Abstract
Converging lines of evidence show that a sizable subset of autism-spectrum disorders (ASDs) is characterized by increased blood levels of serotonin (5-hydroxytryptamine, 5-HT), yet the mechanistic link between these two phenomena remains unclear. The enzymatic degradation of brain 5-HT is mainly mediated by monoamine oxidase (MAO)A and, in the absence of this enzyme, by its cognate isoenzyme MAOB. MAOA and A/B knockout (KO) mice display high 5-HT levels, particularly during early developmental stages. Here we show that both mutant lines exhibit numerous behavioural hallmarks of ASDs, such as social and communication impairments, perseverative and stereotypical responses, behavioural inflexibility, as well as subtle tactile and motor deficits. Furthermore, both MAOA and A/B KO mice displayed neuropathological alterations reminiscent of typical ASD features, including reduced thickness of the corpus callosum, increased dendritic arborization of pyramidal neurons in the prefrontal cortex and disrupted microarchitecture of the cerebellum. The severity of repetitive responses and neuropathological aberrances was generally greater in MAOA/B KO animals. These findings suggest that the neurochemical imbalances induced by MAOAdeficiency (either by itself or in conjunction with lack of MAOB) may result in an array of abnormalities similar to those observed in ASDs. Thus, MAOA and A/B KO mice may afford valuable models to help elucidate the neurobiological bases of these disorders and related neurodevelopmental problems.
Keywords: Animal models, autistic-spectrum disorders, monoamine oxidase
Introduction
Autism spectrum disorders (ASDs) are neurodevelopmental conditions characterized by social deficits, communication impairments and repetitive, restricted behaviours (APA, 2000). Although the neurobiological bases of ASDs remain elusive, cogent anatomical and neuroimaging evidence suggests that the neuropsychiatric perturbations in these disorders are underpinned by aberrant cerebral and cerebellar connectivity (Amaral et al. 2008; Pickett & London, 2005). Several independent lines of research have documented that 30–50% of ASD patients exhibit significant increases in whole-blood levels of serotonin (5-hydroxytryptamine; 5-HT; Anderson et al. 1987, 1990; Mulder et al. 2004; Ritvo et al. 1970; Schain & Freedman, 1961). Although this association is well-documented, it remains unclear whether congenital hyperserotonemia may be inherently conducive to ASD. An attractive approach to verify the possible existence of a causal nexus between these two phenomena is afforded by animal models with congenital abnormalities of 5-HT homeostasis.
The metabolism of 5-HT is served by monoamine oxidases (MAOs; Bortolato et al. 2010), two mitochondrial membrane-bound enzymes that also catalyse the deamination of other neurotransmitters such as norepinephrine (NE), dopamine (DA) and trace amines. The two MAO isoenzymes, A and B, are encoded by abutting X-linked genes (Lan et al. 1989) with 70% identity in amino acid sequence (Bach et al. 1988). MAOA has high affinity for 5-HT and NE, whereas MAOB prefers β-phenylethylamine (PEA) as a substrate (Shih et al. 1999a). In humans and mice, MAOA deficiency results in high 5-HT blood and brain levels, particularly during early post-natal life, as well as extreme predisposition to reactive aggression, repetitive responses and other behavioural perturbations (Bortolato et al. 2008; Brunner et al. 1993a; Cases et al. 1995; Chen et al. 2004; Shih et al. 1999a). Recent lines of evidence have shown a relationship between allelic variants of MAOA gene associated with low catalytic activity and the severity of social, communicative, cognitive and neuromorphological impairments in ASD (Cohen et al. 2003, 2011, Davis et al. 2008; Yirmiya et al. 2002).
In the absence of MAOA, the overlapping substrate specificities of the two MAO iso-enzymes allow MAOB to vicariously contribute to the metabolism of 5-HT and other monoamines (Bortolato et al. 2008). In addition, since MAOB is the only isoenzyme expressed in platelets (Bond & Cundall, 1977), it may play an important function in the regulation of plasma 5-HT levels. As a result, the combined lack of MAO isoenzymes in MAOA/B knockout (KO) mice leads to greater monoamine brain and blood levels than those observed in their MAOA KO counterparts. These alterations are conducive to phenotypic abnormalities distinctively more severe than those encountered in MAOA KO mice, including developmental disability anxiety-like behaviours and other behavioural deficits (Chen et al. 2004). In humans, the same double genetic deficiency has been associated with a clinical presentation featuring severe communication impairments, stereotypical hand movements and profound mental disability (O’Leary et al. 2012; Sims et al. 1989; Whibley et al. 2010). Interestingly, the behavioural abnormalities in patients affected by MAOA and MAOB combined deficiency have been consistently described as ‘ autistic-like ’ or likened to those featured in high-severity pervasive development disorders (Lenders et al. 1996; Sims et al. 1989; Whibley et al. 2010).
Based on these premises, we hypothesized that the congenital hyperserotonemia associated with the deficiency of MAOA, either by itself or in combination with MAOB, might result in ASD-related features. To test for this possibility, in this study we examined whether MAOA and A/B KO mice may exhibit behavioural and neuropathological changes related to ASDs. Our findings reveal that both mutant lines display phenotypic abnormalities strikingly similar to core pathognomonic traits of these disorders.
Materials and method
Animals and husbandry
We used experimentally naive male 129S6 mice (n=241) weighing 25–30 g. MAOA and A/B KO and their wild-type (WT) littermates were generated and genotyped as described elsewhere (Chen et al. 2004; Scott et al. 2008). Animals were housed with ad libitum access to food and water, in facilities maintained at 22 °C on a 12 h–12 h light/dark cycle (lights on 06:00 hours). All in vivo experimental procedures were in compliance with the National Institute of Health guidelines and approved by the Animal Use Committees of Universities of Southern California and Cagliari.
Behavioural testing
All experiments were performed on separate sets of MAOA and A/B KO mice, in comparison to their WT littermates. Values from the two groups of WT were pooled together for the overall analysis, as no significant difference was found between the two groups in any paradigm. With the exception of ultrasonic vocalization testing (which was performed on pups aged 6 d), all experiments were carried out using male mice aged 3–4 months. Light and sound were maintained at 10 lux and 70 dB for all behavioural tests unless otherwise indicated. Behaviours were video recorded and analysed later by a single trained observer, who was blind to the genotype of the mice.
Social interaction
Mice were tested as previously described (Bortolato et al. 2011). Male adult mice were introduced into a neutral, unfamiliar Makrolon cage (20×10 cm), with foreign age- and weight-matched male WT conspecifics (from separate litters). To differentiate between the test mouse and the foreign WT conspecifics, we used odourless yellow acrylic paint markers to colour the tail of the foreign conspecifics. Testing sessions lasted 10 min and were scored for total duration of social interactions. Social behaviours consisted of the frequency and duration of investigative sniffing of the conspecifics towards their facial, abdominal and anogenital areas. To provide a more qualitative assessment of social encounters, the latency to the first social investigation, social approach (initiation of sniffing), social reciprocation (reciprocal social investigation of the test mouse when first approached by the WT conspecific), pinning and following behaviours were scored (Jacome et al. 2011). Non-social behaviours scored included the frequency and duration of grooming and digging.
Social investigation
We used a cylindrical wire cup enclosure (10×6 cm in diameter) that was placed in the centre of a Makrolon cage (20×30 cm). An age- and weight-matched male foreign conspecific was placed inside the cylinder. The test mouse was placed in one of four corners of the cage and allowed to freely explore the apparatus for 10 min. Placement of the test mouse was counterbalanced across genotypes. Behavioural measures include the number of social approaches and the duration of social investigation/interaction. Locomotor activity was analysed by counting the crossings of a grid (5×4 squares) superimposed with a transparent sheet onto the image of each cage in the video monitor.
Ultrasonic vocalizations in pups
Vocalizations were tested in pups on postnatal day 6. Cages were acclimatized to the room for 30 min. Light and background noise were maintained at 5 lux and 70 dB using white noise. Pups were individually placed on the test platform in a sound-proof cabinet. To eliminate any thermal effects on vocalizations, the test platform was placed above a warm water bath to maintain a temperature of approximately 35 °C. Ultrasonic vocalizations were measured for 5 min using an ultrasonic vocalization detector (Med Associates, USA). Behavioural measurements included the frequency and the duration of ultrasonic vocalizations.
Spontaneous alternation in the T-maze
The spontaneous alternation paradigm was performed as previously described (Deacon & Rawlins, 2006). Each session consisted of eight consecutive trials. In each trial, the mouse was placed in the start compartment of a T-maze. After 15 s, the door was lifted and the mouse was left free to explore the two arms of the maze. As soon as the animal entered (with all four paws) one of the two alternative arms (left or right), the door of that compartment was closed for 15 s to confine the animal. The animal was removed between trials and the T-maze was quickly cleaned with 70% alcohol and dried to remove any olfactory cue that may condition the performance in the next trial. If the animal did not enter an arm within 120 s, the animal failed the trial. Mice that recorded two failures were eliminated from testing. In total, one MAOA KO and two MAOA/B KO mice were excluded. The numbers of arm alterations and the trials elapsed before the first alternation were both analysed for each mouse.
Hole-board
Hole-board testing was conducted in a 16-hole square apparatus, as previously described (Bortolato et al. 2009). Each session lasted 10 min and the total number of head pokes was recorded. The occurrence of perseverative behaviours (manifested as the tendency to explore the same holes) was measured via the coefficient of exploratory variation, calculated as the ratio of the S.D. for each different hole over the mean for each mouse. This index (Geyer & Paulus, 1992) has been shown to provide a dependable estimation of the dispersion of the probability distribution for exploratory activity with regard to perseverative behaviours. In addition, we measured the average number of alternations for each hole explored, as well as the average number of head pokes per hole.
