Abstract
Ca2+ sparks are fundamental Ca2+ signaling events arising from ryanodine receptor (RyR) activation, events that relate to contractile and dilatory events in the pulmonary vasculature. Recent studies demonstrate that long-term hypoxia (LTH) can affect pulmonary arterial reactivity in fetal, newborn, and adult animals. Because RyRs are important to pulmonary vascular reactivity and reactivity changes with ontogeny and LTH we tested the hypothesis that RyR-generated Ca2+ signals are more active before birth and that LTH suppresses these responses. We examined these hypotheses by performing confocal imaging of myocytes in living arteries and by performing wire myography studies. Pulmonary arteries (PA) were isolated from fetal, newborn, or adult sheep that lived at low altitude or from those that were acclimatized to 3,801 m for > 100 days. Confocal imaging demonstrated preservation of the distance between the sarcoplasmic reticulum, nucleus, and plasma membrane in PA myocytes. Maturation increased global Ca2+ waves and Ca2+ spark activity, with sparks becoming larger, wider, and slower. LTH preferentially depressed Ca2+ spark activity in immature pulmonary arterial myocytes, and these sparks were smaller, wider, and slower. LTH also suppressed caffeine-elicited contraction in fetal PA but augmented contraction in the newborn and adult. The influence of both ontogeny and LTH on RyR-dependent cell excitability shed new light on the therapeutic potential of these channels for the treatment of pulmonary vascular disease in newborns as well as adults.
Keywords: development, maturation, chronic hypoxia, wire myography, Fluo-4
increases in cytosolic Ca2+ are essential to many functions in vascular myocytes, including contractility as well as gene expression. The Ca2+ signals that govern these and other biological processes depend on global increases in the intracellular Ca2+ concentration as well as localized, subcellular, Ca2+ events. The cellular responses to these Ca2+ signals are dependent upon the spatial and temporal, as well as amplitude aspects of the Ca2+ increases (17, 19, 21, 31, 48, 50). In the pulmonary vasculature, attention has been given to the influence of global Ca2+ to contractility, and the dependence of these signals on Ca2+ influx across the plasma membrane and Ca2+ release from intracellular stores (8, 11, 19, 44). Recent studies also have focused on local Ca2+ signals and their roles to arterial contractility, with specific consideration on rapid Ca2+ release events. Ryanodine receptors (RyRs) are critical to local Ca2+ spark events in skeletal, cardiac, and smooth muscle myocytes (4, 7, 19, 32, 48, 51). In the pulmonary vasculature, RyRs are important during endothelin-1-mediated contractility (47, 48), hypoxic pulmonary vasoconstriction (16), and associated Ca2+ signaling (27, 51).
RyR-generated Ca2+ signals change with development. For instance, in rat cerebral arterial myocytes, global Ca2+ responses to caffeine, a putative RyR agonist, were similar in fetus and adult; however, Ca2+ sparks were not prevalent before birth and, as such, did not affect vascular reactivity (7). Yet, the influence of development on RyR-generated signals may differ in the pulmonary vasculature. Unlike fetal rat cerebral vascular myocytes, fetal rabbit pulmonary arterial (PA) myocytes have abundant Ca2+ sparks. In that latter species, ryanodine causes global Ca2+ responses most prominently in myocytes from fetuses, as opposed to newborns or juveniles (33). Notably, we have shown that caffeine elicits Ca2+ responses in nearly all PA myocytes from fetal sheep, and the events have greater amplitude compared with adults (8). This also occurs in superior cervical ganglion neurons from these fetal sheep (1). Together, these findings provide support that RyRs are functional in utero in sheep PA myocytes and that their function may change following birth.
Long-term hypoxia (LTH) induced by placing animals in a normobaric hypoxia chamber in a laboratory setting or due to living at high altitude can cause numerous changes in the structure and function of the pulmonary vasculature. These alterations may result in pulmonary hypertension in humans and various animal models including rats, cattle, and pigs (39) and elevated pulmonary pressures in newborn sheep (12, 13). Numerous studies have detailed mechanistic changes in global Ca2+ signaling in association with LTH, and the relationship to pulmonary hypertension (2, 14). For instance, LTH reduced endothelin-1-induced Ca2+ signals in rat pulmonary arterial myocytes (37). However, the influence of LTH on RyRs in the developing pulmonary vasculature and potential deregulation in Ca2+ signaling and contribution to pulmonary hypertension are unresolved.
The couplings between RyR-dependent Ca2+ signaling and vascular function led us to design experiments that addressed the role of RyRs in the developing pulmonary vasculature and the influence of LTH on their function. Based on previous studies by others and us (8, 33, 37), the present studies used wire myography and Ca2+ imaging approaches to test the hypotheses that RyR-generated Ca2+ signals are more active before birth and that LTH suppresses RyR-mediated Ca2+ responses.
MATERIALS AND METHODS
All experimental procedures were performed within the regulations of the Animal Welfare Act, the National Institutes of Health Guide for the Care and Use of Laboratory Animals, “The Guiding Principles in the Care and Use of Animals” approved by the Council of the American Physiological Society, and the Animal Care and Use Committee of Loma Linda University, Loma Linda, CA. Ewes were bred at Nebeker Ranch (Lancaster, CA) and were either maintained at low altitude (300 m; arterial PaO2 = 95 ± 5 Torr) throughout gestation and parturition or were taken to high altitude (3,801 m, 12,470 ft, PaO2 = 60 ± 5 Torr for ewe and lamb and 19 ± 3 Torr for fetus) at the Barcroft Laboratory (White Mountain Research Station, Bishop, CA) and acclimatized for the final ∼110 days of gestation (24). High-altitude lambs were delivered spontaneously at the Barcroft Research Station and lived there until 7 to 12 days of age. Lambs with their mothers were then transported to the animal care facilities at Loma Linda University. To maintain hypoxia between the times of arrival at Loma Linda University (altitude 353 m) and experimental study (1 to 3 days), high-altitude newborn sheep were placed into a simulated altitude chamber. The O2 of the chamber was maintained at ∼13–15%, which is equivalent to 3,200–4,358 m, using a molecular sieve and supplemental N2 as necessary (CAT-12; Colorado Altitude Training Louisville, CO). Pregnant and nonpregnant animals maintained at high altitude were also transported to Loma Linda University, and shortly after arrival a tracheal catheter was placed in the ewe through which N2 flowed at a rate adjusted to maintain PaO2 at ∼60 Torr (20), which is equivalent to the oxygen tension measured at the White Mountain Research Station. This PaO2 was maintained until the time of the experimental study. One to five days after delivery to our laboratory at Loma Linda University, the sheep were anesthetized with pentobarbital sodium (10 mg/kg iv), and adult sheep were maintained on aesthesia by endotracheal delivery of a constant flow of isoflurane (1 to 2% in O2). All sheep were then euthanized with an overdose of the proprietary euthanasia solution, Euthasol (pentobarbital sodium 100 mg/kg and phenytoin sodium 10 mg/kg; Virbac, Ft. Worth, Tx). Lungs were removed for pulmonary artery imaging and contractility experiments.
Tissue Preparation
We dissected fourth- or fifth-order PA (∼500 μm diameter) free of parenchyma and cut them into 5-mm long rings in ice-cold phosphate-free balanced salt solution of the following composition (in mM): 126 NaCl, 5 KCl, 10 HEPES, 1 MgCl2, 2 CaCl2, 10 glucose, pH 7.4 (adjusted with NaOH). To avoid complication from endothelium-mediated effects, the endothelium was removed by carefully rotating the artery on the mounting wire (43), a procedure that disrupts endothelial-dependent contraction in this preparation (9, 28). Arteries used for confocal microscopy studies were cut longitudinally prior to dye loading.
Contractility Studies
Pulmonary arterial rings were suspended in organ baths (Radnoti glass instruments, Monrovia, CA) that contained 5 or 10 ml of modified Krebs-Henseleit solution at 4–5°C containing (in mM): 120 NaCl, 4.8 KCl, 1.2 K2HPO4, 25 NaHCO3, 1.2 MgCl2, 2.5 CaCl2, 10 glucose maintained at 37°C and aerated with 95% O2-5% CO2 (pH = 7.4). Arterial rings were suspended between two tungsten wires passed through the lumen. One wire was anchored to the glass hook at the bottom of the organ chamber, and the other connected to a tissue hook attached to a low-compliance force transducer (Radnoti Glass Instruments) for the measurement of isometric force (43). The transducers were connected to an analog-to-digital data interface (PowerLab 16/30; A/D Instruments, Colorado Springs, CO or model MP100; Biopac, Goleta, CA) attached to a computer. Changes in tension were recorded using Chart 5.5 (AD Instruments), or AcqKnowledge 3.9 (Biopac Systems), and the obtained data were stored on magnetic media for later analysis. Vessels were thermally equilibrated at the beginning of each experiment without tension for a minimum of 30 min. Vessel rings were tensioned by progressively stretching the vessels, reaching a resting tension of 0.5 ± 0.01 g (n = 100 vessels from 22 animals) (9, 28, 29, 43). The tension was normalized for comparison to the maximum response obtained with high K (%TKmax), where the NaCl in the Krebs-Henseleit solution was replaced for an equimolar concentration of KCl.
Confocal Microscopy Studies
Ca2+ imaging.
The intracellular Ca2+ concentration was measured in PA myocytes in situ, with the Ca2+ sensitive dye Fluo-4 AM (Invitrogen, Carlsbad, CA) by using an model FV1000 (Olympus, Center Valley, PA) or a model 710 NLO (Carl Zeiss Microscopy, Thornwood, NY) laser scanning confocal imaging workstation with inverted microscopes (Olympus IX81 or Zeiss Axio Observer Z1). Fluo-4 AM was dissolved in DMSO and added from a 1 mM stock to the arterial suspension at a final concentration of 10 μM, along with 0.1% pluronic F127 for 1 to 1.5 h at room temperature in the dark in balanced salt solution. Arterial segments then were washed for 30 min to allow for dye esterification and then cut into linear strips. The arterial segments were pinned to sylgard blocks (Ellsworth Adhesives, Germantown, WI) and placed in an open bath imaging chamber (Warner Instruments, Hamden, CT) mounted on the confocal imaging stage. Cells were illuminated at 488 nm with a krypton argon laser and the emitted light was collected using a photomultiplier tube (PMT). Line scans were imaged at rates from 422 to 822 lines generated every 1 s, depending on line length. Acquisition periods for Ca2+ spark recordings were from 10 to 20 s. To ensure that sparks within the cell were imaged, the pinhole was adjusted to provide an imaging depth of ∼2.5 μm. This depth is roughly equivalent to the width of 50% of the cell based on morphological examination of fixed and live preparations (data not shown). Global Ca2+ responses were acquired at roughly one image per second with an imaging depth of 5 to 10 μm, which is equivalent to one or two cells thick. The sampling depth was either 12-bit (Olympus FV1000) or 16-bit (Zeiss 710). Ca2+ spark recordings on the FV1000 were made using a oil immersion ×60 Apochromat (numerical aperture, NA 1.4) objective. Global Ca2+ responses were made either with a nonimmersion ×20 Plan Apochromat (NA 0.8) or the previously described immersion objectives or a water immersion ×63 c-Apochromat (NA 1.2) objective on the Zeiss 710. Regions of interest were examined post hoc, and analyzed within ImageJ (35). Analysis of time series recordings was achieved by hand, using the time series analyzer plugin for ImageJ. For presentation purposes, the fractional fluorescence intensity was calculated as F/F0 = F − baseline/F0 − baseline, where baseline is the intensity from a region of interest with no cells, F is the fluorescence intensity for the region of interest, and F0 is the fluorescence intensity during a period from the beginning of the recording when there was no Ca2+ activity.
