Abstract
The dorsolateral reticular formation of the caudal medulla, or the lateral tegmental field (LTF), has been classified as the brain's “vomiting center”, as well as an important region in regulating sympathetic outflow. We examined the responses of LTF neurons in cats to rotations of the body that activate vestibular receptors, as well as to stimulation of baroreceptors (through mechanical stretch of the carotid sinus) and gastrointestinal receptors (through the intragastric administration of the emetic compound copper sulfate). Approximately half of the LTF neurons exhibited graviceptive responses to vestibular stimulation, similar to primary afferents innervating otolith organs. The other half of the neurons had complex responses, including spatiotemporal convergence behavior, suggesting that they received convergent inputs from a variety of vestibular receptors. Neurons that received gastrointestinal and baroreceptor inputs had similar complex responses to vestibular stimulation; such responses are expected for neurons that contribute to the generation of motion sickness. LTF units with convergent baroreceptor and vestibular inputs may participate in producing the cardiovascular system components of motion sickness, such as the changes in skin blood flow that result in pallor. The administration of copper sulfate often modulated the gain of responses of LTF neurons to vestibular stimulation, particularly for units whose spontaneous firing rate was altered by infusion of drug (median of 459%). The present results raise the prospect that emetic signals from the gastrointestinal tract modify the processing of vestibular inputs by LTF neurons, thereby affecting the probability that vomiting will occur as a consequence of motion sickness.
Keywords: semicircular canal, otolith organ, vomiting, nausea, motion sickness
the dorsolateral reticular formation of the caudal medulla, typically referred to as the “lateral tegmental field” (LTF) (11), plays a variety of roles in autonomic regulation. The LTF has historically been deemed as the brain stem “vomiting center” in emetic animals (13). Borison and Wang (13) demonstrated that stimulation of the LTF in cats produced vomiting. Damage to this area by implanting a radon pellet into dogs was also shown to eliminate emesis induced by the injection of a copper sulfate solution into the stomach (55). Similar data were obtained by Fukuda, who reported that stimulation within the LTF induced vomiting (19, 20), whereas electrolytic or chemical lesions of the area abolished the capacity to vomit (33). In addition, Fukuda's electrophysiological studies demonstrated that neurons in the LTF had appropriate firing patterns to coordinate the respiratory muscle contractions that result in vomiting (20). Furthermore, administration of emetic drugs or stimulation of gastrointestinal afferents evoked the expression of the intermediate-early gene c-fos by a large number of LTF neurons (12, 26, 27, 36, 41).
However, other investigators have claimed that there is not a compact vomiting center present in the LTF. Instead, they suggested that a larger network of cells distributed through the medullary reticular formation coordinates emesis. This view was based on experiments showing that stimulation in the LTF failed to produce vomiting in cats (35), as was previously demonstrated by others (13, 20). In addition, although large chemical lesions of the lateral reticular formation prevented vomiting, more focal lesions of the LTF did not abolish the response, but the patterning of the respiratory muscle contractions during emesis was altered (35). The apparent discrepancies across studies are likely linked to the size of lesions and the magnitude of stimulus currents that were employed. The “vomiting center” first described by Borison and Wang may, in fact, be just a part of a “vomiting region” distributed over several millimeters of the medullary reticular formation. Nonetheless, there appears to be general agreement in the literature that neurons located in the LTF play an important role in eliciting and coordinating vomiting.
In addition to producing emetic responses, some neurons in the LTF participate in regulating sympathetic nervous system activity and blood pressure. In felines, the LTF appears to play an important role in maintaining tonic spontaneous activity of neurons located in the rostral ventrolateral medulla (RVLM), that, in turn, provide inputs to sympathetic preganglionic neurons in the thoracic spinal cord (7). Consequently, blockade of excitatory amino acid neurotransmission in the LTF produced a significant reduction in sympathetic nerve activity and blood pressure (8). LTF neurons have also been shown to participate in generating some (42, 43), but not all (9), reflex-related changes in sympathetic nerve activity. In particular, LTF neurons serve as an important synaptic relay in the baroreceptor reflex pathway (42).
Among the inputs that LTF neurons receive are those from the vestibular system. Injection of the anterograde tracer Phaseolus vulgaris leucoagglutinin into the caudal aspect of the vestibular nucleus complex resulted in the labeling of axon terminals in the LTF (59). Electrical stimulation of the vestibular nerve affected the firing rate of LTF neurons, including approximately one-third of those whose axons could be antidromically activated from the RVLM (59). However, no previous studies described the responses of LTF neurons to movements of the head that activate receptors in the inner ear. One hypothesis tested in this study was that LTF neurons involved in cardiovascular regulation and LTF neurons that are components of the pattern generator for emesis receive distinct labyrinthine signals, and thus have different responses to natural vestibular stimulation. For this purpose, we assumed that LTF neurons with baroreceptor inputs participated in the regulation of blood pressure, while LTF neurons, whose firing rate was altered by the infusion of the emetic drug copper sulfate into the stomach, contributed to the production of vomiting.
Our previous study showed that the delivery of the emetic drug copper sulfate to the stomach altered the gain of responses of parabrachial nucleus neurons to vestibular stimulation (54). Because the parabrachial nuclei play a key role in relaying visceral inputs to the diencephalon and telencephalon (14), parabrachial neurons with labyrinthine inputs likely participate in generating affective responses and queasiness during motion sickness and related conditions (5). Consequently, our prior data suggested that emetic signals from the gastrointestinal system influence sensations such as nausea elicited by vestibular stimulation. It is useful to determine whether an analogous interaction of gastrointestinal and vestibular inputs occurs in the LTF, since many neurons in this region coordinate vomiting (20), to establish whether the integration of these signals is similar across the brain stem pathways that generate nausea and emesis. Accordingly, the second hypothesis tested in this study was that the responses to body rotations of LTF neurons that contributed to the production of vomiting were altered by the intragastric infusion of copper sulfate.
METHODS
All experimental procedures conformed to the American Physiological Society's “Guiding Principles for the Care and Use of Animals,” as well as the National Research Council Guide for the Care and Use of Laboratory Animals, and were approved by the University of Pittsburgh's Institutional Animal Care and Use Committee. Data were collected from 15 purpose-bred adult male cats (Liberty Research, Waverly, NY), weighing 4–5 kg (median=4.4 kg). Because the procedures employed in this study were similar to those described in two recent articles (53, 54), they are presented in abbreviated fashion below.
Surgical procedures.
Surgery was performed under 1–3% isoflurane anesthesia to cannulate both femoral veins, insert a Millar (Houston, TX) blood pressure transducer through the femoral artery into the abdominal aorta, perform a tracheostomy, and ligate both carotid arteries. The carotid arteries were also dissected free of surrounding tissues and prepared for mechanical stretch of the carotid sinus, as in our prior studies (15, 53, 54). An intragastric catheter was inserted through an esophagostomy to administer copper sulfate (53, 54). Subsequently, the animal was placed in a stereotaxic frame with the head pitched-down 30° to vertically align the vertical semicircular canals, and the body was secured using hip pins and a clamp placed on the dorsal process of the T1 vertebra. The stereotaxic frame was mounted on a servo-controlled hydraulic tilt table (NeuroKinetics, Pittsburgh, PA). A midcollicular decerebration was performed, and a craniotomy was completed to expose the caudal medulla. The cerebellum was gently retracted and partially aspirated to expose the dorsal surface of the brain stem to 3–5 mm rostral to the obex.
Throughout the surgery and subsequent recording session, rectal temperature was maintained at 37–38°C using a DC-powered heating pad and infrared lamp. Fluids, and, if necessary, phenylephrine (0.005–0.01 mg·kg−1·min−1), were infused intravenously to maintain blood pressure > 90 mmHg. Atropine sulfate (0.10–0.15 mg/kg) was administered intramuscularly every 6 h to reduce airway secretions, and dexamethasone (2 mg/kg initial dose, 1 mg/kg subsequent doses) was injected intravenously every 6 h to reduce brain edema. Anesthesia was discontinued after all surgery was complete, and animals were paralyzed using intravenous injections of pancuronium bromide (initial injection of 0.2 mg/kg, maintained by hourly injections of 0.1–0.2 mg/kg), and artificially ventilated with room air. Tidal volume and ventilation frequency were adjusted to maintain end-tidal CO2 at 4–5%. A bilateral pneumothorax was performed to reduce ventilation-related brain movements.
Data recording procedures.
