Summary
Based on data from a large cohort of milk allergic children, our results show that measurement of casein-specific IgE is a helpful diagnostic indicator for predicting reactivity to baked milk, showing the greatest area under the receiver operating characteristic curve of parameters tested.
Keywords: Cow’s milk, baked milk, casein, milk allergy, food allergy, diagnosis, children, hypersensitivity, specific IgE, specific IgG4
To the editor,
Cow’s milk (CM) allergy is the most common food allergy affecting 2–3% of infants (1), half of which is estimated to be IgE-mediated and is responsible for up to 13% of fatal food-induced anaphylaxis (2). The overall prognosis is favorable, although recent studies have shown a later acquisition of tolerance to CM in a subset of patients (3, 4). It was recently reported that the majority (75%) of children with CM allergy tolerate baked milk products (e.g. muffins, waffles, cakes, breads, etc.) (5). Moreover, the addition of baked milk to the diet of children tolerating such foods appears to accelerate the development of regular milk tolerance compared with strict dietary avoidance (6). In contrast, children reactive to baked milk have a more severe phenotype of CM allergy with a higher risk of severe anaphylaxis and a more protracted course. Identification of patients tolerating baked milk is therefore essential, but challenging. Although the clinician-supervised oral food challenge (OFC) is considered to be the gold standard, it is resource consuming, has limited availability and is associated with a risk for anaphylaxis. The diagnostic value of skin-prick tests and serum specific IgE to CM in distinguishing baked milk-reactivity from tolerant children is not well defined. Recently, major interest has focused on component resolved diagnostics in the diagnosis of CM allergy, particularly specific IgE to casein (7). Based on two large cohorts of patients, the aim of this study was to evaluate the clinical utility and added diagnostic value of measurements of specific IgE and IgG4 antibodies to CM, casein and β-lactoglobulin.
We analyzed baseline data from 225 patients evaluated for tolerance to baked milk. Ninety-seven patients were prospectively recruited at the Mount Sinai Allergy clinics between 2004 and 2007 from an original study on tolerance to baked milk (5). A second cohort of 128 milk allergic patients was recruited prospectively between 2008 and 2010 at the Mount Sinai Allergy clinics and was used to compare the results obtained from the initial cohort. Characteristics of patients from both cohorts are shown in supplemental Table I. More detailed clinical characteristics as well as the inclusion and exclusion criteria for both studies are described elsewhere (5, 8) and in the supplementary Appendix A. The studies were approved by the Mount Sinai Institutional Review Board and informed consent was obtained before enrollment. Tolerance to baked milk was determined by OFC and subjects were categorized as baked milk-reactive (group A), tolerant to baked-milk only (group B) and tolerant to both baked and regular milk, i.e. no longer milk allergic (group C).
Table 1.
Specificity, sensitivity, and predictive values for various serum casein- and cow’s milk-specific IgE levels in the combined cohort of milk allergic patients (n=225)
| Casein-specific igb (kUA/L) | Sensitivity | Specificity | Negative predictive value | Positive predictive value |
|---|---|---|---|---|
| 0.94* | 95% | 32% | 96% | 34% |
| 4.95** | 74% | 77% | 89% | 54% |
| 20.2*** | 30% | 95% | 78% | 69% |
| Cow's milk-specific IgE (kUA/L) | Sensitivity | Specificity | Negative predictive value | Positive predictive value |
| 1.21* | 95% | 27% | 94% | 33% |
| 9.97** | 62% | 85% | 86% | 60% |
| 24.5*** | 30% | 95% | 78% | 69% |
Negative decision point defined as the cutoff level producing a sensitivity of 95%
Optimal cutoff point, whereby equal weight is given to sensitivity and specificity
Positive decision point defined as the cutoff level producing a specificity of 95%
At baseline, serum samples were collected from subjects to measure CM-, casein-, and β-lactoglobulin-specific IgE as well as casein- and β-lactoglobulin-specific IgG4 antibody concentrations with the UniCAP system (ThermoFisher Scientific, Phadia US, Portage, MI). Statistical analyses, comparisons of data between the baked milk-reactive (group A) and tolerant patients (group B and C) were performed with the Mann-Whitney U test. A probability level of less than 5% was considered significant. P values were adjusted for multiple testing. Receiver operating characteristic (ROC) curves were generated for specific IgE to CM, casein and β-lactoglobulin. The areas under the curves (AUCs) were estimated and compared using PROC LOGISTIC in SAS.