Ca2+ sparks were analyzed for the percentage of cells with Ca2+ sparks and the frequency of firing by visual inspection. Sparks per 100 micrometer per second and the percentage of cells firing were computed from these observed sparks. Spatial and temporal characteristics of the Ca2+ spark events were made using the SparkMaster plug-in for ImageJ (32, 35); this includes the fractional fluorescence intensity, the full duration at half maximum (FDHM), full duration, time constant for spark recovery, the full width at half maximum (FWHM), full width and time to peak. Before analysis with SparkMaster, the full-size original line scan images were manually cropped for regions that bounded suspected Ca2+ sparks, thus accounting for fluctuations in the basal Ca2+ that occurred during the recordings. The SparkMaster ImageJ plugin was used to automate analysis of these suspected Ca2+ sparks and quantify the changes in fluorescence due to Ca2+ spark activation. In our studies, the threshold for Ca2+ spark event detection was 3.2 times the standard deviation of the background noise above the mean background level. Thus, the criterion level of 3.2 is a measure of sensitivity related to the amplitude of the event above the background signal. We determined empirically that this criterion value maximized the identification of Ca2+ sparks and minimized both false negative and false positive identification. Initial analyses were performed on a representative data subset using a range of criteria from 2.0 to 4.0 where an increase in the criterion value decreases the sensitivity. False positives were manually parsed from the final data set. Notably, the spatial, temporal, and amplitude of the Ca2+ sparks presented here are similar to those analyzed by the SparkMaster plugin for permeabilized ventricular and skeletal muscle (32). The amplitude values for Ca2+ sparks recorded in myocytes from the fetal hypoxic group are near to the detectability limit for SparkMaster. Manual identification and cropping of suspected events enhanced the ability of SparkMaster to detect and analyze these low-amplitude events. The values are somewhat less than those analyzed by the techniques used by other groups for cerebral and pulmonary arterial myocytes (7, 19, 48). These variations in amplitude may reflect differences in how SparkMaster subtracts baseline fluorescence relative to other analysis routines. No additional background subtraction procedures were performed beyond those incorporated into the SparkMaster analysis program.
Structural imaging.
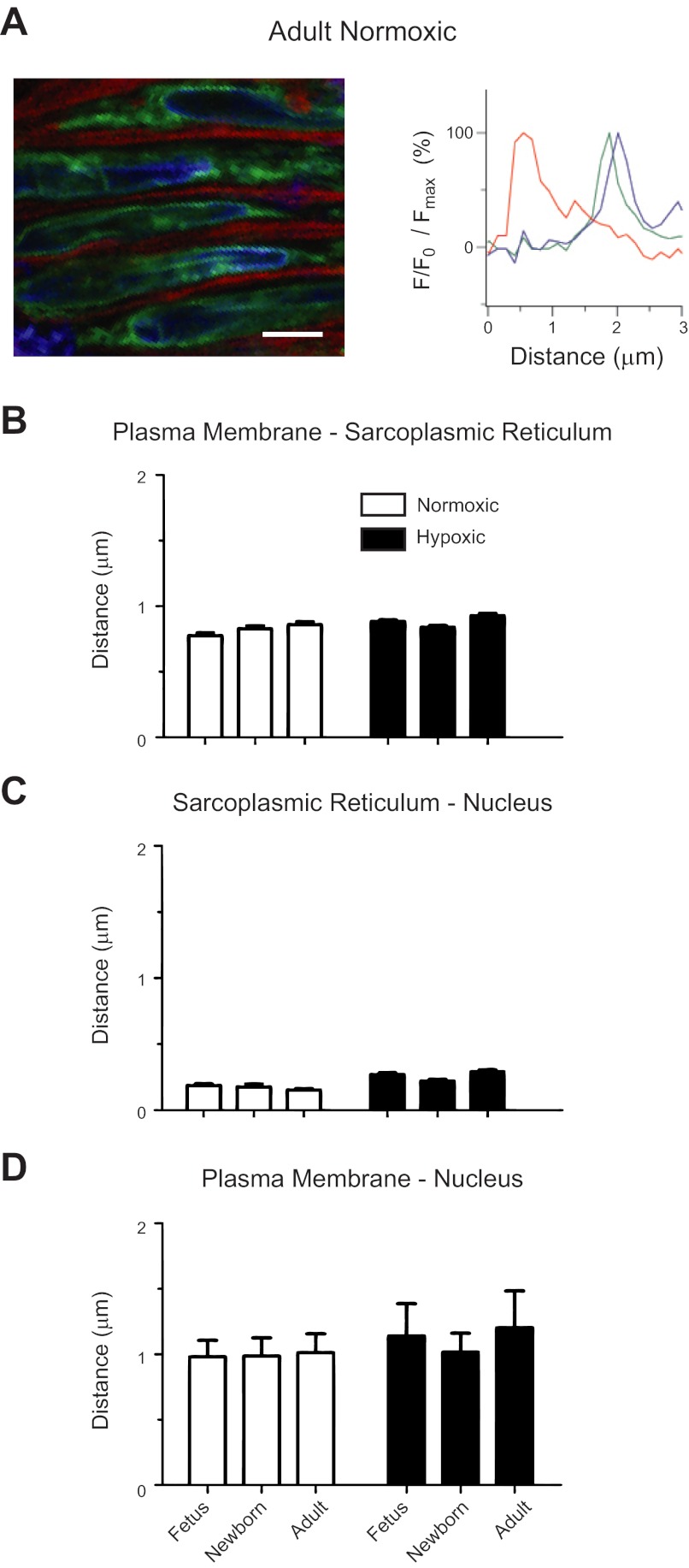
Pulmonary arterial myocyte structure was examined in living tissue using specific fluorophores. Arteries for these studies were isolated, cleaned, and loaded in balanced salt solution to visualize the plasma membrane, endoplasmic reticulum, and nuclei. Specifically, nuclei were labeled with 1.38 μM Hoescht 33258 (Invitrogen) for 1 h; sarcoplasmic-endoplasmic reticulum with 1 μM ER-Tracker Green (Bodipy FL glibenclamide; Invitrogen) for 1 h, and the plasma membrane with 5 μg/ml CellMask Deep Red (proprietary amphipathic molecules; Invitrogen) for 30 min at room temperature in the dark. Images were taken using a Zeiss Axio Observer Z1 inverted LSM 710 NLO laser scanning confocal microscope with a water immersion ×63 c- Plan Apochromat (NA 1.2) objective. Images were 67.41 × 67.41 μm and were 1,024 × 1,024 pixels, with a 16-bit sampling depth. The pinhole was set for an optimal imaging depth, which was ∼1.2 μm. After labeling, images were sequentially taken through the arterial wall from the endothelial to the adventitial layer. Different lasers were used to excite each dye. Hoechst 33258 was excited with a 405-nm DPSS laser, ER-Tracker Green was activated with the 488-nm line of a Kr-Ar multiple laser, and CellMask Red with a 633-nm HeNe laser on. For Hoechst 33258, emitted light was collected at 417–679 nm using a PMT array. For ER-Tracker Green, emitted light was collected with a single PMT at 493–630 nm. For CellMask Red, emitted light was collected with a single PMT at 638–755 nm. The distances between the ER, nucleus, and plasma membrane were measured using profile tools within Zen 2009 (Zeiss Microsystems) with measurements made in the widest portion that encompasses the nuclei and at the axial center of the myocyte. The value recorded for the distance between the structures was recorded by visual inspection of the peak fluorescence signal. For display purposes, the brightness and contrast of the micrographs were digitally adjusted, and the fluorescent intensity values used to generate the line plots in Fig. 1 were baseline subtracted and normalized to the peak intensity value for each emission wavelength. All measurements were made by a single observer to control for experimenter bias, and measurements were made in multiple cells and animals to control for sample variability.
Fig. 1.
Ontogeny and long-term hypoxia (LTH) do not affect the spatial relationships between the sarcoplasmic-endoplasmic reticulum and the plasma membrane or nucleus. A: representative micrograph and associated intensity profiles of the plasma membrane (red), sarcoplasmic-endoplasmic reticulum (green), and nucleus (blue) of myocytes in a living pulmonary artery from normoxic adult, newborns, and fetuses. Bars indicate means ± SE for the distance between the sarcoplasmic endoplasmic reticulum and the plasma membrane (B) as well as nucleus (C–D). The difference in the distances for the various compartments in myocytes of fetal and adult pulmonary arteries of sheep shown in B, C, and D were within the calculated point-spread function for the Zeiss 710. Images were made in arterial segments from 4 fetal, 3 newborn, and 4 adult normoxic sheep and from 7 fetal, 4 newborn, and 6 adult long-term hypoxic animals with a ×63 water immersion c-Apochromat objective. F/F0: difference between peak spark fluorescence and background fluorescence. Scale bar = 5 μm.
Because the distances that were measured between the nucleus, endoplasmic reticulum, and plasma membrane were small, and the differences between the various groups smaller still, we measured the axial point-spread function to ensure that the distances were beyond the lower limit of detectability for our imaging system. The point-spread function was determined using 0.175-μm fluorescent bead samples (Invitrogen) excited with a 488-nm laser and imaged with a water immersion ×63 c- Plan Apochromat (NA 1.2) objective. The acquisition parameters for these calibrations entailed imaging at a frame size of 1,024 × 1,024, 12 bit depth, a zoom factor of three, and the pinhole set to one Airy unit. Individual microspheres were imaged, and data processing was performed using the MetroloJ plug-in for ImageJ (25, 26). The theoretical point-spread function resolution for the microspheres was 0.163 μm for both x and y. The calculated FWHM for x was 0.259 μm, while y was 0.252 μm.
Chemicals and Drugs
Most reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Fluo-4 AM, Hoescht 33258, ER-Tracker Green, CellMask Deep Red, and Pluronic F127 were purchased from Invitrogen (Carlsbad, CA). Ryanodine and dantrolene were from Tocris (Ellisville, MO). Arteries were treated with 10 μM ryanodine for 30 min prior to imaging to ensure that RyR activity was reduced (8, 15, 19, 43, 44).
Statistical Methods
All time series recordings were graphed with IGOR Pro 6.0 (Wavemetrics, Lake Oswego, OR), and the data were presented as means ± SE. Statistical analyses were made using GraphPad Prism 5.0 (La Jolla, CA). Data was evaluated for normality using the D'Agostino and Pearson omnibus normality test prior to any comparative statistical analysis. Statistical difference between groups that were normally distributed was determined with a two-tailed unpaired Student's t-test, while a Mann-Whitney U-test was used for comparisons of nonnormal data. For analysis of the spatial and kinetic properties for Ca2+ sparks and other studies where the data were not normally distributed, comparisons were made within groups using a Kruskal-Wallis one-way ANOVA with a Dunn's multiple comparison test and among groups using a Mann-Whitney U-test. For the contraction data and where the data were normally distributed, a one-way ANOVA and a Newman-Keuls multiple comparison procedure was used to test within groups or a two-way ANOVA and the Bonferroni post hoc analysis was also used to test within and among groups. The n values reported reflect the total number of Ca2+ sparks, or recordings examined. A P value of < 0.05 was accepted as statistically significant. The actual P values for the various analyses are provided with each figure.
RESULTS
We designed the first series of studies to determine the spatial apposition of the sacroplasmic reticulum to the plasma membrane and nucleus in intact arteries. These studies are important as they show that the line scan recordings were made in locations consistent to where Ca2+ sparks usually arise; this being just under the plasma membrane in vascular myocytes (7, 19, 33, 48). Figure 1 shows one representative image and associated plots of the normalized fluorescent intensity of each dye within the cell from normoxic and long-term hypoxic fetal, newborn, and adult sheep stained with vital dyes that marked the plasma membrane, sarcoplasmic reticulum, and nucleus. Figure 1A shows a representative image from a normoxic adult pulmonary artery and the associated fluorescence intensity distribution across the cell. The representative fluorescence intensity plot for the adult normoxic myocytes shows that the distance between the peak intensity for CellMask Red (plasma membrane) and ER-Tracker Green (sarcoplasmic reticulum) is roughly 1 μm, while the distance to Hoescht 33258 (nuclei) was about 1.5 μm. The distance between ER-Tracker Green and Hoescht 33258 was much smaller, being near the resolving limit for our system.
