Abstract
Riboflavin (RF) is essential for the normal metabolic activities of pancreatic β-cells and provides protection against oxidative stress. Very little is known about the mechanism of RF uptake by these cells and how the process is regulated. We addressed these issues using mouse-derived pancreatic β-TC-6 cells and freshly isolated primary mouse and human pancreatic islets. Our results showed 3H-RF uptake by β-TC-6 cells is Na+ independent, cis inhibited by RF-related compounds, trans stimulated by unlabeled RF, and saturable as a function of concentration (apparent Km of 0.17 ± 0.02 μM). The latter findings suggest involvement of a carrier-mediated process. Similarly, RF uptake by primary mouse and human pancreatic islets was via carrier-mediated process. RF transporters 1, 2, and 3 (RFVT-1, -3, and -2) were all expressed in mouse and human pancreatic β-cells/islets, with RFVT-1 being the predominant transporter expressed in the mouse and RFVT-3 in the human. Specific knockdown of RFVT-1 with gene-specific small interfering RNA leads to a significant inhibition in RF uptake by β-TC-6 cells. RF uptake by β-TC-6 cells was also found to be adaptively upregulated in RF deficiency via a transcriptional mechanism(s). Also, the process appears to be under the regulation of a Ca2+/calmodulin-mediated regulatory pathway. Results of these studies demonstrate, for the first time, the involvement of a carrier-mediated process for RF uptake by mouse and human pancreatic β-cells/islets. Furthermore, the process appears to be regulated by extracellular and intracellular factors.
Keywords: riboflavin, pancreatic cells, uptake mechanism, uptake regulation
the micronutrient riboflavin (RF) is essential for normal cellular functions, growth, and development. In its coenzyme forms, i.e., RF-5-phosphate and flavin adenosine dinucleotide, the vitamin plays a key metabolic role in the transfer of electrons in biological oxidation-reduction reactions involving carbohydrate, lipid and amino acid metabolism, as well as the conversion of vitamin B6 and folate into their active forms (7). RF also plays a role in protein folding in the endoplasmic reticulum (34) and reduces cellular oxidative stress (13, 20, 30). Furthermore, RF appears to be able to modulate both innate and immune responses (33) and exhibits powerful anti-inflammatory properties (4, 5, 13). Systemic RF deficiency leads to serious clinical abnormalities that include degenerative changes in the nervous system, anemia, and growth retardation (7, 12). Deficiency and suboptimal levels of the vitamin occur in patients with diabetes mellitus, inflammatory bowel disease, and chronic alcoholism (2, 10, 11, 14, 15, 21, 25). In contrast to the negative effects of RF deficiency, optimizing RF body level appears to have the potential of protecting vital tissues from ischemia-induced oxidative injury (1, 20) and is effective in the treatment of patients with RF-responsive multiple acyl-CoA dehydrogenase deficiency (17, 18).
Like other mammalian cells, pancreatic cells cannot synthesize RF and thus must obtain the vitamin from exogenous sources via transport across the plasma membrane. Nothing, however, is currently known about the mechanism of RF uptake by cells of this important organ, how the uptake process is regulated, or the effect of conditions and factors that affect the process. In this study, we began to address these issues, focusing on pancreatic β-cells. These cells are highly sensitive to oxidative stress and to inflammation (5), and RF has the ability to reduce the level of free radicals and proinflammatory mediators (1, 5), not to mention its essentiality for their normal cellular metabolism. Our studies were conducted using cultured mouse-derived pancreatic β-TC-6 cells and freshly isolated mouse primary pancreatic islets as models. We also extended the study to the human situation and examined aspects of RF uptake by freshly isolated primary human pancreatic islets. Our results showed RF uptake by pancreatic β-cells/islets are via a specific carrier-mediated process. Furthermore, our findings showed RF uptake by these cells to be regulated by extracellular substrate level (via what appears to be a transcriptional mechanism) and also by an intracellular Ca+2-calmodulin regulatory pathway.
MATERIALS AND METHODS
Materials
3H-RF (specific activity 21.2 Ci/mmol) was obtained from Moravek Biochemicals (Brea, CA). All other chemicals, kits, and routine reagents used in the study were either analytic or molecular biology grade and purchased from commercial vendors. Gene-specific primers used in this study were synthesized from Sigma Genosys (Woodlands, TX) and listed in Table 1.
Table 1.
Combination of primers used for PCR
| mRFVT-1 | TGCTGGCCATCACCAA; CGTGAGCACCTGCACA |
| mRFVT-2 | GGATCAGTGGAAGCCAGTG; GACCTGTTAGGCAGGAAGATG |
| mRFVT-3 | CCTCCCTTCCTACCTCTCTG; TAGGAAGGCCACTGAGTACG |
| mβ-actin | CATCCTGCGTCTGGACCT; TAATGTCACGCACGATTTCC |
| hRFVT-1 | AAAAGACCTTCCAGAGGGTTG; AGCACCTGTACCACCTGGAT |
| hRFVT-2 | CCTTTCCGAAGTGCCCATC; AGAAGGTGGTGAGGTAGTAGG |
| hRFVT-3 | CCCTGGTCCAGACCCTA; ACACCCATGGCCAGGA |
| hβ-actin | AGCCAGACCGTCTCCTTGTA; TAGAGAGGGCCCACCACAC |
| mRFVT-1-hnRNA | GGAGGGTGAGTGAAGTGG; AGGCTCCAACCTGCAG |
| mβ-actin-hnRNA | GATTTCTGTCACTCTTCTCTTAGG; TCTCACCAAGCTAAGGATGC |
m, Mouse; h, human; RFVT, RF transporter; hnRNA, heterogeneous nuclear RNA.
Cell Culture and Uptake Studies
Mouse-derived β-TC-6 cells were obtained from ATCC (Rockville, MD) and maintained under standard condition in DMEM growth medium supplemented with 15% FBS (BenchMark). RF-deficient medium was prepared by supplementing custom-made vitamin-deficient medium (DMEM from GIBCO-BRL, Grand Island, NY) with other vitamins, except RF. Uptake studies were performed in Krebs-Ringer (KR) buffer (pH 7.4) at 37°C during initial linear periods (i.e., 5 min), and the cells were processed as described before (22). Total protein content was determined using Bio-Rad Dc protein assay kit (Hercules, CA), and uptake was expressed per milligram protein per 5 min.
