3.1 mV (n = 5 in each group; Fig. 5E). Excitatory junction potentials and IJPs can still be evoked using electrical field stimulation (EFS; 0.5 ms, 20 V, single pulse, n = 3) in acutely and chronically obstructed animals, suggesting that the neuromuscular junction is intact and functional (Fig. 5D). The excitatory junction potential was blocked by atropine (1 μM), and the IJP was blocked by the P2Y receptor antagonist MRS-2500 (1 μM, n = 3) (20).
Fig. 5.
Spontaneous inhibitory junction potentials (IJPs) in whole colons. A: intracellular recordings from circular smooth muscle showing spontaneous IJPs in a control colon. B and C: slow waves, but no IJPs, in acutely and chronically obstructed colon. D: electrical field stimulation (EFS) evokes an excitatory junction potential and IJP in a chronically obstructed colon. E: IJP frequency and amplitude in control, acutely/chronically obstructed, and relieved colon. **P < 0.001. F: spontaneous IJPs in a relieved colon.
Increases in COX-2 Activity in the Colon
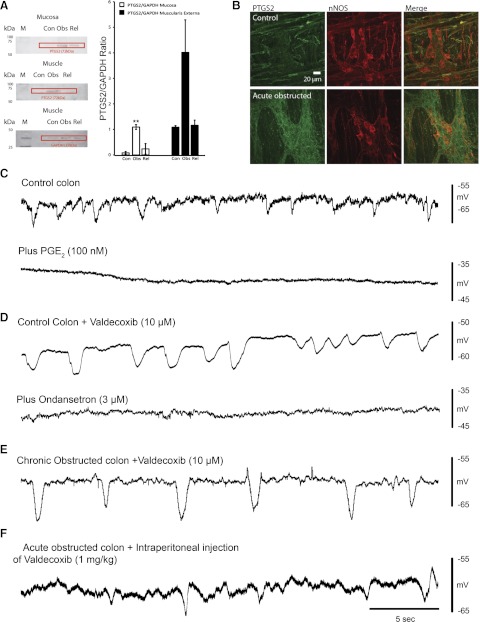
COX-2 (PTGS2)-to-GAPDH ratios increased from 8% (0.08 ± 0.05) in mucosa from control animals to 110% (1.09 ± 0.09) in mucosa from obstructed mice and decreased to 24% (0.24 ± 0.21) in mucosa from relieved animals. The muscularis externa, including the myenteric plexus, exhibited a similar pattern of COX-2-to-GAPDH ratio: increases of 3% (0.03 ± 0.02), 402% (4.02 ± 1.27), and 8% (0.08 ± 0.06) in control, obstructed, and relieved animals, respectively (n = 3; Fig. 6, A and B). Immunohistochemical labeling indicated low COX-2 (PTGS2) immunoreactivity in control colon (n = 3; Fig. 6B). In control preparations, there was little COX-2 immunoreactivity within the myenteric plexus (Fig. 6B); however, in the obstructed colon, COX-2 staining was prominent and observed around many myenteric neurons and throughout the ganglia, suggesting that it may also be located in glial cells. COX-2 appeared to colocalize in some nNOS neurons (n = 4; Fig. 6B).
Fig. 6.
Consequences of cyclooxygenase (COX)-2 inhibition. A: Western blots indicating an increase in prostaglandin-endoperoxide synthase II (PTGS2) levels in muscularis externa (including myenteric plexus) and mucosa of obstructed control (Con), obstructed (Obs), and relieved (Rel) colon (left; M, molecular weight marker) and plot illustrating increased PTGS2-to-GAPDH ratio in muscularis externa and mucosa of obstructed colon (right). **P < 0.001. B: immunohistochemical labeling of myenteric ganglia for COX-2 (PTGS2 in green) and neuronal nitric oxide synthase (nNOS, red) in control and acutely obstructed colon. Merge shows COX-2 labeling of nNOS-positive neurons. C: spontaneous IJPs in control colon before and after PGE2. D: spontaneous IJPs in control colon after valdecoxib and ondansetron. E: spontaneous IJPs in chronically obstructed colon following valdecoxib. F: spontaneous IJPs in acutely obstructed colon following intraperitoneal injection of valdecoxib.
