Abstract
Background & Aims
Amino acid transporters have been proposed as regulators of protein synthesis. The primary aim of this study was to determine whether amino acid transporter expression is increased in human muscle following resistance exercise (RE) coupled with essential amino acid (EAA) ingestion, and whether a differential response occurs with aging. Secondly, we aimed to compare this response to a previous study examining RE alone.
Methods
Young (n=7, 30±2yr) and older men (n=6, 70±2yr) ingested EAA 1h after RE. Muscle biopsies were obtained at rest and 3 and 6h postexercise to examine amino acid transporter mRNA and protein expression.
Results
In both age groups, RE+EAA increased mRNA of L-type amino acid transporter 1 (LAT1)/solute linked carrier (SLC)7A5, sodium-coupled neutral amino acid transporter 2 (SNAT2)/SLC38A2, and cationic amino acid transporter 1/SLC7A1 (p<0.05). SNAT2 protein increased in young at 3 and 6h (p<0.05), whereas old maintained higher LAT1 protein (p<0.05). Compared to RE alone, RE+EAA enhanced amino acid transporter expression only in young (p<0.05).
Conclusions
RE increases muscle amino acid transporter expression in young and older adults, however, postexercise EAA ingestion enhances amino acid transporter expression only in young indicating that aging may influence the function of specific amino acid transporters.
Keywords: mTOR signaling, sarcopenia, nutrition, resistance exercise, muscle protein synthesis
INTRODUCTION
Resistance exercise presents a powerful adaptive stimulus to skeletal muscle, and when performed chronically can lead to gains in muscle size and strength.1,2 The ability of skeletal muscle to adapt to resistance exercise in this manner is likely facilitated through the repeated elevations in the rate of skeletal muscle protein synthesis that occurs following each exercise bout.3 The acute anabolic response to a bout of resistance exercise is enhanced by the presence of amino acids, such that an increase in amino acid availability following a bout of resistance exercise produces an additive increase in muscle protein synthesis rate,4,5 which favors a more robust adaptive response. Furthermore, the coupling of amino acid availability and resistance exercise appears to be more critical in older adults. For instance, we have recently demonstrated that older individuals have an impaired muscle protein anabolic response to resistance exercise compared to young adults,6 however, this age-related impairment can be overcome with the ingestion of essential amino acids shortly following a bout of resistance exercise.7 Consequently this strategy provides an important countermeasure to the loss of muscle mass and function that occurs with advancing age (i.e., sarcopenia). However, while there is little question regarding the potent anabolic response elicited by the combination of resistance exercise and amino acids, the mechanisms through which this effect is elicited remain to be fully elucidated.
The activity of amino acid transporters provides an important link between amino acid availability and the regulation of muscle protein metabolism. Specifically, the function of select amino acid transporters has been shown to stimulate mammalian target of rapamycin complex 1 (mTORC1) activity, which is a central regulator of protein synthesis.4 For instance, the system L amino acid transporter LAT1/solute-linked carrier (SLC)7A5 (which forms a heterodimer complex with CD98/SLC3A28) and the system A amino acid transporter SNAT2/SLC38A2 have been shown to be sensitive to changes in amino acid availability,9,10 and the cooperative activity of these transporters facilitates the transport of large neutral amino acids, such as leucine, into the cell11 to subsequently stimulate mTORC1.10,12,13 In addition, changes in amino acid availability has also been linked to the expression of cationic amino acid transporter 1 (CAT1/SLC7A1) in cell models,14,15 whereas the ability of amino acids to activate mTORC1, and subsequently stimulate protein synthesis, appears to be mediated through the function of proton-assisted transporters (PAT), namely PAT1/SLC36A1.16,17
We have recently demonstrated that the expression levels of select amino acid transporters in human skeletal muscle are responsive to the independent effects of resistance exercise18 and essential amino acid ingestion.9 However, the response of amino acid transporters following the potent combination of resistance exercise and amino acid ingestion remains to be investigated. Consequently, given the apparent association of amino acid transporters with the regulation of protein synthesis, coupled with the enhanced increase in muscle protein synthesis rate when essential amino acids are provided after resistance exercise,7 the primary aims of the current study were 1) to examine the mRNA and protein expression of select amino acid transporters in skeletal muscle following a bout of resistance exercise coupled with essential amino acid ingestion and 2) to determine whether a differential response occurs with aging. A secondary aim of this study was to determine whether the combination of resistance exercise and essential amino acid ingestion stimulates a greater increase in amino acid transporter expression compared to the effects of resistance exercise alone previously reported by our laboratory18. We hypothesized that ingestion of essential amino acids following resistance exercise would increase amino acid transporter expression to a greater extent than resistance exercise alone in both young and older adults.
MATERIALS & METHODS
Subjects
For the primary aim of the study, seven healthy young (30 ± 2 yr) and six healthy older (70 ± 2 yr) men volunteered for this study7. All participants were healthy and considered recreationally active but not engaged in a regularly scheduled exercise-training program. Screening for all participants was performed with clinical history, physical examination, and laboratory tests, including complete blood count with differential, liver and kidney function tests, coagulation profile, fasting blood glucose, oral glucose tolerance test, hepatitis B and C screening, HIV testing, thyroid-stimulating hormone, urinalysis, and drug screening. Maximal knee extensor muscle strength was determined for each subject on two separate occasions using a one-repetition maximum (1RM) performed on a leg extension device (Cybex-VR2, Medway, MA). The first 1RM measurement was obtained during the initial screening and the second 1RM measurement was obtained approximately 1 wk prior to study participation. The highest weight lifted between the two measurements was considered the subject’s 1RM. The mean ± SE 1RM for the young and older groups were 103 ± 8 and 78 ± 5 kg (4.7 ± 0.3 and 4.2 ± 0.4 1RM, kg/Leg Lean Mass, kg), respectively. All participants gave informed written consent prior to participation in the study, which was approved by the Institutional Review Board of the University of Texas Medical Branch (in compliance with the Declaration of Helsinki as revised in 1983).