Preparation of Mouse and Human Pancreatic Islets for Uptake Studies
Mouse primary pancreatic islets were isolated freshly by stationary digestion followed by Ficoll gradient centrifugation, as described by our laboratory previously (22). Briefly, four adult mice were euthanized, and pancreas was removed without fat contamination. Pancreatic tissue was minced, pooled, and digested with collagenase IV (1 mg/ml) containing DNase I (0.1 mg/ml). Following digestion, tissues were filtered through nylon mesh, and islets were isolated by discontinuous Ficoll gradient (25, 23, 20, and 11%, respectively). Islets were taken after centrifuging at 2,500 rpm (for 15 min) from the interface of 20 and 11% of Ficoll. Islets were washed, and uptake studies were performed by rapid filtration technique. This experimental procedure has been approved by Institutional Animal Care and Use Committee of Veterans Affairs Long Beach and University of California Irvine.
Freshly isolated human pancreatic islets were obtained from normal adult organ donors (National Disease Research Interchange, Philadelphia, PA). Islets were centrifuged at 1,500 rpm for 8 min, washed once, and resuspended in KR buffer. Viability of the human islets was determined by Trypan blue and found to be around 70%. Uptake was also done by rapid filtration method, as described before (22).
Real-Time PCR
Total RNA was isolated using Trizol (Invitrogen) method, as described in the manufacturer's protocol. DNA contamination was removed by treating the RNA with DNase (Invitrogen), and first-strand cDNA was synthesized using i-script reverse transcriptase (Bio-Rad). Relative gene expression was quantified in a CFX96 real-time PCR system (Bio-Rad) and gene specific primers (Table 1). Ct values were normalized to respective β-actin following relative relationship method (19).
siRNA Transfection
RF transporter-1 (RFVT-1)-specific small interfering RNA (siRNA; Sigma) (130 nM) was transiently transfected to β-TC-6 cells, and, 48 h posttransfection, cells were used for uptake studies and RNA isolation.
Protein Isolation and Western Blot Analysis
Total protein was isolated from β-TC-6 cells by lysing with RIPA buffer (Sigma) in the presence of protease inhibitor cocktail (Roche). Equal amounts of protein (60 μg) were loaded into NuPAGE 4–12% Bis-Tris gradient minigels (Invitrogen) and were transferred onto polyvinylidene difluoride membrane (Bio-Rad). The membrane was then probed simultaneously with anti-RFVT-1 antibody (raised in rabbit) and anti-β-actin antibody (raised in mice). The blot was incubated with anti-rabbit IR 800 dye and anti-mouse IR 680 dye (LI-COR) secondary antibodies (1:25,000) at room temperature for 1 h, and the florescent intensity was quantified using Odyssey application software (version 3.0) in Odyssey Infrared imaging system (LI-COR).
hnRNA Analysis
Total RNA isolated from β-TC-6 cells maintained in RF deficient and over supplemented medium (72 h) was treated with DNase I to exclude genomic DNA contamination. RNA was reverse transcribed using a kit (Bio-Rad). To ensure heterogeneous nuclear RNA (hnRNA) amplification, primers were designed in the exon-intron junction, as described before (31) (Table 1). Real-time PCR was performed, and relative expression was normalized with β-actin-hnRNA primers. As a negative control, DNase I-treated total RNA was used.
Statistical Analysis
Data shown in this study are means ± SE of at least three independent determinations. Level of significance was judged by the Student's t-test. Uptake by the carrier-mediated process was determined by subtracting the diffusion component from total uptake. The apparent Km and Vmax of the saturable carrier-mediated component were determined by nonlinear regression analysis via fitting the data into Michaelis-Menten equation using Graph Pad Prism software (version 5.03).
RESULTS
Physiological Aspects of RF Uptake by Mouse and Human Pancreatic β-Cells/Islets
The effect of incubation temperature (37, 25, 4°C) on the initial rate of RF (14 nM) uptake by mouse pancreatic β-TC-6 cells was examined, and the results showed a significantly (P < 0.01) higher uptake at 37°C compared with 25 and 4°C (3.80 ± 0.19, 2.31 ± 0.06, and 0.68 ± 0.02 pmol·mg protein−1·5 min−1, respectively). We also examined the effect of changing incubation buffer pH on the initial rate of RF uptake (14 nM) by β-TC-6 cells and observed an increase in uptake with decreasing buffer pH from pH 8 to 6, followed by a slight decrease thereafter (4.83 ± 0.16, 5.03 ± 0.37, 5.25 ± 0.18, 7.13 ± 0.22, and 5.13 ± 0.88 pmol·mg protein−1·5 min−1 for pH 8, 7.4, 6.5, 6, and 5, respectively).
The role of Na+ in the incubation medium in RF uptake was also tested by examining the effect of its isoosmotic replacement with K+ or mannitol on initial rate of RF uptake. No difference in RF uptake was observed in the presence and absence of Na+ (5.13 ± 0.29, 5.07 ± 0.62, and 5.88 ± 0.69 pmol·mg protein−1·5 min−1 in the presence of Na+ and in its absence and presence of K+ and mannitol, respectively).
We next examined the effect of unlabeled RF (1 mM) on the initial rate of 3H-RF (14 nM) uptake by pancreatic β-TC-6 cells and observed a significant (P < 0.01) inhibition in uptake (4.83 ± 0.12 and 0.256 ± 0.021 pmol·mg protein−1·5 min−1 for control and in the presence of 1 mM RF, respectively). We performed the same experiment using freshly isolated primary mouse pancreatic islets and again observed a significant (P < 0.01) inhibition in initial rate of 3H-RF uptake (1.29 ± 0.16 and 0.457 ± 0.08 pmol·mg protein−1·5 min−1 for control and in the presence of 1 mM unlabeled RF, respectively). We further extended the study to the human situation and examined the effect of unlabeled RF (1 mM) on initial rate of 3H-RF uptake. Again, a significant (P < 0.01) inhibition in 3H-RF uptake was observed in the presence of unlabeled RF (3.13 ± 0.35 and 1.18 ± 0.11 pmol·mg protein−1·5 min−1 for control and in the presence of unlabeled RF, respectively). These findings suggest involvement of a carrier-mediated component for RF uptake by mouse and human pancreatic β-cells/islets.