PGE2 (100 nM), a product of the membrane lipid arachidonic acid catalyzed by COX-2, completely abolished spontaneous IJPs and depolarized the circular muscle by 9.0 ± 0.4 mV (n = 4) in control colon (Fig. 6C).
Bath application of valdecoxib (10 μM) for 1–2 h had no significant effect on spontaneous IJPs in control preparations, which were blocked by the 5-HT3 antagonist ondansetron (3 μM, n = 2; Fig. 6D) (12, 18). Valdecoxib (10 μM) also had no significant effect on the amplitude and propagation of CMMCs (data not shown; n = 3). After bath application of valdecoxib (10 μM) for 1–2 h in acutely obstructed (n = 2) and chronically obstructed (n = 3) colons, the resting membrane potential (−55 ± 4 mV), spontaneous IJPs (frequency 12 ± 2.8 min−1), and amplitude of IJPs (8.8 ± 2.5 mV, n = 5) returned toward control levels (n = 5; Fig. 6E). Spontaneous IJPs were also observed in acutely obstructed mice that had received one daily injection of valdecoxib (1 mg/kg ip) throughout the obstruction period (Fig. 6F).
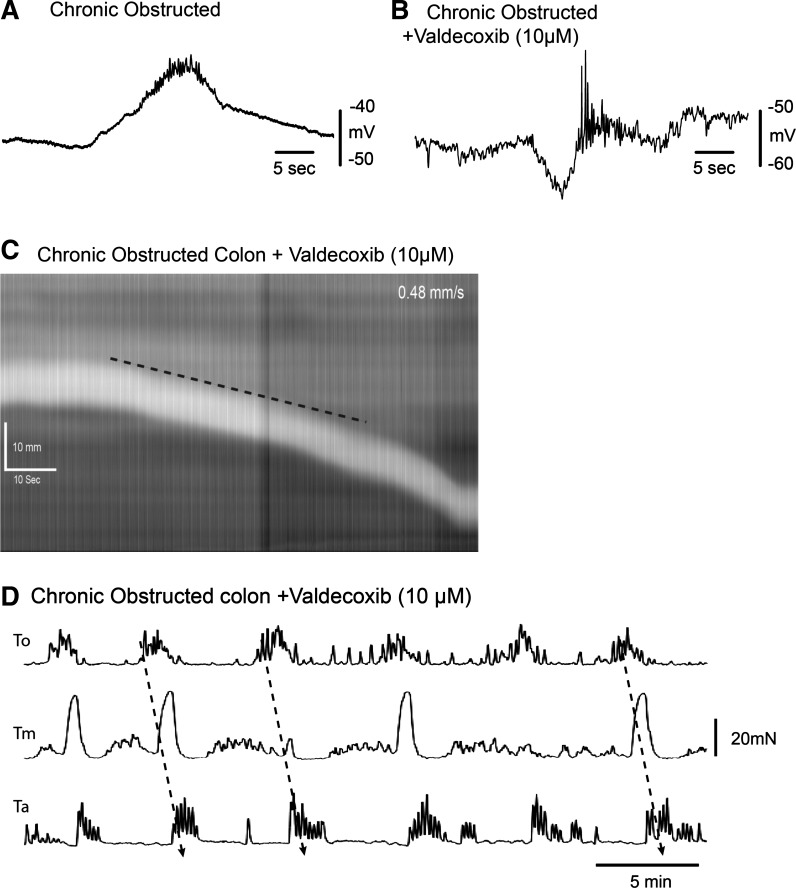
Spontaneous CMMCs in acutely/chronically obstructed colons lacked the preceding inhibition, which was restored following valdecoxib (10 μM; Fig. 7, A and B). Valdecoxib (10 μM, a COX-2 antagonist), which had no detectable effect on control CMMCs (n = 3), increased the rate of pellet propagation to 0.5 ± 0.1 mm/s (n = 6) through the isolated obstructed colon (Fig. 7C). After valdecoxib, the propagation direction, amplitude, and frequency of CMMCs in obstructed mice were similar to those in control mice: 26.7 ± 2.7, 32.9 ± 4.1, and 20.1 ± 1.9 mN at To, Tm, and Ta, and To, respectively; 0.3 ± 0.01 min−1, and 81% (n = 5) (Fig. 7D), presumably because of restored preceding inhibition.
