Abstract
Objectives
To examine the relationship between depressive symptoms and subjective and objective sleep in older women.
Design
Cross-sectional.
Setting
Four US clinical centers.
Participants
3045 community-dwelling women ≥70 years.
Measurements
Depressive symptoms were assessed with the Geriatric Depression Scale categorizing participants as “normal” (0–2, referent), “some depressive symptoms” (3–5), or “depressed” (≥6). Subjective sleep quality and daytime sleepiness were assessed using the Pittsburgh Sleep Quality Index (PSQI) and Epworth Sleepiness Scale (ESS). Objective sleep measures were assessed with wrist actigraphy.
Results
In multivariable-adjusted models, there were graded associations between increased level of depressive symptoms and both worse subjective sleep quality and more subjective daytime sleepiness (p-trends <0.001). Women with some depressive symptoms (OR 1.82, CI 1.48–2.24) and depressed (OR 2.84, CI 2.08–3.86) women had greater odds of reporting poor sleep (PSQI>5). Women with some depressive symptoms (OR 1.97, CI 1.47–2.64) and depressed women (OR 1.70, CI 1.12–2.58) had greater odds of reporting excessive daytime sleepiness (ESS>10). There were also graded associations between increased level of depressive symptoms and objectively measured wake after sleep onset (WASO) (p-trend = 0.030) and long wake episodes >5 minutes (p-trend 0.006). Depressed women had modestly increased odds of WASO ≥1 hour (OR 1.37, CI 1.03–1.83). Women with some depressive symptoms (OR 1.49, CI 1.19–1.86) and depressed women (OR 2.04, CI 1.52–2.74) had greater odds of being in the highest quartile for number of nap episodes >5 minutes. No associations between depressive symptom level and prolonged sleep latency, reduced sleep efficiency, or reduced or increased total sleep time were found.
Conclusion
Greater depressive symptom levels were associated with more subjective sleep disturbance and objective evidence of sleep fragmentation and napping.
Keywords: Depression, sleep, actigraphy, elderly, age
INTRODUCTION
Depression is a common complaint in older adults. The estimated prevalence of significant depressive symptoms in community-dwelling older adults is 15% (1) and depression in this age group has been associated with a number of adverse outcomes including functional impairment (2, 3), medical illnesses (4), disability (5, 6), and increased health services utilization (7–9).
Poor sleep is also common among older adults. In one survey of 9000 adults aged 65 years or older, over 80% of those surveyed reported at least one problem with sleep while more than half reported that at least one sleep complaint occurred nearly all the time (10). Further, older adults may under-report the severity of sleep disturbances compared with objective assessments by polysomnography (PSG) (11). Insomnia has been associated with poor outcomes in older adults with depression (12) and poor subjective sleep quality is a risk factor for suicide in older adults (13).
Although insomnia in depressed patients was initially conceptualized as a symptom of depression, recent evidence supports a bidirectional relationship between insomnia and depression. Longitudinal studies have shown that subjective sleep disturbance is a major risk factor for future development of both first-onset and recurrent depressive episodes in both younger and older adults (12, 14–16). However, the existing literature regarding the relationship between depression and sleep disturbances in older adults has largely focused on subjective assessments of sleep disturbances. There is therefore little data regarding the relationship between depression and objectively measured sleep disturbances in this age group. This relationship was recently explored using actigraphy to assess objective sleep disturbances in a group of community-dwelling older men. Although a strong, graded association between increased level of depressive symptoms and subjective sleep disturbance was found, objective assessment of sleep revealed only a modest association between increased level of depressive symptoms and increased sleep latency (17). To our knowledge there are no studies looking at the relationship between depression and objectively measured sleep in older women.
We examined this relationship in a large group of community-dwelling older women. We hypothesized that older women who endorsed more depressive symptoms would be more likely to report subjective sleep disturbances and more likely to have objective evidence of disturbed night time sleep and increased daytime napping.
METHODS
Participants
Participants were women enrolled in the Study of Osteoporotic Fractures (SOF), an ongoing, multi-center, prospective, observational cohort study of primarily Caucasian, community-dwelling women aged 65 years and older from four metropolitan areas (Portland, OR; Minneapolis, MN; Pittsburgh, PA/Monongahela Valley, PA; Baltimore, MD). Women gave informed consent prior to enrollment in the study. Between September 1986 and October 1988 the 9,704 participants making up the original cohort were recruited via community listings and mailed announcements. Between February 1997 and February 1998, 662 African American women were also recruited. Women were excluded from participation if they required assistance with ambulation or had undergone bilateral hip replacement. Details regarding the study have been published previously (18, 19). The current analyses focused on women participating in SOF visit 8 (approximately 15 years after baseline assessment) which occurred between January 2002 and February 2004. There were 4727 participants at visit 8, representing 84% of active survivors. Of these women, 3052 had objective assessment of sleep by actigraphy and 3045 also returned completed Geriatric Depression Scale (GDS) questionnaires. The analyses of night time sleep were preformed on this subset of 3045 women. Fifty of these women did not have usable daytime data either because they removed their actigraph for more than 10% of the wake-time or because there were not two nights of data surrounding the day. Hence, the analyses of number of naps was performed on 2995 subjects.
Subjective Measures
Demographic information (age, ethnicity, education) was recorded at baseline. At visit 8, participants completed questionnaires regarding health status, smoking, alcohol consumption, caffeine intake, exercise, and medical history. Medications were recorded and categorized according to a computerized coding dictionary. Cognition was assessed using the Mini-Mental State Exam (MMSE) and cognitive impairment was defined as MMSE ≤ 24 (19).
Depression was assessed using the Geriatric Depression Scale (GDS), a 15-item validated self-report questionnaire commonly used for assessment of depressive symptoms in older adults (20). Women were categorized into three groups [0–2 (none to few depressive symptoms), 3–5 (some depressive symptoms), ≥ 6 (many depressive symptoms)]. To remain consistent with prior publications using this strategy(17), these groups are labeled as “normal” (0–2), “some depressive symptoms” (3–5), and “depressed” (≥ 6) throughout the remainder of the manuscript. A standard cut-off of ≥6 has been shown to have a sensitivity of 91% and specificity of 65% for diagnosis of a major depressive episode compared with the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (21).
Subjective sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI), a validated19-item self-report questionnaire. Global PSQI scores range from 0–21 with higher scores reflecting worse sleep. A total score >5 has a sensitivity of 89.6% and specificity of 86.5% for distinguishing good sleepers from poor sleepers (22). Subjective daytime sleepiness was measured using the Epworth Sleepiness Scale (ESS), a self-administered questionnaire that asks subjects to rate how likely they are to doze off in different situations. Scores on the ESS range from 0–24 with a standard cut-off of greater than 10 indicating excessive daytime sleepiness (23, 24).