We also investigated possible trans-stimulation in RF transport across the plasma membrane of pancreatic β-TC-6 cells by examining the effect of adding unlabeled RF to the incubation medium on the efflux of 3H-RF from preloaded β-TC-6 cells. The results showed a significantly (P < 0.01) lower cellular content of radioactivity when cells were incubated in the presence of unlabeled RF than in its absence (4.98 ± 0.13 and 1.68 ± 0.09 pmol·mg protein−1·5 min−1, respectively). This trans-stimulation in RF transport further supports the above suggestion of involvement of a carrier-mediated process in RF transport by pancreatic β-cells.
In other studies, we investigated the specificity of the RF uptake process by pancreatic β-TC-6 cells. This was done by examining the effect of the RF structural analogs lumiflavin and lumichrome, as well as that of the structurally unrelated biotin (all at 500 μM) on the initial rate of 3H-RF (14 nM) uptake. The results showed a significant (P < 0.01) inhibition in 3H-RF uptake in the presence of lumiflavin and lumichrome, but not in the presence of biotin (3.69 ± 0.18, 1.63 ± 0.41, 1.38 ± 0.40, and 3.63 ± 0.06 pmol·mg protein−1·5 min−1 for control and in the presence of lumiflavin, lumichrome, and biotin, respectively). These findings demonstrate specificity of the pancreatic β-cell RF uptake process.
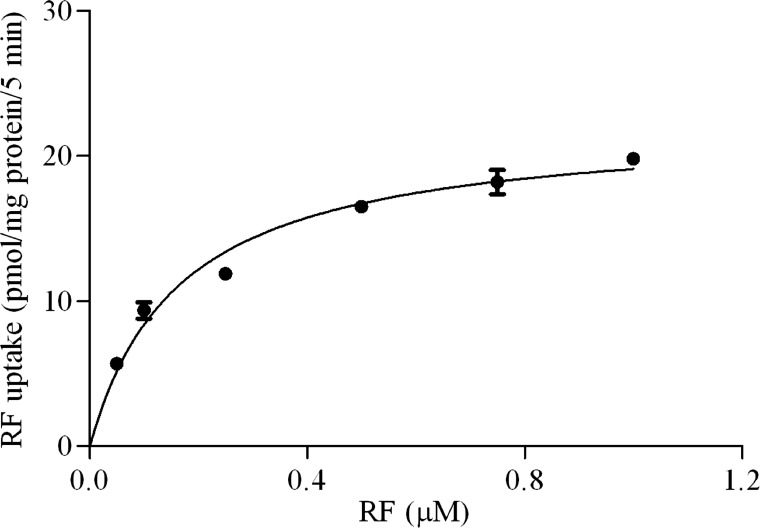
Finally, we examined the saturability in the RF uptake process of pancreatic β-TC-6 cells as a function of concentration and observed clear saturation, further indicating the involvement of a carrier-mediated process. Kinetic parameters of the saturable process were then calculated, as described in materials and methods, and found to be 0.17 ± 0.02 μM and 22.26 ± 0.80 pmol·mg protein−1·5 min−1 for the apparent Km and Vmax, respectively (Fig. 1).
Fig. 1.
Initial rate of riboflavin (RF) uptake by pancreatic β-TC-6 cells as a function of substrate concentration. Cells were incubated at 37°C in Krebs-Ringer buffer (pH 7.4) in the presence of different concentrations of unlabeled RF, and uptake (5 min) by the saturable component was determined. Values are means ± SE of 3 independent experiments. When not visible, the error bars are smaller than the symbol.
Molecular Aspects of the RF Uptake Process of Pancreatic β-Cells
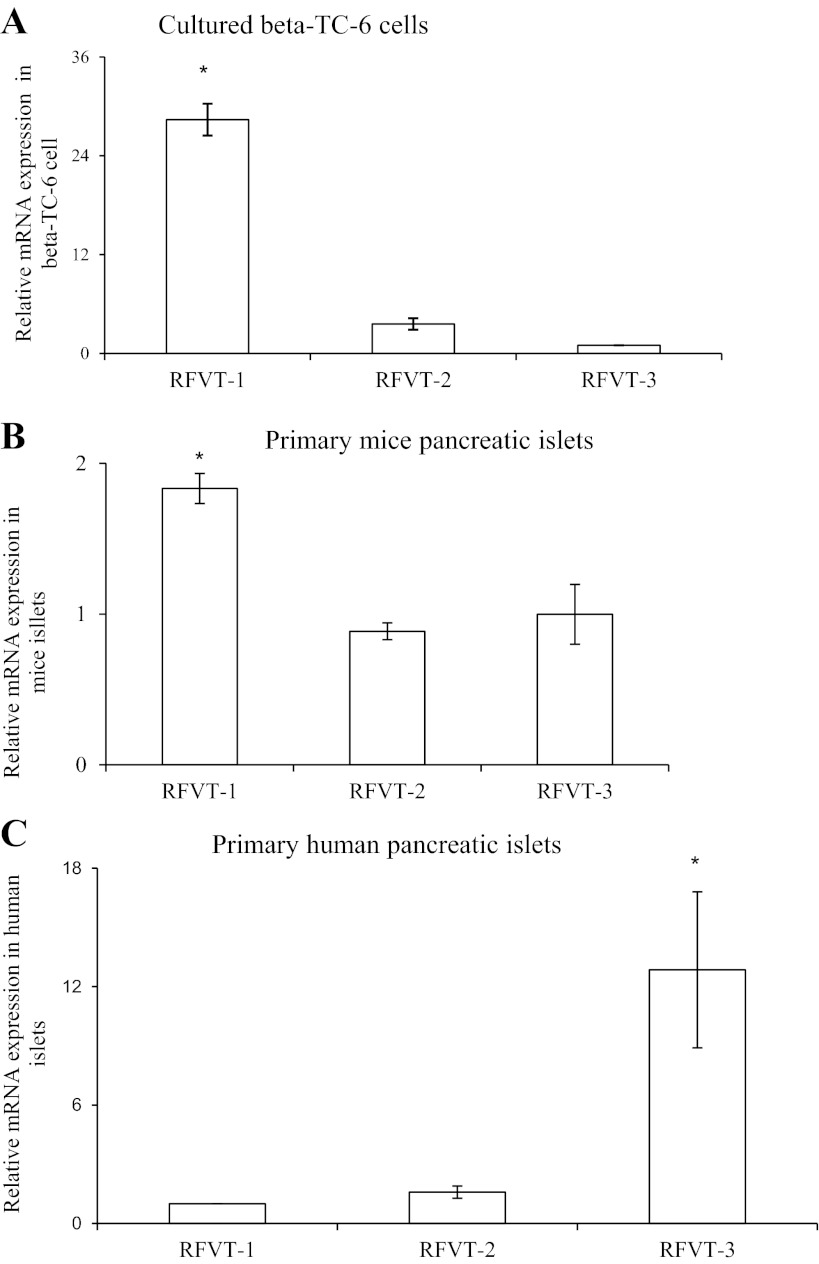
Three RF membrane transport systems (RFVT-1, RFVT-2, and RFVT-3) have been recently identified in mammalian cells and have been shown to display a different level of expression in different tissues (35–37). Thus we investigated whether these transporters are expressed in mouse and human pancreatic β-cells and determined their relative level of expression. The results showed that all three transporters are expressed in mouse and human pancreatic β-cells/islets. However, in the mouse, the level of expression of RFVT-1 was found to be considerably (P < 0.01) higher than that of RFVT-2 and RFVT-3 (Fig. 2, A and B), while in the human the level of expression of RFVT-3 was the highest (P < 0.05) (Fig. 2C). These findings demonstrate the existence of species variability in the type of the RF transport systems expressed in pancreatic β-cells between mouse and human.
