Abstract
Background
Rat studies have demonstrated that exposure to environments associated with alcohol intake reinstates alcohol seeking after extinction of alcohol-reinforced responding in a different context. However, extinction is limited as an abstinence model because humans typically abstain because of negative consequences associated with excessive drinking. It is currently unknown whether alcohol-associated contexts can provoke relapse to alcohol seeking after alcohol-taking behavior is suppressed by adverse consequences in a different context.
Methods
Alcohol-preferring P rats were first given home-cage access to 20% ethanol. Next, they were trained to self-administer 20% ethanol in one context (context A). Subsequently, all rats continued to self-administer alcohol in a different context (context B). For one group, 50% of alcohol-reinforced responses were punished by mild footshock; two other groups either received non-contingent shocks or no shock. A fourth group was given extinction training in context B. All rats were then tested for relapse to alcohol seeking under extinction conditions in contexts A and B.
Results
In Context B, alcohol-taking behavior was suppressed by contingent shock (punishment) and extinction training but not by non-contingent shock. In Context A, relapse to alcohol seeking was reliably observed in the punished and extinction groups; a context switch had no effect on alcohol seeking in the no-shock or non-contingent shock groups.
Conclusions
Our data indicate that punishment-induced suppression of alcohol-taking behavior is context-dependent. We propose that our procedure can be used to explore mechanisms of context-induced relapse to alcohol seeking after alcohol-taking behavior is suppressed by adverse consequences.
Keywords: alcohol, P rats, intermittent access, punishment, context, relapse, extinction
Introduction
In humans, places or contexts previously associated with alcohol use often provoke relapse during abstinence (1–5). In rats, studies using the ABA renewal procedure (6, 7) have demonstrated that exposure to the alcohol self-administration context after extinction of the alcohol-reinforced responding in a different context reinstates alcohol seeking (8–11). In this procedure, rats are trained to self-administer alcohol in one context. Next, lever-responding is extinguished in a different (non-alcohol) context. Subsequently, context-induced reinstatement (renewal) of alcohol seeking is assessed by testing rats in the alcohol-associated context.
In the context-induced reinstatement procedure, alcohol abstinence is achieved by operant extinction training. During extinction, alcohol is unavailable and the previously alcohol-reinforced responding declines over time. In humans, however, alcohol is often available and abstinence is typically initiated because of the negative consequences associated with excessive drinking (12, 13). Therefore, one limitation of the context-induced reinstatement procedure (14), and other extinction-reinstatement procedures (15), is the lack of homology between the animal model and the human condition (16–20). Our goal here was to develop an animal model of context-induced relapse after alcohol intake is suppressed by negative consequences.
One way to impose negative consequences on drug use in animal models is to introduce a punishment contingency during self-administration training. In most rats, response-contingent shock punishment suppresses operant responding for both drug (21–25) and non-drug (26, 27) rewards. Response-contingent shock causes rapid suppression of morphine, amphetamine, and cocaine self-administration (23, 28). The magnitude of suppression of drug self-administration is correlated with shock intensity (28, 29) and nonpunished cocaine-reinforced responses are chosen over punished cocaine-reinforced responses (30, 31).
It is well known that punishment of food-reinforced responding does not permanently suppress responding (26, 27). Furthermore, studies using the Geller-Seifert procedure indicate that punishment-induced response suppression during food-reinforced sessions is under discriminative stimulus control (27, 32). However, there are surprisingly few studies on recovery of drug or alcohol seeking after punishment-induced abstinence. In an early study, Smith and Davis (23) found that morphine priming injections do not provoke ‘relapse’ to morphine seeking after severe punishment (high intensity shock). However, more recently, Panlilio et al. (22) reported that priming injections of remifentanil (an opiate), after punishment-induced abstinence led to resumption of remifentantil self-administration (relapse). Subsequently, Panlilio et al. (33) reported that priming injections of lorazepam (a benzodiazepine) or heroin provoke relapse to remifentanil seeking, as assessed in extinction tests, after punishment-induced abstinence. This finding demonstrates that punished drug seeking can be recovered in the absence of drug-reinforced responding during ‘relapse’ testing.
In previous studies relapse to drug seeking after punishment was assessed after drug priming injections. Here we assessed whether alcohol-associated contexts can provoke relapse to alcohol seeking after alcohol-seeking is punished in a different context. For this purpose, we adapted the ABA context-induced reinstatement procedure (6, 7, 34) and suppressed alcohol seeking by punishment in a different context. We then assessed relapse to alcohol seeking in extinction tests in both the punishment and the original alcohol self-administration contexts. We used the selectively bred alcohol- preferring P rats, because these rats are a useful model for voluntary self-administration of alcohol (35). Finally, for comparison purposes, we also examined context-induced reinstatement of alcohol seeking after extinction, using the established ABA renewal procedure (14, 34).
Methods
Subjects
Male alcohol-preferring P rats (~30 days old, total n=44) were obtained from Indiana University Medical Center. Rats were quarantined for ~3 weeks. The rats were housed singly under a reversed 12-h light/dark cycle (8:00 A.M. light off). The experimental procedures followed the Guide for the Care and Use of Laboratory Animals (eighth edition) and were approved by the Animal Care and Use Committee.
Apparatus
See Supplement.
Procedure
In Exp. 1, three groups were distinguished by the manipulation during the context B training phase. These groups either received footshock that was contingent on lever-presses (Punished, n=15), footshock that was not contingent on lever-presses (Non-contingent, n=8), or no shock (Unpunished, n=11). Exp. 1 timeline is provided in Fig. 1A. In Exp. 2, one group (Extinction, n=8) was used to demonstrate context-induced reinstatement of alcohol seeking after extinction of lever-presses in a different context.