Study Design
All subjects were admitted to the Institute for Translation Sciences Clinical Research Center (ITS-CRC) of the University of Texas Medical Branch the evening prior to the exercise study, and a dual-energy X-ray absorptiometry scan (Hologic QDR 4500W, Bedford, MA) was performed to measure body composition and lean mass. The subjects were then fed a standard dinner and a snack at 2200 h. All subjects were studied following an overnight fast under basal conditions and refrained from exercise for 24 h prior to study participation. All subjects were studied during the same time of day to avoid potential circadian changes. Details regarding tracer infusions (for determination of blood and muscle intracellular leucine enrichment) can be found elsewhere.7
The morning of the experimental trial, a basal muscle biopsy was obtained under sterile procedures and local anesthesia (1% lidocaine) from the lateral portion of the vastus lateralis using a 5-mm Bergström biopsy needle19 with suction. The muscle tissue was immediately blotted and frozen in liquid nitrogen and stored at −80°C until analysis. After two hours of rest, subjects were escorted to a Cybex leg extension machine and performed eight sets of 10 repetitions of bilateral leg extension resistance exercise (RE) equivalent to ~70% of their predetermined 1RM with 3 min of rest between sets as we have previously described.6,7 Upon completion of the RE bout, the subjects were transported back to their hospital bed and rested supine for the remainder of the study.
At 1 h post exercise, subjects ingested a solution (500 ml) that contained 20 g of essential amino acids (EAA) enriched in leucine in the following composition: histidine (8%), isoleucine (8%), leucine (35%), lysine (12%), methionine (3%), phenylalanine (14%), threonine (10%), and valine (10%) (Ajinomoto/Sigma Aldrich, Raleigh, NC).7 At 3 h post exercise (2 h post EAA ingestion) another muscle biopsy was collected from a new incision site ~7 cm proximal to the first incision and a final muscle biopsy was collected at 6 h post exercise from the second incision site with the biopsy needle angled ~5 cm proximal from the preceding biopsy. Blood samples were also collected for determination of blood leucine concentration throughout the experimental trial.
Resistance Exercise Only Comparison
A secondary aim of this study was to determine whether the combination of resistance exercise and essential amino acid ingestion (RE+EAA) stimulates a greater increase in amino acid transporter expression compared to the effects of RE only (RE alone). Specifically, our goal was to compare the findings from the current study to the response of a cohort of male subjects obtained from a previous study conducted by our laboratory18. This cohort included 8 young men (27 ± 3 yr, 177 ± 3 cm, 79 ± 4 kg) and 8 older men (70 ± 2 yr, 173 ± 3 cm, 76 ± 3 kg) who completed the identical RE protocol as described above, however, they remained fasted throughout the post exercise study period. The data presented herein for these men have not been published separately as our previous publication included both men and women in the overall data set (n=13 for both young and old). The mean ± SE 1RM for the young and older RE alone cohorts were 124 ± 6 kg and 81 ± 4 kg (5.9 ± 0.2 and 4.3 ± 0.2 1RM, kg/Leg Lean Mass, kg), respectively. All muscle biopsies were obtained at the same time points examined in the current study and were obtained from the vastus lateralis in a manner identical to that utilized in the current investigation. Due to tissue limitations as described previously18, LAT1 and SNAT2 protein expression data in the older RE alone cohort are presented from n = 6, and n = 7, respectively. All data analyses for this group were completed with identical laboratory techniques (described below).
RNA Extraction and Semiquantitative real-time PCR
RNA isolation, cDNA synthesis, and real-time qPCR were performed as we have previously described.18,20 Total RNA was isolated by homogenizing 30–40 mg tissue with a hand-held homogenizing dispenser (T10 Basic Ultra Turrax, IKA, Wilmington, NC) in 1 ml of Tri reagent. The RNA was separated into an aqueous phase using 0.2 ml of chloroform and subsequently precipitated from the aqueous phase using 0.5 ml of isopropanol. RNA was washed with 1 ml of 75% ethanol, dried, and suspended in a known amount of nuclease-free water. RNA concentration was determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE) and RNA was DNase-treated using a commercially available kit (DNA-free, Ambion, Austin, TX). A total of 1 μg of RNA was reverse transcribed into cDNA according to the directions provided by the manufacturer (iScript, BioRad, Hercules, CA). Real-time qPCR was carried out with an iQ5 Multicolor Real Time PCR cycler (BioRad). cDNA was analyzed with SYBR green fluorescence (iQ SYBR green supermix; BioRad). Primer sequences for the current investigation have been previously published 9. β2-Microglobulin was utilized as a normalization/housekeeping gene, as this gene product was unchanged across time or between groups. Relative fold changes were determined from the Ct values using the 2 −ΔΔCt method.21
Immunoblot Analysis
Immunoblot analysis was performed as previously detailed.22 Briefly, frozen tissue was homogenized, centrifuged for 10 min at 4°C, and the supernatant collected. Total protein concentrations were determined using the Bradford assay (Smartspec Plus, BioRad, Hercules, CA, USA). The supernatant was diluted (1:1) in a 2X sample buffer mixture containing 125mM Tris, pH 6.8, 25% glycerol, 2.5% SDS, 2.5% β-mercaptoethanol and 0.002% bromophenol blue and then boiled for 3 min at 100°C. Equal amounts of total protein (50 μg) were loaded into each lane and the samples were separated by electrophoresis (150 V for 60 min) on a 15% polyacrylamide gel (Criterion, BioRad). Each sample was loaded in duplicate and each gel contained an internal loading control and molecular weight ladder (Precision Plus, BioRad). In addition, all samples from a given experimental trial were loaded onto the same gel and each gel contained samples from both the young and older group. Following electrophoresis, protein was transferred to a polyvinylidene difluoride membrane (BioRad) at 50 V for 60 min. Blots were then blocked for 1 h in 5% non fat dry milk and incubated in primary antibody overnight at 4°C (see Antibodies below). The next morning, blots were incubated in secondary antibody for 1 h at room temperature. Blots were then incubated in a chemiluminescent solution (ECL plus, Amersham BioSciences, Piscataway, NJ, USA) for 5 min and optical density measurements were obtained with a phosphoimager (ChemiDoc, BioRad) and densitometric analysis was performed using Quantity One 4.5.2 software (BioRad). Immunoblot data were normalized to an internal loading control, which was loaded on all gels for comparison across blots, and data are adjusted to represent fold change from basal. Antibodies utilized were LAT1/SLC7A5 (Abcam, Cambridge, MA) and SNAT2/SLC38A2 (Santa Cruz Biotechnologies, Santa Cruz, CA).