Fig. 2.
Relative expression of RF transporter-1 (RFVT-1), RFVT-2, and RFVT-3 in mouse-derived pancreatic β-TC-6 cells (A), freshly isolated primary mouse pancreatic islets (B), and freshly isolated human pancreatic islets (C). Total RNA were isolated from cultured β-TC-6 cells (A), freshly isolated primary mouse (B), and human pancreatic islets (C) and were subjected to quantitative real-time PCR analysis using RFVT-1, -2, and -3 gene-specific primers (Table 1). Levels are expressed relative to β-actin. Values are means ± SE of at least 3 independent experiments. *P < 0.01.
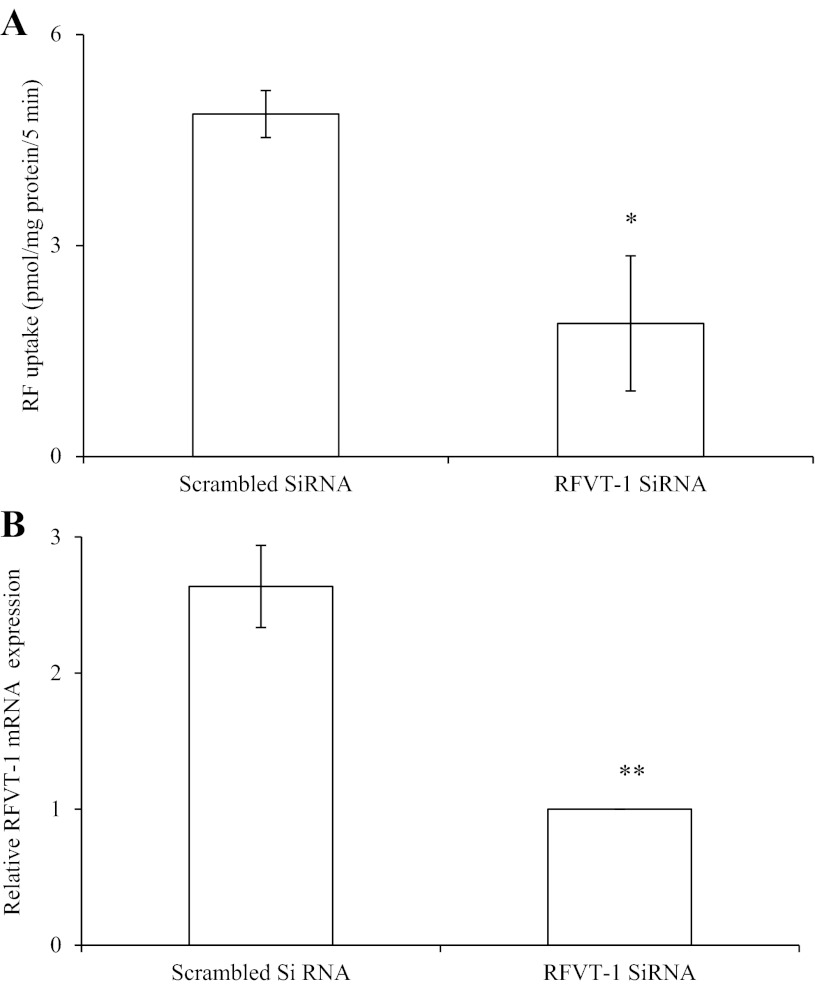
Since RFVT-1 appears to be the predominant RFVT system expressed in mouse pancreatic β-cells, we examined its relative contribution toward total carrier-mediated RF uptake. For this, we knocked down RFVT-1 of pancreatic β-TC-6 cells using gene-specific siRNA and then examined the effect of that knockdown on initial rate of 3H-RF (14 nM) uptake (Fig. 3A). The results showed a significant (P < 0.05) inhibition in RF uptake by cells treated with the gene-specific siRNA compared with those treated with scrambled siRNA (i.e., control). Effectiveness of the gene-specific RFVT-1 siRNA was demonstrated by observing a significant (P < 0.01) reduction in the level of expression of RFVT-1 mRNA compared with control (Fig. 3B).
Fig. 3.
Effect of knocking down RFVT-1 of pancreatic β-TC-6 cells on RF uptake. A: 3H-RF uptake was examined in β-TC-6 cells treated with RFVT-1 gene-specific and scrambled (control) small interfering RNA (siRNA). B: quantitative real-time PCR showing relative expression of RFVT-1 mRNA; data were normalized relative to β-actin. Values are means ± SE of at least 3 independent experiments. *P < 0.05. **P < 0.01.
