Abstract
Gonadotrophin-releasing hormone (GnRH) pulsatility is required for optimal luteinizing hormone (LH) secretion, but whether LH pulsatility is required for physiological testosterone (T) secretion is not known. To test the postulate that pulses of recombinant human (rh) LH stimulate greater T secretion than continuous infusion of the same dose, a potent selective GnRH antagonist was administered overnight to 19 healthy men ages 18–49 yr. Subjects then received saline or rhLH intravenously continuously or as 6-min pulses intravenously every 1 or 2 h at the same total dose. Blood was sampled every 10 min for 10 h to quantify T responses. For the four interventions, the descending rank order of mean LH and mean T concentrations was 1-h = 2-h rhLH pulses > continuous rhLH > saline (P < 10−3). Plateau LH and T concentrations correlated positively (R2 = 0.943, P = 0.029) as did LH concentrations and LH half-lives (R2 = 0.962, P = 0.019). Percentage pulsatile T secretion assessed by deconvolution analysis (Keenan DM, Takahashi PY, Liu PY, Roebuck PD, Nehra AX, Iranmanesh A, Veldhuis JD. Endocrinology 147: 2817–2828, 2006) was the highest (P = 0.019), and half-time to attain peak T concentrations was the shortest (P < 10−6), for 1-h rhLH pulses. Approximate entropy (a pattern-regularity measure) revealed more orderly T secretion for 1- than 2-h rhLH pulses (P = 0.0076). Accordingly, a pulsatile LH signal, while not obligatory to maintain mean T concentrations, controls the mean plasma LH concentration and determines quantifiable patterns of T secretion. These data introduce the question whether blood T patterns in turn supervise distinctive target-tissue responses.
Keywords: pulse, gonadotropin, human, testis, testosterone
low testosterone (T) concentrations increase the clinical risk of sarcopenia, osteopenia, diminished libido and potentia, visceral adiposity, decreased aerobic capacity, and (possibly) impaired cognitive function (30). Reduced T availability may result from reduced gonadotrope stimulation by hypothalamic gonadotrophin-releasing hormone (GnRH), attenuated biosynthesis of luteinizing hormone (LH), heightened T clearance, greater T negative feedback, and/or blunted testicular responses to LH (32, 33). In addition, hypoandrogenemia in some conditions such as aging is associated with disorderly LH secretion patterns (30–32, 36, 51) and smaller more frequent circhoral LH pulses (18, 32, 36). Whether small frequent LH pulses or continuous LH delivery into the bloodstream is less effective than larger infrequent LH pulses in driving T secretion is not known. The issue is central to the broader theme of pulsatility regulation and action in endocrine systems (17, 20, 54). Indeed, growth hormone (GH), ACTH, parathyroid hormone (PTH), insulin, and oxytocin drive certain target-tissue responses in a pulse-dependent fashion (54). In addition, pulsatile LH delivery may be more effective than continuous LH delivery in stimulating ovarian steroidogenesis (49).
The present study tests the hypothesis that infusion of LH continuously or as low-amplitude, frequent LH pulses typical of older men (21) is less effective than high-amplitude, infrequent LH pulses characteristic of young men (22) in stimulating Leydig-cell T secretion. Selected analytical tools (deconvolution analysis, exponential T recovery rate, and approximate entropy) were used to quantify T responses more precisely than mean T concentrations alone (19, 20, 29).
METHODOLOGY
Overall rationale.
The experimental paradigm comprised intravenous infusions of saline versus recombinant human (rh) LH either continuously over 12 h or as distinct pulses every 1 or 2 h (32). The same total dose of rhLH was used in each of the three active LH infusion sessions, so that the independent variable was time mode of infusion rather than the infused dose of LH. To limit confounding by endogenous LH, a potent selective GnRH-receptor antagonist (ganirelix) was administered before each infusion session at a dose shown to reduce endogenous LH and T by >80% for 24–28 h.
Human subjects.
Volunteers provided written informed consent approved by Mayo Institutional Review Board. Preliminary outpatient screening consisting of a complete history, physical examination, and normal biochemical measures of endocrine [LH, follicular stimulating hormone (FSH), thyroid-stimulating hormone (TSH), insulin-like growth factor (IGF-I), prolactin, testosterone, estradiol, and sex hormone-binding globulin (SHBG)], metabolic (glucose, electrolytes, calcium, albumin), hepatic, renal, and hematologic function.
Criteria for exclusion included structural hypothalamo-pituitary-gonadal disease; recent use of psychotropic or neuroactive drugs (within five biological half-lives); body mass index (BMI) > 32.5 kg/m2; anemia (hemoglobin < 12.9 g/dl); drug or alcohol abuse, psychosis, depression, mania, anorexia/bulimia, or severe anxiety; prostate cancer; elevated prostate-specific antigen (PSA) by age; obstructive uropathy; acute or chronic organ system or inflammatory disease; endocrinopathy, other than primary thyroidal failure receiving replacement; nightshift work or recent transmeridian travel (exceeding 3 time zones within 7 days of admission); acute weight change (loss or gain of >2 kg in 6 wk); abnormal hepatorenal function; anabolic steroid use or reproductive hormone therapy; allergy to administered compounds; and/or unwillingness to provide written informed consent.
Protocol.
Eligible subjects each participated in four prospectively randomized, placebo-controlled, crossover interventions in the Mayo Center for Translational Science Activities. Sessions were separated by at least 10 days. Volunteers were admitted to the Center at or before 1500 h on each occasion, and two indwelling intravenous catheters were placed in (contralateral) forearm veins by 1600 h, one for sampling and the other for infusions. Ganirelix (1 mg sc) was given at 1700 h on the night of admission to suppress pulsatile LH secretion over the next 24–28 h (6, 27, 37, 55). The last mentioned (55) is a sequel, wherein the same subjects received an additional 6 h of fixed-dose synthetic LH infusion (identical pulses in all 4 groups) as a novel test of possible testis hyporesponsiveness. Thus the present paper represents part of a two-step study. The ganirelix dose was obtained from prior dose-response data in men (28). Based upon pilot data, a pulsatile rhLH dose was identified that yielded normal adult mean LH concentrations of 1.8–8.6 IU/l (45). Given this estimate, commencing at 2300 h, subjects received randomly ordered intravenous infusions of 1) saline continuously; 2) rhLH (9.4 IU/h) continuously for 12 h; 3) 2-h pulses of rhLH (18.75 IU/6 min) for a total of 6 doses; or 4) 1-h pulses of rhLH (9.4 IU/6 min) for a total of 12 doses. The total amount of rhLH delivered was thus held constant at 112.5 IU in the three active infusion regimens. Blood samples (1.2 ml) were withdrawn every 10 min for 10 h overnight beginning at 0100 h. Patients were allowed eucaloric breakfast and lunch and ambulation to the lavatory only. Daytime sleep, caffeine, and alcohol or tobacco were disallowed. Serum was frozen for later assay of LH and T concentrations.
Investigational drugs.
Ganirelix is a FDA-approved decapeptide analog of GnRH, a single injection of which inhibits LH secretion rapidly (within 4–6 h) and for up to 28 h. The drug is administered daily in controlled ovulation-induction regimens to suppress endogenous LH (6, 37, 47). Ganirelix and rhLH were used here under a Food and Drug Administration-reviewed new drug number.
