Abstract
Apolipoprotein E (apoE) is a 34-kDa glycoprotein that is important in lipoprotein metabolism both peripherally and centrally. Because it is primarily produced in the liver, apoE observed in the brain or cerebrospinal fluid (CSF) could have originated in the periphery; i.e., circulating apoE may cross the blood-brain barrier (BBB) and/or enter CSF and be taken up by brain cells. To determine whether this occurs, a second-generation adenovirus encoding human apoE3 was administered intravenously (iv) to C57BL/6J mice, and the detection of human apoE3 in the CSF was used as a surrogate measure of central availability of this protein utilizing an improved method for sampling CSF from mice. This improved technique collects mouse CSF samples with a 92% success rate and consistently yields relatively large volumes of CSF with a very low rate of blood contamination, as determined by molecular assessment of apolipoprotein B, a plasma-derived protein that is absent in the central nervous system. Through this improved method, we demonstrated that in mice receiving the administered apoE3 adenovirus, human apoE3 was expressed at high levels in the liver, leading to high levels of human apoE3 in mouse plasma. In contrast, human apoE3 levels in the CSF, as assessed by a sensitive ELISA, were essentially undetectable in human apoE3 adenovirus-treated mice, and comparable to levels in LacZ adenovirus-treated control mice. These data indicate that apoE in the CSF cannot be derived from the plasma pool and, therefore, must be synthesized locally in the brain.
Keywords: cerebrospinal fluid collection, cisternum magnum, adenovirus, apolipoprotein E
apolipoprotein e (apoe) is a 299-amino acid protein with a 34-kDa molecular weight (33). Although it is synthesized in several areas of the body, the liver accounts for most circulating apoE (19). ApoE plays a key role in lipid transport and lipoprotein metabolism (18). It mediates the uptake and degradation of chylomicron and very low-density lipoprotein remnants by acting as a ligand for the low-density lipoprotein (LDL) receptor and the LDL receptor-related protein 1 (LRP1) (1, 18). Because apoE is involved directly in the uptake and distribution of plasma lipids, it is not surprising that it has been implicated in cardiovascular disease, and apoE deficiency is associated with high serum cholesterol and triacylglycerol levels and leads to premature artherosclerosis (23).
ApoE is also present in the brain. Central apoE is the most abundant component of HDL-like lipoproteins in the cerebrospinal fluid (CSF) (22), and it has a pivotal role in brain lipid transport and the maintenance of cell membranes, myelin, and neural networks under normal conditions (3, 11). Recently, we demonstrated that central apoE is important in the control of food intake and body weight (27, 28), and these effects might be mediated by the LRP1 receptor (16). Because apoE is present in both blood and CSF, any apoE found in the brain could have originated in the periphery; i.e., circulating apoE may cross the blood-brain barrier (BBB) and/or enter CSF and be taken up by brain cells. One of main goals of the current studies was to determine whether apoE enters the brain from the blood as assessed by changes in CSF levels when its plasma levels are increased.
While murine apoE is monomorphic, apoE occurs in three major common isoforms in humans. ApoE3 (112 Cys, 158 Arg) is the most common isoform and is considered the wild type. ApoE4 (112 Arg, 158 Arg) and apoE2 (112 Cys, 158 Cys) differ from apoE3 by single amino acid substitutions. The homology of mouse and human apoE is ∼70%, but human and mouse apoE have similar molecular weights (34 vs. 33 kDa) (35). We used a second-generation adenovirus to express human apoE3 uniquely in the liver of C57BL/6J mice. When human apoE3 is induced in mouse liver, it is secreted into the blood, and its levels can be assessed differentially from native mouse apoE. If this human apoE3 appears in the CSF, it is, therefore, likely that it came from the circulation, either directly via passage across the choroid plexus or indirectly by passage through the BBB followed by diffusion/convection transport from the brain interstitial fluid to the CSF (26). We reasoned that assessing the CSF for human apoE3 in apoE3 adenovirus-treated mice would enable us to ascertain possible transport. If both the plasma and CSF have human apoE3 protein, and synthesis is restricted to the liver, it would imply that the apoE enters the brain from the blood. If, on the other hand, human apoE3 is only present in blood but not present in the CSF, it would imply that apoE does not cross the BBB and that native mouse apoE that is found in the CSF is likely made within the brain.
