Abstract
Chorioamnionitis and antenatal corticosteroids mature the fetal lung functionally but disrupt late-gestation lung development. Because Sonic Hedgehog (Shh) signaling is a major pathway directing lung development, we hypothesized that chorioamnionitis and antenatal corticosteroids modulated Shh signaling, resulting in an altered fetal lung structure. Time-mated ewes with singleton ovine fetuses received an intra-amniotic injection of lipopolysaccharide (LPS) and/or maternal intramuscular betamethasone 7 and/or 14 days before delivery at 120 days gestational age (GA) (term = 150 days GA). Intra-amniotic LPS exposure decreased Shh mRNA levels and Gli1 protein expression, which was counteracted by both betamethasone pre- or posttreatment. mRNA and protein levels of fibroblast growth factor 10 and bone morphogenetic protein 4, which are important mediators of lung development, increased 2-fold and 3.5-fold, respectively, 14 days after LPS exposure. Both 7-day and 14-day exposure to LPS changed the mRNA levels of elastin (ELN) and collagen type I alpha 1 (Col1A1) and 2 (Col1A2), which resulted in fewer elastin foci and increased collagen type I deposition in the alveolar septa. Corticosteroid posttreatment prevented the decrease in ELN mRNA and increased elastin foci and decreased collagen type I deposition in the fetal lung. In conclusion, fetal lung exposure to LPS was accompanied by changes in key modulators of lung development resulting in abnormal lung structure. Betamethasone treatment partially prevented the changes in developmental processes and lung structure. This study provides new insights into clinically relevant prenatal exposures and fetal lung development.
Keywords: lung development, lung maturation, secondary septation, bronchopulmonary dysplasia
bronchopulmonary dysplasia (BPD), a disease of impaired lung development, is the most common adverse lung outcome of preterm birth (2, 30). BPD is associated with fetal lung inflammation, which can be initiated by chorioamnionitis, an intrauterine bacterial infection of the placental membranes and amniotic fluid that is often clinically silent (21). Chorioamnionitis can induce a potentially harmful inflammatory response in the immature fetal lungs that disrupts lung septation and vascular development, leading to a decreased lung surface area (16).
Antenatal corticosteroids are given to mothers at risk of imminent preterm birth to induce lung maturation in the fetus, which increases neonatal survival but does not decrease BPD (4, 14). Because the incidence of chorioamnionitis is ∼60% for very preterm babies, the administration of maternal antenatal corticosteroids in the presence of chorioamnionitis is common and standard of care (3). Although antenatal corticosteroids cause functional lung maturation, they also can inhibit lung development (54). As a result, a large number of premature infants are exposed in utero to both pro- and anti-inflammatory stimuli that each alter normal fetal lung development and might predispose the infants to the development of BPD (10). The molecular mechanisms by which chorioamnionitis and antenatal corticosteroids influence these lung developmental processes are largely unknown.
Sonic Hedgehog (Shh) signaling is critical for lung development since Shh-null mice have hypoplastic lungs and die from respiratory failure (32). During lung development, Shh expression is localized to the epithelium and activates Gli transcriptional activators Gli1, Gli2, and Gli3 (5). The Shh pathway regulates the expression of lung growth factors such as fibroblast growth factor 10 (FGF10) and bone morphogenetic protein 4 (BMP4), which both mediate branching and myofibroblast differentiation (52).
We hypothesized that chorioamnionitis and/or antenatal corticosteroids modulate Shh signaling to alter fetal lung structural development. We evaluated this signaling pathway after lipopolysaccharide (LPS)-induced chorioamnionitis in a 120-day gestational age (GA) preterm lamb model during a stage of early alveolar septation. Fetal sheep were exposed in utero to intra-amniotic LPS from gram-negative bacteria and/or antenatal betamethasone (Beta), a corticosteroid used clinically to induce lung maturation (28, 41). We correlated Shh signaling components with markers for lung damage [heat shock protein 70 (HSP70)], cell proliferation (Ki67), and changes in the lung structural proteins elastin and collagen, which are crucial for alveolar septation (9, 25, 49).
METHODS
Animal model and sampling protocol.
All studies were approved by the Animal Ethics Committees at The University of Western Australia and Cincinnati Children's Hospital Medical Center (animal ethics protocol RA/3/100/830). The experimental design of this study was published previously (29). Time-mated Merino ewes with singleton fetuses were randomly assigned to one of six treatment groups to receive an intra-amniotic injection of LPS (10 mg Escherichia coli 055:B5, Sigma Chemical, St. Louis, MO) and/or an intramuscular injection of Beta [Celestone Soluspan, Schering-Plough, North Ryde, New South Wales (NSW), Australia, 0.5 mg/kg maternal weight] and/or an equivalent injection of saline for control animals at 107 days and/or 114 days GA. All ewes in this study received a single intramuscular injection of 150 mg medroxyprogesterone acetate (Depo-Provera, Kenral, NSW, Australia) at 100 days GA to prevent preterm birth induced by Beta treatment. Lambs were surgically delivered at 120 days GA (term = 150 days GA) and euthanized after birth. Lung tissue from the right lower lobe (RLL) was snap frozen, and the right upper lobe (RUL) was inflation fixed in 10% buffered formalin for 24 h.
RNA extraction and real-time PCR.
Total RNA was extracted from frozen lung tissue of the RLL by using the SV Total RNA Isolation system (Z3100, Promega, Madison, WI) according to the manufacturer's instructions. Genomic DNA contamination was removed by treatment with RQ1 DNase (M610A, Promega) and the RNA was tested for the presence of genomic GAPDH, a housekeeping gene. Briefly, PCR amplification for the detection of genomic DNA was performed with DNA Taq Polymerase (M124B, Promega) at 95°C for 5 min followed by 40 cycles at 95°C for 30 s, 55°C for 45 s, and 72°C for 30 s. Total RNA was used as a template. PCR products were analyzed on a 1.5% agarose gel. Total RNA was reverse transcribed with the First Strand cDNA synthesis kit (4379012001, Roche Applied Science, Mannheim, Germany) according to manufacturer's instructions by using anchored oligo primers. Primers for real-time PCR (RT-PCR) were constructed based on published ovine or bovine cDNA sequences (Table 1). Dilution experiments were performed to ensure similar PCR amplification efficiency of the primers. RT-PCR reactions were performed in duplicate with the LightCycler 480 SYBR Green I Master mix (4707516001, Roche-Applied) on a LightCycler 480 Instrument according to the manufacturer's instructions. RT-PCR results were normalized to cyclophilin A, a housekeeping gene, and mean fold changes in mRNA expression were calculated by the ΔΔCt method (33).