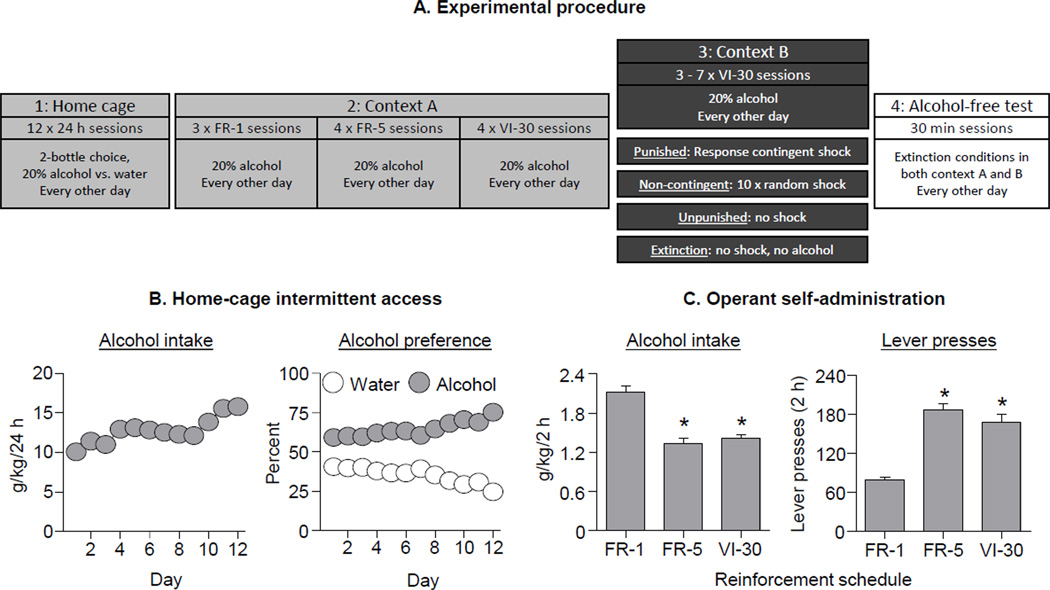
Figure 1. Intermittent free-choice home-cage access to 20% alcohol leads to high alcohol intake and robust acquisition of operant self-administration.
(A) Graphical representation of the experimental design, see Methods for details. (B) Mean±SEM alcohol intake in g/kg (left panel) and preference for 20% alcohol over water (right panel) during the 12 sessions of free-choice home-cage access to 20% alcohol. The standard errors for these data points are smaller than the symbol size. (C) Mean±SEM alcohol intake in g/kg (left panel) and active lever-presses (right panel) during the three different 20% alcohol self-administration schedules: FR-1, 3 sessions, FR-5, 4 sessions, VI-30, 4 sessions (n=42).
Phase 1: Intermittent home-cage access
We used the intermittent access 20% alcohol procedure (36, 37). Upon arrival to the animal facility from quarantine, rats were handled for 2 days. All rats received 12 × 24-h sessions of access to 1 bottle of 20% ethanol v/v in water and 1 bottle of water. Food was freely available. The session started around 9:00 am. After 24-h, the alcohol bottle was replaced with a second water bottle, and the no-alcohol session also lasted for 24-h. The following day, the 2nd water bottle was replaced with a 20% ethanol bottle and the location of the alcohol bottle was alternated from the previous session. The majority of no alcohol sessions were 24 h, however, some no alcohol sessions were 48 h. The volume consumed in each session was calculated as the weight difference between the start and end of the sessions, minus 2 grams for spillage, multiplied by 0.97 (density of 20% ethanol). Two rats with the lowest g/kg consumption were excluded.
Phase 2: Operant self-administration in context A
This phase began 48-h after the final home-cage access session. Sessions were 2-h long and run every other day. The first 2 sessions were 2-h ‘autoshaping’ sessions during which 0.1 ml of alcohol was delivered non-contingently every 5 min. Alcohol delivery was accompanied by a 2-sec compound tone-light cue. Subsequently, the rats were trained for 3 sessions to self-administer 0.1 ml deliveries of alcohol on a fixed-ratio-1 (FR-1) 2-sec timeout reinforcement schedule. Lever-presses on one lever (an active, retractable lever) resulted in presentation of the 2-sec tone-light cue and activated the infusion pump for 2 sec. Lever-presses on the other lever (an inactive, stationary lever) were recorded but had no programmed consequences. Subsequently, rats were trained on an FR-5 2-sec timeout reinforcement schedule for 4 sessions. Following this, rats were trained on a variable-interval 30-sec (VI-30) reinforcement schedule for 4 sessions. During these sessions, alcohol delivery was available after an active lever press at random intervals (range: 1-sec to 59-sec) after the preceding alcohol delivery. The variable interval began immediately after an alcohol-reinforced response. Responses during the timeout interval had no consequences.
Phase 3: Punishment (Exp. 1) or extinction (Exp. 2) in context B
Exp. 1
During this phase, the rats continued alcohol self-administration every other day (2-h sessions) under the VI-30 reinforcement schedule. For group Punished, 50% of the reinforced lever-presses, which occurred after the VI-30 response requirement was met, delivered a 0.5-sec, 0.45 mA footshock through the grid floor. Responses during the timeout interval were recorded, but had no consequences. During the punished responses, the tone-light cue was presented and 0.1 ml of 20% alcohol was delivered. There was a minimum of 3 punishment sessions; subsequently, rats in group Punished that made more than 20-responses/session were given additional sessions with increasing shock intensity until this criterion was met. The final shock intensity was 1.09 mA on the 7th session. For rats in group Non-contingent Shock, ten 0.5-sec 0.45 mA footshocks were delivered randomly throughout the 2-h sessions; we chose to deliver 10 footshocks, because this was the approximate mean number of shocks/session given to the rats in group Punished during the first 3 sessions. For rats in group Unpunished, conditions were identical to the previous sessions in context A. Rats in groups Unpunished and Non-contingent were given 3–7 sessions in context B, to match their context B exposure to group Punished. The n for each context B session for each group was:; ‘Punished’: session 1–3=15, session 4=9, session 5=7, session 6=7, and session 7=3; ‘Unpunished’: session 1–3=11, session 4=6, session 5=4, session 6=3, and session 7=2; ‘Non-contingent’: session 1–3=8, session 4=5, session 5=5, session 6=2, and session 7=2.