Key to such experiments is the ability to obtain CSF samples from mice that have absolutely no blood contamination. Because mice are small, making it difficult to obtain blood-free CSF samples in sufficient quantities for assessing its constituents, a reliable approach had to be devised. Therefore, we modified a technique for obtaining relatively large samples and developed a novel method to screen CSF samples for blood contamination. Using this improved technique, we demonstrated that the human apoE3 induced by adenovirus does not exist in mouse CSF, indicating that apoE is not able to cross the BBB, and the central apoE is produced within the brain.
MATERIALS AND METHODS
Animals.
Adult male C57BL/6J mice (22–28 g, Jackson Laboratory, Bar Harbor, ME) were housed in a temperature- and humidity-controlled vivarium on a 12:12-h light-dark cycle (lights on at 0600) with ad libitum access to pelleted laboratory chow (Teklad 7912; Madison, WI) and water. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati.
Materials.
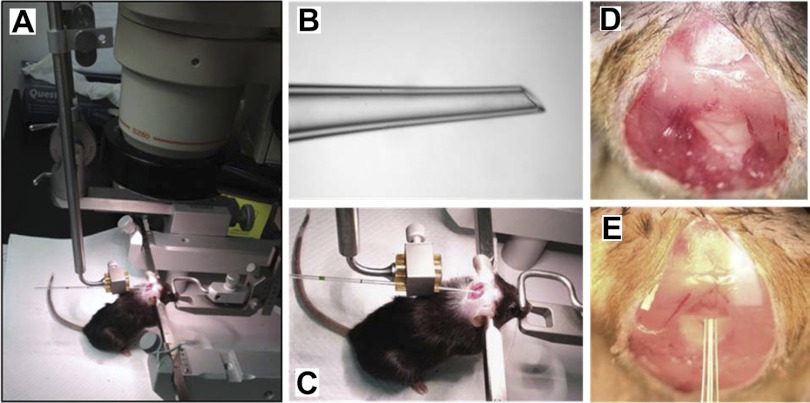
A second-generation recombinant adenovirus encoding human apoE3 (AdhapoE3) was constructed by Tsukamoto et al. (31). Previous studies have demonstrated that this adenovirus sustains expression of human apoE3 uniquely in the liver of apoE-deficient mice (31, 32). An electrode holder with a 90° bend at its distal end (model 1769, Kopf, Tujunga, CA) (Fig. 1A) was attached to a rat stereotaxic instrument (Stoelting, Wood Dale, IL). A glass capillary tube (cat. no. 53508–466; VWR, Radnor, PA) was heated and pulled manually. The tapered end of the glass capillary had an approximate diameter of 0.5 mm and was smoothed with a fine whetstone to provide a sharp tip (Fig. 1B). The rabbit polyclonal antibody against rodent anti-apoB, reported previously (17), was obtained from Dr. Patrick Tso's laboratory. The chemicals were purchased from Sigma (St. Louis, MO).
Fig. 1.
A: photo of the stereotaxic instrument with the electrode holder bent at 90° at its distal end. B: an amplified view of the tapered and sharpened tip of the capillary tube. C: position of the mouse during the procedure. The incisor bar in the stereotaxic device is positioned so that the mouse's neck is maximally ventroflexed. D and E: Close-up views of the membrane of exposed cisternum magnum before and after penetration of the capillary tube.
Procedures.
The CSF sampling procedure is a modification and improvement of a previously reported technique (14), and we additionally included a sensitive molecular method for identifying and thus discarding any blood-contaminated samples. To collect CSF, a straight, pulled, and sharpened glass capillary tube was mounted onto the electrode holder and adjusted, so that it was perpendicular to the ear bars and thus parallel to the long axis of the stereotaxic apparatus (Fig. 1A). Each mouse was anesthetized with intraperitoneal (ip) ketamine (87 mg/kg body wt) plus xylazine (13 mg/kg), and the area between the shoulders and below the skull was shaved. The mouse was then placed prone on the stereotaxic instrument and secured in the ear bars. The incisor bar was adjusted to the lowest possible position so that the mouse's head was maximally ventroflexed (Fig. 1C).
