Abstract
Pulmonary fibrosis, the end stage of a variety of fibroproliferative lung diseases, is usually induced after repetitive or chronic lung injury or inflammation. The mechanisms of fibroproliferation are poorly understood. Insulin-like growth factor-I (IGF-I) is significantly elevated in patients with pulmonary fibrosis and fibroproliferative acute respiratory distress syndrome. However, we showed that IGF-I overexpression alone in wild-type mouse lungs does not cause fibroproliferation. We therefore questioned whether IGF-I, acting together with active TGF-β1, a known profibrotic cytokine, enhances pulmonary fibroproliferation caused by active TGF-β1. A unique sequential adenoviral transgene mouse model was used expressing AdEmpty/AdTGF-β1 or AdhIGF-IB/AdTGF-β1 transgenes. IGF-IB plus active TGF-β1 transgene expression synergistically increased collagen deposition in the lung parenchyma compared with active TGF-β1 expression alone. The enhanced fibrosis was accompanied by an increased recruitment of macrophages and lymphocytes into the bronchoalveolar lavage fluid (BALF) and inflammatory cells in the lungs. α-Smooth muscle actin expression, a marker of myofibroblast proliferation and differentiation, was also increased. Finally, fibroblasts exposed ex vivo to BALF isolated from AdhIGF-IB/AdTGF-β1-transduced mice showed synergistic collagen induction compared with BALF from AdEmpty/AdTGF-β1-transduced mice. This study provides the first direct evidence that IGF-I is able to synergistically enhance pulmonary fibroproliferation in cooperation with TGF-β1.
Keywords: pulmonary fibrosis, mouse model, adenovirus
pulmonary fibrosis (PF), the final step of a group of lung disorders known as interstitial lung diseases, has been described to follow three stages: injury, inflammation, and tissue repair (38). Injury and inflammation cause the destruction of the alveolar epithelium, which activates lung fibroblasts, leading to their proliferation and differentiation into myofibroblasts (20). The fibroproliferative process is precisely regulated by a plethora of cytokines and growth factors, such as TGF-β1, IL-1β, TNF-α, PDGF, GM-CSF, and CTGF (5, 31, 38). Failure to regulate this process leads to excessive extracellular matrix production and accumulation in lung connective tissue, resulting in PF.
IGF-I expression has been documented in the lungs of patients with idiopathic pulmonary fibrosis (IPF) (2, 4, 35) and fibroproliferative acute respiratory distress syndrome (ARDS) (22). IGF-I stimulates the proliferation and differentiation of lung fibroblasts into myofibroblasts, increases collagen synthesis (9), and protects myofibroblasts from apoptosis and thus may directly or indirectly increase the overall quantity of myofibroblast-produced extracellular matrix in the lungs (9). In addition, in vitro studies by Harrison et al. (16) clearly demonstrated a role for IGF-I in fibroblast proliferation in patients with systemic sclerosis. Importantly, inhibition of the type I IGF tyrosine kinase receptor in an in vivo murine model of bleomycin-induced lung injury resulted in less fibrosis (10). We, however, demonstrated in a noninjury mouse model that overexpression of hIGF-IB caused pulmonary inflammation but did not, by itself, induce PF (23). Others have shown that chronic overexpression of a different isoform of IGF-I, IGF-IA, also did not cause PF (14).
TGF-β1 is an important pleiotropic cytokine regulating tissue morphogenesis through effects on cell proliferation, differentiation, apoptosis, and extracellular matrix production (7). TGF-β1 is upregulated in lungs of patients with IPF (11). Furthermore, overexpression of active TGF-β1 resulted in PF characterized by extensive deposition of the extracellular matrix proteins collagen, fibronectin, and elastin and by the emergence of cells with a myofibroblast phenotype (32). In a bleomycin murine model, fibrosis is diminished when a soluble TGF-β1 receptor (37) or a TGF-β1 kinase inhibitor (6) are used to inhibit TGF-β1 activity, demonstrating the importance of TGF-β1 in the pathogenesis of PF.
Bloor et al. (4) showed that fibroblasts recovered from the bronchoalveolar lavage (BAL) of patients with IPF expressed high levels of IGF-I, which could be further increased by stimulation with TGF-β. We thus hypothesize that in a milieu of cytokines/growth factors that occur in inflammation, IGF-I and TGF-β1 interact and contribute to the pathogenic process of PF. To shed some light on the potential role of IGF-I acting in concert with TGF-β1 in pulmonary fibroproliferation, we used adenoviral-mediated gene transfer technology to express both hIGF-IB and TGF-β1(active) sequentially in mouse lungs in vivo. We found that mice transduced first with AdhIGF-IB followed by AdTGF-β1(active) induced greater fibroproliferation than control (AdEmpty/AdTGF-β1-transduced) mice. In this model, expression of the hIGF-IB transcript synergistically enhances the pulmonary fibroproliferation induced by TGF-β1 in vivo.
MATERIALS AND METHODS
Recombinant adenovirus.
AdhIGF-IB and AdEmpty vectors were prepared as previously described (23). Active porcine TGF-β1 adenovirus was a generous gift from Dr. Jack Gauldie. Active porcine TGF-β1 adenovirus (AdTGF-β1C223S/C225S, referred to in this paper as AdTGF-β1) is a point-mutated constitutively active form of TGF-β1 where residues Cys223 and Cys225 have been mutated to Ser223 and Ser225 residues (32). AdTGF-β1 was amplified and titrated by plaque assay using the same technique as AdhIGF-IB and AdEmpty vectors (23).
Animal treatment.
Male C57BL/6 mice 5–7 wk old (Charles River Laboratories, Montreal, PQ, Canada) were treated according to the Canadian Council of Animal Care guidelines. Ethics approval was obtained from the University of Calgary Animal Care Committee. Mice were anesthetized by inhalation of isoflurane. AdhIGF-IB or AdEmpty adenovirus [2 × 109 plaque-forming units (pfu)] in 50 μl of sterile PBS were injected into mouse lungs by intratracheal intubation as previously described (23). Two days after the AdhIGF-IB or AdEmpty intratracheal injection, the same mice were transduced with 5 × 107 pfu of AdTGF-β1 by the same intratracheal injection technique (Fig. 1). This dose of TGF-β1 showed minimal PF in a dose-response assay (data not shown).
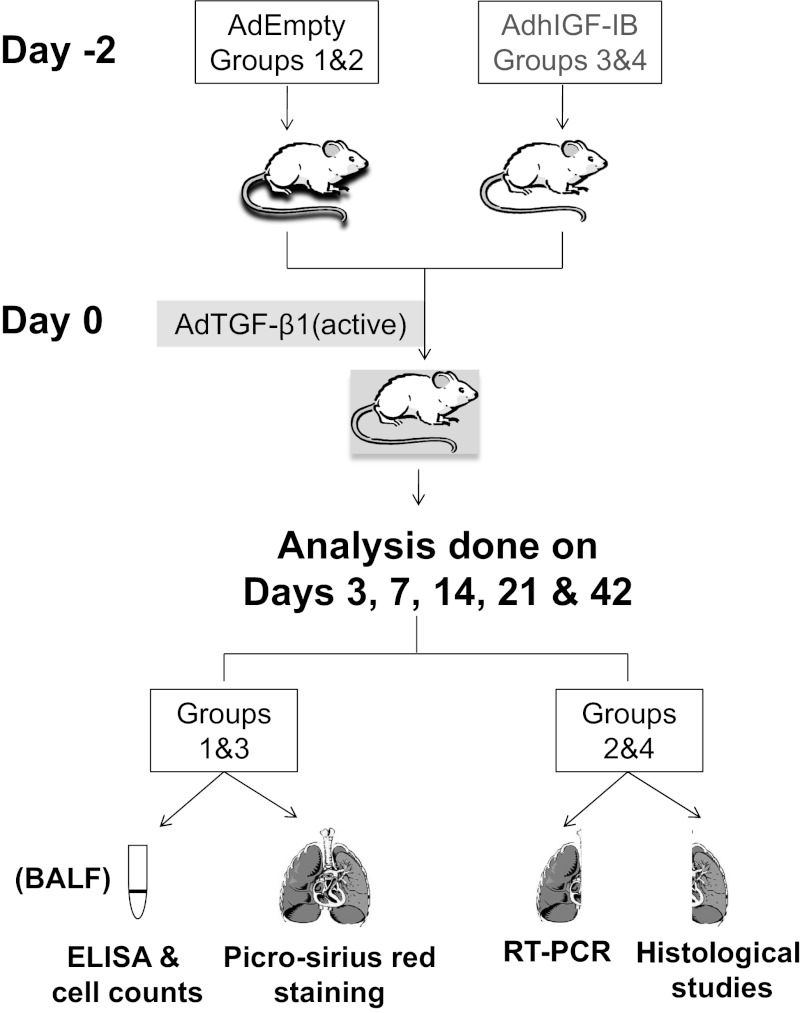
Fig. 1.