Marble burying
Marble burying was conducted using a variation of the protocol previously described (Bortolato et al. 2009). The trial included a 10-min habituation phase, in which the mouse was placed in the cage, which was filled with sawdust. At the end of this phase, the mouse was briefly removed and 24 glass marbles were placed on the surface of the cage at even distances. The animal was then reintroduced into the cage and its behaviour was monitored for the following 10 min. The number of digging bouts, the overall digging duration and the number of buried marbles were scored. A marble was considered buried if at least two-thirds of its surface area was covered in sawdust.
Water maze
Visual training, spatial learning and reversal learning were performed as described previously (Labrie et al. 2009; Xu et al. 2009). We used the Morris water maze, which consisted of a large circular metal tub (83 cm × 150 cm in diameter) filled with opaque water maintained at approximately 30 °C. The maze was divided into four equal quadrants. For spatial reference, six unique visual cues were placed on the walls of the maze at equal distances. Testing was performed in three phases: (1) visual training; (2) spatial learning; (3) reversal learning.
Visual training
Mice were individually exposed to the water for a 2-min probe trial. A 32-cm platform (14 cm in diameter) was placed 1 cm above the water in the middle of a quadrant. Mice were individually placed in the water and allowed to freely explore until they reached the platform. A 2-min cut-off time was used to minimize fatigue. Mice that failed to reach the platform by the cut-off time were gently guided to the platform. On the five subsequent trials, the position of the platform was randomly rotated.
Spatial learning
At the start of the spatial memory phase, mice were exposed to a 2-min probe trial without the presence of any platform. The platform was added and placed 1 cm below the water in the middle of a quadrant. Mice were individually placed in the water and allowed to freely explore until they reached the platform. A 2 min cut-off time was used to minimize fatigue. Mice that failed to reach the platform were gently guided to it. A total of five training trials were conducted every day until the mice were able to consistently locate the platform within 20 s. Mice were then subjected to a 2-min probe trial without the presence of a platform. Locomotor activity and time spent in the target quadrant were recorded and scored using behavioural tracking software (Ethovision; Noldus, Netherlands). Mice that were unable to locate the platform during the final trial (one WT, two MAOA KO and two MAOA/B KO mice) were excluded from further testing.
Reversal learning
Reversal learning was conducted 24 h after the conclusion of the spatial learning test. For the reversal acquisition phase, the platform was placed in the adjacent quadrant and submerged to 1 cm below the water surface. Animals performed four trials per day with a 60-min inter-trial interval for 5 consecutive days. Each mouse was individually placed in the maze periphery at pseudorandom locations and allowed free exploration until they reached the platform. A 2 min cut-off time was used to minimize fatigue. Mice that failed to reach the platform were gently guided to it. Mice were allowed to remain on the platform for 30 s. Spatial learning and reversal learning were measured by the percent change in time to locate the platform in comparison to the first day. Reversal learning was assessed in a 2-min probe trial that occurred 24 h after the last acquisition trial. In the probe trial, the platform was removed and mice were placed in the periphery of the quadrant opposite to the platform. Reversal learning was measured by the time spent in the platform-associated quadrant, as well as the percent locomotor activity in the target quadrant (defined as the distance travelled in the target quadrant over the total distance travelled).
Sticky tape test
Deficits in sensorimotor integration were measured using the sticky tape test as previously described (Bouet et al. 2009). Mice were briefly restrained and a circular piece of tape (5 mm in diameter) was placed on the bottom of each forepaw. Animals were then released and the latency to remove each piece of tape was recorded. A 5-min cut-off time was used.
Hot plate
Mice were individually exposed to a hot plate (IITC; Life Science, USA) at 52.5 °C. The latency to lick their paws was measured. A cut-off time for each trial was set at 40 s.
Beam-walking task
Testing was performed as previously described (Stanley et al. 2005). Mice were individually placed on the middle of a metal rod (60 cm in length and 2 cm in diameter), elevated 30 cm above the floor. Mice were allowed to walk on platforms located at each end of the beam. Each trial consisted of 10 repetitions of the assay and the number of foot slips (with one or both hindlimbs slipping from the beam) was counted.
Morphological and histological testing
Golgi histology and dendritic analyses
Adult male MAOA KO, MAOA/B KO and WT littermates were overdosed with urethane and transcardially perfused with saline. Brains were removed and processed for Golgi histology using Glaser and van der Loos’ modified Golgi stain (Glaser & van der Loos, 1981). Briefly, tissue was immersed in Golgi-Cox solution for 14 d. Brains were then dehydrated, infiltrated with a graded series of celloidins and embedded in 8% celloidin. Coronal sections were cut at 180 μM on a sliding microtome (AO 860; American Optics, USA). Free-floating sections were alkalinized, developed in Kodak D-19 (Tokyo, Japan), fixed in Ilford Rapid Fix (Ilford Photo, UK), dehydrated, mounted, and coverslipped.
Pyramidal neurons within the ventral and lateral orbitofrontal cortex were reconstructed. This region of interest was selected in view of its well-characterized involvement in social interaction and repetitive behaviours in ASD (Amaral et al. 2008). The orbitofrontal cortex was identified by its position on the ventrum of the rostral pole of the forebrain (Paxinos & Franklin, 2001). Pyramidal neurons were defined by the presence of a distinct, single apical dendrite, two or more basilar dendritic trees extending from the base of the soma and dendritic spines. Neurons selected for reconstruction were located in the middle third of the section, did not have truncated branches and were unobscured by neighbouring neurons and glia, with dendrites that were easily discriminable by focusing through the depth of the tissue. For each mouse, 6–12 neurons meeting the above criteria were randomly selected and drawn. Neurons were drawn at a final magnification of 600× and morphology of apical and basilar arbours was quantified in three dimensions using a computer-based neuron tracing system (Neurolucida; MBF Bioscience, USA) with the experimenter blind to the genotypes. Some of the present data were also used in previous analyses (Bortolato et al. 2011).
Corpus callosum thickness
Thickness of corpus callosum was examined at eight equal distance locations (+1,+0.5 mm, Bregma, −0.5, −1.0, −1.5, −2.0 and −2.5 mm). The brains were sliced into 40 μM coronal sections using a freezing microtome with every other section stained with Cresyl Violet (Nissl stain). For each animal, 2–3 stions were selected from the identified location according to the Allen Mouse Brain Atlas (http://mouse.brainmap.org) (Lein et al. 2007). The corpus callosal thickness was measured using a Nikon light microscope (Nikon Instrument, USA) equipped with a colour video camera that connected to a computer with Stereo Investigator 10 software (MicroBrightField, USA). The thickness was recorded at the midline using 10× objective and the average corpus callosal thickness was calculated.
Cerebellum molecular and granular layers thickness and Purkinje cell number measurements
The thickness of molecular and granular layers from lobular I, II and III of Nissl stained cerebellum were measured from photomicrographs (two from each area) and then averaged as the final number per case. For the Purkinje cell number analysis, at least two sections from a width of 870 μM containing Purkinje cell layer per case were counted and then averaged.
Barrel fields
In order to view the rodent barrel organization, the somatosensory cortex (~4×6 mm area) was trimmed out first and flat mounted on a freezing microtome and then cut tangentially from the pia surface at 40 μM. The sections were Nissl stained and photographed with a Nikon (E800) microscope equipped with a digital camera system.
Statistical analyses
Normality and homoscedasticity of data distribution were verified by using Kolmogorov–Smirnov and Bartlett’s tests. Parametric analyses were performed with one-way ANOVAs followed by Tukey’s test (with Spjøtvoll–Stoline correction) for post-hoc comparisons. Non-parametric analyses were performed by Kruskal–Wallis test, followed by Nemenyi’s test for post-hoc testing. Significance threshold was set at 0.05.
Results
Behavioural alterations
Although the behavioural profiles of MAOA and A/B KO mice have been partially described in previous reports (Cases et al. 1995; Chen et al. 2004; Shih et al. 1999a, b), neither line has been characterized with regard to ASD-related traits. The present behavioural survey was conducted based on the comprehensive methodological guidelines provided by Moy and colleagues for the phenotyping of ASD murine models (Moy et al. 2007). In particular, our assessment was focused on the three core symptomatic domains of ASDs, namely, social deficits, communication impairments and perseverative, restricted behaviours. In addition, since ASD patients are often reported to display abnormalities in tactile sensitivity and gait, we studied the haptic, nociceptive and motor functions of both mutant lines.
Social deficits
We studied the performance of MAOA and A/B KO mice in the social interaction paradigm (Jacome et al. 2011). We also used a simplified version of the sociability task (Moy et al. 2007) within an open, familiar chamber. This modification was implemented since preliminary testing revealed that both mutant lines exhibited a marked reduction in the number of transitions between adjacent compartments of a multi-chamber sociability maze (Moy et al. 2007), irrespective of the presence of other mice or empty cages.