Table 1.
Primers used for RT-PCR
| Gene | Sequence (5′-3′) | Amplicon Size, bp | Tm | Accession Code (RefSeq) |
|---|---|---|---|---|
| Col1A1 | ||||
| Forward | GAAGAAGACATCCCACCAG | 125 | 60°C | NM_001034039.1 |
| Reverse | GTCCTTAAGTTCGTCGCAG | |||
| Col1A2 | ||||
| Forward | GGCTCAACCTGAAGACATCC | 150 | 59°C | EF114225.1 |
| Reverse | TCTCCTACCCAGACATGCTTC | |||
| ELN | ||||
| Forward | CCAAATTCGGTGCTGCTG | 144 | 60°C | NM_175772.1 |
| Reverse | ACTCCAACACCTGGGACTC | |||
| Shh | ||||
| Forward | ACTGGAGCGGACCGGCTGAT | 82 | 68°C | XM_614193.3 |
| Reverse | CCGGCCACTGGCTCATCAC | |||
| Gli1 | ||||
| Forward | AATCTGAAGACGCACCTG | 137 | 60°C | NM_001099000.1 |
| Reverse | GTAGGGCTTCTCATTGGA | |||
| Gli2 | ||||
| Forward | CCTGGAGAACCTGAAGAC | 147 | 60°C | NM_001192250.1 |
| Reverse | GATGTAGGGTTTCTCGTTGG | |||
| Gli3 | ||||
| Forward | AGAAGCCTCACAAATGCAC | 197 | 60°C | XM_002686896.1 |
| Reverse | ACACATATGGTTTCTCGTTGG | |||
| FGF10 | ||||
| Forward | TGCCCGTACAGTATCCTG | 220 | 60°C | NM_001009230.1 |
| Reverse | GCCACATACATTTGCCTC | |||
| BMP4 | ||||
| Forward | ACCACGAAGAACATCTGGAG | 173 | 61°C | NM_001110277.1 |
| Reverse | TTATACGATGAAAGCCCTGC | |||
| Cyclophilin A | ||||
| Forward | TTATAAAGGTTCCTGCTTTCACAGAA | 93 | 60°C | NM_178320.2 |
| Reverse | ATGGACTTGCCACCAGTACCA |
Tm, melt temperature; RefSeq, reference sequence.
Protein extraction and ELISA of HSP70.
Frozen RLL lung tissue was homogenized (PRO Quick Connect Generators part no. 02-07095; PRO Scientific, Oxford, CT) in ice-cold RIPA buffer (R0278, Sigma Aldrich) containing 0.1% protease inhibitors (p9599, Sigma Aldrich) and subsequently centrifuged at 12 × relative centrifugal force for 5 min at 4°C (31). Heat shock protein 70 (HSP70) was measured with an R&D DuoSet ELISA development kit (human/mouse/rat total HSP70: DYC1663, R&D Systems, Minneapolis, MN) according to manufacturer's instructions. HSP70 protein concentrations were calculated per kilogram body weight.
Immunohistochemistry.
Paraffin-embedded RUL lung sections (4 μm, transverse) were stained for Ki67 (M7240, DAKO, Glostrup, Denmark), Gli1 (ab49314, Abcam, Cambridge, UK) and BMP4 (sc-6896, Santa Cruz Biotechnology, Santa Cruz, CA). Briefly, the sections were deparaffinized in an ethanol series and endogenous peroxidase activity was blocked by incubation with 0.5% H2O2 in 1 × PBS (pH 7.4). Antigen retrieval was performed by incubating the sections in heated citrate buffer (10 mM, pH 6.0) for 30 min. To block specific binding, the slides were incubated with 20% normal goat serum in PBS. Sections were incubated overnight at 4°C with the diluted primary antibody (Ki67 1:50, Gli1 1:500, BMP4 1:500). After incubation with the appropriate secondary antibody, immunostaining was enhanced with Vectastain ABC Peroxidase Elite kit (PK-6200, Vector Laboratories, Burlingame, CA) and stained with nickel sulfate-diaminobenzidine. Subsequently, the sections were rinsed in Tris-saline and incubated with Tris-cobalt. After being counterstained with 0.1% Nuclear Fast Red, the sections were washed, dehydrated, and coverslipped. All slides were stained at the same time under the same conditions.
Evaluation was performed by light microscopy (Axioskop 40, Zeiss, Germany) with LeicaQWin Pro v.3.4.0 software (Leica Microsystems, Germany). Alveolar Ki67 and Gli1 staining was scored by blinded observers with a semiquantitative scoring system: 1, little staining; 2, some staining; and 3, heavy staining. BMP4 staining was semiquantitatively scored in three representative bronchioli using Image J software (W. S. Rasband, Image J, US National Institutes of Health, Bethesda, MD) and represented as a percentage of the entire bronchiole surface area.
Elastin and collagen staining.
Elastin and collagen stainings were performed each on four paraffin sections of the RUL per animal (4 μm, transverse). For the visualization of elastin, the sections were deparaffinized in an ethanol series and incubated in Hart's staining solution [70% ethanol, 10% Weigerts Resorcine-Fuchsine (2E 030, Chroma, Münster, Germany) and 2% hydrochloric acid] overnight at room temperature. After rinse with water, the sections were incubated in 0.25% acetic acid for 3 min at room temperature. Subsequently the sections were washed and dehydrated. For the detection of collagen fibers, the sections were deparaffinized and incubated in 0.2% phosphomolybdic acid for 5 min. Sections were placed in a Sirius red solution for 90 min in the dark. After rinse with 0.01 M HCl for 3 min, the sections were washed, dehydrated, and coverslipped. Evaluation was performed by light microscopy (Zeiss, Axioskop 40) with LeicaQWin Pro v.3.4.0 software. The number of elastin foci and the percentage of collagen fibers in the total lung surface area were quantified by using four paraffin sections per animal, 12 representative images per section across septa at ×200 magnification by a blinded observer using specialized LeicaQWin Pro v.3.4.0 software.