Schematic of mouse transduction and sample collection. N = 8 for each of the 2 study cohorts (AdEmpty/AdTGF-β1 and AdhIGF-IB/AdTGF-β1) at each time point; however, each cohort was split into 2 groups of 4 animals for sample processing. Group 1 and 2 mice (AdEmpty/AdTGF-β1) were transduced first with AdEmpty virus and 2 days later with AdTGF-β1(active). Group 3 and 4 mice (AdhIGF-IB/AdTGF-β1) were first transduced with AdhIGF-IB and 2 days later with AdTGF-β1(active). All sampling was performed on days 3, 7, 14, 21, and 42 after AdTGF-β1 transduction. Bronchoalveolar lavage fluid (BALF) recovered from mice in groups 1 and 3 was used for ELISA and inflammatory cell counts while the lungs were subjected to collagen quantification (Sirius red staining). The lungs of mice in groups 2 and 4 were used for RT-PCR and histological studies.
Measurement of hIGF-IB and TGF-β1 mRNA transgene expression in the lung.
Human IGF-IB and porcine TGF-β1(active) transgene mRNA expression in the lung tissue were detected by RT-PCR using a SuperScript One-Step RT-PCR kit (Invitrogen). Human IGF-IB-specific primer pair (forward: ATTGCTCTCAACATCTCCCATC; reverse: TTCCGTTTTCTCCATGTTTCTT) and porcine TGF-β1(active)-specific primer pair (forward: TTCATGAACCCAAGGGCTAC; reverse: TAAATACAGCCCCGGTGAG) were used to detect transgenic hIGF-IB and porcine TGF-β1(active) mRNA expression, respectively; 0.5 μg of total lung RNA treated with DNase and a DNA-free kit (Ambion) were used in each reaction. Reverse transcription was done by 30-min incubation at 50°C, followed by 35 PCR cycles. Mouse GAPDH primer pair (forward: CAACTTTGGCATCGTGGAAGG; reverse: CAACGGATACATTGGGGGTAG) was used in a separate reaction to serve as internal control. The adenoviral-transduced mRNA positivity occurred throughout 42 days. This prolonged mRNA expression has been shown previously (23) despite the fact that translation of the protein is limited to ∼14 days posttransduction.
Measurement of hIGF-I and active TGF-β1 protein expression in the BALF.
The supernatant of the first milliliter aliquot of BAL fluid (BALF) was used to measure hIGF-I and active TGF-β1 proteins. Recombinant human IGF-I and porcine active TGF-β1 were obtained from R&D Systems (Minneapolis, MN). Human IGF-I protein was measured by using a Quantikine human IGF-I immunoassay kit (R&D Systems) that does not cross-react with mouse IGF-I. TGF-β1 protein was measured with a Quantikine human TGF-β1 immunoassay kit (R&D Systems) that does not cross-react with latent (inactive) TGF-β1 but does cross-react with active mouse TGF-β1.
Total and differential cell counts in BAL.
Total and differential cell counts in BAL were performed as previously described (23).
α-SMA staining and quantification.
Lung tissue was processed for histology as previously described (23). Lung sections were deparaffinized, hydrated, and stained with an α-SMA antibody (Fisher, Toronto, ON, Canada). The stain was revealed by standard peroxidase immune reaction and counterstained with Gill II hematoxylin. Quantification of the positive α-SMA staining was done with Image J 1.43u software (National Institutes of Health), following a protocol previously described (http://rsb.info.nih.gov/ij/docs/examples/stained-sections/index.html) modified to exclude vessels and airways.
Sirius red staining, image analysis, and quantification.
Collagen deposition in the lungs was visualized by picrosirius (Sirius) red staining, as previously described (23). Sirius red-stained lung sections were observed under polarized light by use of a Zeiss Axioplan microscope with a ×10 objective to visualize the collagen deposition (23). The image was converted to black and white, inverted and then ten 100 × 100 μm2 areas were quantified per section in four different animals in each group using Image J software. Quantification was done independently by two blinded individuals. Collagen content from the AdEmpty alone-transduced mice were quantified from pooled day 21 and day 42 (n = 4) mice as they showed very minimal collagen content.
Histology.
The right lung was inflated with 10% buffered formalin for 24 h before being processed, embedded in paraffin, cut in 4-μm-thick sections and stained with hematoxylin and eosin (H&E). The inflammatory cells in tissue sections of each lobe were evaluated in a blinded fashion by a pulmonary pathologist (M. M. Kelly) using the following eight-point scoring method.
Scoring for inflammation.
This modified semiquantitative histological scoring system was based on two previously published methods (3, 36). The scoring system had eight grades and, therefore, a maximal inflammatory grade of 8 was possible in this grading system (Table 1). The scores from each of the three lobes of the right lung were combined into a total score and then divided by three to yield an average score for each lobe of the right lung. The repeatability of measurement for the semiquantitative scoring system (expressed as the intraclass correlation coefficient) was >0.99.
Table 1.
Grading system used for pulmonary inflammation
| Grade | Perivascular/Peribronchial Inflammation | Interstitial Inflammation |
|---|---|---|
| Zero | Nil | Nil |
| 1 | Scanty, only occasional cells present | Nil |
| 2 | Scattered small groups of cells, <3 cells depth | Nil |
| 3 | Infiltrates present around most bronchi and vessels, easily seen at magnification of ×400 | Nil/very scanty |
| 4 | Infiltrates present around all bronchi and vessels, <5 cells depth | Nil/very scanty |
| 5 | Infiltrates present around all bronchi and vessels, 5–10 cells depth | Mild interstitial inflammation, <20% area involved |
| 6 | Infiltrates present around all bronchi and vessels, >10 cells depth | Mild interstitial inflammation, <50% area involved |
| 7 | Infiltrates present around all bronchi and vessels, >10 cells depth | Moderate interstitial inflammation, 50–80% area involved |
| 8 | Infiltrates present around all bronchi and vessels, >10 cells depth | Marked interstitial inflammation, >80% area involved |
Measurement of ex vivo type I and III collagen-α1 expression in fibroblasts.
HFL-1 cells (human lung fibroblast cell line-1) were obtained from ATCC (Manassas, VA) and cultured in DMEM, 10% FBS, and PSG (penicillin 100 U/ml, streptomycin 100 μg/ml, and l-glutamine 2 mmol/l; Invitrogen, Burlington, ON, Canada). Recombinant human IGF-I, porcine active TGF-β1, monoclonal anti-human IGF-I receptor-1 (IGF-IR) antibody and isotype control (mouse IgG1) were obtained from R&D Systems. The TGF-βRI inhibitor SD-208 {2-(5-chloro-2-fluorophenyl)-4[(4-pyridyl)amino]pteridine} was purchased from Tocris Biosciences (Ellisville, MO).