Assays.
LH and T concentrations in sera collected every 10 min were measured in duplicate by automated immunochemiluminometry (ACS Corning, Bayer, Tarrytown, NY). All samples from a single volunteer were measured in one assay. Concentration-dependent intraassay coefficients of variation (CVs) for LH averaged 5.5, 4.7, 3.5, and 3.8% and interassay CVs 6.5, 5.2, 3.7, and 4.7% at LH concentrations of 1.3, 4.4, 18, and 38 IU/l, respectively. Procedural sensitivity was 0.02 IU/l using the World Health Organization Second International Reference Preparation 80/552 as standard. In this assay, 75 IU Serono rhLH equals 30 IU. Median intra- and interassay CVs for T were 6.8% and 8.3%, respectively, and the sensitivity was 18 ng/dl. T measurements obtained by immunochemiluminometry correlate strongly (r2 = 0.98) at nearly unit slope with those determined by gas chromatography-mass spectrometry (27).
Serum SHBG and albumin were respectively measured by Immulite 2000 (Diagnostic Products, Los Angeles, CA) (9) and Roche/Hitachi 912 (Roche Diagnostics, Basal, Switzerland) (23). Inter- and intra-assay CVs were <6.0% at SHBG concentrations ranging from 5.4 to 96 nmol/l. Inter- and intra-assay CVs were < 2.0% at albumin concentrations from 2.5 and 4.6 g/dl.
Free and bioavailable T concentrations were measured by equilibrium dialysis and ammonium-sulfate precipitation, as described earlier (45).
Liquid chromatography-tandem mass spectrometry (LC-MS/MS, API 5000, Applied Biosystems-MDS Sciex, Foster City, CA) was employed for baseline screening measurements of T and estradiol (E2). Testosterone was prepared by acetonitrile precipitation and high-throughput liquid chromatography extraction. Analysis was performed by MS/MS equipped with a heated nebulizer ion source. For E2 measurements by LC-MS/MS, organic extraction was performed with methylene chloride to remove water-soluble conjugates and concentrate the specimen. After derivatization with dansyl chloride, an aliquot was processed by high-pressure liquid chromatography. Deuterated d5-17β-estradiol and d3-testosterone served as internal standards. Values as low as 1 ng/dl for T and 1.25 pg/ml for E2 were detectable by this method. For T values of 8, 4, 2, and 1 ng/dl, respective CVs were 7.5, 2.2, 6.3, and 28.8%. For E2 values of 10, 5, 2.5, and 1.25 pg/ml, respective CVs were 2.3, 6.1, 14.5, and 13.4% (14, 42).
Deconvolution analysis.
Overnight 10-min LH and T concentration time series were analyzed by way of a recently developed variable-waveform deconvolution method. The automated Matlab program first detrends and normalizes concentrations to the unit interval [0, 1]. Successive potential pulse-time sets are created by an incremental smoothing process (a nonlinear adaptation of the heat-diffusion equation), which deletes the least significant nadir one at a time (26). Maximum-likelihood expectation parameter estimation is used to calculate secretion and elimination rates simultaneously for each candidate pulse-time set. The model specifies basal secretion (β0), a rapid (α1) and slow-phase half-life (α2), secretory-burst mass (η0, η1), random effects on burst mass (σA), procedural/measurement error (σε), and a three-parameter flexible gamma probability distribution to embody secretory-burst waveform (β1, β2, β3). The rapid phase half-life of LH was fixed at 6.93 min while the slow component was estimated from each profile allowing 63% fast-decay amplitude (54). Analogously, the rapid phase for T was assumed to be 1.4 min representing 33% of total decay and the slow phase 27 min (53). The Akaike information criterion is applied to distinguish objectively among candidate pulse-time sets (26). Units of parameters are total, basal, and pulsatile T secretion rates (concentration units/10 h).
Approximate entropy.
Approximate entropy (ApEn), (1, 20%) provides a scale- and model-independent regularity statistic to quantify the orderliness of serial measurements (35). ApEn was applied to the 10-h T concentration series. Higher ApEn values denoted greater relative randomness or disorderliness of subpatterns. Mathematical models and clinical experiments establish that reduced pattern orderliness is a barometer of increased feedforward and/or decreased feedback coupling within a neuroendocrine axis with high sensitivity and specificity (both > 90%).
Biostatistics.
In a pilot study, observed (means ± SD) T concentrations were 337 ± 87 ng/dl after combined subcutaneous ganirelix administration and a total of six consecutive intravenous pulses of rhLH 50 IU infused every 2 h in 8 young men. The saline control values were 85 ± 12 ng/dl, thus verifying inhibitory efficacy of ganirelix. Therefore, based upon the simplest analysis via a paired two-tailed Student's t-test, approximate statistical power was estimated as >90% for detecting a proposed >33% contrast between T concentrations during pulsatile and continuous rhLH infusions if 18 individuals were studied at penalized P < 0.01. The penalty reflects the hypothesis that either one or both (1 or 2 h) of the rhLH-pulse paradigms differ from continuous saline or continuous rhLH stimulation (34). A secondary unpowered hypothesis was that age would negatively determine T responses to any of the three active rhLH infusion types as assessed by linear regression (P penalized at 0.0167 by Bonferroni adjustment).
Exponential curve fitting (monoexponential ascending curve) and kinetic analysis were used to estimate the rate of rise of measured T concentrations and the metabolic clearance rate of infused LH, respectively (50, 52). Deconvolution analysis was applied to assess the half-life of disappearance of infused LH (26). The plateau T response was defined as the mean of the last 90-min T concentrations and the peak T response as the maximal (single) T concentration achieved in each session. The mean T response was the arithmetic average T concentration over each 10 h of sampling.
One-way ANOVA, using the incremental T response to an active rhLH infusion minus that to saline as the variable, was applied to test for T-response differences among infusion protocols (34). Post hoc comparisons of means were made by Tukey's honestly significant difference test at experiment-wise P < 0.05.
Linear or quadratic regression analysis was used to evaluate response correlations (10).
Data are given as means ± SD, except where indicated otherwise in the text.
RESULTS
Subject characteristics and baseline screening endocrine data are summarized in Table 1. The range of age was 18 to 49 yr and BMI was 18 to 31 kg/m2. LH, FSH, T, E2 (mass spectrometry), free T (by dialysis), bioavailable T (ammonium-sulfate precipitation), SHBG, and albumin were all normal for age. SHBG rose with age (P < 0.05). Three patients reported local injection-site pain or redness.
Table 1.
Human subject characteristics at baseline
| Mean | SD | Median | Normal | |
|---|---|---|---|---|
| Age, yr | 33 | 9.6 | 34 | |
| BMI, kg/m2 | 26 | 3.5 | 26 | |
| LH, IU/l | 3.0 | 0.88 | 3.0 | 1.8–8.6 |
| FSH, IU/l | 3.9 | 1.8 | 3.3 | 1.0–18 |
| T, ng/dl* | 485 | 114 | 481 | 240–950 |
| Free T, ng/dl† | 18 | 6.6 | 18 | 8–33 |
| Bioavailable T, ng/dl‡ | 198 | 79 | 185 | 72–235 |
| E2, pg/ml* | 17 | 4.4 | 16 | 6–35 |
| SHBG, nmol/l | 20 | 6.6 | 20 | 10–60 |
| Albumin, g/dl | 3.8 | 0.44 | 3.8 | 3–5 |
Data are from 19 healthy men. SD defines ± SD. BMI, body mass index; LH, luteinizing hormone; FSH, follicular stimulating hormone; T, testosterone; E2, estradiol; SHBG, sex hormone-binding globulin; Multiple T by 0.0347 and E2 by 3.67 to obtain respective SI units of nmol/l and pmol/l.