Exp. 2
Rats were placed into context B and each response that met the VI-30 reinforcement schedule resulted in the presentation of the tone-light cue, but no alcohol. There were 13 (2-h) extinction sessions; the first 10 sessions were every other day and the final 3 extinction sessions were given daily to minimize the influence of spontaneous recovery—the recovery of extinguished conditioned responses after the passage of time (34)—on lever-presses.
Phase 4: Relapse tests
All rats were tested for alcohol seeking (operationally defined as active lever responding under extinction conditions) with no footshock punishment, in both context A and context B in 30-min extinction sessions. In Exp. 1, the test order in each context was counterbalanced between and within groups. The first test was 48 h after each rat’s final context B training session, and tests were separated by 48 h. In Exp. 2, the rats were tested 24 h after the last extinction session and the tests were 24 h apart. The order of testing in contexts A and B was counterbalanced.
Statistical analysis
Data were analyzed separately for the 4 phases: home-cage access, context A training, context B punishment or extinction, and relapse tests. For the home-cage access phase, the dependent measures were 20% alcohol preference and g/kg alcohol intake. For the training and punishment phases, the dependent measures were total active lever-presses and g/kg alcohol intake. For the relapse test, the dependent measures were total (non-reinforced) responses on the previously active lever and inactive lever responses. Group differences in these measures were analyzed by ANOVAs and Tukey post-hoc tests. The groups were matched for their home-cage alcohol intake context A lever-presses during training. Inactive lever-presses were very low (mean of less than 4 presses session; Table S1); thus, these data were only used as covariates in the analyses.
Results
Home-cage alcohol intake and initial operant training in context A (Exp. 1–2)
Figure 1B shows alcohol intake (g/kg) and preference during the home-cage access phase (see Figure S1 for data from each group in Exp. 1). Mean alcohol intake during this phase was 12.8±0.2 g/kg/24-h. The rats increased their alcohol intake and preference over time. The analyses showed significant effects of Session for alcohol intake (g/kg) and preference (F1,41=41.5 and 42.6, p<0.01).
All rats acquired alcohol self-administration of in context A (Fig. 1C), as indicated by a reliable (>90%) discrimination between the active and inactive levers, and mean intake >0.5 g/kg/session. The switch from the FR-1 schedule to the intermittent reinforcement schedules (FR-5 and VI-30) caused decreased alcohol intake (g/kg) (F1,41=70.9, p<0.01) and increased active lever-presses (F1,38=38.1, p<0.01).
Exp. 1: Relapse to alcohol seeking after punishment
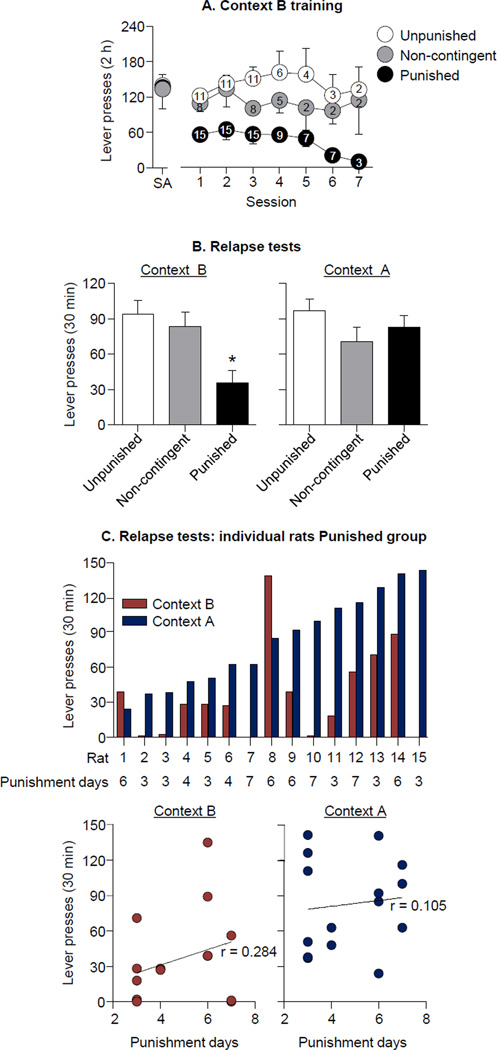
Figure 2A shows the mean (±SEM) number of active lever-presses in the final self-administration session and the context B training sessions. The Punished group, but not in the Non-Contingent Shock or Unpunished (no shock) groups, decreased their active lever-presses over time. All rats were given at least 3 sessions in context B. After the 3rd session, rats in group Punished that responded above the suppression criteria (<20 responses/2-h) were given additional sessions with footshock intensity increased by 0.15 mA/session for up to 7 sessions. Some rats from groups Unpunished and Non-contingent were also given additional sessions in context B. ANCOVA of active lever-presses during the first 3 punishment days (with the final selfadministration day as a covariate) showed a main effect of Group (F2,30=9.0; p<0.05), but no Group × Day interaction (p>0.05). During context B training, no alcohol remained in the receptacles of the Punished rats, indicating that alcohol earned during these sessions was consumed.