Actigraphy
Objective sleep parameters were measured using wrist actigraphy, a previously validated (25–27) non-invasive tool which provides information about sleep and wake patterns via an accelerometer that detects wrist movement. Actigraphs (SleepWatch-O, Ambulatory Monitoring, inc., Ardsley, NY) were worn on participants’ non-dominant wrist for at least 3 consecutive 24 hour periods. Movements were recorded and summarized in 1-minute epochs. The data were collected using the proportional integration mode (PIM) (26) and analyzed using ActionW-2 software (Ambulatory Monitoring, Inc., Ardsley, NY). The University of California San Diego algorithm was used to distinguish sleep from wake (28, 29). Participants also completed sleep diaries for the time period they wore the actigraph. The diaries included information about times participants got into and out of bed and times the actigraph was removed. This information was used in editing the actigraphy data files to set intervals for when the participant was in bed trying to sleep (after “lights off”), and to delete time when the actigraph was removed. In the SOF study, inter-scorer reliability for editing the actigraphy data files has been previously found to be high (TST intra-class coefficient = 0.95), and TST estimated via actigraphy has been shown to have good concordance with TST assessed by PSG (25, 26).
The following variables were used in the analyses: total sleep time (TST, minutes sleep between bed time and wake time), sleep efficiency (SE, percent of time asleep while in bed), sleep onset latency (SOL, minutes between bed time and the first block of inactivity after bed time), time awake after sleep onset (WASO, minutes awake between sleep onset and wake time), number of long wake episodes (number of wake episodes between sleep onset and wake time exceeding 5 minutes), and number nap episodes (number of inactive episodes between wake time and sleep onset exceeding 5 minutes). Daytime data was excluded from the analysis if the participant took the actigraph off for greater than 10% of the wake period. Actigraphy data were collected for an average of 4.1 (+/−0.83) days. Data for each variable was averaged over the recorded time.
Statistical Analyses
Differences in the characteristics of the participants according to level of depressive symptoms were assessed using a chi-squared test for categorical variables, analysis of variance for normally distributed continuous data, and Kruskal-Wallis tests for skewed continuous data (caffeine intake and number of medical conditions). Pearson’s correlations were preformed to determine the relationship between subjective and objective sleep variables in the entire sample and in each subgroup of women by level of depressive symptoms.
Objective and subjective sleep measures were analyzed as continuous variables using linear regression models and the least-squared means strategy was used to estimate the mean and 95% confidence interval (CI) for each sleep parameter by level of depressive symptoms. Tests of linear trend were performed to detect graded associations. Because the distributions of SE, SOL, WASO, and number nap episodes were skewed, the data was transformed for normality [LN (P) for WASO and SOL, LN (100-P) for SE, LN (P+1) for number nap episodes > 5 minutes.
Sleep variables were also expressed as dichotomous outcomes. Specific categories included, PSQI > 5 vs. PSQI ≤ 5, and ESS > 10 vs. ESS ≤ 10, SE <85% vs. SE ≥85%, SOL ≥30 minutes vs. SOL <30 minutes, WASO ≥1 hour vs. WASO <1 hour, number long wake episodes ≥8 vs. number long wake episodes <8. TST was expressed as a three level outcome including <5 hours (short sleep duration), 5–8 hours (normal sleep duration), and >8 hours (prolonged sleep duration). Because there were no previously described clinically relevant cut-points for number nap episodes, quartiles were used and the women in the highest quartile were compared with the rest of the sample. Associations between depressive symptoms and dichotomous sleep variables were assessed using logistic regression and a test for linear trend. The association between depressive symptoms and TST was assessed using a multinomial regression model with normal sleepers serving as the referent category.
Models adjusted for age and site and multivariable adjusted models were assessed. Covariates were included in multivariable models if they were known correlates of sleep disturbance or depression or if they were related to the level of depressive symptoms in this population (p ≤ 0.10). These included age, race, site, smoking status, body mass index (BMI), self reported health status, education, exercise, reported medical conditions, impairments in activities of daily living, cognitive impairment, antidepressant use, benzodiazapene use, and use of medications for sleep.
RESULTS
Characteristics of the Study Population
Characteristics of the sample are shown in Table 1. Ages ranged from 70–99 years with a mean age of 83.6 (± 3.8) years. The majority of the women were Caucasian (89.3%), rated their health status as “excellent” or “good” (75.4%), lived alone (60.4%), were not smokers (97.2%), and reported drinking two or less alcoholic beverages per week (86.9%). The group was diverse with respect to education with 40.1% having graduated college, 41.9% having completed high school and 18.0% having completed less education. The average number of medical conditions reported was 2.46 (+/− 1.61). A minority of the sample population reported use of medications for sleep (14.0%) or antidepressants (13.7%). The rate of cognitive impairment (MMSE ≤24) was low in the study population (5.6%) and the mean MMSE score was 27.85 +/− 2.01. More than half (52.0%) of the population reported subjectively poor sleep (PSQI >5) and a significant portion (11.0%) reported excessive daytime sleepiness (ESS>10).
Table 1. Characteristics of Participants According to Level of Depressive Symptoms.
GDS (Geriatric Depression Scale); MMSE (Mini Mental State Exam); IADL (Instrumental Activities of Daily Living); SD (Standard Deviation). Reported medical conditions includes stroke, diabetes, Parkinson’s disease, chronic obstructive pulmonary disease, congestive heart failure, myocardial infarction, thyroid disease, hypertension, other neurological condition, other cardiac condition, osteoarthritis, rheumatoid arthritis, osteoporosis, and cancer.