Leucine Concentrations
Concentrations of leucine were determined in blood and muscle intracellular fluid using [1-13C]leucine tracer enrichments and L-[5,5,5-2H3]leucine as the internal standard, as previously described.23 All tracer measurements were determined via gas chromatography-mass spectrometry (GCMS, 6890 Plus GC, 5973N MSD, 7683 autosampler, Agilent Technologies, Palo Alto, CA, USA). Basal blood leucine concentrations, taken as the average of two pre exercise blood samples, were subtracted from all time points such that data are presented as absolute change from basal blood leucine concentration. In addition the ratio of intracellular leucine concentration to blood leucine concentration was calculated at basal and 3 h and 6 h post resistance exercise by dividing the intracellular leucine concentration by the blood leucine concentration at each respective time point.
Statistical Analysis
A 2-way ANOVA with repeated measures on the time factor was used to test time by group differences for the effect of age on amino acid transporter expression following the combination of resistance exercise and essential amino acid ingestion. The effect of RE+EAA vs. RE alone on amino acid transporter expression was examined independently for the young and older groups using a 2-way ANOVA with repeated measures on the time factor. A Fisher LSD post hoc analysis was used when necessary to determine specific differences within an ANOVA. All data were analyzed using SigmaStat v.11.0 (Systat Software). Significance for all analyses was set to p<0.05. Data are presented as mean ± SE.
RESULTS
Blood and Intracellular Leucine Concentrations Following Resistance Exercise and Essential Amino Acid Ingestion
Blood and intracellular leucine concentrations for RE+EAA are presented as absolute change from basal in Table 1. Blood leucine concentrations dropped at 1 h post resistance exercise in both age groups (p<0.05), but were elevated above basal in both young and older men at 2, 3, 4, 5, and 6 h post exercise (p<0.05), with the older men having a greater increase than the young at 4 h post resistance exercise (p<0.05). Both the young and older men experienced increases in muscle intracellular leucine concentration at 3 and 6 h post resistance exercise (p<0.05). The ratio of intracellular to blood leucine concentration for RE+EAA is presented in Table 2. Compared to basal, both the young and older men experienced a reduction in the ratio of intracellular to blood leucine concentration at 3 h (p<0.05), with the older men having a more pronounced reduction in this ratio compared to the young (p<0.05). At 6 h, only the older men had a reduction in this ratio (p<0.05).
Table 1.
Absolute change (μmol·L−1) from basal for blood and muscle intracellular leucine concentrations in response to resistance exercise and ingestion of 20 g of essential amino acids in young and older men.
| Group | Time (h) Post Resistance Exercise
|
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Blood Leucine, Absolute Change from Basal, μmol·L−1 | ||||||
| Young | −21 ± 5* | 503 ± 111* | 497 ± 49* | 204 ± 25*# | 111 ± 21 | 80 ± 17* |
| Older | −11 ± 2* | 652 ± 125* | 694 ± 120* | 346 ± 12* | 157 ± 24* | 128 ± 19* |
| Muscle Intracellular Leucine, Absolute Change from Basal, μmol·L−1 | ||||||
| Young | 402 ± 46* | 79 ± 31* | ||||
| Older | 514 ± 47* | 100 ± 15* | ||||
Data are mean±SE, expressed absolute change from Basal (μmol·L−1). Young, n = 7; Older n = 6.
Significant change from basal (p<0.05);
Significant group difference (p<0.05). Note: essential amino acids ingested at 1 h post resistance exercise.
Table 2.
The ratio of muscle intracellular leucine to blood leucine concentration in response to resistance exercise and ingestion of 20 g of essential amino acids in young and older men.
| Group | Basal | 3h Post RE | 6h Post RE |
|---|---|---|---|
| Young | 1.30 ± 0.08 | 0.94 ± 0.08* | 1.20 ± 0.07 |
| Older | 1.24 ± 0.03 | 0.66 ± 0.12*# | 1.01 ± 0.10* |
Data are mean±SE, calculated by dividing the intracellular leucine concentration by the blood leucine concentration at each respective time point. Young, n = 7; Older n = 6.
Significant change from basal (p<0.05);
Significant group difference (p<0.05). Note: essential amino acids were ingested at 1 h post resistance exercise.