Figure 2. Relapse to alcohol seeking when rats returned to the original training context after response-contingent punishment in an alternative context.
(A) Mean±SEM reinforced active lever-presses during the final self-administration session in the initial training context (context A) and all self-administration sessions in the punishment context (context B). The numbers within the symbol represent n per group for each session. After the 3rd session, rats in group Punished with more than 20 active lever-presses in the 2-h session were given additional sessions with increased shock intensity; rats from the other groups were given additional sessions to match this. (B) Mean±SEM non-reinforced active lever-lever-presspresses during the relapse tests in context B (left panel) and context A (right panel). Lever-presses of the Punished rats (n=15) were higher in context A than in context B. Context switch had no effect on non-reinforced responding in the Unpunished (n=11) and Non-contingent Shock (n=8) groups. (C) The top panel shows active lever-presses during testing in each context for individual rats in group Punished. The bottom panel shows correlation plots of active lever-presses during testing as a function of the number of context B punishment sessions. * Different from the other experimental conditions, p<0.05.
Figure 2B shows the mean (±SEM) number of lever-presses on the previously active lever during the relapse test in context B (left panel) and context A (right panel). In the Punished group, non-reinforced active lever responding was higher in context A than in context B, and similar to that of the Non-contingent Shock and Unpunished Groups. Lever-presses in the latter two groups was similar in contexts A and B. ANCOVA of total active lever-presses showed a Group × Context interaction (F2,29=7.0; p<0.05). Post-hoc group differences are shown in Fig. 2.
Figure 2C shows the lever-presses during testing for each individual rat in group Punished. There were no significant correlations between the number of context B punishment sessions and lever-presses during testing in either context A (r=0.105; p>0.05) or context B (r=0.284; p>0.05).
Exp. 2: Context-induced reinstatement of alcohol seeking after extinction
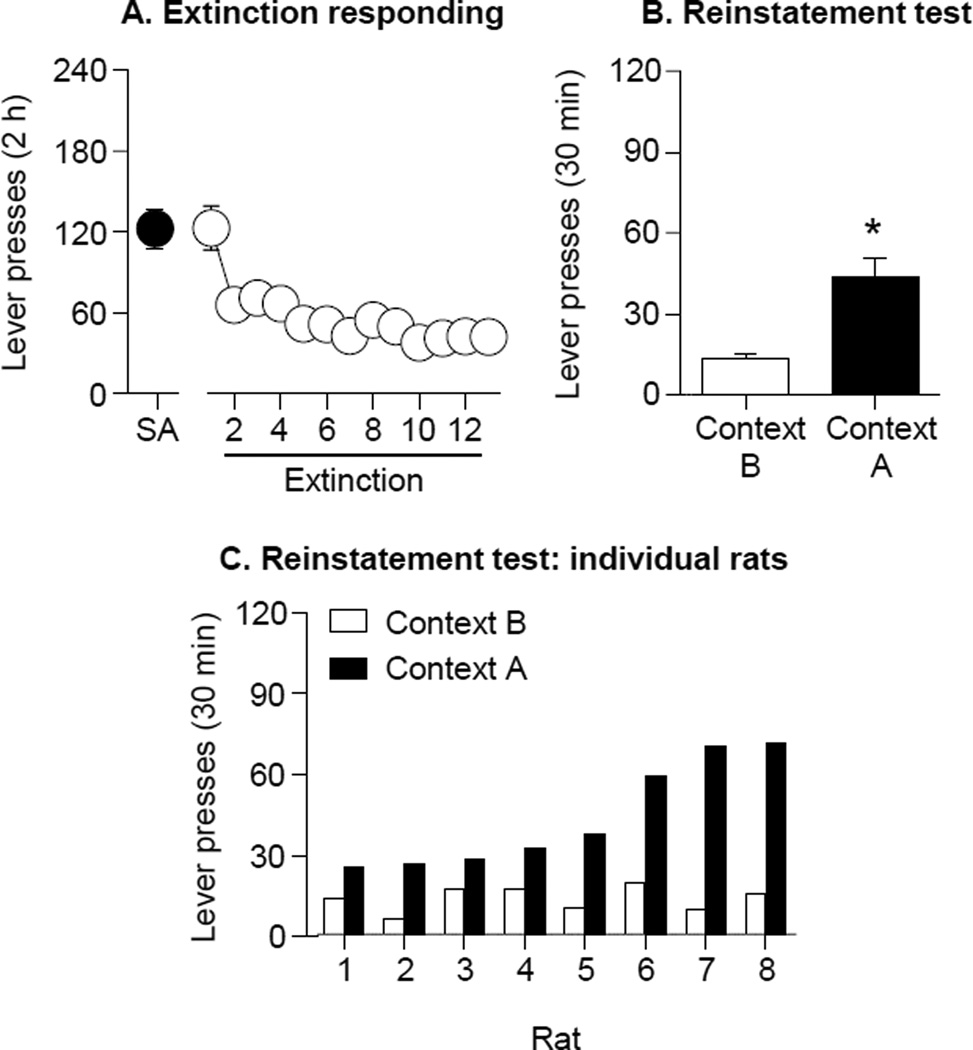
Figure 3A shows the mean (±SEM) active lever-presses in the final self-administration session and the context B extinction sessions. The rats decreased their active lever-presses over time, as indicated by a main effect of Extinction Session (F12,84=12.1, p<0.01). Figure 3B shows the mean (±SEM) number of lever-presses on the previously active lever during the reinstatement tests in contexts A and B. The rats reinstated lever-presses in context A after extinction in context B, as indicated by a main effect of Context (F1,7=18.6; p<0.05).
