Abstract
Vascular smooth muscle contraction occurs following an initial response to an increase in intracellular calcium concentration and a sustained response following increases in the sensitivity of contractile proteins to calcium (calcium sensitization). This latter process is regulated by the rhoA/rho kinase pathway and activated by serotonin. In multiple cell types, signaling molecules compartmentalize within caveolae to regulate their activation. We hypothesized that serotonin differentially compartmentalizes rhoA within caveolar versus noncaveolar lipid rafts to regulate sustained vascular contractions. To test this hypothesis, we measured aortic contractions in response to serotonin in wild-type (WT) and cav-1-deficient mice (cav-1 KO). RhoA-dependent contractions in response to serotonin were markedly augmented in arteries from cav-1 KO mice despite a modest reduction in rhoA expression compared with WT. We found that under basal conditions, rhoA in WT arteries was primarily localized within high-density sucrose gradient fractions but temporally shifted to low-density fractions in response to serotonin. In contrast, rhoA in cav-1 KO arteries was primarily in low-density fractions and shifted to high-density fractions in a similar timeframe as that seen in WT mice. We conclude that localization of rhoA to caveolar versus noncaveolar lipid rafts differentially regulates its activation and contractions to rhoA-dependent agonists with greater activation associated with its localization to noncaveolar rafts. Disruption of rhoA localization within caveolae may contribute to increased activation and enhanced vascular contractions in cardiovascular disease.
Keywords: lipid rafts, caveolae, rho kinase
alterations in rhoa/rho kinase (ROCK) pathway have been implicated in the development of a variety of cardiovascular diseases, including hypertension (3, 35), atherosclerosis (14, 30), and cerebral and coronary vasospasm (10, 26). In all of these disease states, rhoA and ROCK activity, which are determinants of the calcium sensitivity of contractile proteins, is increased, leading to enhanced resting vascular tone and serotonin-induced vasoconstriction. The mechanisms underlying the increased rhoA and ROCK activity in these diseases have not been fully elucidated. We have demonstrated that, in response to serotonin, both the rho/ROCK-dependent component of vascular contractions and the activity of rhoA and ROCK are increased in the absence of changes in protein expression in models of diabetes (19, 20). Thus, mechanisms other than changes in protein expression regulate activation of rhoA and its downstream target, ROCK, resulting in changes in vascular function in diabetes.
One method for regulating activation of signaling pathways is the congregation of proteins within specialized membrane compartments or lipid rafts. Although there is some debate regarding the functional significance of lipid rafts, their role in the regulation of cell function has recently gained increasing support (16). Lipid rafts are rich in tightly packed sphingolipids and cholesterol (32), which act to compartmentalize membrane proteins, such as receptors and downstream molecules involved in signal transduction (5, 9). For example, serotonergic receptors (5HT2A), which mediate serotonin-induced contractions (21), are localized within lipid rafts in the plasma membrane (2). Disruption of these microdomains can alter vascular smooth muscle (VSM) function as demonstrated by studies by Dreja et al. (5), showing that serotonin-induced contractions were attenuated following chemical disruption of lipid rafts with methyl-β-cyclodextrin. Cyclodextrin, however, cannot discern between specific populations of lipid rafts in vessels from wild-type mice. One subpopulation of lipid rafts, caveolae, is abundant in both endothelium and vascular smooth muscle, where they compartmentalize and regulate signaling molecules such as G protein-coupled and tyrosine kinase-associated receptors, thus converging multiple signaling processes (5, 7, 29). Caveolins, the hallmark scaffolding protein of caveolae, form oligomeric structures that bind cholesterol and represent the functional assembly units of caveolae (8, 9, 23, 32, 36). Loss of caveolin-1 (cav-1), the predominant caveolin isoform in smooth muscle, results in loss of caveolar microdomains (4, 28).
Caveolins are not only structural components of caveolae microdomains, but also regulate assembly and activation of a variety of receptors and signaling proteins (12, 22). It has been hypothesized that caveolin inhibits enzyme activity by stabilizing proteins in an inactive conformation within caveolae (7, 29). In this regard, inactive rhoA is localized within cytosol and following activation of G protein-coupled receptors, translocates to the plasma membrane where it activates ROCK. Both rhoA and 5HT2A receptors associate with cav-1 in lipid rafts (2). Thus, the association of rhoA and/or ROCK with caveolins may act to regulate their activity. We hypothesized that compartmentalization of rhoA and ROCK within membrane lipid rafts of smooth muscle cells determines their activation and the subsequent, activation of downstream signaling pathways that regulate VSM contractions. To address this hypothesis, we compared membrane localization of rhoA and ROCK with cav-1 in aortas from wild-type and cav-1-deficient mice (cav-1 KO) in relation to vascular responses to serotonin.
MATERIALS AND METHODS
Animal model.
Male caveolin-1-deficient (Cav1tm1Mls/J, cav-1 KO) and wild-type (WT, B6129SF2/J or C57BL6/J) mice were obtained from Jackson Laboratories. Animal protocols were approved by the Animal Care and Use Committee of the Iowa City Veterans Affairs Health Care System and complied with the “Guiding Principles for Research Involving Animals and Human Beings.” Mice were euthanized with pentobarbital sodium (150 mg/kg ip), and the thoracic aorta was obtained for measurement of functional responses, protein expression, and isolation of lipid rafts.
Measurement of vascular reactivity.
Studies were performed in the aorta, since it allowed us to compare vascular reactivity with biochemical analyses in the same vessel. Thoracic aortas were removed, placed in ice-cold Krebs buffer (mmol/l: 118.3 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, and 5 glucose), cut into rings (3–4 mm in length), and mounted on wires connected to a force transducer in an organ bath filled with Krebs buffer (37°C aerated with 20% O2, 5% CO2, and balance N2). Tension was increased to 0.75 g over 60 min. Contractions in response to serotonin (10 nM to 10 μM) and KCl (25–100 mM) were obtained under basal conditions and expressed as tension in grams. In general, two aortic rings from each animal were used as controls, and two rings were treated with a cell-permeable rhoA inhibitor CT04 (cell-permeable exoenzyme C3 transferase, Cytoskeleton, 10 μg/ml for 2 h at 37°C), ROCK inhibitor H1152 (1 μM), or denuded of endothelium by gently rubbing the inner surface with silk. A minimum of 0.200 g developed tension in response to 75–100 mM KCL was used as an acceptable criterion for inclusion in this study.
Cholesterol extraction.