The surgical site was swabbed with 10% povidone, followed by 70% ethanol. Under a dissection microscope, a midsagittal incision of the skin, ∼1.0 cm in length, was made posterior to the occipital crest. The subcutaneous tissue and occipital muscles were carefully separated by blunt dissection with cotton swabs to expose the glistening surface of the dura covering the cisternum magnum (Fig. 1D). The surrounding area was then gently cleaned with sterile saline-soaked cotton swabs to remove any residual blood or interstitial fluid. The glass capillary tube was then advanced slowly in an anterior direction by the worm-gear-driven drives. When it reached the surface of the membrane, increased resistance occurred as the tip of the capillary slowly penetrated the dura mater, and the resistance diminished as the capillary entered the cisternum magnum by a fraction of 1 mm. CSF flow was then spontaneously initiated, and CSF was collected by capillary action. This method typically allowed collection of 10–15 μl of CSF within a 3-min period when it was used as a terminal procedure, and the CSF was transferred to a microtube and placed on dry ice.
Western blot of mouse apoB.
Two-fold serial dilutions of plasma, corresponding to 2.5–0.078% plasma in water (vol/vol), were prepared as standards using mouse plasma. Twenty microliters of the standards and individual mouse CSF samples (5 μl, 1:4 dilution) were heat-denatured, and separated by 4 to 15% SDS-PAGE gradient gels (Bio-Rad Life Sciences, Hercules, CA) and then electro-transferred to PVDF membranes (Millipore, Bedford, MA). The membranes were then incubated with a polyclonal rabbit anti-apoB antibody (1:5,000 dilution) (17) overnight at 4°C with gentle shaking. After washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (1:10,000, Dako, Carpinteria, CA). The amount of immune complexes was quantified using an enhanced chemiluminescence detection system (Millipore, Billerica, MA). The reacted membranes were exposed to X-ray film (Kodak Scientific, Rochester, NY).
Method to assess circulating human apoE3 in CSF.
Mice were divided into two groups (n = 10 mice/group) with equal body weight. Via intravenous (iv) administration at the retroorbital sinus (4), group 1 received 100 μl of AdhapoE3 (1 × 1010 particles/ml), and group 2 received 100 μl of control adenovirus encoding LacZ cDNA (AdLacZ, 1 × 1010 particles/ml). Three days after intravenous injection, CSF was withdrawn. The still-anesthetized mice were then killed, and their blood and a piece of liver were collected. The CSF, plasma, and liver were stored at −80°C for subsequent molecular studies.
Measurements of human apoE3 in plasma and CSF by ELISA.
Human apoE3 levels in both plasma and CSF were determined by a sandwich ELISA from Mabtech (Cincinnati, OH). Briefly, plasma and CSF samples were diluted 1:1,000 and 1:50, respectively. Maxisorp 96-well plates (Nunc, Rochester, NY) were coated with mAB-E276 (1:250) overnight. Samples were then added and sandwiched with antibody mAb-E887-biotin (1:500) for 1 h. Streptavidin-ALP was then added and detected by adding p-nitrophenyl phosphate, disodium salt (PNPP) (Thermo Fisher Scientific, Rockford, IL), and the absorbance was measured at 405 nm.
Western blot of human apoE3 in mouse liver.
Eighty micrograms total protein extracted from mouse liver was heat-denatured, and separated by 4 to 15% SDS-PAGE gradient gels and then electro-transferred to PVDF membranes (Millipore). After incubating in blocking buffer, membranes were incubated with a polyclonal rabbit anti-human apoE antibody (1:1,000, Dako) overnight at 4°C with gentle shaking. After washing with PBS, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (1:5,000; Dako). The amount of immune complexes was quantified using an enhanced chemiluminescence detection system (Millipore). The reacted membranes were exposed to X-ray film (Kodak Scientific).
Statistics.
All data are presented as means ± SE. Differences between sets of data were determined using Student's t-test for comparison of treatments. P values less than 0.05 were considered statistically significant.
RESULTS
CSF sampling procedure parameters.
As depicted in Fig. 1, a straight and sharpened glass capillary tube was mounted onto the electrode holder and adjusted by the worm-gear-driven drives of stereotaxic instrument to penetrate the cisternum magnum. This method typically allowed collection of 10–15 μl of CSF within 6 min per mouse, including 3 min from the time of insertion of the tip of capillary tube to completion of CSF sampling. The temporal limitation of 3 min was imposed to minimize the possibility that continued drainage of the CSF might affect its composition.
Determination of blood contamination in CSF samples.