| Level of Depressive Symptoms
|
|||||
|---|---|---|---|---|---|
| Population Characteristics | All Participants | Normal (GDS 0–2) | Some Depressive Symptoms (GDS 3–5) | Depressed (GDS ≥ 6) | P-value |
| Total number of participants | 3045 | 1966 | 718 | 361 | |
| Age group n(%) | <.001 | ||||
| 70–80 years | 527 (17.3) | 390 (19.8) | 96 (13.4) | 41 (11.4) | |
| 81–82 years | 853 (28.0) | 585 (29.8) | 192 (26.7) | 76 (21.1) | |
| 83–85 years | 833 (27.4) | 535 (27.2) | 192 (26.7) | 106 (29.4) | |
| 86–100 years | 832 (27.3) | 456 (23.2) | 238 (33.1) | 138 (38.2) | |
| African-American, n(%) | 325 (10.7) | 231 (11.7) | 60 (8.4) | 34 (9.4) | 0.030 |
| Self-reported health status, n(%) | <.001 | ||||
| Excellent or good | 2295 (75.4) | 1690 (86.0) | 452 (63.0) | 153 (42.4) | |
| Fair, poor, or very poor | 750 (24.6) | 276 (14.0) | 266 (37.0) | 208 (57.6) | |
| Lives alone, n(%) | 1840 (60.4) | 1167 (59.4) | 443 (61.7) | 230 (63.7) | 0.217 |
| Education, n(%) | <.001 | ||||
| Less than high school diploma | 547 (18.0) | 325 (16.5) | 133 (18.6) | 89 (24.7) | |
| High school diploma | 1275 (41.9) | 800 (40.7) | 316 (44.1) | 159 (44.2) | |
| College/Graduate School | 1221 (40.1) | 841 (42.8) | 268 (37.4) | 112 (31.1) | |
| Alcohol use (Drinks per week) n(%) | 0.024 | ||||
| 0 – 2 drinks per week | 2647 (86.9) | 1682 (85.6) | 636 (88.6) | 329 (91.1) | |
| 3 – 13 drinks per week | 341 (11.2) | 243 (12.4) | 72 (10.0) | 26 (7.2) | |
| > 13 drinks per week | 57 (1.9) | 41 (2.1) | 10 (1.4) | 6 (1.7) | |
| Smoking status, n(%) | 0.002 | ||||
| Never Smoked | 1935 (63.6) | 1279 (65.1) | 447 (62.3) | 209 (57.9) | |
| Former Smoker | 1024 (33.6) | 644 (32.8) | 248 (34.6) | 132 (36.6) | |
| Current Smoker | 85 (2.8) | 43 (2.2) | 22 (3.1) | 20 (5.5) | |
| Caffeine intake, mean +/− SD, mg/day | 151.08 +/− 154.13 | 152.48 +/− 156.11 | 147.72 +/− 151.15 | 150.19 +/− 149.4 | 0.829 |
| Current antidepressant use, n(%) | 417 (13.7) | 181 (9.2) | 138 (19.3) | 98 (27.1) | <.001 |
| Current benzodiazepine use, n(%) | 222 (7.3) | 110 (5.6) | 59 (8.2) | 53 (14.7) | <.001 |
| Current non-benzodiazepine anxiolytic/hypnotic use n(%) | 33 (1.1) | 16 (0.8) | 10 (1.4) | 7 (1.9) | 0.108 |
| Reported use of sleep medication, n(%) | 427 (14.0) | 230 (11.7) | 114 (15.9) | 83 (23.0) | <.001 |
| Cognitively impaired (MMSE< 24), n(%) | 163 (5.7) | 87 (4.6) | 45 (6.9) | 31 (9.8) | 0.004 |
| Body mass index (BMI), n(%) | 0.002 | ||||
| Underweight or normal weight (BMI <25) | 1108 (37.2) | 691 (35.5) | 269 (38.82) | 148 (43.79) | |
| Overweight (BMI 25–30) | 1131 (38.0) | 780 (40.0) | 253 (36.51) | 98 (28.99) | |
| Obese (BMI ≥30) | 740 (24.8) | 477 (24.5) | 171 (24.68) | 92 (27.22) | |
| Takes walks for exercise, n(%) | 1119 (37.2) | 831 (42.7) | 203 (28.63) | 85 (23.94) | <.001 |
| IADL impairments, n(%) | 1609 (53.1) | 814 (41.6) | 500 (70.03) | 295 (82.17) | <.001 |
| Number of selected medical conditions, mean +/− SD | 2.46 +/− 1.61 | 2.2 +/− 1.49 | 2.85 +/− 1.7 | 3.09 +/− 1.72 | <.001 |
The sample was stratified into three groups according to the level of depressive symptoms. 1966 (64.6%) reported 0–2 depressive symptoms (“normal”), 718 (23.6%) reported 3–5 (“some depressive symptoms”), and 361 (11.9%) women reported ≥6 symptoms (“depressed”). Women with greater levels of depressive symptoms were more likely to be older, to smoke, to be cognitively impaired, to report poor health, to be less educated, and to report use of antidepressants, benzodiazapines, and medications for sleep. They were also more likely to report impairment in instrumental activities of daily living (IADLs), less likely to walk for exercise, more likely to be obese, and reported more medical problems.
In general subjective and objective sleep measures were either weakly correlated or not significantly correlated in this sample. For example there was a small but significant inverse correlation between PSQI and SE (r= −0.12, p=<0.001) and small but significant direct correlations between PSQI and both WASO (r= 0.13. p=<0.001) and SOL (r= 0.08, p=<0.001) while PSQI and TST were not significantly correlated. There were also small but significant correlations between ESS and TST (r=0.17, p<001), and WASO (R=0.07, r<0.001) in the sample. These relationships were similar in all groups of women with differing levels of depressive symptoms. There was no significant correlation between ESS and either SE or SOL either in the total sample or in any of the groups of women by depressive symptom level.
Level of Depressive Symptoms and Subjective Sleep Measures
Regardless of level of depressive symptoms, the estimated mean PSQI scores for all groups of women were greater than the clinical cut-point of 5, suggesting poor subjective sleep quality (Table 2). After adjustment for age and site, there was a strong graded association between greater level of depressive symptoms and worse subjective sleep quality (p-trend < 0.001). There was also a significant association between greater level of depressive symptoms and increased subjective daytime sleepiness (ESS; p-trend < 0.001). However, the absolute differences in mean ESS scores were small and means were in the normal range for all three groups. These associations remained significant after adjustment for multiple potential confounders.
Table 2. Comparison Subjective Sleep Parameters by Level of Depressive Symptoms.