Data analysis.
Results are given as means ± SE. The groups were compared by one-way ANOVA with Dunnett's or Tukey's test for post hoc analysis as appropriate. Statistical analysis was performed by GraphPad Prism v5.0. Significance was accepted at P < 0.05.
RESULTS
Lung damage and cell proliferation.
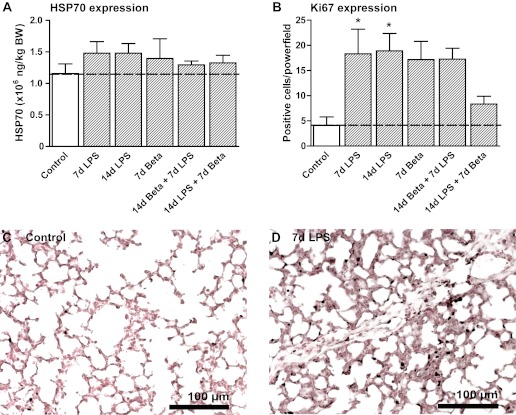
Characteristics of the animals and the pulmonary inflammatory and maturation response to LPS-induced chorioamnionitis and/or antenatal corticosteroids were reported previously (29). Lung injury due to the exposure to LPS was assessed by measurement of HSP70 in the lung tissue. HSP70 protein expression was not increased in any of the experimental groups compared with control (Fig. 1A). To assess cell proliferation, lung tissue was stained for Ki67, a marker of mitotic cells. There were increased proliferating cells, which by morphological evaluation could be discerned as immune cells, 7 and 14 days after the exposure to LPS (Fig. 1B). Representative images are shown for controls (Fig. 1C) and 7-day LPS-exposed lungs (Fig. 1D).
Fig. 1.
Lung injury and cell proliferation. A: protein levels of heat shock protein 70 (HSP70) did not change in homogenates of LPS and/or betamethasone (Beta) exposed fetal lungs. B: the number of Ki67-positive cells in the alveoli increased after LPS exposure. Pretreatment and particularly posttreatment with Beta partially prevented this increase. Alveolar Ki67 expression in controls (C) and 7-day (7d) LPS-exposed animals (D). BW, body weight; 14d, 14-day. *P < 0.05 vs. controls by 1-way ANOVA with Tukey's post hoc test.
Changes in Shh signaling after intrauterine LPS exposure.
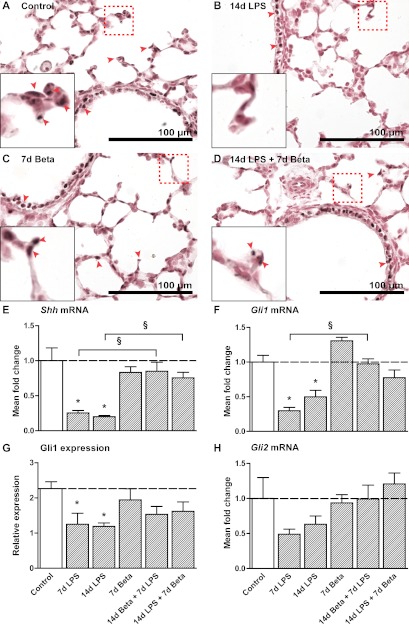
Shh mRNA levels decreased to less than 25% of control value after 7 and 14 days of LPS exposure (Fig. 2E). Beta pre- or posttreatment prevented the decrease in Shh mRNA. In addition, we analyzed the expression of Gli1 and Gli2, which are components of the Shh pathway. Gli1 mRNA expression had a similar decreased expression at 7 and 14 days following LPS exposure (Fig. 2F). Gli1 protein expression was mainly detected in the bronchiolar and alveolar epithelium in controls (Fig. 2A). Exposure to LPS for 7 or 14 days selectively decreased Gli1 protein expression in the alveolar epithelium (Fig. 2B). Beta pre- or posttreatment again prevented this decline (Fig. 2G). Representative images are shown for controls (Fig. 2A), 14-day LPS-exposed lungs (Fig. 2B), 7-day-Beta-exposed lungs (Fig. 2C), and 14-day LPS and 7-day Beta-exposed lungs (Fig. 2D). Gli2 mRNA expression had similar trends toward declines after LPS exposure (Fig. 2H).
Fig. 2.
Inhibition of the Sonic Hedgehog (Shh) pathway. Gli1 expression in alveolar and bronchial tissue as seen in controls (A), 14 days after LPS exposure (B), 7 days after Beta treatment (C), and after a combination of 14d LPS followed by posttreatment with Beta (D). E: expression of Shh was decreased after 7d and 14d LPS exposure. Both pre- and posttreatment with Beta normalized Shh mRNA levels compared with controls. F: Gli1 mRNA levels were decreased in LPS-exposed lungs. Both pre- and posttreatment with Beta normalized Gli1 mRNA levels compared with controls. G: Gli1 protein expression as scored in the alveoli in lung sections decreased after 7d and 14d LPS exposure. Pre- or posttreatment could partially attenuate this decrease. H: levels of Gli2 mRNA in experimental groups did not differ significantly from controls. *P < 0.05 vs. controls and §P < 0.05 between experimental groups by 1-way ANOVA with Tukey's post hoc test.