Electrode penetrations were made using 4–6 MΩ tungsten microelectrodes (FHC, Bowdoin, ME), from 8.5–12 mm posterior to stereotaxic zero and 2–5 mm lateral to the midline, to record activity from LTF neurons. Single-unit activity, blood pressure, and tilt table position were sampled using a Micro1401–2 data collection system and Spike2 version 6 software (Cambridge Electronic Design, Cambridge, UK), as discussed in our previous papers (15, 53, 54). In addition, the electrocardiogram was recorded using leads placed on each side of the chest and sampled at 1,000 Hz. During some tracks, we tested all units for responses to whole body rotations, and during others, we delivered a 0.2-Hz wobble search stimulus, as described below, to target those neurons whose activity was modulated by the rotations. Clockwise wobble stimuli were generated by driving the pitch axis of the tilt table with a sine wave, while simultaneously driving the roll axis with a cosine wave. During this stimulus, the animal's body (viewed from above) appeared to wobble, taking in succession nose-down, right ear-down, nose-up, and left ear-down positions. When the signal to the pitch axis of the tilt table was inverted, the stimulus vector rotated in the counterclockwise direction. We typically delivered 0.2-Hz wobble stimuli at 5°-7.5° to determine whether a unit responded to vertical vestibular stimulation. As discussed in results, responses to wobble stimulation were used to calculate the response vector orientation, or plane of tilt that produced a maximum modulation of neuronal firing rate. The response vector orientation was then confirmed by comparing the responses to 0.2-Hz sinusoidal tilts in the roll and pitch planes. Subsequently, tilts in a fixed plane near this orientation were used to study the dynamics of the vestibular response (response gain and phase across stimulus frequencies). Response dynamics were customarily determined over the frequency range of 0.05–0.5 Hz; for some units, 0.02 or 1-Hz rotations were also delivered. The amplitude of these stimuli was routinely 2.5°-7.5°; occasionally 10° amplitudes were employed.
In addition to recording responses of neurons to whole body rotations, we sampled the spontaneous firing rate of each unit over a 60-s period along with blood pressure, to determine whether a cell had activity that was synchronized to a phase of the cardiac cycle. Furthermore, we determined the change in the unit's firing rate during the decrease in blood pressure and heart rate produced by mechanical stretch of the carotid sinus (15, 53, 54). Subsequently, 83 mg of copper sulfate dissolved in 10 ml of distilled water was injected into the stomach, as in our recent studies (53, 54). We recorded the effects of copper sulfate injection on the unit's firing rate for 5 min, and then repeated the vestibular testing protocol outlined above. When stimuli were completed, the copper sulfate solution was aspirated from the stomach, and six washes were performed, each using 10 ml of distilled water.
Data analysis procedures.
All data were subjected to offline spike sorting to ensure that counts of neuronal activity were accurate. During this process, neural activity recorded during every run was inspected to ensure that action potential size and shape did not change systematically during whole body rotations or other manipulations, such that spike counts were inaccurate. If we were not certain about the fidelity of data collected during a trial, that run was discarded. Neural activity recorded during whole body rotations was binned (500 bins/cycle) and averaged over the sinusoidal stimulus period. Sine waves were fitted to responses with the use of a least-squares minimization technique (51). The response sinusoid was characterized by two parameters: phase shift from the stimulus sinusoid (subsequently referred to as phase) and amplitude relative to the stimulus sinusoid (subsequently referred to as gain). The signal-to-noise ratio for responses was also calculated (51). We used one primary criterion and two secondary criteria to determine whether neuronal activity was modulated by rotations (15, 29, 53, 61). First, responses were considered significant only when the signal-to-noise ratio was >0.5. Data meeting this criterion were considered to represent real modulation of neuronal activity if only the first harmonic was prominent and the responses were consistent from trial to trial.
Statistical analyses were performed using Prism 5 software (GraphPad Software, San Diego, CA). Pooled data are presented as means ± SE. Statistical significance was assumed if P < 0.05.
Histological procedures.
Near the end of the recording session, lesions were made at defined coordinates by passing a 0.1–0.2 mA negative current through the recording electrode for 60 s. Animals were subsequently killed using Euthasol Euthanasia Solution, and the brain stem was removed and fixed in 10% formaldehyde solution. A freezing microtome was used to cut the brain stem transversely at 100-μm thickness, and tissue sections were stained using thionine. Recording sites were reconstructed on drawings of sections with reference to the locations of electrolytic lesions, the relative positions of electrode tracks, and microelectrode depths.
RESULTS
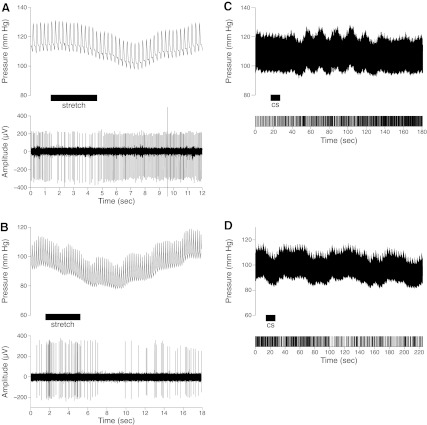
The responses of 93 neurons to whole body tilts were recorded; 45 of these cells were tested for the effects of vestibular stimulation before and after the intragastric infusion of copper sulfate. Forty-two percent of the neurons (39/93) either had activity that was synchronized to a phase of the cardiac cycle (n = 2), or firing that decreased (n = 12) or increased (n = 25) over 30% during stretch of the carotid sinus (as shown in Fig. 1, A and B). The firing rate of 17 cells either increased (n = 9) or decreased (n = 8) over 30% following the intragastric infusion of copper sulfate, as indicated in Fig. 1, C and D; the onset of these alterations in activity typically occurred a minute or more after copper sulfate was injected into the stomach. Approximately half (9/17) of these units did not respond to baroreceptor stimulation and were classified as gastrointestinal neurons. Eight neurons responded to both cardiovascular and gastrointestinal stimulation and were classified as convergent neurons. Although transient changes in blood pressure often occurred after delivery of copper sulfate (52, 53), the sustained changes in firing rate elicited by infusion of the compound were not synchronized with these blood pressure perturbations. The 31 cells with cardiac-related activity or responses to carotid stretch that were not deemed to be convergent units were classified as cardiovascular neurons. The remaining cells (45, 48%) were classified as unknown units.
Fig. 1.
Activity of lateral tegmental field (LTF) neurons during mechanical stimulation of baroreceptor afferents (A and B) and associated with the infusion of copper sulfate (cs) into the stomach (C and D). A and B: each panel contains a record of blood pressure (top, sampled at 100 Hz) and a recording of unit activity (bottom, sampled at 25,000 Hz), indicating the associated firing pattern of an LTF unit. When the carotid sinus was stretched (indicated by horizontal bar), the activity of the unit in A decreased, whereas that in B increased. A silencing of unit activity occurred in B following the carotid stretch, as blood pressure declined as a consequence of the baroreceptor reflex. Because spike size and shape did not change appreciably during the trial, it is likely that this reduction in unit activity resulted from the unloading of baroreceptors, and not a mechanical artifact. C and D: effect of copper sulfate infusion into the stomach (indicated by horizontal bar) on arterial blood pressure (top) and activity of an LTF unit (bottom raster plot). In C, a sustained increase in unit firing occurred after copper sulfate was present in the stomach for over a minute. A transient perturbation in blood pressure was also observed, which was not synchronized with the activity of the cell. In D, administration of copper sulfate resulted in a sustained reduction in the activity of an LTF unit, with a latency >1 min.