Figure 3. Context-induced reinstatement of alcohol seeking after extinction training.
(A) Mean±SEM reinforced active lever-presses during the final self-administration session (context A) and non-reinforced lever-presses (extinction) in context B. (B) Mean±SEM non-reinforced active lever-presses during the reinstatement tests in context A and context B. All rats were tested in both contexts and the order of testing was counterbalanced. (C) Total active lever-presses during test in each context for individual rats (n=8). * Different from Context B, p<0.05.
Discussion
We found that exposure to the original alcohol self-administration environment (context A) after punishment-induced suppression of alcohol seeking in a different environment (context B) provoked relapse to alcohol seeking in alcohol-preferring P rats. Non-reinforced lever-presses in context A (the operational measure of alcohol seeking) of the previously punished rats was similar in magnitude to that of non-punished rats or rats that received non-contingent shock in context B, which had no effect on alcohol self-administration. Additionally, while comparison across procedures should be done with caution, context-induced relapse to alcohol seeking after punishment appears as robust as context-induced reinstatement after extinction in the traditional ABA renewal procedure (14). We propose that our procedure can be used to study mechanisms of relapse to alcohol seeking under conditions that more closely mimic the human condition.
Context-induced relapse to alcohol seeking after punishment
Our aim was to develop a procedure in which relapse occurs after active alcohol-taking behavior is suppressed by adverse consequences. We have developed this alternative procedure because in current animal models of relapse abstinence is achieved by extinction training and/or by imposing a forced abstinence period (15, 38–40). These relapse models, however, do not fully capture the human condition where abstinence is often initiated because of a desire to avoid the negative consequences associated with excessive use (12, 13, 19, 20). This lack of homology between the animal models and the human condition has been identified as a potential limitation of current relapse models (17, 19, 20).
One issue to consider regarding the validity of our procedure is that response-contingent punishment is also somewhat limited, because time-locked contingent punishment of alcohol use is likely not a common reason for abstinence in alcoholics. Furthermore, we do not propose that human abstinence, imposed by punishment or negative consequences of alcohol use, exclusively occurs within a specific physical context. While we have used a physical environment to signal punishment, or adverse consequences of alcohol seeking, contextual control over the expression of learned behavior is not limited to physical environments. For example, internal states, induced by drug effects, mood, or other factors can lead to state-dependent learning. Consequently, memory retrieval and expression of learned behaviors can become dependent on those internal states (41–43). Furthermore, passage of time can also act as a ‘temporal context’, with learned behaviors reemerging after an extended time period (44). More importantly, we have shown that punishment-induced suppression of alcohol seeking can be encoded as a context-specific memory, with alcohol seeking recovered by exposure to the original training context. At the very least, we argue that our procedure more closely mimics factors leading to human abstinence prior to relapse, or more formally, it has adequate face validity--the degree to which the animal model appears to reflect the human condition it has been designed to model (45–47). However, as argued elsewhere, face validity alone is not a sufficient criterion for a valid animal model (48). Thus, a future research question is our procedure’s predictive validity, which can be determined by assessing the effect of the FDA-approved alcoholism medication naltrexone (49) on context-induced relapse after punishment.
What psychological mechanisms mediate relapse after punishment in an alternative context? Our results demonstrate that response-contingent punishment does not permanently erase or prevent the expression of the previously established action (lever response)-outcome (alcohol delivery) associations. Indeed, it has been known for some time that punishment training does not permanently reduce the strength of the action-outcome associations after the termination of the punishment contingencies (26). Punishment may share similar features to other procedures that lead to suppression of instrumental responding, such as extinction training. Several lines of evidence suggest that extinction training causes the formation of new associations that inhibit, or ‘mask’, the original associations (44, 46, 50). Furthermore, other procedures used to suppress learned responses, such as differential-reinforcement-of-other-behavior, and non-contingent reward delivery, are also context-dependent (51, 52). Thus, a common feature of different response suppression procedures is that they do not erase the original learned associations, and the context-dependent suppression is thought to be mediated by new inhibitory associations (53). It is tempting to speculate that punishment training, like extinction training, causes the formation of new, inhibitory, associations that serve to suppress alcohol seeking in the punishment context. One potential test of this hypothesis is the demonstration of ‘relapse’ in context B after training and punishment in context A (an ‘AAB’ condition). The outcome of such experiment is difficult to predict, however, because while ‘AAB’ renewal is commonly observed in Pavlovian conditioning (53), evidence for ‘AAB’ renewal of extinguished instrumental responding is weak (7, 54, 55). On the other hand, a potential learning mechanism for our results is that contexts A and B act as ‘occasion setters’ (14, 56), and serve as retrieval cues that signal whether or not alcohol-reinforced responding will be punished.
Finally, we believe that it is unlikely that motivational effects of alcohol dependence (withdrawal relief) (57, 58) played a role in context-induced relapse after punishment under our experimental procedure. This is because alcohol intake of our rats (1–2 g/kg every other day) during training is not sufficient to induce alcohol dependence, and consequently, to allow rats to associate alcohol self-administration with withdrawal relief, as demonstrated in studies using alcohol vapor chambers (59). Future studies, combining the vapor chamber procedure with our punishment-relapse procedure, are needed to determine the impact of alcohol dependence on both suppression of alcohol self-administration by punishment in context B and relapse in context A.