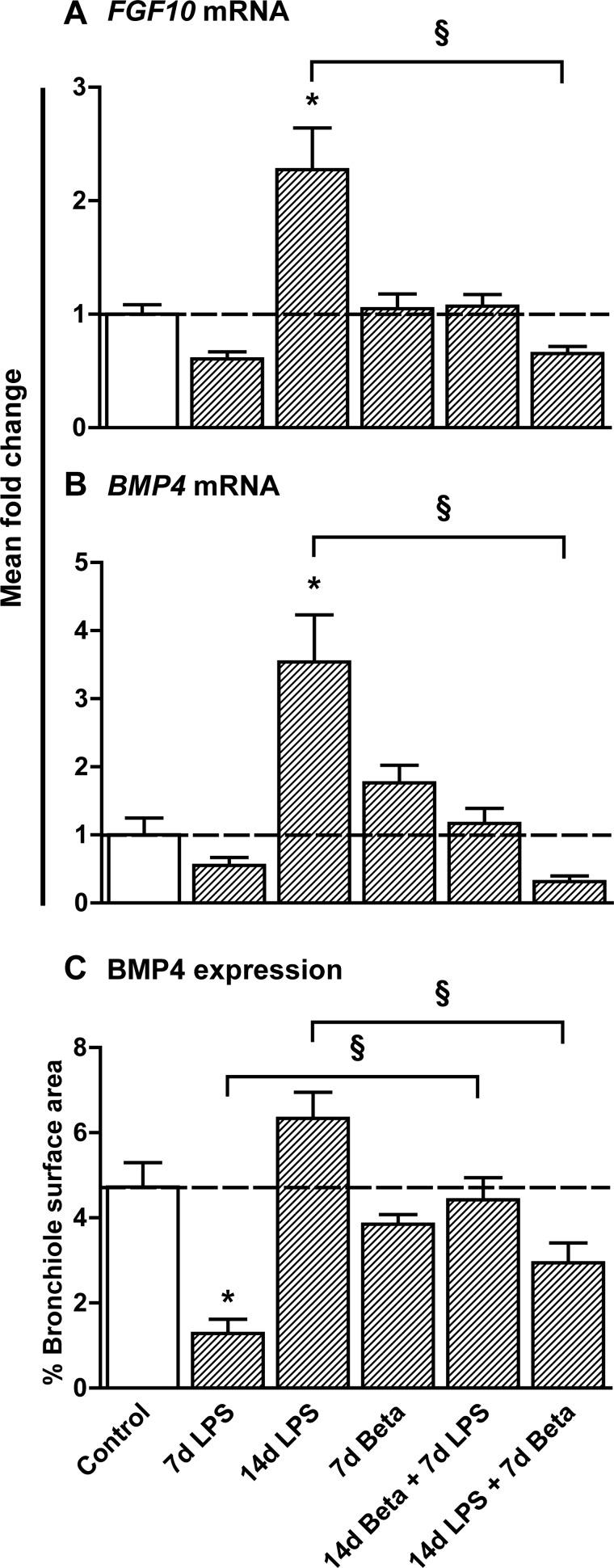
Levels of FGF10 and BMP4, which are two important Shh-regulated mediators of lung development, were also assessed. Both FGF10 and BMP4 mRNA increased 14 days after LPS exposure, by 2-fold and 3.5-fold, respectively (Fig. 3, A and B). Exposure to Beta after LPS exposure lowered FGF10 and BMP4 mRNA. BMP4 protein expression was mainly localized in the bronchial epithelial cells, which corresponds with recent data obtained in adult lung tissue (35, 42). Immunohistochemical analysis of BMP4 expression in bronchioli revealed that BMP4 was decreased 7 days after LPS exposure and showed a trend toward increased expression at 14 days after LPS exposure (Fig. 3C). Treatment with Beta before LPS exposure prevented the decrease in BMP4 levels seen after 7-day LPS exposure only. Treatment with Beta 7 days after the LPS exposure decreased BMP4 levels.
Fig. 3.
Expression of fibroblast growth factor 10 (FGF10) and bone morphogenetic protein 4 (BMP4). A: mRNA levels of FGF10 were increased 2-fold 14 days after LPS exposure. Posttreatment with Beta normalized FGF10 levels compared with controls. B: mRNA levels of BMP4 were increased 3.5-fold 14 days after LPS exposure. Posttreatment with Beta normalized BMP4 levels compared with controls. C: immunohistochemical analysis of BMP4 expression in bronchioli decreased after 7 days of LPS exposure but showed a recovery of BMP4 14 days after LPS exposure. Pretreatment with Beta before LPS exposure prevented a drop in BMP4 levels. *P < 0.05 vs. controls and §P < 0.05 between experimental groups by 1-way ANOVA with Tukey's post hoc test.
Expression of lung structural proteins.
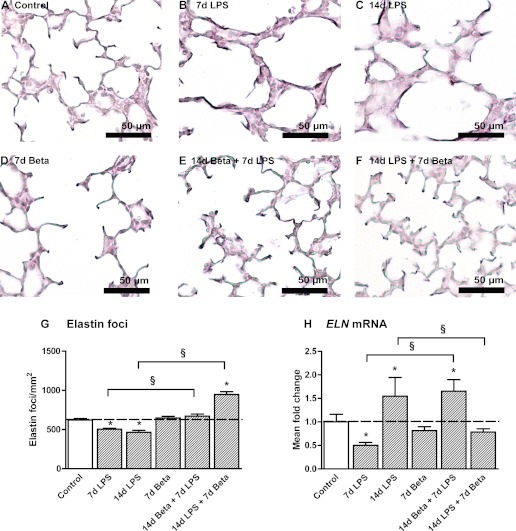
Elastin foci were quantified in lung sections as an assessment of secondary septation. Representative images are shown for control (Fig. 4A), 7-day LPS (Fig. 4B), 14-day LPS (Fig. 4C), 7-day Beta (Fig. 4D), 14-day Beta + 7-day LPS (Fig. 4E), and 14-day LPS + 7-day Beta (Fig. 4F) lambs. The number of elastin foci decreased in the lungs of LPS-exposed groups lambs (Fig. 4G). Pretreatment with Beta minimized the decrease in elastin foci. Posttreatment with Beta after LPS exposure increased elastin foci in the fetal lung. ELN mRNA first decreased by 50% 7 days after LPS exposure followed by a 50% increase 14 days after LPS exposure compared with controls (Fig. 4H). Beta pretreatment followed by 7-day LPS exposure increased ELN mRNA by 50% compared with controls. No change in ELN mRNA was detected in the 14-day LPS + 7-day Beta animals.
Fig. 4.
Altered expression of elastin. Elastin deposition in the alveoli of control (A), 7d LPS (B), 14d LPS (C), 7d Beta (D), 14d Beta + 7d LPS (E), and 14d LPS + 7d Beta (F) lambs. G: the number of elastin foci per mm2 tissue was decreased in LPS-exposed lambs. Pretreatment with Beta could prevent a significant decrease in elastin foci. Posttreatment with Beta after LPS exposure increased the number of elastin foci. H: ELN mRNA levels decreased by 50% in 7d LPS lambs but increased in 14d LPS-exposed lambs. Pretreatment with Beta before LPS exposure resulted in a 60% increase in ELN mRNA levels. Posttreatment with Beta prevented the increase in ELN mRNA. *P < 0.05 vs. controls and §P < 0.05 between experimental groups by 1-way ANOVA with Tukey's post hoc test.