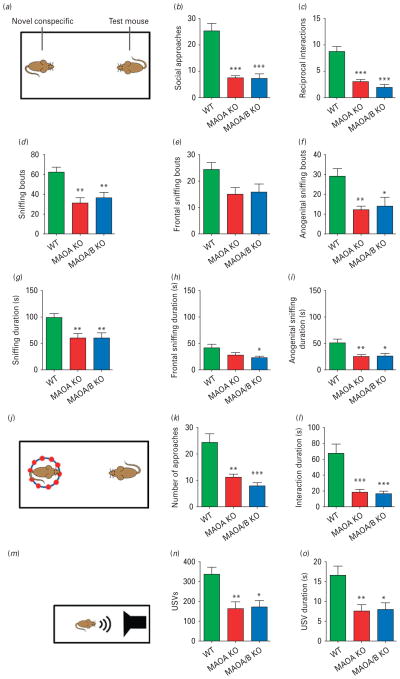
In the social interaction test (Fig. 1a), both MAOA and A/B KO mice displayed significant reductions in the number of social approaches (Fig. 1b; F2,21=28.54; p<0.001) and engaged in fewer reciprocal social responses (Fig. 1c; F2,20=24.97; p<0.001) to the foreign WT conspecifics compared to controls. Both mutant lines exhibited a decrease in frequency (Fig. 1d; F2,21=8.75; p<0.01) and overall duration (Fig. 1g; F2,22=7.00; p<0.01) of sniffing episodes in comparison to their WT littermates. Specifically, reductions were most evident in the number (Fig. 1f; ; F2,22=7.48; p<0.01) and duration (Fig. 1i; F2,22=6.84; p<0.01) of anogenital sniffing episodes. Furthermore, both MAOA and A/B KO mice exhibited significant decreases in following, pinning and grooming behaviour, but not in digging, locomotion or abdominal sniffing responses (Supplementary Fig. S1).
Fig. 1.
Monoamine oxidase (MAO)A knockout (KO) and MAOA/B KO mice display social and communication impairments. (a) Schematic diagram of the social interaction paradigm. (b, c) MAOA- and MAOA/B-deficient mice exhibit a significant reduction in social approaches and reciprocal interactions towards the foreign conspecifics compared to wild-type (WT) mice. (d–i) MAO mutant genotypes also displayed a marked decrease in overall sniffing behaviour in both number and duration in comparison to their WT counterparts. While this decrease was most pronounced in anogenital sniffing, MAOA/B-deficient mice also spent significantly less time in frontal sniffing than WT animals. (j–l) In the social investigation paradigm, both MAO mutant genotypes displayed a significant decrease in number and duration of social approaches. (m–p) Maternal separation-induced ultrasonic vocalizations (USVs) were markedly lower in MAOA KO and MAOA/B KO pups than WT pups. Values are displayed as means±S.E.M. *p<0.05, **p<0.01 and ***p<0.001 compared to WT mice. For social interactions n=7–10; social investigation n=8–10; maternal separation-induced USVs n=13–20.
In the social investigation test (Fig. 1j), both MAO-deficient lines exhibited a similar reduction in duration (H2=14.02; p<0.001) and number (H2=12.90; p<0.01) of investigative approaches towards the caged foreign counterparts (Fig. 1k, l), with no differences in locomotion (WT=52.2±4.8; MAOA KO=49.3±6.7; MAOA/B KO=47.2±7.2 crossings). This phenomenon contrasted with the lack of differences in investigative approach towards the empty cage in separate experiments.
Communication deficits
Communication was tested with the maternal separation- induced ultrasonic vocalization test, conducted in 6-d-old pups (Fig. 1m; Bader et al. 2011). Both MAO-deficient male mice displayed a significant reduction in the number (Fig. 1n; F2,42=7.25; p<0.01) and duration (Fig. 1o; F2,42=6.56; p<0.01) of ultrasonic vocalizations.
Perseverative behaviours
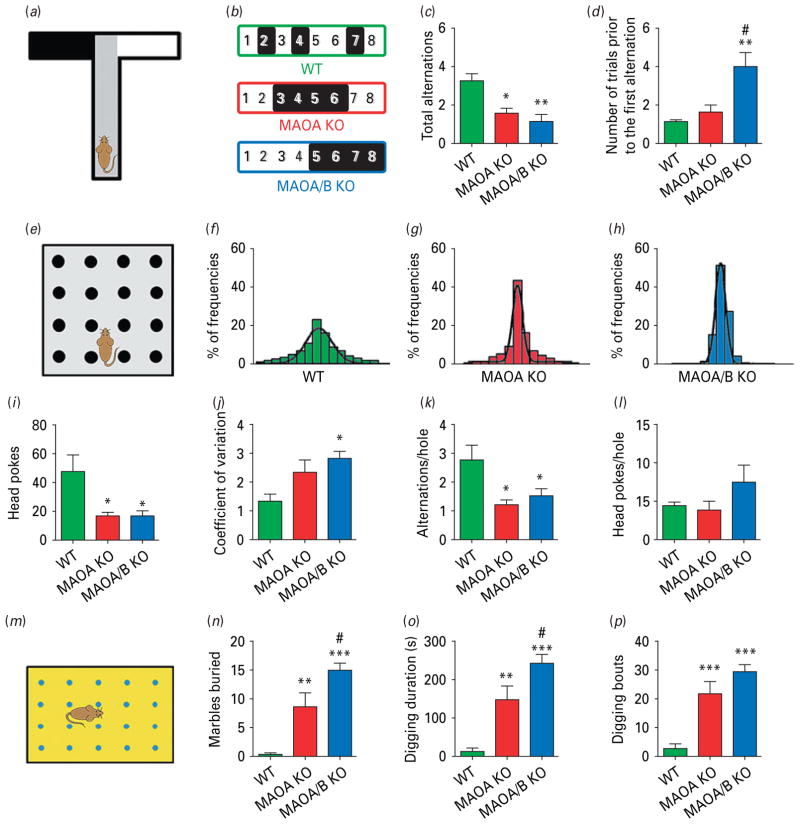
Repetitive and ritualistic behaviours are one of the core symptoms of the ASD diagnostic triad. To capture the insistence on sameness in MAOA and A/B KO mice, we assessed their exploratory responses in a novel T-maze (Zhao et al. 2010) upon eight consecutive presentations. In each trial, the animals were placed in the central compartment and tested for their preference towards one of the two perpendicular arms (Fig. 2a). Both mutant lines showed a significant tendency to repeat the exploration of the same arms in consecutive trials (Fig. 2b), which was reflected by their lower number of alternations between arms (Fig. 2c; F2,17=9.97; p<0.01). In addition, the number of entries in the same arm prior to the first alternation was significantly higher in MAOA/B KO mice (Fig. 2d; H2=10.63; p<0.01).
Fig. 2.
Monoamine oxidase (MAO)A knockout (KO) and MAOA/B KO mice exhibit perseverative behavioural responses. (a) Schematic representation of the T-maze paradigm. (b) Example of a typical set of alternations for each genotype where the change from black (left arm) to white (right arm) or vice versa indicates an alternation. (c, d) While both MAOA and MAOA/B KO mice displayed a significant reduction in spontaneous alternations compared to wild-type (WT) mice, MAOA/B-deficient mice engaged in a higher number of repetitive arm entries prior to the first alternation compared to the other genotypes. (e) Schematic representation of the hole-board task. (f–h) The 16-bar histograms depict the representative cases of percent relative dispersion of head pokes across the 16 holes of the board for each genotype. (i–l) Both MAO mutants had significantly fewer overall head pokes, as well as a lower average number of alternations per hole, but not head pokes per hole compared to WT mice. MAOA/B KO, but not MAOA KO mice, also exhibited a significantly higher coefficient of variation than their WT counterparts, signifying greater perseverative hole exploration. (m–p) In the marble-burying task, both transgenic lines displayed higher marble-burying and digging activity than WT mice; however, MAOA/B KO responses to marbles were significantly more robust than MAOA KO mice. Values are displayed as means±S.E.M. *p<0.05, **p<0.01 and ***p<0.001 compared to WT mice and #p<0.05 compared to MAOA KO mice. For spontaneous alternations in T-maze n=5–8; hole-board n=8; marble burying n=8–9.
The perseverative behaviour of MAO mutants was corroborated in the hole board paradigm (Fig. 2e; Geyer & Paulus, 1992; Kalueff et al. 2007). The head pokes of both MAO-deficient lines were significantly fewer (Fig. 2i; H2=7.96; p<0.05), but typically addressed towards the same holes of the apparatus (Fig. 2 f–h), as revealed by the higher coefficient of variation of head pokes across the board (Fig. 2j; F2,21=4.98; p<0.05). Furthermore, the average number of alternations (Fig. 2k; H2=8.91; p<0.05), but not total head pokes (Fig. 2l; H2=3.06; n.s.) in each hole was significantly lower in MAOA and A/B KO mice than WT littermates, although all genotypes showed comparable locomotor activity (data not shown).
The inclination of both MAO-deficient lines to engage in perseverative behaviours was finally confirmed in the marble-burying assay (Fig. 2m; Thomas et al. 2009). Both mutant lines buried a significantly higher number of marbles (Fig. 2n; H2=17.27; p<0.001) and displayed increases in digging duration (Fig. 2o; H2=16.16; p<0.001) and frequency (Fig. 2p; H2=14.78; p<0.001). No significant difference in locomotor activity was detected.
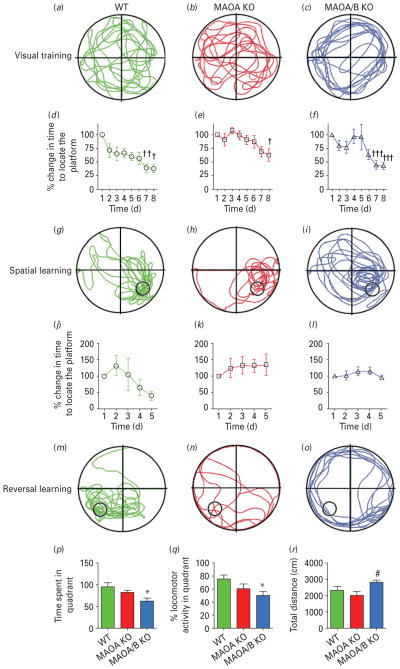
Water-maze and reversal learning
Cognitive inflexibility is another well-described characteristic trait in ASDs. To study this phenomenon in MAOA and A/B KO mice, we tested their ability to reverse spatial learning in the water maze (Fig. 3; Coldren & Halloran, 2003). No spatial bias was observed during the initial probe trial in any genotype (Fig. 3a–c). In the acquisition phase, MAOA and A/B KO mice learned to identify the location of the hidden platform in 7 d, in a fashion comparable to their WT littermates (genotype×time: F12,108=0.84; n.s.; Fig. 3d–i). During the reversal phase, WT learned the new location of the platform in 5 d on average (genotype×time: F8,64=2.95; p<0.01), while MAOA and A/B KO mice did not (Fig. 3j–l). In the final probe test (Fig. 3m–o), MAOA/B, but not A KO mice spent significantly less time (Fig. 3p; F2,18=4.88; p<0.05) and displayed lower percent activity (Fig. 3q; F2,18=4.48; p<0.05) in the platform-associated quadrant than WT controls (p<0.05 for both parameters). A significant change in locomotor activity was detected (F2,17=4.23; p<0.05) between MAOA and A/B KO mice (Fig. 3r; p<0.05), but not between any of these genotypes and WT counterparts.