WASO (wake after sleep onset). Multivariable models adjusted for age, race, site, education, exercise, BMI, smoking status, alcohol intake, self reported health status, IADL impairments, cognitive impairment, number of reported medical conditions, and current use of antidepressants, benzodiazepines, and medications for sleep.
| Level of Depressive Symptoms
|
||||
|---|---|---|---|---|
| Subjective Sleep Parameters, mean (95% Confidence Interval) | Normal (GDS 0–2) | Some Depressive Symptoms (GDS 3–5) | Depressed (GDS ≥ 6) | P-trend |
| Pittsburgh Sleep Quality Index (0–21) | ||||
| Age and site adjusted (N=3045) | 5.6 (5.4–5.8) | 7.1 (6.8–7.3) | 8.6 (8.2–8.9) | < .001 |
| Multivariable adjusted (N=2785) | 5.8 (5.7–6.0) | 6.9 (6.6–7.1) | 8.1 (7.7–8.5) | < .001 |
| Epworth Sleepiness Scale (0–24) | ||||
| Age and site adjusted (N=3045) | 5.3 (5.1–5.5) | 6.4 (6.1–6.7) | 6.1 (5.8–6.5) | < .001 |
| Multivariable adjusted (N=2785) | 5.3 (5.2–5.5) | 6.3 (6.0–6.5) | 6.2 (5.7–6.6) | < .001 |
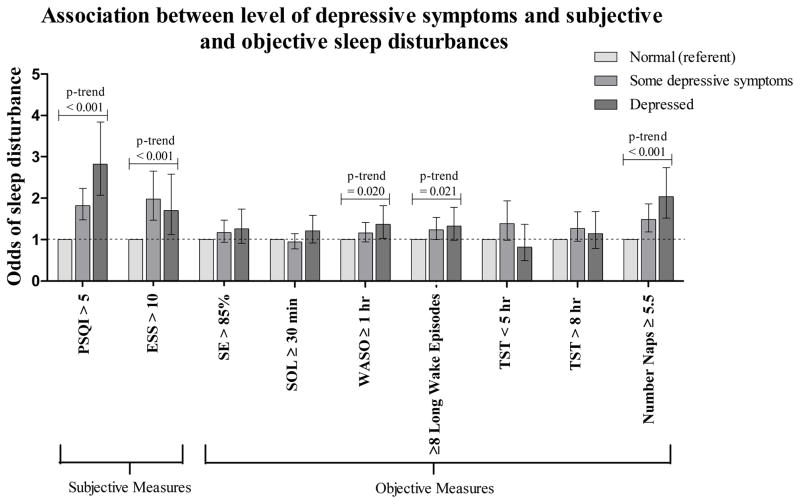
The odds of subjective sleep disturbances were increased for women with greater levels of depressive symptoms (Figure 1 and Table 4). In age- and site- adjusted models, the odds of endorsing subjective sleep disturbance were significantly increased for the group with some depressive symptoms (OR 2.01, CI 1.69–2.40) and further increased for the depressed group (OR 3.36, CI 2.61–4.32) compared with the normal group. The odds of subjective excessive daytime sleepiness were increased for women with some depressive symptoms (OR 2.16, CI 1.67–2.79) and for those in the depressed group (OR 1.90, CI 1.35–2.67) compared with women in the normal group. These associations remained statistically significant after adjusting for multiple potential confounders.
Figure 1.
Odds ratios and 95% confidence intervals (95% CI) for subjective and objective sleep disturbances are shown for groups of women with differing levels of depressive symptoms compared with the “normal” (referent) group. Models represented in the graph are adjusted for multiple possible confounders including age, race, site, education, exercise, body mass index, smoking status, alcohol intake, self-reported health status, instrumental activities of daily living impairments, cognitive impairment, and current use of antidepressants, benzodiazapines, and medications for sleep.
Table 4. Association Between Depressive Symptoms and Subjective and Objective Sleep Disturbances.
Multivariable models adjusted for age, race, site, education, exercise, body mass index, smoking status, alcohol intake, self reported health status, instrumental activities of daily living impairments, cognitive impairment, number of reported medical conditions, and current use of antidepressants, benzodiazapines, and medications for sleep.
| Level of Depressive Symptoms
|
||||
|---|---|---|---|---|
| Sleep Disturbance, Odds Ratio (95% Confidence Interval) | Normal (GDS 0–2) | Some Depressive Symptoms (GDS 3–5) | Depressed (GDS ≥ 6) | P-trend |
| Subjective Sleep Disturbances | ||||
| Pittsburgh Sleep Quality Index > 5 | ||||
| Age and site adjusted (N=3044) | 1.0 (referent) | 2.01 (1.69–2.40) | 3.36 (2.61–4.32) | <.001 |
| Multivariable adjusted (N=2985) | 1.0 (referent) | 1.82 (1.48–2.24) | 2.84 (2.08–3.86) | <.001 |
| Epworth Sleepiness Scale > 10 | ||||
| Age and site adjusted (N=3044) | 1.0 (referent) | 2.16 (1.67–2.79) | 1.90 (1.35–2.67) | <.001 |
| Multivariable adjusted (N=2985) | 1.0 (referent) | 1.97 (1.47–2.64) | 1.70 (1.12–2.58) | <.001 |
| Objective Sleep Disturbances | ||||
| Sleep Efficiency < 85% | ||||
| Age and site adjusted (N=3044) | 1.0 (referent) | 1.38 (1.13–1.68) | 1.53 (1.17–2.01) | <.001 |
| Multivariable adjusted (N=2985) | 1.0 (referent) | 1.18 (0.94–1.47) | 1.27 (0.92–1.74) | 0.078 |
| Sleep Onset Latency ≥ 30 minutes | ||||
| Age and site adjusted (N=3044) | 1.0 (referent) | 1.12 (0.94–1.33) | 1.38 (1.09–1.73) | 0.005 |
| Multivariable adjusted (N=2985) | 1.0 (referent) | 0.95 (0.78–1.15) | 1.21 (0.92–1.59) | 0.390 |
| Wake After Sleep Onset ≥ 1 hour | ||||
| Age and site adjusted (N=3044) | 1.0 (referent) | 1.45 (1.22–1.73) | 1.73 (1.36–2.19) | <.001 |
| Multivariable adjusted (N=2985) | 1.0 (referent) | 1.16 (0.95–1.42) | 1.37 (1.03–1.83) | 0.019 |
| ≥8 Long Wake Episodes | ||||
| Age and site adjusted (N=3044) | 1.0 (referent) | 1.55 (1.29–1.86) | 1.84 (1.45–2.33) | <.001 |
| Multivariable adjusted (N=2985) | 1.0 (referent) | 1.25 (1.01–1.55) | 1.34 (1.00–1.79) | 0.019 |
| Total Sleep Time < 5 hours (short sleeper) | ||||
| Age and site adjusted (N=3044) | 1.0 (referent) | 1.82 (1.36–2.43) | 1.30 (0.86–1.96) | 0.006 |
| Multivariable adjusted (N=2985) | 1.0 (referent) | 1.39 (0.99–1.95) | 0.88 (0.50–1.37) | 0.877 |
| Total Sleep Time > 8 hours (long sleeper) | ||||
| Age and site adjusted (N=3044) | 1.0 (referent) | 1.46 (1.15–1.86) | 1.58 (1.17–2.15) | <.001 |
| Multivariable adjusted (N=2985) | 1.0 (referent) | 1.26 (0.96–1.67) | 1.15 (0.79–1.68) | 0.223 |
| Number Nap Episodes ≥ 5.5 | ||||
| Age and site adjusted (N=2995) | 1.0 (referent) | 1.71 (1.40–2.08) | 2.57 (2.02–3.27) | <.001 |
| Multivariable adjusted (N=2742) | 1.0 (referent) | 1.49 (1.19–1.86) | 2.04 (1.52–2.74) | <.001 |
Level of Depressive Symptoms and Objective Sleep Measures
After adjustment for age and site, there were graded associations between greater levels of depressive symptoms and decreased SE (p-trend < 0.001), increased SOL (p-trend =0 .003), increased WASO (p-trend < 0.001), and greater number of long wake episodes (Table 3; p-trend < 0.001). After adjustment for multiple variables, graded associations between greater level of depressive symptoms and increased WASO and number of long wake episodes continued to be significant. There were no association between level of depressive symptoms and either short or long TST.