The averaged measurements for the spatial data are provided in Fig. 1, B–D for normoxic fetal (n = 40 myocytes), newborn (n = 30), and adult (n = 33) sheep as well as for long-term hypoxic fetal (n = 221), newborn (n = 70), and adult (n = 121) sheep. As shown in Figure 1B, the data suggest that in each age and altitude group the sarcoplasmic reticulum and the plasma membrane are spatially separated, with the peak intensities being ∼0.75 μm apart. This spatial separation would provide discrete signaling in the subsarcolemmal region (19). Figure 1C shows that the sacroplasmic reticulum nearly overlaps the nucleus, while Fig. 1D shows the distance from the plasma membrane to the nucleus is as expected, this being roughly equivalent to the sum total of the distance from the plasma membrane to the sarcoplasmic reticulum (Fig. 1B) and the distance from the sarcoplasmic reticulum to the nucleus (Fig. 1D). Furthermore, the impact of both postnatal maturation and LTH on the distance between the cellular structures was within the theoretical point spread function for the system used for these studies (see materials and methods). Therefore, to adequately address more than gross changes in sarcoplasmic reticulum ultrastructure, which do not appear to occur, more detailed morphological studies of peripheral and junctional sarcoplasmic reticulum are required.
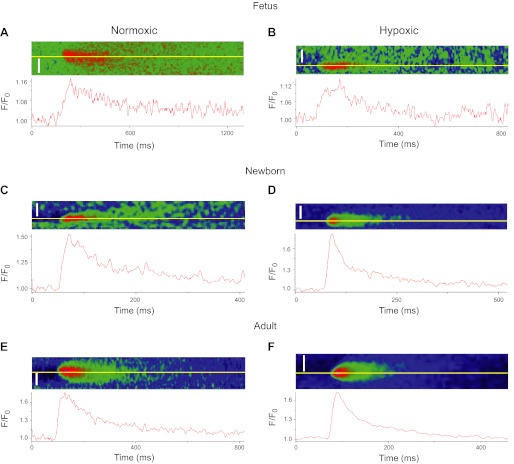
We then designed experiments to address whether or not maturation or LTH increases or decreases the prevalence of spontaneous and evoked Ca2+ sparks in the subsarcolemmal space of PA myocytes. Figure 2 shows representative Ca2+ sparks and time series of the fractional fluorescence recorded in single pulmonary arterial myocytes of intact arteries from fetal (Fig. 2, A and B), newborn (Fig. 2, C and D), and adult (Fig. 2, E and F) normoxic and LTH sheep. Overall, these Ca2+ sparks display the characteristic swift rise in amplitude from a single point source with a slower, monoexponential decay.
Fig. 2.
Ca2+ sparks from sheep pulmonary arterial myocytes. A–F: pseudo color filtered line scan images and associated time series profiles recorded in individual myocytes for pulmonary arteries from normoxic and long-term hypoxic adults, newborns, and fetuses. Line plots show the relative fluorescent intensity profile for the line (yellow) shown in each micrograph. Calcium spark and wave recordings for Figs. 2–8 were obtained from recordings performed in a total of 12 fetal, 8 newborn, and 8 adult normoxic sheep and from 9 fetal, 9 newborn, and 7 adult long-term hypoxic sheep with a ×60 oil immersion Apochromat objective. Scale bars = 5 μm.
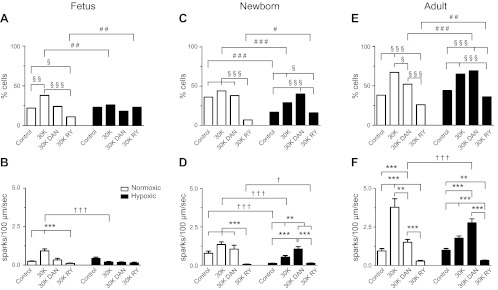
We then visually examined the line scan images and found that both developmental maturation and LTH influenced Ca2+ spark occurrence and activity, as demonstrated in Fig. 3. Overall, we analyzed a total of 4,615 line scan images. This included 749 records from fetal, 926 from newborn, and 758 from adult normoxic and 598 from fetal, 690 from newborn. and 894 from adult hypoxic sheep. These line scan recordings were analyzed for the presence or absence of Ca2+ sparks and for their frequency of incidence in each responsive myocyte. With developmental maturation, the number of myocytes with spontaneous Ca2+ sparks increased significantly, while LTH reduced their occurrence in newborn vessels. In fetal and adult normoxic myocytes (Fig. 3A), evoking Ca2+ sparks by depolarizing the plasma membrane with 30 mM K+ increased the percent of cells firing. Selective inhibition of RyR1 and RyR3 with 10 μM dantrolene, as well as pan-RyR inhibition with 10 μM ryanodine, suppressed 30 mM K+-evoked Ca2+ sparks in most normoxic groups (15, 27, 41, 44). Figure 3A shows that 30 mM K+ failed to increase the incidence of Ca2+ sparks in myocytes from hypoxic fetuses. In addition, dantrolene did not curb Ca2+ sparks in myocytes from normoxic newborns or those from hypoxic sheep, regardless of age (Fig. 3, A, C, and E). In fact, dantrolene may even accentuate spark activity in LTH newborns and adults, even when ryanodine effectively reduced Ca2+ sparks.
Fig. 3.
Ca2+ spark activity in pulmonary arterial myocytes and the influence of ontogeny and LTH. A, C, and E: bars are % of myocytes with sparks. B, D, and F: bars are number of sparks/100 μm/s. Ca2+ spark activity was determined by visual inspection of line scans. Each trial was ∼10 s. Measurements were made in 58–262 records from a minimum of 3 animals in each condition. §,†,#P < 0.05; **,§§,††,##P < 0.01; ***,§§§,†††,###P < 0.001 denotes significant difference within (*) groups based on the treatment using a Kruskal-Wallis 1-way ANOVA on ranks with a Dunn's multiple comparison test and (†) between groups based on their altitude using a Mann-Whitney U-test. The symbol § shows difference within groups and the symbol #, between groups based on a χ2 test.
We then calculated the firing frequency for Ca2+ sparks by analyzing all records, whether or not there were any Ca2+ sparks in the recording. These findings are presented in Fig. 3, B, D, and F and parallel those of the percentage of cells firing. Thirty millimolar of potassium increased the Ca2+ spark firing rate in most groups, while ryanodine reduced activity. There were, however, notable exceptions. Figure 3B shows that in LTH fetuses Ca2+ spark activity was unaffected by 30 mM K+, ryanodine, or dantrolene. In a similar manner, Fig. 3, D and F show that dantrolene did not suppress the Ca2+ spark frequency in myocytes from all newborns as well as LTH adults. Finally, postnatal maturation increased Ca2+ spark frequency, where newborns and adults (Figs. 3, D and F) had a greater Ca2+ spark frequency compared with fetuses (Fig. 3B).
The spatial and temporal aspects of the Ca2+ spark events were measured using SparkMaster (32). This analysis is important because the spatial-temporal relationships of the Ca2+ sparks influence the local interaction between the Ca2+ released and activity of downstream targets (3, 15, 19, 27). We chose to use the automated SparkMaster analysis plugin for ImageJ because it allows for rapid unbiased analysis of line scan images (32), as opposed to more laborious and subjective analysis procedures such as we have performed previously (19). These measurements were made in myocytes depolarized with 30 mM K+, a common method of facilitating spark activity so that average responses can be determined (18).
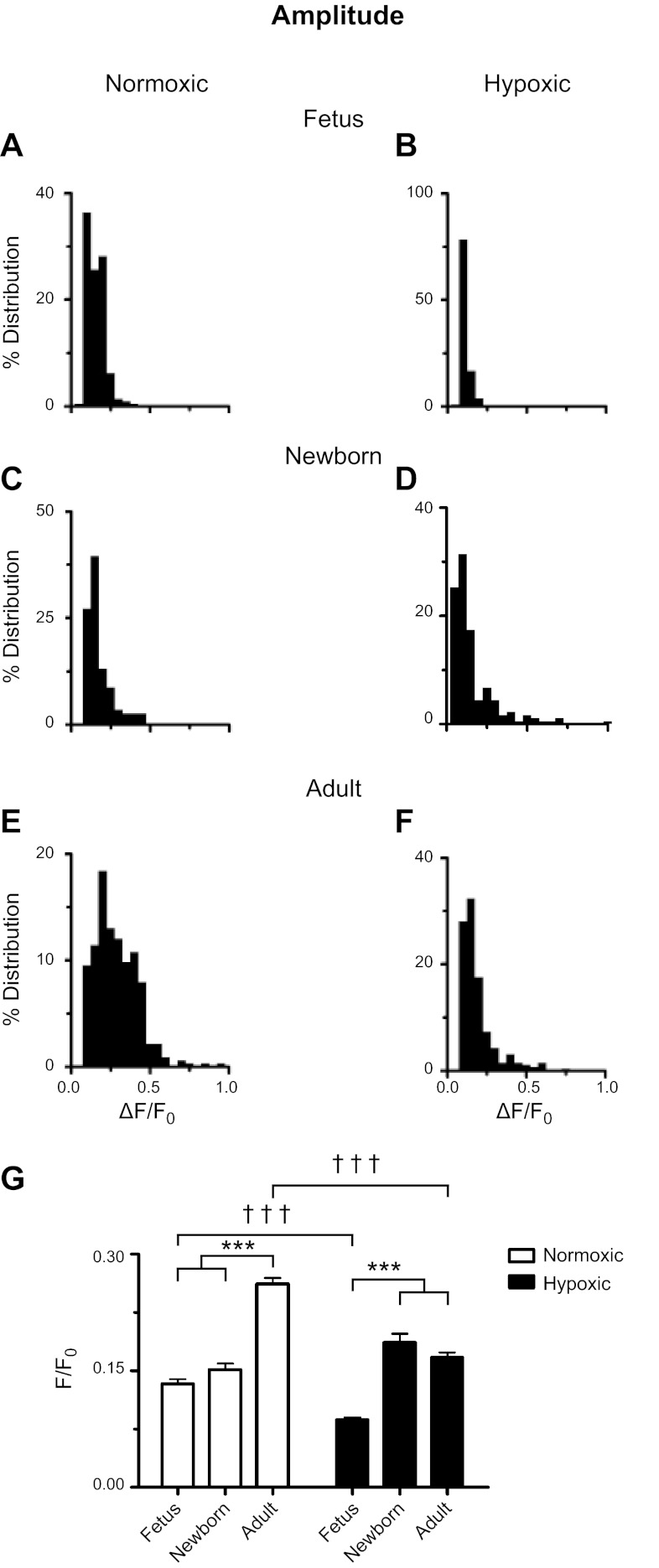
Figures 4–7 show the various spatial and temporal analyses for Ca2+ sparks recorded in myocytes isolated from fetal, newborn, and adult normoxic and long-term hypoxic sheep. These analyses were made using a criteria of 3.2 for Ca2+ sparks recorded from myocytes of normoxic fetal (n = 206, Figs. 4A–7A), newborn (n = 114, Figs. 4C–7C), and adult (n = 315, Figs. 4E–7E) and LTH fetal (n = 102, Figs. 4B–7B), newborn (n = 178, Figs. 4D–7D) and adult (n = 256, Figs. 4F–7F) sheep. The general relationships of Ca2+ sparks and summarized histogram plots were similar for each group and across the measured parameters.