Punishment-based models of addiction
To our knowledge, our study is the first to demonstrate context-induced relapse to alcohol seeking after punishment-induced abstinence. As mentioned above, Panlilio et al., (22, 33) reported drug-priming-induced relapse to opiate seeking after punishment of the opiate-reinforced response. Additionally, using a conflict procedure (60, 61) in which the rats had to cross a shock barrier to gain access to the cocaine-reinforced lever, Cooper et al. (17) reported cue-induced relapse to drug seeking when rats were exposed to a discrete cue previously paired with cocaine infusions after suppression of drug seeking by shock exposure.
Recent studies have identified an ‘addiction’ phenotype in rats, operationally defined as resistance to punishment, after extended cocaine self-administration training (21, 24, 25). We also observed individual differences in sensitivity to footshock punishment. While this suggests that a sub-set of the punished rats has an ‘addicted’ phenotype, we found that increased shock intensity suppressed alcohol self-administration in all rats. The lack of correlation between number of punishment sessions and test responding in both contexts (Fig. 2C) suggests that individual differences in shock-induced suppression of alcohol seeking do not predict relapse in our procedure.
Our procedure is conceptually similar to addiction models that involve punishment or the introduction of an aversive consequence associated with drug intake. The primary focus of previous alcohol models has been to assess whether alcohol consumption itself is resistant to taste adulteration as an experimental procedure to study ‘compulsive’ alcohol use. Extended access to alcohol in the home-cage results in alcohol intake that is insensitive to adulteration with quinine (62–65). Other investigators have proposed that continuation of drug self-administration in the presence of response-contingent shock punishment can serve as an experimental procedure to study of compulsive drug use (21, 24, 25). These studies show that extended cocaine selfadministration results in ‘compulsive’ cocaine use in a sub-group (approximately 20%) of the rats. We found that a larger proportion of rats (approximately 50%) continued alcohol self-administration during punishment in the first 3 sessions with a mild shock intensity (Fig. 2A). We are hesitant to describe this as ‘compulsive’ alcohol self-administration, because of variable shock intensities across our operant chambers. However, we did find that different rats displayed different thresholds of shock intensity to suppress alcohol selfadministration below our suppression criterion. As such, in future studies, it will be of interest to determine whether a sub-group of alcohol-preferring P rats would demonstrate resistance to punishment under more controlled experimental conditions and can be labeled as ‘compulsive’.
Finally, one advantage of the context-induced relapse after punishment procedure is that the majority of rats are sensitive to the experimental manipulation (Fig 2C). As mentioned above, ‘compulsive’ cocaine selfadministration is typically observed in only a small sub-group of punishment-resistant rats (~20%) (21, 24, 25). In the conflict procedure (17, 66), presentation of the cocaine-associated discrete cue causes highly variable relapse responding in about 50% of the rats, and no relapse-like responding in other rats. Therefore, these procedures are more suitable to study individual differences in drug-taking behavior. On the other hand, our procedure enables more straightforward studies on neuropharmacological mechanisms of relapse after punishment-induced abstinence, because most rats demonstrate reliable relapse behavior during testing.
Conclusions and future directions
In humans, short- or long-term abstinence often occurs because the negative consequences associated excessive alcohol use outweigh the drug rewarding effects, and during abstinence, relapse often occurs after exposure to alcohol-associated contexts. We describe a rat model of context-induced relapse after punishment that mimics, to some degree, this clinical scenario. An attractive feature of our procedure is the reliability of the relapse responding across subjects, which appears as robust as the relapse/reinstatement response in the established context-induced reinstatement after extinction procedure.
Finally, like with any new relapse procedure, there are many outstanding questions that warrant further investigation, some of which we list below. One question that has implications for medication development is whether similar or different neuropharmacological mechanisms mediate context-induced relapse after punishment versus context-induced reinstatement after extinction. Another question is the extent to which our findings generalize to other drugs and other manipulations that cause reinstatement of extinguished drug and alcohol seeking. Based on previous research with different drugs on context-induced reinstatement after extinction (8, 14), it is likely that context-induced relapse after punishment would occur with other abused drugs. It is also likely that context-induced relapse after punishment would generalize to food rewards. This is because phenomena observed in drug relapse models such as priming-induced, cue-induced, and stress-induced reinstatement (15), as well as incubation of craving (67) and deprivation effects (40, 68) are also observed with food rewards (69–72). Additionally, while we have used a physical context to signal punishment, our procedure can also likely be adapted for the use of discriminative stimuli (73, 74) to signal punishment or non-punishment. Finally, It will be interesting to determine whether punished alcohol seeking recovers after the passage of time (spontaneous recovery) or by alcohol priming (40, 46).
Supplementary Material
Acknowledgments
The work was supported by the Intramural Research Program of the National Institute on Drug Abuse. We thank Drs. Leigh Panlilio and Markus Heilig for helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: The authors are employed by the US federal government and report no biomedical financial interests or potential conflicts of interest.