Fig. 7.
Effect of COX-2 inhibition on CMMCs in obstructed colon. A and B: electrical recording of CMMC in obstructed colon before and after valdecoxib (10 μM), where CMMC is preceded by a hyperpolarization. C and D: valdecoxib (10 μM) increases transit of a fecal pellet (C) and causes propagation of CMMCs in obstructed colon (D).
Effect of Mucosa Removal and Mucosal Reflexes in Obstructed Mice
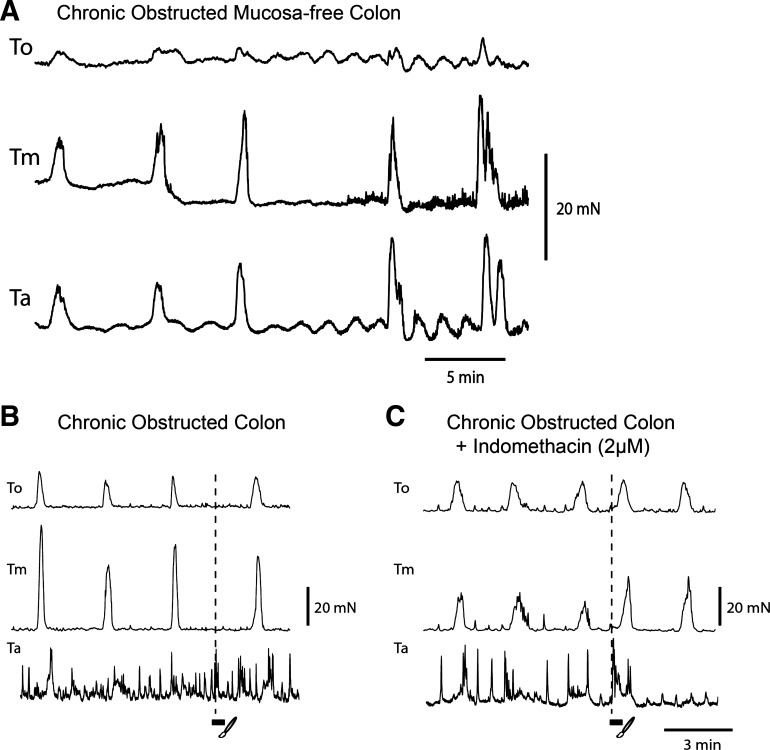
Previously we reported that removal of the mucosa abolishes CMMCs (1, 12, 13). However, when the mucosa was removed from obstructed mice with high COX-2 levels, spontaneous CMMC activity, at a frequency of 0.40 ± 0.02 min−1, was observed (n = 6; Fig. 8A).
Fig. 8.
Effect of mucosa removal and mucosal reflexes in obstructed colon. A: tension recordings from a mucosa-free chronically obstructed colon (note CMMCs occurred simultaneously along the colon). B and C: mucosal stimulation (3 strokes) of the rectum before (B, no response) and after (C) indomethacin. Note premature CMMC at oral recording sites.
In acutely and chronically isolated colons, stroking of the mucosa rarely (1 in ∼20 stimuli, n = 6) evoked a CMMC (Fig. 8B), as shown for normal colons (1–3, 18). However, after addition of indomethacin (2 μM), a robust premature CMMC was evoked following mucosal stimulation (n = 3; Fig. 8C).
DISCUSSION
Our mouse model of STC induced by partial outlet obstruction requires a simple local procedure and only 15 min for an experienced investigator, as opposed to invasive surgical intervention. Our model shows a number of features that are phenotypically similar to those observed in some humans with STC induced by outlet obstruction; these include reduced fecal output, an impacted elongated colon, slowed transit, and enhanced slow-wave activity resulting from a lack of tonic inhibition, CMMCs, which are still present but do not propagate in an oral-to-anal direction, and muscle hypertrophy.
Many of these changes appear to result from an upregulation of COX-2 activity in the mucosa and muscularis externa, including nNOS-positive myenteric neurons and possibly glia.
Interestingly, muscle hypertrophy along the large intestine of obstructed mice was similar to the gradient in muscle thickness observed in the Hirschsprung's mouse model, which has an aganglionic constricted rectum resulting from lack of tonic inhibition (35).