Amino Acid Transporter mRNA and Protein Expression Following Resistance Exercise and Essential Amino Acid Ingestion
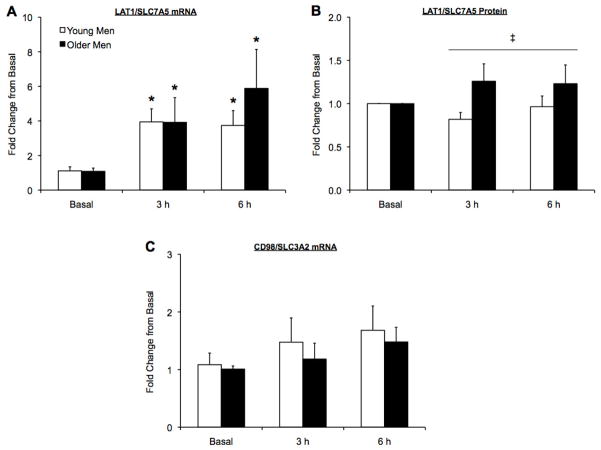
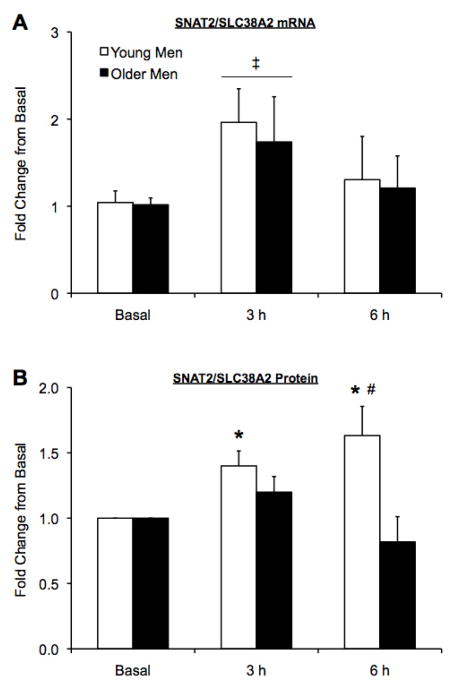
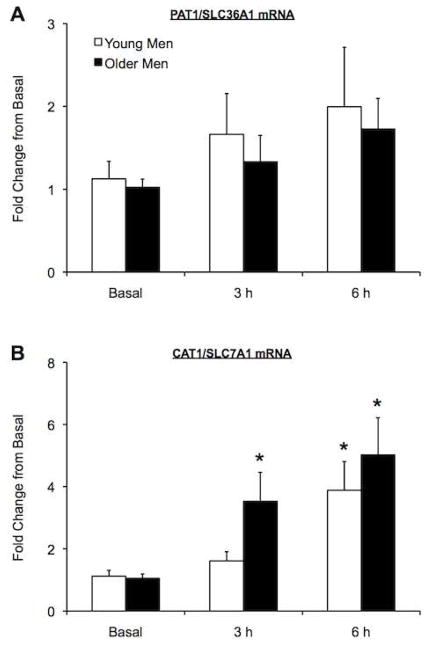
Tissue quantities limited data analysis for two young subjects, such that on the tissue from one of these young subjects only mRNA expression was performed, whereas only protein expression was performed on the tissue from the other young subject. Therefore, all mRNA and protein analyses were completed using n = 6 for young. LAT1/SLC7A5 mRNA expression was elevated above basal in both age groups at 3 and 6 h post exercise (p<0.05) (Fig 1A). LAT1 protein expression was not statistically elevated in either group following exercise and amino acid ingestion (p>0.05), however, older men maintained a higher LAT1 protein expression post exercise compared with the young (main effect of group, p<0.05) (Fig 1B). CD98/SLC3A2 mRNA expression was similar between age groups at all time points and no increases following exercise and amino acid ingestion were observed at any time point for young or older men (p>0.05) (Fig 1C). Independent of age group, SNAT2/SLC38A2 mRNA expression was elevated above basal at 3 h post exercise (main effect, p<0.05), but returned to basal values at 6 h post exercise for both age groups (p>0.05) (Fig 2A). SNAT2 protein expression in the older men was not different from basal at any time point following exercise and amino acid ingestion (Fig 2B). In contrast, young men exhibited a 40% and 63% increase in SNAT2 protein expression at 3 and 6 h post exercise (p<0.05), respectively, and young men also had a greater SNAT2 protein expression at 6 h post exercise compared with older men (p<0.05) (Fig 2B). PAT1/SLC36A1 mRNA expression was similar between age groups at all time points and no increases following exercise and amino acid ingestion were observed at any time point for young or older men (p>0.05) (Fig 3A). CAT1/SLC7A1 mRNA expression increased at 6 h post exercise in the young men (p<0.05), whereas CAT1/SLC7A1 mRNA expression was elevated at 3 and 6 h in the older men, (p<0.05) (Fig 3B).
Figure 1.
L-type amino acid transporter 1 (LAT1)/solute-linked carrier (SLC)7A5 mRNA (A) and protein (B) expression and CD98/SLC3A2 mRNA expression (C) in the skeletal muscle of young and older men. Subjects performed a bout of resistance exercise and ingested 20 g of essential amino acids following the exercise bout. Time points along the x-axis correspond to pre exercise (basal) and 3 and 6 h post exercise. Data are mean ± SE and represent fold change from basal. Young Men, n = 6; Older Men n = 6. *Significantly different from basal (p<0.05); ‡ Significant main effect of group (p<0.05).
Figure 2.
Sodium-coupled neutral amino acid transporter 2 (SNAT2)/solute-linked carrier (SLC)38A2 mRNA (A) and protein (B) expression in the skeletal muscle of young and older men. Subjects performed a bout of resistance exercise and ingested 20 g of essential amino acids following the exercise bout. Time points along the x-axis correspond to pre exercise (basal) and 3 and 6 h post exercise. Data are mean ± SE and represent fold change from basal. Young Men, n = 6; Older Men n = 6. ‡Significant time effect vs. basal (p<0.05); *Significantly different from basal (p<0.05); #Significant group difference (p<0.05).
Figure 3.
Proton-assisted amino acid transporter 1 (PAT1)/solute-linked carrier (SLC)36A1 mRNA (A) and cationic amino acid transporter 1 (CAT1)/SLC36A1 mRNA (B) expression in the skeletal muscle of young and older men. Subjects performed a bout of resistance exercise and ingested 20 g of essential amino acids following the exercise bout. Time points along the x-axis correspond to pre exercise (basal) and 3 and 6 h post exercise. Data are mean ± SE and represent fold change from basal. Young Men, n = 6; Older Men n = 6. *Significantly different from basal (p<0.05).
Resistance Exercise and Essential Amino Acid Ingestion vs. Resistance Exercise Alone
The effect of RE+EAA compared to RE alone on amino acid transporter mRNA and protein expression in young adults is presented in Table 3. In the young adults, LAT1/SLC7A5 mRNA expression was increased in both the RE+EAA and RE alone groups at 3 h (p<0.05), however, only the RE+EAA group had an increase at 6 h (p<0.05), and this value was greater than the RE alone group (p<0.05). The RE alone group did not increase SNAT2/SLC38A2 mRNA expression at any time point (p>0.05), whereas the RE+EAA group had an increase at 3 h (p<0.05). Both the RE+EAA and RE groups experienced similar responses for PAT1/SLC36A1, CAT1/SLC7A1, and CD98/SLC3A3 mRNA expression (Table 3). LAT1 protein was increased at 6 h only in the RE alone group (p<0.05), and the RE alone group had higher LAT1 protein expression at both 3 and 6 h (p<0.05). In contrast, SNAT2 protein was increased only in the RE+EAA group at both 3 and 6 h (p<0.05), and these values were greater than the RE alone group (p<0.05).