Table 3. Comparison of Objective Sleep Parameters by Level of Depressive Symptoms.
Multivariable models adjusted for age, race, site, education, exercise, body mass index, smoking status, alcohol intake, self reported health status, instrumental activity of daily living impairment, cognitive impairment, number of reported medical conditions, and current use of antidepressants, benzodiazepines, and medications for sleep. Data transformations: 1) LN (P) for WASO and SOL, 2) LN (100-P) for SE, 3) LN (P+1) for number nap episodes > 5 minutes.
| Level of Depressive Symptoms
|
||||
|---|---|---|---|---|
| Objective Sleep Parameters, mean (95% Confidence Interval) | Normal (GDS 0–2) | Some Depressive Symptoms (GDS 3–5) | Depressed (GDS ≥ 6) | P-trend |
| Sleep Efficiency (%) | ||||
| Age and site adjusted (N=3045) | 80.59 (80.16–81.01) | 78.42 (77.62–79.19) | 78.20 (77.04–79.29) | < .001 |
| Multivariable adjusted (N=2785) | 80.44 (79.99–80.87) | 79.62 (78.83–80.38) | 79.76 (78.57–80.89) | 0.120 |
| Sleep Onset Latency (minutes) | ||||
| Age and site adjusted (N=3044) | 28.16 (27.09–29.27) | 30.24 (28.36–32.24) | 31.89 (29.13–34.92) | 0.003 |
| Multivariable adjusted (N=2785) | 28.39 (27.28–29.54) | 28.11 (26.26–30.09) | 29.60 (26.72–32.78) | 0.628 |
| Wake After Sleep Onset (minutes) | ||||
| Age and site adjusted (N=3044) | 61.68 (60.04–63.36) | 71.02 (67.92–74.25) | 74.67 (70.11–79.52) | < .001 |
| Multivariable adjusted (N=2785) | 62.42 (60.72–64.18) | 65.83 (62.78–69.02) | 67.04 (62.44–71.99) | 0.030 |
| Number Long Wake Episodes > 5 minutes | ||||
| Age and site adjusted (N=3045) | 6.53 (6.39–6.67) | 7.26 (7.03–7.50) | 7.58 (7.25–7.91) | < .001 |
| Multivariable adjusted (N=2785) | 6.58 (6.44–6.72) | 6.90 (6.65–7.14) | 7.07 (6.70–7.44) | 0.006 |
| Total Sleep Time (hours) | ||||
| Age and site adjusted (N=3045) | 6.75 (6.69–6.81) | 6.71 (6.62–6.81) | 6.87 (6.74–7.01) | 0.278 |
| Multivariable adjusted (N=2785) | 6.75 (6.69–6.80) | 6.74 (6.65–6.84) | 6.87 (6.72–7.02) | 0.233 |
| Number Nap Episodes > 5 minutes | ||||
| Age and site adjusted (N=2995) | 2.82 (2.71–2.93) | 3.57 (3.36–3.79) | 3.98 (3.66–4.33) | < .001 |
| Multivariable adjusted (N=2742) | 2.84 (2.73–2.96) | 3.43 (3.21–3.66) | 3.60 (3.26–3.97) | < .001 |
In models adjusted for age and site, the odds of some objectively measured sleep disturbances increased with level of depressive symptoms (Figure 1 and Table 4). Compared to women with no depressive symptoms, the odds of having reduced SE <85% were greater for women with some depressive symptoms (OR 1.38, CI 1.13–1.68), and for the depressed group (OR 1.53, CI 1.17–2.01) compared with those in the normal group. Similarly, the odds of having WASO ≥1 hour were increased for those women with some depressive symptoms (OR 1.45, CI 1.22–1.73) and for the depressed group (OR 1.73, CI 1.36–2.19). The odds of having ≥8 long wake episodes were also greater for women with some depressive symptoms (OR 1.55, CI 1.29–1.86) and for the depressed group (OR 1.84, CI 1.45–2.33). The odds of being a long sleeper (>8 hours sleep per night), as opposed to a normal sleeper (5–8 hours sleep per night), were greater for women with some depressive symptoms (OR 1.46, 1.15–1.86) and for the depressed group (OR 1.58, 1.17–2.15). The odds of being a short sleeper (< 5 hours sleep per night), as opposed to a normal sleeper, were greater for women with some depressive symptoms (OR 1.82, CI 1.36–2.43) while there was no association for the depressed group (OR 1.30, CI 0.86–1.96). The odds of prolonged sleep latency ≥30 minutes was not significantly increased for women with some depressive symptoms (OR 1.12, CI 0.94–1.33) but was slightly increased for those in the depressed group (OR 1.38, 1.09–1.17). When models were adjusted for multiple possible confounders, there continued to be significant graded associations between level of depressive symptoms and WASO ≥1 hour and ≥8 long wake episodes only (Figure 1 and Table 4).
Level of Depressive Symptoms and Daytime Napping
There was a significant graded association between level of depressive symptoms and number of objectively assessed daytime naps (Table 3). After adjustment for age and site, women in the depressed group had an average of 3.98 (CI 3.66–4.33) nap episodes exceeding 5 minutes in length compared with 3.57 (CI 3.36–3.79) for women with some depressive symptoms, and 2.82 (CI 2.71–2.93) for those classified as normal (p-trend < 0.001). Compared to women in the normal group, the odds of having ≥ 5.5 daytime nap episodes were increased for women with some depressive symptoms (OR 1.71, CI 1.40–2.08) and for those in the depressed group (OR 2.57, CI 2.02–3.27) (Figure 1 and Table 4). These associations remained statistically significant after adjusting for multiple variables.