Tandem mass spectrometry;
Equilibrium dialysis;
Ammonium-sulfate precipitation.
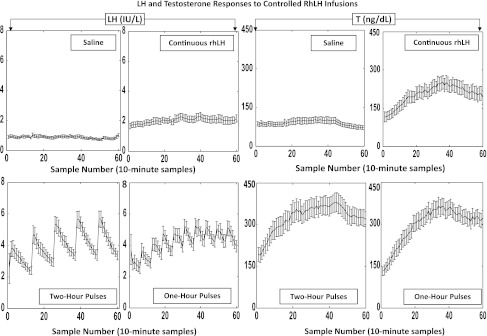
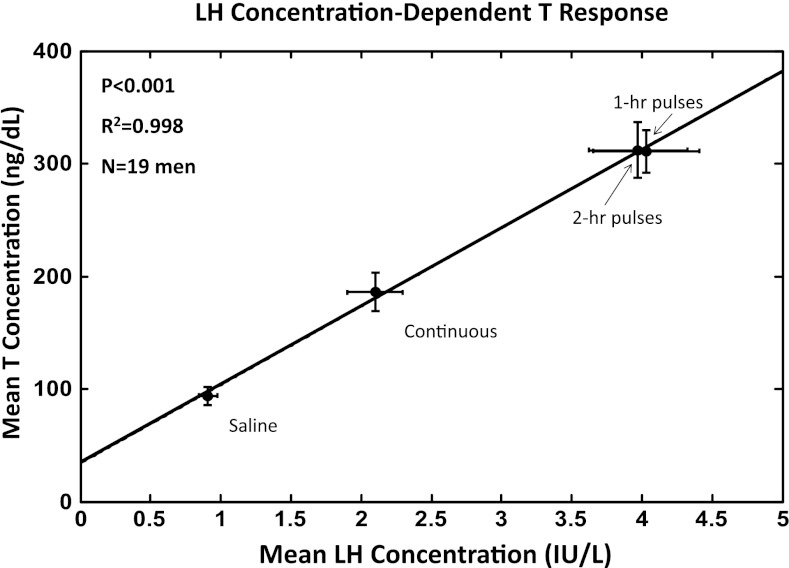
The last 10 h of the 12-h overnight infusions of saline or rhLH were evaluated by 10-min sampling in all four sessions in all 19 men (4,636 samples in total). The time courses of the mean (10-h algebraic average) LH (left) and T-concentration (right) responses are presented in Fig. 1. The paradigms were successful in achieving four distinct time patterns of LH in each subject. In particular, infused rhLH pulses were not distinguishable from normal endogenously secreted LH pulses with respect to mean amplitude (21, 22). In the control ganirelix/saline session, 10-h mean LH and T concentrations were 0.88 IU/l and 93 ng/dl, respectively, reflecting marked suppression by the GnRH antagonist compared with screening baseline (uninfused) 0800 h concentrations of LH and T, viz., 3.0 ± 0.88 IU/l and 485 ± 114 ng/dl (means ± SD, N = 19), respectively. Continuous rhLH infusion after ganirelix elevated mean (10 h) concentrations of LH by 2.0-fold and of T by 2.0-fold compared with saline (Fig. 2). Pulsatile rhLH infusions every hour and every 2 h further increased 10-h mean LH concentrations by an additional 2.1- to 2.3-fold over continuous LH infusion and thus by 4.2- to 4.8-fold over saline. Mean LH concentrations were statistically comparable for the 1- and 2-h rhLH pulse frequencies (P = 0.67). Mean total T concentrations followed the same pattern as mean LH concentrations. Overall treatment effects were significant at P < 10−14 for mean LH and P < 10−13 for mean T concentrations. The descending rank order of 10-h mean LH and 10-h mean T concentration responses was the following: rhLH pulses every 1 h = rhLH pulses every 2 h > continuous rhLH > saline, as assessed by post hoc Tukey's test. The linear correlation between 10-h mean LH and T concentrations was significant at P < 10−3 with R2 = 0.998, indicating that 10-h mean T concentrations are strongly dependent on mean LH concentrations (Fig. 2).
Fig. 1.
Mean (± SD) 10-min profiles of luteinizing hormone (LH) (left two columns) and testosterone (T) (right two columns) concentrations over 10 h in 19 healthy men during overnight gonadotrophin-releasing hormone (GnRH) antagonist (ganirelix) administration. Ganirelix injection was followed by intravenous infusion of saline (top left) and recombinant human (rh)LH continuously (top right), rhLH pulses every 2 h (bottom left), or every 1 h (bottom right) at the same total dose of rhLH (see methodology). Sample zero (x axis) was obtained at 0100 h, which is 6 h after ganirelix injection.
Fig. 2.
Arithmetic mean (± SD) 10-h serum LH (x axis) and T (y axis) concentrations (61 measurements of each hormone in each study session in each person) attained in 19 healthy men studied as described in the legend of Fig. 1. The overall R2 and P values for the linear regression are shown.
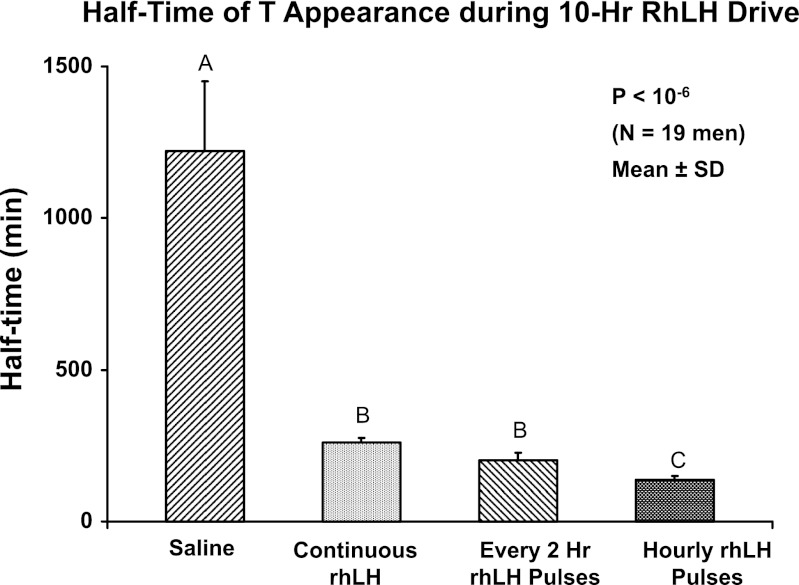
The time profile of total T concentrations was nearly flat during saline/ganirelix treatment corresponding to a very long half-time of the diurnal rise of endogenously driven T in the control setting. In contrast, T levels exhibited a strong exponential rise in all three rhLH-infused/ganirelix-treatment sessions with stable maximal values reached in 360–400 min in all conditions. The median half-time (min) of T rise defined by 0.693/k, where k is derived from a monoexponential fit over the first 6 h of sampling, was shortest (most rapid) for 1-h rhLH pulses (viz., 139 min), intermediate for both continuous and 2-h rhLH pulse infusions (260 and 203 min), and longest for saline infusion (1,220 min) (overall P < 10−6; Fig. 3). Thus the most rapid initial increase in total T concentrations was achieved by 1-h rhLH pulses.