Fig. 3.
Monoamine oxidase (MAO)A/B, but not MAOA-deficient mice show a significant impairment in learning reversal. (a–c) Typical locomotor pathways during the initial probe trial prior to training. (d–i) All genotypes exhibited a significant decrease in the percent time to reach the platform for spatial learning. (j–o) After reversal training, only wild-type (WT) mice displayed a significant reduction in the percent time to locate the platform, signifying learning acquisition. (p–r) MAOA/B knockout (KO) mice showed a significant reduction in both the total duration and the percent locomotor activity in the platform-associated target quadrant compared to WT mice. This was not caused by alterations in locomotor activity as no changes were found between MAOA/B KO and WT mice. Values are displayed as means±S.E.M. *p<0.05, **p<0.01 and ***p<0.001 compared to controls. #p<0.05 compared to MAOA KO mice. † p<0.05, †† p<0.01 and ††† p<0.001 compared to first trial within subject. For reversal learning n=6–9.
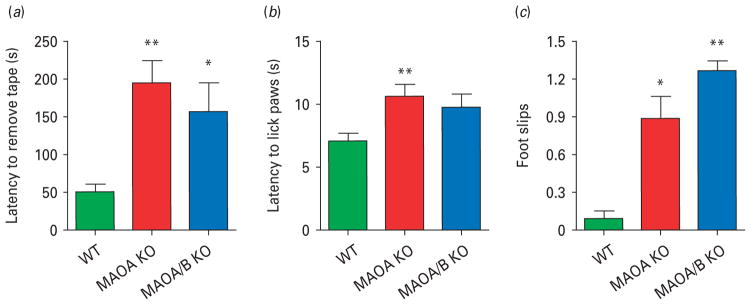
Tactile and motor disturbances
Disturbances in somatosensory and motor regulation, albeit not diagnostic, are common among ASD patients (APA, 2000; Ming et al. 2007). MAOA and A/B KO mice were tested in the sticky tape assay to assess their tactile sensitivity and sensorimotor control. Both mutant lines exhibited impairments in these functions, as indicated by the significant increase in latency to remove the tape from their forepaw (Fig. 4a; H2=9.79; p<0.01). A reduction in thermal nociception was also found in the hot plate test (Karl et al. 2003), as MAOA KO, but not MAOA/B KO, animals displayed an increase in their latency to lick their paw (Fig. 4b; F2,33=5.74; p<0.01). In the beam-walking assay (Crawley, 2007), both genotypes exhibited a typical crawling posture (Salichon et al. 2001) and subtle gait deficits, indicated by a significant increase in footslips in comparison to WT mice (Fig. 4c; F2,27=7.25; p<0.01).
Fig. 4.
Monoamine oxidase (MAO)A knockout (KO) and MAOA/B KO mice exhibit sensory and motor alterations. (a–c) Both MAO mutants display a significant reduction in haptic stimulation, as well as impairments in motor function compared to wild-type (WT) counterparts in the sticky tape and the balance beam tests respectively. In comparison to WT mice, MAOA KO, but not MAOA/B KO mice show an increase in the latency to lick paws in the hot plate test, indicating thermal insensitivity. Values are displayed as means±S.E.M. *p<0.05, **p<0.01 and ***p<0.001 compared to WT mice. For sticky-tape test n=9–10; hot-plate n=9–16; balance beam n=10.
Neuropathological alterations
Extensive neuropathological research has highlighted that the brain of a large subset of ASD patients features alterations in the connectivity of cerebral and cerebellar cortices. In view of the similarity between ASD symptoms and the behavioural manifestations of MAO-deficient mice, we further explored whether the brain of these mutants may also exhibit morphological changes akin to well-documented abnormalities in ASDs.
Connectivity of the cerebral cortex
To verify whether MAOA and A/B KO mice may display alterations in cortical connectivity, we first examined the size and shape of their corpus callosum, as this structure consists of axonal fibres projecting from pyramidal neurons throughout the cortex to the contralateral side. Notably, callosal alterations are a very well-characterized feature of ASDs (Frazier & Hardan, 2009). Our analyses showed that both MAO-deficient lines exhibited a significant reduction of callosal thickness (Fig. 5a–d; p<0.05 for MAOA KO vs. WT; p<0.01 for MAOA/B KO vs. WT), but the impairments were significantly more pronounced in MAOA/B KO than in MAOA KO mice (p<0.05). The decrease in callosal thickness was restricted to the rostral portion of the corpus callosum, suggesting that the frontal and prefrontal cortices exhibited the most remarkable deficits in output connectivity.
Fig. 5.
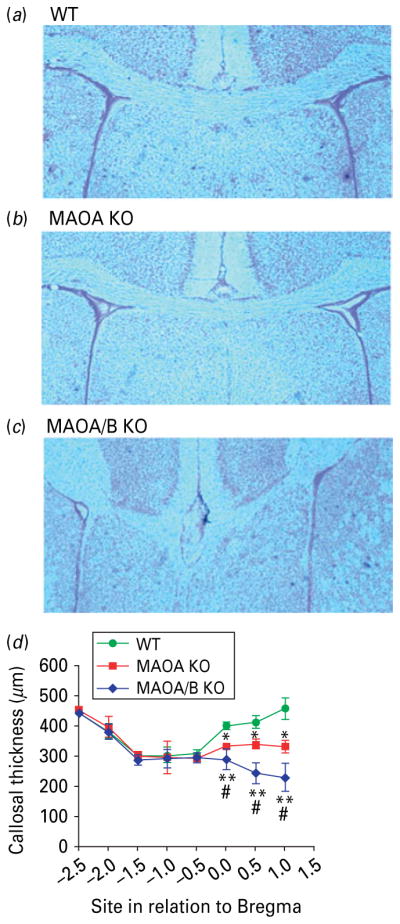
Monoamine oxidase (MAO)A knockout (KO) and MAOA/B KO mice show a reduction of callosal thickness. (a–c) Coronal images of the corpus callosum in wild-type (WT), MAOA KO, and MAOA/B KO mice. (d) MAOA- and MAOA/B-deficient mice show a significant reduction in thickness in the rostral level of the corpus callosum. Values are displayed as means±S.E.M. * p<0.05 and ** p<0.01 compared to WT mice and #p<0.05 compared to MAOA KO mice. For callosal thickness n=12–18.
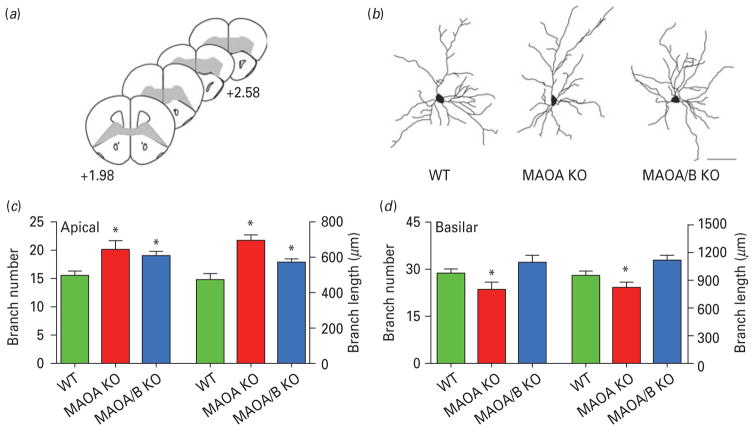
To verify whether these changes may also be accompanied by local alterations in input connections, we examined the dendritic tree of the pyramidal neurons in the orbitofrontal cortex (Fig. 6a–d). The number and total length of apical branches were significantly increased by 33 and 38% in MAOA KO mice and 26 and 23% in MAOA/B KO mice relative to WT controls (Fig. 6c; p<0.05 for all comparisons). In both genotypes, the increased dendritic material was more pronounced in the distal apical arbour (MAOA KO vs. WT, p<0.05 for radii ≥70 μM; MAOA/B vs. WT, p<0.05 for 10–20, 70–80 and 110–120 μM from the soma; MAOA KO vs. MAOA/B, p<0.05 for radii ≥140 μM from the soma). Conversely, MAOA KO mice displayed a significant reduction in basilar branch number (Fig. 6d; 19%; p<0.05) and length (21%; p<0.05) as compared to WT. No difference in these parameters was found between MAOA/B KO and WT mice.
Fig. 6.