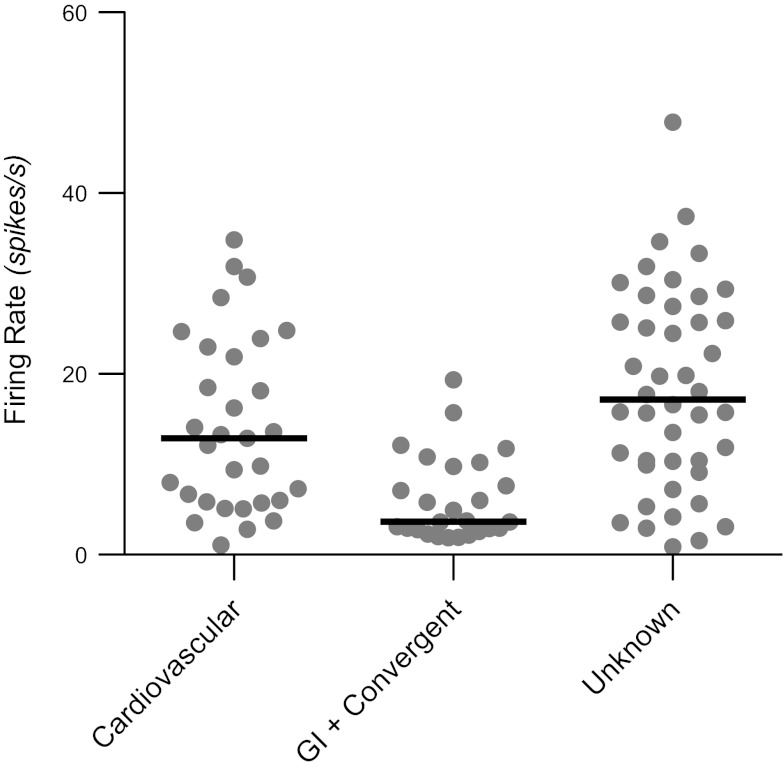
The spontaneous firing rates of each of the unit types are shown in Fig. 2. All of the neurons whose firing rate was altered by the administration of copper sulfate (gastrointestinal and convergent units) were pooled together for this analysis. The median firing rate for neurons with gastrointestinal inputs (3.7 spikes/s) was significantly lower (Kruskal-Wallis test, P < 0.01) than for cardiovascular (12.9 spikes/s) or unknown (17.2 spikes/s) neurons.
Fig. 2.
Spontaneous firing rates of different unit types tested for responses to vertical vestibular stimulation. Gray circles indicate data for each neuron, whereas black horizontal lines designate median firing rates. GI, gastrointestinal.
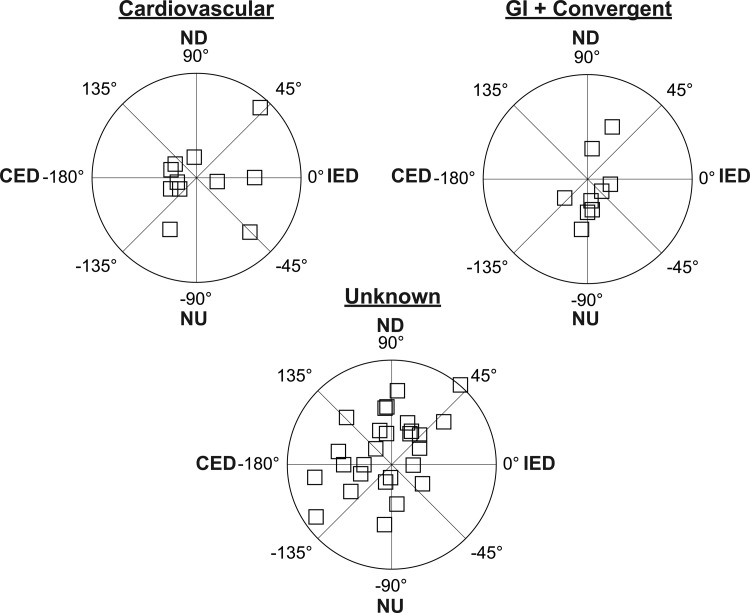
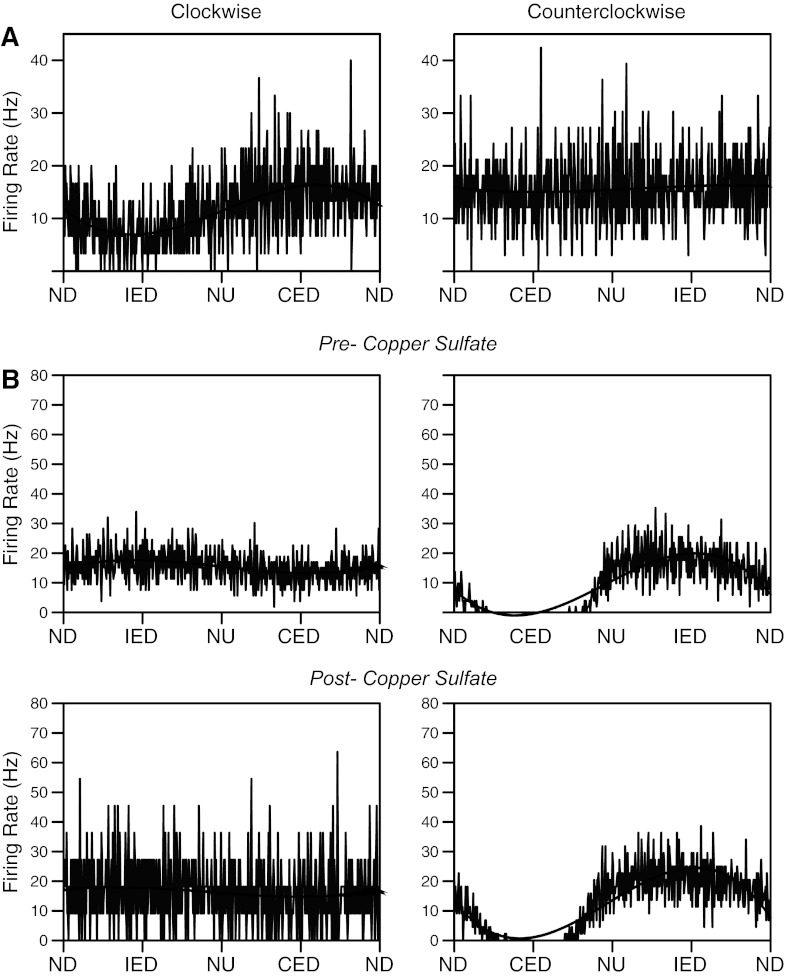
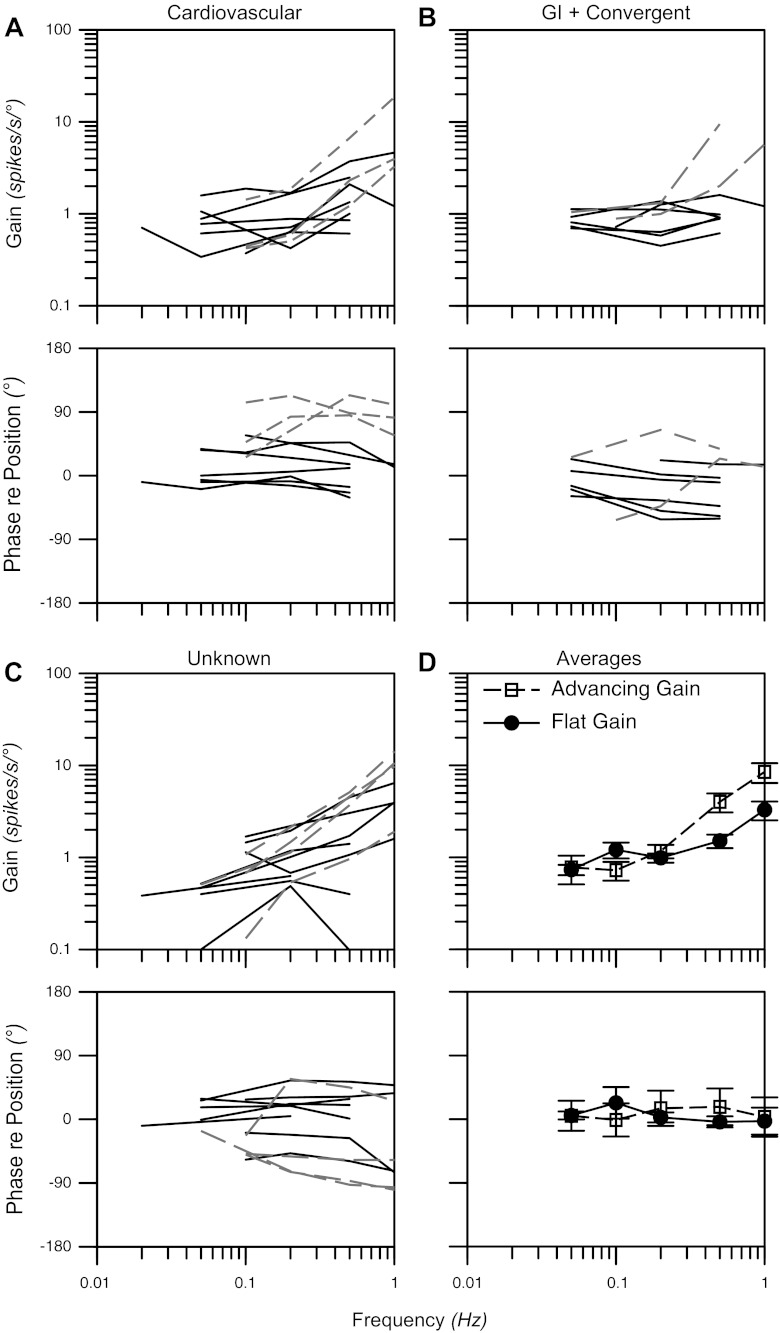
Fifty-five neurons in our sample responded to whole-body rotations ≤7.5° in amplitude, including 14 cardiovascular, five gastrointestinal, six convergent, and 30 unknown units. The response vector orientations for each of the unit types are indicated in Fig. 3. These response vector orientations were determined by considering the responses to clockwise and counterclockwise wobble stimuli, as in our previous research (15, 29, 53, 54, 61), because phase shifts between stimuli and responses are typically reversed during the two directions of rotation (51). However, Fig. 3 excludes eight neurons (three cardiovascular, one gastrointestinal, one convergent, three unknown) for which we could not calculate a response vector orientation, since they responded to only one direction of wobble rotation, as illustrated in Fig. 4. Such a response pattern has been attributed to the convergence of inputs with different spatial and temporal properties (for instance, the convergence of inputs from semicircular canal afferents activated by roll rotations and otolith organ afferents activated by pitch rotations) and has been termed “spatiotemporal convergence” (STC) behavior (2, 50, 51). Even when neurons responded to both clockwise and counterclockwise wobble stimuli, it was common for one response to be much larger than the other. For two cardiovascular, one gastrointestinal, one convergent, and five unknown units, rotations in one direction elicited responses that were over twice as large as those produced by rotations in the other direction. Consequently, pronounced STC behavior (50) was exhibited by almost one-third (17/55) of the LTF neurons that responded to rotations in vertical planes.