Table 3.
Comparison of mRNA and protein expression of amino acid transporters in response to resistance exercise coupled with essential amino acid ingestion or resistance exercise alone in skeletal muscle of young men.
| Study Group | Basal | 3h Post RE | 6h Post RE |
|---|---|---|---|
| LAT1/SLC7A5 mRNA, Fold Change | |||
| RE+EAA | 1.11 ± 0.23 | 3.95 ± 0.75* | 3.74 ± 0.86*# |
| RE | 1.01 ± 0.16 | 2.64 ± 0.49* | 1.84 ± 0.35 |
| SNAT2/SLC38A2 mRNA, Fold Change | |||
| RE+EAA | 1.04 ± 0.13 | 1.96 ± 0.39* | 1.21 ± 0.37 |
| RE | 1.23 ± 0.35 | 1.54 ± 0.18 | 1.19 ± 0.32 |
| PAT1/SLC36A1 mRNA, Fold Change | |||
| RE+EAA | 1.13 ± 0.21 | 1.66 ± 0.49 | 2.00 ± 0.72‡ |
| RE | 1.02 ± 0.22 | 1.01 ± 0.20 | 2.22 ± 0.48 |
| CAT1/SLC7A1 mRNA, Fold Change | |||
| RE+EAA | 1.12 ± 0.19 | 1.61 ± 0.29 | 3.88 ± 0.93* |
| RE | 1.27 ± 0.19 | 1.85 ± 0.20 | 4.84 ± 1.09* |
| CD98/SLC3A2 mRNA, Fold Change | |||
| RE+EAA | 1.08 ± 0.20 | 1.48 ± 0.42 | 1.68 ± 0.42‡ |
| RE | 1.20 ± 0.24 | 0.91 ± 0.12 | 1.89 ± 0.37‡ |
| LAT1 Protein, Fold Change | |||
| RE+EAA | 1.00 | 0.84 ± 0.09* | 0.96 ± 0.12 |
| RE | 1.00 | 1.12 ± 0.07# | 1.25 ± 0.07*# |
| SNAT2 Protein, Fold Change | |||
| RE+EAA | 1.00 | 1.40 ± 0.11*# | 1.63 ± 0.22*# |
| RE | 1.00 | 1.01 ± 0.01 | 1.01 ± 0.01 |
Data are mean±SE. RE+EAA = resistance exercise followed by ingestion of 20 g of essential amino acids 1h following exercise; RE = resistance exercise without EAA ingestion. RE+EAA, n = 6; RE, n = 8.
Significant change from basal (p<0.05);
Significant group difference (p<0.05);
Significant time effect vs. basal (p<0.05).
The effect of RE+EAA compared to RE alone on amino acid transporter mRNA and protein expression in the older adults is presented in Table 4. In the older adults, similar responses were observed between the RE+EAA and the RE alone groups for LAT1/SLC7A5, SNAT2/SLC38A2, and CAT1/SLC7A1 mRNA expression (Table 4). PAT1/SLC36A1 and CD98/SLC3A2 mRNA expression were increased at 6 h only in the RE alone group (p<0.05). Neither the RE+EAA and RE alone groups experienced an increase in LAT1 or SNAT2 protein expression at any time point (p>0.05).
Table 4.
Comparison of mRNA and protein expression of amino acid transporters in response to resistance exercise coupled with essential amino acid ingestion or resistance exercise alone in skeletal muscle of older men.
| Study Group | Basal | 3h Post RE | 6h Post RE |
|---|---|---|---|
| LAT1/SLC7A5 mRNA, Fold Change | |||
| RE+EAA | 1.09 ± 0.19 | 3.92 ± 1.43* | 5.88 ± 2.25* |
| RE | 1.33 ± 0.31 | 4.08 ± 0.82* | 4.06 ± 1.40* |
| SNAT2/SLC38A2 mRNA, Fold Change | |||
| RE+EAA | 1.02 ± 0.08 | 1.74 ± 0.52‡ | 1.21 ± 0.37 |
| RE | 1.01 ± 0.16 | 1.63 ± 0.35‡ | 1.08 ± 0.40 |
| PAT1/SLC36A1 mRNA, Fold Change | |||
| RE+EAA | 1.02 ± 0.10 | 1.33 ± 0.32 | 1.73 ± 0.37 |
| RE | 1.29 ± 0.38 | 1.63 ± 0.14 | 2.71 ± 0.64* |
| CAT1/SLC7A1 mRNA, Fold Change | |||
| RE+EAA | 1.05 ± 0.14 | 3.53 ± 0.93‡ | 5.02 ± 1.20‡ |
| RE | 1.27 ± 0.18 | 3.79 ± 1.15‡ | 5.37 ± 1.70‡ |
| CD98/SLC3A2 mRNA, Fold Change | |||
| RE+EAA | 1.01 ± 0.05 | 1.18 ± 0.28 | 1.48 ± 0.25 |
| RE | 1.35 ± 0.27 | 1.50 ± 0.21 | 2.04 ± 0.33* |
| LAT1 Protein, Fold Change | |||
| RE+EAA | 1.00 | 1.27 ± 0.20 | 1.24 ± 0.21 |
| RE | 1.00 | 1.10 ± 0.14 | 0.90 ± 0.13 |
| SNAT2 Protein, Fold Change | |||
| RE+EAA | 1.00 | 1.20 ± 0.11 | 0.82 ± 0.19 |
| RE | 1.00 | 1.00 ± 0.01 | 0.99 ± 0.01 |
Data are mean±SE. RE+EAA = resistance exercise followed by ingestion of 20 g of essential amino acids 1h following exercise; RE = resistance exercise without EAA ingestion. RE+EAA, n = 6; RE, n = 8 (mRNA) and n = 7 (SNAT2 protein) and n = 6 (LAT1 protein).