Fig. 4.
Ontogeny increases, while LTH reduces, Ca2+ spark amplitude. A–F: histograms of Ca2+ spark amplitudes for recordings made from fetal, newborn, and adult sheep pulmonary arterial myocytes housed in normoxic or long-term hypoxic conditions. G: bars are for fetal, newborn, and adult normoxic (open) and long-term hypoxic (solid) conditions. ***, ††† P < 0.001 denotes significant difference within (*) groups based on their age using a Kruskal-Wallis 1-way ANOVA on ranks with a Dunn's multiple comparison test and between (†) groups based on their altitude using a Mann-Whitney U-test.
Fig. 5.
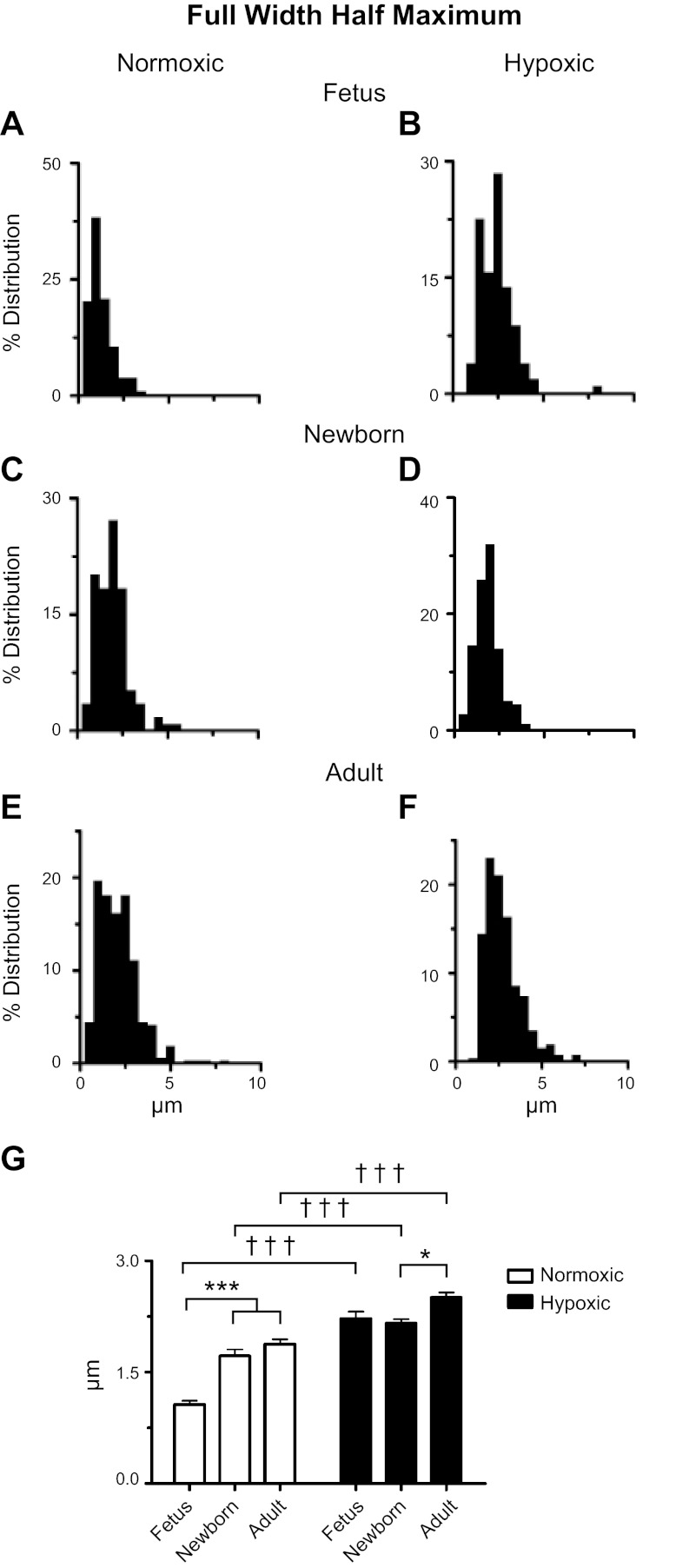
Ontogeny and LTH increase the full width at half maximum (FWHM) amplitude for Ca2+ sparks. A–F: histograms of Ca2+ spark FWHM for SparkMaster analyses of recordings made from fetal, newborn, and adult sheep pulmonary arterial myocytes housed in normoxic or long-term hypoxic conditions. G: bars are for fetal, newborn, and adult normoxic (open) and long-term hypoxic (solid) conditions. *P < 0.05 and ***,†††P < 0.001 denote significant difference within (*) groups based on their age using a Kruskal-Wallis 1-way ANOVA on ranks with a Dunn's multiple comparison test and between (†) groups based on their altitude using a Mann-Whitney U-test.
Fig. 6.
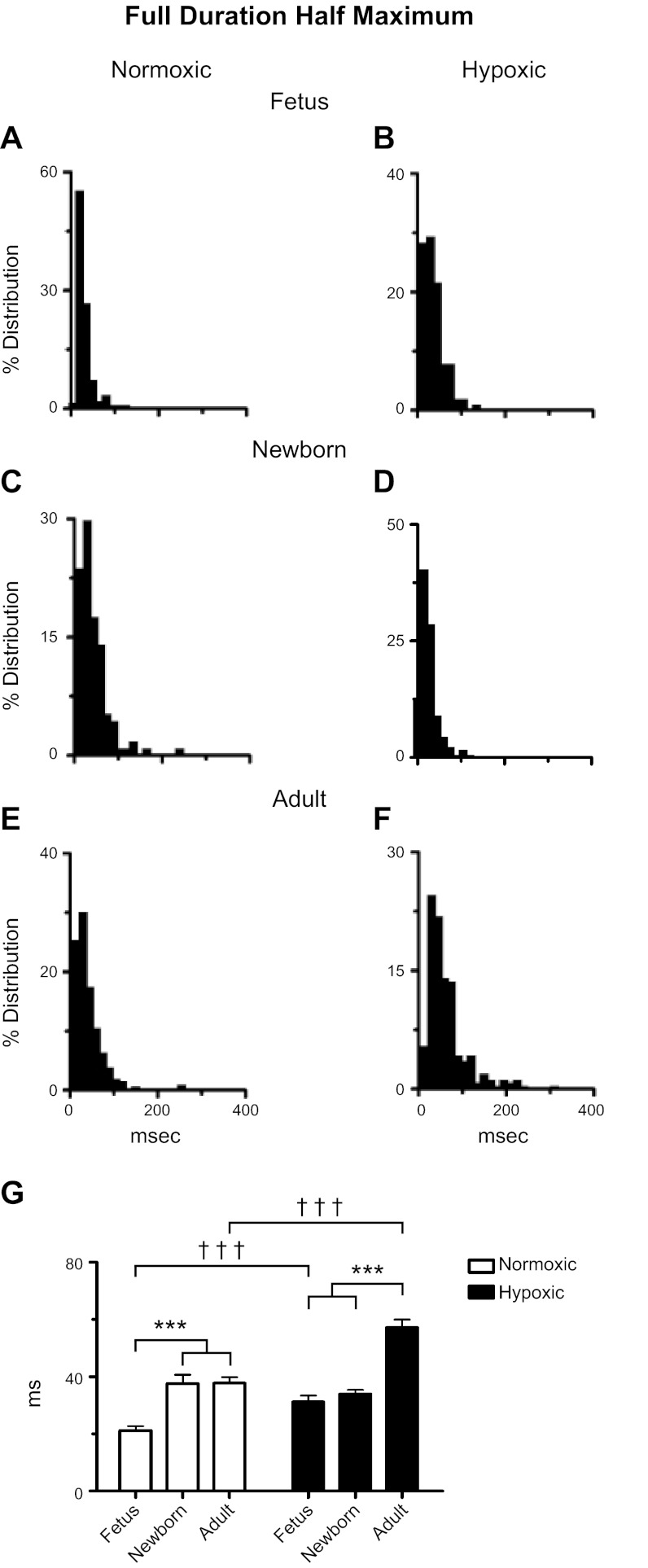
Ontogeny and LTH increase the FDHM amplitude for Ca2+ sparks. A–F: histograms of Ca2+ spark FDHM for SparkMaster analyses of recordings made from fetal, newborn, and adult sheep pulmonary arterial myocytes housed in normoxic or long-term hypoxic conditions. G: bars are for fetal, newborn, and adult normoxic (open) and long-term hypoxic (solid) conditions. ***,††† P < 0.001 denotes significant difference within (*) groups based on their age using a Kruskal-Wallis 1-way ANOVA on ranks with a Dunn's multiple comparison test and between (†) groups based on their altitude using a Mann-Whitney U-test.
Fig. 7.
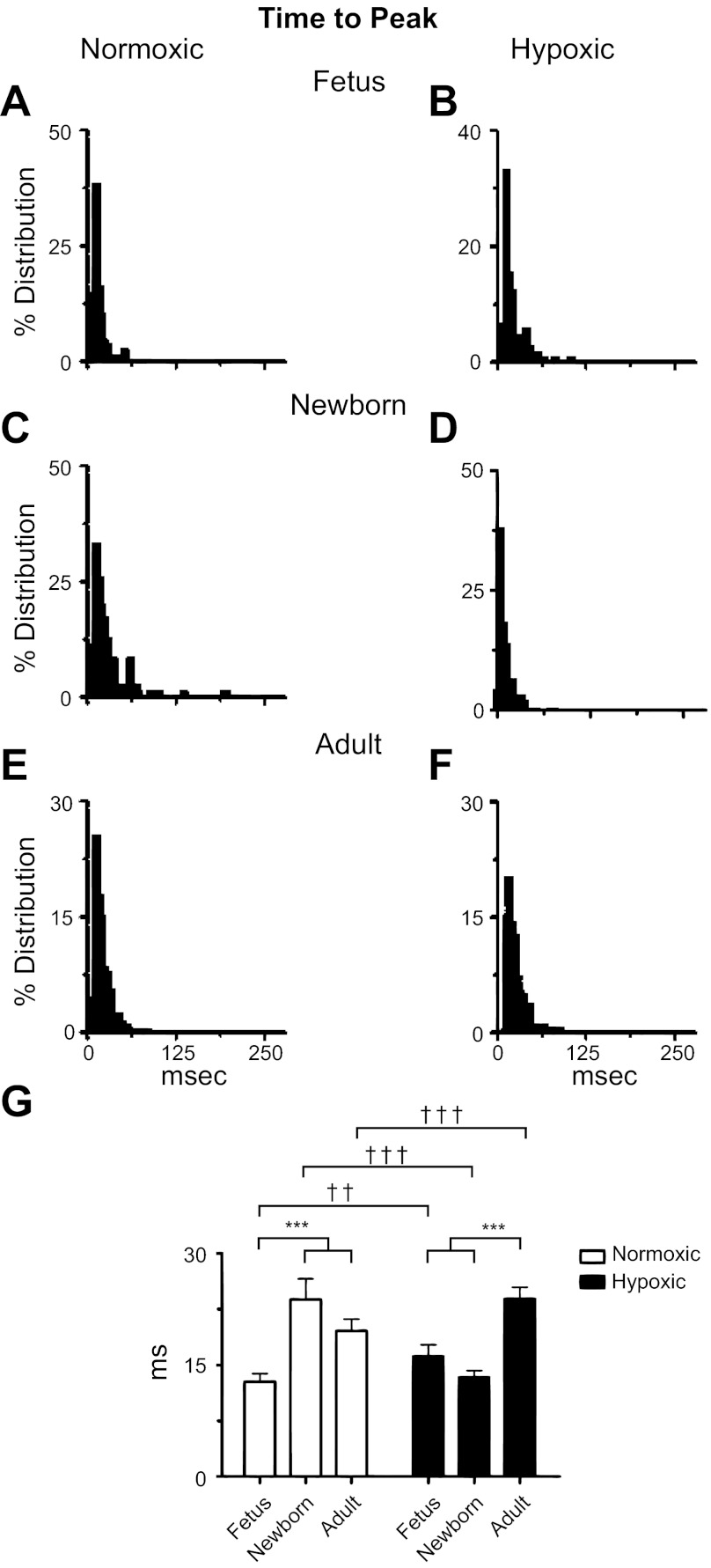
Ontogeny increases the time to the peak of Ca2+ sparks. A–F: histograms of time to the peak from Ca2+ spark events for SparkMaster analyses of recordings made from fetal, newborn, and adult sheep pulmonary arterial myocytes housed in normoxic or long-term hypoxic conditions. G: bars are for fetal, newborn, and adult normoxic (open) and long-term hypoxic (solid) conditions. ††P < 0.01; ***,†††P < 0.001 denotes significant difference within (*) groups based on their age using a Kruskal-Wallis 1-way ANOVA on ranks with a Dunn's multiple comparison test and between (†) groups based on their altitude using a Mann-Whitney U-test.