Monoamine oxidase (MAO)A knockout (KO) and MAOA/B KO mice show significant disturbances in dendritic arbourization in the orbitofrontal cortex. (a) Schematic diagram of coronal sections through mouse prefrontal cortex. The coordinates indicate position relative to bregma (Paxinos & Franklin, 2001). (b) Computer-assisted reconstructions of Golgi-stained neurons in the orbitofrontal cortex in wild-type (WT), MAOA KO and MAOA/B KO mice. Scale bar: 50 μm. (c) Both MAOA and MAOA/B KO mice display an increase in the number and length of apical dendritic branches of pyramidal neurons in the orbitofrontal cortex. (d) Conversely, MAOA KO, but not MAOA/B KO mice exhibit a significant reduction in the number and length of basilar dendritic branches in the pyramidal neurons of the orbitofrontal cortex compared to their WT counterparts. Values are displayed as means±S.E.M. *p<0.05 compared to WT mice. For dendritic analyses n=6–7.
Connectivity of the cerebellar cortex
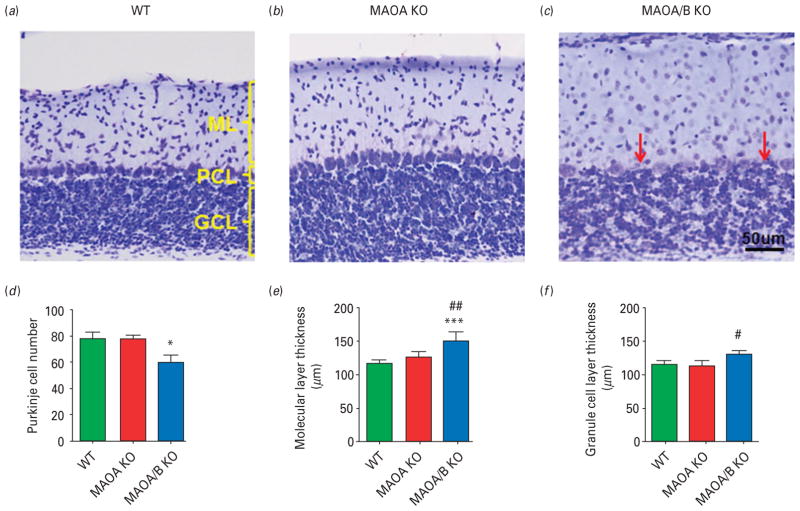
Abnormalities of the cerebellar cortex have been widely documented in ASDs (Allen et al. 2004). ANOVA statistical analysis followed by Tukey’s post-hoc test revealed that MAOA/B KO mice showed a significant loss of Purkinje cells (F2,18=13.30; p<0.001) and increased thickness of the molecular (F2,7=8.30; p<0.05), but not granular (F2,7=6.87; p<0.05; Fig. 7a–f) layer compared to WT controls. In addition, these mutants featured no distinct separation between the Purkinje cell layer and the granular layer, reflecting the fact that Purkinje cells were often embedded/intermingled with granules and Golgi cells. Conversely, MAOA KO mice did not exhibit any overt morphological abnormalities of the cerebellar cortex in comparison to WT counterparts.
Fig. 7.
Monoamine oxidase (MAO)A/B knockout (KO) mice exhibit morphological abnormalities in the cerebellum. (a–c) Representative photographs of Nissl stained cerebellar layers. Cerebellar layers are labelled in yellow and red arrows represent the Purkinje cells. (d–f) MAOA/B KO animals have significantly fewer Purkinje cells than wild-type (WT) and MAOA KO mice. Moreover, MAOA/B KO mice show an increase in the molecular layer (ML) thickness compared to WT mice and in the granule cell layer (GCL) thickness compared to MAOA KO mice. Values are displayed as means±S.E.M. *p<0.05 and ***p<0.001 compared to WT mice. #p<0.05 and ##p<0.01 compared to MAOA KO mice. Cerebellar layers n=5–8. PCL, Purkinje cell layer.
Discussion
The results of the present study have shown that male MAOA and A/B KO mice exhibit a spectrum of abnormal phenotypes highly reminiscent of those observed in ASD patients and other rodent models of these disorders (Silverman et al. 2010; Volkmar & Cohen, 1994). In particular, the behavioural alterations observed in both lines are closely related to the three core diagnostic domains of ASDs, social impairments, communication deficits and repetitive, restricted behaviour, as well as somatosensory and motor alterations typically observed in this condition (Volkmar & Cohen, 1994). These behavioural aberrances were accompanied by neuropathological changes previously documented in ASD, including significant reductions in the thickness of the rostral portion of the corpus callosum and increases in apical dendritic arbourization of the pyramidal cells in the prefrontal cortex, suggesting that both lines of mutants feature abnormalities of cortical connectivity, a well-characterized phenomenon in ASDs. Finally, MAOA/B KO mice displayed disruptions of cerebellar cortex reminiscent of the histological changes documented in ASDs (Palmen et al. 2004).
The identification of autistic-like behavioural abnormalities in MAOA and MAOA/B KO mice supports and extends our previous findings on the high levels of reactive aggression and behavioural perturbations in these two mutant lines (Cases et al. 1995; Chen et al. 2004; Godar et al. 2011; Kim et al. 1997). Our research has shown that MAOA and MAOA/B KO mice exhibit anxiety- and fear-related responses in select paradigms (Chen et al. 2004; Kim et al. 1997), as well as dysfunctional defensive reactivity to novel environmental cues (Godar et al. 2011). In keeping with this notion, the behavioural abnormalities reported in this study may be reflective of a general inability to properly process and integrate contextual information, a well-documented deficit in male carriers of low-activity MAOA haplotypes (Lee & Ham, 2008; Williams et al. 2009). This idea is further supported by our recent finding that MAOA KO mice feature marked impairments in the biophysical properties of N-methyl D-aspartate glutamate receptors in the prefrontal cortex, which may contribute to their deficits in the emotional processing of social stimuli (Bortolato et al. 2012).
Although the behavioural abnormalities displayed by MAOA and A/B KO mice appear to match all core features of ASDs, it cannot be excluded that they may reflect other behavioural problems. For example, some of the observed deficits may be contributed by the subtle sensory and postural impairments documented in this study. In addition, a number of aberrant responses, such as the reductions in ultrasonic vocal emissions and social approaches or the increase in marble burying may denote anxiety-related perturbations. These possibilities, however, are tempered by a number of observations. First, in all tested paradigms, locomotor activity of MAO-deficient lines was always equivalent to that of WT counterparts; second, the interpretation of the reduced ultrasonic vocalizations in MAO-deficient pups as a sign of milder separation anxiety is in contrast to the excessive marble burying and reduced social interactions, which are generally posited to reflect heightened emotional reactivity.
The spectra of neurobehavioural alterations exhibited by MAOA and MAOA/B KO mice diverged with regard to some characteristic aspects. Specifically, MAOA/B KO mice displayed a greater severity of perseverative responses and callosal aberrances, as well as unique cytoarchitectural abnormalities in the cerebellar cortex. It is likely that these differences may be ascribed to the higher degree of monoaminergic imbalances in MAOA/B KO mice as compared to MAOA KO counterparts (Chen et al. 2004). Alternatively, the elevation in PEA levels consequent to MAOB deficiency may be responsible for a more pronounced expressivity of repetitive behaviours and neuropathological changes in MAOA/B KO mice. This last interpretation is partially challenged by previous reports documenting the lack of overt pathological abnormalities in patients with isolated MAOB deficiency (Lenders et al. 1996). In addition, we recently characterized that the behavioural changes in MAOB KO mice are largely antithetical to those displayed by MAOA/B KO conspecifics, including increased novelty seeking and reduced anxiety-like behaviours (Bortolato et al. 2009). For example, while our studies show that MAOA/B KO mice displayed significantly greater levels of marble-burying responses than MAOA KO and WT counterparts, previous studies performed by our group indicated that this phenomenon is typically ablated in MAOB KO mice (Bortolato et al. 2009).
In contrast to the greater neurochemical imbalances in MAOA/B KO mice, the severity of social and communicative deficits exhibited by this line was unexpectedly comparable to that of MAOA KO animals. Although the biological bases for this phenomenon remain unclear, it should be noted that MAOA has been shown to play a predominant role in social and communicative deficits in ASD patients (Cohen et al. 2011). Thus, the observed similarity between MAOA and A/B KO mice with regard to these domains may depend on ‘ceiling effects’ caused by the profound impairments associated with MAOA deficiency, which may leave limited room for further behavioural exacerbations caused by MAOB deficiency in MAOA/B KO mice.
Similar to other rodent models of ASDs (Markram et al. 2008; Tabuchi et al. 2007), MAOA and A/B KO mice displayed significant deficits in reversal learning. This impairment is likely to signify behavioural and cognitive rigidity, a consistent feature in ASD (Lopez et al. 2005), rather than deficits in mnemonic encoding and retrieval, as shown by the lack of significant changes in the acquisition of spatial memory in the water maze. Notably, the lack of altered responses in the last task is consistent with clinical reports showing that spatial memory is generally unaffected or even enhanced in ASD patients (Bennetto et al. 1996).
Our morphological analyses revealed that MAOA and MAOA/B KO mice featured a reduction of the rostral portion of corpus callosum and increased apical arbourization of pyramidal cells in the orbitofrontal cortex – a region highly implicated in social impairments and repetitive behaviours in ASD (Amaral et al. 2008). Although no clear and consistent pathological features have emerged for autism, morphological alterations of the corpus callosum have been extensively documented in ASD (Amaral et al. 2008), in association with impairments in social skills, pragmatics of language and emotional processing (Paul et al. 2007). Similarly, aberrances of the apical dendrites of prefrontal pyramidal cells have also been reported in ASDs (Hutsler & Zhang, 2010). The spatial arrangement of the apical dendrites of pyramidal neurons is a critical determinant of the geometric organization of the minicolumns, the functional units of the cerebral cortex (Jones, 2000), suggesting that both MAOA and A/B deficiency may result in irregularities of these formations. This possibility is supported by the lack of barrel formation in both MAOA and A/B KO mice (Cases et al. 1995; Supplementary Fig. S2), a division of the rodent somatosensory cortex that affords one of the best-characterized examples of minicolumnar organization (Bruno et al. 2003). Indeed, abnormalities of the minicolumnar organization are posited to be one of the primary determinant factors in ASD pathophysiology (Casanova et al. 2002, 2006).