Cells were plated at a density of 0.2 × 106 cells per well in 24-well plates. At the time of plating, the cells were transferred to a low-serum-containing medium (DMEM, 0.5% fetal bovine serum, and PSG) and were maintained in this medium for the duration of the experiment. Cells were treated 24 h after plating with 1) 1 ng/ml of active TGF-β1, 2) 50 ng/ml of hIGF-I, 3) both 1 ng/ml of active TGF-β1 and 50 ng/ml of hIGF-I, 4) BALF obtained at day 3 posttransduction of mice with AdEmpty/TGF-β1, or 5) BALF obtained at day 3 posttransduction of mice with AdhIGF-IB/AdTGF-β1 for another 24 h before isolating RNA. In some experiments cells were pretreated with the inhibitors anti-IGF-IR antibody or mouse IgG1 (10 μg/ml) or SD-208 (200 nM) for 1 h before stimulation with the growth factors or with the BALF for 24 h. At 24 h posttreatment the cells were rinsed once with PBS at 37°C and total RNA was extracted by using a Purelink RNA microkit (Invitrogen) according to the manufacturer's instructions. Total RNA was quantified via a Nanodrop spectrophotometer (Thermo Fisher Scientific, Nepean, ON, Canada). cDNA was generated from 0.5 μg of total RNA by using the RT2 first Strand Kit (C-03, SABiosciences, Frederick, MD) according to manufacturer's instructions. Real-time quantitative PCR was performed using the following TaqMan gene expression assays (Applied Biosystems, Foster City, CA) COL1A1 Hs00164004_m1 for collagen 1, COL3A1 Hs00164103_m1 for collagen 3, and the reference gene Eukaryotic 18SrRNA (part number 4314913E). Samples were run on a 7900HT Fast real-time PCR system (Applied Biosystems) and the cycling parameters were set as follows: 50°C for 2 min, 95°C for 10 min (95°C for 15 s, 60°C for 1 min) × 40 cycles. The data was analyzed with the RQ manager (version 1.2) software.
Measurement of cytokines in BALF.
The BALF samples were analyzed for 22 inflammation-relevant cytokines and chemokines by use of a Luminex 200 apparatus (Applied Cytometry Systems) and a multiplex mouse cytokine/chemokine kit from Millipore according to manufacturer's instructions. The panel included G-CSF, GM-CSF, IFN-γ, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-9, IP-10, KC, MCP-1, MIP-1α, RANTES, and TNF-α. The data were analyzed with the StarStation V.2.3 software from Applied Cytometry Systems. Only cytokines showing changes were presented.
Statistical analysis.
All results are reported as means ± SE. GraphPad Prism 4.03 software was used for all statistical analysis. One-way ANOVA was used to compare the means of all five time points of control or treatment groups with Tukey's posttest correction. A two-tailed unpaired t-test was used to compare the results of control and treatment groups at any one time point. Differences were considered statistically significant at P < 0.05. A sample size of eight animals was chosen for each time point and each treatment arm to allow appropriate processing of samples leaving four animals in each group for each analysis (Fig. 1).
RESULTS
hIGF-IB and TGF-β1(active) transgenes are expressed in mouse lungs.
The hIGF-IB and TGF-β1 mRNA expression were confirmed by RT-PCR of total RNA extracted from mouse lungs at 3, 7, 14, 21, and 42 days posttransduction. Days posttransduction refers to the days after transduction of the AdTGF-β1 vector. For transfection and experimental schematic, see Fig. 1. AdEmpty/AdTGF-β1-transduced mice expressed TGF-β1 mRNA in each mouse at all time points (Fig. 2). In addition, the mice transduced with AdhIGF-IB/AdTGF-β1 expressed both hIGF-IB and TGF-β1 mRNA in each mouse at all of the studied time points (Fig. 2).
Fig. 2.
TGF-β1(active) and hIGF-IB transgene mRNA expression in the lungs of transduced mice. Mice were transduced with AdEmpty/AdTGF-β1 or AdhIGF-IB/AdTGF-β1 for 3, 7, 14, 21, and 42 days. At these times the lungs were collected to measure TGF-β1 and hIGF-IB transgene mRNA expression by RT-PCR (materials and methods). This representative RT-PCR shows mRNA from 2 AdEmpty/AdTGF-β1-transduced mice and adjacent GAPDH and TGF-β1 lanes in lanes 1 and 2 and mRNA from 2 AdhIGF-IB/AdTGF-β1-transduced mice and adjacent GAPDH, TGF-β1, and hIGF-IB in lanes 3 and 4. NC, negative control; PT, positive control for TGF-β1; PI, positive control for hIGF-IB; GAPDH, glyceraldehyde 3-phosphate dehydrogenase (used as an internal control). The black vertical line denotes the groupings of images from different parts of the same gel.
Active TGF-β1 protein was induced and secreted into the BALF of both groups of mice (Fig. 3A) in equivalent amounts. Expression of active TGF-β1 protein was detected at day 3, peaked at day 7, and declined to baseline levels by day 14 (Fig. 3A). The timing and level of TGF-β1 expression were not affected by hIGF-IB expression (Fig. 3A). In addition, based on our previous work where AdhIGF-IB alone was transduced into mouse lungs (23), AdTGF-β1 did not affect the timing or level of AdhIGF-IB expression (Fig. 3B and Ref. 23). hIGF-I protein (IGF-I) was induced and secreted into BALF as early as day 3. By day 7 the production of IGF-I had declined more than 50%, returning to baseline levels by day 14 (Fig. 3B). The level of induction of IGF-I expression is comparable to the levels induced previously (23).
Fig. 3.
Protein expression in BALF of transduced mice. Mice were transduced with AdEmpty/AdTGF-β1 (open bars) or AdhIGF-IB/AdTGF-β1 (solid bars) for 3, 7, 14, 21, and 42 days. At these times BALF was collected to determine TGF-β1 (A) and IGF-I protein levels (B) (see materials and methods). Data are expressed as means ± SE of n = 4 in each group. *P < 0.05 comparing IGF-I levels in AdEmpty/TGF-β1-transduced mice with AdhIGF-IB/AdTGF-β1 mice. There was no statistical difference in TGF-β1 levels between these 2 groups.
Inflammatory cell recruitment in the BALF of both AdEmpty/AdTGF-β1- and AdhIGF-IB/AdTGF-β1-transduced mice.
Lung damage is believed to be caused by inflammatory mediators secreted by infiltrating neutrophils, monocytes, and macrophages. To determine whether IGF-I acting together with TGF-β1 recruited a different number or type of inflammatory cells than TGF-β1 alone, total and differential cell counts were performed on BALF isolated from AdEmpty/AdTGF-β1- and AdhIGF-IB/AdTGF-β1-transduced mice. Table 2 shows that both transduced groups of mice had increased inflammatory cell accumulation over time in the BALF, which returned to the values observed at day 3 after 21 days. Although there were no statistical differences in total cell numbers recruited between the two groups of mice, AdhIGF-IB/AdTGF-β1-transduced mice resulted in a trend toward higher cell recruitment at virtually all of the studied time points compared with AdEmpty/AdTGF-β1-transduced mice (Table 2). Mice transduced with AdhIGF-IB/AdTGF-β1 showed a significant increase in macrophage recruitment in the BALF at every time point except for day 7, an increase in lymphocyte population at days 14 and 21, but significant decreases in neutrophil counts at days 7 and 14 compared with the AdEmpty/AdTGF-β1-transduced mice. These data demonstrate that AdhIGF-IB/AdTGF-β1 transduction induced a significant change in the different leukocyte populations recruited to the lungs at specific time points compared with AdEmpty/AdTGF-β1-transduced mice.
Table 2.