To acutely remove cholesterol and lipid rafts from membranes, aortas from WT and cav-1 KO mice were treated with methyl-β-cyclodextrin (MβCD) (5). Following measurement of control responses to agonists, vessels were treated with MβCD (10 mM, 1 h at 37°C). Previous studies have demonstrated that caveolae were absent in vascular smooth muscle following MβCD treatment (5). Measurements of responses to agonists were repeated following MβCD treatment.
Isolation of lipid rafts.
Thoracic aortas were placed in ice-cold Krebs buffer (in mMol/l: 118.3 NaCl, 4.7 KCl, 2.25 CaCl2, 1.2 MgSO4, 1.2 KPO4, 25 NaHCO3, and 5 glucose) and cleaned of adherent fat. Then the entire segment (18–20 mm in length) was mounted on metal rings connected to a force transducer in an organ bath filled with Krebs buffer warmed to 37°C and aerated with 5% CO2 and 20% O2, balanced with N2. Tension was increased to 0.75 g over 45–60 min. Aortas from WT and cav-1 KO mice were flash frozen with liquid nitrogen under basal conditions (untreated) or following serotonin-contraction (5-HT, 1.0 μM at 1 or 5 min), and pooled (four aortas per sample) for isolation of lipid rafts.
Lipid raft fractions were isolated using a modified method by Song et al. (33) with sodium carbonate buffer. Samples were dounced in liquid nitrogen before addition of NaCO3 (200–300 μl, 500 mM, at pH 11) containing a complete protease inhibitor cocktail tablet (Roche) and phosphatase inhibitors (Sigma-Aldrich). Samples were dounced an additional 10 times, sonicated (50% power, 10 s, three times), and centrifuged (2,000 rpm, 5 min at 4°C). An aliquot of supernatant was adjusted to 45% sucrose, and a sucrose gradient was prepared by layering 35% and 5% sucrose solutions [0.25 mM NaCO3 in 24 mM 2-(N-morpholino)-ethanesulfonic acid, at pH 6.5 and 150 mM NaCl]. The gradient was centrifuged (54,000 rpm, 20–24 h, 4°C). Equal fractions were collected for Western immunoblotting from lightest (fraction 1) to heaviest (fraction 10) density fractions.
Western immunoblots.
Protein expression in whole cell lysates was compared in WT and cav-1 KO aortas. Vessels were removed, cleaned of connective tissue, and individually flash frozen. Samples were dounced in liquid nitrogen, a denaturing lysis buffer containing Tris·HCl (50 mM), EDTA (0.1 mM), EGTA (0.1 mM), SDS (0.1%), NP40 (1%), deoxycholic acid (2.4 mM); protease and phosphate inhibitors were added, and samples were sonicated three times on ice. Samples were centrifuged at 4°C, 14,000 rpm 10–15 min, and protein concentration of whole cell lysates was determined using the bicinchoninic acid method.
For protein analysis in whole cell lysates and lipid raft fractions, an aliquot of each lipid raft fraction or equal amounts of whole cell lysate were subjected to SDS PAGE analysis. After blocking, immunoblotting was performed using anti-rhoA (1:100, no. 610991; BD Sciences), anti-ROCK1, and -ROCK2 (1:500, nos. 611136 and 610623; BD Biosciences), anti-cav-1 (1:500, no. 610407; BD Biosciences) and anti-β-actin (1:500, no. A2228; Sigma-Aldrich) followed by secondary antibodies conjugated with horseradish peroxidase. Immunoreactivity was visualized with enhanced chemiluminescence and analyzed using National Institutes of Health ImageJ.
Coimmunoprecipitation.
Whole cell lysate of aortas from WT mice (200 μg protein per sample) in the lysis buffer was initially precleared and then incubated overnight at 4°C with either 3 μg of anti-caveolin-1 (no. 610407; BD Biosciences) or anti-rhoA (no. 610991; BD Bioscience) and 50 μl of anti-mouse IgG-conjugated agarose beads (eBiosciences). The agarose beads were washed three times with lysis buffer and pelleted by centrifugation (10,000 g, 3 min). The bound proteins were eluted from the beads by boiling for 5 min in SDS sample buffer, analyzed by SDS-PAGE, and transferred to nitrocellulose membrane followed by immunoblotting with anti-rhoA (1:200, ab86297; Abcam) or anti-caveolin-1 (1:500, no. 3238; Cell Signaling), as described above.
Chemicals.
All chemicals were obtained from Sigma-Aldrich with the exception of H1152 (Alexis Chemical).
Statistical analysis.
Data are presented as means ± SE. Responses of multiple rings from a given animal treated similarly were averaged, and n represents numbers of mice per group. Concentration response curves were compared by repeated-measures two-way ANOVA followed by Bonferroni post hoc test. All n's for lipid rafts and Western immunoblots indicate the number of pooled samples. Western immunoblots were compared using Student's t-tests. Significance was defined as P ≤ 0.05.
RESULTS
Contractions of arteries to serotonin are mediated by rhoA and ROCK.
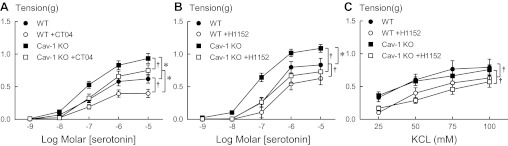
We have previously demonstrated in other WT mouse strains that contractions of arteries in response to serotonin are mediated, in part, through activation of rhoA and ROCK (19–21). Because mouse strain plays an important role in vascular reactivity (25), we first addressed the role of rhoA and rho kinase in responses to serotonin in these mice. To confirm the contribution of rhoA in response to serotonin, we compared contractions of arteries from WT mice in response to serotonin before and after inhibition of rhoA with a cell-permeable exoenzyme C3 transferase, CT04. Serotonin produced concentration-dependent contractions of aortas from these WT mice with a maximal response of 0.620 ± 0.066 g at 10 μM (Fig. 1A). The contractions to serotonin in arteries from WT mice were reduced by inhibition of rhoA with CT04 (Fig. 1A). The role of rho kinase was confirmed by a similar reduction in contractions to serotonin with H1152, a specific rho kinase inhibitor (Fig. 1B). Because endothelium-derived relaxing or contracting factors can modify contractions to serotonin in some vascular beds, we tested the effect of endothelial removal. In contrast to studies in C57BL/6J mice (20), mechanical removal of endothelium of aorta from these WT mice had no effect on contractions to serotonin or the inhibition with H1152 (n = 4; 5HT 1 μM intact 0.87 ± 0.07 g, denuded 0.70 ± 0.07 g, following H1152 intact 0.50 ± 0.14 g, denuded 0.52 ± 0.14 g) but did abolish relaxation to ACh (data not shown), as expected.