References
- 1.O'Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- 2.Staiger PK, White JM. Cue reactivity in alcohol abusers: Stimulus specificity and extinction of the responses. Addictive Behaviors. 1991;16:211–221. doi: 10.1016/0306-4603(91)90014-9. [DOI] [PubMed] [Google Scholar]
- 3.Wikler A. Dynamics of Drug Dependence: Implications of a Conditioning Theory for Research and Treatment. Arch Gen Psychiatry. 1973;28:611–616. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- 4.Collins BN, Brandon TH. Effects of extinction context and retrieval cues on alcohol cue reactivity among nonalcoholic drinkers. J Consult Clin Psychol. 2002;70:390–397. [PubMed] [Google Scholar]
- 5.McCusker CG, Brown K. Alcohol-predictive cues enhance tolerance to and precipitate "craving" for alcohol in social drinkers. J Stud Alcohol. 1990;51:494–499. doi: 10.15288/jsa.1990.51.494. [DOI] [PubMed] [Google Scholar]
- 6.Bouton ME, Bolles RC. Contextual control of the extinction of conditioned fear. Learn Motiv. 1979;10:445–466. [Google Scholar]
- 7.Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- 8.Janak PH, Chaudhri N. The potent effect of environmental context on relapse to alcohol-seeking after extinction. Open Addiction J. 2010;3:76–87. doi: 10.2174/1874941001003010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zironi I, Burattini C, Aicardi G, Janak PH. Context is a trigger for relapse to alcohol. Behav Brain Res. 2006;167:150–155. doi: 10.1016/j.bbr.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Hamlin AS, Blatchford KE, McNally GP. Renewal of an extinguished instrumental response: neural correlates and the role of D1 dopamine receptors. Neuroscience. 2006;143:25–38. doi: 10.1016/j.neuroscience.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Burattini C, Gill TM, Aicardi G, Janak PH. The ethanol self-administration context as a reinstatement cue: acute effects of naltrexone. Neuroscience. 2006;139:877–887. doi: 10.1016/j.neuroscience.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Burman S. The challenge of sobriety: Natural recovery without treatment and self-help groups. Journal of Substance Abuse. 1997;9:41–61. doi: 10.1016/s0899-3289(97)90005-5. [DOI] [PubMed] [Google Scholar]
- 13.Klingemann HKH. The motivation for change from problem alcohol and heroin use. British Journal of Addiction. 1991;86:727–744. doi: 10.1111/j.1360-0443.1991.tb03099.x. [DOI] [PubMed] [Google Scholar]
- 14.Crombag H, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Trans R Soc Lond B: Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed SH. Validation crisis in animal models of drug addiction: Beyond non-disordered drug use toward drug addiction. Neuroscience & Biobehavioral Reviews. 2010;35:172–184. doi: 10.1016/j.neubiorev.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Cooper A, Barnea-Ygael N, Levy D, Shaham Y, Zangen A. A conflict rat model of cue-induced relapse to cocaine seeking. Psychopharmacology. 2007;194:117–125. doi: 10.1007/s00213-007-0827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epstein D, Preston K. The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology. 2003;168:31–41. doi: 10.1007/s00213-003-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epstein D, Preston K, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- 21.Pelloux Y, Everitt BJ, Dickinson A. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology. 2007;194:127–137. doi: 10.1007/s00213-007-0805-0. [DOI] [PubMed] [Google Scholar]
- 22.Panlilio L, Thorndike E, Schindler C. Reinstatement of punishment-suppressed opioid selfadministration in rats: an alternative model of relapse to drug abuse. Psychopharmacology. 2003;168:229–235. doi: 10.1007/s00213-002-1193-0. [DOI] [PubMed] [Google Scholar]
- 23.Smith SG, Davis WM. Punishment of amphetamine and morphine self-administration behavior. The Psychological Record. 1974;24:477–480. [Google Scholar]
- 24.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for Addiction-like Behavior in the Rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 25.Belin D, Balado E, Piazza PV, Deroche-Gamonet V. Pattern of Intake and Drug Craving Predict the Development of Cocaine Addiction-like Behavior in Rats. Biol Psychiatry. 2009;65:863–868. doi: 10.1016/j.biopsych.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 26.Estes WK. An Experimental Study of Punishment. Psychological Monographs. 1944;57:1–40. [Google Scholar]
- 27.Azrin NH, Holz WC. Punishment. In: Honig WK, editor. Operant behavior: areas of research and application. Englewood Cliffs, NJ: Prentice-Hall; 1966. pp. 380–447. [Google Scholar]
- 28.Grove RN, Schuster CR. Suppression of cocaine self-administration by extinction and punishment. Pharmacol Biochem Behav. 1974;2:199–208. doi: 10.1016/0091-3057(74)90053-7. [DOI] [PubMed] [Google Scholar]
- 29.Bergman J, Johanson CE. The effects of electric shock on responding maintained by cocaine in rhesus monkeys. Pharmacol Biochem Behav. 1981;14:423–426. doi: 10.1016/0091-3057(81)90413-5. [DOI] [PubMed] [Google Scholar]
- 30.Johanson CE. The effects of electric shock on responding maintained by cocaine injections in a choice procedure in the rhesus monkey. Psychopharmacology. 1977;53:277–282. doi: 10.1007/BF00492364. [DOI] [PubMed] [Google Scholar]
- 31.Johanson CE. Pharmacological and Environmental Variables Affecting Drug Preference in Rhesus Monkeys. Pharmacological Reviews. 1975;27:343–355. [PubMed] [Google Scholar]
- 32.Geller I, Seifter J. The effects of meprobamate, barbiturates, d-amphetamine and promazine on experimentally induced conflict in the rat. Psychopharmacology. 1960;1:482–492. [Google Scholar]
- 33.Panlilio LV, Thorndike EB, Schindler CW. Lorazepam reinstates punishment-suppressed remifentanil self-administration in rats. Psychopharmacology. 2005;179:374–382. doi: 10.1007/s00213-004-2040-2. [DOI] [PubMed] [Google Scholar]
- 34.Bouton ME, Swartzentruber D. Sources of relapse after extinction in Pavlovian and instrumental learning. Clin Psychol Rev. 1991;11:123–140. [Google Scholar]
- 35.Li TK, Lumeng L, McBride WJ, Waller MB, Hawkins TD. Progress toward a voluntary oral consumption model of alcoholism. Drug and Alcohol Dependence. 1979;4:43–60. doi: 10.1016/0376-8716(79)90040-1. [DOI] [PubMed] [Google Scholar]