Regulatory Aspects of the RF Uptake Process of Pancreatic β-Cells
Effect of substrate level on RF uptake by pancreatic β-cells.
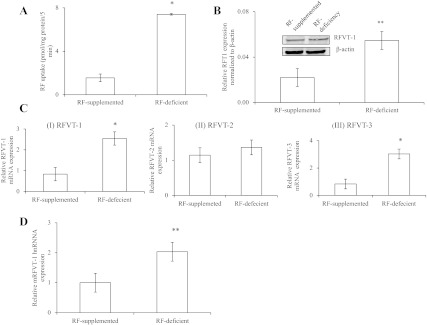
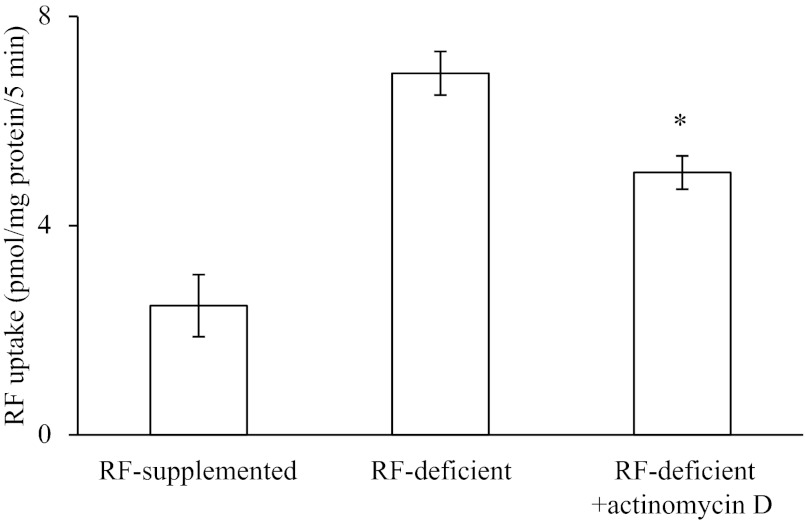
In this study, we examined whether the pancreatic β-cell RF uptake process is adaptively regulated by substrate level in the surrounding environment. This was done by examining the effect of maintaining pancreatic β-TC-6 cells (for 72 h) in a growth medium that lacks RF or contains 100 μM unlabeled RF, followed testing of the initial rate of 3H-RF (14 nM) uptake. The results showed a significantly (P < 0.01) higher 3H-RF uptake by cells maintained in RF-deficient medium compared with those maintained in RF-supplemented medium (Fig. 4A), indicating that the uptake process is adaptively regulated by substrate level. This adaptive upregulation in RF uptake by cells maintained in the RF-deficient medium compared with those maintained in the supplemented medium was associated with a significant induction in expression of RFVT-1 (the predominant RF transporter in mouse pancreatic β-cells) at the protein (P < 0.05) and mRNA (P < 0.01) levels (Fig. 4, B and C). The latter findings suggest possible involvement of transcriptional mechanism(s) in the adaptive upregulatory effect. To further test this possibility, we determined the level of expression of RFVT-1 hnRNA in cells maintained in RF-deficient growth medium compared with those maintained in RF-supplemented medium [the level of hnRNA of a given gene can be used as a measure of transcriptional activity of that gene (9, 26, 32)]. The results showed a significantly (P < 0.05) higher level of expression of RFVT-1 hnRNA in cells maintained in the former growth medium (RF deficient) compared with the latter (Fig. 4D). In a related study, we examined the effect of pretreating the cells maintained in RF-deficient medium with the transcriptional inhibitor actinomycin D on their ability to upregulate RF uptake and observed a significant (P < 0.05) blunting in the level of induction in RF uptake compared with cells maintained in RF-deficient medium, but without actinomycin D (Fig. 5) [actinomycin D also caused a significant (P < 0.01) reduction in level of expression of the RFVT-1 mRNA; data not shown]. These findings further confirm the possible involvement of a transcriptional mechanism(s) in the adaptive regulation of RF uptake by substrate level.
Fig. 4.
Effect of extracellular RF level on RF uptake by pancreatic β-TC-6 cells. A: effect on RF uptake. β-TC-6 cells were maintained (72 h) in growth medium containing no added RF (deficient) or that supplemented with 100 μM unlabeled RF, followed by determination of initial rate of 3H-RF uptake. *P < 0.01. B: effect on level of expression of RFVT-1 protein. Western blotting was performed using equal amounts of total protein (60 μg) harvested from β-TC-6 cells maintained under RF-deficient and supplemented conditions and specific polyclonal antibodies. Expression of RFVT-1 was normalized relative to corresponding β-actin. Inset: results of a representative Western blot. **P < 0.05. C: effect on level of expression of RFVT-1 (and RFVT-2 and -3) mRNA. Total RNA was isolated from β-TC-6 cells maintained in RF-deficient and supplemented growth medium. Quantitative real-time PCR was performed using gene-specific primers. *P < 0.01. All results presented here are means ± SE of at least 3 independent experiments. D: effect on the level of expression of RFVT-1 heterogeneous nuclear RNA (hnRNA). RFVT-1 hnRNA level was determined as an index of transcriptional control using gene-specific primers. mRFVT-1, mouse RFVT-1. Values are means ± SE of 3 independent experiments. **P < 0.05.
Fig. 5.
Effect of the transcriptional inhibitor actinomycin D on the observed induction in RF uptake in RF deficiency. β-TC-6 cells were maintained (48 h) in RF-deficient growth medium in the presence and absence of actinomycin D, followed by determination of initial rate of RF (14 nM) uptake. Values are means ± SE of 3 independent experiments. *P < 0.05.
Potential role of intracellular signaling mechanisms in the regulation of RF uptake by pancreatic β-cells.