Altogether, these neuropathological findings are likely to signify the existence of alterations in input and output connectivity in the anterior cortex. In particular, these disturbances may contribute to the behavioural deficits in MAOA and A/B KO mice, such as social impairments, perseverative behaviours, reactive aggression and defects in environmental reactivity and risk assessment (Godar et al. 2011), in view of the prominent role of the prefrontal cortex in these functions (Goto et al. 2010; Volman et al. 2011). Moreover, perturbations in the orbitofrontal cortex, a key neural correlate of reversal learning (Boulougouris et al. 2007; Chamberlain et al. 2008; Chudasama & Robbins, 2003; Schoenbaum et al. 2003), may underpin some of the compulsive and repetitive responses observed in these lines. Carriers of low-activity MAOA polymorphic variants have been shown to display volumetric and functional alterations of the orbitofrontal cortex, as well as antisocial traits and impairments in facial affect processing (Lee & Ham, 2008; Meyer-Lindenberg et al. 2006).
We showed that MAOA/B KO mice displayed significant alterations in the cytoarchitectonics of the cerebellar cortex, including the loss of Purkinje cells and the enlargement of the molecular layer, which likely reflects the increase in dendritic arbourization of the Purkinje cells. These impairments were accompanied by ataxia in the beam-walking test. Interestingly, the developmental loss of Purkinje cells was also noted in the brain of a child affected by MAOA/B double deficiency (Whibley et al. 2010). Alterations in gait, posture and cerebellar morphology have been extensively documented in ASDs (Rinehart et al. 2006). In particular, the developmental loss of Purkinje cells has often been observed in the majority of post-mortem samples from ASD patients (Amaral et al. 2008; Whitney et al. 2009) and may be linked to behavioural rigidity (Dickson et al. 2010; Martin et al. 2010).
Translational implications: is there a link between MAO deficiency and ASDs?
The striking overlap between ASD-associated characteristics and altered phenotypes in MAOA and A/B KO mice may point to an involvement of these enzymes in the pathophysiology of autism or at least of specific perturbations observed in this disorder. Accordingly, recent lines of evidence have shown that genetic variants associated with low MAOA activity increased the vulnerability to higher severity of social, communicative and neuromorphological impairments in male ASD patients (Cohen et al. 2003, 2011; Davis et al. 2008; Yirmiya et al. 2002). Furthermore, it should be noted that previous reports have likened the social and cognitive outcomes of MAOA/B dual deficiency in humans with autism and other pervasive developmental disorders (Lenders et al. 1996; Sims et al. 1989; Whibley et al. 2010).
In contrast with our findings, the nosographic description of Brunner syndrome – the X-linked condition caused by MAOA human deficiency (Brunner et al. 1993a, b), has not been specifically associated with ASD characteristics, although several of its symptoms overlap with well-characterized alterations in ASD patients, including antisocial and aggressive behaviour, withdrawn personality, stereotyped movements, night terrors, maladaptive emotional responses to sudden stressors and mild cognitive alterations (Brunner et al. 1993a, b). Indeed, the question of the possible presence of autistic disturbances in MAOA-deficient individuals remains open, in view of the suboptimal clinical characterization of this syndrome (Hebebrand & Klug, 1995) and the lack of published studies on this condition in children.
The bulk of research has shown that MAOA plays a central role in the pathophysiology of reactive aggression in males. In particular, recent findings indicate that the severity of this trait is inversely correlated with the catalytic activity of MAOA in the brain (Alia-Klein et al. 2008), thereby highlighting this target as a potential biomarker for specific subsets of antisocial personality disorder. Notably, current theoretical perspectives posit that MAOA’s role in the genetic vulnerability to reactive aggression reflect alterations in corticolimbic connectivity, leading to alterations in the emotional processing of social and environmental stimuli, which could predispose to aggressive and antisocial reactions in response to ambiguous cues (Buckholtz & Meyer-Lindenberg, 2008). Of note, ASD patients display a general inability to process social cues, spatio-temporal relationships and other contextual information (Dawson et al. 2004; Gepner & Tardiff, 2006; Krysko & Rutherford, 2009; Philip et al. 2010). Anxiety, novelty avoidance, irritability and explosive aggression are highly frequent in ASD (Evans et al. 2005; Muris et al. 1998) and are generally enacted as maladaptive strategies to cope with unpredictable situational changes (Groden et al. 1994; Volkmar & Cohen, 1994).
The neuropathological alterations of MAO mutant mice described in the present report collectively point to abnormalities of cortical and cerebellar connectivity and are in line with several theories positing that the cognitive and behavioural deficits in ASD are underpinned by hypofunctional integrative brain circuitries (Just et al. 2007; Kleinhans et al. 2008).
In spite of the numerous elements of analogy between the abnormal phenotypes in MAOA and A/B KO mice and ASD patients, extreme caution should be advocated with regard to the translational value of these findings in relation to ASDs, in view of the intrinsic limitations of murine models in reproducing human illnesses. Moreover, it should be stressed that the similarity between the observed behavioural deficits and ASD symptoms does not signify that the two phenomena necessarily bear actual pathophysiological relations. Further studies will be needed to understand whether the isomorphism of behavioural changes in MAO-deficient mice and ASD symptoms are actually supported by common mechanisms. Finally, even if future research substantiated a connection between MAO deficiency and ASDs, it is likely that this link may be only pertinent to a very restricted of pathological phenomena within this spectrum of disorders.
Are the abnormal phenotypes in MAOA and A/B KO mice caused by high 5-HT?
The central hypothesis supporting the rationale of this study was that MAOA and A/B KO mice may exhibit autistic-like behaviours, insofar as they both exhibit congenital high 5-HT blood (and brain) levels. Our study provided only an initial phenomenological description of a number of autistic-like characteristics in male MAOA and A/B KO mice, but did not address mechanistic questions on the role of 5-HT in the ontogenesis of these alterations. While the investigation of the role of 5-HT in the behavioural and morphological changes in MAO-deficient mice was outside the scope of this study, the possibility that some phenotypic changes observed in MAOA and A/B KO mice may be caused by high 5-HT levels in early developmental stages is in line with recent findings linking ASD-like behaviours and hyperserotonemia in a novel line of transgenic mice featuring a gain-of-function mutation of the 5-HT transporter (Veenstra-VanderWeele et al. 2012). The possibility that excessive stimulation of 5- HT receptors may underpin the neuropathological changes in MAOA and A/B KO mice is also in keeping with the well-documented role of this neurotransmitter as a regulator of neuronal maturation, dendritic formation and synaptogenesis (Chubakov et al. 1986; Lauder, 1990; Whitaker-Azmitia, 2001). Notably, previous studies have shown that the alterations in barrel fields in MAOA-deficient mice are supported by the high levels of 5-HT in early developmental stages (Cases et al. 1996), through the excessive stimulation of cortical 5-HT1B/D receptors (Salichon et al. 2001).
If the abnormalities exhibited by MAOA and A/B KO mice reported in the present study were actually related to ASD manifestations, our results may support a direct implication of high blood and/or brain 5- HT concentrations in the pathophysiology of autism. Future research is warranted to test this intriguing possibility and parse out the specific contributions of 5-HT and its receptors in the ASD-like morphological and behavioural alterations of MAOA and A/B KO mice.
Although our hypothesis focuses on high 5-HT as a likely mechanism for the ASD-related disturbances in MAOA and A/B mutant mice, we cannot rule out that NE and DA may contribute to these phenotypical abnormalities. It should be noted, however, that the current evidence on the role of these catecholamines in ASDs is still preliminary and poorly supported (Ernst et al. 1997; Gillberg & Svennerholm, 1987; Lake et al. 1977; Lam et al. 2006; Launay et al. 1987; Martineau et al. 1992; Minderaa et al. 1994).
Irrespective of the neurochemical mechanisms implicated in the phenotypical aberrances of MAO-deficient mice, the results of the present study afford an interesting platform for further preclinical studies on the impact of developmental alterations of monoaminergic systems with regard to ASDs and other related neuropsychiatric disorders.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Health grants [R01MH39085 to J. C. S., R21HD070611 to M. B.], Boyd and Elsie Welin Professorship to J. C. S. and USC Zumberge Research Individual Grant to M. B. We are grateful to Anna L. Scott, Simone Tambaro, Felix Li and Paradai Adisayathepkul for their valuable support in the execution of the experiments.
Footnotes
Statement of Interest
None.
For supplementary material accompanying this paper, visit http://dx.doi.org/10.1017/S1461145712000715.