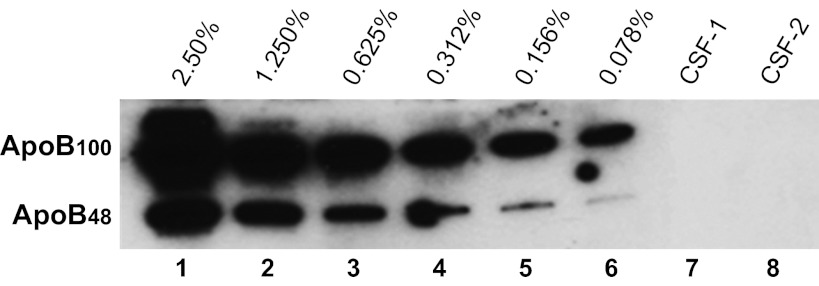
CSF was collected from 86 mice. By visual inspection, more than 97% of the CSF samples were clear; i.e., appeared to have no blood contamination. This was further evaluated using Western blotting for apoB, a protein found in high concentration in plasma but absent in the central nervous system (CNS) (29). We conducted Western blot analysis using plasma diluted in water, spanning a range of concentrations corresponding to 2.5–0.078%, as standards. As depicted in Fig. 2, this immunoassay was able to detect apoB levels as low as 0.078% in aqueous solutions. The lack of apoB-immunoreactive bands in the two depicted CSF samples (Fig. 2) indicates the absence of detectable plasma contamination. Any CSF samples with detectable apoB were not used (around 5% of samples).
Fig. 2.
Determination of apolipoprotein B (apoB) levels by Western blot analysis. Twofold serial dilutions of plasma, corresponding to 2.5–0.078% plasma in water (vol/vol), were prepared as standards using the plasma from one mouse. These standards were run in parallel. A level as low as 0.078% blood contamination (lane 6) was observed as clear bands. Two cerebrospinal fluid (CSF) samples (lanes 7 and 8, 1:4 dilution, 20 μl/lane) from two individual mice were used as examples to determine potential blood contamination. No apoB-immunoreactive bands (apoB100 and apoB48) were observed, indicating no blood contamination in either CSF sample.
Liver is the primary organ expressing the human apoE3.
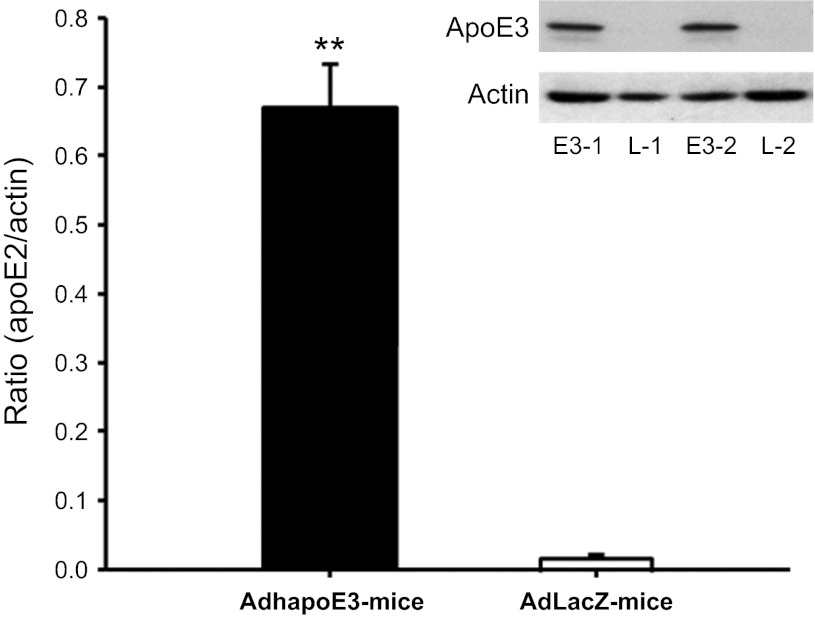
To confirm the expression of the human apoE3 in vivo, total protein was extracted from the livers, and the contents of human apoE3 were determined by Western blot analysis. As depicted in Fig. 3, the administration of AdhapoE3 induced significant expression of human apoE3 in mouse liver. The human apoE3 bands were observed only in the liver of AdhapoE3-treated mice, and almost none was detected in that of AdLacZ-treated controls. These data are consistent with a previous report (31, 32) and confirm in vivo expression of the expected human apoE3 protein in mouse liver.
Fig. 3.