Total and differential cell counts (× 105 cells/ml) in BALF of AdEmpty/AdTGF-β1- and AdhIGF-IB/AdTGF-β1-transduced mice
| Days Posttransduction | 3 | 7 | 14 | 21 | 42 |
|---|---|---|---|---|---|
| Total Cell Count | |||||
| AdEmpty/AdTGF-β1 | 3.12 ± 0.41 | 6.01 ± 1.52 | 5.53 ± 1.60 | 4.73 ± 1.04 | 3.54 ± 0.03 |
| AdhlGF-IB/AdTGF-β1 | 4.63 ± 0.75 | 5.63 ± 0.67 | 10.09 ± 2.47 | 7.68 ± 0.75 | 5.16 ± 0.88 |
| Macrophages | |||||
| AdEmpty/AdTGF-β1 | 1.80 ± 0.06 | 3.40 ± 0.19 | 3.30 ± 0.20 | 4.50 ± 0.15 | 3.42 ± 0.04 |
| AdhlGF-IB/AdTGF-β1 | 3.11 ± 0.12† | 3.30 ± 0.23 | 6.90 ± 0.24† | 5.30 ± 0.08† | 4.99 ± 0.05† |
| Lymphocytes | |||||
| AdEmpty/AdTGF-β1 | 0.75 ± 0.09 | 2.07 ± 0.23 | 2.14 ± 0.31 | 0.48 ± 0.15 | 0.11 ± 0.03 |
| AdhlGF-IB/AdTGF-β1 | 1.18 ± 0.13 | 2.30 ± 0.21 | 3.12 ± 0.23* | 2.35 ± 0.08* | 0.14 ± 0.03 |
| Neutrophils | |||||
| AdEmpty/AdTGF-β1 | 0.54 ± 0.09 | 0.52 ± 0.10 | 0.70 ± 0.01 | 0.00 | 0.00 |
| AdhlGF-IB/AdTGF-β1 | 0.39 ± 0.07 | 0.06 ± 0.01† | 0.04 ± 0.02† | 0.01 ± 0.01 | 0.02 ± 0.02 |
Data are expressed as means ± SE of n = 4 in each group. 0.00: Undetectable.
P < 0.05,
P < 0.01 in AdhIGF-IB/AdTGF-β1- vs. AdEmpty/AdTGF-β1-transduced mice.
Lung histology reveals that hIGF-IB combined with TGF-β1 transgene expression induces lung parenchymal inflammatory cell accumulation.
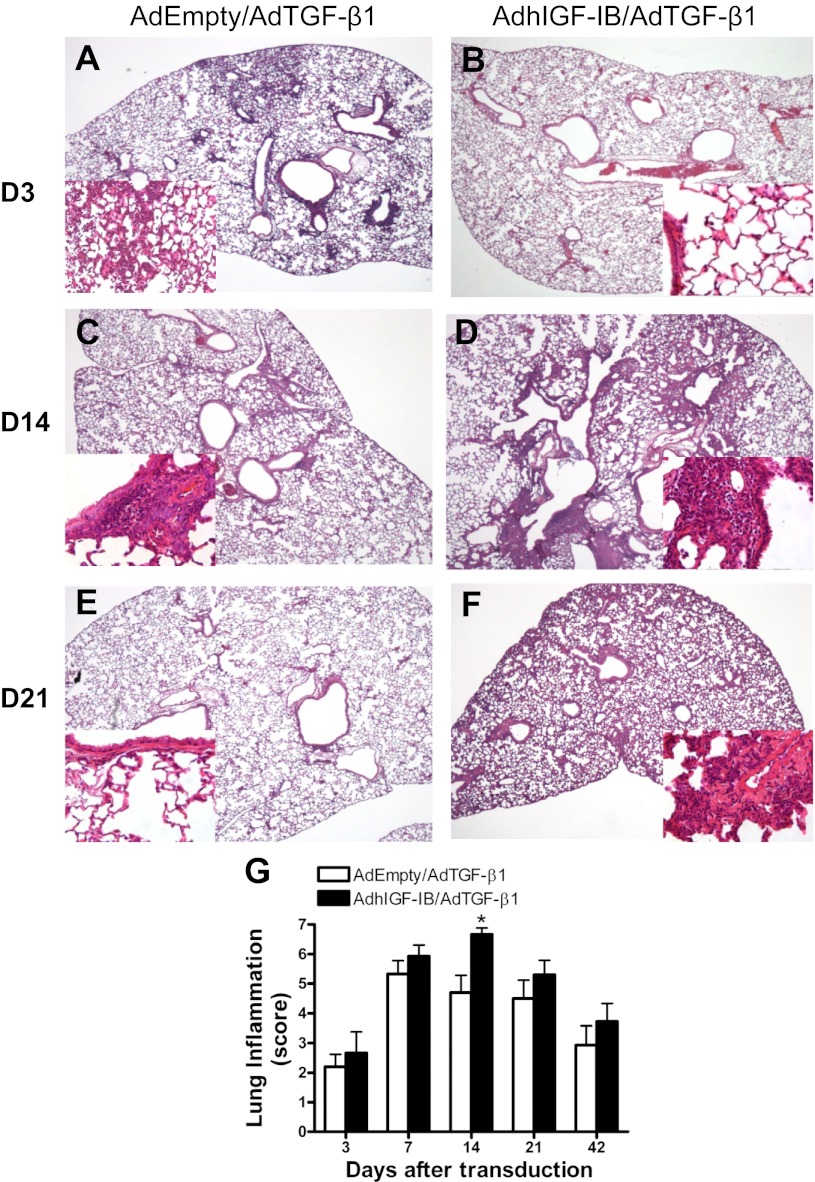
We examined the lung parenchyma for evidence of inflammation using lung histology. Figure 4 shows representative H&E-stained lung sections of AdEmpty/AdTGF-β1- and AdhIGF-IB/AdTGF-β1-transduced mice over time. To quantify the level of inflammation seen in the lung parenchyma, slides were analyzed by a lung pathologist (M. M. Kelly) using a modified inflammation score (materials and methods). As shown, inflammatory cell infiltrates are observed in peribronchial/perivascular and interstitial distributions. Lungs from AdhIGF-IB/AdTGF-β1-transduced mice had more inflammation in the peribronchial and perivascular tissue as well as the interstitial parenchymal areas at virtually all studied time points, reaching statistical significance on day 14 (Fig. 4G; P = 0.013). Most of the inflammatory cells observed in the lung inflammation were lymphocytes (Fig. 4, A–F, higher power insets) and as the days progressed lymphocytes were indeed the majority of the infiltrating cells.
Fig. 4.
Representative photomicrographs of hematoxylin and eosin (H&E)-stained mouse lungs from AdEmpty/AdTGF-β1 or AdhIGF-IB/AdTGF-β1 at different times. Mice were transduced with AdEmpty/AdTGF-β1 or AdhIGF-IB/AdTGF-β1 for 3, 7, 14, 21, and 42 days. The lungs were collected at these times for histology and stained with H&E. Representative lung sections are shown from AdEmpty/AdTGF-β1-transduced mice at day 3 (A), day 14 (C), and day 21 (E) and from AdhIGF-IB/AdTGF-β1-transduced mice at day 3 (B), day 14 (D), and day 21 (F). Inflammatory cell infiltrates are demarcated by blue-staining nuclei and are present in a peribronchial/perivascular and interstitial distribution. Magnification of all sections is ×100. High-power insets highlight areas of inflammatory infiltration in the lungs (×400 magnification). G: lung tissue inflammation grading over time. Lung tissue inflammation grade values are expressed as means ± SE; n = 3–5 at each time point in each treatment group. Lung sections were prepared as described in materials and methods. A lung pathologist blinded to treatment conditions graded the intensity of lung inflammation based on an 8-tier scale as described in materials and methods. *P = 0.0128 (Fisher exact test) comparing AdEmpty/AdTGF-β1 with AdhIGF-IB/AdTGF-β1 at 14 days after transduction.
AdhIGF-IB/AdTGF-β1 transgene expression causes synergistic collagen deposition in the lungs.