In both cohorts of patients, the levels of specific IgE to CM, casein and β-lactoglobulin were significantly higher in baked milk-reactive patients (group A) compared to baked milk-tolerant subjects (group B and C), P<0.05 (supplemental Table I). As previously reported for the first cohort (5), casein- and β-lactoglobulin-specific IgG4 levels did not differ significantly between subjects reactive and tolerant to baked milk. In the second cohort, only casein-specific IgG4 levels were greater in reactive subjects, with borderline significance (P=0.045). Casein- and β-lactoglobulin-specific IgE/IgG4 ratios were significantly higher in baked milk-reactive subjects in comparison to baked milk-tolerant subjects in both cohorts (P<0.05). However, the ratio IgE/IgG4 seems to be driven mostly by levels of specific IgE itself, based on the analysis of covariance (ANCOVA) using ranks.
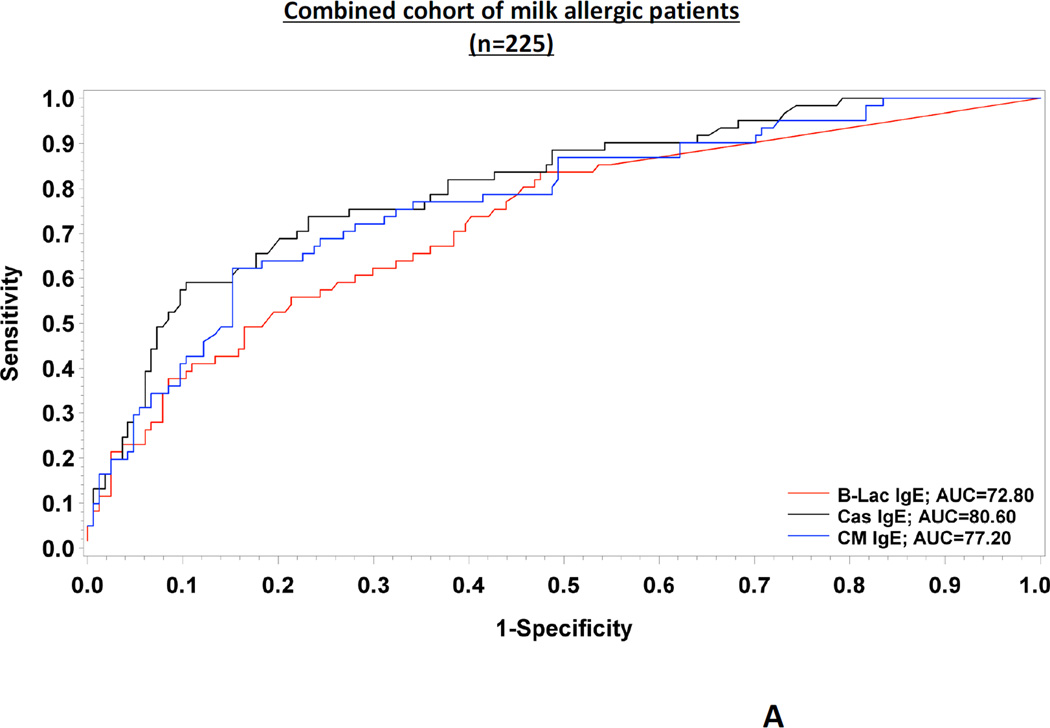
Regarding the performance of tests evaluated in the combined cohort of patients (n=225), casein-specific IgE had a significantly greater accuracy for predicting baked milk reactivity, compared to specific IgE to CM (P=0.01) and to β-lactoglobulin (P=0.02) (Fig 1.A.). All patients with undetectable levels of specific IgE to casein (n=25, 11.1%) tolerated baked milk. Various cutoff levels of specific IgE to CM, casein and β-lactoglobulin have been analyzed (Table I). We used specific IgE levels representing the 95% specificity of the tests as the positive decision points and levels representing the 95% sensitivity as the negative decision points (9). We chose these values since they are not influenced by prevalence of the disease. The positive decision point for reactivity to baked milk was 20.2 kUA/L for casein-specific IgE (UniCAP) based on the combined cohort. In practice, this means that patients having casein-specific IgE antibodies greater than this value are unlikely to pass a baked milk challenge and baked milk products should be avoided. In contrast, a concentration of less than approximately 0.94 kUA/L (negative decision point) indicates a very low risk of reacting to baked milk, even though the patient might very well react to regular milk. When giving equal weight to sensitivity and specificity, the optimal cutoff point was 4.95 kUA/L.