Fig. 3.
Half-times (min) of exponential rise of serum T concentrations after ganirelix suppression in 19 healthy men. Saline and 3 active rhLH-infusion schedules were used. Differing (unshared) alphabetic superscripts denote significantly different means (± SD) by post hoc Tukey's test.
Deconvolution analysis was applied to estimate 10-h overnight T secretion rates (basal, pulsatile, and total) in the four interventions. Total (basal plus pulsatile) T secretion values differed significantly (P < 10−12) by intervention, as follows: rhLH pulses every 2 h = rhLH pulses every 1 h > continuous rhLH > saline. Less striking differences existed for both basal and pulsatile T secretion (P < 10−8 and P < 10−4, respectively). The percentage of total T secretion that was pulsatile varied significantly with intervention (P = 0.019), as follows: rhLH pulses every 1 h (44 ± 20%) > rhLH pulses every 2 h (24 ± 13%) = continuous rhLH (28 ± 17%) = saline infusion (29 ± 18%). Thus the highest percentage pulsatile T secretion was achieved by the 1-h rhLH-pulse paradigm and the lowest percentage by 2-h pulses.
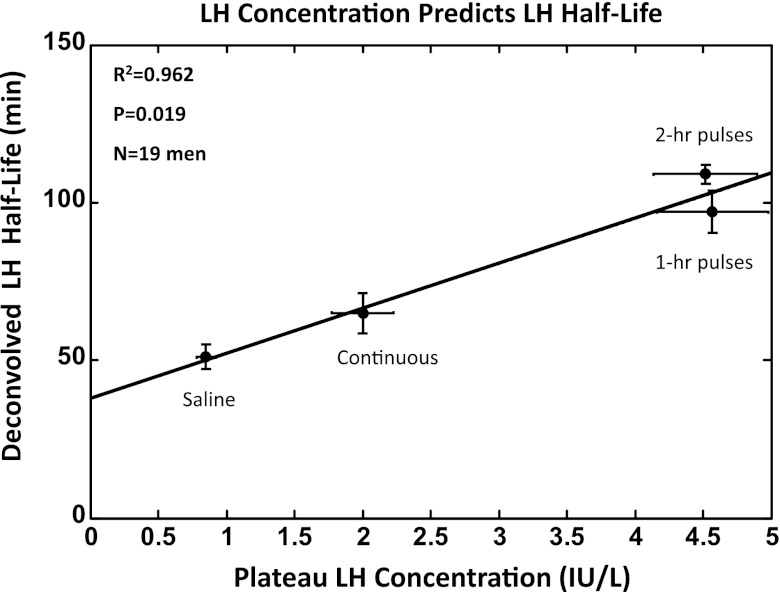
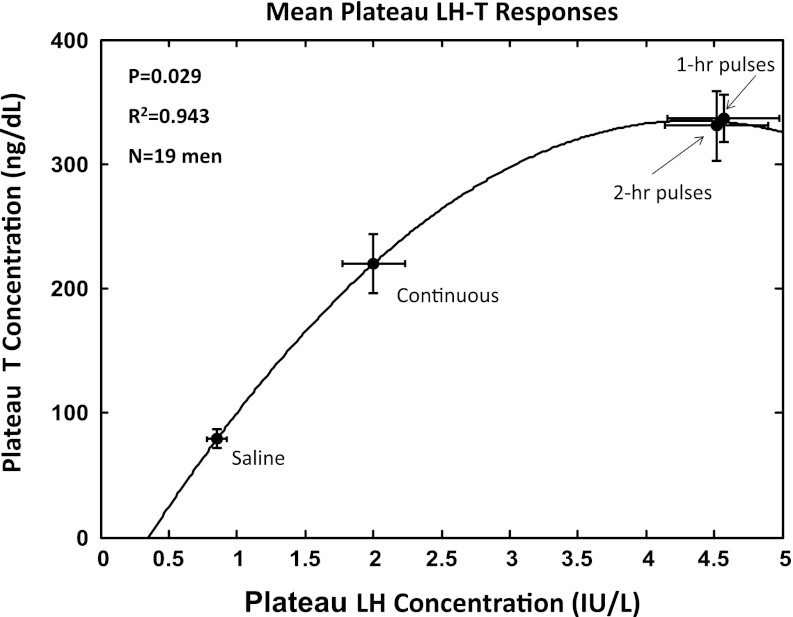
Deconvolution analysis of LH-concentration profiles revealed a graded increase in estimated LH half-life of elimination with increasing mean LH concentrations (Table 2). The square of the correlation coefficient for this relationship was R2 = 0.962 with P = 0.019 (Fig. 4). Concomitantly, higher mean LH concentrations were associated with lower LH metabolic clearance rates (P < 10−3) and smaller LH distribution volumes (P < 10−3). The half-life of endogenous LH (ganirelix/saline condition) did not differ from that of exogenous LH during any of the three separate rhLH infusion paradigms. The plateau (mean of last 90 min) T concentration was a simple quadratic function of mean LH concentration over the same interval (R2 = 0.943, P = 0.029; Fig. 5). Accordingly, all four of the following attributes, LH half-life, metabolic clearance rate, distribution volume, and plateau T concentrations, were determined by mean LH concentrations.
Table 2.
Estimated LH kinetics and plateau LH concentrations
| t1/2, min | k,min−1 | MCR, l/day | LH, IU/l | Vol Dis, liter | |
|---|---|---|---|---|---|
| Endogenous LH (saline) | 51 ± 3.9a | 0.0136a | NA | 0.85 ± 0.07a | NA |
| rhLH continuous | 65 ± 6.3a | 0.0108a | 112.5a | 2.00 ± 0.23b | 7.2 ± 0.83b |
| rhLH 1-h pulses | 97 ± 6.7b | 0.0071b | 49.2b | 4.57 ± 0.38c | 4.8 ± 0.40b |
| rhLH 2-h pulses | 109 ± 3.1b | 0.0064b | 49.8b | 4.52 ± 0.41c | 5.4 ± 0.49a,b |
Data are means ± SD; n = 19 men. Assuming metabolic clearance rate (MCR) = infusion rate/[LH] and k = 0.693/t1/2.; NA, not applicable (ganirelix plus saline infusion). rh, recombinant human; Significant (P < 0.05) differences within columns are denoted by different (unshared) alphabetic superscripts. Plateau was defined as the last 90 min of the 10-h sampling session. The half-life was estimated by deconvolution analysis (see methodology).
Fig. 4.
Linear regression of deconvolution-estimated LH half-life of elimination on plateau (mean of last 90 min in the 10-h sampling session) LH concentrations. See legend of Fig. 2.
Fig. 5.
Quadratic curve relating the plateau T (last 90-min mean) and LH concentrations.