Fig. 3.
Polar plots showing response vector orientations and gains of cardiovascular units, units with gastrointestinal (GI) inputs (both GI and convergent neurons), and unknown units. Response vector orientations were determined using wobble stimuli, usually delivered at 0.2 Hz. The maximal radius of each plot designates a response gain of 3 spikes·s−1·deg−1. The response vector orientations were plotted using a head-centered coordinate system, with 0° corresponding to ipsilateral ear-down (IED) roll tilt, 90° corresponding to nose-down (ND) pitch, 180° corresponding to contralateral ear-down (CED) roll, and −90° corresponding to nose-up (NU) pitch.
Fig. 4.
Averaged responses of two STC neurons whose activity was modulated by only one direction of wobble stimulation. Responses illustrated were elicited by 0.2-Hz wobble stimulation delivered at 7.5°. Averaged unit activity is indicated by traces, whereas overlain black curves are sine waves fit to the responses. A: responses of one unit prior to the administration of copper sulfate. The unit responded to clockwise (CW) wobble stimulation, but not counterclockwise (CCW) rotations (the signal-to-noise ratio was 0.82 for the CW response, and 0.01 for the CCW response). B: responses of a different unit before (top) and after (bottom) the administration of copper sulfate. During both conditions, the unit responded to CCW wobble, but not CW rotations. The response to CCW stimuli was so powerful that the activity of the unit was completely abolished during one phase of the rotation (contralateral ear down). The response gain for CCW trials was similar before (2.3 spikes·s−1·deg−1) and after (2.2 spikes·s−1·deg−1) copper sulfate administration. See Fig. 3 for definitions of abbreviations. The number of sweeps averaged to generate each trace was A: 33 (CW), 30 (CCW); B, top: 53 (CW), 51 (CCW); B, bottom: 11 (CW), 44 (CCW).
Approximately half of the neurons (22/47) whose response vector orientations could be determined responded better to roll (ear-down) than to pitch (nose- or tail- down) rotations, as indicated in Fig. 3. Among these cells, 14/22 were excited by contralateral ear-down rotations, and eight were excited by ipsilateral ear-down tilts. Of the 25/47 units that responded better to pitch than to roll rotations, 15 were excited by nose-down tilt and 10 were excited by nose-up tilt. These data show that any direction of vertical tilt can activate a population of LTF neurons, although rotations in the contralateral ear-down and nose-down directions are particularly effective.
Subsequently, we delivered sinusoidal rotations in a fixed plane aligned closely to the response vector orientation, to determine how the gain and phase of responses with respect to tilt table position changed as a function of rotational frequency. Such an analysis was not performed for STC neurons that only responded to one direction of wobble rotation, since we could not calculate a response vector orientation for these units. Examples of responses of two neurons to sinusoidal tilts in the roll plane are illustrated in Fig. 5. Bode plots indicating the gains and phases of the neuronal responses to single-plane tilts are shown in Fig. 6. Data are available for 30 neurons whose responses to rotations were established for a 10-fold range of frequencies, usually 0.05–0.5 Hz. The response gains for most of the neurons (21/30) increased less than five-fold per stimulus decade (mean of 2.3 ± 0.4), and these units were classified as having a “flat gain”. The phase difference between tilt table position and the responses of most (16/21) units with flat gains was <45° at all frequencies. However, the response phases for four of the neurons with flat gains lagged stimulus position more than 45° at higher stimulus frequencies (0.5–1 Hz), and the response phases for one neuron led stimulus position by 50°–55° at higher stimulus frequencies. The response gains for the remaining nine units increased over five-fold per stimulus decade (mean of 11 ± 1, range of 7.3–14.4); these cells were classified as having “gain advances”. One-third of the cells with gain advances had response phases that remained within 45° of stimulus position at all frequencies, another one-third developed phase lags with increasing stimulus frequency, and the responses of the remaining gain-advancing units led stimulus position >45° when tilt table rotations were delivered at frequencies ≥0.2 Hz.
Fig. 5.
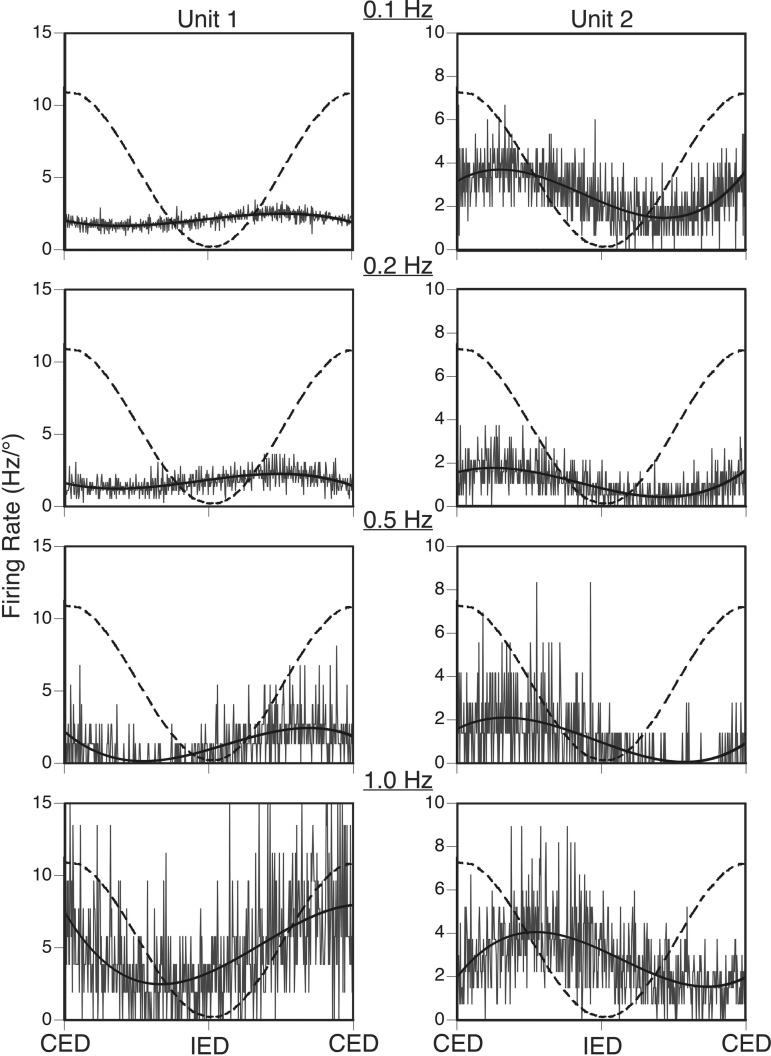
Averaged responses of two neurons to sinusoidal tilts in the roll plane at frequencies of 0.1–1 Hz. Both units were excited by CED and inhibited by IED. Averaged unit activity is indicated by gray traces, overlain solid black curves are sine waves fit to the responses, and dashed curves indicate tilt table position. The following tilt amplitudes were used for each trial: Unit 1, 7.5° at 0.1 Hz, 5° at 0.2–0.5 Hz, and 2.5° at 1 Hz; Unit 2, 7.5° at 0.1–0.2 Hz, 5° at 0.5 Hz, and 2.5° at 1 Hz. Because smaller tilt amplitudes were used for higher-frequency rotations, firing rate is expressed in Hz/° to facilitate comparisons. The response gain for Unit 1 increased ∼10-fold as the stimulus frequency advanced from 0.1 to 1 Hz, and the response phase led stimulus position by ∼90° at all frequencies. In contrast, the response gain for Unit 2 was relatively flat across stimulus frequencies, whereas the response phase lagged stimulus position by 50–75° across the range of frequencies tested.