- 36.Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. Intermittent Access to 20% Ethanol Induces High Ethanol Consumption in Long–Evans and Wistar Rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacology. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- 38.Tran-Nguyen TL, Fuchs RA, Coffey GP, O'Dell LE, Baker DA, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and dopamine overflow in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- 39.Adams CL, Short JL, Lawrence AJ. Cue-conditioned alcohol seeking in rats following abstinence: involvement of metabotropic glutamate 5 receptors. British Journal of Pharmacology. 2010;159:534–542. doi: 10.1111/j.1476-5381.2009.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le A, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol Ther. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 41.Cunningham C. Alcohol as a cue for extinction: State dependency produced by conditioned inhibition. Learning & Behavior. 1979;7:45–52. [Google Scholar]
- 42.Bouton ME, Kenney FA, Rosengard C. State-dependent fear extinction with two benzodiazepine tranquilizers. Behav Neurosci. 1990;104:44–55. doi: 10.1037//0735-7044.104.1.44. [DOI] [PubMed] [Google Scholar]
- 43.Overton DA. Experimental methods for the study of state-dependent learning. Fed Proc. 1974;33:1800–1813. [PubMed] [Google Scholar]
- 44.Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 45.Willner P. The validity of animal models of depression. Psychopharmacology. 1984;83:1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- 46.Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 47.Markou A, Weiss F, Gold LH, Caine B, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology. 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- 48.Sarter M, Bruno JP. Animal models in biological psychiatry. In: D'haenen H, den Boer JA, Willner P, editors. Biol Psychiatry. Hoboken, NJ: Johns Willey & Sons Ltd; 2002. pp. 1–8. [Google Scholar]
- 49.O'Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278:66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- 50.Rescorla RA. Experimental Extinction. In: Mowrer RM, Klein SB, editors. Handbook of contemporary learning theories. Mahwah, NJ: Erlbaum; 2001. pp. 119–154. [Google Scholar]
- 51.Kearns DN, Weiss SJ. Contextual renewal of cocaine seeking in rats and its attenuation by the conditioned effects of an alternative reinforcer. Drug and Alcohol Dependence. 2007;90:193–202. doi: 10.1016/j.drugalcdep.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Nakajima S, Urushihara K, Masaki T. Renewal of operant performance formerly eliminated by omission or noncontingency training upon return to the acquisition context. Learn Motiv. 2002;33:510–525. [Google Scholar]
- 53.Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 54.Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cueinduced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakajima S, Tanaka S, Urushihara K, Imada H. Renewal of Extinguished Lever-Press Responses upon Return to the Training Context. Learn Motiv. 2000;31:416–431. [Google Scholar]
- 56.Holland PC. Occasion Setting in Pavlovian Conditioning. In: Douglas LM, editor. Psychology of Learning and Motivation. Academic Press; 1992. pp. 69–125. [Google Scholar]
- 57.Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addiction Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 59.Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- 60.Warden CJ. Animal motivation: experimental studies on the albino rat. New York: Columbia University Press; 1931. [Google Scholar]
- 61.Jenkins TN, Warner LH, Warden CJ. Standard apparatus for the study of animal motivation. J Comp Psychol. 1926;6:361–382. [Google Scholar]
- 62.Wolffgramm J. An ethopharmacological approach to the development of drug addiction. Neuroscience & Biobehavioral Reviews. 1991;15:515–519. doi: 10.1016/s0149-7634(05)80142-3. [DOI] [PubMed] [Google Scholar]
- 63.Wolffgramm J, Heyne A. From controlled drug intake to loss of control: the irreversible development of drug addiction in the rat. Behav Br Res. 1995;70:77–94. doi: 10.1016/0166-4328(95)00131-c. [DOI] [PubMed] [Google Scholar]
- 64.Lesscher HMB, Van Kerkhof LWM, Vanderschuren LJMJ. Inflexible and Indifferent Alcohol Drinking in Male Mice. Alcohol Clin Exp Res. 2010;34:1219–1225. doi: 10.1111/j.1530-0277.2010.01199.x. [DOI] [PubMed] [Google Scholar]
- 65.Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol Clin Exp Res. 2010;34:1565–1573. doi: 10.1111/j.1530-0277.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barnea-Ygael N, Yadid G, Yaka R, Ben-Shahar O, Zangen A. Cue-induced reinstatement of cocaine seeking in the rat “conflict model”: Effect of prolonged home-cage confinement. Psychopharmacology. 2012;219:875–883. doi: 10.1007/s00213-011-2416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- 69.Pickens C, Cifani C, Navarre B, Eichenbaum H, Theberge F, Baumann M, et al. Effect of fenfluramine on reinstatement of food seeking in female and male rats: implications for the predictive validity of the reinstatement model. Psychopharmacology. 2012:1–13. doi: 10.1007/s00213-011-2585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nair SG, Navarre BM, Cifani C, Pickens CL, Bossert JM, Shaham Y. Role of Dorsal Medial Prefrontal Cortex Dopamine D1-Family Receptors in Relapse to High-Fat Food Seeking Induced by the Anxiogenic Drug Yohimbine. Neuropsychopharmacology. 2011;36:497–510. doi: 10.1038/npp.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Wit H. Priming effects with drugs and other reinforcers. Exp Clin Psychopharmacol. 1996;4:5–10. [Google Scholar]
- 72.Pinel JPJ, Rovner LI. Saccharin elation effect. Bull Psychonom Soc. 1977;9:275–278. [Google Scholar]
- 73.Katner SN, Magalong JG, Weiss F. Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology. 1999;20:471–479. doi: 10.1016/S0893-133X(98)00084-0. [DOI] [PubMed] [Google Scholar]
- 74.Martin-Fardon R, Weiss F. Modeling Relapse in Animals. Current Topics Behav Neurosci. 2012 doi: 10.1007/7854_2012_202. (online) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.