ApEn analysis was used as an objective measure of feedforward (stimulatory) LH-T coupling. ApEn is a concentration-independent pattern-irregularity statistic (methodogly). Lower ApEn denotes greater regularity or orderliness of T-concentration patterns. Based upon mean (± SD) T ApEn values calculated over 10 h in each of the 4 interventions, the decreasing rank order of T ApEn was significant for 2-h rhLH pulses > 1-h rhLH pulses (P = 0.0076). Neither infused pulse mode differed from continuous rhLH or saline infusion. Thus rhLH pulses every 1 h drove more orderly (reproducible) T patterns than rhLH pulses every 2 h.
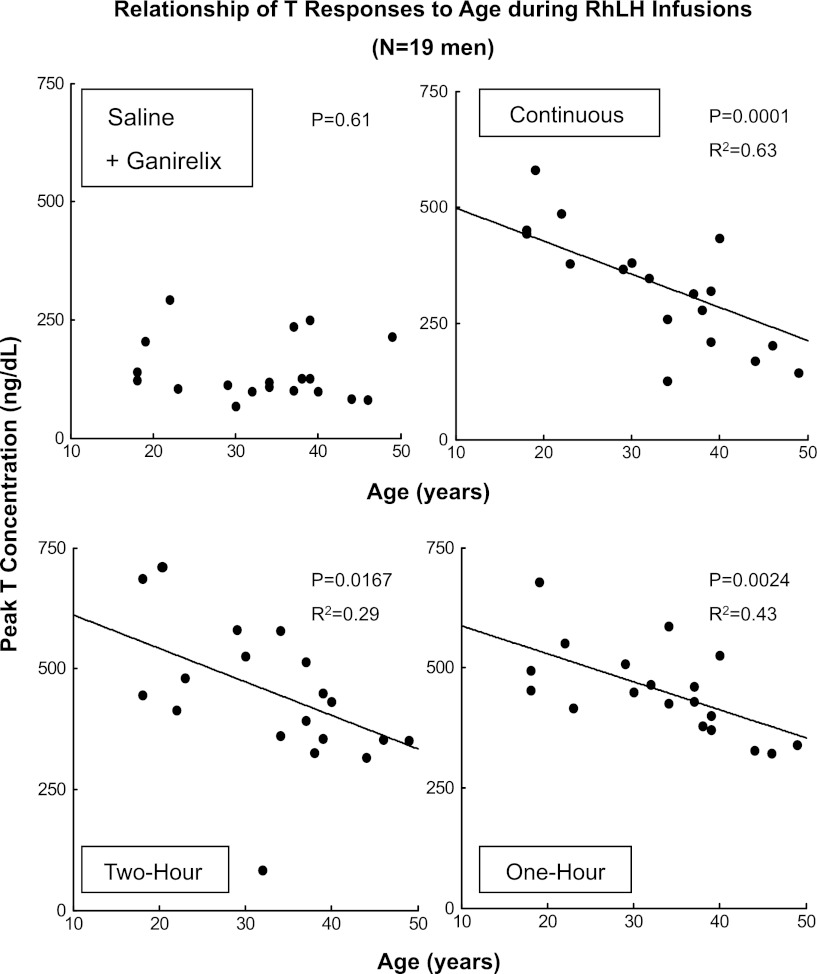
As an exploratory measure, univariate linear regression was applied to test for possible effects of BMI or age on peak total T responses in each of the four interventions. There was no significant effect of BMI (P > 0.30). By stepwise regression, BMI did not interact significantly with age in determining T responses to LH. Rather, as shown in Fig. 6, age alone explained ∼63% of the variance in peak total T concentrations driven by continuous rhLH infusion (regression P = 0.0001), and ∼43% and 29% of the variance in T responses to 1- and 2-h rhLH pulses, respectively (both P ≤ 0.0167). Slopes for total T on age are given in appendix Table A1. Similarly strong effects (P < 0.0167, R2 = 0.31 to 0.53) were observed for peak free T and peak bioavailable T concentrations regressed on age under continuous rhLH infusion and to a lesser degree during 2- and 1-h pulsatile rhLH pulses (P < 0.05) but not saline infusion (P > 0.05) in these 19 subjects. There were no effects of age on mean, peak, or plateau LH concentrations in any of the four interventions.
Fig. 6.
Univariate linear regression of peak (maximal single value achieved over 10 h) total T concentrations on age in 19 men studied as described in Fig. 1. R2 is the square of the corresponding correlation coefficient. The matching P value is indicated. Comparable plots were obtained for both free and bioavailable T (see results).
Appendix Table 1.
Univariate linear regressions of peak T concentration responses on age
| Infusion Type | Linear Coefficient (95% C.I.) | Intercept on y-Axis | One-tailed P Value | R2 Value |
|---|---|---|---|---|
| Saline | NS | NS | 0.61 | NS |
| Continuous rhLH | −10.3 (−15.8 to −6.0) | 667 | 0.0001 | 0.63 |
| 2-h rhLH pulses | −9.1 (−16 to −1.5) | 746 | 0.0167 | 0.29 |
| 1-h rhLH pulses | −6.3 (−10 to −1.3) | 661 | 0.0024 | 0.43 |
Data are from (10-h) overnight sampling (n = 19) using peak total T concentrations. NS, not significant (P > 0.05). There was no significant effect of BMI (results).
DISCUSSION
The present investigation in young and middle-aged men utilizes the concept of a combined GnRH antagonist and pulsatile LH clamp to show that a pulsatile LH signal, albeit not required for mean T output, strongly determines T secretion patterns. If verified, this outcome would motivate investigation of the impact of pulsatile T-dependent signaling patterns on target-tissues of androgen action. In this regard, glucocorticoid pulsatility has recently been shown to mediate distinct central nervous system effects (25, 38, 46). Analogously, in the only such study available, pulsatile and continuous T feedback exerted distinct effects of LH secretion (58). Unlike the present LH-clamp model, in the T clamp, continuous T infusion yielded higher mean T concentrations than pulsatile T infusion. Together, the data indicate that the time mode of hormone delivery (secretion or infusion) into the bloodstream determines serum hormone concentrations and certain tissue actions.
Although many studies have verified the importance of pulsatility of the GnRH signal to gonadotrope cells (16), to our knowledge only one earlier human investigation has appraised LH/ human chorionic gonadotropin (hCG) pulsatility effects. In that study, five once-daily injections of hCG maintained higher T concentrations than a single injection of the same total hCG dose in healthy men (43). The present analyses differ by quantifying gonadal T responses to three distinct intravenous LH stimulation patterns during overnight GnRH antagonist-induced hypogonadotropism. The different design used here is important because high doses of hCG can downregulate Leydig-cell steroidogenesis (8); hCG has a nearly 20-fold longer half-life than LH (48); endogenous LH may confound hCG effects and thus, in the present study, was depleted first by injecting a potent selective GnRH receptor antagonist; a suitable negative control is requisite, which here was achieved by imposing saline compared with continuous and pulsatile rhLH infusions on separate days in randomized order in the same subject; and more detailed analysis of (10-min) T concentration profiles than simple averaging is necessary as accomplished using deconvolution analysis, exponential curve fitting, and approximate entropy (ApEn, a pattern regularity statistic). Thereby we show that 1- and 2-h rhLH pulses both evoke higher mean LH and T concentrations than does continuous infusion of the same total amount of rhLH; the mean concentration differences are due to strong kinetic effects of pulsatile versus continuous LH infusions, wherein pulses yield a longer half-life, lower metabolic clearance, and smaller distribution volume; and the effects of 1- and 2-h rhLH pulse frequencies, despite conferring comparable mean LH concentrations, differ in distinct dynamic ways: 1) the rapidity of the T concentration rise is maximal for 1-h pulses compared with each of the other three paradigms; 2) the percentage of pulsatile vis-à-vis total T secretion is highest (44%) for 1-h pulses; and 3) the orderliness of T secretion patterns as quantified by ApEn is greater for 1-h than 2-h pulses. The ensemble outcomes allow the postulates that mean LH and thereby mean T concentrations are controlled by the time mode of LH's delivery into the circulation at a given LH secretion (infusion) rate, whereas specific T dynamics (rate of T rise, percentage pulsatile T secretion, and T ApEn) are dependent on the frequency of LH pulses at comparable mean LH concentrations.