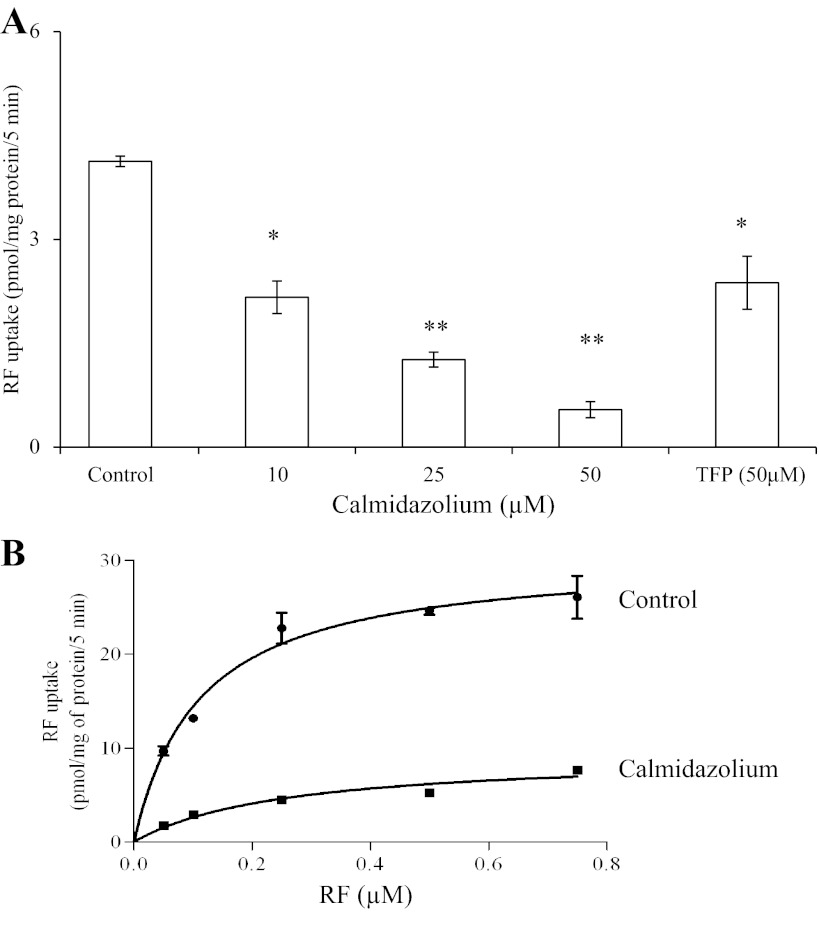
In these investigations, we examined the potential involvement of certain intracellular regulatory pathways in the regulation of RF uptake by mouse pancreatic β-cells. We specifically focused on examining the role of Ca2+-calmodulin, protein kinase A (PKA), and protein kinase C (PKC)-mediated pathways, since a role for these pathways in the regulation of membrane transport of other substrates has been well documented (3, 6, 8, 23, 24). We used specific modulators of these pathways in our investigations. The results showed that pretreating (1 h) β-TC-6 cells with calmidazolium (an inhibitor of the Ca2+-calmodulin-mediated pathway) leads to a significant and concentration-dependent reduction in the initial rate of RF uptake (P < 0.05 for 10 μM; P < 0.01 for 25 and 50 μM calmidazolium, respectively) (Fig. 6A). Similarly, trifluoperazine (50 μM), another inhibitor of the Ca2+-calmodulin-mediated pathway, showed a significant (P < 0.05) inhibition in RF uptake. We also tested the effect of calmidazolium on kinetic parameters of pancreatic β-cells RF uptake process and observed a significant (P < 0.01) reduction in the Vmax (30.49 ± 1.142 and 9.36 ± 0.871 pmol·mg protein−1·5 min−1 for control and calmidazolium-treated samples, respectively) and a significant (P < 0.05) increase in the apparent Km (0.111 ± 0.014 to 0.254 ± 0.06 μM) of the RF uptake process (Fig. 6B). Treatment of cells with IBMX, forskolin, and dibutyryl cAMP (compounds that increase intracellular level of cAMP and thus activate PKA), however, did not affect RF uptake (data not shown). Similarly, pretreatment of β-TC-6 cells with phorbol 12-myristate 13-acetate, which modulates the activity of PKC, was without an effect on RF uptake (data not shown).
Fig. 6.
A: effect of modulators of the Ca2+/calmodulin-mediated pathway on RF uptake by pancreatic β-TC-6 cells. β-TC-6 cells were incubated for 1 h in the absence and presence of different concentrations of calmidazolium (10, 25, and 50 μM) or with trifluoperazine (TFP; 50 μM), followed by determination of initial rate of 3H-RF uptake. Values are means ± SE of 3 independent experiments. *P < 0.05. **P < 0.01. B: effect of calmidazolium on kinetic parameters of RF uptake as a function of RF concentration. β-TC-6 cells were incubated in the presence and absence of calmidazolium (for 1 h), and initial rate of uptake of different concentrations of RF was examined. Values are means ± SE of 3 independent experiments.
DISCUSSION
The aim of this investigation was to examine the mechanism and regulation of RF uptake by pancreatic β-cells and islets. RF is essential for normal metabolic activities of pancreatic β-cells, and its deficiency increases the oxidative stress burden on cells (5, 20, 30). We used cultured mouse-derived pancreatic β-TC-6 cells and freshly isolated mouse primary pancreatic islets, as well as human primary pancreatic islets obtained from organ donors. Our findings demonstrated that RF uptake by pancreatic β-TC-6 cells is temperature dependent but Na+ independent in nature and involves a specific carrier-mediated process. Evidence supporting the latter includes the significant cis-inhibition caused by unlabeled RF and its closely related structural analogs lumiflavin and lumichrom on the initial rate of 3H-RF uptake, the trans-stimulation in the 3H-RF uptake by unlabeled RF, and the saturation in substrate uptake as a function of concentration. Similarly, RF uptake by mouse primary pancreatic islets was found to be carrier mediated, thus providing physiological relevance to our in vitro findings with β-TC-6 cells. The same was seen with human primary pancreatic islets with regards to existence of a carrier-mediated process for RF uptake.
After establishing the physiological mechanism of involvement of a carrier-mediated process in RF uptake by pancreatic β-cells, we investigated molecular aspects of the uptake process by determining which of the three known mammalian RF transporters (RFVT-1, -2, and -3) are expressed in these cells. The results showed that all three systems were expressed in both mouse and human pancreatic β-cells/islets. However, some differences were also observed between the two species in that, in the mouse, RFVT-1 was found to be the predominantly expressed RF transporter, while RFVT-3 was the predominantly expressed system in the human pancreatic islets. It is relevant to mention here that the three RF transport systems identified thus far have different functional characteristics (36). Specifically, hRFVT-3 appears to be more active in transporting RF than the other two systems and is acidic pH dependent (36). Also, the three RF uptake systems display a different pattern of tissue distribution (36).