Fig. 6.
Bode plots illustrating the dynamic properties of responses of LTF neurons to rotations in a fixed plane at multiple frequencies. Response gain and phase are plotted with respect to stimulus position. Bode plots for cardiovascular neurons (A), neurons with gastrointestinal (GI) inputs (gastrointestinal and convergent unit types) (B), and unknown units (C) are provided separately. Solid lines designate data for flat-gain neurons, whereas dashed lines show responses for advancing gain neurons. D: average Bode plots for all flat and advancing gain neurons; error bars designate SE.
We evaluated the effects of the intragastric infusion of copper sulfate on the responses to vertical vestibular stimulation of 20 cardiovascular and 15 unknown units, as well as 10 units with gastrointestinal inputs (four convergent and six gastrointestinal units; seven were excited, and three were inhibited after the drug was given). Sixteen of the 45 neurons did not initially respond to whole-body rotations; 13 of these units remained unresponsive following the administration of copper sulfate, but three acquired STC responses. Twenty-nine neurons whose firing rate was modulated by vestibular stimulation prior to the administration of copper sulfate, including six with STC behavior, were also tested for the effects of the drug on their responses to whole body rotations. Although the responses to rotations of approximately half (14/29) of these cells were relatively unaffected by the delivery of copper sulfate, the responses of the other half were appreciably altered. Four neurons, including one STC cell, became unresponsive to vestibular stimulation after copper sulfate was injected into the stomach, and the responses to wobble stimulation of four other cells were attenuated over 30% (median of 53%). In contrast, four neurons responded over 30% stronger to wobble stimulation (median of 49%) following infusion of copper sulfate. One neuron that responded to roll and two others that responded to pitch tilts acquired STC behavior after copper sulfate was administered, such that only one direction of wobble stimulation was effective in generating responses.
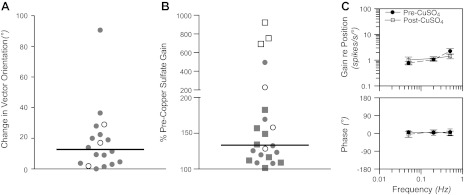
The effects of copper sulfate administration on the responses of LTF neurons to vestibular stimulation are shown in Fig. 7. Fig. 7A indicates the change in response vector orientation determined for the neurons for which this parameter could be calculated both before and after copper sulfate was injected. In the majority of cases, response vector orientation was similar before and after drug administration; the median change was 13°. In contrast, copper sulfate infusion produced a large alteration in response gain for an appreciable number of neurons, as indicated in Fig. 7B. The average gain of a unit's responses to clockwise and counterclockwise wobble stimuli is indicated by each symbol in Fig. 7B, including responses that became insignificant after copper sulfate was delivered, although response gains for STC neurons that responded to only one direction of wobble stimulation were excluded. Fig. 7B depicts the absolute value of the change in gain (both increases and decreases in gain are designated as a value >100%), to facilitate comparisons between units whose responses were strengthened or weakened by copper sulfate infusion. The median change in response gain was 135%, but the value (459%) for neurons whose firing rate was altered by the injection of copper sulfate (convergent and gastrointestinal units, open symbols) was significantly larger (P < 0.01, Mann-Whitney U-test) than for unknown and cardiovascular units (126%, filled symbols). Fig. 7C illustrates mean Bode plots for the 11 neurons whose response dynamics were determined before and after the intragastric injection of copper sulfate. This panel shows that response dynamics for the LTF neuronal population as a whole were relatively unaffected when copper sulfate was provided to an animal.
Fig. 7.
Effects of injection of copper sulfate into the stomach on the responses of LTF neurons to vestibular stimulation. A: changes in response vector orientation produced by administration of copper sulfate. Data are restricted to the subset of units for which a response vector orientation could be calculated before and after copper sulfate was provided. Open symbols designate values for neurons with gastrointestinal inputs, whereas filled symbols designate cardiovascular and unknown units. Horizontal lines show median values. B: Changes in response gain produced by delivery of copper sulfate. The absolute value of the percent change in gain following injection of the compound is indicated. Squares designate units whose response gain decreased after copper sulfate was infused, while circles indicate units whose response gain increased. C: bode plots comparing average response dynamics for neurons before and after copper sulfate administration. Error bars indicate means ± SE.
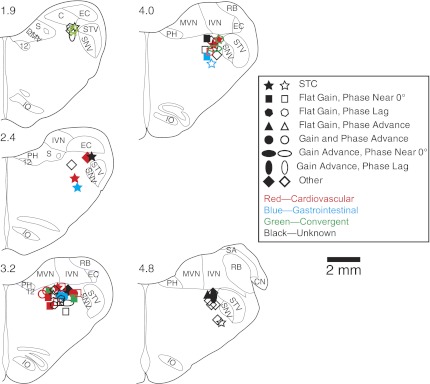
The locations of the neurons that responded to vestibular stimulation either before or after the injection of copper sulfate into the stomach are shown in Fig. 8. Different symbols are used to indicate whether the responses had a flat or increasing gain with advancing stimulus frequency, and phases that remained near stimulus position or which lagged or led stimulus position >45° at higher stimulus frequencies. Filled symbols show the locations of cells whose response characteristics changed appreciably following copper sulfate administration (i.e., response gains increased or decreased >30%, or the neurons acquired or lost STC behavior). In addition, the color of each symbol indicates whether the unit's spontaneous firing rate changed during the stimulation of baroreceptors and/or gastrointestinal receptors. In general, different unit types were interspersed in the LTF. However, a two-way ANOVA (P < 0.05, factors were the presence of cardiovascular inputs and the coordinates of unit location) revealed that neurons that received cardiovascular inputs were more caudal (median of 2.7 mm rostral to the obex) than other units (median of 3.3 mm rostral to the obex).
Fig. 8.
Locations of neurons that responded to vertical vestibular stimulation, either before or after the intragastric administration of copper sulfate. Unit locations, which were generated through reference to Berman's atlas (11), are plotted on standard transverse sections through the caudal medulla. Numbers above each section designate distance in millimeters rostral to the obex. Distinct symbols are used to designate the characteristics of responses to tilts at different frequencies: STC behavior (responds to only one direction of wobble rotations); flat gain (increasing <5-fold per stimulus decade) and phase near (within 45° of) stimulus position; flat gain and a phase lag (>45°) from stimulus position at higher stimulus frequencies; flat gain and a phase advance (>45°) from stimulus position at higher frequencies; both a gain advance (>5-fold per stimulus decade) and phase advance (>45° from stimulus position) at higher frequencies; a gain advance and a phase near (within 45° of) stimulus position at all frequencies; a gain advance and a phase lag (>45°) from stimulus position at higher frequencies; or others (including neurons that were lost before response dynamics could be determined). Different colors are used to designate cardiovascular, gastrointestinal, convergent, and unknown unit types. Solid symbols indicate neurons whose response characteristics changed after administration of copper sulfate (response gains changed over 30%, or which developed or lost STC behavior). 12, hypoglossal nucleus; C, cuneate nucleus; CN, cochlear nucleus; DMV, dorsal motor nucleus of the vagus; EC, external cuneate; IO, inferior olivary nucleus; PH, prepositus hypoglossi; IVN, inferior vestibular nucleus; MVN, medial vestibular nucleus; RB, restiform body; S, solitary nucleus; SA, stria acoustica; SNV, spinal trigeminal nucleus; STV, spinal trigeminal tract.