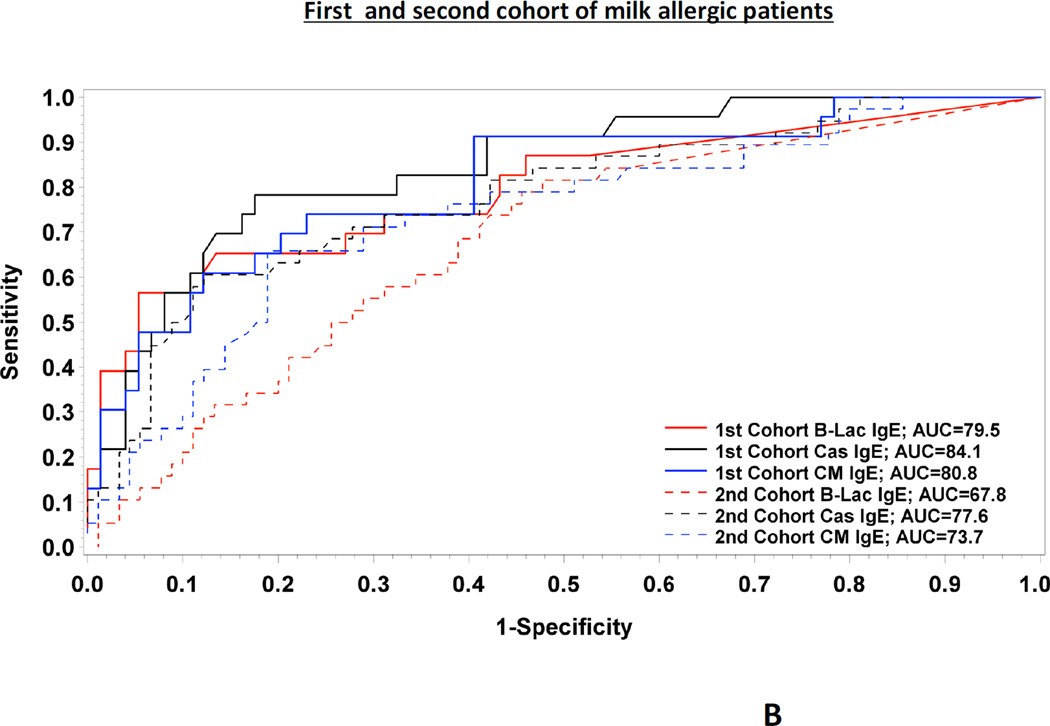
FIG. 1.
Full ROC curves for the three predictors tested (Cow’s milk-, casein- and β- lactoglobulin specific IgE) in the combined cohort of milk allergic patients (n=225) (FIG.1.A.) as well as in each cohort (FIG.1.B.)
In addition, we analysed both cohorts separately (supplemental Table II). Again, the AUCs for specific IgE to casein were greater than the AUCs for specific IgE to CM, although the difference was not statistically significant in the first cohort of patients (Fig 1.B.). Moreover, we found very similar values for the positive and the negative decision point, i.e. 21.4 kUA/L and 1.0 kUA/L in the first cohort and 20.2 kUA/L and 0.7 kUA/L in the second cohort, respectively (supplemental Table II). Based on cross-validation analysis, similar results for AUCs were obtained in both cohorts of patients, which suggest adequate reliability of these cutoffs. The distribution of casein-specific IgE is shown in supplemental Fig 1.