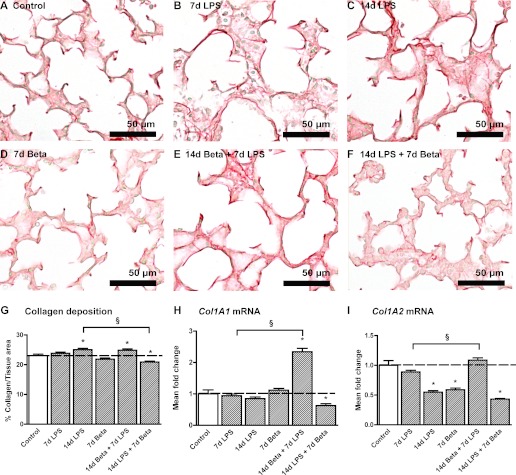
Representative images of the collagen deposition in the fetal lungs are shown for controls (Fig. 5A), 7-day LPS (Fig. 5B), 14-day LPS (Fig. 5C), 7-day Beta (Fig. 5D), 14-day Beta + 7-day LPS (Fig. 5E), and 14-day LPS + 7-day Beta (Fig. 5F) lambs. Col1A1 mRNA increased more than twofold after combined 14-day Beta and 7-day LPS exposure (Fig. 5H). Collagen type I deposition increased after 14 days of LPS exposure (Fig. 5G). In contrast, 14-day LPS exposure followed by Beta posttreatment resulted in a significant decrease of collagen type I deposition. Beta pretreatment followed by 7-day LPS exposure increased collagen type I deposition in the fetal lung. In contrast, 14-day LPS exposure followed by Beta posttreatment significantly decreased mRNA levels of Col1A1 similar to the collagen deposition in the lung. mRNA levels of collagen type I gene Col1A2 decreased to 50% after 14 days of LPS exposure irrespective of Beta posttreatment (Fig. 5I). Interestingly, 7-day Beta exposure only also decreased Col1A2 mRNA by ∼40%. Further computerized morphometric analyses are reported elsewhere and did not show differences (29).
Fig. 5.
Collagen I expression. Collagen I deposition in the alveoli of control (A), 7d LPS (B), 14d LPS (C), 7d Beta (D), 14d Beta + 7d LPS (E), and 14d LPS + 7d Beta (F) lambs. G: 14d LPS exposure and 14d Beta + 7d LPS exposure increased the percentage of collagen type I tissue in the fetal lung. Posttreatment with Beta after LPS exposure decreased collagen type I expression. H: mRNA levels of Col1A1 increased 2-fold after combined exposure to 14d Beta + 7d LPS; 14d LPS exposure followed by Beta treatment decreased Col1A1 mRNA by 40%. I: Col1A2 mRNA levels decreased in 14d LPS, 7d Beta and 14d LPS + 7d Beta lambs by 50%. *P < 0.05 vs. controls and §P < 0.05 between experimental groups using a 1-way ANOVA with Tukey's post hoc test.
DISCUSSION
LPS exposure leads to changes in Shh signaling in the fetal lung.
In the context of developmental biology research, less is known about later fetal lung development than early organogenesis. Later lung development is, however, an area of human biology where clinical care interfaces with development since survival after very preterm birth at 60% of gestation is now frequent. We used an animal model with similarities to late-gestation human lung development to test two very common clinical exposures, chorioamnionitis and antenatal steroids. We used 7- and 14-day intrauterine periods of exposure based on our previous findings of a delay in alveolar development after 7 and 14 days of LPS-induced inflammation (25, 54). Here we show that fetal lung exposure to LPS-induced inflammation (29) is accompanied by changes in the Shh pathway, which is crucial for early lung development. In addition, we demonstrated that a maternal intramuscular injection of Beta attenuated the effects of LPS on this developmental pathway. We therefore provide some molecular insights into the observational data from clinical practice that maternal corticosteroids are beneficial despite the inflammation of chorioamnionitis (20).
Exposure to intra-amniotic LPS has been shown to cause severe lung inflammation and damage leading to structural changes in the fetal lung that mimic pulmonary changes seen in BPD patients (26). Although in this study we did not measure an increased expression of HSP70, an indicator of oxidative-stress mediated lung damage, previous work from our group showed that LPS-induced chorioamnionitis causes fetal lung injury as early as 5 h after the exposure (26). Tissue remodeling was further characterized by increased proliferation seen up to 14 days after the LPS exposure and maturation of alveolar type II cells (26, 29).
As evidence is accumulating that the Shh pathway is involved during aberrant lung development and disease (24, 51), we asked whether intra-amniotic LPS exposure altered Shh signaling in the fetal lung. Recently, LPS was shown to downregulate Shh in vitro in pulmonary microvascular endothelial cells (55). In our study, LPS strongly downregulated mRNA levels of Shh and its signaling components Gli1 and Gli2 in the ovine fetal lung. LPS exposure also selectively decreased Gli1 protein expression in the distal epithelial tips where Shh signaling in the lung is mainly localized (57). Shh expression can be induced by retinoic acid (40), which is decreased in babies who develop BPD (44). The downregulation of Shh is in line with a previous study from our group, in which LPS-induced chorioamnionitis decreased retinoic acid in fetal sheep lungs (25). Therefore, the LPS-induced decrease of retinoic acid may have decreased Shh mRNA.
The decreases in mRNA and protein expression of Shh signaling pathway components were accompanied by two- to threefold increases in FGF10 and BMP4 expression 14 days after LPS exposure, following a slight decrease 7 days after LPS exposure. The initial decrease in FGF10 expression, which has also been measured in the lung tissue of infants with BPD (8), might be due to the activation of TLR2 and 4 by LPS, which can suppress FGF10 through binding of NF-κB to the FGF10 promoter (7). Since FGF10 induces BMP4 expression in the developing lung (52), the inhibition of FGF10 may indirectly decrease BMP4 expression. The continuous suppression of Shh measured at 7 and 14 days after LPS exposure, which normally downregulates FGF10 (6), may have caused FGF10 and BMP4 levels to rise.