Additional Analyses
To determine whether increased fragmentation of sleep accounted for the strong associations observed between a greater level of depressive symptoms and self reported sleep outcomes including sleep quality (PSQI) and daytime sleepiness (ESS), WASO and number of long wake episodes were added separately to the multivariable model with PSQI (and then ESS) as a dependent variable. After further adjusting for WASO or number of long wake episodes, the associations between greater level of depressive symptoms and both PSQI and ESS remained unchanged (data not shown). To further assess the effect of current use of antidepressants, benzodiazapines, and medications for sleep on these analyses, the analyses were repeated excluding 594 participants who reported use of these medications and the results were similar (data not shown).
DISCUSSION
Our results suggest a strong, graded association between increased level of depressive symptoms and poorer self-reported sleep quality in community-dwelling older women, a finding that is consistent with previously published reports (10, 17, 30–33). We also found significant associations between greater levels of depressive symptoms and measures of sleep fragmentation (increased WASO and number of long wake episodes). Paudel and colleagues recently preformed a similar analysis of actigraphic sleep in a cohort of community-dwelling older men. The authors found a modest association between more depressive symptoms and increased sleep latency but no significant associations between level of depressive symptoms and objective measures of sleep fragmentation (17). It is possible that this difference is related to the populations being tested since study population assessed by Paudel and colleagues was male and was younger (mean age 76.4 +/− 5.5 years) than the women in the SOF study.
After adjusting for multiple potential confounders, our objective assessment did not show any association between level of depressive symptoms and TST, SE, or SOL in this group. This is in contrast to other studies which have found objective evidence of decreased TST and SE as well as increased SOL in depressed patients by both subjective assessment (34) and PSG (35, 36). This difference may be related to the method of sleep disturbance assessment. Further, those studies focused on individuals who either had a diagnosis of Major Depressive Disorder or who met DSM-IV criteria for a Major Depressive Episode whereas our analysis focuses on level of subjectively reported depressive symptoms. Alternatively, it may be that these specific sleep disturbances are less prominent in elderly depressed women compared to other groups of depressed individuals. A recent study comparing actigraphic sleep measures in elderly men and women found elderly men to have shorter and more fragmented sleep when compared to elderly women (37). To our knowledge gender differences have not been examined in depressed older adults.
Objective sleep disturbances accounted for very little of the association between increased level of depressive symptoms and increased subjective sleep disturbance in this population. There are several possible explanations for this observation. Additional factors that were not assessed in this analysis may also play a role in the increased subjective perception of poor sleep quality. It is also possible that, in older community-dwelling women, women with and without depressive symptoms experience and report their sleep problems differently. Paudel and colleagues’ analysis in a cohort of community-dwelling older men found a similar discrepancy between the association of depressive symptoms with subjective as compared to objective sleep disturbances (17). In particular, older men with depressive symptoms were much more likely to report poor sleep quality compared to non-depressed counterparts while depression was more modestly associated with objectively measured sleep disturbance. To our knowledge, there are no other studies evaluating the association between depressive symptoms and both subjectively and objectively assessed sleep disturbances specifically in older adults.
Several studies comparing subjective reports of sleep quality using the PSQI with objective sleep measurements by PSG in healthy older adults have found that, in that population, subjective reports of sleep quality were not as poor as might be expected considering the objective sleep disturbances that were observed by PSG. This suggests that healthy older adults may under-report their sleep disturbances or adapt their perception of acceptable sleep quality compared with younger adults (11, 38). The data presented here suggest that older women with depressive symptoms do not share this tendency to under-report their problems with sleep. Indeed, it has been previously shown that among depressed subjects, older adults are more likely to underestimate their total sleep when compared to younger adults when subjective assessments are compared to PSG (39).
In this population, women with depressive symptoms were almost twice as likely to report excessive subjective daytime sleepiness compared with those in the normal category. Several studies in non-elderly populations have shown a similar relationship between depression and excessive daytime sleepiness (40, 41). Objective measurements also revealed an association between more depressive symptoms and daytime inactivity raising the possibility that there may be an increase in napping in women with more depressive symptoms. Although actigraphy is not able to distinguish definitively between quiet restfulness and sleep, previous studies have found similar associations between depressive symptoms and self-reported napping (42). Further, Goldman and colleagues recently showed an association between objectively measured increased fragmentation of sleep and increased subjective daytime napping in older adults (43). Studies employing PSG will be required to clarify this relationship.
The estimated point prevalence of major depressive disorder (MDD) in older adults has been reported to range from 3–10 % (44–47) while “less than major” depressive syndromes (including dysthymia, minor depression and subsyndromal depression) are nearly twice as common with a reported prevalence of 9–24% (46, 48–51). The women in the SOF study did not undergo any formal psychiatric evaluation to determine diagnosis. However, the prevalence of differing levels of depressive symptoms based on GDS score in this cohort suggests a similar pattern. Given that subsyndromal depressive syndromes make up the majority of depressive syndromes in older adults, it is interesting that having “some depressive symptoms” conferred a similar increased risk for subjectively assessed poor sleep quality and excessive daytime sleepiness compared with being in the “depressed” group. Although there were significant trends suggesting graded associations between increasing levels of depressive symptoms and objective measures of sleep fragmentation in multivariable adjusted models, specific associations between having “some depressive symptoms” and objective measures of sleep fragmentation observed in age and site adjusted models were no longer significant in multivariable adjusted models. Future studies designed to better explore the relationship between subsyndromal depression and objective sleep disturbances would be useful to further investigate this relationship.
Our analyses add to the growing body of literature suggesting that both subjective sleep disturbance and excessive daytime sleepiness are common in older adults. More than half of the women in this study endorsed subjectively poor sleep (PSQI >5) and more than 10% of the women endorsed excessive daytime sleepiness. This is similar to what has been reported in other similar populations including the National Sleep Foundation’s Sleep in America survey in which more than 50% of respondents reported a sleep complaint occurring nearly every night. Similarly, in the Osteoporotic Fractures in Men study, the prevalence of subjective poor sleep (PSQI >5) in a large cohort of community dwelling older men was reported to be 44.2% while the prevalence of excessive daytime sleepiness (ESS >10) was12.7%. Subtle differences in the age and other covariates (e.g. burden of medical illness) of these different samples may explain small differences in the prevalence rates of these subjectively reported problems.
This study has several strengths. The sample size is large and is collected from four separate United States locations and the women were not selected based on depressive symptoms or on their report of sleep disturbances. Unlike most previous studies examining the relationship between depression and sleep disturbances in older adults, both subjective and objective measures of sleep disturbance and daytime sleep were assessed.