Figure 4 shows Ca2+ spark amplitudes. The histogram plots in Fig. 4, A–F show that all of the groups display a Poisson distribution, similar to the original report describing SparkMaster and that examined populations of Ca2+ spark events (32). First, the summary bar graph shown in Fig. 4G illustrates that Ca2+ sparks from the adult normoxic group had a greater amplitude than Ca2+ sparks from immature myocytes. Second, in LTH sheep the Ca2+ spark amplitude of fetal myocytes was nearly half the height of Ca2+ sparks compared with newborns and adults. Furthermore, LTH reduced the amplitude of Ca2+ sparks in myocytes from fetal and adult sheep.
Figure 5 presents computations of the FWHM for Ca2+ sparks. With the exception of the fetal LTH group, the histograms presented in Fig. 5, A–F show that Ca2+ spark widths display Poisson distributions. The summary data in Fig. 5G show that Ca2+ sparks were approximately twofold wider in normoxic newborn and adult myocytes, compared with fetuses, and in LTH adults the FWHM was also ∼10–15% wider than in immature myocytes from newborns. LTH, on the other hand, caused modest to substantial increases in the FWHM (Fig. 5G) of all age groups.
Figure 6 shows the FDHM. The histogram plots of Fig. 6, A–F show that responses of normoxic fetuses cluster to short time periods, while those of newborn and adult normoxic and adult LTH myocytes have longer periods and somewhat wider variability. Maturation dramatically influenced the rate of Ca2+ spark decay as shown in the summary bar graph data of Fig. 6G. Ca2+ sparks of normoxic sheep fetal myocytes had faster decay rates, compared with those in myocytes from newborns or adults. In long-term hypoxic sheep, the decay rate remained short in fetal and newborn myocytes, compared with those of adults. However, LTH lengthened the decay for Ca2+ sparks in myocytes from fetal and adult sheep, while the decay was unchanged in newborns.
Figure 7 shows the time required to reach the peak intensity from F/F0. The histograms in Fig. 7, A–F show the same general Poisson relationship for each group, and yet the mean values differed. The summary bar graph data of Fig. 7G show that postnatal maturation increased the time to peak in normoxic newborns and adults relative to fetuses. The summarized data presented in Fig. 7G also show that LTH newborns and fetuses had similar rates of rise, and Ca2+ increased more rapidly in adults. LTH caused Ca2+ sparks to rise more rapidly in newborns, but slowed the responses in fetuses and adults.
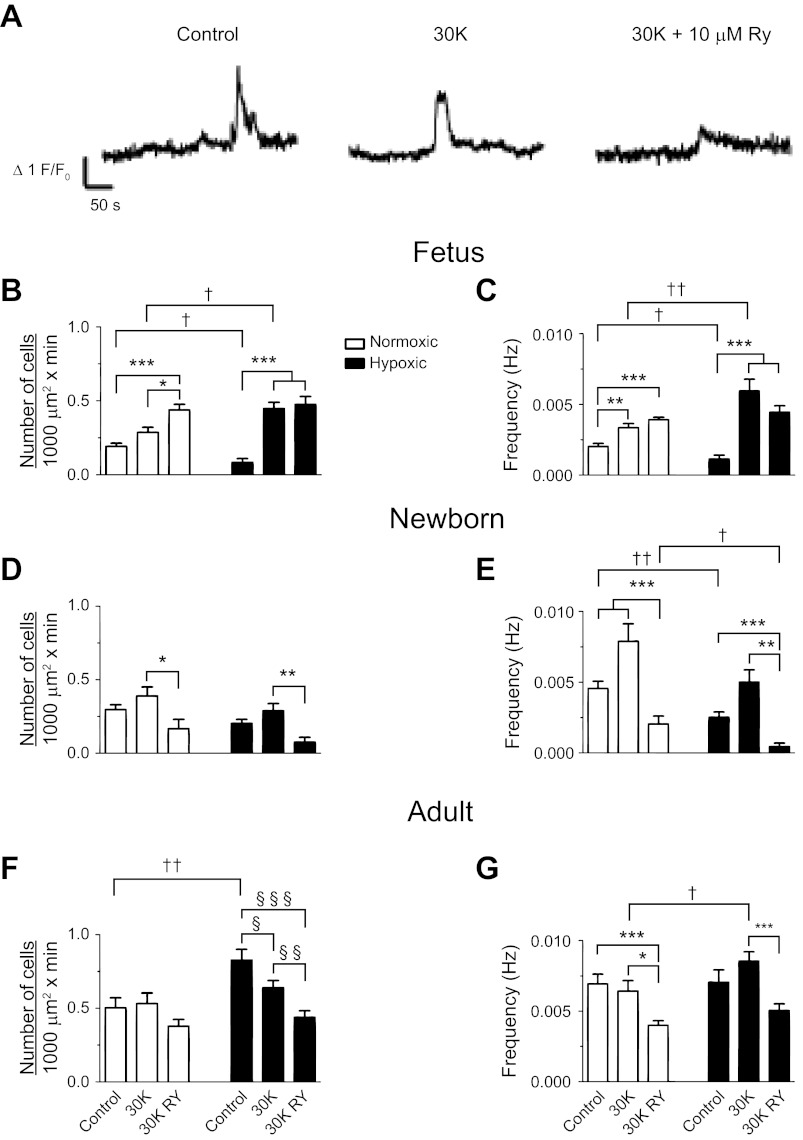
Classically, membrane depolarization with potassium increases Ca2+ spark frequency (18), and coordinated RyR activation elicits global Ca2+ responses in sheep PA myocytes (8). Thus, we next examined global Ca2+ responses to 30 mM K+ and caffeine. Figure 8 shows the influence of 30 mM K+ and ryanodine on global Ca2+ responses in adult PA. Figure 8A shows the Ca2+ signaling behavior of individual myocytes expressed as the average baseline subtracted fractional fluorescence intensity tracing (F/F0) over time in the absence and presence of 30 mM K+ and ryanodine. We then counted the number of basal and stimulated myocytes in each 1,000 μm2 of the pulmonary arterial wall. This was performed in 24–30 regions of interest for fetal, 12–16 for newborn, and 14–19 for adult normoxic vessels and for 9–12 fetal, 6–9 newborn, and 12–21 adult long-term hypoxic arteries. In addition, the frequency of firing for multiple cells was determined within those arteries for 59–90 individual myocytes from fetal, 40–45 from newborn, and 43–62 adult normoxic sheep and from 30–38 myocytes from fetal, 20–31 from newborn, and 41–70 from adult hypoxic animals. The 30 mM K+ increased the frequency in fetal but not newborn or adult myocytes, regardless of oxygenation status and 30 mM K+ also increased the number of active fetal LTH myocytes. In the newborn and adult groups, myocyte activity was relatively high under basal conditions, which we believe prevented further increases in Ca2+ wave activity due to membrane depolarization. Addition of 10 μM ryanodine to 30 mM K+ decreased the number of cells with Ca2+ waves and their frequency in the postnatal groups tested except for those from adult normoxic where it failed to reduce the number of cells with Ca2+ waves. Furthermore, ryanodine failed to reduce Ca2+ waves in fetal myocytes and had mixed responses on adult normoxic myocytes where it reduced the frequency of activity but not the number of cells that were firing. LTH in the fetus reduced the number of cells with basal Ca2+ wave activity, while in adults more cells were active. These basal Ca2+ waves had a slower frequency in LTH fetus. The 30 mM K+ increased the number of cells with Ca2+ wave activity and wave frequency more appreciably in LTH fetus, while only wave frequency was increased in LTH adult.
Fig. 8.
Effect of 30 mM K+ on whole-cell spatial and temporal Ca2+ signaling characteristics recorded in situ. A: baseline-subtracted fractional fluorescence (F/Fo) traces for Fluo-4 fluorescence are shown for individual pulmonary arterial myocytes from a normoxic adult sheep. Recordings were made in the absence (control) or presence of 30 mM K+ (30K) with or without 10 μM ryanodine (30K RY). Bars indicate means ± SE for normoxic (open) and long-term hypoxic (solid) conditions. B, D, and F: number of myocytes with Ca2+ responses each minute in a 1,000 μm2 area. C, E, and G: frequency of Ca2+ events *,§,†P < 0.05; **,§§,††P < 0.01; or ***,§§§P < 0.001, where the symbol * denotes significant difference within groups based on treatment using a Kruskal-Wallis 1-way ANOVA on ranks with a Dunn's multiple comparison procedure, the symbol § denotes significant difference within groups based on a 1-way ANOVA and an SNK multiple comparison procedure, and the symbol † denotes between groups based on their altitude using a Mann-Whitney U-test. Images were made with a ×60 Apochromat oil immersion objective.
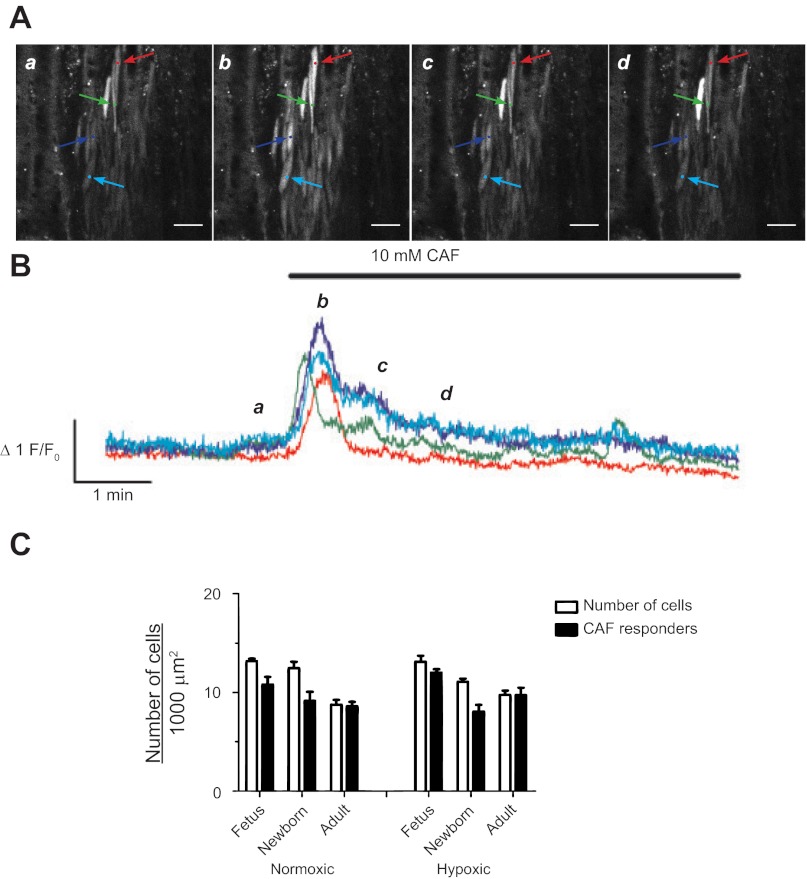
We next focused on the global responses to caffeine because it directly activates all RyRs as opposed to 30 mM K+ that indirectly activates RyRs by depolarizing the plasma membrane (18). Figure 9 shows Ca2+ responses in myocytes recorded before and during caffeine administration. The representative data trace in Fig. 9A shows images of myocytes in the arterial wall for various time points for the representative fluorescence intensity tracing in Fig. 9B. Caffeine rapidly increased cytosolic Ca2+ in virtually every pulmonary arterial myocyte of this adult hypoxic sheep, which then decayed back to baseline. Figure 9C summarizes the caffeine-mediated Ca2+ responses showing the number of cells with Ca2+ responses as well as the total number of cells in the arterial wall. Caffeine-elicited Ca2+ responses in nearly all myocytes from the six animal groups studied. Based on visual analysis of the images, the proportion of myocytes with Ca2+ responses was unaffected by maturation or LTH.