The changes in these pivotal developmental pathways were accompanied by changes in mRNA levels and deposition of structural proteins that are known to direct alveolar septation (9, 25, 49). In a normally developing lung, focal expression of elastin identifies sites for alveolar budding (13). In the lungs of the 7-day LPS-exposed lambs, mRNA levels of ELN and the numbers of elastin foci decreased. Persistent exposure to LPS did not only result in less elastin foci but also increased collagen deposition along the alveolar wall. These observations of dysregulated elastin and collagen deposition in the fetal lung are consistent with ventilation-induced (1, 9, 12) and inflammation-induced (25, 27) animal models of BPD and histology reports of BPD patients (46, 48). Although Shh signaling has been implicated in the activation of fibroblasts and production of extracellular matrix (ECM) proteins such as collagen (19, 23, 51), Shh seems to act mainly through regulation of FGF10 to direct ECM deposition in the developing lung. Shh expression at the pulmonary epithelial tips controls FGF10 expression, which in turn controls bud size and shape (37). Both overexpression and inhibition of FGFs lead to inhibition of lung branching and alterations in ECM protein expression (15, 38, 45).
Betamethasone and lung development.
Antenatal corticosteroids are routinely administered to mothers who are at risk of preterm birth to mature the fetal organs (4). A secondary benefit may be suppression of inflammation (4). Antenatal steroids also reduce adverse neonatal outcome after preterm birth associated with chorioamnionitis (20), which constitute the majority of early-gestational preterm births (4). The effect of these combined pro- and anti-inflammatory stimuli on pathways that are crucial for the developing lung are, however, unknown.
Recently, we showed that Beta treatment before the LPS exposure suppressed fetal lung inflammation by an unknown priming or conditioning mechanism of the fetal immune system (29). As such little pulmonary damage was inflicted by the exposure to LPS and no changes in the developmental pathways that we studied were detected. Very little is known about the effect of maternal corticosteroids on Shh, FGF10, and BMP4. Corticosteroids can inhibit Shh-mediated neural development and as such can have a detrimental effect on the neonatal developing brain (22). We found that maternal corticosteroids alone did not change the expression of these factors in the fetal lung compared with controls. Moreover, the effects of LPS on these factors were neutralized by maternal Beta, irrespective of whether Beta was given 7 days before or after LPS.
At the lung structural level, corticosteroid treatment before LPS exposure could mitigate the decrease in elastin foci and increase in ELN mRNA levels. Corticosteroid posttreatment even increased elastin foci, which is in line with previous reports showing that corticosteroids can stimulate tropoelastin production in a dose-dependent manner (34, 39), most likely through a TGF-β3 mediated mechanism (56). Here we show that corticosteroid treatment can counteract the negative effects of the LPS exposure on elastin deposition.
Furthermore, treatment with only Beta 7 days before delivery decreased Col1A2 mRNA levels. Whether this resulted in a decreased collagen deposition in a later stage remains to be investigated. However, combined exposure to 7-day Beta treatment with 14-day LPS exposure did decrease collagen deposition. Several studies have shown beneficial effects of corticosteroid treatment on attenuating fibrotic processes following lung injury (17, 36, 50). Preterm infants at risk of BPD patients showed a significant reduction in markers of collagen synthesis after prenatal corticosteroid treatment up to 7 days after administration (18, 43). Surprisingly, corticosteroid treatment before the inflammatory stimulus stimulated collagen deposition although little pulmonary inflammation was noticed in these animals (29). Brenner et al. (11) demonstrated in vitro that corticosteroids do not uniformly suppress the fibrotic activity of lung fibroblasts in vitro. This could explain our contradictory results and the diverse outcomes of lung morphology and function of BPD patients after corticosteroid treatment.
In this study, we modeled a common clinical exposure to provide new insights into the effects of exposures on fetal lung development (47, 54). However, there are limitations, such as exposures at different time points, for different intervals and to a single dose or repeated doses of corticosteroids during fetal development may have different outcomes. The effects of pro- and anti-inflammatory stimuli on the developing lung are clearly complex. It remains to be determined whether the observed changes are the result of direct or indirect effects of LPS and Beta with these developmental pathways. On the basis of these results, intervention studies using inhibitors of the Shh pathway [e.g., cyclopamine (53)] would be helpful. The developing lung is plastic in that it is continually changing over gestation. Because chorioamnionitis is often clinically silent, the duration of exposure of the lung to chorioamnionitis and the subsequent inflammatory response are unknown. It is generally unknown whether antenatal maternal corticosteroids are administered before or after the onset of chorioamnionitis (4).
In conclusion, this report shows that LPS-induced chorioamnionitis can disturb Shh signaling during early alveolar lung development, which is partially mitigated by Beta exposure. Therefore, this report provides some insights into the complicated interactions that can alter lung structure during the maturation phase of lung development where clinical intervention may already occur.
GRANTS
This study was supported by NIH HD-57869 (S. G. Kallapur) from the National Institutes of Health, the National Health and Medical Research Council of Australia, the Women and Infants Research Foundation, Western Australia, Veni BWK 016.096.141 from the Dutch Scientific Research Organization and the Research School for Oncology and Developmental Biology (GROW), Maastricht University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.J.C., E.K., I.N., J.J.P., G.R.P., M.W.K., J.P.N., S.G.F., S.G.K., A.H.J., and B.W.K. conception and design of research; J.J.C., E.K., I.N., J.J.P., G.R.P., M.W.K., J.P.N., S.G.F., S.G.K., and A.H.J. performed experiments; J.J.C., E.K., and J.P.C. analyzed data; J.J.C., E.K., and B.W.K. interpreted results of experiments; J.J.C. and E.K. prepared figures; J.J.C., E.K., and B.W.K. drafted manuscript; J.J.C., E.K., I.N., J.J.P., G.R.P., M.W.K., J.P.N., J.P.C., S.G.F., S.G.K., A.H.J., and B.W.K. edited and revised manuscript; J.J.C., E.K., I.N., J.J.P., G.R.P., M.W.K., J.P.N., S.G.F., S.G.K., A.H.J., and B.W.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Monique Willems, Dennis Kruk, Nynke van den Hoogen, Richard Dalton, Joe Derwort, Masatoshi Saito, Clare Berry, Carryn McLean, Shaofu Li, and Jennifer Henderson for excellent technical support.
Present address of I. Nitsos and G. R. Polglase: The Ritchie Centre, Monash Institute of Medical Research, Melbourne, NSW, Australia.