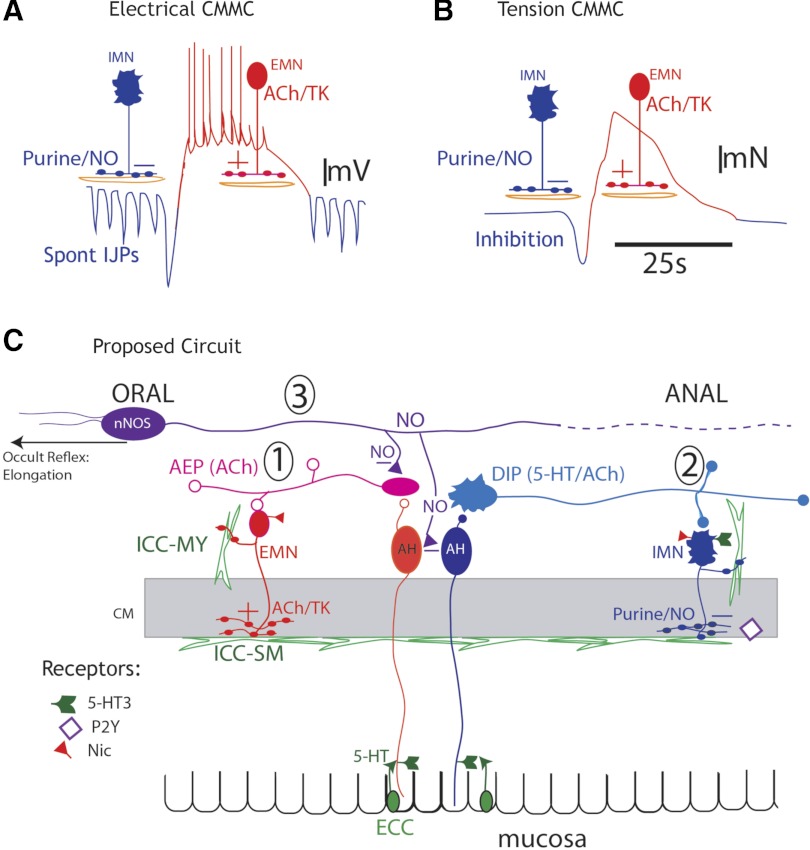
The large intestine is always under intrinsic neural activity. Between CMMCs, descending inhibitory nerve pathways exhibit ongoing activity (tonic inhibition); however, during the CMMC, some inhibitory motor neurons reduce their activity, and ascending excitatory nerve pathways are strongly activated (11, 19, 37) (Fig. 9, circuit 1).
Fig. 9.
Hypothetical nerve circuit controlling colonic motility. A and B: electrical and tension recordings of CMMCs. A: ongoing tonic inhibition, consisting of spontaneous inhibitory junction potentials (IJPs, blue downward deflections) in circular muscle and inhibition of pacemaker interstitial cells of Cajal, between CMMCs. Before the CMMC, there can be an increased burst of IJPs/relaxation that determines its oral-to-anal direction of propagation and fecal pellet propulsion. A CMMC consists of activation of excitatory motor neurons (EMNs), which produce a slow depolarization, upon which are superimposed fast oscillations that trigger action potentials and contraction (red). IMN, inhibitory motor neurons; TK, tachydinin; NO, nitric oxide. B: between CMMCs, there is little muscle tone because of ongoing tonic inhibition. When a fecal pellet is present, a small relaxation, due to increased IJP activity (blue), can precede the CMMC. C: spontaneous release of 5-HT from enterochromaffin cells (ECC) activates 5-HT3 receptors on projections of afterhyperpolarizing (AH) neurons in the mucosa (circuit 1); these neurons synapse with ascending interneurons in the ascending excitatory pathway (AEP), which synapse with excitatory motor neurons that are responsible for the CMMC (A and B). When fecal pellets are present, they are more likely to activate different AH sensory neurons (located around the pellet) by circumferential stretch or by mucosal distortion, which releases 5-HT onto the 5-HT3 receptors of AH neurons in the mucosa (circuit 2); these neurons synapse with descending serotonergic interneurons in the descending inhibitory pathway (DIP), which synapse with inhibitory motor neurons (A and B). Elongation activates mechanosensitive descending nNOS-positive interneurons, which release NO to inhibit ascending interneurons and AH sensory neurons, thereby decreasing colonic motility (circuit 3, occult reflex); partial outlet obstruction increases prostaglandins, which depress the DIP (circuit 2), by inhibiting serotonergic neurons or IMNs; in addition, increased prostaglandins may inhibit 5-HT release from ECC in the mucosa and block mucosal reflexes. Nic, nicotinic receptor; ICC-MY, ICCs in the myenteric region; ICC-SM, ICCs along the submucosal surface.