To determine whether AdEmpty/AdTGF-β1- and AdhIGF-IB/AdTGF-β1 transduction induced a fibrotic response in the lungs of these mice, we quantified the amount of collagen deposited using Sirius red staining of lung sections. This staining has enabled us to differentially measure the collagen deposited in the peribronchial/perivascular area from that deposited in the lung parenchyma (19). Our data focuses on the lung parenchyma. We have previously shown that mice transduced with AdhIGF-IB alone showed no difference in lung parenchymal collagen content compared with AdEmpty-transduced mice (23). Figure 5, A and D, shows representative Sirius red stains of lungs as seen under direct light microscopy from AdEmpty/AdTGF-β1- and AdhIGF-IB/AdTGF-β1-transduced mice at day 14, respectively. Figure 5, B and E, shows representative Sirius red stains of lungs as seen through a polarized lens from AdEmpty/AdTGF-β1- and AdhIGF-IB/AdTGF-β1-transduced mice at day 14, respectively. Figure 5, C and F, shows representative Sirius red stains of lung sections with the polarized images converted to black and white and then inverted for quantification of dark pixels from AdEmpty/AdTGF-β1- and AdhIGF-IB/AdTGF-β1-transduced mice, respectively, at day 14. The dark pixels in Fig. 5, C and F, represent collagen content within the lung section. Quantification of the Sirius red staining shows a significant increase in the collagen content in lung parenchyma at days 21 and 42 in AdEmpty/AdTGF-β1-transduced mice vs. AdEmpty-transduced mice. In addition, a significant increase in the collagen content in the lung parenchyma is observed at days 14, 21, and 42 in AdhIGF-IB/AdTGF-β1-transduced mice vs. AdEmpty alone (Fig. 5G). Figure 5G confirms that TGF-β1 expression alone did induce pulmonary fibroproliferation in vivo (32). Furthermore, we now show a synergistic increase in the amount of collagen deposited in the lungs in AdhIGF-IB/AdTGF-β1-transduced mice compared with AdEmpty/AdTGF-β1-transduced mice at days 14, 21, and 42 (Fig. 5). This clearly demonstrates that IGF-I synergistically enhances pulmonary fibrosis in vivo when coexpressed with TGF-β1.
Fig. 5.
Collagen quantification by Sirius red staining of lungs transduced with AdEmpty/AdTGF-β1 or AdhIGF-IB/AdTGF-β1 over time. Mice were transduced with AdEmpty/AdTGF-β1 or AdhIGF-IB/AdTGF-β1 for 3, 7, 14, 21, and 42 days. The lungs were harvested at these times and processed to measure collagen content by Sirius red staining. Sirius red-stained sections were observed under polarized microscopy. The tissue illuminated under polarized light reflected collagen associated with the Sirius red stain. Representative lung sections stained with Sirius red stain at day 14 are shown from AdEmpty/AdTGF-β1 (A–C) and AdhIGF-IB/AdTGF-β1 (D–F)-transduced mice. A and D show the images of the Sirius red-stained sections under normal light illumination. B and E show the polarized light Sirius red images. C and F show the Sirius red polarized images obtained after conversion to black and white and then inverted so that the black pixels represent stained collagen. Black pixels are then quantified by using Image J software reflecting collagen content. G: data are expressed as means ± SE of 10 measures of Sirius red-stained and polarized light quantified images of 100 × 100 μm2 areas of lung parenchymal (n = 4 from each group) measured by 2 independent, blinded, individuals. AdhIGF-IB/AdTGF-β1-transduced lungs induced synergistic collagen deposition in the lung parenchyma at days 14, 21, and 42 compared with AdEmpty/AdTGF-β1 transduced mouse lungs (*P < 0.05, **P < 0.01). #P < 0.05 vs. AdEmpty. °P < 0.01 vs. AdEmpty. By contrast, neither AdhIGF-IB nor AdEmpty transduction alone results in any significant collagen induction (23). AdEmpty alone transduced mice are represented in the far left column.
AdhIGF-IB/AdTGF-β1 induced increased α-SMA expression in the lungs compared with AdEmpty/AdTGF-β1-transduced lungs.
A key event in both repair and pathological fibrosis is the activation, proliferation, and differentiation of fibroblasts into myofibroblasts characterized by de novo expression of α-SMA (12). Of note, mice transduced with AdhIGF-IB alone showed no increase in α-SMA expression compared with AdEmpty-transduced mice (23). By contrast, mice transduced with either AdEmpty/AdTGF-β1 or AdhIGF-IB/AdTGF-β1 showed a significant increase in α-SMA expression at days 7 and 14 posttransduction compared with day 3 (P < 0.01, Fig. 6), proving that TGF-β1 itself induces α-SMA expression. Importantly, mice transduced with AdhIGF-IB/AdTGF-β1 expressed significantly more α-SMA than AdEmpty/AdTGF-β1-transduced mice on days 3, 7, and 14 (Fig. 6E). This proves that fibroblast differentiation/proliferation is significantly increased by IGF-I plus TGF-β1 compared with TGF-β1 transduction alone.
Fig. 6.
α-Smooth muscle actin (α-SMA) immunohistochemistry and quantification in AdEmpty/AdTGF-β1 or AdhIGF-IB/AdTGF-β1-transduced mice over time. Mice were transduced with AdEmpty/AdTGF-β1 or AdhIGF-IB/AdTGF-β1 for 3, 7, 14, 21, and 42 days. At these times the lungs were harvested and snap frozen. The frozen lungs were embedded in formaldehyde and processed for histology. The slides were stained with α-SMA antibody (see materials and methods). A and B: representative pictures of α-SMA expression at day 7 (D7) in the lungs of AdEmpty/AdTGF-β1- and AdhIGF-IB/AdTGF-β1-transduced mice, respectively. C and D: representative pictures of α-SMA expression at day 14 (D14) in the lungs of AdEmpty/AdTGF-β1- and AdhIGF-IB/AdTGF-β1-transduced mice, respectively. Brown staining reflects α-SMA. Low-power images are at ×200 magnification; high-power insets in each panel show the stained image magnified at ×400. E: quantification of α-SMA expression. Data are expressed as means ± SE of n = 2- 5 in each group. *P < 0.01 comparing the 2 groups. #P < 0.01 vs. AdEmpty/AdTGF-β1 day 3 and °P < 0.01 vs. AdhIGF-IB/AdTGF-β1 vs. day 3. Of note, AdhIGF-IB-transduced mice showed no increase in α-SMA staining (23).
BALF from AdhIGF-IB/AdTGF-β1-transduced mice induces the transcription of collagen A1 and A3 in fibroblasts ex vivo.
We previously demonstrated that BALF from AdhIGF-IB-transduced mice did not increase collagen mRNA in fibroblasts ex vivo (23). We therefore questioned whether IGF-I would add to the fibroproliferative process when expressed in an environment where fibrosis is already initiated by TGF-β1. Human fibroblasts (HFL-1 cells) were incubated with mouse BALF recovered at day 3 posttransduction of both AdhIGF-IB/AdTGF-β1- and AdEmpty/AdTGF-β1-transduced mice. BALF from both transduced groups significantly increased collagen A1 and A3 induction in HFL-1 cells (Fig. 7, P < 0.01) compared with control. Of note, the BALF of AdhIGF-IB/AdTGF-β1-transduced mice induced significantly higher levels of collagen A1 and A3 compared with the BALF of AdEmpty/AdTGF-β1-transduced mice (P < 0.01). These data suggest that IGF-I induction in the presence of TGF-β1 increases collagen induction in a synergistic manner (Fig. 7). Interestingly, a TGF-β1 receptor I kinase inhibitor (SD-208) was able to significantly reduce the amount of collagen A1 and A3 induced by the BALF of AdEmpty/AdTGF-B1 to control values. However, this inhibitor was not able to completely abolish the amount of collagen A1 induced by AdhIGF-IB/AdTGF-β1 vs. control (Fig. 7, P = 0.0016), showing that the increase in collagen is also dependent on the action of IGF-I. On the other hand, although the use of an IGF-IR blocking antibody ex vivo was able to block the effects of IGF-I-spiked BALF from PBS-treated mice, it did not reduce the amount of collagen A1 or A3 induced by the BALF of AdhIGF-IB/AdTGF-β1-transduced mice (data not shown), suggesting that interaction of IGF-I with its receptor is not directly responsible for this increase. This points to a secondary factor being induced by IGF-I, which would stimulate HFL-1 cells to produce collagen A1 and A3 or to a synergistic action of IGF-I and TGF-β1 through an IGF-I receptor-independent mechanism.