During overnight GnRH receptor antagonist-induced depletion of LH and T concentrations, adding back rhLH by pulsatile intravenous infusions yielded higher LH concentrations during either 2- or 1-h rhLH pulses than during continuous rhLH delivery. This effect was due to demonstrably longer LH half-life, smaller LH distribution volume, and lower LH metabolic clearance rate for pulsatile than continuous rhLH infusions. These outcomes would be consistent with saturable mechanisms of LH's metabolism or removal, thus attenuating clearance of infused LH peaks per se. The notion was proposed earlier for high doses of pituitary LH extracts infused in men as well as for large GH pulses injected in women (13, 41, 50). The present data indicate that even within the physiologically normal range of LH concentrations in men (1.8–8.6 IU/l), the metabolic clearance of LH from the circulation falls by 50% as LH concentrations double. Concurrently, T concentrations also increase by nearly twofold (190 to 320 ng/dl). Thus a fundamental mechanism by which pulsatile LH secretion maintains higher T concentrations than does continuous LH delivery is via a kinetic effect of LH pulses to elevate mean LH concentrations more effectively within the physiological range of LH clearance. This mechanism was probably overlooked earlier because hCG/LH doses exceeded physiological addback.
Deconvolution-based estimates of total and basal T secretion under the GnRH antagonist delineated a marked stimulatory effect of continuous rhLH stimulation over that of saline infusion, viz. a 2.5-fold effect. Pulsatile rhLH infusions elevated total and basal T secretion even more over saline, namely by 3.8-fold (2-h pulses) and 3.6-fold (1-h pulses). For these particular end points, the effects of the two rhLH-pulse paradigms were not different. A possible caveat is that no current methods of deconvolution fully distinguish pulsatile and basal T secretion (54). Another possibility is that acute GnRH receptor antagonism impairs testis responses to rhLH. However, this is unlikely in humans (5, 48) and is not observed by in vitro bioassay of LH using dispersed Leydig cells (7).
The exponential rate of rise of T concentrations from ganirelix-suppressed values during rhLH stimulation was most rapid (shortest half-time of rise) during 1-h rhLH pulses. The rate of response to 2-h rhLH pulses was more delayed and similar to that of continuous rhLH drive, even though mean LH concentrations were indistinguishable during 1- and 2-h LH pulses. If confirmed in a larger cohort of subjects (here, N = 19), the data would indicate that the pattern of LH-pulse delivery to the testis influences T response latency. Pulse-frequency distinctions were also made for LH's effects on approximate entropy and percentage pulsatile T secretion, wherein 1-h rhLH pulses elicited more orderly patterns of T secretion and higher ratios of pulsatile to total T secretion than 2-h rhLH pulses. Because mean and plateau LH concentrations did not differ during 1- and 2-h pulses of rhLH, we infer that the dynamics of T production at a given mean LH concentration depend on LH pulse frequency. Whether such dynamics convey specific regulatory information to T-target cells is not known (48). However, in one analysis, pulses of T yielded lower mean T levels and suppressed LH secretion less than continuous T infusions in young men (58).
In GnRH-immunized rams, intravenous infusion of low-amplitude, high-frequency LH pulses as well as of LH continuously for 6 days stimulates higher mean and peak T concentrations than high-amplitude, low-frequency LH pulses (4). In experiments in male rats, 2-h pulsatile and continuous LH infusions for 10 days achieved similar total T concentrations (12). These outcomes clearly differ from the present overnight data. However, one cannot determine whether species, duration of LH infusion, or other methodological factors explain the differences noted.
The central nervous system coordinates oscillations among GnRH, LH, FSH, prolactin, testosterone, nocturnal penile tumescence, and sleep stage in healthy young men (36, 51). Coordinated patterns are disrupted in middle-aged and older men consistent with impaired hypothalamic regulation (30–32, 36, 51). In addition, at the hypothalamo-pituitary levels, older men exhibit lower amplitude and higher frequency LH pulses than young counterparts (19, 20, 30–32, 44, 51). The two extremes of mean LH pulse frequency and size were represented faithfully in the present paradigms (Fig. 2). Our analyses show negative effects of middle age on peak T concentrations in each of the three rhLH-addback protocols (Fig. 6). Thus acute differences in the mean time mode of LH delivery could not fully explain the negative effect of middle age on total, free, and bioavailable T concentrations (22, 45). Although longer-term LH infusions and pulses delivered with random variability are needed to verify this inference, the present outcomes would be consistent with animal experiments showing reduced gonadal T secretion in the middle-aged male (30). In principle, the paradigm and analyses presented here could have application to other in vivo studies of partial Leydig-cell failure in humans and animals.
Perspectives and Significance
Much has been learned in the last decade concerning dynamic neuroendocrine interactions that generate pituitary hormone pulses (15, 38, 54, 56). Far less is known about the peripheral (postsecretory) regulation and implications of pulsatile hormone delivery to target tissues (3, 11, 39, 57). Delineating the target gland's mechanisms of decoding a frequency- and amplitude-varying hypophyseal signal has fundamental physiological significance in regulatory biology (24, 40) and has the potential to improve diagnostic identification of early pathological failure of neuroendocrine pathways (both feedforward and feedback), enhance therapeutic interventional strategies, and refine mechanistic understanding. Therefore, future investigations will need to dissect more precisely the impact of pulsatile peripheral (systemic) signals generated by endocrine glands, such as testis T, adrenal aldosterone, and ovarian estradiol and progesterone pulses, as well as the manner in which circadian time keeping modulates peripheral pulse generation and action (1, 2).
GRANTS
This study was supported in part via the Center for Translational Science Activities (CTSA) Grant Number 1 UL 1 RR-024150 from the National Center for Research Resources (Rockville, MD), RO1 AG-031763, and DK-050456 (Metabolic Studies Core of the Minnesota Obesity Center) from the National Institutes of Health (Bethesda, MD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health. Matlab versions of the deconvolution methodology are available from Veldhuis.johannes@mayo.edu.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.D.V., P.Y.L., and P.Y.T. conception and design of research; J.D.V., P.Y.L., P.Y.T., S.M.W., and J.R.W. performed experiments; J.D.V. analyzed data; J.D.V. interpreted results of experiments; J.D.V. prepared figures; J.D.V. drafted manuscript; J.D.V. edited and revised manuscript; J.D.V. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jill Smith for support of manuscript preparation, Sandra Cabral for data analysis and graphics, the Mayo Immunochemical Laboratory for assay assistance, and the Mayo research nursing staff for implementing the protocol.