Fig. 1.
Inhibition of rhoA with cell-permeable exoenzyme C3 transferase, CT04 (A), or rho kinase with H1152 (1 μM, B), reduced contractions to serotonin in both wild-type (WT) and cav-1 knockout mice (cav-1 KO). Contractions to serotonin did not differ between WT and cav-1 KO following H1152. Contractions to KCl were inhibited by H1152 (C) but not CT04 (not shown). Values are expressed as means ± SE. *P < 0.05 vs. WT. †P < 0.05 vs. respective control in the absence of inhibitor; n = 3–12.
In aortas isolated from cav-1 KO mice, contractions in response to serotonin were markedly augmented compared with WT mice (Fig. 1A). Similar to WT, serotonin-induced contractions of arteries from cav-1 KO mice were reduced by both CT04 (Fig. 1A) and H1152 (Fig. 1B) but only H1152-normalized contractions in arteries between WT and cav-1 KO mice. Contractions of arteries to KCl did not differ between WT and cav-1 KO mice and were unaffected by CT04 (data not shown). Contractions to KCl were, however, reduced in arteries from both WT and cav-1 KO mice by H1152 (Fig. 1C). Thus, contractions of arteries to serotonin are mediated by activation of rhoA and rho kinase. Furthermore, the absence of caveolin-1 enhanced contractions to serotonin, suggesting that signaling pathways regulating serotonin-induced contractions are suppressed by caveolin-1 and caveolae.
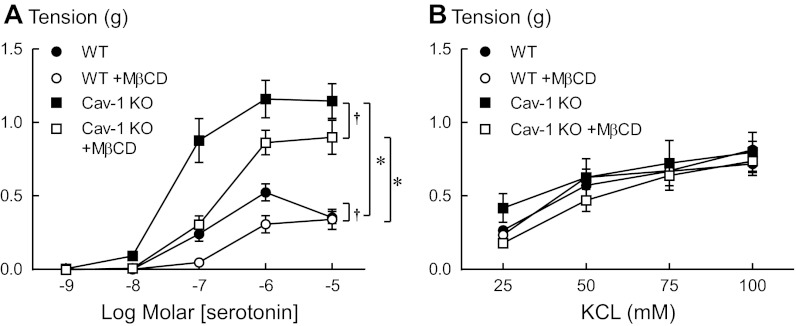
To further address the contribution of both caveolar and noncaveolar lipid rafts in contractions to serotonin, arteries were treated with MβCD to deplete membrane cholesterol, a key component of all lipid rafts. Similar to a previous study in rat tail arteries (5), disrupting both caveolar and noncaveolar lipid rafts with MβCD (10 mM for 1 h) specifically reduced contractions of arteries from WT mice in response to serotonin (Fig. 2A), while not affecting contractions to KCl (Fig. 2B). These data are in contrast to the augmented contraction measured when the gene for cav-1 was deleted and only caveolae were disrupted.
Fig. 2.
Disruption of lipid rafts with methyl-β-cyclodextrin (MβCD, 10 mM) reduced contractions to serotonin (A) but not to KCl (B) in arteries from both wild-type (WT) and cav-1 knockout mice (cav-1 KO). Values are expressed as means ± SE. *P < 0.05 vs. WT. †P < 0.05 vs. respective control in the absence of MβCD; n = 3–6.
To determine whether noncaveolar lipid rafts in aortas of cav-1 KO mice regulate contractions to serotonin, responses of cav-1-deficient aortas to serotonin were measured before and after cholesterol extraction with MβCD. Similar to effects of MβCD in WT aortas, MβCD reduced contractions to serotonin in aortas of cav-1 KO mice but not to the level observed in WT mice (Fig. 2A). Contractions of arteries from cav-1 KO mice in response to KCl were not affected by MβCD (Fig. 2B). Taken together, these data demonstrate the opposing effects of caveolar and noncaveolar lipid rafts in regulating contractions to serotonin. Specifically, whereas the absence of caveolin-1 and caveolae increases rhoA/ROCK-mediated contractions to serotonin, disrupting noncaveolar lipid rafts decreases contractions to serotonin.
RhoA and ROCK expression in WT and cav-1 KO mice.
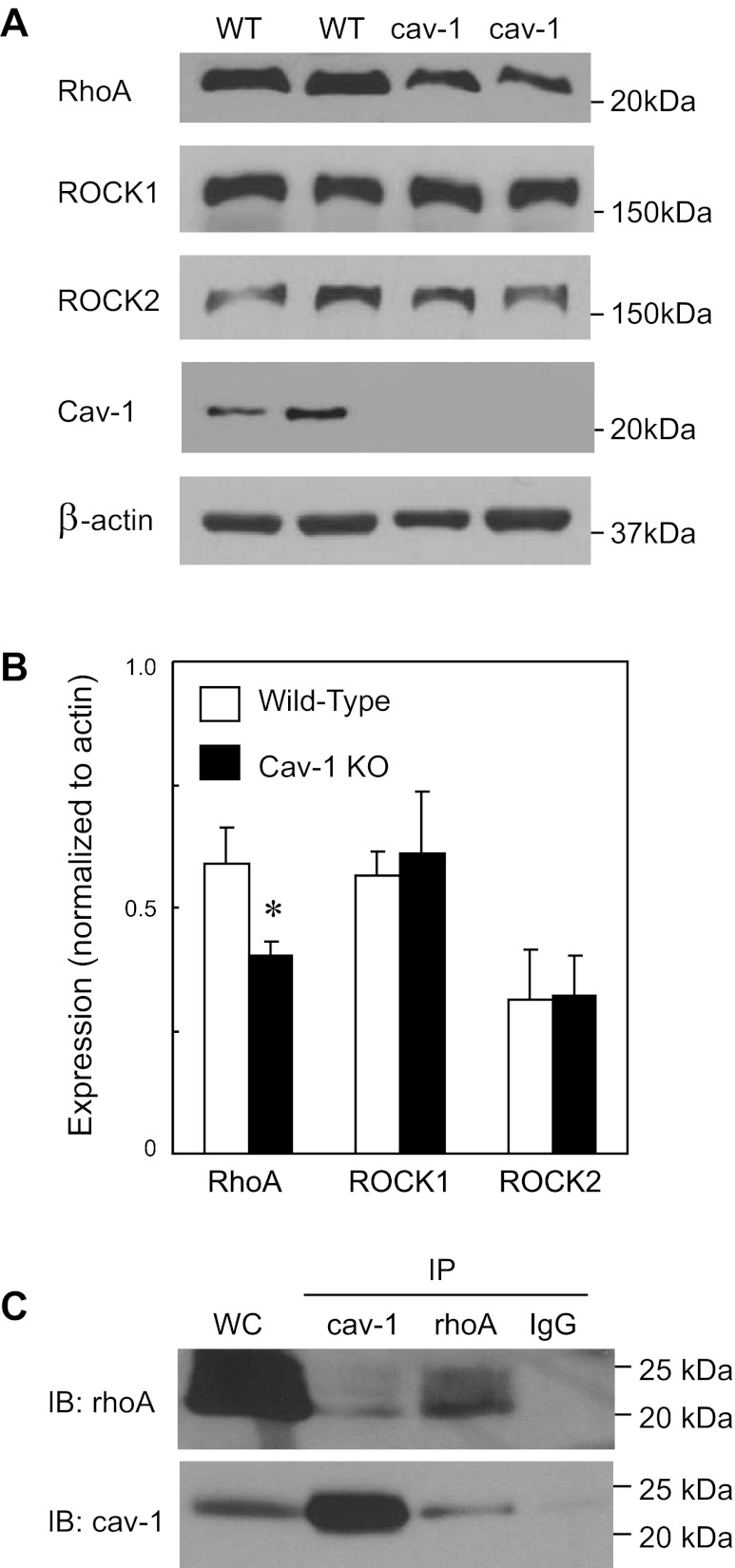