The functional contribution of mouse RFVT-1 in RF uptake by the mouse-derived β-TC-6 cells was also tested, utilizing RNA interference approach. The results showed a significant (∼60%) inhibition in carrier-mediated RF uptake by the siRNA-treated cells compared with those treated with scrambled siRNA, thus demonstrating the important role played by this transporter in the vitamin uptake by mouse pancreatic β-cells.
We also investigated certain regulatory aspects of RF uptake by pancreatic β-cells. Thus effect of changes in extracellular RF level on the vitamin uptake was tested, and evidence was obtained to indicate that the uptake process is adaptively regulated by substrate availability. This regulation involves parallel changes in level of expression of the RFVT-1 at the protein, RNA, and hnRNA levels. The latter findings suggest that a transcriptional mechanism(s) may be involved in this adaptive regulatory response. This suggestion was further supported by the finding that treating cells maintained in RF-deficient medium with the transcriptional inhibitor actinomycin D led to a significant blunting in the induction in RF uptake seen in RF deficiency. Further studies are needed to delineate the exact mechanism involved in this apparent transcriptional regulation.
The other RF uptake process of pancreatic β-TC-6 cells was also found to be under the regulation of an intracellular Ca+2/calmodulin-mediated regulatory pathway, as suggested by the findings of significant and concentration-dependent inhibition of RF uptake by modulators (calmidazolium and trifluoperazine) of this pathway. The effect of calmidazolium appeared to be mediated via a significant inhibition in the Vmax of the RF uptake process and an increase in its apparent Km, suggesting that the effect is mediated via a decrease in the activity of the RF uptake system and a decrease in its affinity, respectively. The exact mechanism(s) through which the Ca+2/calmodulin-mediated pathway exerts its regulatory effect on RF uptake is not clear and is in need of further investigations. A similar role for Ca+2/calmodulin-mediated pathway in regulating RF uptake in other cell types has also been observed previously (16, 28, 29). No role for the PKA (and PKC)-mediated pathway in the regulation of RF uptake by pancreatic β-cells was observed. This is in contrast to the apparent regulation of RF uptake by intestinal epithelial cells by a PKA-mediated pathway (27).
In conclusion, our study shows, for first time, the existence of a specific, carrier-mediated process for RF uptake by mouse and human pancreatic β-cells/islets. The study also shows that the uptake process is adaptive regulated by substrate level and by an intracellular Ca+2/calmodulin-mediated pathway.
GRANTS
This study was supported by grants from the Department of Veterans Affairs and the National Institutes of Health (AA 018071, DK56061, DK58057).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.G. and H.M.S. conception and design of research; A.G. performed experiments; A.G. and H.M.S. analyzed data; A.G. and H.M.S. interpreted results of experiments; A.G. prepared figures; A.G. and H.M.S. drafted manuscript; A.G. and H.M.S. edited and revised manuscript; A.G. and H.M.S. approved final version of manuscript.
REFERENCES
- 1. Betz AL, Ren XD, Ennis SR, Hultquist DE. Riboflavin reduces edema in focal cerebral ischemia. Acta Neurochir Suppl (Wien) 60: 314–317, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Bonjour JP. Vitamins and alcoholism. V. Riboflavin, VI. Niacin, VII. Pantothenic acid, and VIII. Biotin. Int J Vitam Nutr Res 50: 425–440, 1980 [PubMed] [Google Scholar]
- 3. Brandsch M, Miyamoto Y, Ganapathy V, Leibach FH. Regulation of taurine transport in human colon carcinoma cell lines (HT-29 and Caco-2) by protein kinase C. Am J Physiol Gastrointest Liver Physiol 264: G939–G946, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Cai F, Li CR, Wu JL, Chen JG, Liu C, Min Q, Yu W, Ouyang CH, Chen JH. Theaflavin ameliorates cerebral ischemia-reperfusion injury in rats through its anti-inflammatory effect and modulation of STAT-1. Mediators Inflamm 2006: 30490, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cobianchi L, Fornoni A, Pileggi A, Molano RD, Sanabria NY, Gonzalez-Quintana J, Bocca N, Marzorati S, Zahr E, Hogan AR, Ricordi C, Inverardi L. Riboflavin inhibits IL-6 expression and p38 activation in islet cells. Cell Transplant 17: 559–566, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Cohen ME, Reinlib L, Watson AJ, Gorelick F, Rys-Sikora K, Tse M, Rood RP, Czernik AJ, Sharp GW, Donowitz M. Rabbit ileal villus cell brush border Na+/H+ exchange is regulated by Ca2+/calmodulin-dependent protein kinase II, a brush border membrane protein. Proc Natl Acad Sci U S A 87: 8990–8994, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cooperman JM, Lopez R. (Editors). Riboflavin. In: Handbook of Vitamins: Nutrional, Biochemical and Clinical Aspects. New York: Dekker, 1984, p. 299–327 [Google Scholar]
- 8. Donowitz M, Montgomery JL, Walker MS, Cohen ME. Brush-border tyrosine phosphorylation stimulates ileal neutral NaCl absorption and brush-border Na+-H+ exchange. Am J Physiol Gastrointest Liver Physiol 266: G647–G656, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Elferink CJ, Reiners JJ., Jr Quantitative RT-PCR on CYP1A1 heterogeneous nuclear RNA: a surrogate for the in vitro transcription run-on assay. Biotechniques 20: 470–477, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Fennelly J, Frank O, Baker H, Leevy CM. Peripheral neuropathy of the alcoholic. I. Aetiological role of aneurin and other B-complex vitamins. Br Med J 2: 1290–1292, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernandez-Banares F, Abad-Lacruz A, Xiol X, Gine JJ, Dolz C, Cabre E, Esteve M, Gonzalez-Huix F, Gassull MA. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol 84: 744–748, 1989 [PubMed] [Google Scholar]