REFERENCES
- 1. Allison BJ, Crossley KJ, Flecknoe SJ, Davis PG, Morley CJ, Harding R, Hooper SB. Ventilation of the very immature lung in utero induces injury and BPD-like changes in lung structure in fetal sheep. Pediatr Res 64: 387–392, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Bancalari E, Claure N, Sosenko IR. Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin Neonatol 8: 63–71, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Been J, Degraeuwe P, Kramer B, Zimmermann L. Antenatal steroids and neonatal outcome after chorioamnionitis: a meta-analysis. BJOG 118: 113–122, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Behrman RE, Stith Butler A, editors. Preterm Birth: Causes, Consequences and Prevention. Washington, DC: National Academies Press, 2007 [PubMed] [Google Scholar]
- 5. Bellusci S, Furuta Y, Rush MG, Henderson R, Winnier G, Hogan BL. Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development 124: 53–63, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 124: 4867–4878, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Benjamin JT, Carver BJ, Plosa EJ, Yamamoto Y, Miller JD, Liu JH, van der Meer R, Blackwell TS, Prince LS. NF-kappaB activation limits airway branching through inhibition of Sp1-mediated fibroblast growth factor-10 expression. J Immunol 185: 4896–4903, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benjamin JT, Smith RJ, Halloran BA, Day TJ, Kelly DR, Prince LS. FGF-10 is decreased in bronchopulmonary dysplasia and suppressed by Toll-like receptor activation. Am J Physiol Lung Cell Mol Physiol 292: L550–L558, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Bland RD, Xu L, Ertsey R, Rabinovitch M, Albertine KH, Wynn KA, Kumar VH, Ryan RM, Swartz DD, Csiszar K, Fong KS. Dysregulation of pulmonary elastin synthesis and assembly in preterm lambs with chronic lung disease. Am J Physiol Lung Cell Mol Physiol 292: L1370–L1384, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Bourbon J, Boucherat O, Chailley-Heu B, Delacourt C. Control mechanisms of lung alveolar development and their disorders in bronchopulmonary dysplasia. Pediatr Res 57: 38R–46R, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Brenner RE, Felger D, Winter C, Christiansen A, Hofmann D, Bartmann P. Effects of dexamethasone on proliferation, chemotaxis, collagen I, and fibronectin-metabolism of human fetal lung fibroblasts. Pediatr Pulmonol 32: 1–7, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Brew N, Hooper SB, Allison BJ, Wallace MJ, Harding R. Injury and repair in the very immature lung following brief mechanical ventilation. Am J Physiol Lung Cell Mol Physiol 301: L917–L926, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Burri PH. Structural aspects of postnatal lung development — alveolar formation and growth. Biol Neonate 89: 313–322, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Carlo WA, McDonald SA, Fanaroff AA, Vohr BR, Stoll BJ, Ehrenkranz RA, Andrews WW, Wallace D, Das A, Bell EF, Walsh MC, Laptook AR, Shankaran S, Poindexter BB, Hale EC, Newman NS, Davis AS, Schibler K, Kennedy KA, Sanchez PJ, Van Meurs KP, Goldberg RN, Watterberg KL, Faix RG, Frantz ID, 3rd, Higgins RD. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks' gestation. JAMA 306: 2348–2358, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chailley-Heu B, Boucherat O, Barlier-Mur AM, Bourbon JR. FGF-18 is upregulated in the postnatal rat lung and enhances elastogenesis in myofibroblasts. Am J Physiol Lung Cell Mol Physiol 288: L43–L51, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Collins JJ, Kallapur SG, Knox CL, Nitsos I, Polglase GR, Pillow JJ, Kuypers E, Newnham JP, Jobe AH, Kramer BW. Inflammation in fetal sheep from intra-amniotic injection of Ureaplasma parvum. Am J Physiol Lung Cell Mol Physiol 299: L852–L860, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Corbel M, Lagente V, Theret N, Germain N, Clement B, Boichot E. Comparative effects of betamethasone, cyclosporin and nedocromil sodium in acute pulmonary inflammation and metalloproteinase activities in bronchoalveolar lavage fluid from mice exposed to lipopolysaccharide. Pulm Pharmacol Ther 12: 165–171, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Crofton PM, Shrivastava A, Wade JC, Stephen R, Kelnar CJH, McIntosh N, Lyon AJ. Effects of dexamethasone treatment on bone and collagen turnover in preterm infants with chronic lung disease. Pediatr Res 48: 155–162, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Ding H, Zhou D, Hao S, Zhou L, He W, Nie J, Hou FF, Liu Y. Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J Am Soc Nephrol 23: 801–813, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goldenberg RL, Andrews WW, Faye-Petersen OM, Cliver SP, Goepfert AR, Hauth JC. The Alabama preterm birth study: corticosteroids and neonatal outcomes in 23- to 32-week newborns with various markers of intrauterine infection. Am J Obstet Gynecol 195: 1020–1024, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 371: 75–84, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gulino A, De Smaele E, Ferretti E. Glucocorticoids and neonatal brain injury: the hedgehog connection. J Clin Invest 119: 243–246, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jung IH, Jung DE, Park YN, Song SY, Park SW. Aberrant Hedgehog ligands induce progressive pancreatic fibrosis by paracrine activation of myofibroblasts and ductular cells in transgenic zebrafish. PLoS One 6: e27941, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katoh Y, Katoh M. Hedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr Mol Med 9: 873–886, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Kramer BW, Albertine KH, Moss TJ, Nitsos I, Ladenburger A, Speer CP, Newnham JP, Jobe AH. All-trans retinoic acid and intra-amniotic endotoxin-mediated effects on fetal sheep lung. Anat Rec (Hoboken) 291: 1271–1277, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kramer BW, Kramer S, Ikegami M, Jobe AH. Injury, inflammation, and remodeling in fetal sheep lung after intra-amniotic endotoxin. Am J Physiol Lung Cell Mol Physiol 283: L452–L459, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Kramer BW, Ladenburger A, Kunzmann S, Speer CP, Been JV, van Iwaarden JF, Zimmermann LJ, Gantert M, Garnier Y. Intravenous lipopolysaccharide-induced pulmonary maturation and structural changes in fetal sheep. Am J Obstet Gynecol 200: 