We compared expression levels of cav-1, rhoA, and ROCK in whole cell lysates from aortas of WT and cav-1 KO mice. Confirming previous studies (4, 17), aortas from cav-1 KO mice did not express cav-1 (Fig. 3A). Interestingly, despite an increased rhoA/ROCK-mediated contractile response, rhoA expression was lower in aortas from cav-1 KO mice compared with WT controls (Fig. 3, A and B). Neither isoform of rho kinase (ROCK1 or ROCK2) differed between WT and cav-1 KO mice (Fig. 3, A and B). Thus, in the absence of cav-1, the rhoA/rho kinase-dependent contractile responses were augmented despite reduced expression of rhoA. These surprising differences between protein expression and function suggest that caveolin-1 may stabilize rhoA protein and/or its activation-inactivation.
Fig. 3.
Representative (A) and mean (B) levels of protein expression of rhoA, rho kinase (ROCK1 and ROCK2), caveolin-1 (Cav-1), and β-actin in whole cell lysate of aorta from wild-type (WT) and cav-1 KO mice. Values are expressed as means ± SE of 4 aorta. *P < 0.05 vs. WT. C: representative immunoblot (IB) from immunoprecipitations (IP) using cav-1, rhoA, or IgG in aorta from WT mice. Lane 1 shows positive control using whole cell lysate from aorta (WC); lane 2 shows IP with cav-1; lane 3 shows IP with rhoA; and lane 4: shows IP with IgG. (n = 2 for each IP).
To determine whether rhoA and cav-1 associate, we performed coimmunoprecipitation studies in whole cell lysate of mouse aorta under basal conditions. Immunoprecipitation with either cav-1 or rhoA demonstrated an association between rhoA or cav-1 (n = 2 for each IP with cav-1 or rhoA, Fig. 3C). No signal was detected in the absence of a primary antibody or with an IgG control (Fig. 3C). These coimmunoprecipitation experiments suggest that rhoA and cav-1 associate in mouse aorta under basal conditions, confirming previous results in rat mesenteric arteries (6).
RhoA and ROCK localization in WT mice.
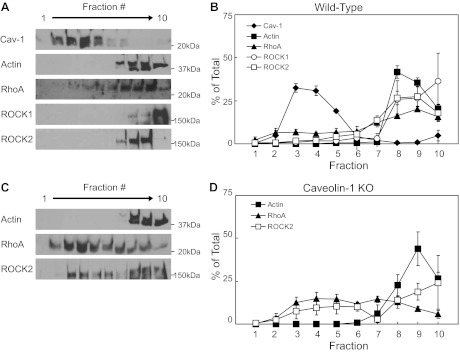
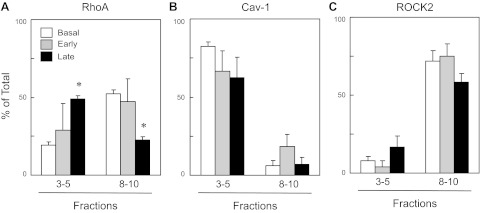
We next examined the localization of cav-1, rhoA, and ROCK within low- and high-density membrane fractions obtained by sucrose density fractionation. Under basal conditions, localization of cav-1 within low-density membrane fractions of aortas from WT mice was characteristic of caveolae with enriched lipid content and resistance to solubilization with sodium carbonate at low temperature (Fig. 4, A and B). Approximately, 80% of the total cav-1 was found in the low-density fractions (fractions 3–5) of aorta from WT mice (Figs. 4, A and B, and 5B). β-actin, which does not associate with lipid rafts, was primarily localized to the high-density membrane fractions (∼90% of the total in fractions 8–10, Figs. 4). Low levels of rhoA (∼20% of the total) and both isoforms of ROCK (∼4 and ∼8% % of the total ROCK1 and ROCK2, respectively) were found in the low-density cav-1 containing fractions (3–5) from unstimulated aortas (Figs. 4, A and B, and 5). Thus, under basal conditions, the majority of rhoA and ROCK was not associated with cav-1 in lipid rafts of vascular plasma membranes.
Fig. 4.
Under basal conditions, neither rhoA nor ROCK1 nor ROCK2 localized with caveolin-1 (cav-1) in low-density fractions in the aorta from wild-type mice (A, representative blots, B, levels as percent of total; values are expressed as means ± SE; n = 3–6). Conversely, in aorta from cav-1 KO mice, levels of rhoA and ROCK2 were higher in low-density fractions compared with wild-type mice (C, representative blots, D, levels as percent of total, mean ± SE; n = 3 or 4 pooled samples).