Our studies suggest that the motility dysfunction resulting from partial outlet obstruction is due to suppression of the descending (serotonergic) inhibitory nerve pathway (Fig. 9, circuit 2). This nerve pathway is necessary for not only tonic inhibitory drive to the muscle and interstitial cells of Cajal (ICC) in the myenteric region (ICC-MY) between CMMCs, but also for propagating CMMCs (1, 2, 18, 37). Since CMMC activity is observed in obstructed mice, it is clear that the ascending excitatory neural pathways that generate the CMMC are intact (1–3, 12, 13, 37) (Fig. 9, circuit 1). Additionally, ICC-MY, which contact both muscle layers and are activated by excitatory motor neurons during the CMMC, must also be viable, since they are largely responsible for the fast oscillations and slow depolarization phase of the CMMC in the muscle (2, 13) (Fig. 9A). This complex electrical event appears to conduct from ICC-MY into the longitudinal and circular muscle layers to generate muscle action potentials and synchronous contraction of both muscle layers during the CMMC (1, 2, 35). Occult reflexes appear to be normal in the obstructed mice, since colonic elongation still suppresses the CMMC (19); therefore, mechanosensitive descending (nNOS) interneurons appear to be unaffected by partial outlet obstruction (Fig. 9, circuit 3).
Correlation of the Murine Model With STC in Human Patients
CMMCs in the isolated human colon, which were surgically removed (colectomy) from patients with colon cancer or chronic STC, have a duration and frequency similar to CMMCs in mice (38, 42). CMMCs in the empty isolated colons of cancer patients (controls) can propagate in any direction, similar to those in control mice without a fixed fecal pellet (18). In contrast, colons removed from patients with chronic STC exhibit CMMCs that do not propagate but appear to occur synchronously over long lengths of colon (38, 42), similar to CMMCs in our obstructed mice. Importantly, intraluminal distension of the colon from patients with STC evokes an ascending excitatory neural reflex (premature CMMC) but apparently no descending inhibitory reflex, i.e., anal relaxation, which was observed in control colons (38). It is likely that the lack of propagation of CMMCs in patients with STC is due to the depression of descending inhibitory nerve pathways, similar to our obstructed mice.
Occult Reflexes in Elongated Colons
Our partial outlet obstruction model would predict a powerful suppression of motility by the occult reflex due to increased elongation, reduced mucosal reflexes, and spontaneous CMMCs in mucosa-free preparations.
Contractions along the impacted obstructed colon are of low amplitude because of the release of NO, and emptying is extremely slow compared with that of full control colons. This suggests that the NO may largely derive from the inhibitory occult reflex pathways (10, 11, 19), which are clearly intact and likely strongly activated because of the increased colonic elongation. Elongated colons are frequently observed in humans with chronic constipation (33, 39). NO is an ideal inhibitory neurotransmitter for the occult reflex, since mechanosensitive interneurons underlying this neural reflex are always likely to be continuously firing while the colon is elongated. NO is generated by the Ca2+-dependent enzyme nNOS in axonal varicosities and, unlike most other inhibitory neurotransmitters stored in vesicles, is unlikely to be depleted following bursts of action potentials that depolarize varicosities causing Ca2+ influx (3) (Fig. 9, circuit 3).