- 12. Goldsmith GA. Riboflavin deficiency. In: Riboflavin; New York: Plenum, 1975, p. 221–244 [Google Scholar]
- 13. Iwanaga K, Hasegawa T, Hultquist DE, Harada H, Yoshikawa Y, Yanamadala S, Liao H, Visovatti SH, Pinsky DJ. Riboflavin-mediated reduction of oxidant injury, rejection, and vasculopathy after cardiac allotransplantation. Transplantation 83: 747–753, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Kodentsova VM, Vrzhesinskaia OA, Sokol'nikov AA, Alekseeva IA, Spirichev VB. [Metabolism of riboflavin and B group vitamins functionally bound to it in insulin-dependent diabetes mellitus]. Vopr Med Khim 39: 33–36, 1993 [PubMed] [Google Scholar]
- 15. Kodentsova VM, Vrzhesinskaia OA, Trofimenko EV, Sokol'nikov AA, Beketova NA, Blazheevich NV, Isaeva VA, Aleinik SI, Trofimenko LS, Dronova VI, et al. [Vitamin status of children with diabetes mellitus]. Vopr Med Khim 40: 45–48, 1994 [PubMed] [Google Scholar]
- 16. Kumar CK, Yanagawa N, Ortiz A, Said HM. Mechanism and regulation of riboflavin uptake by human renal proximal tubule epithelial cell line HK-2. Am J Physiol Renal Physiol 274: F104–F110, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Law LK, Tang NL, Hui J, Fung SL, Ruiter J, Wanders RJ, Fok TF, Lam CW. Novel mutations in ETFDH gene in Chinese patients with riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency. Clin Chim Acta 404: 95–99, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Liang WC, Ohkuma A, Hayashi YK, Lopez LC, Hirano M, Nonaka I, Noguchi S, Chen LH, Jong YJ, Nishino I. ETFDH mutations, CoQ10 levels, and respiratory chain activities in patients with riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency. Neuromuscul Disord 19: 212–216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Mack CP, Hultquist DE, Shlafer M. Myocardial flavin reductase and riboflavin: a potential role in decreasing reoxygenation injury. Biochem Biophys Res Commun 212: 35–40, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Majumdar SK, Shaw GK, Thomson AD. Vitamin utilization status in chronic alcoholics. Int J Vitam Nutr Res 51: 54–58, 1981 [PubMed] [Google Scholar]
- 22. Mee L, Nabokina SM, Sekar VT, Subramanian VS, Maedler K, Said HM. Pancreatic beta cells and islets take up thiamin by a regulated carrier-mediated process: studies using mice and human pancreatic preparations. Am J Physiol Gastrointest Liver Physiol 297: G197–G206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piper RC, James DE, Slot JW, Puri C, Lawrence JC., Jr GLUT4 phosphorylation and inhibition of glucose transport by dibutyryl cAMP. J Biol Chem 268: 16557–16563, 1993 [PubMed] [Google Scholar]
- 24. Rood RP, Emmer E, Wesolek J, McCullen J, Husain Z, Cohen ME, Braithwaite RS, Murer H, Sharp GW, Donowitz M. Regulation of the rabbit ileal brush-border Na+/H+ exchanger by an ATP-requiring Ca++/calmodulin-mediated process. J Clin Invest 82: 1091–1097, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosenthal WS, Adham NF, Lopez R, Cooperman JM. Riboflavin deficiency in complicated chronic alcoholism. Am J Clin Nutr 26: 858–860, 1973 [DOI] [PubMed] [Google Scholar]
- 26. Ryu DY, Levi PE, Fernandez-Salguero P, Gonzalez FJ, Hodgson E. Piperonyl butoxide and acenaphthylene induce cytochrome P450 1A2 and 1B1 mRNA in aromatic hydrocarbon-responsive receptor knock-out mouse liver. Mol Pharmacol 50: 443–446, 1996 [PubMed] [Google Scholar]
- 27. Said HM, Ma TY, Grant K. Regulation of riboflavin intestinal uptake by protein kinase A: studies with Caco-2 cells. Am J Physiol Gastrointest Liver Physiol 267: G955–G959, 1994 [DOI] [PubMed] [Google Scholar]
- 28. Said HM, Ortiz A, Ma TY, McCloud E. Riboflavin uptake by the human-derived liver cells Hep G2: mechanism and regulation. J Cell Physiol 176: 588–594, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Said HM, Ortiz A, Moyer MP, Yanagawa N. Riboflavin uptake by human-derived colonic epithelial NCM460 cells. Am J Physiol Cell Physiol 278: C270–C276, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Seekamp A, Hultquist DE, Till GO. Protection by vitamin B2 against oxidant-mediated acute lung injury. Inflammation 23: 449–460, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Subramanian VS, Subramanya SB, Said HM. Chronic alcohol exposure negatively impacts the physiological and molecular parameters of the renal biotin reabsorption process. Am J Physiol Renal Physiol 300: F611–F617, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Subramanian VS, Subramanya SB, Tsukamoto H, Said HM. Effect of chronic alcohol feeding on physiological and molecular parameters of renal thiamin transport. Am J Physiol Renal Physiol 299: F28–F34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Toyosawa T, Suzuki M, Kodama K, Araki S. Highly purified vitamin B2 presents a promising therapeutic strategy for sepsis and septic shock. Infect Immun 72: 1820–1823, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tu BP, Ho-Schleyer SC, Travers KJ, Weissman JS. Biochemical basis of oxidative protein folding in the endoplasmic reticulum. Science 290: 1571–1574, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Yamamoto S, Inoue K, Ohta KY, Fukatsu R, Maeda JY, Yoshida Y, Yuasa H. Identification and functional characterization of rat riboflavin transporter 2. J Biochem 145: 437–443, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Yao Y, Yonezawa A, Yoshimatsu H, Masuda S, Katsura T, Inui K. Identification and comparative functional characterization of a new human riboflavin transporter hRFT3 expressed in the brain. J Nutr 140: 1220–1226, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Yonezawa A, Masuda S, Katsura T, Inui K. Identification and functional characterization of a novel human and rat riboflavin transporter, RFT1. Am J Physiol Cell Physiol 295: C632–C641, 2008 [DOI] [PubMed] [Google Scholar]