There are also several limitations to this analysis. The study is cross-sectional and it is therefore not possible to draw any conclusions about causality. The sample is made up only of community dwelling women, the vast majority of whom were white and over 80 years old, and the findings may not be applicable to other populations. Depressive symptoms were assessed by questionnaire only rather than a clinical diagnostic interview, so conclusions about psychiatric diagnosis cannot be made. Actigraphy relies on self-reported times in and out of bed which may be inaccurate and could introduce errors into those measurements such as sleep onset latency. However, these errors would not affect WASO and number of long wake episodes at night which we found to be associated with depressive symptoms. Additionally, actigraphy cannot definitively distinguish between daytime sleep and periods of quiet restfulness. Finally, the impact of adding multiple covariates to the many of the multivariable adjusted models underscores the complexity of the relationship between these covariates, sleep, and depression in this population. Our analyses were not designed to fully explore the role these covariates play in the relationship between depressive symptoms and sleep disturbances. Future studies designed specifically to address this issue would be useful, particularly since some of these covariates may be amenable to intervention.
In conclusion, these analyses add to the body of literature supporting an association between depressive symptoms and subjective sleep disturbance in older adults. Further, these data suggest an association between more depressive symptoms and fragmentation of sleep as well as increased daytime napping in older women. Longitudinal analyses will now be required to determine whether objectively measured sleep disturbances predict changes in depression over time.
Acknowledgments
The authors would like to thank the Study of Osteoporotic Fractures Research Group. We also thank Loki Natarajan, Ph.D., and Feng He M.S. for thoughtful comments regarding the statistical analysis used in this study.
Support: Dr. Maglione was supported by NIH R25MH7450, and a Mini-Grant in Aging provided by the UCSD Academic Geriatric Resource Center (09SD-A7-1-24). Dr Ancoli-Israel is supported by NIA AG08415. The Study of Osteoporotic Fractures (SOF) is also supported by National Institutes of Health funding under the following grant numbers: AG05407, AR35582, AG05394, AR35584, AR35583, R01 AG005407, R01 AG027576-22, 2 R01AG005394-22A1, 2 R01 AG027574-22A1, AG05407, AR35582, AG05394, AR35584, AR35583, AG026720, R01 MH086498.
Footnotes
Meetings: A portion of the data in this manuscript was presented at the Associated Professional Sleep Societies annual meeting, Sleep 2010.
Author Contributions: Maglione JE: analysis and interpretation of data, preparation of manuscript. Ancoli-Israel S: study concept and design, interpretation of data, critical review of manuscript. Peters KW: analysis and interpretation of data, critical review of manuscript. Paudel ML: critical review of manuscript. Yaffe K: study concept and design, critical review of manuscript. Ensrud KE: study concept and design, interpretation of data, critical review of manuscript. Stone KL: study concept and design, interpretation of data, critical review of manuscript.
Conflicts of Interest: This was not an industry supported study. Dr. Ancoli-Israel has been a consultant to Ferring Pharmaceuticals Inc., GlaxoSmithKline, Merck, NeuroVigil, Inc., Pfizer, Philips Respironics, Purdue Pharma LP The other authors have indicated no financial conflicts of interest.
References
- 1.Blazer DG. Depression in late life:Review and commentary. J Gerontol A Biol Sci Med Sci. 2003;58:249–265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- 2.Hays JC, Krishnan KR, George LK, et al. Psychosocial and physical correlates of chronic depression. Psychiatry Res. 1997;72:149–159. doi: 10.1016/s0165-1781(97)00105-4. [DOI] [PubMed] [Google Scholar]
- 3.Bruce ML. Depression and disability in late life: directions for future research. Am J Geriatr Psychiatry. 2001;9:102–112. [PubMed] [Google Scholar]
- 4.Frasure-Smith N, Lesperance F. Depression--a cardiac risk factor in search of a treatment. JAMA. 2003;289:3171–3173. doi: 10.1001/jama.289.23.3171. [DOI] [PubMed] [Google Scholar]
- 5.Alexopoulos GS, Vrontou C, Kakuma T, et al. Disability in geriatric depression. Am J Psychiatry. 1996;153:877–885. doi: 10.1176/ajp.153.7.877. [DOI] [PubMed] [Google Scholar]
- 6.Zeiss AM, Lewinsohn PM, Rohde P, et al. Relationship of physical disease and functional impairment to depression in older people. Psychol Aging. 1996;11:572–581. doi: 10.1037//0882-7974.11.4.572. [DOI] [PubMed] [Google Scholar]
- 7.Huang BY, Cornoni-Huntley J, Hays JC, et al. Impact of depressive symptoms on hospitalization risk in community-dwelling older persons. J Am Geriatr Soc. 2000;48:1279–1284. doi: 10.1111/j.1532-5415.2000.tb02602.x. [DOI] [PubMed] [Google Scholar]
- 8.Unutzer J, Patrick DL, Simon G, et al. Depressive symptoms and the cost of health services in HMO patients aged 65 years and older. A 4-year prospective study. JAMA. 1997;277:1618–1623. doi: 10.1001/jama.1997.03540440052032. [DOI] [PubMed] [Google Scholar]
- 9.Luber MP, Meyers BS, Williams-Russo PG, et al. Depression and service utilization in elderly primary care patients. Am J Geriatr Psychiatry. 2001;9:169–176. [PubMed] [Google Scholar]
- 10.Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: An epidemiologic study of three communities. Sleep. 1995;18:425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 11.Vitiello MV, Larsen LH, Moe KE. Age-related sleep change: Gender and estrogen effects on the subjective-objective sleep quality relationships of healthy, noncomplaining older men and women. J Psychosom Res. 2004;56:503–510. doi: 10.1016/S0022-3999(04)00023-6. [DOI] [PubMed] [Google Scholar]
- 12.Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci. 2008;10:473–481. doi: 10.31887/DCNS.2008.10.4/plfranzen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turvey CL, Conwell Y, Jones MP, et al. Risk factors for late-life suicide: A prospective, community-based study. Am J Geriatr Psychiatry. 2002;10:398–406. [PubMed] [Google Scholar]
- 14.Livingston G, Blizard B, Mann A. Does sleep disturbance predict depression in elderly people? A study in inner London. Br J Gen Pract. 1993;43:445–448. [PMC free article] [PubMed] [Google Scholar]