Fig. 7.
Collagen A1 and collagen A3 induction measured by quantitative RT-PCR in human fibroblasts stimulated ex vivo with BALF from AdEmpty/AdTGF-β1 or AdhIGF-IB/AdTGF-β1-transduced mice. Human fibroblasts (HFL-1 cells) were incubated with BALF from control mice (mice transduced with AdGFP, control) or mice transduced with AdEmpty/AdTGF-β1 or AdhIGF-IB/AdTGF-β1 for 3 days to measure the induction of collagen A1 and A3 (see materials and methods). In some experiments the HFL-1 cells were pretreated with SD-208 (200 nM, a TGF-β1 receptor I kinase inhibitor) for 1 h before stimulation with BALF from transduced mice. Data are expressed as the mean fold RNA induction ± SE of n = 3–6 in each group. *P < 0.01 vs. control Collagen A1, #P < 0.01 vs. control collagen A3, **P < 0.01 vs. AdEmpty/AdTGF-β1 collagen A1, ##P < 0.01 vs. AdEmpty/AdTGF-β1 Collagen A3. °P < 0.05 vs. AdEmpty/AdTGF-β1 or control Collagen A1.
AdhIGF-IB/AdTGF-β1 vs. AdEmpty/AdTGF-β1-transduced mice induce differential cytokine production in BALF.
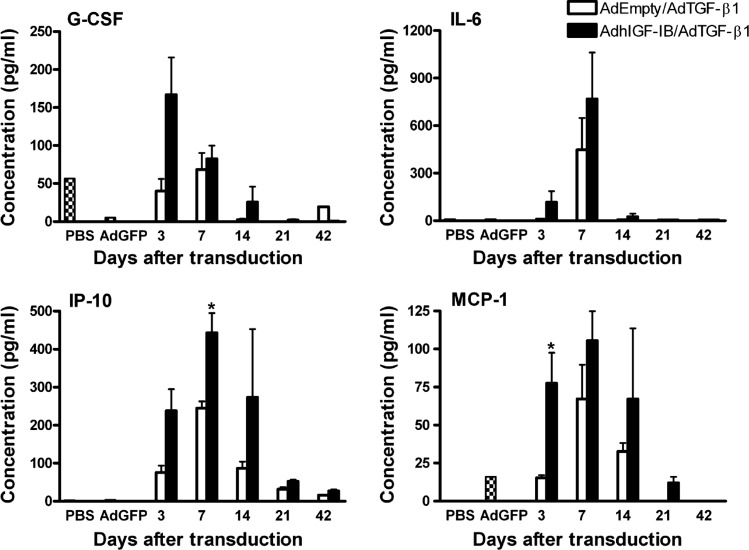
Since inhibition of the IGF-I receptor did not directly reduce fibroblast induction of collagen ex vivo, we asked whether other mediators known to be involved in collagen induction are differentially expressed in the BALF of the AdhIGF-IB/AdTGF-β1-transduced mice compared with AdEmpty/AdTGF-β1. We measured 22 cytokines/chemokines in the BALF of mice. G-CSF, IFN-γ, IP-10, MCP-1, KC, MIP-1α, RANTES, and TNF-α were increased in both group of mice, of which only G-CSF, IL-6, IP-10, and MCP-1 were differentially expressed between AdhIGF-IB/AdTGF-β1- and AdEmpty/AdTGF-β1-transduced mice (Fig. 8). MCP-1 and IP-10 levels reached statistical significance on days 3 and 7, respectively. Both have been previously identified as important factors in PF (33, 34), suggesting that IGF-I may contribute to fibrosis through upregulation of these cytokines.
Fig. 8.
Cytokine/chemokine levels in BALF from AdhIGF-IB/AdTGF-β1 vs. AdEmpty/AdTGF-β1-transduced mice. Mice were transduced with AdEmpty/AdTGF-β1 or AdhIGF-IB/AdTGF-β1 for 3, 7, 14, 21, and 42 days. At these times BALF was collected to determine cytokine and chemokine levels by use of a Luminex 200 apparatus (see materials and methods). Expression of G-CSF, IL-6, IP-10, and MCP-1 are shown. Data are expressed as means ± SE of n = 3 in each group. *P < 0.05 comparing BALF from AdhIGF-IB/AdTGF-β1 vs. AdEmpty/AdTGF-β1. PBS represents cytokine levels in BALF from PBS intratracheally injected control mice and AdGFP reflects cytokine levels from AdGFP-transduced control mice.
DISCUSSION
In this study we questioned whether IGF-I expressed sequentially with TGF-β1 influenced pulmonary fibroproliferation in a noninjury mouse model of PF. We delivered AdTGF-β1 into the lungs of mice already transduced with AdhIGF-IB. At all studied time points, AdhIGF-IB/AdTGF-β1-transduction resulted in a trend toward more inflammatory cell recruitment than AdEmpty/AdTGF-β1-transduction. Significantly increased numbers of macrophages and lymphocytes were detected in the BALF and significantly more lymphocytes in the lung tissue of AdhIGF-IB/AdTGF-β1-transduced mice. Interestingly, in the lungs of these mice, more inflammation, collagen deposition, and α-SMA expression were also observed. In addition, the BALF of AdhIGF-IB/AdTGF-β1-transduced mice induced more collagen A1 and A3 expression in cultured fibroblasts ex vivo than the BALF of AdEmpty/AdTGF-β1-transduced mice. These data reveal that although IGF-I is unable to initiate fibroproliferation on its own (23), it can synergistically contribute to fibroproliferation when induced just prior to TGF-β1 expression.
Since the timing of growth factor presence in the lungs may be important in the fibroproliferative process, we attempted to emulate the peak induction of IGF-I and TGF-β1(active) relative to one another by examining the timing of growth factor presence in BALF isolated from patients with fibroproliferative ARDS. In these patients, peak IGF-I levels appear either before or very near peak levels in TGF-β1 (data not shown) (25, 30). This timing has also been shown in animal models of lung fibrosis (24, 26). Thus we transduced AdhIGF-IB 2 days prior to transducing mice with AdTGF-β1 (Fig. 1) and the transduced protein levels for IGF-I peaked prior to TGF-β1 protein levels as shown in Fig. 3. In the AdhIGF-IB/AdTGF-β1-transduced mice, IGF-I and TGF-β1 proteins both peak between 3 and 7 days posttransduction and are essentially back to background levels by 14 days posttransduction. The IGF-I and TGF-β1 time course are particularly significant to the data presented here because all of the fibroproliferative processes that we observed, namely inflammatory cell recruitment, collagen deposition rate, lung α-SMA induction, and cytokine induction, peaked by day 14 posttransduction. Many of these differences abate after day 14, strongly supporting the direct effects on fibroproliferation of the two induced growth factors (IGF-I and TGF-β1) and the synergy induced by IGF-I when expressed with TGF-β1.
Fibroblasts/myofibroblasts are critical cells in the fibroproliferative process because they produce collagen (and other matrix elements) that are deposited in the lung parenchyma. Figure 6 shows a synergistic increase in α-SMA immunostaining in the lungs through day 14 posttransduction with AdhIGF-IB/AdTGF-β1 compared with AdEmpty/AdTGF-β1. As previously indicated, a key event in both repair and pathological fibrosis is the activation of fibroblasts and their proliferation and differentiation into myofibroblasts characterized by the de novo expression of α-SMA (12). The peak of α-SMA immunostaining follows the peak of IGF-I and active TGF-β1 levels and coincides directly with the peak levels of lung inflammation (Fig. 4). This could be explained by the fact that IGF-I (8, 9) and active TGF-β1 (21) enhance the transition from fibroblast to myofibroblast and enhance myofibroblast proliferation. It is noteworthy that α-SMA immunostaining in both groups of transduced mice decreases after day 14 posttransduction but collagen deposition does continue, albeit at a lesser rate. It is possible that the myofibroblasts either decrease in number (increase in apoptosis, see paragraph below) or mature to a fibroblast-like state making less α-SMA when the influence of IGF-I and TGF-β1 disappears over time (i.e., after day 14).