Changes in Motility of Partially Obstructed Mice
Increases and decreases in motility have been observed in humans with STC (8). Our obstructed colons appear to be adapting to upregulate motility to expel fecal content against an increased resistance (hypertrophy), since tonic inhibition is absent and slow-wave activity is prominent. As demonstrated in our murine model, acute and chronic obstructions result in a loss of spontaneous IJPs in the circular muscle layer, although IJPs can be readily evoked by EFS, suggesting that the neuromuscular junction is unaffected. Where in the descending inhibitory nerve pathway the depression occurs is unclear. Prostaglandins may inhibit activity in descending serotonergic interneurons or inhibitory (nNOS-positive) motor neurons (2, 10) (Fig. 9). Because this descending inhibitory nerve pathway is depressed, CMMCs do not propagate in an oral-to-anal direction (18, 37) and pellet transit is inhibited in the obstructed colon.
Removal of tonic inhibition cannot be responsible for generating the CMMC, as proposed previously (31), since CMMCs are still present in obstructed mice. They are more likely generated primarily by the activation of ascending excitatory nerve pathways, which activate excitatory motor neurons that release ACh and tachykinins onto pacemaker ICC and the muscle (1, 2, 6, 13, 37) (Fig. 9, circuit 1).
COX-2 in Partially Obstructed Mice
Typically, colitis and diarrhea are associated with increases in COX-2, which is found in enteric neuronal cell populations (23–25). Increases in COX-2, but decreases in COX-1, occur in patients with chronic STC (7). We found that COX-2 inhibitors restored IJP activity, suggesting that the descending inhibitory nerve pathway was only suppressed by prostaglandins. PGE2 was found to block spontaneous IJPs and depolarize the circular muscle, suggesting that it was removing tonic inhibition. COX-2 and nNOS were colocalized to a small population of nNOS neurons; similar results have been shown in gastric nNOS neurons, suggesting that COX-2 can be constitutively expressed in some guinea pig myenteric neurons and human colonic tissue (16, 25). Additionally, prostaglandins such as PGE2 have been shown to regulate myenteric neuron activity during inflammation (23, 24).
CMMCs Without the Mucosa and Mucosal Reflexes
The role of the mucosa in generating CMMCs is controversial (22, 31). We have observed that administration of ondansetron (a 5-HT3 antagonist) or removal of the mucosa abolishes spontaneous CMMCs and CMMCs evoked by mechanical stimulation of the mucosa in control mice (1, 12, 13, 18). AH neurons can be activated by ondansetron-sensitive 5-HT release from the mucosa following local stimulation (1). These observations lead to the suggestion that 5-HT release from enterochromaffin cells in the mucosa was necessary for initiating the CMMC via activation of AH neurons, which are certainly active during the CMMC (1, 12, 19).
Others observed spontaneous CMMCs in mucosa-free preparations (22) and no mucosal reflexes in control mice (41). It was suggested that the dissection technique we used to remove the mucosa damaged the myenteric plexus and that mucosal reflexes are due to mechanical distortion of the myenteric plexus (41, 42). Both of these suggestions are unlikely, because 1) we observed ongoing neural activity in myenteric neurons, including nNOS-positive inhibitory motor neurons, and spontaneous IJPs in the circular muscle of mucosa-free preparations (1, 12, 13); 2) mucosal stimulation evokes an ondansetron-sensitive CMMC, as well as responses in AH neurons that were also ondansetron-sensitive, suggesting that 5-HT was released from the mucosa during stimulation (1, 19); and 3) EFS evoked an ondansetron-insensitive CMMC in our mucosa-free preparations (12, 13), suggesting that the neural circuitry is functional.
In our partially obstructed mice, which have higher prostaglandin levels, we observed spontaneous CMMCs in mucosa-free preparations and could not readily evoke mucosal reflexes in partially obstructed mice until we inhibited prostaglandin synthesis with indomethacin. We have found that AH neurons in partially obstructed colons are more excitable (unpublished observations). The difference between our results and the findings reported by others is that their mice may exhibit higher levels of prostaglandins, likely due to environmental conditions, stress, diet, or dissection.
Outlet obstruction increases COX-2, which leads to a hyperexcitable colon due to loss of tonic inhibition. The absence of mucosal reflexes in obstructed mice suggests that transit would rely on higher-threshold stretch reflexes, rather than mucosal reflexes (19). The lack of rectal sensitivity in some patients with STC may also be a consequence of a lack of mucosal reflexes.