195.e1–10, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Kramer BW, Moss TJ, Willet KE, Newnham JP, Sly PD, Kallapur SG, Ikegami M, Jobe AH. Dose and time response after intraamniotic endotoxin in preterm lambs. Am J Respir Crit Care Med 164: 982–988, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Kuypers E, Collins JJ, Kramer BW, Ofman G, Nitsos I, Pillow JJ, Polglase GR, Kemp MW, Newnham JP, Gavilanes AW, Nowacki R, Ikegami M, Jobe AH, Kallapur SG. Intra-amniotic LPS and antenatal betamethasone: inflammation and maturation in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol 302: L380–L389, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laughon M, Bose C, Allred EN, O'Shea TM, Ehrenkranz RA, Van Marter LJ, Leviton A. Antecedents of chronic lung disease following three patterns of early respiratory disease in preterm infants. Arch Dis Child Fetal Neonatal Ed 96: F114–F120, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee AJ, Lambermont VA, Pillow JJ, Polglase GR, Nitsos I, Newnham JP, Beilharz MW, Kallapur SG, Jobe AH, Kramer BW. Fetal responses to lipopolysaccharide-induced chorioamnionitis alter immune and airway responses in 7-week-old sheep. Am J Obstet Gynecol 204: 364.e17–24, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet 20: 58–61, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Loehle M, Schwab M, Kadner S, Maner KM, Gilbert JS, Brenna JT, Ford SP, Nathanielsz PW, Nijland MJ. Dose-response effects of betamethasone on maturation of the fetal sheep lung. Am J Obstet Gynecol 202: 186.e1–7, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Masterson JC, Molloy EL, Gilbert JL, McCormack N, Adams A, O'Dea S. Bone morphogenetic protein signalling in airway epithelial cells during regeneration. Cell Signal 23: 398–406, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Miller M, Cho JY, McElwain K, McElwain S, Shim JY, Manni M, Baek JS, Broide DH. Corticosteroids prevent myofibroblast accumulation and airway remodeling in mice. Am J Physiol Lung Cell Mol Physiol 290: L162–L169, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol 8: 1083–1086, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Perl AK, Gale E. FGF signaling is required for myofibroblast differentiation during alveolar regeneration. Am J Physiol Lung Cell Mol Physiol 297: L299–L308, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pierce RA, Mariencheck WI, Sandefur S, Crouch EC, Parks WC. Glucocorticoids upregulate tropoelastin expression during late stages of fetal lung development. Am J Physiol Lung Cell Mol Physiol 268: L491–L500, 1995 [DOI] [PubMed] [Google Scholar]
- 40. Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 75: 1401–1416, 1993 [DOI] [PubMed] [Google Scholar]
- 41. Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 3: CD004454, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Rosendahl A, Pardali E, Speletas M, Ten Dijke P, Heldin CH, Sideras P. Activation of bone morphogenetic protein/Smad signaling in bronchial epithelial cells during airway inflammation. Am J Respir Cell Mol Biol 27: 160–169, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Saarela T, Risteli J, Koivisto M. Effects of short-term dexamethasone treatment on collagen synthesis and degradation markers in preterm infants with developing lung disease. Acta Paediatr 92: 588–594, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Shenai JP, Chytil F, Stahlman MT. Vitamin A status of neonates with bronchopulmonary dysplasia. Pediatr Res 19: 185–188, 1985 [DOI] [PubMed] [Google Scholar]
- 45. Srisuma S, Bhattacharya S, Simon DM, Solleti SK, Tyagi S, Starcher B, Mariani TJ. Fibroblast growth factor receptors control epithelial-mesenchymal interactions necessary for alveolar elastogenesis. Am J Respir Crit Care Med 181: 838–850, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Starcher B. Mechanical ventilation and elastic fiber assembly. Am J Physiol Lung Cell Mol Physiol 294: L1–L2, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Sweet DG, Huggett MT, Warner JA, Moss TJ, Kloosterboer N, Halliday HL, Newnham JP, Kallapur SG, Jobe AH, Kramer BW. Maternal betamethasone and chorioamnionitis induce different collagenases during lung maturation in fetal sheep. Neonatology 94: 79–86, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Thibeault DW, Mabry SM, Ekekezie II, Truog WE. Lung elastic tissue maturation and perturbations during the evolution of chronic lung disease. Pediatrics 106: 1452–1459, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Thibeault DW, Mabry SM, Ekekezie II, Zhang X, Truog WE. Collagen scaffolding during development and its deformation with chronic lung disease. Pediatrics 111: 766–776, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Wang XQ, Zhou X, Zhou Y, Rong L, Gao L, Xu W. Low-dose dexamethasone alleviates lipopolysaccharide-induced acute lung injury in rats and upregulates pulmonary glucocorticoid receptors. Respirology 13: 772–780, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature 422: 313–317, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Weaver M, Dunn NR, Hogan BL. BMP4 and FGF10 play opposing roles during lung bud morphogenesis. Development 127: 2695–2704, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Welch KD, Panter KE, Lee ST, Gardner DR, Stegelmeier BL, Cook D. Cyclopamine-induced synophthalmia in sheep: defining a critical window and toxicokinetic evaluation. J Appl Toxicol 29: 414–421, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Willet KE, Jobe AH, Ikegami M, Newnham J, Brennan S, Sly PD. Antenatal endotoxin and glucocorticoid effects on lung morphometry in preterm lambs. Pediatr Res 48: 782–788, 2000 [DOI] [PubMed] [Google Scholar]
- 55. Yang Y, Li Q, Deng Z, Zhang Z, Xu J, Qian G, Wang G. Protection from lipopolysaccharide-induced pulmonary microvascular endothelial cell injury by activation of hedgehog signaling pathway. Mol Biol Rep 38: 3615–3622, 2011 [DOI] [PubMed] [Google Scholar]
- 56. Yee W, Wang J, Liu J, Tseu I, Kuliszewski M, Post M. Glucocorticoid-induced tropoelastin expression is mediated via transforming growth factor-β3. Am J Physiol Lung Cell Mol Physiol 270: L992–L1001, 1996 [DOI] [PubMed] [Google Scholar]
- 57. Zhang M, Wang H, Teng H, Shi J, Zhang Y. Expression of SHH signaling pathway components in the developing human lung. Histochem Cell Biol 134: 327–335 [DOI] [PubMed] [Google Scholar]