Because localization within caveolae can affect either activation or inactivation of signaling molecules and enzymes, we compared rhoA and ROCK localization during the early phase of serotonin-induced contraction of WT aortas (30–60 s post 1 μM serotonin, primarily associated with calcium-dependent phase of contraction) versus the sustained maximal contraction (5 min after serotonin, associated with the calcium sensitization component of the contraction) (1, 13, 18). During the early phase of the response and despite near-maximal contraction, there was no significant change in rhoA localization in WT aortas following serotonin stimulation (Fig. 5A). In contrast, after 5 min, a significant portion of rhoA was localized with cav-1 in low-density membrane fractions (∼50% of the total, Fig. 5, A and B). Cav-1 localization did not change during serotonin-induced contractions (Fig. 5B). Although the amount of ROCK2 tended to increase in low-density membrane fractions, there were no significant changes in the localization of either ROCK1 or ROCK2 in aortas with serotonin stimulation (Fig. 5C, ROCK1 not shown). Thus, during the calcium-independent phase of the sustained contraction to serotonin, rhoA shifts its localization from high- to low-density fractions.
Fig. 5.
Levels of RhoA (A), cav-1 (B), and ROCK2 (C), measured in low-density fractions (3–5) versus high-density fractions (8–10) from sucrose density centrifugation of aorta from wild-type mice under basal conditions, and early (30–60 s) and late (5 min) after the addition of serotonin (1 μM). Values are expressed as means ± SE; n = 3–6 pooled samples. *P < 0.05 vs. basal.
RhoA and ROCK localization in cav-1 KO mice.
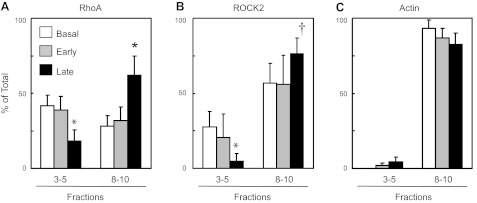
Having determined the localization pattern of rhoA and ROCK within lipid fractions from WT aorta, we next wanted to determine whether loss of cav-1 would affect rhoA and ROCK membrane localization. We confirmed the absence of cav-1 in aorta from cav-1 KO mice (Fig. 3A). β-Actin localization was primarily limited to high-density fractions similar to WT (Figs. 4 and 6C). In contrast to the WT mice, in cav-1 KO mice under basal conditions, rhoA was slightly higher within the low-density noncaveolar fractions (fractions 3–5) than in the high-density fractions (fractions 8–10), yet this was not statistically significant (Figs. 4D and 6A). ROCK2 expression pattern was similar to aortas obtained from WT mice (Figs. 4D and 6B).
Fig. 6.
Levels of RhoA (A), ROCK2 (B), and β-actin (C) measured in low-density fractions (3–5) versus high-density fractions (8–10) from sucrose density centrifugation of aorta from caveolin-1 KO mice. Samples were flash frozen under basal conditions, and early (30–60 s) and late (5 min) after the addition of serotonin (1 μM). Values are expressed as means ± SE; n = 3–5 pooled samples. *P ≤ 0.05 vs. basal. †P < 0.07 vs. basal.
We next compared the effect of serotonin on localization of rhoA and ROCK2 within noncaveolar lipid rafts of cav-1 KO mice. Similar to results obtained in aortas from WT mice, there were no significant changes in localization of rhoA or ROCK2 early after serotonin administration in aorta from cav-1 KO mice; however, in contrast to WT mice, during the sustained contraction to serotonin, levels of both rhoA and ROCK2 decreased in low-density fractions and increased in the high-density membrane fractions (Fig. 6, A and B) in aorta from cav-1 KO mice.
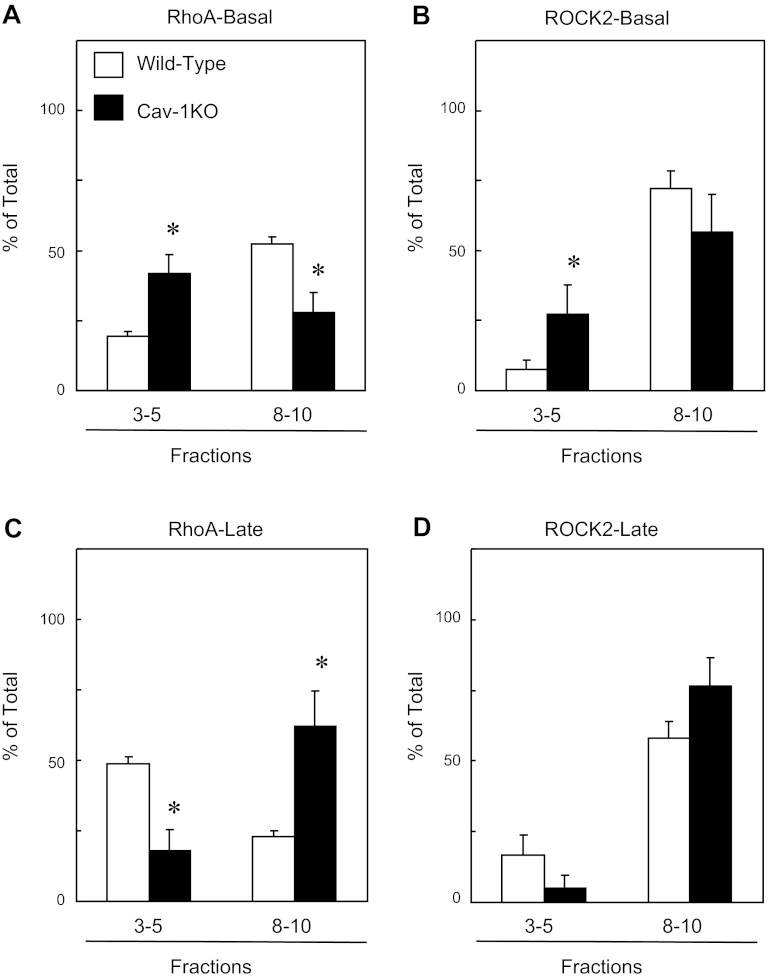
To determine the role of noncaveolar and caveolar rafts, we compared densities between WT vs. cav-1 KO mice for rhoA and ROCK2 under basal conditions. Under basal conditions, the percentage of rhoA in low-density fractions was significantly higher in cav-1 KO mice (∼20% vs. 42%) and significantly lower in high-density fractions (∼52% vs. 28%, Fig. 7A). There were also greater levels of ROCK-2 localized in low-density fractions compared with WT (∼8 vs. 27%, Fig. 7B). During the sustained contractions, only rhoA localization shifted in both WT and cav-1 KO mice but in opposing directions. Relatively higher percent of rhoA was localized within the low-density fraction in WT (∼49 vs. 18) and in the higher-density fraction in cav-1 KO mice (∼23 vs. 62%, Fig. 7C). ROCK2 was not significantly different in WT compared with cav-1 KO mice (Fig. 7D).