Significant change from basal (p<0.05);
Significant time effect vs. basal (p<0.05).
DISCUSSION
The primary goal of the present study was to expand on our previous work examining the link between amino acid transporter expression and stimuli known to increase protein synthesis rate in human skeletal muscle.9,18 The novel findings from the current investigation are that the combination of resistance exercise and amino acid ingestion stimulates an increase in the mRNA expression levels of several amino acid transporters (LAT1/SLC7A5, SNAT2/SLC38A2, CAT1/SLC7A1) in skeletal muscle of young and older men. However, in young men this was accompanied by an increase in the protein expression of SNAT2, whereas the older men maintained a higher protein expression of LAT1 following resistance exercise and amino acid ingestion. Secondly, in young men we found that the combination of RE and EAA ingestion enhanced the expression of select amino acid transporters when compared to RE alone, whereas in older men the increase in EAA availability after RE did not influence amino acid transporter expression beyond that seen with RE alone. These data add important new information regarding the cellular response of amino acid transporters in skeletal muscle of young and older adults to a potent anabolic stimulus. Further, the age-related differences may indicate that young and older adults rely differently on the function of specific amino acid transporters in skeletal muscle in response to the combination of RE and EAA ingestion.
SNAT2/SLC38A2 and LAT1/SLC7A5 mRNA and Protein Expression
While both young and older men displayed similar increases in mRNA expression of amino acid transporters following RE+EAA, an interesting finding in the current study is the age-related differences in the protein expression of SNAT2 and LAT1 in response to RE+EAA. Specifically, young men experienced an increase in SNAT2 protein, whereas the older men maintained higher levels of LAT1 protein. The premise for these age-related differences is unclear, but it is understood that these two transporters work in concert with one another to increase intracellular leucine delivery,11 and these data suggest that young and older adults may rely differently on the activity of one amino acid transporter over the other for increased cooperative transport. Further, in addition to the evidence suggesting the transporter function of these two amino acid transporters is important for protein synthesis and cell growth,10,24 recent data suggest that SNAT2/SLC38A2 may also have a role as an amino acid sensing “transceptor”,25 such that SNAT2/SLC38A2 appears capable of sensing an increase in amino acid availability, and subsequently signals downstream events leading to the stimulation of protein synthesis.26 In support of this theory, we have previously demonstrated in young adults that SNAT2 protein expression is increased in the immediate hours following EAA ingestion.9 Further, SNAT2 protein was not elevated in the young or older men following RE alone, whereas SNAT2 protein was elevated in the young men in response to RE+EAA. However, this increase in SNAT2 protein following RE+EAA in the young men did not correlate with a greater absolute accumulation of intramuscular leucine compared to the older men, suggesting the increased SNAT2 protein in young men may not necessarily contribute to greater leucine transport. Instead, the increase in SNAT2 protein occurring only in young men in response to RE+EAA may represent an age-related difference in the function of, or reliance on, SNAT2/SLC38A2 as an amino acid sensor in response to an elevation in amino acid availability following exercise.
It has recently been suggested that older adults require a higher intracellular amino acid concentration to stimulate muscle protein synthesis in response to exercise and amino acids as compared to young adults.27 Although not statistically evident, the absolute increase in both the blood and intracellular leucine concentration following RE+EAA in the current study appeared to be of greater magnitude in the older adults compared to the young (Table 1). LAT1/SLC7A5 directly transports leucine across the cell membrane, and therefore this response could be manifested through higher expression of LAT1 protein post exercise. Consequently, the higher expression of LAT1 protein in the older men could represent an important mechanistic response allowing older adults to increase intracellular leucine to a level necessary to stimulate muscle protein synthesis following RE+EAA.7 On the other hand, we cannot discount that the higher LAT1 protein expression in the older men may instead be a mechanism to export amino acids as a consequence of an increased stress response to the exercise as we have previously discussed18, which could contribute to the lower ratio of intracellular to plasma leucine concentrations (Table 2). This latter scenario may also explain the delay in the muscle protein synthesis response previously reported in older individuals following RE+EAA.7 Future work is needed to more precisely determine the role of LAT1/SLC7A5 following RE+EAA in young and older adults.
PAT1/SLC36A1 Expression
Recent data have implicated PAT1/SLC36A1 as an important modulator of mTORC1 activity17 and cell growth16 in the presence of elevated amino acids. RE alone increased skeletal muscle PAT1/SLC36A1 mRNA expression in both the younger and older men, however, surprisingly RE+EAA failed to stimulate a significant increase in either age group. Consequently, the time-course of skeletal muscle PAT1/SLC36A1 mRNA expression following RE+EAA could be different than that elicited by RE only, and perhaps more closely mirrors the transient response following EAA ingestion,9 which may have been missed with the biopsy schedule of the current investigation. Regardless, given the role of PAT1/SLC36A1 in the regulation of mTORC1 activity and protein metabolism in other models,16,17 additional investigation is clearly warranted to better understand the role of PAT1/SLC36A1 in the regulation of human skeletal muscle protein metabolism.
CAT1/SLC7A1 Expression
Following RE+EAA CAT1/SLC7A1 amino acid transporter expression was increased in both young and older men, although the increase was delayed in the young relative to the older men. Further, the response of CAT1/SLC7A1 mRNA expression following RE+EAA was very similar to that following RE alone for both age groups.18 Whereas previous studies have described a link between amino acid availability and CAT1/SLC7A1 expression in cell models,14,15 these data collectively suggest that ingesting EAA following resistance exercise does not further stimulate CAT1/SLC7A1 mRNA expression in human skeletal muscle. Instead, it is interesting to speculate that the increase in skeletal muscle CAT1/SLC7A1 mRNA expression stimulated by RE (with or without EAA) may have more of a role in regulating post exercise nutritive muscle blood flow, and hence amino acid delivery to the muscle, through increasing arginine availability,28,29 a major regulator of local blood flow. However, such an aim is beyond the scope of the current study and therefore further research is necessary to more clearly define the role of increased human skeletal muscle CAT1/SLC7A1 expression following exercise.