Fig. 9.
Caffeine causes whole cell Ca2+ responses in pulmonary arterial myocytes. A: representative fluorescent images at time points (a, b, c, and d) corresponding to the lower case italicized letters for the traces (B) of fractional Fluo-4 fluorescence shown for four pulmonary arterial myocyte from a LTH adult sheep. Colored arrows on images in A correspond to the placement of regions of interest for the traces of the same color in B. The 10 mM caffeine (CAF) was present during the time period denoted by the horizontal bar. C: number of myocytes in a 1,000 μm2 area measured in the multicolor images for Fig. 1 (open bars) and the number of myocytes with Ca2+ responses in the presence (solid bars) of 10 mM caffeine. Bars indicate means ± SE. No significant differences were noted between the number of myocytes responding to caffeine in each recording vs. the total number of myocytes in the arterial wall. Caffeine-elicited calcium responses were obtained in 3 fetal, 4 newborn, and 4 adult normoxic sheep and in 4 fetal, 4 newborn, and 3 adult LTH sheep. Images were made with a ×20 nonimmersion Apochromat or a ×63 water immersion c-Apochromat objective. Images in A were made with a ×63 objective. Scale bar = 20 μm.
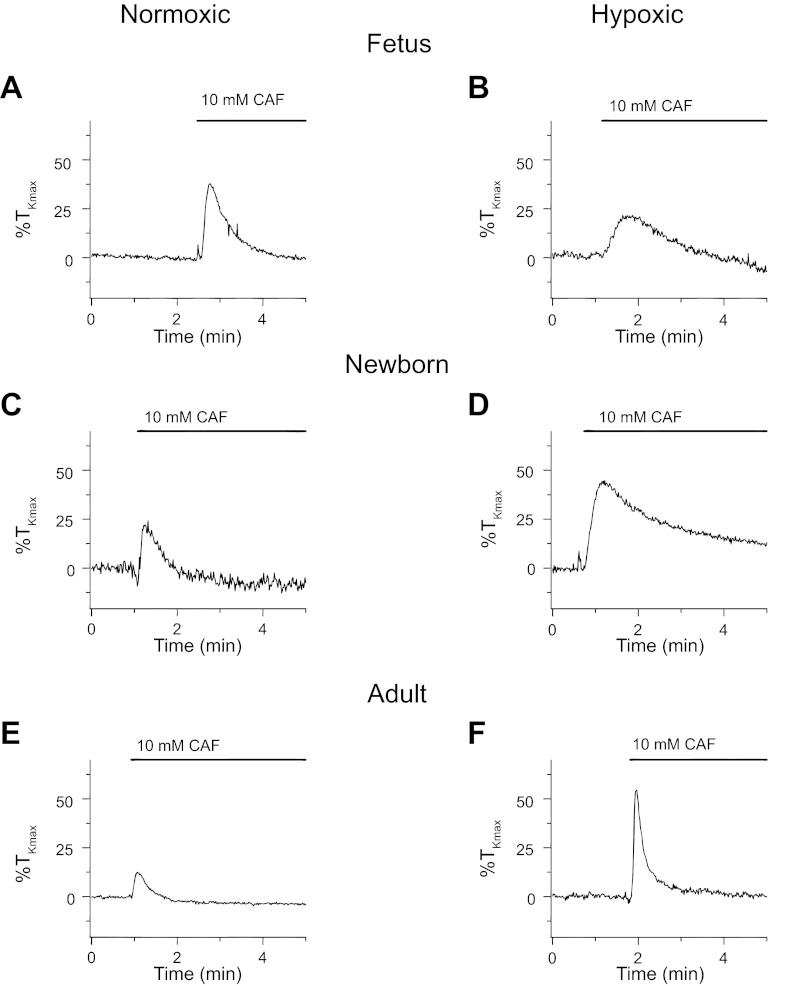
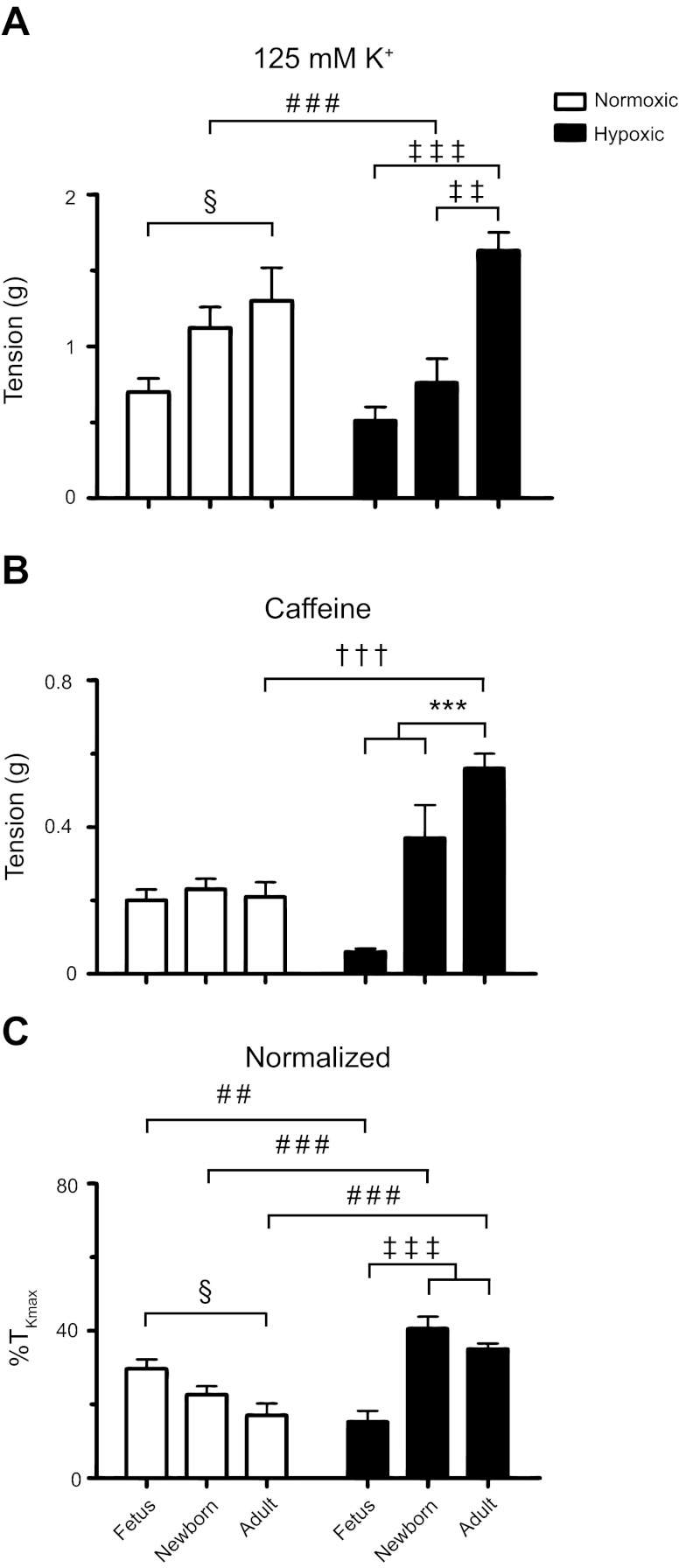
In view of the relationship between increases in the cytosolic Ca2+ concentration and the ability of the arteries to generate force (43), we performed additional studies. Figure 10 shows isometric contraction responses to 10 mM caffeine. Figure 10 shows representative tracings of caffeine-elicited contraction in PA segments isolated from normoxic and LTH fetal, newborn, and adult sheep. In all groups, caffeine elicited a transient contraction that corresponded with the short-lived Ca2+ responses due to caffeine stimulation shown in Fig. 9 (8, 15). As indicated in these representative traces, the contraction amplitude differed based on the animal age and exposure to LTH. Figure 11 shows the summarized responses that illustrate the effect of maturation and LTH on high K- and caffeine-elicited contraction. Figure 11A shows that in both normoxic and LTH PA, the contraction to high K was dependent on the level of maturity. We found that high K-force generation was lower in the fetus compared with the adult. The contraction responses of arteries from newborn sheep were intermediate to fetal and adult animals, and LTH reduced contraction only in this newborn group. These data confirm and extend our previous studies performed in PA from fetal and adult normoxic and LTH sheep where we have examined high K-contraction responses (9, 28, 29). Interestingly, Fig. 11B shows that force generated by caffeine increased following birth in LTH arteries but was preserved across all age groups in normoxic PA. LTH increased caffeine-elicited tension in vessels from adults, but not in the other two groups. Figure 11C shows the tension due to caffeine stimulation normalized to that of high K. These data show that in normoxic PA, the caffeine-elicited contraction was greater in the fetus compared with the adult. Concerning LTH, fetal arteries had reduced contraction to caffeine relative to vessels from newborn and adult hypoxic sheep. Overall, the reduced caffeine-elicited contraction in arteries from LTH fetal sheep follows from the very low Ca2+ spark (Fig. 4) but not from the well-maintained caffeine-elicited global Ca2+ responses (Fig. 9). This suggests postnatal maturation leads to complex changes in the ability of the arteries to contract in response to various stimuli. In addition, the absolute (Fig. 11B) and normalized (Fig. 11C) contractile responses to caffeine were markedly elevated in LTH newborn and adult arteries relative to arteries from their normoxic counterparts. This is distinct from the reduced Ca2+ spark amplitude in adult (Fig. 4) and equivalent caffeine elicited Ca2+ responses (Fig. 9). Again, these data suggest LTH causes complex changes in the regulation of RyR function that extend beyond alterations in RyR expression.
Fig. 10.
Caffeine causes transient contraction of pulmonary arterial rings in fetus, newborn, and adult. A–F: representative traces of arterial tension relative to %TKmax in response to 10 mM caffeine for arterial rings from normoxic and LTH sheep.
Fig. 11.
Contraction of pulmonary arterial rings to high K and caffeine are affected by maturation and LTH. Average tension generated by 125 mM K+ (A), 10 mM caffeine (B), and the normalized (C) contraction response relative to %TKmax in vessels from fetal, newborn, and adult sheep housed in normoxic (open) and LTH (solid) conditions. Bars indicate means ± SE. §P < 0.05; ‡‡P < 0.01; ***,†††,###,‡‡‡P < 0.001 denote significant difference (*) within and (†) between groups based on their age or altitude using a 2-way ANOVA and a Bonferroni multiple comparison procedure, (§) within groups using a 1-way ANOVA and a Newman-Keuls multiple comparison procedure, and (‡) using a Kruskal-Wallis 1-way ANOVA based on ranks and a Dunn's multiple comparison procedure, and (#) between groups using a Mann-Whitney U-test. Caffeine-induced contractile responses were examined in 4 fetal, 3 newborn, and 3 adult normoxic and in 4 fetal, 4 newborn, and 4 adult LTH sheep.