Fig. 7.
Levels of RhoA (A and C) and ROCK2 (B and D) measured in wild-type and cav-1 KO low-density fractions (3–5) versus high-density fractions (8–10) of aorta under basal conditions (A and B) and late (5 min, C and D) after addition of serotonin (1 μM). Values are expressed as means ± SE. *P < 0.05 vs. wild-type; n = 3–6 pooled samples.
DISCUSSION
Our results suggest that cav-1 dampens arterial contractions in response to serotonin by regulating rhoA localization and activation. In WT arteries under basal conditions, the majority of rhoA and ROCK was localized to high-density fractions, but upon activation with serotonin, rhoA translocated to low-density fractions in association with cav-1. The shift in localization of rhoA to low-density lipid rafts occurred late during the sustained contraction, which is associated with a calcium sensitization mechanisms and not during development of the contraction, which is associated with an increase in intracellular calcium concentration (1, 13, 18). Although expression of rhoA was slightly reduced in arteries from cav-1-deficient mice compared with WT mice, a larger percentage of rhoA was localized to the low-density fractions under basal conditions. These low-density fractions likely represent noncaveolar lipid rafts since caveolae do not form in vascular smooth muscle in the absence of cav-1 (4). Greater localization of rhoA to low-density noncaveolar rafts was associated with greater serotonin-stimulated contractions in cav-1 KO mice. But in contrast to WT aorta, in which serotonin stimulated rhoA to associate within lipid raft fractions, the absence of cav-1 caused rhoA to decrease in the low-density fractions during the sustained portion of the serotonin-induced contraction. Depletion of noncaveolar lipid rafts in cav-1 KO mice with MβCD reduced serotonin-induced contractions. These data suggest that first, localization of rhoA with cav-1 is not involved in the calcium-dependent initiation of contraction since the shift in localization occurred late during the sustained contraction. Second, cav-1 negatively regulates rhoA since the absence of cav-1 and caveolae increased contractions. Third, noncaveolar lipid rafts positively regulate rhoA since depleting noncaveolar lipid rafts in cav-1 KO mice reduced contractions. Finally, cav-1 may stabilize rhoA protein to prevent its degradation since in its absence, levels of rhoA were reduced. On the basis of our observations, we propose that contractions to serotonin are regulated by compartmentalization of rhoA within multiple subpopulations of lipid rafts in the plasma membrane.
RhoA and ROCK in caveolae.
Caveolins are not only structural components of caveolae that assemble a variety of signaling proteins, but they also regulate enzyme activity. Caveolins inhibit the activity of tyrosine and serine/threonine kinases, including ERK, PKC, and PKA (12, 22, 40). It has been hypothesized that caveolins inhibit these enzymes by stabilizing them in an inactive conformation within caveolae (23). We initially hypothesized that association of both rhoA and ROCK with caveolin would regulate their activation; however, there was no association between cav-1 and ROCK2, the isoform primarily involved in vascular contractions in WT mice (27, 37), under basal or stimulated conditions. Total ROCK2 protein levels were also unaffected by cav-1 deficiency. Moreover, only localization of rhoA was shifted within lipid rafts during serotonin-induced contractions in WT aorta. Taken together, our data suggest that cav-1 stabilizes rhoA within caveolae to prevent inactivation and/or degradation but does not function to regulate the activity or degradation of rho kinase. However, noncaveolar rafts may have a greater impact on rho kinase.
Noncaveolar lipid rafts.
Vascular smooth muscle and endothelium from cav-1-deficient mice are devoid of caveolae (4). Thus, the functional difference between caveolae and noncaveolar lipid microdomains in serotonin-induced rhoA activation can be determined by comparing cav-1 KO to WT. The divergent role of caveolar versus noncaveolar lipid rafts in the regulation of receptor and enzyme activation is recognized but has received less attention. Studies of localization of phosphatidylinositol bisphosphate (PIP2) (15), glucose transporter-4 (GLUT-4) (41), epidermal growth factor receptors (EGFR) (38 and 39), and endothelial nitric oxide synthase (eNOS) (34) within distinct membrane compartments suggest an important role for noncaveolar lipid rafts in regulating their activation. PIP2, GLUT-4, EGFR, and eNOS all partition to both caveolar and noncaveolar lipid rafts, but the functional consequences of their localization within these sites differ in magnitude and/or direction. For example, while PIP2 is found in both caveolar and noncaveolar lipid rafts, only PIP2 localized within caveolae is subject to activation by α1-adrenergic receptor stimulation (15). In contrast, insulin-stimulated glucose uptake by GLUT-4 occurs in both caveolar and noncaveolar lipid rafts (41), while EGFR is activated only when localized within noncaveolar lipid rafts (38).
The use of caveolin-deficient mice in combination with MβCD allows us to dissect the contribution of caveolae versus noncaveolar lipid rafts in the contraction to serotonin in these arteries (34). Despite lower levels of rhoA in the absence of cav-1, a greater percentage was localized within low-density lipid raft fractions under basal conditions. Greater amounts of rho kinase were localized within these low-density noncaveolar lipid rafts as well. These low-density fractions in cav-1 KO mice likely represent noncaveolar lipid rafts (34). Localization of rhoA to low-density noncaveolar fractions was associated with higher levels of basal rho activation under basal condition and following serotonin treatment (data not shown) and greater sustained contractions to serotonin. Subsequent removal of the noncaveolar lipid rafts with MβCD in arteries from cav-1 KO mice reduced contractions, while those induced by KCl were maintained. These data suggest that noncaveolar lipid rafts are involved in the serotonin-induced contractions. We propose that rhoA localization within noncaveolar lipid rafts promotes activation, whereas caveolar localization negatively regulates rhoA activation of downstream targets, such as rho kinase and vascular contractions. The differential regulation of the signaling molecules within lipid rafts suggests that multiple pools of rhoA and rho kinase are localized within the plasma membranes to regulate their function.
Regulation of rhoA activation.