DISCUSSION
This study showed that a subset of LTF neurons, including those with baroreceptor inputs and those whose firing rate, was modulated by the intragastric infusion of the emetic drug copper sulfate, responded to whole-body rotations that activate vestibular receptors. In contrast to vestibular nucleus neurons, the majority of which in felines have responses to body rotations that resemble those of otolith organ or semicircular canal afferents, or a simple summation of these responses (16, 30, 38), many LTF neurons had complex responses to vestibular stimulation. In particular, one-third of LTF neurons exhibited pronounced STC behavior, and most of the advancing gain neurons (25% of the neurons for which Bode plots were generated) had response phases that were near or lagged stimulus position, unlike vestibular afferents (1, 17, 18). Such complex responses likely reflect the convergence of a variety of labyrinthine inputs onto single LTF neurons. We also demonstrated that the administration of copper sulfate often produced a change in the gain of responses of LTF neurons to vestibular stimulation, particularly for units whose spontaneous firing rate was altered by infusion of drug. In addition, some neurons acquired STC behavior after copper sulfate was provided, indicating that the complement of vestibular inputs to these cells was modified. Since the LTF participates in coordinating vomiting (19, 20), it is reasonable to assume that labyrinthine signals to this region serve, in part, to elicit motion sickness-related emesis. Consequently, the present results raise the prospect that emetic signals from the gastrointestinal tract alter the processing of vestibular inputs by LTF neurons, thereby affecting the probability that vomiting will occur as a consequence of motion sickness.
Emesis can be evoked in animals with all portions of the nervous system removed except the caudal medulla and spinal cord (19, 35), indicating that the pattern generator that coordinates the respiratory muscle contractions that generate vomiting is located in the caudal medulla. In contrast, a variety of experimental approaches have indicated that an ascending pathway from the brain stem through the parabrachial nucleus to the hypothalamus, limbic system, and perhaps other cortical areas is responsible for nausea and the affective responses that precede and accompany emesis (4, 21, 22, 47–49, 57, 58). To better appreciate the neural mechanisms that generate motion sickness, it is useful to contrast the processing of vestibular signals by the pathways responsible for producing nausea and vomiting. We recently used methodology similar to that in the current study to examine the responses of parabrachial nucleus neurons to vestibular stimulation in vertical planes, both before and after intragastric copper sulfate administration (54). Unlike LTF units, parabrachial nucleus neurons rarely exhibited STC behavior. Instead, over 70% of parabrachial neurons had graviceptive responses to vertical vestibular stimulation, similar to those of otolith organ afferents: relatively flat gains across stimulus frequencies, and phases that remained near stimulus position or lagged stimulus position (16, 30, 38). Consequently, less convergence of inputs from different vestibular end organs seemingly occurs in the parabrachial nucleus, as in the LTF of cats. Complex head movements that activate a variety of labyrinthine receptors are required to elicit a maximal firing rate of units with STC behavior (50), such that many LTF neurons would only rarely attain a maximal firing rate, when complicated movements are executed. These findings are in alignment with previous observations that vomiting occurs less readily than nausea during motion sickness (39).
Although conflicting sensory information from different vestibular end organs can induce motion sickness (6, 37), the condition typically results from conflicting information from the visual and vestibular systems regarding spatial orientation, particularly when these inputs deviate from those expected based on previous experience (46). It is unknown where this sensory integration occurs in the brain and whether the sensory conflict signal has differential effects on neural regions that coordinate nausea and vomiting. Recordings in conscious animals will be required to determine whether neurons in the nausea-generating pathway, including those in the parabrachial nucleus, are preferentially activated over those in the emetic pathway when vestibular-visual sensory conflicts are present.
For neurons in both the LTF and parabrachial nuclei (54), the gain of responses to vestibular stimulation of units receiving gastrointestinal inputs was appreciably altered when copper sulfate was present in the stomach, with the responses of some cells being attenuated and those of others being strengthened. These findings raise the prospect that the LTF and parabrachial nuclei receive inputs from a common brain region that integrates vestibular and gastrointestinal signals. Nucleus tractus solitarius (NTS) relays visceral signals to both the parabrachial nuclei and LTF (10, 23, 24, 31, 32, 34, 40, 44, 45, 56) and additionally receives inputs from the vestibular nuclei (53, 60). However, the responses to vestibular stimulation of neurons in NTS were relatively unaffected by the administration of copper sulfate (53), such that the NTS neuronal responses do not mirror those observed in the parabrachial nuclei and LTF. The parabrachial nuclei have reciprocal connections with LTF (24), which could be at least partially responsible for the characteristics of the responses to gastrointestinal and vestibular stimulation in the two areas. For example, convergence of inputs from multiple parabrachial nucleus neurons onto single LTF neurons could generate the STC responses noted in the latter area, whose expression may be dependent on whether gastrointestinal inputs are also present. Another possibility is that the responses of LTF neurons to combined labyrinthine and gastrointestinal inputs reflect the integration of signals directly from the vestibular nuclei. The caudal aspect of the vestibular nucleus complex that projects to the LTF (59) receives disynaptic inputs from NTS (28), and neurons located in this region respond to electrical stimulation of the vagus nerve (29). However, it has yet to be determined whether gastrointestinal inputs modulate the processing of vestibular signals in the caudal vestibular nuclei, as has been observed in the LTF and parabrachial nuclei (54). Consequently, additional experiments will be needed to ascertain the brain regions that contribute to shaping the responses of LTF neurons to combined stimulation of gastrointestinal and vestibular receptors.
In addition to coordinating vomiting, the LTF participates in regulating sympathetic nerve activity and blood pressure in felines (7–9, 42, 43). Most neurons in the RVLM of decerebrate cats that received baroreceptor inputs had graviceptive responses to vestibular stimulation (15), like a subset of LTF neurons that we identified in the current study. However, the response vector orientations differed between the two populations of neurons, with a larger fraction of units in the RVLM having a response vector orientation near nose-up pitch. Neurons in brain regions other than the LTF, including the caudal vestibular nuclei (25) and caudal ventrolateral medulla (52), participate in relaying labyrinthine signals to the RVLM. Thus, it is likely that the responses of neurons in the RVLM to vestibular stimulation reflect the integration of inputs from multiple sources. The role in cardiovascular regulation of LTF neurons with STC and other complex responses during rotations of the body in vertical planes is less clear. One possibility is that these neurons could participate in triggering the cardiovascular responses that typically accompany motion sickness (39), although additional studies will be needed to test this hypothesis.
Potential problems and pitfalls.
During data analysis, we verified that the shape and amplitude of action potentials had not changed systematically during rotations of the animal or during and following copper sulfate administration or carotid sinus stretch, such that counts of neuronal firing were inaccurate. We also excluded neurons from the sample if there were any concerns that movement of the brain stem during respiration or alterations in blood pressure affected the fidelity of the data collected from the cells. Administration of copper sulfate often elicited transient changes in blood pressure, as shown in Fig. 1, C and D. However, changes in spontaneous neuronal activity resulting from the administration of the drug usually developed after a minute or more, and they were not synchronized with the blood pressure fluctuations. Furthermore, over half of the cells whose spontaneous activity was modified by copper sulfate administration failed to respond to mechanical stretch of the carotid sinus, which should have provided a powerful stimulus to baroreceptors. Consequently, we are confident that changes in neuronal activity elicited by copper sulfate administration were not triggered by fluctuations in blood pressure.
Since copper sulfate is a potent emetic agent (53, 54, 55), we assumed that prolonged changes in the firing rate of an LTF neuron after the intragastric administration of this drug indicated that the cell participated in the production of vomiting. However, this conclusion is not certain, since copper sulfate irritates the stomach lining and thus provides a noxious stimulus. Thus, it is impossible to definitely ascertain whether LTF neuronal responses to copper sulfate administration were due to the noxious or emetic qualities of the compound.