DISCUSSION
The present studies are the first to investigate the combined contributions of developmental maturation and acclimatization to high-altitude LTH on RyR activity in ovine pulmonary arterial myocytes. The major findings are as follows: 1) maturation increases global Ca2+ waves and Ca2+ spark activity; 2) LTH preferentially depresses Ca2+ spark activity in immature, but not mature, pulmonary arterial myocytes; 3) both maturation and LTH alter markedly Ca2+ spark firing characteristics; and 4) caffeine-elicited contraction but not Ca2+ signals are suppressed by LTH in the fetus, while contraction is augmented in the newborn and the adult.
Potassium-mediated depolarization of the plasma membrane is a common way to increase Ca2+ spark activity in smooth muscle (18) through activation of voltage-dependent Ca2+ entry (5). Furthermore, coordination among Ca2+ sparks is, at least, partly responsible for the global Ca2+ responses (48, 49). Based on these relations, we anticipated that both maturation and LTH would be associated with parallel changes in Ca2+ sparks and global Ca2+ responses. Rather, in LTH fetal PA, membrane depolarization of myocytes failed to enhance Ca2+ spark activity, but increased global Ca2+ wave activity. The lack of association between Ca2+ sparks and global Ca2+ responses following maternal LTH may result from hypoxia impeding communication between the plasma membrane and the sarcoplasmic reticulum. One possibility is that there is a gross structural rearrangement between the sacroplasmic reticulum and the plasma membrane. However, our morphological studies argue against such a phenomenon, even though we cannot discount the potential for alterations in peripheral and junctional sarcoplasmic reticulum. Instead, the division between Ca2+ sparks and global Ca2+ signals is most likely due to complexities in the interaction between voltage-dependent Ca2+ influx and RyR-dependent Ca2+ release (sparks). Within this scope, potential changes in the function of either Ca2+ influx or release compound these differences.
Our data indicate clearly that myocytes from LTH fetal sheep have voltage-dependent Ca2+ influx, as K+ stimulation increased global Ca2+ and caused arterial contraction. This and our previous studies argue against the idea that the lack of activation of Ca2+ sparks by 30 mM K+ in LTH fetus is due to reduced L-type Ca2+ channel expression. Comparisons of data from fetal, newborn, and adult sheep indicate that the depolarization-dependent pathways are more prominent following birth (9, 28, 29). Yet, our studies also illustrate that LTH does not alter high K-induced contraction in PA from fetal or adult sheep, while decreasing contraction in newborn vessels (9, 28). Together these data suggest L-type Ca2+ channel expression is preserved following LTH in the fetus and adult. The lack of change in L-type Ca2+ channel expression would be distinct from piglets in which postnatal LTH increased channel expression and currents (14). The finding that LTH reduces high K-induced contraction in the newborn, however, is more similar to the LTH-induced reduction of L-type Ca2+ channel function in pulmonary arterial myocytes from rats (37).
We anticipated that ryanodine would inhibit Ca2+ spark activity, a result confirming that RyRs are responsible for Ca2+ sparks. An unexpected and notable discovery, however, was that ryanodine failed to inhibit Ca2+ sparks in LTH fetal vessels. Nevertheless, Ca2+ spark activity was already at low levels in these arteries. One possibility is that ryanodine failed to inhibit Ca2+ events because the events we recorded are mediated by Ca2+ puffs due to localized release by inositol 1,4,5-trisphosphate [Ins(1,4,5)P3] receptors and are not due to RyRs (10, 30, 40). We believe this is unlikely, however, because we did not observe Ins(1,4,5)P3-driven Ca2+ puffs in our earlier studies on canine pulmonary arterial myocytes (19) and serotonin failed to increase Ca2+ spark-like events in pulmonary arterial myocytes from adult normoxic sheep (data not shown). These data and their interpretation indicate that LTH impedes RyR-dependent signaling in immature, but not adult, pulmonary arterial myocytes. Further support for this premise is the finding that LTH reduced caffeine-elicited contractions in fetal PA. A second possibility is that these changes are due to suppression of sarcoplasmic reticulum Ca2+ function, as we have recently shown that serotonin-generated Ca2+ signals are also reduced in myocytes of LTH fetal sheep (9). The discovery that LTH hinders RyR and potentially sarcoplasmic reticulum function in the fetus is exciting because it opens new avenues of research into these important regulators of pulmonary vascular tone.
The LTH-associated reduction in RyR activity in fetal pulmonary arterial myocytes could, presumably, be associated with alterations in one or more of the three RyR isoforms that are believed to be expressed in PA myocytes (21, 22, 49–51). Previous evidence shows that RyR2 is highly expressed in fetal cerebral arterial myocytes (7) and that PA myocytes from rats have all three RyR isoforms (46). Using ryanodine, our data confirm that in fetal and adult pulmonary arterial myocytes one or more of the three isoforms are important to Ca2+ spark activity and contraction. Unfortunately, ryanodine restricts the activity of all three RyR isoforms and, therefore, does not distinguish pharmacologically one isoform from another. In an attempt to resolve which RyR isoforms are important to Ca2+ sparks, we tested dantrolene, which preferentially inhibits RyR1 and RyR3 (41). The finding that dantrolene mainly reduced Ca2+ sparks in normoxic adults, but may actually enhance activity in hypoxic newborns and adults but not in the other groups, suggests that RyR1 and RyR3 have a more limited role. This implies that RyR2 predominates and that RyR1 and RyR3 function is labile and influenced by both development and LTH.
We did not anticipate that dantrolene would enhance Ca2+ spark activity in myocytes from long-term hypoxic newborns and adults. This augmentation in Ca2+ spark activity could be due to inhibition of a passive leak of Ca2+ from the sarcoplasmic reticulum mediated by tonic RyR activation. Such RyR dysfunctions are associated with malignant hyperthermia in skeletal muscle (45) and various cardiomycyte dysfunctions (36). Dantrolene treatment and the resultant inhibition of RyR activity potentially retards the tonic Ca2+ leak and recovers channel function (36, 45).
The established view is that membrane depolarization activates L-type Ca2+ channels, which in turn triggers type 1 and type 2 RyRs that are commonly expressed in pulmonary vascular smooth muscle (3, 5, 6). Based on this premise, we anticipated that K+-induced depolarization would increase both Ca2+ sparks and global Ca2+ responses. The influence of membrane depolarization on Ca2+ sparks and global responses did not follow this pattern, however. Most profound were PA myocytes from LTH fetuses, in which membrane depolarization increased global Ca2+ oscillations but not spark activity. Several possible reasons may account for this. One is that gestational hypoxia may reduce RyR expression, while depolarization-dependent processes remain intact. Secondly, gestational hypoxia may restrict the indirect coupling between L-type Ca2+ channels and RyRs, as this process requires diffusion of Ca2+ across a junctional cleft (5). This is in opposition to the tight coupling between the L-type Ca2+ channels and RyR in skeletal muscle (3). Finally, potentially there is reduced Ca2+ flux through L-type Ca2+ channels (5, 6) or increased cytosolic Ca2+ buffering (38, 42), each of which would impede Ca2+ activation of the RyR.
Our finding that Ca2+ spark activity increased following birth in PA myocytes also was unexpected. The most definitive study to date has shown that ∼20% of fetal rabbit PA myocytes had Ca2+ sparks (33). In contrast, the present findings appear similar to the influence of ontogeny on Ca2+ spark activity in rat cerebral arteries (7). Nonetheless, in the present studies the magnitude of the increases in Ca2+ spark activity following birth were greater than that observed in cerebral arteries. Our studies also demonstrate there are prominent changes in the spatial and temporal aspects to Ca2+ sparks that extend beyond fundamental changes in overall cellular Ca2+ spark activity. That is, postnatal maturation leads to significantly larger and more long-lived Ca2+ sparks, which in turn should enhance their influence on cell excitability.
The changes in the spatial and temporal characteristics of the Ca2+ sparks due to maturation and LTH were surprising. Changes in Ca2+ spark profiles likely occur as a consequence of alterations in the number of release sites or the frequency of activation. Yet, spatial and temporal changes in Ca2+ spark activity also could explain these differences. Namely, postnatal maturation leads to incremental changes that caused sparks to become larger, wider, and slower. In comparison, LTH caused Ca2+ sparks to become smaller, wider, and slower. Multiple cellular changes could contribute to these spatial and temporal changes in Ca2+ sparks. There could be modifications in the expression, as well as activity, of individual RyRs, Ca2+ buffering mechanisms, and changes in the ability of the Ca2+ stores to sequester Ca2+ (3). Delineating the importance of the cellular processes that govern Ca2+ spark activity will require systematic evaluation.
Perspective and Conclusions
The present study provides details regarding the combined influence of postnatal maturation and long-term perinatal and postnatal hypoxia on RyR activity. Presumably, the changes in RyR activity would modify ion channel activity and alter cell excitability and pulmonary arterial function through this interface and interaction. Conceivably, the depression in Ca2+ spark activity observed in LTH fetal and newborn PA myocytes could help protect newborn sheep from exacerbated pulmonary hypertension in a hypoxic environment following birth (12, 13). Alternatively, antenatal LTH could compromise oxygen-mediated pulmonary arterial relaxation at birth, a process also dependent on RyRs (23, 34).
GRANTS
A portion of this material was performed in the Loma Linda University School of Medicine Advanced Imaging and Microscopy Core that is supported by the National Science Foundation under Major Research Instrumentation, Division of Biological Infrastructure Grant 0923559 (to S. M. Wilson) and the Loma Linda University School of Medicine, Loma Linda, CA. The work was also supported in part by United States Public Health Services Grants HD/HL-03807 and HD-31226 (to L. D. Longo) and HD-069746 (to S. M. Wilson). S. R. Hadley was supported by a research fellowship from the Walter E. Macpherson Society at Loma Linda University. B. Lumbard was a scholar in the Undergraduate Training Program in the Center for Health Disparities and Molecular Medicine and was supported by Oakwood University and Howard Hughes Medical Institute under Grant 52006309 (to A. D. Paul) and by National Institutes of Health Grant 5-P20-MD-001632 (to M. DeLeón).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.R.H., Q.B., M.R., E.W., B.L., P.L., and S.M.W. analyzed data; S.R.H., Q.B., M.R., E.W., B.L., P.L., L.D.L., J.N.B., and S.M.W. interpreted results of experiments; S.R.H., Q.B., M.R., B.L., and S.M.W. prepared figures; S.R.H., Q.B., M.R., E.W., B.L., P.L., L.D.L., J.N.B., and S.M.W. edited and revised manuscript; S.R.H., Q.B., M.R., E.W., B.L., P.L., L.D.L., J.N.B., and S.M.W. approved final version of manuscript; Q.B., M.R., E.W., B.L., and S.M.W. performed experiments; L.D.L., J.N.B., and S.M.W. conception and design of research; J.N.B. and S.M.W. drafted manuscript.
ACKNOWLEDGMENTS
We thank Travis T. Merritt and Rachael H. Wilson for technical assistance and Jeff E. Angermann at the University of Nevada, Reno, NV for use of the CAT-12 hypoxic generator.