Effect of Age on RE+EAA vs. RE alone
A secondary aim of this study was to determine any potential mechanistic role for amino acid transporters in the enhanced protein synthesis response that is observed in young and older adults when EAA are ingested following RE4–7. Specifically, we compared the response of amino acid transporter expression following RE+AA to a cohort of younger and older men from our previous RE alone study18. Relative to RE alone, we found that RE+EAA appeared to enhance the overall response of LAT1/SLC7A5 and SNAT2/SLC38A2 in the young subjects. In particular, relative to RE alone, RE+EAA in the young produced: 1) a more prolonged elevation of LAT1/SLC7A5 mRNA expression; 2) an increase in SNAT2/SLC38A2 mRNA expression; and 3) an increase in SNAT2 protein expression. The enhanced and/or prolonged molecular response targeted to LAT1/SLC7A5 and SNAT2/SLC38A2 in the muscle of young adults is of keen interest with respect to protein synthesis. Specifically, these two transporters work to accumulate intracellular leucine11, a known stimulator of protein synthesis,30,31 and are associated with enhanced mTORC1 signaling9,10,13,24,32, cell growth,24,33 and protein synthesis10,13. Thus, the increased function/activity of these two amino acid transporters may have a role in the enhanced increase in muscle protein synthesis following RE+EAA in younger adults.
In contrast to the young, RE+EAA and RE alone produced very similar responses in amino acid transporter expression in the skeletal muscle of older men. The inability of EAA ingestion after RE to enhance the expression of these amino acid transporters in older adults could be related to potential age-specific mechanisms leading to increased amino acid transporter expression after RE. Specifically, we have previously suggested that increased amino acid transporter expression following RE in older adults may be manifested through an enhanced exercise-induced stress response, mediated through STAT318, and thus ingesting EAA after RE may not further stimulate this stress-related expression of amino acid transporters. On the other hand, we have recently demonstrated that EAA ingestion (without exercise) does increase amino acid transporter expression in older adults34 indicating that the exercise-induced stress response in older adults may cause a maximal expression of amino acid transporters that is not influenced by an increase in amino acid availability. This suggests that the ability of EAA ingestion to restore the normal increase in muscle protein synthesis following RE in older adults is due to enhanced amino acid uptake and activation of mTORC1 signaling independent of a further increase in amino acid transporter expression.
Summary
We have demonstrated that a bout of RE followed by EAA ingestion stimulates an increase in the mRNA expression of several amino acid transporters (LAT1/SLC7A5, SNAT2/SLC38A2, CAT1/SLC7A1) in the skeletal muscle of young and older men. Furthermore, we identified age-related differences in SNAT2 and LAT1 protein expression, such that only the young experienced an increase in SNAT2 protein expression whereas the old maintained higher expression levels of LAT1 protein. We have also demonstrated that ingesting EAA after RE enhances the response of LAT1/SLC7A5 and SNAT2/SLC38A2 in younger men, whereas amino acid transporter expression does not appear to be further stimulated by post exercise EAA ingestion in older men. These data suggest that both young and older adults increase skeletal muscle amino acid transporter expression following the potent combination of RE and EAA ingestion, however, young and older adults may rely differently on the function of specific amino acid transporters to stimulate metabolic processes in response to this stimulus.
Acknowledgments
We thank the subjects for their participation. We also thank Junfung Hao for technical assistance. This study was supported by the NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R01AR049877, NIH/National Institute on Aging P30AG024832, R01AG018311, and T35AG038048, and National Institute on Disability and Rehabilitation Research H133P110012. This study was conducted with the support of the Institute for Translational Sciences at the University of Texas Medical Branch, supported in part by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences, National Institutes of Health.
Abbreviations
- 1RM
one-repetition maximum
- CAT1
cationic amino acid transporter 1
- EAA
essential amino acids
- LAT1
system L amino acid transporter
- mTORC1
mammalian target of Rapamycin complex 1
- PAT1
proton-assisted amino acid transporter 1
- RE
resistance exercise
- SLC
solute-linked carrier
- SNAT2
sodium-coupled neutral amino acid transporter 2
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
STATEMENT OF AUTHORSHIP
BBR, EV, JMD, and MJD designed the research; JMD, MJD, JRC conducted research, collected data, and reviewed the manuscript; JMD, MJD, JRC, EV, and BBR analyzed data; JMD and BBR wrote the manuscript and had primary responsibility for final content. All authors read and approved the final draft of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jared M. Dickinson, Email: jadickin@utmb.edu.
Micah J. Drummond, Email: micah.drummond@hsc.utah.edu.
Jennifer R. Coben, Email: jrcoben@utmb.edu.
Elena Volpi, Email: evolpi@utmb.edu.
Blake B. Rasmussen, Email: blrasmus@utmb.edu.