Another potential concern is that fluid shifts and the consequent activation of baroreceptors during whole body rotations could have resulted in the modulation of the firing rate of LTF neurons. However, we previously demonstrated that the small-amplitude tilts used in this study usually do not result in blood pressure alterations (53, 54). Ligation of the common carotid arteries, as part of the experimental protocol also diminished the possibility that baroreceptors were activated by whole body tilts, since both carotid sinuses were isolated from the blood supply. Carotid artery ligation in these studies could also explain why only two neurons in our sample exhibited cardiac-related firing patterns. In addition, most neurons responded as well or better to higher-frequency and lower-amplitude rotations than to lower-frequency and larger-amplitude tilts. The latter movements should have provided a better opportunity for fluid displacements to occur in the body, and thus for the stimulation of baroreceptors. It is, therefore, unlikely that LTF neuronal responses to whole-body rotations resulted from the stimulation of cardiovascular receptors.
The region of the LTF defined by Barman and colleagues (7, 8, 9, 42, 43) as regulating sympathetic activity and blood pressure is concentrated ventral and caudal to Borison and Wang's “vomiting center” (13). The focus of the present study on the “vomiting center” might be another explanation for why so few neurons in our sample had a cardiac-related firing pattern. It is also possible that neurons in the ventral and caudal area of the LTF have somewhat different responses to vestibular stimulation than the units studied in these experiments. Additional work will be needed to explore this possibility.
Perspectives and Significance
This study is the first to consider the integrative effects of vestibular and visceral inputs on the activity of neurons in the LTF, a brain region that participates in both the regulation of blood pressure and the production of vomiting. We demonstrated that although many LTF neurons have simple graviceptive responses to vestibular stimulation, the responses of almost half were complex, with one-third of the cells exhibiting pronounced STC behavior. These complex responses likely arise from the convergence of inputs from multiple vestibular receptors, such that the neurons would only respond maximally during complex head movements that engage a variety of vestibular end organs. Accordingly, the responses of many LTF units to rotations in vertical planes are appropriate for neurons that elicit vomiting associated with motion sickness (39, 46). LTF neurons with baroreceptor inputs and complex responses to vestibular stimulation may participate in producing the cardiovascular components of motion sickness, such as the changes in skin blood flow that result in pallor (39). The gain of responses to vestibular stimulation of LTF neurons with gastrointestinal inputs was altered by the intragastric placement of the emetic compound copper sulfate, showing that the presence of one emetic input affects the processing of another. A similar gating of vestibular signals by gastrointestinal inputs occurs in the parabrachial nucleus (54), which participates in the generation of nausea (3, 4). These observations suggest that although separate brain pathways are responsible for the production of nausea and vomiting, they are activated under similar conditions. However, during motion sickness, nausea and affective responses almost always precede vomiting (39), indicating that the nausea pathway is activated preferentially. Often, vomiting is absent during a bout of motion sickness that results in severe malaise and affective responses, although in other instances, it occurs soon after motion sickness is triggered (39). Further studies conducted in conscious animal models will be needed to discern the neural mechanisms responsible for the relative timing of nausea and vomiting during motion sickness and other conditions such as poisoning that evoke emetic responses.
GRANTS
This work was supported by Grant R01-DC03732 from the National Institute on Deafness and Other Communication Disorders (NIDCD) of the National Institutes of Heath (NIH). Jennifer Moy was supported by NIH, NIDCD Training Grant T32 DC000066.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.D.M., D.J.M., M.F.C., B.M.B., S.W.O., L.A.C., B.J.Y., and A.A.M. performed experiments; J.D.M., D.J.M., M.F.C., B.M.B., S.W.O., L.A.C., B.J.Y., and A.A.M. analyzed data; J.D.M., B.J.Y., and A.A.M. interpreted results of experiments; J.D.M., D.J.M., M.F.C., B.M.B., S.W.O., B.J.Y., and A.A.M. edited and revised manuscript; J.D.M., D.J.M., M.F.C., B.M.B., S.W.O., L.A.C., B.J.Y., and A.A.M. approved final version of manuscript; B.J.Y. and A.A.M. conception and design of research; B.J.Y. prepared figures; B.J.Y. and A.A.M. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Michael Kashaf, Janera Koehler, Sonya Puterbaugh, Nevin Sastry, Kunal Shah, Ryan Toth, and Mike Yoder, and for assistance in data collection and analysis.
REFERENCES
- 1.Anderson JH, Blanks RHI, Precht W. Response characteristics of semicircular canal and otolith systems in the cat. I Dynamic responses of primary vestibular fibers. Exp Brain Res 32: 491–507, 1978 [DOI] [PubMed] [Google Scholar]
- 2.Baker J, Goldberg J, Hermann G, Peterson B. Spatial and temporal response properties of secondary neurons that receive convergent input in vestibular nuclei of alert cats. Brain Res 294: 138–143, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Balaban CD. Vestibular autonomic regulation (including motion sickness and the mechanism of vomiting). Curr Opin Neurol 12: 29–33, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Balaban CD. Vestibular nucleus projections to the parabrachial nucleus in rabbits: implications for vestibular influences on the autonomic nervous system. Exp Brain Res 108: 367–381, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Balaban CD, Jacob RG, Furman JM. Neurologic bases for comorbidity of balance disorders, anxiety disorders and migraine: neurotherapeutic implications. Exp Rev Neurother 11: 379–394, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bard P, Woolsey CN, Snider RS, Mountcastle VB, Bromiley RB. Delimitation of central nervous mechanisms involved in motion sickness. Fed Proc 6: 72, 1947 [PubMed] [Google Scholar]
- 7.Barman SM, Gebber GL. Lateral tegmental field neurons of cat medulla: a source of basal activity of ventrolateral medullospinal sympathoexcitatory neurons. J Neurophysiol 57: 1410–1424, 1987 [DOI] [PubMed] [Google Scholar]
- 8.Barman SM, Gebber GL, Orer HS. Medullary lateral tegmental field: an important source of basal sympathetic nerve discharge in the cat. Am J Physiol Regul Integr Comp Physiol 278: R995–R1004, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Barman SM, Orer HS. Rostral ventrolateral medullary but not medullary lateral tegmental field neurons mediate sympatho-sympathetic reflexes in cats. Am J Physiol Regul Integr Comp Physiol 299: R1269–R1278, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckstead RM, Morse JR, Norgren R. The nucleus of the solitary tract in the monkey: projections to the thalamus and brain stem nuclei. J Comp Neurol 190: 259–282, 1980 [DOI] [PubMed] [Google Scholar]
- 11.Berman AI. The Brain Stem of the Cat. Madison, WI: University of Wisconsin, 1968 [Google Scholar]
- 12.Billig I, Yates BJ, Rinaman L. Plasma hormone levels and central c-Fos expression in ferrets after systemic administration of cholecystokinin. Am J Physiol Regul Integr Comp Physiol 281: R1243–R1255, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Borison HL, Wang SC. Functional localization of central coordinating mechanism for emesis in cat. J Neurophysiol 12: 305–313, 1949 [DOI] [PubMed] [Google Scholar]
- 14.Cechetto DF. Central representation of visceral function. Fed Proc 46: 17–23, 1987 [PubMed] [Google Scholar]
- 15.Destefino VJ, Reighard DA, Sugiyama Y, Suzuki T, Cotter LA, Larson MG, Gandhi NJ, Barman SM, Yates BJ. Responses of neurons in the rostral ventrolateral medulla (RVLM) to whole-body rotations: comparisons in decerebrate and conscious cats. J Appl Physiol 110: 1699–1707, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Endo K, Thomson DB, Wilson VJ, Yamaguchi T, Yates BJ. Vertical vestibular input to and projections from the caudal parts of the vestibular nuclei of the decerebrate cat. J Neurophysiol 74: 428–436, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. III. Response dynamics. J Neurophysiol 39: 996–1008, 1976 [DOI] [PubMed] [Google Scholar]
- 18.Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol 34: 661–675, 1971 [DOI] [PubMed] [Google Scholar]