REFERENCES
- 1. Albers N. Overview of pulse actions in the human. Growth Horm IGF Res 11, Suppl A: S39–S42, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Bonnefont X. Circadian timekeeping and multiple timescale neuroendocrine rhythms. J Neuroendocrinol 22: 209–216, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Charloux A, Brandenberger G, Piquard F, Geny B. Dysregulation of pulsatility in aging. IV. Pulsatile signaling and cardiovascular aging: functions and regulation of natriuretic peptide signaling. Ageing Res Rev 7: 151–163, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Chase DJ, Schanbacher BD, Lunstra DD. Effects of pulsatile and continuous luteinizing hormone (LH) infusions on testosterone responses to LH in rams actively immunized against gonadotropin-releasing hormone. Endocrinology 123: 816–826, 1988 [DOI] [PubMed] [Google Scholar]
- 5. Chi L, Zhou W, Prikhozhan A, Flanagan C, Davidson JS, Golembo M, Illing N, Millar RP, Sealfon SC. Cloning and characterization of the human GnRH receptor. Mol Cell Endocrinol 91: R1–R6, 1993 [DOI] [PubMed] [Google Scholar]
- 6. de Jong D, Macklon NS, Mannaerts BM, Coelingh Bennink HJT, Fauser BC. High dose gonadotrophin-releasing hormone antagonist (ganirelix) may prevent ovarian hyperstimulation syndrome caused by ovarian stimulation for in vitro fertilization. Hum Reprod 13: 573–575, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Dufau ML, Veldhuis JD. Pathophysiological relationships between the biological and immunological activities of luteinizing hormone. In: Bailliere's Clinical Endocrinology and Metabolism, edited by Burger HG. Philadelphia, PA: Saunders, 1987, p. 153–176 [DOI] [PubMed] [Google Scholar]
- 8. Dufau ML, Winters CA, Hattori M, Aquilano D, Baranao JL, Nozu K, Baukal A, Catt KJ. Hormonal regulation of androgen production by the Leydig cell. J Steroid Biochem 20: 161–173, 1984 [DOI] [PubMed] [Google Scholar]
- 9. Elmlinger MW, Kuhnel W, Wormstall H, Doller PC. Reference intervals for testosterone, androstenedione and SHBG levels in healthy females and males from birth until old age. Clin Lab 51: 625–632, 2005 [PubMed] [Google Scholar]
- 10. Fisher LD, van Belle G. Descriptive statistics. In: Biostatistics: A Methodology for the Health Sciences. New York: Wiley, 1996, p. 58–74 [Google Scholar]
- 11. Gan EH, Quinton R. Physiological significance of the rhythmic secretion of hypothalamic and pituitary hormones. Prog Brain Res 181: 111–126, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Gibson-Berry KL, Chase DJ. Continuous and pulsatile infusions of luteinizing hormone have identical effects on steroidogenic capacity and sensitivity of Leydig cell in rats passively immunized against gonadotropin-releasing hormone. Endocrinology 126: 3107–3115, 1990 [DOI] [PubMed] [Google Scholar]
- 13. Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev 19: 717–797, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Guo T, Gu J, Soldin OP, Singh RJ, Soldin SJ. Rapid measurement of estrogens and their metabolites in human serum by liquid chromatography-tandem mass spectrometry without derivatization. Clin Biochem 41: 736–741, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hauffa BP. Clinical implications of pulsatile hormone signals. Growth Horm IGF Res 11, Suppl A: S1–S8, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology 138: 1224–1231, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Keenan DM, Alexander S, Irvine CH, Veldhuis JD. Quantifying nonlinear interactions within the hypothalamo-pituitary-adrenal axis in the conscious horse. Endocrinology 150: 1941–1951, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keenan DM, Chattopadhyay S, Veldhuis JD. Composite model of time-varying appearance and disappearance of neurohormone pulse signals in blood. J Theor Biol 236: 242–255, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Keenan DM, Clarke IJ, Veldhuis JD. Non-invasive analytical estimation of endogenous gonadotropic-releasing hormone (GnRH) drive: analysis using graded competitive GnRH-receptor antagonism and a calibrating pulse of exogenous GnRH. Endocrinology 152: 4882–4893, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keenan DM, Takahashi PY, Liu PY, Roebuck PD, Nehra AX, Iranmanesh A, Veldhuis JD. An ensemble model of the male gonadal axis: illustrative application in aging men. Endocrinology 147: 2817–2828, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Keenan DM, Veldhuis JD. Disruption of the hypothalamic luteinizing-hormone pulsing mechanism in aging men. Am J Physiol Regul Integr Comp Physiol 281: R1917–R1924, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Keenan DM, Veldhuis JD. Age-dependent regression analysis of male gonadal axis. Am J Physiol Regul Integr Comp Physiol 297: R1215–R1227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ledue TB, Collins MF, Ritchie RF. Development of immunoturbidimetric assays for fourteen human serum proteins on the Hitachi 912. Clin Chem Lab Med 40: 520–528, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Leitolf H, Szkudlinski MW, Hoang-Vu C, Thotakura NR, Von Zur MA, Brabant G, Weintraub BD. Effects of continuous and pulsatile administration of pituitary rat thyrotropin and recombinant human thyrotropin in a chronically cannulated rat. Horm Metab Res 27: 173–178, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Lightman SL, Wiles CC, Atkinson HC, Henley DE, Russell GM, Leendertz JA, McKenna MA, Spiga F, Wood SA, Conway-Campbell BL. The significance of glucocorticoid pulsatility. Eur J Pharmacol 583: 255–262, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Liu PY, Keenan DM, Kok P, Padmanabhan V, O'Byrne KT, Veldhuis JD. Sensitivity and specificity of pulse detection using a new deconvolution method. Am J Physiol Endocrinol Metab 297: E538–E544, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu PY, Pincus SM, Takahashi PY, Roebuck PD, Iranmanesh A, Keenan DM, Veldhuis JD. Aging attenuates both the regularity and joint synchrony of LH and testosterone secretion in normal men: analyses via a model of graded GnRH receptor blockade. Am J Physiol Endocrinol Metab 290: E34–E41, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Liu PY, Takahashi PY, Roebuck PD, Iranmanesh A, Veldhuis JD. Age-specific changes in the regulation of LH-dependent testosterone secretion: assessing responsiveness to varying endogenous gonadotropin output in normal men. Am J Physiol Regul Integr Comp Physiol 289: R721–R728, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Liu PY, Takahashi PY, Roebuck PD, Iranmanesh A, Veldhuis JD. Aging in healthy men impairs recombinant human LH-stimulated testosterone secretion monitored under a two-day intravenous pulsatile LH clamp. J Clin Endocrinol Metab 90: 5544–5550, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Liu PY, Veldhuis JD. The hypothalamo-pituitary unit, testis and male accessory organs. In: Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management, edited by Barbieri R, Strauss J. Philadelphia, PA: Elsevier, 2009, p. 283–298 [Google Scholar]