IPF results from failure to regulate the fibroproliferative process leading to excessive extracellular matrix production and accumulation in lung tissue. Some authors support the concept of the “apoptosis paradox,” whereby alveolar epithelial cells appear to be undergoing apoptosis and the myofibroblasts appear to be resistant to apoptosis. In fact, blockade of the IGF pathway with an antibody against the IGF-I receptor increased fibroblast apoptosis and resulted in resolution of PF (10). In addition, the IGF-I pathway, acting through the type 1 IGF-receptor, repressed apoptosis of lung fibroblasts but not lung epithelial cells (30). It is possible that IGF-I is not only involved in myofibroblast proliferation but also delays myofibroblasts apoptosis which, when IGF-I disappears, myofibroblasts numbers decrease. Interestingly, by day 21 and day 42 when IGF-I protein levels are low, lung tissue apoptosis increased when examined by TUNEL assay (data not shown), suggesting that IGF-I plays a role in delaying cell apoptosis.
Our data also strongly suggests that the synergistic increase in lung fibroproliferation is dependent on secondary factor(s) activated by IGF-I since an IGF-I receptor inhibitor did not decrease collagen induction ex vivo. It is notable that a direct interaction cannot be entirely discarded because our results are based on BALF from mice stimulated for 3 days, time for many factors unmeasured to be induced. In addition, the increase in collagen induction is only partially mediated by TGF-β1 interaction with its receptor (Fig. 7) since the synergistic induction of collagen A1 is not entirely inhibited by the inhibitor of the TGF-β1 receptor. We therefore looked for secondary mediators by examining cytokines that were induced at a greater level by AdhIGF-IB/AdTGF-β1 than with AdEmpty/AdTGF-β1 alone. Indeed, MCP-1 (monocyte chemoattractant protein 1) and IP-10 (CXCL10) were found to be significantly elevated at day 3 and day 7, respectively, in the AdhIGF-IB/AdTGF-β1-transduced mice compared with the AdEmpty/AdTGF-β1-transduced mice (Fig. 8).
MCP-1 is commonly upregulated in the lungs of patients with inflammatory conditions such as ARDS, PF, and systemic sclerosis (1, 13, 15, 29). It also correlates with the level of lung injury in the later stages of ARDS (15). MCP-1 probably plays a role in the late phase of fibrogenesis since, in a mouse model, overexpressing an anti-MCP-1 gene at days 10-14, but not at earlier times after intratracheal instillation of bleomycin, resulted in decreased DNA damage, apoptosis, and PF (17). Here, we show that the sequential expression of IGF-I and TGF-β1 is essential to induce a significant increase (fivefold) in the level of MCP-1 on day 3 posttransduction, reaching a maximal level of expression on day 7, a timing similar to what is observed in another mouse lung fibrosis model (33). This rise in MCP-1 correlates with the increased recruitment of macrophages in the BALF of the AdhIGF-IB/AdTGF-β1-transduced mice. Even though IGF-I has not been shown to induce MCP-1 on its own, this result clearly demonstrates a synergistic amplification of the expression of MCP-1 driven by the conditioning provided by AdhIGF-IB/AdTGF-β1 transduction.
IP-10, on the other hand, has been shown to be involved in the resolution phase of lung repair by limiting the development of fibrosis following a bleomycin challenge. After bleomycin the maximum mRNA expression of IP-10 was observed at days 3 and 5, returning to basal levels in the second and third week posttreatment (34). Even though we were able to demonstrate a significant increase in the level of IP-10 protein in the AdhIGF-IB/AdTGF-β1 vs. AdEmpty/AdTGF-β1 group only on day 7, the sustained trend toward an elevated expression of IP-10 in the AdhIGF-IB/AdTGF-β1 group points to IGF-I, or a downstream product of IGF-I, as an important factor in IP-10 upregulation in this model. Importantly, these results also suggest that IGF-I preferentially expressed in the BALF of fibrotic patients is not responsible for their concomitant decreased expression of IP-10 as suggested previously (28) since, in the experiments presented here, IGF-I expression induced an increase in IP-10. Furthermore, our results shed doubts on the role of IP-10 in suppressing fibrosis, since AdhIGF-IB/AdTGF-β1-transduced mice had not only increased IP-10 levels in the BALF but also more extensive fibroproliferation compared with the AdEmpty/AdTGF-β1-transduced mice.
A potential limitation of our approach is the adenovirus itself. Adenoviruses have been previously shown to induce an innate immune response (18, 39). Muruve et al. (27) showed that intravenous infection with various adenovirus vectors increased both the mRNA and protein levels of MIP-2, MCP-1, and IP-10 in the liver. In our experimental setting, we minimized the adenoviral effects by using a lower dose of adenovirus (100 times less) compared with the original study of AdTGF-β1-induced fibrosis (32). In addition, we used a local adenoviral infection vs. a potentially more inflammatory systemic route of administration (27). Furthermore, as previously shown (23), in our hands and at the dose used empty adenovirus does not cause fibroproliferation and is only responsible for a minimal and transient recruitment of inflammatory cells into the BALF (23). In addition, when we transduced mice with AdGFP, the adenovirus did not have any significant effect on the 22 cytokines measured in the BALF at day 3 posttransduction compared with mice that received control buffer (PBS) (Fig. 8 and data not shown). Therefore, we are satisfied that the vector is not responsible for the inflammatory and fibroproliferative responses we are describing in this study.
For many years, IGF-I has been suspected to play a role in pulmonary fibrosis but direct in vivo evidence of its role did not exist. In our model, IGF-I clearly and synergistically participates, with TGF-β1, in the progression of fibroproliferation. There is no doubt that these two growth factors induce other cytokines and growth factors that may contribute to the fibroproliferative process. Dissecting these molecular pathways should help to better understand the exact mechanisms of the fibroproliferative response as well as identify novel therapeutic avenues for the treatment of fibrotic lung diseases.
GRANTS
B. Winston was funded by an Alberta Heritage Foundation for Medical Research (AHFMR) scholarship. M. M. Kelly was funded by an AHFMR clinical investigator award. This research was funded by grants from the Canadian Institutes of Health Research, The Lung Association of Alberta and NWT, the Canadian Intensive Care Foundation, by an equipment and infrastructure grant from the Canadian Foundation for Innovation (CFI) and the Alberta Science and Research Authority, and a grant from the Department of Critical Care Medicine, Faculty of Medicine, University of Calgary.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.A., A.N., C.L., M.M.K., J.F.W., and A.J. performed experiments; G.A., A.N., C.L., M.M.K., J.F.W., and B.W.W. analyzed data; G.A. and B.W.W. interpreted results of experiments; G.A. and C.L. prepared figures; G.A. and C.L. drafted manuscript; G.A. and B.W.W. edited and revised manuscript; B.W.W. conception and design of research; B.W.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank The Snyder Translational Laboratory in Critical Care Medicine for use of their facilities, Carol Gwozd for technical assistance, and Darren Xu, a Heritage Youth Research Summer Program (HYRS) student, for collaboration in this project.