Based on the largest cohort of baked milk allergic children to date, we conclude that quantitative measurements of casein-specific IgE antibodies as measured by UniCAP is useful in the management of CM allergy. Casein is a major allergen in CM and has been previously identified as the best performing diagnostic component in the diagnosis of CM allergy (7). This is likely due to the fact that casein retains its allergenicity after extensive heating, as opposed to whey proteins that have low heat stability (10). Combined with clinical history and the expertise of the physician, the use of cut-off decision points for specific IgE to casein could identify the optimal candidates for baked milk OFCs and improve management of children with suspected CM allergy (Table I). The choice of a cut-off that a physician wishes to use will be based on individual risk assessment, which may vary according to circumstances under which the challenges are being offered. For example, a casein-specific IgE cutoff of 0.94 kUA/L (negative decision point) had a negative predictive value of 96% but a specificity of 32%. Of note, this testing should not replace OFCs since some patients might still react to baked milk. On the other hand, using a cutoff of 20.2 kUA/L (positive decision point) would decrease the negative predictive value to approximately 78%, a number that may still be acceptable to some clinicians; however the specificity would increase to 95%, thus capturing most children tolerant to baked milk. In addition to enhancing the quality of life by removing unnecessary dietary restrictions, consumption of baked milk could change the natural evolution of milk allergy by promoting development of tolerance to regular CM (6). Although promising, our results should be validated in other populations and age groups before applying them in clinical practice.
Sincerely,
Jean Christoph Caubet, MD1
Anna Nowak-Węgrzyn, MD1
Erin Moshier, MS2
James Godbold, PhD2
Wang Julie, MD1
Hugh A. Sampson, MD1
1Division of Pediatric Allergy & Immunology and Jaffe Institute for Food Allergy, Department of Pediatrics; 2 Division of Biostatistics, Department of Preventive Medicine, The Mount Sinai School of Medicine, New York, NY, USA
Acknowledgments
Funding sources: NIAID AI44236; NCRR RR026134
Abbreviations
- CM
Cow’s milk
- OFC
Oral food challenge
- ROC
Receiver operating characteristic
- AUC
Area under the curve
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflicts of interest: none
References
- 1.Branum AM, Lukacs SL. Food allergy among children in the united states. Pediatrics. 2009 Dec;124(6):1549–1555. doi: 10.1542/peds.2009-1210. [DOI] [PubMed] [Google Scholar]
- 2.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol. 2007 Apr;119(4):1016–1018. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 3.Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow's milk allergy. J Allergy Clin Immunol. 2007 Nov;120(5):1172–1177. doi: 10.1016/j.jaci.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Cantani A, Micera M. Natural history of cow's milk allergy. an eight-year follow-up study in 115 atopic children. Eur Rev Med Pharmacol Sci. 2004 Jul-Aug;8(4):153–164. [PubMed] [Google Scholar]
- 5.Nowak-Wegrzyn A, Bloom KA, Sicherer SH, Shreffler WG, Noone S, Wanich N, et al. Tolerance to extensively heated milk in children with cow's milk allergy. J Allergy Clin Immunol. 2008 Aug;122(2) doi: 10.1016/j.jaci.2008.05.043. 342,7, 347.e1-e2. [DOI] [PubMed] [Google Scholar]
- 6.Kim JS, Nowak-Wegrzyn A, Sicherer SH, Noone S, Moshier EL, Sampson HA. Dietary baked milk accelerates the resolution of cow's milk allergy in children. J Allergy Clin Immunol. 2011 Jul;128(1) doi: 10.1016/j.jaci.2011.04.036. 125,131.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Urbano LE, Pellegrino K, Artesani MC, Donnanno S, Luciano R, Riccardi C, et al. Performance of a component-based allergen-microarray in the diagnosis of cow's milk and hen's egg allergy. Clin Exp Allergy. 2010 Oct;40(10):1561–1570. doi: 10.1111/j.1365-2222.2010.03568.x. [DOI] [PubMed] [Google Scholar]
- 8.Ford LS, Bloom KA, Nowak-Wegrzyn A, Shreffler WG, Masilamani M, Sampson HA. Basophil Reactivity, Wheal Size and Immunoglobulin Levels Distinguish Degree of Cow’s Milk Tolerance. J Allergy Clin Immunol. 2012 doi: 10.1016/j.jaci.2012.06.003. Submitted, in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ando H, Moverare R, Kondo Y, Tsuge I, Tanaka A, Borres MP, et al. Utility of ovomucoid-specific IgE concentrations in predicting symptomatic egg allergy. J Allergy Clin Immunol. 2008 Sep;122(3):583–588. doi: 10.1016/j.jaci.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Gjesing B, Osterballe O, Schwartz B, Wahn U, Lowenstein H. Allergen-specific IgE antibodies against antigenic components in cow milk and milk substitutes. Allergy. 1986 Jan;41(1):51–56. doi: 10.1111/j.1398-9995.1986.tb00275.x. [DOI] [PubMed] [Google Scholar]