- 19.Fukuda H, Koga T. The Bötzinger complex as the pattern generator for retching and vomiting in the dog. Neurosci Res 12: 471–485, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Fukuda H, Koga T. Non-respiratory neurons in the Bötzinger complex exhibiting appropriate firing patterns to generate the emetic act in dogs. Neurosci Res 14: 180–194, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Gallo M, Marquez SL, Ballesteros MA, Maldonado A. Functional blockade of the parabrachial area by tetrodotoxin disrupts the acquisition of conditioned taste aversion induced by motion-sickness in rats. Neurosci Lett 265: 57–60, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Gieroba ZJ, Blessing WW. Vasopressin secretion after stimulation of abdominal vagus in rabbit: role of A1 norepinephrine neurons. Am J Physiol Regul Integr Comp Physiol 266: R1885–R1890, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Granata AR. Ascending and descending convergent inputs to neurons in the nucleus parabrachialis of the rat: an intracellular study. Brain Res 600: 315–321, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol 293: 540–580, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Holstein GR, Friedrich VL, Jr, Kang T, Kukielka E, Martinelli GP. Direct projections from the caudal vestibular nuclei to the ventrolateral medulla in the rat. Neuroscience 175: 104–117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito H, Nishibayashi M, Kawabata K, Maeda S, Seki M, Ebukuro S. Induction of Fos protein in neurons in the medulla oblongata after motion- and X-irradiation-induced emesis in musk shrews (Suncus murinus). Auton Neurosci 107: 1–8, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Ito H, Nishibayashi M, Maeda S, Seki M, Ebukuro S. Emetic responses and neural activity in young musk shrews during the breast-feeding/weaning period: comparison between the high and low emetic response strains using a shaking stimulus. Exp Anim 54: 301–307, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Jian BJ, Acernese AW, Lorenzo J, Card JP, Yates BJ. Afferent pathways to the region of the vestibular nuclei that participates in cardiovascular and respiratory control. Brain Res 1044: 241–250, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Jian BJ, Shintani T, Emanuel BA, Yates BJ. Convergence of limb, visceral, and vertical semicircular canal or otolith inputs onto vestibular nucleus neurons. Exp Brain Res 144: 247–257, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Kasper J, Schor RH, Wilson VJ. Response of vestibular neurons to head rotations in vertical planes. I. Response to vestibular stimulation. J Neurophysiol 60: 1753–1764, 1988 [DOI] [PubMed] [Google Scholar]
- 31.King GW. Topology of ascending brainstem projections to nucleus parabrachialis in the cat. J Comp Neurol 191: 615–638, 1980 [DOI] [PubMed] [Google Scholar]
- 32.Kobashi M, Adachi A. Projection of nucleus tractus solitarius units influenced by hepatoportal afferent signal to parabrachial nucleus. J Auton Nerv Syst 16: 153–158, 1986 [DOI] [PubMed] [Google Scholar]
- 33.Koga T, Qu R, Fukuda H. The central pattern generator for vomiting may exist in the reticular area dorsomedial to the retrofacial nucleus in dogs. Exp Brain Res 118: 139–147, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Leslie RA, Gwyn DG. Neuronal connections of the area postrema. Fed Proc 43: 2941–2943, 1984 [PubMed] [Google Scholar]
- 35.Miller AD, Nonaka S, Jakus J. Brain areas essential or non-essential for emesis. Brain Res 647: 255–264, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Miller AD, Ruggiero DA. Emetic reflex are revealed by expression of the immediate-early gene c-Fos in the cat. J Neurosci 14: 871–888, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller AD, Wilson VJ. Vestibular-induced vomiting after vestibulocerebellar lesions. Brain Behav Evol 23: 26–31, 1983 [DOI] [PubMed] [Google Scholar]
- 38.Miller DM, Cotter LA, Gandhi NJ, Schor RH, Cass SP, Huff NO, Raj SG, Shulman JA, Yates BJ. Responses of caudal vestibular nucleus neurons of conscious cats to rotations in vertical planes, before and after a bilateral vestibular neurectomy. Exp Brain Res 188: 175–186, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Money KE. Motion sickness. Physiol Rev 50: 1–39, 1970 [DOI] [PubMed] [Google Scholar]
- 40.Norgren R. Projections from the nucleus of the solitary tract in the rat. Neuroscience 3: 207–218, 1978 [DOI] [PubMed] [Google Scholar]
- 41.Onishi T, Mori T, Yanagihara M, Furukawa N, Fukuda H. Similarities of the neuronal circuit for the induction of fictive vomiting between ferrets and dogs. Auton Neurosci 136: 20–30, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Orer HS, Barman SM, Gebber GL, Sykes SM. Medullary lateral tegmental field: an important synaptic relay in the baroreceptor reflex pathway of the cat. Am J Physiol Regul Integr Comp Physiol 277: R1462–R1475, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Orer HS, Gebber GL, Phillips SW, Barman SM. Role of the medullary lateral tegmental field in reflex-mediated sympathoexcitation in cats. Am J Physiol Regul Integr Comp Physiol 286: R451–R464, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Otake K, Ezure K, Lipski J, Wong She RB. Projections from the commissural subnucleus of the nucleus of the solitary tract: an anterograde tracing study in the cat. J Comp Neurol 324: 365–378, 1992 [DOI] [PubMed] [Google Scholar]
- 45.Papas S, Ferguson AV. Electrophysiological characterization of reciprocal connections between the parabrachial nucleus and the area postrema in the rat. Brain Res Bull 24: 577–582, 1990 [DOI] [PubMed] [Google Scholar]
- 46.Reason J. Motion sickness: Some theoretical and practical considerations. Appl Ergonom 9: 163–167, 1978 [DOI] [PubMed] [Google Scholar]
- 47.Sakai N, Yamamoto T. Conditioned taste aversion and c-fos expression in the rat brainstem after administration of various USs. NeuroReport 8: 2215–2220, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Sakai N, Yamamoto T. Role of the medial and lateral parabrachial nucleus in acquisition and retention of conditioned taste aversion in rats. Behav Brain Res 93: 63–70, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Scalera G, Spector AC, Norgren R. Excitotoxic lesions of the parabrachial nuclei prevent conditioned taste aversions and sodium appetite in rats. Behav Neurosci 109: 997–1008, 1995 [PubMed] [Google Scholar]
- 50.Schor RH, Angelaki DE. The algebra of neural response vectors. Ann NY Acad Sci 656: 190–204, 1992 [DOI] [PubMed] [Google Scholar]
- 51.Schor RH, Miller AD, Tomko DL. Responses to head tilt in cat central vestibular neurons. I. Direction of maximum sensitivity. J Neurophysiol 51: 136–146, 1984 [DOI] [PubMed] [Google Scholar]
- 52.Steinbacher BC, Yates BJ. Processing of vestibular and other inputs by the caudal ventrolateral medullary reticular formation. Am J Physiol Regul Integr Comp Physiol 271: R1070–R1077, 1996 [DOI] [PubMed] [Google Scholar]
- 53.Sugiyama Y, Suzuki T, Destefino VJ, Yates BJ. Integrative responses of neurons in nucleus tractus solitarius to visceral afferent stimulation and vestibular stimulation in vertical planes. Am J Physiol Regul Integr Comp Physiol 301: R1380–R1390, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki T, Sugiyama Y, Yates BJ. Integrative responses of neurons in parabrachial nuclei to a nauseogenic gastrointestinal stimulus and vestibular stimulation in vertical planes. Am J Physiol Regul Integr Comp Physiol 302: R965–R975, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang SC, Borison HL. The vomiting center; its destruction by radon implantation in dog medulla oblongata. Am J Physiol 166: 712–717, 1951 [DOI] [PubMed] [Google Scholar]
- 56.Williams JB, Murphy DM, Reynolds KE, Welch SJ, King MS. Demonstration of a bilateral projection from the rostral nucleus of the solitary tract to the medial parabrachial nucleus in rat. Brain Res 737: 231–237, 1996 [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto T, Shimura T, Sako N, Azuma S, Bai WZ, Wakisaka S. C-fos expression in the rat brain after intraperitoneal injection of lithium chloride. NeuroReport 3: 1049–1052, 1992 [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto T, Shimura T, Sako N, Yasoshima Y, Sakai N. Neural substrates for conditioned taste aversion in the rat. Behav Brain Res 65: 123–137, 1994 [DOI] [PubMed] [Google Scholar]
- 59.Yates BJ, Balaban CD, Miller AD, Endo K, Yamaguchi Y. Vestibular inputs to the lateral tegmental field of the cat: potential role in autonomic control. Brain Res 689: 197–206, 1995 [DOI] [PubMed] [Google Scholar]
- 60.Yates BJ, Grelot L, Kerman IA, Balaban CD, Jakus J, Miller AD. Organization of vestibular inputs to nucleus tractus solitarius and adjacent structures in cat brain stem. Am J Physiol Regul Integr Comp Physiol 267: R974–R983, 1994 [DOI] [PubMed] [Google Scholar]
- 61.Yates BJ, Jian BJ, Cotter LA, Cass SP. Responses of vestibular nucleus neurons to tilt following chronic bilateral removal of vestibular inputs. Exp Brain Res 130: 151–158, 2000 [DOI] [PubMed] [Google Scholar]