References
- Alia-Klein N, Goldstein RZ, Kriplani A, Logan J, et al. Brain monoamine oxidase A activity predicts trait aggression. Journal of Neuroscience. 2008;28:5099–5104. doi: 10.1523/JNEUROSCI.0925-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G, Muller RA, Courchesne E. Cerebellar function in autism: functional magnetic resonance image activation during a simple motor task. Biological Psychiatry. 2004;56:269–278. doi: 10.1016/j.biopsych.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends in Neuroscience. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Freedman DX, Cohen DJ, Volkmar FR, et al. Whole blood serotonin in autistic and normal subjects. Journal of Child Psychology and Psychiatry. 1987;28:885–900. doi: 10.1111/j.1469-7610.1987.tb00677.x. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Horne WC, Chatterjee D, Cohen DJ. The hyperserotonemia of autism. Annals of the New York Academy of Sciences. 1990;600:331–340. doi: 10.1111/j.1749-6632.1990.tb16893.x. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Bach AW, Lan NC, Johnson DL, Abell CW, et al. cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties. Proceedings of the National Academy of Sciences USA. 1988;85:4934–4938. doi: 10.1073/pnas.85.13.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader PL, Faizi M, Kim LH, Owen SF, et al. Mouse model of Timothy syndrome recapitulates triad of autistic traits. Proceedings of the National Academy of Sciences USA. 2011;108:15432–15437. doi: 10.1073/pnas.1112667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetto L, Pennington BF, Rogers SJ. Intact and impaired memory functions in autism. Child Development. 1996;67:1816–1835. [PubMed] [Google Scholar]
- Bond PA, Cundall RL. Properties of monoamine oxidase (MAO) in human blood platelets, plasma, lymphocytes and granulocytes. Clinica Chimica Acta. 1977;80:317–326. doi: 10.1016/0009-8981(77)90039-0. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Chen K, Godar SC, Chen G, et al. Social deficits and perseverative behaviors, but not overt aggression, in MAO A hypomorphic mice. Neuropsychopharmacology. 2011;36:2674–2688. doi: 10.1038/npp.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Chen K, Shih JC. Monoamine oxidase inactivation: from pathophysiology to therapeutics. Advances in Drug Delivery Review. 2008;60:1527–1533. doi: 10.1016/j.addr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Chen K, Shih JC. The degradation of serotonin: role of MAO. In: Müller C, Jacobs B, editors. Handbook of Behavioral Neurobiology of Serotonin. Amsterdam, The Netherlands: Elsevier; 2010. pp. 203–218. [Google Scholar]
- Bortolato M, Godar SC, Davarian S, Chen K, et al. Behavioral disinhibition and reduced anxiety-like behaviors in monoamine oxidase B-deficient mice. Neuropsychopharmacology. 2009;34:2746–2757. doi: 10.1038/npp.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Godar SC, Melis M, Soggiu A, et al. NMDA receptors mediate the role of monoamine oxidase A in pathological aggression. Journal of Neuroscience. 2012;20:8574–8582. doi: 10.1523/JNEUROSCI.0225-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouet V, Boulouard M, Toutain J, Divoux D, et al. The adhesive removal test: a sensitive method to assess sensorimotor deficits in mice. Nature Protocols. 2009;4:1560–1564. doi: 10.1038/nprot.2009.125. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behavioral Brain Research. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, et al. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993a;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Brunner HG, Nelen MR, van Zandvoort P, Abeling NG, et al. X-linked borderline mental retardation with prominent behavioral disturbance: phenotype, genetic localization, and evidence for disturbed monoamine metabolism. American Journal of Human Genetics. 1993b;52:1032–1039. [PMC free article] [PubMed] [Google Scholar]
- Bruno RM, Khatri V, Land PW, Simons DJ. Thalamocortical angular tuning domains within individual barrels of rat somatosensory cortex. Journal of Neuroscience. 2003;23:9565–9574. doi: 10.1523/JNEUROSCI.23-29-09565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. MAOA and the neurogenetic architecture of human aggression. Trends in Neurosciences. 2008;31:120–129. doi: 10.1016/j.tins.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Casanova MF, van Kooten IA, Switala AE, van Engeland H, et al. Minicolumnar abnormalities in autism. Acta Neuropathologica. 2006;112:287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, De Maeyer E, et al. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Menzies L, Hampshire A, Suckling J, et al. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science. 2008;321:421–422. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- Chen K, Holschneider DP, Wu W, Rebrin I, et al. A spontaneous point mutation produces monoamine oxidase A/B knock-out mice with greatly elevated monoamines and anxiety-like behavior. Journal of Biological Chemistry. 2004;279:39645–39652. doi: 10.1074/jbc.M405550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubakov AR, Gromova EA, Konovalov GV, Sarkisova EF, et al. The effects of serotonin on the morpho-functional development of rat cerebral neocortex in tissue culture. Brain Research. 1986;369:285–297. doi: 10.1016/0006-8993(86)90537-8. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. Journal of Neuroscience. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen IL, Liu X, Lewis ME, Chudley A, et al. Autism severity is associated with child and maternal MAOA genotypes. Clinical Genetics. 2011;79:355–362. doi: 10.1111/j.1399-0004.2010.01471.x. [DOI] [PubMed] [Google Scholar]
- Cohen IL, Liu X, Schutz C, White BN, et al. Association of autism severity with a monoamine oxidase A functional polymorphism. Clinical Genetics. 2003;64:190–197. doi: 10.1034/j.1399-0004.2003.00115.x. [DOI] [PubMed] [Google Scholar]
- Coldren JT, Halloran C. Spatial reversal as a measure of executive functioning in children with autism. Journal of Genetic Psychology. 2003;164:29–41. doi: 10.1080/00221320309597501. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathology. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LK, Hazlett HC, Librant AL, Nopoulos P, et al. Cortical enlargement in autism is associated with a functional VNTR in the monoamine oxidase A gene. American Journal of Medical Genetics. 2008;147B:1145–1151. doi: 10.1002/ajmg.b.30738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, Carver L, Panagiotides H, McPartland J. Young children with autism show atypical brain responses to fearful vs. neutral facial expressions of emotion. Developmental Science. 2004;7:340–359. doi: 10.1111/j.1467-7687.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Rawlins JN. T-maze alternation in the rodent. Nature Protocols. 2006;1:7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- Dickson PE, Rogers TD, Del Mar N, Marin LA, et al. Behavioral flexibility in a mouse model of developmental cerebellar Purkinje cell loss. Neurobiology of Learning and Memory. 2010;94:220–228. doi: 10.1016/j.nlm.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, et al. Low medial prefrontal dopaminergic activity in autistic children. Lancet. 1997;350:638. doi: 10.1016/s0140-6736(05)63326-0. [DOI] [PubMed] [Google Scholar]
- Evans DW, Canavera K, Kleinpeter FL, Maccubbin E, et al. The fears, phobias and anxieties of children with autism spectrum disorders and Down syndrome: comparisons with developmentally and chronologically age matched children. Child Psychiatry and Human Development. 2005;36:3–26. doi: 10.1007/s10578-004-3619-x. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Hardan AY. A meta-analysis of the corpus callosum in autism. Biological Psychiatry. 2009;66:935–941. doi: 10.1016/j.biopsych.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepner B, Tardiff C. Autism, movement, time and thought. In: Vanchevsky M, editor. E-motion Mis-Sight and Other Temporo-Spatial Processing Disorders in Autism. New York: Nova Science Publishers; 2006. pp. 1–30. [Google Scholar]
- Geyer MA, Paulus MP. Multivariate and nonlinear approaches to characterizing drug effects on the locomotor and investigatory behavior of rats. In: Frascella J, Brown RM, editors. Neurobiological Approaches to Brain-Behavior Interaction. Rockville, MD: National Institute on Drug Abuse; 1992. pp. 203–235. [PubMed] [Google Scholar]
- Gillberg C, Svennerholm L. CSF monoamines in autistic syndromes and other pervasive developmental disorders of early childhood. British Journal of Psychiatry. 1987;151:89–94. doi: 10.1192/bjp.151.1.89. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Van der Loos H. Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high clarity Golgi-Nissl stain. Journal of Neuroscience Methods. 1981;4:117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
- Godar SC, Bortolato M, Frau R, Dousti M, et al. Maladaptive defensive behaviours in monoamine oxidase A-deficient mice. International Journal Neuropsychopharmacology. 2011;14:1195–1207. doi: 10.1017/S1461145710001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Yang CR, Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biological Psychiatry. 2010;67:199–207. doi: 10.1016/j.biopsych.2009.08.026. [DOI] [PubMed] [Google Scholar]
- Groden J, Cautela JR, Prince S, Berryman J. The impact of stress and anxiety on individuals with autism and other developmental disabilities. In: Schopler E, Mesibov G, editors. Behavioral Issues in Autism. New York: Plenum Press; 1994. pp. 177–194. [Google Scholar]
- Hebebrand J, Klug B. Specification of the phenotype required for men with monoamine oxidase type A deficiency. Human Genetics. 1995;96:372–376. doi: 10.1007/BF00210430. [DOI] [PubMed] [Google Scholar]
- Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Research. 2010;1309:83–94. doi: 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- Jacome LF, Burket JA, Herndon AL, Deutsch SI. Genetically inbred Balb/c mice differ from outbred Swiss Webster mice on discrete measures of sociability: relevance to a genetic mouse model of autism spectrum disorders. Autism Research. 2011;4:393–400. doi: 10.1002/aur.218. [DOI] [PubMed] [Google Scholar]