References
- 1.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol. 2006;101:531–544. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 2.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. Jama. 1990;263:3029–3034. [PubMed] [Google Scholar]
- 3.Burd NA, Tang JE, Moore DR, Phillips SM. Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences. J Appl Physiol. 2009;106:1692–1701. doi: 10.1152/japplphysiol.91351.2008. [DOI] [PubMed] [Google Scholar]
- 4.Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol. 2009;106:1374–1384. doi: 10.1152/japplphysiol.91397.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfe RR. Protein supplements and exercise. Am J Clin Nutr. 2000;72:551S–557S. doi: 10.1093/ajcn/72.2.551S. [DOI] [PubMed] [Google Scholar]
- 6.Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skeletal Muscle. 2011;1:11. doi: 10.1186/2044-5040-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008;104:1452–1461. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanagida O, Kanai Y, Chairoungdua A, Kim DK, Segawa H, Nii T, Cha SH, Matsuo H, Fukushima J, Fukasawa Y, Tani Y, Taketani Y, Uchino H, Kim JY, Inatomi J, Okayasu I, Miyamoto K, Takeda E, Goya T, Endou H. Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta. 2001;1514:291–302. doi: 10.1016/s0005-2736(01)00384-4. [DOI] [PubMed] [Google Scholar]
- 9.Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E1011–1018. doi: 10.1152/ajpendo.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans K, Nasim Z, Brown J, Butler H, Kauser S, Varoqui H, Erickson JD, Herbert TP, Bevington A. Acidosis-sensing glutamine pump SNAT2 determines amino acid levels and mammalian target of rapamycin signalling to protein synthesis in L6 muscle cells. J Am Soc Nephrol. 2007;18:1426–1436. doi: 10.1681/ASN.2006091014. [DOI] [PubMed] [Google Scholar]
- 11.Baird FE, Bett KJ, MacLean C, Tee AR, Hundal HS, Taylor PM. Tertiary active transport of amino acids reconstituted by coexpression of System A and L transporters in Xenopus oocytes. Am J Physiol Endocrinol Metab. 2009;297:E822–829. doi: 10.1152/ajpendo.00330.2009. [DOI] [PubMed] [Google Scholar]
- 12.Christie GR, Hajduch E, Hundal HS, Proud CG, Taylor PM. Intracellular sensing of amino acids in Xenopus laevis oocytes stimulates p70 S6 kinase in a target of rapamycin-dependent manner. J Biol Chem. 2002;277:9952–9957. doi: 10.1074/jbc.M107694200. [DOI] [PubMed] [Google Scholar]
- 13.Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J. 2005;19:461–463. doi: 10.1096/fj.04-2284fje. [DOI] [PubMed] [Google Scholar]
- 14.Hyatt SL, Aulak KS, Malandro M, Kilberg MS, Hatzoglou M. Adaptive regulation of the cationic amino acid transporter-1 (Cat-1) in Fao cells. J Biol Chem. 1997;272:19951–19957. doi: 10.1074/jbc.272.32.19951. [DOI] [PubMed] [Google Scholar]
- 15.Lopez AB, Wang C, Huang CC, Yaman I, Li Y, Chakravarty K, Johnson PF, Chiang CM, Snider MD, Wek RC, Hatzoglou M. A feedback transcriptional mechanism controls the level of the arginine/lysine transporter cat-1 during amino acid starvation. Biochem J. 2007;402:163–173. doi: 10.1042/BJ20060941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goberdhan DC, Meredith D, Boyd CA, Wilson C. PAT-related amino acid transporters regulate growth via a novel mechanism that does not require bulk transport of amino acids. Development. 2005;132:2365–2375. doi: 10.1242/dev.01821. [DOI] [PubMed] [Google Scholar]
- 17.Heublein S, Kazi S, Ogmundsdottir MH, Attwood EV, Kala S, Boyd CA, Wilson C, Goberdhan DC. Proton-assisted amino-acid transporters are conserved regulators of proliferation and amino-acid-dependent mTORC1 activation. Oncogene. 2010;29:4068–4079. doi: 10.1038/onc.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drummond MJ, Fry CS, Glynn EL, Timmerman KL, Dickinson JM, Walker DK, Gundermann DM, Volpi E, Rasmussen BB. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol. 2011;111:135–142. doi: 10.1152/japplphysiol.01408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergstrom J. Muscle electrolytes in man. Scand J Med Sci Sports. 1962;68:1–110. [Google Scholar]
- 20.Drummond MJ, Miyazaki M, Dreyer HC, Pennings B, Dhanani S, Volpi E, Esser KA, Rasmussen BB. Expression of growth-related genes in young and older human skeletal muscle following an acute stimulation of protein synthesis. J Appl Physiol. 2009;106:1403–1411. doi: 10.1152/japplphysiol.90842.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolfe RR, Chinkes DL. Isotopic Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. 2. Hoboken: John Wiley & Sons, Inc; 2005. [Google Scholar]
- 24.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyde R, Cwiklinski EL, MacAulay K, Taylor PM, Hundal HS. Distinct sensor pathways in the hierarchical control of SNAT2, a putative amino acid transceptor, by amino acid availability. J Biol Chem. 2007;282:19788–19798. doi: 10.1074/jbc.M611520200. [DOI] [PubMed] [Google Scholar]
- 26.Pinilla J, Aledo JC, Cwiklinski E, Hyde R, Taylor PM, Hundal HS. SNAT2 transceptor signalling via mTOR: a role in cell growth and proliferation? Front Biosci (Elite Ed) 2011;3:1289–1299. doi: 10.2741/e332. [DOI] [PubMed] [Google Scholar]
- 27.Durham WJ, Casperson SL, Dillon EL, Keske MA, Paddon-Jones D, Sanford AP, Hickner RC, Grady JJ, Sheffield-Moore M. Age-related anabolic resistance after endurance-type exercise in healthy humans. FASEB J. 2010;24:4117–4127. doi: 10.1096/fj.09-150177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hyde R, Taylor PM, Hundal HS. Amino acid transporters: roles in amino acid sensing and signalling in animal cells. Biochem J. 2003;373:1–18. doi: 10.1042/bj20030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 2004;447:532–542. doi: 10.1007/s00424-003-1086-z. [DOI] [PubMed] [Google Scholar]
- 30.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000;130:139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- 31.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 32.Liu XM, Reyna SV, Ensenat D, Peyton KJ, Wang H, Schafer AI, Durante W. Platelet-derived growth factor stimulates LAT1 gene expression in vascular smooth muscle: role in cell growth. FASEB J. 2004;18:768–770. doi: 10.1096/fj.03-0886fje. [DOI] [PubMed] [Google Scholar]
- 33.Kim CH, Park KJ, Park JR, Kanai Y, Endou H, Park JC, Kim do K. The RNA interference of amino acid transporter LAT1 inhibits the growth of KB human oral cancer cells. Anticancer Res. 2006;26:2943–2948. [PubMed] [Google Scholar]
- 34.Drummond MJ, Dickinson JM, Fry CS, Walker DK, Gundermann DM, Reidy PT, Timmerman KL, Markofski MM, Paddon-Jones D, Rasmussen BB, Volpi E. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab. 2012;302:E1113–1122. doi: 10.1152/ajpendo.00603.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]