- 15.Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4:104–113. doi: 10.1207/s15402010bsm0402_3. [DOI] [PubMed] [Google Scholar]
- 16.Roberts RE, Shema SJ, Kaplan GA, et al. Sleep complaints and depression in an aging cohort: A prospective perspective. Am J Psychiatry. 2000;157:81–88. doi: 10.1176/ajp.157.1.81. [DOI] [PubMed] [Google Scholar]
- 17.Paudel ML, Taylor BC, Diem SJ, et al. Association between depressive symptoms and sleep disturbances in community-dwelling older men. J Am Geriatr Soc. 2008;56:1228–1235. doi: 10.1111/j.1532-5415.2008.01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72–75. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Brink TL. Clinical Gerontology: A Guide to Assessment and Intervention. New York: Howarth Press; 1986. [Google Scholar]
- 21.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: A study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14:858–865. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 23.Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 24.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 25.Blackwell T, Ancoli-Israel S, Gehrman PR, et al. Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep. 2005;28:1599–1605. doi: 10.1093/sleep/28.12.1599. [DOI] [PubMed] [Google Scholar]
- 26.Blackwell T, Redline S, Ancoli-Israel S, et al. Comparison of sleep parameters from actigraphy and polysomnography in older women: The SOF Study. Sleep. 2008;31:283–291. doi: 10.1093/sleep/31.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ancoli-Israel S, Cole R, Alessi C, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 28.Cole RJ, Kripke DF, Gruen W, et al. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–469. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 29.Jean-Louis G, Kripke DF, Mason WJ, et al. Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods. 2001;105:185–191. doi: 10.1016/s0165-0270(00)00364-2. [DOI] [PubMed] [Google Scholar]
- 30.Foley DJ, Monjan A, Simonsick EM, et al. Incidence and remission of insomnia among elderly adults: An epidemiologic study of 6,800 persons over three years. Sleep. 1999;22 (Suppl 2):S366–372. [PubMed] [Google Scholar]
- 31.Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Newman AB, Enright PL, Manolio TA, et al. Sleep disturbance, psychosocial correlates, and cardiovascular disease in 5201 older adults: The Cardiovascular Health Study. J Am Geriatr Soc. 1997;45:1–7. doi: 10.1111/j.1532-5415.1997.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 33.Reid KJ, Martinovich Z, Finkel S, et al. Sleep: a marker of physical and mental health in the elderly. Am J Geriatr Psychiatry. 2006;14:860–866. doi: 10.1097/01.JGP.0000206164.56404.ba. [DOI] [PubMed] [Google Scholar]
- 34.van den Berg JF, Luijendijk HJ, Tulen JH, et al. Sleep in depression and anxiety disorders: A population-based study of elderly persons. J Clin Psychiatry. 2009;70:1105–1113. doi: 10.4088/JCP.08m04448. [DOI] [PubMed] [Google Scholar]
- 35.Benca RM, Obermeyer WH, Thisted RA, et al. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49:651–668. doi: 10.1001/archpsyc.1992.01820080059010. discussion 669–670. [DOI] [PubMed] [Google Scholar]
- 36.Benca RM, Peterson MJ. Insomnia and depression. Sleep Med. 2008;9(Suppl 1):S3–9. doi: 10.1016/S1389-9457(08)70010-8. [DOI] [PubMed] [Google Scholar]
- 37.van den Berg JF, Miedema HM, Tulen JH, et al. Sex differences in subjective and actigraphic sleep measures: A population-based study of elderly persons. Sleep. 2009;32:1367–1375. doi: 10.1093/sleep/32.10.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–338. [PubMed] [Google Scholar]
- 39.Tsuchiyama K, Nagayama H, Kudo K, et al. Discrepancy between subjective and objective sleep in patients with depression. Psychiatry Clin Neurosci. 2003;57:259–264. doi: 10.1046/j.1440-1819.2003.01114.x. [DOI] [PubMed] [Google Scholar]
- 40.Pallesen S, Nordhus IH, Omvik S, et al. Prevalence and risk factors of subjective sleepiness in the general adult population. Sleep. 2007;30:619–624. doi: 10.1093/sleep/30.5.619. [DOI] [PubMed] [Google Scholar]
- 41.Chellappa SL, Araujo JF. Excessive daytime sleepiness in patients with depressive disorder. Rev Bras Psiquiatr. 2006;28:126–129. doi: 10.1590/s1516-44462006000200010. [DOI] [PubMed] [Google Scholar]
- 42.Foley DJ, Vitiello MV, Bliwise DL, et al. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: Findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15:344–350. doi: 10.1097/01.JGP.0000249385.50101.67. [DOI] [PubMed] [Google Scholar]
- 43.Goldman SE, Hall M, Boudreau R, et al. Association between nighttime sleep and napping in older adults. Sleep. 2008;31:733–740. doi: 10.1093/sleep/31.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borson S, Barnes RA, Kukull WA, et al. Symptomatic depression in elderly medical outpatients. I. Prevalence, demography, and health service utilization. J Am Geriatr Soc. 1986;34:341–347. doi: 10.1111/j.1532-5415.1986.tb04316.x. [DOI] [PubMed] [Google Scholar]
- 45.Schulberg HC, Mulsant B, Schulz R, et al. Characteristics and course of major depression in older primary care patients. Int J Psychiatry Med. 1998;28:421–436. doi: 10.2190/G23R-NGGN-K1P1-MQ8N. [DOI] [PubMed] [Google Scholar]
- 46.Lyness JM, King DA, Cox C, et al. The importance of subsyndromal depression in older primary care patients: Prevalence and associated functional disability. J Am Geriatr Soc. 1999;47:647–652. doi: 10.1111/j.1532-5415.1999.tb01584.x. [DOI] [PubMed] [Google Scholar]
- 47.Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: The Cache County study. Arch Gen Psychiatry. 2000;57:601–607. doi: 10.1001/archpsyc.57.6.601. [DOI] [PubMed] [Google Scholar]
- 48.Judd LL, Akiskal HS. The clinical and public health relevance of current research on subthreshold depressive symptoms to elderly patients. Am J Geriatr Psychiatry. 2002;10:233–238. [PubMed] [Google Scholar]
- 49.Judd LL, Akiskal HS, Maser JD, et al. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry. 1998;55:694–700. doi: 10.1001/archpsyc.55.8.694. [DOI] [PubMed] [Google Scholar]
- 50.Judd LL, Akiskal HS, Maser JD, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50:97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- 51.Olfson M, Broadhead WE, Weissman MM, et al. Subthreshold psychiatric symptoms in a primary care group practice. Arch Gen Psychiatry. 1996;53:880–886. doi: 10.1001/archpsyc.1996.01830100026004. [DOI] [PubMed] [Google Scholar]