- Jones EG. Microcolumns in the cerebral cortex. Proceedings of the National Academy of Sciences USA. 2000;97:5019–5021. doi: 10.1073/pnas.97.10.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, et al. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Wheaton M, Murphy DL. What’s wrong with my mouse model? Advances and strategies in animal modeling of anxiety and depression. Behavioral Brain Research. 2007;179:1–18. doi: 10.1016/j.bbr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Karl T, Pabst R, von Horsten S. Behavioral phenotyping of mice in pharmacological and toxicological research. Experimental and Toxicologic Pathology. 2003;55:69–83. doi: 10.1078/0940-2993-00301. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Shih JC, Chen K, Chen L, et al. Selective enhancement of emotional, but not motor, learning in monoamine oxidase A-deficient mice. Proceedings of the National Academy Sciences USA. 1997;94:5929–5933. doi: 10.1073/pnas.94.11.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Muller RA, Cohen DN, Courchesne E. Atypical functional lateralization of language in autism spectrum disorders. Brain Research. 2008;1221:115–125. doi: 10.1016/j.brainres.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysko KM, Rutherford MD. A threat-detection advantage in those with autism spectrum disorders. Brain and Cognition. 2009;69:472–480. doi: 10.1016/j.bandc.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Labrie V, Duffy S, Wang W, Barger SW, et al. Genetic inactivation of D-amino acid oxidase enhances extinction and reversal learning in mice. Learning and Memory. 2009;16:28–37. doi: 10.1101/lm.1112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake CR, Ziegler MG, Murphy DL. Increased norepinephrine levels and decreased dopamine-beta-hydroxylase activity in primary autism. Archives of General Psychiatry. 1977;34:553–556. doi: 10.1001/archpsyc.1977.01770170063005. [DOI] [PubMed] [Google Scholar]
- Lam KS, Aman MG, Arnold LE. Neurochemical correlates of autistic disorder: a review of the literature. Research in Developmental Disabilities. 2006;27:254–289. doi: 10.1016/j.ridd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Lan NC, Heinzmann C, Gal A, Klisak I, et al. Human monoamine oxidase A and B genes map to Xp 11.23 and are deleted in a patient with Norrie disease. Genomics. 1989;4:552–559. doi: 10.1016/0888-7543(89)90279-6. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Ontogeny of the serotonergic system in the rat: serotonin as a developmental signal. Annals of the New York Academy of Sciences. 1990;600:297–313. doi: 10.1111/j.1749-6632.1990.tb16891.x. [DOI] [PubMed] [Google Scholar]
- Launay JM, Bursztejn C, Ferrari P, Dreux C, et al. Catecholamines metabolism in infantile autism: a controlled study of 22 autistic children. Journal of Autism and Developmental Disorders. 1987;17:333–347. doi: 10.1007/BF01487064. [DOI] [PubMed] [Google Scholar]
- Lee BT, Ham BJ. Monoamine oxidase A-uVNTR genotype affects limbic brain activity in response to affective facial stimuli. Neuroreport. 2008;19:515–519. doi: 10.1097/WNR.0b013e3282f94294. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Lenders JW, Eisenhofer G, Abeling NG, Berger W, et al. Specific genetic deficiencies of the A and B isoenzymes of monoamine oxidase are characterized by distinct neurochemical and clinical phenotypes. Journal of Clinical Investigations. 1996;97:1010–1019. doi: 10.1172/JCI118492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of autistic disorder. Journal of Autism and Developmental Disorders. 2005;35:445–460. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- Markram K, Rinaldi T, La Mendola D, Sandi C, et al. Abnormal fear conditioning and amygdala processing in an animal model of autism. Neuropsychopharmacology. 2008;33:901–912. doi: 10.1038/sj.npp.1301453. [DOI] [PubMed] [Google Scholar]
- Martin LA, Goldowitz D, Mittleman G. Repetitive behavior and increased activity in mice with Purkinje cell loss: a model for understanding the role of cerebellar pathology in autism. European Journal of Neuroscience. 2010;31:544–555. doi: 10.1111/j.1460-9568.2009.07073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau J, Barthelemy C, Jouve J, Muh JP, et al. Monoamines (serotonin and catecholamines) and their derivatives in infantile autism: age-related changes and drug effects. Developmental Medicine and Child Neurology. 1992;34:593–603. doi: 10.1111/j.1469-8749.1992.tb11490.x. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana BR, Hariri A, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proceedings of the National Academy of Sciences USA. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minderaa RB, Anderson GM, Volkmar FR, Akkerhuis GW, et al. Noradrenergic and adrenergic functioning in autism. Biological Psychiatry. 1994;36:237–241. doi: 10.1016/0006-3223(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Ming X, Brimacombe M, Wagner GC. Prevalence of motor impairment in autism spectrum disorders. Brain Development. 2007;29:565–570. doi: 10.1016/j.braindev.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behavioral Brain Research. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder EJ, Anderson GM, Kema IP, de Bildt A, et al. Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:491–499. doi: 10.1097/00004583-200404000-00016. [DOI] [PubMed] [Google Scholar]
- Muris P, Steerneman P, Merckelbach H, Holdrinet I, et al. Comorbid anxiety symptoms in children with pervasive developmental disorders. Journal of Anxiety Disorders. 1998;12:387–393. doi: 10.1016/s0887-6185(98)00022-x. [DOI] [PubMed] [Google Scholar]
- O’Leary RE, Shih JC, Hyland K, Kramer N, et al. De novo microdeletion of Xp11.3 exclusively encompassing the monoamine oxidase A and B genes in a male infant with episodic hypotonia: a genomics approach to personalized medicine. European Journal of Medical Genetics. 2012;55:349–353. doi: 10.1016/j.ejmg.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- Paul LK, Brown WS, Adolphs R, Tyszka JM, et al. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nature Reviews Neuroscience. 2007;8:287–299. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- Philip RC, Whalley HC, Stanfield AC, Sprengelmeyer R, et al. Deficits in facial, body movement and vocal emotional processing in autism spectrum disorders. Psychological Medicine. 2010;40:1919–1929. doi: 10.1017/S0033291709992364. [DOI] [PubMed] [Google Scholar]
- Pickett J, London E. The neuropathology of autism: a review. Journal of Neuropathology and Experimental Neurology. 2005;64:925–935. doi: 10.1097/01.jnen.0000186921.42592.6c. [DOI] [PubMed] [Google Scholar]
- Rinehart NJ, Tonge BJ, Bradshaw JL, Iansek R, et al. Gait function in high-functioning autism and Asperger’s disorder: evidence for basal-ganglia and cerebellar involvement? European Child and Adolescent Psychiatry. 2006;15:256–264. doi: 10.1007/s00787-006-0530-y. [DOI] [PubMed] [Google Scholar]
- Ritvo ER, Yuwiler A, Geller E, Ornitz EM, et al. Increased blood serotonin and platelets in early infantile autism. Archives of General Psychiatry. 1970;23:566–572. doi: 10.1001/archpsyc.1970.01750060086009. [DOI] [PubMed] [Google Scholar]
- Salichon N, Gaspar P, Upton AL, Picaud S, et al. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase A and 5-ht transporter knock-out mice. Journal of Neuroscience. 2001;21:884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schain RJ, Freedman DX. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. Journal of Pediatrics. 1961;58:315–320. doi: 10.1016/s0022-3476(61)80261-8. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, et al. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learning and Memory. 2003;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AL, Bortolato M, Chen K, Shih JC. Novel monoamine oxidase A knock out mice with human-like spontaneous mutation. Neuroreport. 2008;19:739–743. doi: 10.1097/WNR.0b013e3282fd6e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annual Reviews of Neuroscience. 1999a;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih JC, Ridd MJ, Chen K, Meehan WP, et al. Ketanserin and tetrabenazine abolish aggression in mice lacking monoamine oxidase A. Brain Research. 1999b;835:104–112. doi: 10.1016/s0006-8993(99)01478-x. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nature Reviews in Neuroscience. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims KB, de la Chapelle A, Norio R, Sankila EM, et al. Monoamine oxidase deficiency in males with an X chromosome deletion. Neuron. 1989;2:1069–1076. doi: 10.1016/0896-6273(89)90231-6. [DOI] [PubMed] [Google Scholar]
- Stanley JL, Lincoln RJ, Brown TA, McDonald LM, et al. The mouse beam walking assay offers improved sensitivity over the mouse rotarod in determining motor coordination deficits induced by benzodiazepines. Journal of Psychopharmacology. 2005;19:221–227. doi: 10.1177/0269881105051524. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, et al. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berlin) 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, et al. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proceedings of the National Academy of Sciences USA. 2012;109:5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR, Cohen DJ. Autism: current concepts. In: Volkmar FR, editor. Psychoses and Pervasive Developmental Disorders. Philadelphia: WB Saunders; 1994. pp. 43–52. [Google Scholar]
- Volman I, Roelofs K, Koch S, Verhagen L, et al. Anterior prefrontal cortex inhibition impairs control over social emotional actions. Current Biology. 2011;21:1766–1770. doi: 10.1016/j.cub.2011.08.050. [DOI] [PubMed] [Google Scholar]
- Whibley A, Urquhart J, Dore J, Willatt L, et al. Deletion of MAOA and MAOB in a male patient causes severe developmental delay, intermittent hypotonia and stereotypical hand movements. European Journal of Human Genetics. 2010;18:1095–1099. doi: 10.1038/ejhg.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Research Bulletin. 2001;56:479–485. doi: 10.1016/s0361-9230(01)00615-3. [DOI] [PubMed] [Google Scholar]
- Whitney ER, Kemper TL, Rosene DL, Bauman ML, et al. Density of cerebellar basket and stellate cells in autism: evidence for a late developmental loss of Purkinje cells. Journal of Neuroscience Research. 2009;87:2245–2254. doi: 10.1002/jnr.22056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Gatt JM, Kuan SA, Dobson-Stone C, et al. A polymorphism of the MAOA gene is associated with emotional brain markers and personality traits on an antisocial index. Neuropsychopharmacology. 2009;34:1797–1809. doi: 10.1038/npp.2009.1. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. Journal of Neuroscience. 2009;29:3676–3684. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya N, Pilowsky T, Tidhar S, Nemanov L, et al. Family-based and population study of a functional promoter-region monoamine oxidase A polymorphism in autism: possible association with IQ. American Journal of Medical Genetics. 2002;114:284–287. doi: 10.1002/ajmg.10189. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Fung C, Shin D, Shin BC, et al. Neuronal glucose transporter isoform 3 deficient mice demonstrate features of autism spectrum disorders. Molecular Psychiatry. 2010;15:286–299. doi: 10.1038/mp.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.