REFERENCES
- 1. Antoniades HN, Neville-Golden J, Galanopoulos T, Kradin RL, Valente AJ, Graves DT. Expression of monocyte chemoattractant protein 1 mRNA in human idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA 89: 5371–5375, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aston C, Jagirdar J, Lee TC, Hur T, Hintz RL, Rom WN. Enhanced insulin-like growth factor molecules in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 151: 1597–1603, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Blanchet MR, Israel-Assayag E, Cormier Y. Modulation of airway inflammation and resistance in mice by a nicotinic receptor agonist. Eur Respir J 26: 21–27, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Bloor CA, Knight RA, Kedia RK, Spiteri MA, Allen JT. Differential mRNA expression of insulin-like growth factor-1 splice variants in patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am J Respir Crit Care Med 164: 265–272, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Bonniaud P, Margetts PJ, Kolb M, Haberberger T, Kelly M, Robertson J, Gauldie J. Adenoviral gene transfer of connective tissue growth factor in the lung induces transient fibrosis. Am J Respir Crit Care Med 168: 770–778, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Bonniaud P, Margetts PJ, Kolb M, Schroeder JA, Kapoun AM, Damm D, Murphy A, Chakravarty S, Dugar S, Higgins L, Protter AA, Gauldie J. Progressive transforming growth factor beta1-induced lung fibrosis is blocked by an orally active ALK5 kinase inhibitor. Am J Respir Crit Care Med 171: 889–898, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med 331: 1286–1292, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Chetty A, Cao GJ, Nielsen HC. Insulin-like growth factor-I signaling mechanisms, type I collagen and alpha smooth muscle actin in human fetal lung fibroblasts. Pediatr Res 60: 389–394, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Chetty A, Faber S, Nielsen HC. Epithelial-mesenchymal interaction and insulin-like growth factors in hyperoxic lung injury. Exp Lung Res 25: 701–718, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Choi JE, Lee SS, Sunde DA, Huizar I, Haugk KL, Thannickal VJ, Vittal R, Plymate SR, Schnapp LM. Insulin-like growth factor-I receptor blockade improves outcome in mouse model of lung injury. Am J Respir Crit Care Med 179: 212–219, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coker RK, Laurent GJ, Jeffery PK, du Bois RM, Black CM, McAnulty RJ. Localisation of transforming growth factor beta1 and beta3 mRNA transcripts in normal and fibrotic human lung. Thorax 56: 549–556, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 63: 21–29, 1990 [PubMed] [Google Scholar]
- 13. Emad A, Emad V. Elevated levels of MCP-1, MIP-alpha and MIP-1 beta in the bronchoalveolar lavage (BAL) fluid of patients with mustard gas-induced pulmonary fibrosis. Toxicology 240: 60–69, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Frankel SK, Moats-Staats BM, Cool CD, Wynes MW, Stiles AD, Riches DW. Human insulin-like growth factor-IA expression in transgenic mice promotes adenomatous hyperplasia but not pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 288: L805–L812, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, Kunkel SL, Walz A, Hudson LD, Martin TR. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med 154: 602–611, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Harrison NK, Cambrey AD, Myers AR, Southcott AM, Black CM, du Bois RM, Laurent GJ, McAnulty RJ. Insulin-like growth factor-I is partially responsible for fibroblast proliferation induced by bronchoalveolar lavage fluid from patients with systemic sclerosis. Clin Sci (Lond) 86: 141–148, 1994 [DOI] [PubMed] [Google Scholar]
- 17. Inoshima I, Kuwano K, Hamada N, Hagimoto N, Yoshimi M, Maeyama T, Takeshita A, Kitamoto S, Egashira K, Hara N. Anti-monocyte chemoattractant protein-1 gene therapy attenuates pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 286: L1038–L1044, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Jooss K, Chirmule N. Immunity to adenovirus and adeno-associated viral vectors: implications for gene therapy. Gene Ther 10: 955–963, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11: 447–455, 1979 [DOI] [PubMed] [Google Scholar]
- 20. Katzenstein AL, Zisman DA, Litzky LA, Nguyen BT, Kotloff RM. Usual interstitial pneumonia: histologic study of biopsy and explant specimens. Am J Surg Pathol 26: 1567–1577, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Khalil N, Xu YD, O'Connor R, Duronio V. Proliferation of pulmonary interstitial fibroblasts is mediated by transforming growth factor-beta1-induced release of extracellular fibroblast growth factor-2 and phosphorylation of p38 MAPK and JNK. J Biol Chem 280: 43000–43009, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Krein PM, Sabatini PJ, Tinmouth W, Green FH, Winston BW. Localization of insulin-like growth factor-I in lung tissues of patients with fibroproliferative acute respiratory distress syndrome. Am J Respir Crit Care Med 167: 83–90, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Leger C, Ni A, Andonegui G, Wong JF, Mowat C, Winston BW. Adenovirus-mediated gene transfer of hIGF-IB in mouse lungs induced prolonged inflammation but no fibroproliferation. Am J Physiol Lung Cell Mol Physiol 298: L492–L500, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Maeda A, Hiyama K, Yamakido H, Ishioka S, Yamakido M. Increased expression of platelet-derived growth factor A and insulin-like growth factor-I in BAL cells during the development of bleomycin-induced pulmonary fibrosis in mice. Chest 109: 780–786, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Martinet Y, Menard O, Vaillant P, Vignaud JM, Martinet N. Cytokines in human lung fibrosis. Arch Toxicol Suppl 18: 127–139, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol 40: 362–382, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muruve DA, Barnes MJ, Stillman IE, Libermann TA. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther 10: 965–976, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Pignatti P, Brunetti G, Moretto D, Yacoub MR, Fiori M, Balbi B, Balestrino A, Cervio G, Nava S, Moscato G. Role of the chemokine receptors CXCR3 and CCR4 in human pulmonary fibrosis. Am J Respir Crit Care Med 173: 310–317, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Schmidt K, Martinez-Gamboa L, Meier S, Witt C, Meisel C, Hanitsch LG, Becker MO, Huscher D, Burmester GR, Riemekasten G. Bronchoalveoloar lavage fluid cytokines and chemokines as markers and predictors for the outcome of interstitial lung disease in systemic sclerosis patients. Arthritis Res Ther 11: R111, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schnapp LM, Donohoe S, Chen J, Sunde DA, Kelly PM, Ruzinski J, Martin T, Goodlett DR. Mining the acute respiratory distress syndrome proteome: identification of the insulin-like growth factor (IGF)/IGF-binding protein-3 pathway in acute lung injury. Am J Pathol 169: 86–95, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sime PJ, Xing Z, Foley R, Graham FL, Gauldie J. Transient gene transfer and expression in the lung. Chest 111: 89S–94S, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 100: 768–776, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith RE, Strieter RM, Zhang K, Phan SH, Standiford TJ, Lukacs NW, Kunkel SL. A role for C-C chemokines in fibrotic lung disease. J Leukoc Biol 57: 782–787, 1995 [DOI] [PubMed] [Google Scholar]
- 34. Tager AM, Kradin RL, LaCamera P, Bercury SD, Campanella GS, Leary CP, Polosukhin V, Zhao LH, Sakamoto H, Blackwell TS, Luster AD. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am J Respir Cell Mol Biol 31: 395–404, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Uh ST, Inoue Y, King TE, Jr, Chan ED, Newman LS, Riches DW. Morphometric analysis of insulin-like growth factor-I localization in lung tissues of patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 158: 1626–1635, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Voynow JA, Fischer BM, Malarkey DE, Burch LH, Wong T, Longphre M, Ho SB, Foster WM. Neutrophil elastase induces mucus cell metaplasia in mouse lung. Am J Physiol Lung Cell Mol Physiol 287: L1293–L1302, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Wang Q, Wang Y, Hyde DM, Gotwals PJ, Koteliansky VE, Ryan ST, Giri SN. Reduction of bleomycin induced lung fibrosis by transforming growth factor beta soluble receptor in hamsters. Thorax 54: 805–812, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol 2: 103–121, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zaiss AK, Muruve DA. Immune responses to adeno-associated virus vectors. Curr Gene Ther 5: 323–331, 2005 [DOI] [PubMed] [Google Scholar]