A variety of mechanisms have been proposed to regulate rhoA activation. Inactive rhoA resides in the cytosol bound to GDP. Following agonist binding to G protein-coupled receptors, rhoA translocates to the membrane where GDP is exchanged with GTP.
These separate but integrated events are under the control of a family of regulatory proteins, including guanine exchange factors (GEFs), GTPase-activating proteins (GAPs), and guanine dissociation inhibitors (GDIs). GEFs control the exchange of GDP for GTP, whereas GAPs stimulate intrinsic activity and inactivate rhoA, and GDIs block spontaneous rhoA activation (27). Expression and/or activation of these regulatory proteins and their association with rhoA may also be regulated by their localization to caveolar and noncaveolar membrane pools. A major regulator of rhoA activity is p190rhoGAP-A, a GTPase-activating protein that facilitates rhoA-GTP hydrolysis (31). In cav-1-deficient mice, tyrosine nitration of p190rhoGAP-A increased rhoA activation and thrombin-induced endothelial permeability (31). Some of these alternative mechanisms may be involved in the increased responses to serotonin in our studies.
Alternative regulation of serotonin-mediated smooth muscle function.
Previous work by Shakirova et al. demonstrated that although rhoA activation is augmented in the absence of caveolae in ileal smooth muscle from cav-1-deficient mice, responses to serotonin were not affected (28). These discrepant findings in the response to serotonin between ileal and vascular tissue highlight the unique regulation of smooth muscle function, depending on organ and/or agonist. Contractions to serotonin are complex, mediated by two primary mechanisms on separate time frames: 1) the initial contraction as a result of an increase in calcium influx and release of calcium from SR stores, and 2) the sustained contraction as a result of calcium sensitization process (1, 13, 18). The second phase regulated by activation of rhoA and rho kinase and/or PKC is the focus of our study (1). A shift in the localization of rhoA from caveolar to noncaveolar lipid rafts could, in part, determine the magnitude of this calcium sensitization mechanism. We propose that in caveolin-1-deficient mice, higher levels of rhoA were localized within noncaveolar lipid rafts under basal conditions, promoting its activation and rho kinase-mediated contractions. In femoral arteries from cav-1-deficient mice, contractile responses to selective α1 receptor activation were increased, in part, via enhanced PKC (28). Although the relative contribution of rhoA/rho kinase and PKC varies between vascular beds and stimulus, at least in the cerebral circulation the G protein-coupled calcium sensitization was primarily due to activation of rhoA and rho kinase (1). However, in caveolin-1-deficient mice, augmented PKC activity may, in part, underlie the increased arterial contractions. Alternative mechanisms include altered endothelial function (nitric oxide or endothelium-derived hyperpolarizing factor) or vascular remodeling (24, 31). In cav-1 KO mice, activation of nitric oxide synthase is markedly enhanced (31), which would be expected to decrease serotonin-mediated contractile responses. Paradoxically, in the arteries from cav-1 KO mice, we observed markedly augmented contractions. Alternatively, endothelium-derived hyperpolarizing factor, which is reduced in the absence of caveolae, could increase contractile responses (24).
Perspectives and Significance
Lipid rafts regulate a variety of signaling pathways that may be modulated by diet and vascular disease (11). Our previous studies addressing the role of rhoA and ROCK in the regulation of vascular function in models of diabetes suggest that their increased activity is not mediated by changes in protein expression (19, 20). In two models of diabetes, activity of rhoA and ROCK was increased in the absence of a change in protein expression (19, 20). In addition to an increased role of rhoA and ROCK in contractile dysfunction in diabetes, numerous studies have shown upregulation of PKC that also affects calcium sensitization of contractile proteins in vascular smooth muscle. Activation of multiple signaling pathways that regulate vascular function may depend on compartmentalization of their components within membrane lipid rafts. The relative contribution of specific subpopulation of membrane lipid rafts in the regulation of vascular function needs further investigation but will require the advancement of techniques to isolate and identify these different membrane compartments.
GRANTS
These studies were supported by resources from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development awarded to Kathryn G. Lamping (Grant BX000543-03) and the National Institute of Child Health and Human Development (Grant HD-037831) awarded to Sarah K. England and the use of facilities at the Iowa City Veterans Affairs Health Care System, Iowa City, IA 52246.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.W.N., S.K.E., and K.G.L. conception and design of research; D.W.N. and K.G.L. performed experiments; D.W.N. and K.G.L. analyzed data; D.W.N. and K.G.L. interpreted results of experiments; D.W.N., S.K.E., and K.G.L. edited and revised manuscript; D.W.N., S.K.E., and K.G.L. approved final version of manuscript; K.G.L. prepared figures; K.G.L. drafted manuscript.
ACKNOWLEDGMENTS
The contents of this manuscript are the sole responsibility of the authors and do not necessarily represent the official views of the granting agencies.