- 31. Mulligan T, Iranmanesh A, Gheorghiu S, Godschalk M, Veldhuis JD. Amplified nocturnal luteinizing hormone (LH) secretory burst frequency with selective attenuation of pulsatile (but not basal) testosterone secretion in healthy aged men: possible Leydig cell desensitization to endogenous LH signaling–a clinical research center study. J Clin Endocrinol Metab 80: 3025–3031, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Mulligan T, Iranmanesh A, Kerzner R, Demers LW, Veldhuis JD. Two-week pulsatile gonadotropin releasing hormone infusion unmasks dual (hypothalamic and Leydig-cell) defects in the healthy aging male gonadotropic axis. Eur J Endocrinol 141: 257–266, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Mulligan T, Iranmanesh A, Veldhuis JD. Pulsatile intravenous infusion of recombinant human LH in leuprolide-suppressed men unmasks impoverished Leydig-cell secretory responsiveness to midphysiological LH drive in the aging male. J Clin Endocrinol Metab 86: 5547–5553, 2001 [DOI] [PubMed] [Google Scholar]
- 34. O'Brien PC. The appropriateness of analysis of variance and multiple-comparison procedures. Biometrics 39: 787–794, 1983 [PubMed] [Google Scholar]
- 35. Pincus SM. Irregularity and asynchrony in biologic network signals. Methods Enzymol 321: 149–182, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Pincus SM, Mulligan T, Iranmanesh A, Gheorghiu S, Godschalk M, Veldhuis JD. Older males secrete luteinizing hormone and testosterone more irregularly, and jointly more asynchronously, than younger males. Proc Natl Acad Sci USA 93: 14100–14105, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rabinovici J, Rothman P, Monroe SE, Nerenberg C, Jaffe RB. Endocrine effects and pharmacokinetic characteristics of a potent new gonadotropin-releasing hormone antagonist (Ganirelix) with minimal histamine-releasing properties: studies in postmenopausal women. J Clin Endocrinol Metab 75: 1220–1225, 1992 [DOI] [PubMed] [Google Scholar]
- 38. Sarabdjitsingh RA, Joels M, de Kloet ER. Glucocorticoid pulsatility and rapid corticosteroid actions in the central stress response. Physiol Behav 106: 73–80, 2012 [DOI] [PubMed] [Google Scholar]
- 39. Schmitz O, Rungby J, Edge L, Juhl CB. On high-frequency insulin oscillations. Ageing Res Rev 7: 301–305, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Seely EW, Conlin PR, Brent GA, Dluhy RG. Adrenocorticotropin stimulation of aldosterone: prolonged continuous versus pulsatile infusion. J Clin Endocrinol Metab 69: 1028–1032, 1989 [DOI] [PubMed] [Google Scholar]
- 41. Shah N, Aloi J, Evans WS, Veldhuis JD. Time-mode of growth hormone (GH) entry into the bloodstream and steady-state plasma GH concentrations rather than sex, estradiol, or menstrual-cycle stage primarily determine the GH elimination rate in healthy young women and men. J Clin Endocrinol Metab 84: 2862–2869, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Singh RJ. Validation of a high throughput method for serum/plasma testosterone using liquid chromatography tandem mass spectrometry (LC-MS/MS). Steroids 73: 1339–1344, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Smals AG, Pieters GF, Boers GH, Raemakers JM, Hermus AR, Benraad TJ, Kloppenborg PW. Differential effect of single high dose and divided small dose administration of human chorionic gonadotropin on Leydig cell steroidogenic desensitization. J Clin Endocrinol Metab 58: 327–331, 1984 [DOI] [PubMed] [Google Scholar]
- 44. Takahashi PY, Liu PY, Roebuck PD, Iranmanesh A, Veldhuis JD. Graded inhibition of pulsatile LH secretion by a selective GnRH-receptor antagonist in healthy men: evidence that age attenuates hypothalamic GnRH outflow. J Clin Endocrinol Metab 90: 2768–2774, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Takahashi PY, Votruba P, Abu-Rub M, Mielke K, Veldhuis JD. Age attenuates testosterone secretion driven by amplitude-varying pulses of recombinant human luteinizing hormone during acute gonadotrope inhibition in healthy men. J Clin Endocrinol Metab 92: 3626–3632, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress 14: 398–406, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. The European Recombinant Human LH Study Group Recombinant human luteinizing hormone (LH) to support recombinant human follicle-stimulating hormone (FSH)-induced follicular development in LH- and FSH-deficient anovulatory women: a dose-finding study The European Recombinant Human LH Study Group. J Clin Endocrinol Metab 83: 1507–1514, 1998 [DOI] [PubMed] [Google Scholar]
- 48. Urban RJ, Evans WS, Rogol AD, Kaiser DL, Johnson ML, Veldhuis JD. Contemporary aspects of discrete peak detection algorithms. I. The paradigm of the luteinizing hormone pulse signal in men. Endocr Rev 9: 3–37, 1988 [DOI] [PubMed] [Google Scholar]
- 49. Urbanski HF, Ojeda SR. In vitro simulation of prepubertal changes in pulsatile luteinizing hormone release enhances progesterone and 17 beta-estradiol secretion from immature rat ovaries. Endocrinology 117: 638–643, 1985 [DOI] [PubMed] [Google Scholar]
- 50. Veldhuis JD, Fraioli F, Rogol AD, Dufau ML. Metabolic clearance of biologically active luteinizing hormone in man. J Clin Invest 77: 1122–1128, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Veldhuis JD, Iranmanesh A, Mulligan T, Pincus SM. Disruption of the young-adult synchrony between luteinizing hormone release and oscillations in follicle-stimulating hormone, prolactin, and nocturnal penile tumescence (NPT) in healthy older men. J Clin Endocrinol Metab 84: 3498–3505, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Veldhuis JD, Johnson ML. Analysis of nonequilibrium facets of pulsatile sex-steroid secretion in the presence of plasma binding proteins. Meth Enzymol 321: 239–263, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Veldhuis JD, Keenan DM, Liu PY, Takahashi PY. Kinetics of removal of intravenous testosterone pulses in normal men. Eur J Endocrinol 162: 787–794, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Veldhuis JD, Keenan DM, Pincus SM. Motivations and methods for analyzing pulsatile hormone secretion. Endocr Rev 29: 823–864, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Veldhuis JD, Liu PY, Takahashi PY, Keenan DM. Dynamic testosterone responses to near-physiological LH pulses are determined by the time-pattern of prior intravenous LH infusion. Am J Physiol Endocrinol Metab 303: E720–E728, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev 27: 101–140, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Walker JJ, Terry JR, Tsaneva-Atanasova K, Armstrong SP, McArdle CA, Lightman SL. Encoding and decoding mechanisms of pulsatile hormone secretion. J Neuroendocrinol 22: 1226–1238, 2010 [DOI] [PubMed] [Google Scholar]
- 58. Zwart AD, Iranmanesh A, Veldhuis JD. Disparate serum free testosterone concentrations and degrees of hypothalamo-pituitary-LH suppression are achieved by continuous versus pulsatile intravenous androgen replacement in men: a clinical experimental model of ketoconazole-induced reversible hypoandrogenemia with controlled testosterone add-back. J Clin Endocrinol Metab 82: 2062–2069, 1997 [DOI] [PubMed] [Google Scholar]