GRANTS
This study was funded National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1 DK-45713 (T. K. Smith). Imaging and molecular biology were performed in a Core Laboratory funded by National Institutes of Health Grant COBRE 8P20GM103513-09.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.J.H. and T.K.S. are responsible for conception and design of the research; D.J.H., N.G., and C.J.M. performed the experiments; D.J.H., N.G., and C.J.M. the analyzed data; D.J.H., N.G., and T.K.S. interpreted the results of the experiments; D.J.H. and T.K.S. prepared the figures; D.J.H. and T.K.S. drafted and revised the manuscript.
ACKNOWLEDGMENTS
We thank Drs. John Hasenau and Walt Mandenville (Laboratory Animal Medicine, University of Nevada, Reno) for assistance in developing this model and the necessary surgical techniques. We also thank Dr. Okamoto for advice with immunohistochemistry.
REFERENCES
- 1. Bayguinov PO, Hennig GW, Smith TK. Calcium activity in different classes of myenteric neurons underlying the migrating motor complex in the murine colon. J Physiol 588: 399–421, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bayguinov PO, Hennig GW, Smith TK. Ca2+ imaging of activity in ICC-MY during local mucosal reflexes and the colonic migrating motor complex in the murine large intestine. J Physiol 588: 453–474, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bayguinov PO, Broadhead MJ, Okamoto T, Hennig GW, Smith TK. Activity in varicosities within the myenteric plexus between and during the colonic migrating motor complex in the isolated murine large intestine. Neurogastroenterol Motil 24: e185–e201, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol 24: 99–143, 1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bayliss WM, Starling EH. The movements and innervation of the large intestine. J Physiol 26: 107–118, 1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brierley SM, Nichols SK, Grasby DJ, Waterman SA. Neural mechanisms underlying migrating motor complex formation in the mouse isolated colon. Br J Pharmacol 132: 507–517, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cong P, Pricolo V, Biancani P, Behar J. Abnormalities of prostaglandins and cyclooxygenase enzymes in female patients with slow-transit constipation. Gastroenterology 133: 445–453, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Connell AM. The motility of the pelvic colon. II. Paradoxical motility in diarrhoea and constipation. Gut 3: 342–348, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D'Hoore A, Penninckx F. Obstructed defecation. Colorectal Dis 5: 280–287, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Dickson EJ, Spencer NH, Hennig GW, Bayguinov PO, Ren J, Heredia DJ, Smith TK. An enteric occult reflex underlies accommodation and slow transit in the distal large bowel. Gastroenterology 132: 1912–1924, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Dickson EJ, Hennig GW, Heredia DJ, Lee HT, Bayguinov PO, Spencer NH, Smith TK. Polarized intrinsic neural reflexes in response to colonic elongation. J Physiol 586: 4225–4240, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dickson EJ, Heredia DJ, Smith TK. Critical role of 5-HT1A, 5-HT3 and 5-HT7 receptor subtypes in the initiation, generation, and propagation of the murine colonic migrating motor complex. Am J Physiol Gastrointest Liver Physiol 299: G144–G157, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dickson EJ, Heredia DJ, Hennig GW, Smith TK. Mechanisms underlying the colonic migrating motor complex in both wild-type and nNOS knockout mice. Am J Physiol Gastrointest Liver Physiol 298: G222–G232, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dinning PG, Smith TK, Scott SM. Pathophysiology of colonic causes of chronic constipation. Neurogastroenterol Motil 21 Suppl 2: 20–30, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology 20: 1377–1390, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Fornai M, Blandizzi C, Colucci R, Antonioli L, Bernardini N, Segnani C, Baragatti B, Barogi S, Berti P, Spisni R, Del Tacca M. Role of cyclooxygenases 1 and 2 in the modulation of neuromuscular functions in the distal colon of humans and mice. Gut 54: 608–616, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghoshal UC. Review of pathogenesis and management of constipation. Trop Gastroenterol 28: 91–95, 2007 [PubMed] [Google Scholar]