REFERENCES
- 1. Behringer EJ, Leite LD, Buchholz NE, Keeney MG, Pearce WJ, Vanterpool CK, Wilson SM, Buchholz JN. Maturation and long-term hypoxia alters Ca2+-induced Ca2+ release in sheep cerebrovascular sympathetic neurons. J Appl Physiol 107: 1223–1234, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonnet S, Hyvelin JM, Bonnet P, Marthan R, Savineau JP. Chronic hypoxia-induced spontaneous and rhythmic contractions in the rat main pulmonary artery. Am J Physiol Lung Cell Mol Physiol 281: L183–L192, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Cheng H, Lederer WJ. Calcium sparks. Physiol Rev 88: 1491–1545, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science 262: 740–744, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Collier ML, Ji G, Wang Y, Kotlikoff MI. Calcium-induced calcium release in smooth muscle: loose coupling between the action potential and calcium release. J Gen Physiol 115: 653–662, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collier ML, Thomas AP, Berlin JR. Relationship between L-type Ca2+ current and unitary sarcoplasmic reticulum Ca2+ release events in rat ventricular myocytes. J Physiol 516: 117–128, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gollasch M, Wellman GC, Knot HJ, Jaggar JH, Damon DH, Bonev AD, Nelson MT. Ontogeny of local sarcoplasmic reticulum Ca2+ signals in cerebral arteries: Ca2+ sparks as elementary physiological events. Circ Res 83: 1104–1114, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Goyal R, Creel KD, Chavis E, Smith GD, Longo LD, Wilson SM. Maturation of intracellular calcium homeostasis in sheep pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 295: L905–L914, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goyal R, Papamatheakis DG, Loftin M, Vrancken K, Dawson A, Osman NJ, Blood AB, Pearce WJ, Longo LD, Wilson SM. Long-term maternal hypoxia: the role of extracellular Ca2+ entry during serotonin-mediated contractility in fetal ovine pulmonary arteries. Reprod Sci 18: 948–962, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Groff JR, Smith GD. Calcium-dependent inactivation and the dynamics of calcium puffs and sparks. J Theor Biol 253: 483–499, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Guibert C, Marthan R, Savineau JP. Angiotensin II-induced Ca2+-oscillations in vascular myocytes from the rat pulmonary artery. Am J Physiol Lung Cell Mol Physiol 270: L637–L642, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Herrera EA, Pulgar VM, Riquelme RA, Sanhueza EM, Reyes RV, Ebensperger G, Parer JT, Valdez EA, Giussani DA, Blanco CE, Hanson MA, Llanos AJ. High-altitude chronic hypoxia during gestation and after birth modifies cardiovascular responses in newborn sheep. Am J Physiol Regul Integr Comp Physiol 292: R2234–R2240, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Herrera EA, Riquelme RA, Ebensperger G, Reyes RV, Ulloa CE, Cabello G, Krause BJ, Parer JT, Giussani DA, Llanos AJ. Long-term exposure to high-altitude chronic hypoxia during gestation induces neonatal pulmonary hypertension at sea level. Am J Physiol Regul Integr Comp Physiol 299: R1676–R1684, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hirenallur SD, Haworth ST, Leming JT, Chang J, Hernandez G, Gordon JB, Rusch NJ. Upregulation of vascular calcium channels in neonatal piglets with hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L915–L924, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hume JR, McAllister CE, Wilson SM. Caffeine inhibits InsP3 responses and capacitative calcium entry in canine pulmonary arterial smooth muscle cells. Vascul Pharmacol 50: 89–97, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jabr RI, Toland H, Gelband CH, Wang XX, Hume JR. Prominent role of intracellular Ca2+ release in hypoxic vasoconstriction of canine pulmonary artery. Br J Pharmacol 122: 21–30, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol 278: C235–C256, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Jaggar JH, Stevenson AS, Nelson MT. Voltage dependence of Ca2+ sparks in intact cerebral arteries. Am J Physiol Cell Physiol 274: C1755–C1761, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Janiak R, Wilson SM, Montague S, Hume JR. Heterogeneity of calcium stores and elementary release events in canine pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 280: C22–C33, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Kamitomo M, Longo LD, Gilbert RD. Cardiac function in fetal sheep during two weeks of hypoxemia. Am J Physiol Regul Integr Comp Physiol 266: R1778–R1785, 1994 [DOI] [PubMed] [Google Scholar]
- 21. Li XQ, Zheng YM, Rathore R, Ma J, Takeshima H, Wang YX. Genetic evidence for functional role of ryanodine receptor 1 in pulmonary artery smooth muscle cells. Pflügers Arch 457: 771–783, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liao B, Zheng YM, Yadav VR, Korde AS, Wang YX. Hypoxia induces intracellular Ca2+ release by causing ROS-mediated dissociation of FKBP12.6 with ryanodine receptor 2 in pulmonary artery myocytes. Antioxid Redox Signal 14: 37–47, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Linden BC, Resnik ER, Hendrickson KJ, Herron JM, O'Connor TJ, Cornfield DN. Chronic intrauterine pulmonary hypertension compromises fetal pulmonary artery smooth muscle cell O2 sensing. Am J Physiol Lung Cell Mol Physiol 285: L1354–L1361, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Longo LD, Ueno N, Zhao Y, Zhang L, Pearce WJ. NE-induced contraction, α1-adrenergic receptors, and Ins(1,4,5)P3 responses in cerebral arteries. Am J Physiol Heart Circ Physiol 270: H915–H923, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Matthews C, Cordelières FP. MetroloJ: an ImageJ plugin to help monitor microscopes' health. ImageJ User and Developer Conference, Mondorf-les-Bains, Luxembourg, 2010. [Google Scholar]
- 26.Matthews C, Cordelières FP.MetroloJ URL. http://imagejdocu.tudor.lu/doku.php?id=plugin:analysis:metroloj:start[2010].
- 27. Ng LC, Wilson SM, McAllister CE, Hume JR. Role of InsP3 and ryanodine receptors in the activation of capacitative Ca2+ entry by store depletion or hypoxia in canine pulmonary arterial smooth muscle cells. Br J Pharmacol 152: 101–111, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Papamatheakis DG, Vemulakonda S, Blood Q, Goyal R, Vrancken K, Bennett AM, Dawson A, Osman NJ, Blood AB, Pearce WJ, Longo LD, Wilson SM. Preservation of serotonin-mediated contractility in adult sheep pulmonary arteries following long-term, high-altitude hypoxia. High Alt Med Biol 12: 253–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Papamatheakis DG, Vemulakonda S, Patel J, Blood Q, Merritt T, Longo LD, Wilson SM. Depolarization-dependent contraction increase after birth and preservation following long-term hypoxia in sheep pulmonary arteries. Pulm Circ 2: 41–53, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parker I, Yao Y. Calcium puffs in Xenopus oocytes. Ciba Found Symp 188: 50–60, 1995 [DOI] [PubMed] [Google Scholar]
- 31. Perez JF, Sanderson MJ. The contraction of smooth muscle cells of intrapulmonary arterioles is determined by the frequency of Ca2+ oscillations induced by 5-HT and KCl. J Gen Physiol 125: 555–567, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Picht E, Zima AV, Blatter LA, Bers DM. SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol Cell Physiol 293: C1073–C1081, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Porter VA, Reeve HL, Cornfield DN. Fetal rabbit pulmonary artery smooth muscle cell response to ryanodine is developmentally regulated. Am J Physiol Lung Cell Mol Physiol 279: L751–L757, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Porter VA, Rhodes MT, Reeve HL, Cornfield DN. Oxygen-induced fetal pulmonary vasodilation is mediated by intracellular calcium activation of KCa channels. Am J Physiol Lung Cell Mol Physiol 281: L1379–L1385, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Rasband WS. ImageJ. Bethesda, MD: National Institutes of Health, 2011 [Google Scholar]
- 36. Sacherer M, Sedej S, Wakula P, Wallner M, Vos MA, Kockskamper J, Stiegler P, Sereinigg M, von Lewinski D, Antoons G, Pieske BM, Heinzel FR. JTV519 (K201) reduces sarcoplasmic reticulum Ca2+ leak and improves diastolic function in vitro in ouabain-induced cellular Ca2+ overload in murine and human non-failing myocardium. Br J Pharmacol 167: 493–504, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shimoda LA, Sham JS, Shimoda TH, Sylvester JT. L-type Ca2+ channels, resting [Ca2+]i, and ET-1-induced responses in chronically hypoxic pulmonary myocytes. Am J Physiol Lung Cell Mol Physiol 279: L884–L894, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Smith GD, Wagner J, Keizer J. Validity of the rapid buffering approximation near a point source of calcium ions. Biophys J 70: 2527–2539, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tucker A, McMurtry IF, Reeves JT, Alexander AF, Will DH, Grover RF. Lung vascular smooth muscle as a determinant of pulmonary hypertension at high altitude. Am J Physiol 228: 762–767, 1975 [DOI] [PubMed] [Google Scholar]
- 40. Ullah G, Parker I, Mak DO, Pearson JE. Multi-scale data-driven modeling and observation of calcium puffs. Cell Calcium 52: 152–160, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vaithianathan T, Narayanan D, Asuncion-Chin MT, Jeyakumar LH, Liu J, Fleischer S, Jaggar JH, Dopico AM. Subtype identification and functional characterization of ryanodine receptors in rat cerebral artery myocytes. Am J Physiol Cell Physiol 299: C264–C278, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wagner J, Keizer J. Effects of rapid buffers on Ca2+ diffusion and Ca2+ oscillations. Biophys J 67: 447–456, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wilson SM, Mason HS, Ng LC, Montague S, Johnston L, Nicholson N, Mansfield S, Hume JR. Role of basal extracellular Ca2+ entry during 5-HT-induced vasoconstriction of canine pulmonary arteries. Br J Pharmacol 144: 252–264, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wilson SM, Mason HS, Smith GD, Nicholson N, Johnston L, Janiak R, Hume JR. Comparative capacitative calcium entry mechanisms in canine pulmonary and renal arterial smooth muscle cells. J Physiol 543: 917–931, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang T, Esteve E, Pessah IN, Molinski TF, Allen PD, Lopez JR. Elevated resting [Ca2+]i in myotubes expressing malignant hyperthermia RyR1 cDNAs is partially restored by modulation of passive calcium leak from the SR. Am J Physiol Cell Physiol 292: C1591–C1598, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Yang XR, Lin MJ, Yip KP, Jeyakumar LH, Fleischer S, Leung GP, Sham JS. Multiple ryanodine receptor subtypes and heterogeneous ryanodine receptor-gated Ca2+ stores in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 289: L338–L348, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Zhang WM, Lin MJ, Sham JS. Endothelin-1 and IP3 induced Ca2+ sparks in pulmonary arterial smooth muscle Cells. J Cardiovasc Pharmacol 44, Suppl 1: S121–S124, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Zhang WM, Yip KP, Lin MJ, Shimoda LA, Li WH, Sham JS. ET-1 activates Ca2+ sparks in PASMC: local Ca2+ signaling between inositol trisphosphate and ryanodine receptors. Am J Physiol Lung Cell Mol Physiol 285: L680–L690, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Zheng YM, Mei QB, Wang QS, Abdullaev I, Lai FA, Xin HB, Kotlikoff MI, Wang YX. Role of FKBP12.6 in hypoxia- and norepinephrine-induced Ca2+ release and contraction in pulmonary artery myocytes. Cell Calcium 35: 345–355, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Zheng YM, Wang QS, Liu QH, Rathore R, Yadav V, Wang YX. Heterogeneous gene expression and functional activity of ryanodine receptors in resistance and conduit pulmonary as well as mesenteric artery smooth muscle cells. J Vasc Res 45: 469–479, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zheng YM, Wang QS, Rathore R, Zhang WH, Mazurkiewicz JE, Sorrentino V, Singer HA, Kotlikoff MI, Wang YX. Type-3 ryanodine receptors mediate hypoxia-, but not neurotransmitter-induced calcium release and contraction in pulmonary artery smooth muscle cells. J Gen Physiol 125: 427–440, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]