REFERENCES
- 1. Akopov SE, Zhang L, Pearce WJ. Regulation of Ca2+ sensitization by PKC and rho proteins in ovine cerebral arteries: effects of artery size and age. Am J Physiol Heart Circ Physiol 275: H930–H939, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Bhatnagar A, Sheffler DJ, Kroeze WK, Compton-Toth B, Roth BL. Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Galphaq-coupled protein receptors. J Biol Chem 279: 34614–34623, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Chrissobolis S, Sobey C. Evidence that rho-kinase activity contributes to cerebral vascular tone in vivo and is enhanced during chronic hypertension. Circ Res 88: 774–779, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293: 2449–2452, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Dreja K, Voldstedlund M, Vinten J, Tranum-Jensen J, Hellstrand P, Sward K. Cholesterol depletion disrupts caveolae and differentially impairs agonist-induced arterial contraction. Arterioscler Thromb Vasc Biol 22: 1267–1272, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Dubroca C, Loyer X, Retailleau K, Loirand G, Pacaud P, Feron O, Balligand JL, Levy BI, Heymes C, Henrion D. RhoA activation and interaction with caveolin-1 are critical for pressure-induced myogenic tone in rat mesenteric resistance arteries. Cardiovasc Res 73: 190–197, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Feron O, Kelly RA. The caveolar paradox: suppressing, inducing, and terminating eNOS signaling. Circ Res 88: 129–131, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Galbiati F, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell 106: 403–411, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Harder T, Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol 9: 534–542, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Kandabashi T, Shimokawa H, Miyata K, Kunihiro I, Kawano Y, Fukata Y, Higo T, Egashira K, Takahashi S, Kaibuchi K, Takeshita A. Inhibition of myosin phosphatase by upregulated rho-kinase plays a key role for coronary artery spasm in a porcine model with interleukin-1β. Circulation 101: 1319–1323, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Lemaire-Ewing S, Lagrost L, Neel D. Lipid rafts: a signalling platform linking lipoprotein metabolism to atherogenesis. Atherosclerosis 221: 303–310, 2012 [DOI] [PubMed] [Google Scholar]
- 12. Li S, Couet J, Lisanti MP. Src tyrosine kinases, Gα subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem 271: 29182–29190, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Masu K, Saino T, Kuroda T, Matsuura M, Russa AD, Ishikita N, Satoh Y. Regional differences in 5-HT receptors in cerebral and testicular arterioles of the rat as revealed by Ca2+ imaging of real-time confocal microscopy: variances by artery size and organ specificity. Arch Histol Cytol 71: 291–302, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Miyata K, Shimokawa H, Kandabashi T, Higo T, Morishige K, Eto Y, Egashira K, Kaibuchi K, Takeshita A. Rho-kinase is involved in macrophage-mediated formation of coronary vascular lesions in pigs in vivo. Arterioscler Thromb Vasc Biol 20: 2351–2358, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Morris JB, Huynh H, Vasilevski O, Woodcock EA. Alpha1-adrenergic receptor signaling is localized to caveolae in neonatal rat cardiomyocytes. J Mol Cell Cardiol 41: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Munro S. Lipid rafts: elusive or illusive? Cell 115: 377–388, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Murata T, Lin M, Huang Y, Yu J, Bauer P, Giordano F, Sessa W. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med 204: 2373–2382, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ng LC, Wilson SM, McAllister CE, Hume JR. Role of InsP3 and ryanodine receptors in the activation of capacitative Ca2+ entry by store depletion or hypoxia in canine pulmonary arterial smooth muscle cells. Br J Pharmacol 152: 101–111, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nuno D, Lamping K. The role of rho kinase in sex-dependent vascular dysfunction in Type 1 diabetes. Exp Diabetes Res 2010: 176361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nuno DW, Harrod JS, Lamping KG. Sex-dependent differences in Rho activation contribute to contractile dysfunction in type 2 diabetic mice. Am J Physiol Heart Circ Physiol 297: H1469–H1477, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Nuno DW, Korovkina VP, England SK, Lamping KG. RhoA activation contributes to sex differences in vascular contractions. Arterioscler Thromb Vasc Biol 27: 1934–1940, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Oka N, Yamamoto M, Schwencke C, Kawabe J, Ebina T, Ohno S, Couet J, Lisanti MP, Ishikawa Y. Caveolin interaction with protein kinase C. Isoenzyme-dependent regulation of kinase activity by the caveolin scaffolding domain peptide. J Biol Chem 272: 33416–33421, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem 273: 5419–5422, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Rahman A, Sward K. The role of caveolin-1 in cardiovascular regulation. Acta Physiol (Oxf) 195: 231–245, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Ryan MJ, Didion SP, Davis DR, Faraci FM, Sigmund CD. Endothelial dysfunction and blood pressure variability in selected inbred mouse strains. Arterioscler Thromb Vasc Biol 22: 42–48, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Sato M, Tani E, Fujikawa H, Kaibuchi K. Involvement of rho-kinase-mediated phosphorylation of myosin light chain in enhancement of cerebral vasospasm. Circ Res 87: 195–200, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Satoh KMD, Fukumoto Y, Shimokawa H. Rho-kinase: important new therapeutic target in cardiovascular diseases. Am J Physiol Heart Circ Physiol 301: H287–H296, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Shakirova Y, Bonnevier J, Albinsson S, Adner M, Rippe B, Broman J, Arner A, Sward K. Increased Rho activation and PKC-mediated smooth muscle contractility in the absence of caveolin-1. Am J Physiol Cell Physiol 291: C1326–C1335, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Shaul PW, Anderson RG. Role of plasmalemmal caveolae in signal transduction. Am J Physiol Lung Cell Mol Physiol 275: L843–L851, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Shimokawa H, Morishige K, Miyata K, Kandabashi T, Eto Y, Ikegaki I, Asano T, Kaibuchi K, Takeshita A. Long-term inhibition of rho-kinase induces a regression of arteriosclerotic coronary lesions in a porcine model in vivo. Cardiovasc Res 51: 169–177, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Siddiqui MR, Komarova YA, Vogel SM, Gao X, Bonini MG, Rajasingh J, Zhao YY, Brovkovych V, Malik AB. Caveolin-1-eNOS signaling promotes p190RhoGAP-A nitration and endothelial permeability. J Cell Biol 193: 841–850, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Simons K, Ikonen E. Functional rafts in cell membranes. Nature 387: 569–572, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J Biol Chem 271: 9690–9697, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Sowa G, Pypaert M, Sessa WC. Distinction between signaling mechanisms in lipid rafts vs. caveolae. Proc Natl Acad Sci USA 98: 14072–14077, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a rho-associated protein kinase in hypertension. Nature 389: 990–994, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Volonte D, Galbiati F, Li S, Nishiyama K, Okamoto T, Lisanti MP. Flotillins/cavatellins are differentially expressed in cells and tissues and form a hetero-oligomeric complex with caveolins in vivo. Characterization and epitope-mapping of a novel flotillin-1 monoclonal antibody probe. J Biol Chem 274: 12702–12709, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Wang Y, Zheng XR, Riddick N, Bryden M, Baur W, Zhang X, Surks HK. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ Res 104: 531–540, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Waugh MG, Lawson D, Hsuan JJ. Epidermal growth factor receptor activation is localized within low-buoyant density, non-caveolar membrane domains. Biochem J 337: 591–597, 1999 [PMC free article] [PubMed] [Google Scholar]
- 39. Waugh MG, Minogue S, Anderson JS, dos Santos M, Hsuan JJ. Signalling and non-caveolar rafts. Biochem Soc Trans 29: 509–511, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Yamamoto M, Toya Y, Jensen RA, Ishikawa Y. Caveolin is an inhibitor of platelet-derived growth factor receptor signaling. Exp Cell Res 247: 380–388, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Yuan T, Hong S, Yao Y, Liao K. Glut-4 is translocated to both caveolae and non-caveolar lipid rafts, but is partially internalized through caveolae in insulin-stimulated adipocytes. Cell Res 17: 772–782, 2007 [DOI] [PubMed] [Google Scholar]