- 18. Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW, Smith TK. Localized release of serotonin (5-hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology 136: 1328–1338, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW, Smith TK. Colonic elongation inhibits pellet propulsion and migrating motor complexes in the murine large bowel. J Physiol 588: 2919–2934, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hwang SJ, Durnin L, Swyer L, Rhee PL, Ward SM, Koh SD, Sanders KM, Mutafova-Yambolieva VN. β-Nicotinamide adenine dinucleotide is an enteric inhibitory neurotransmitter in human and nonhuman primate colons. Gastroenterology 140: 608–617, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keating DJ, Spencer NJ. Release of 5-hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology 138: 659–670, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Linden DR, Sharkey KA, Ho W, Mawe GM. Cyclooxygenase-2 contributes to dysmotility and enhanced excitability of myenteric AH neurones in the inflamed guinea pig distal colon. J Physiol 557: 191–205, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manning BP, Sharkey KA, Mawe GM. Effects of PGE2 in guinea pig colonic myenteric ganglia. Am J Physiol Gastrointest Liver Physiol 283: G1388–G1397, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Porcher C, Horowitz B, Bayguinov O, Ward SM, Sanders KM. Constitutive expression and function of cyclooxygenase-2 in murine gastric muscles. Gastroenterology 122: 1442–1454, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Raahave D, Christensen E, Loud FB, Knudsen LL. Correlation of bowel symptoms with colonic transit, length, and faecal load in functional faecal retention. Dan Med Bull 56: 83–88, 2009 [PubMed] [Google Scholar]
- 27. Ragg J, McDonald R, Hompes R, Jones OM, Cunningham C, Lindsey I. Isolated colonic inertia is not usually the cause of chronic constipation. Colorectal Dis 13: 1299–1302, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Sanmiguel CP, Soffer EE. Constipation caused by functional outlet obstruction. Curr Gastroenterol Rep 5: 414–418, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Schiller LR. New and emerging treatment options for chronic constipation. Rev Gastroenterol Disord 4 Suppl 2: S43–S51, 2004 [PubMed] [Google Scholar]
- 30. Smith TK, Spencer NJ, Hennig GW, Dickson EJ. Recent advances in enteric neurobiology: mechanosensitive interneurons. Neurogastroenterol Motil 19: 869–878, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Smith TK, Dickson EJ, Heredia DJ, Hennig GW, Bayguinov PO. Controversies involving the role of 5-hydroxytryptamine (5-HT) in generating colonic migrating motor complexes: what is spontaneous? Gastroenterology 138: 659–670, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Southwell BR, Clarke MC, Sutcliffe J, Hutson JM. Colonic transit studies: normal values for adults and children with comparison of radiological and scintigraphic methods. Pediatr Surg Int 25: 559–572, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Southwell BR. Colon lengthening slows transit: is this the mechanism underlying redundant colon or slow transit constipation? J Physiol 588: 1833–1843, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spencer NJ, Bywater RA, Taylor GS. Disinhibition during myoelectric complexes in the mouse colon. J Auton Nerv Syst 71: 37–47, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Spencer NJ, Bayguinov P, Hennig GW, Park KJ, Lee HT, Sanders KM, Smith TK. Activation of neural circuitry and Ca2+ waves in longitudinal and circular muscle during CMMCs and the consequences of rectal aganglionosis in mice. Am J Physiol Gastrointest Liver Physiol 292: G546–G555, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Spencer NJ, Smith TK. Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig distal colon. J Physiol 533: 787–799, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spencer NJ, Hennig GW, Dickson E, Smith TK. Synchronization of enteric neuronal firing during the murine colonic MMC. J Physiol 564: 829–847, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spencer NJ, Kyloh M, Wattchow DA, Thomas A, Sia TC, Brookes SJ, Nicholas SJ. Characterization of motor patterns in isolated human colon: are there differences in patients with slow-transit constipation? Am J Physiol Gastrointest Liver Physiol 302: G34–G43, 2012 [DOI] [PubMed] [Google Scholar]
- 39. Tomita R, Howard ER. Clinical studies on anorectal myectomy for chronically constipated patients with outlet obstruction in childhood. Hepatogastroenterology 55: 1600–1605, 2008 [PubMed] [Google Scholar]
- 41. Zagorodnyuk VP, Spencer NJ. Localization of the sensory neurons and mechanoreceptors required for stretch-evoked colonic migrating motor complexes in mouse colon. Front Physiol 2: 98, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zarate N, Spencer NJ. Chronic constipation: lessons from animal studies. Best Pract Res Clin Gastroenterol 25: 59–71, 2011 [DOI] [PubMed] [Google Scholar]