Intravenous administration of adenovirus encoding human apoE3 protein (AdhapoE3) induced a high level of human apoE3 in mouse liver, when compared with adenovirus encoding LacZ cDNA (AdLacZ). Representative bands from Western blot revealed that human apoE3 was in the liver of AdhapoE3-treated mice (E3–1 and E3–2) but not in that of AdLacZ-treated controls (L-1 and L-2). Values are expressed as means ± SE; n = 4–5. **P < 0.01, compared with AdLacZ-treated mice.
Human apoE3 levels in plasma and CSF following intravenous adenovirus administration.
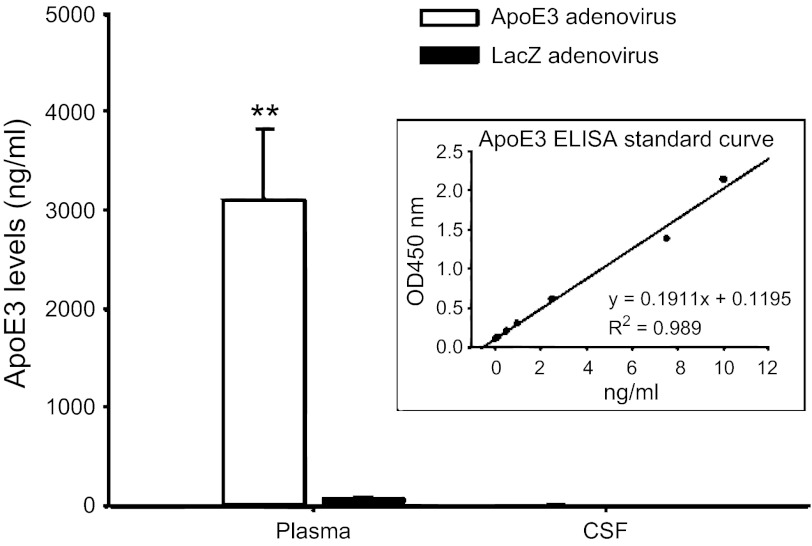
As depicted in Fig. 4, high levels of human apoE3 in plasma were found in AdhapoE3-treated mice, compared with AdLacZ-treated controls. In contrast, human apoE3 levels in CSF were low, and there was no significant difference in CSF apoE3 levels between AdhapoE3- and AdLacZ-treated mice (4.8 ± 0.61 vs. 3.31 ± 0.29 ng/ml). The box within Fig. 3 depicts our ELISA standard curve. Such extremely low content of apoE3 in the CSF could be considered as background level because normal native mouse apoE in CSF was reported more than 1,100 ng/ml (34).
Fig. 4.
Intravenous administration of adenovirus encoding human apoE3 protein induced a high level of human apoE3 in plasma, but not in CSF, when compared with LacZ adenovirus. Values are expressed as means ± SE; n = 9–10, **P < 0.01, compared with LacZ adenovirus-treated mice. The box within the figure depicts the ELISA standard curve.
DISCUSSION
CSF is frequently analyzed in clinical populations for assessing biomarkers of drug efficacy or disease progression in the CNS (12). However, obtaining CSF from preclinical species can be challenging because of small body sizes and, consequently, low volumes of CSF. In recent decades, the advances in the development of highly sensitive detection techniques, such as ELISA and RIA, have made it feasible to detect biological compounds in the CSF of small animals, including mice. Several methods for sampling CSF from the mouse have been reported (10, 14, 25). Compared with those methods, the techniques that we describe have proven to be superior in several aspects: 1) there is a higher success rate (92%); 2) larger volumes (up to 15 μl) of CSF can be obtained within a short operation window (6 min total; 3 min of actual sampling time); 3) there is a lower blood contamination rate (∼5%) of CSF samples; and 4) a molecular screen for apoB can be used to detect minute blood contamination of mouse CSF samples, allowing the ability to exclude blood-contaminated CSF samples and, thereby, avoid misinterpretation of experimental data.
The cisternum magnum has the largest volume of CSF in the CNS of the adult mouse. In 1971, Carp et al. (5) first introduced a method of withdrawing CSF from mice by means of a puncture of the cisternum magnum. After exposing the surface of the dura covering the cisternum magnum, the authors simply made a small hole in the membrane with the tip of a 26-gauge needle, and CSF was aspirated into a 20-RI pipette attached to a syringe (5). A critical pitfall that could lead to erroneous or invalid results was the contamination of the obtained CSF by residual blood and interstitial fluid in the surrounding area of the punched hole.
In 1983, Fleming et al. (10) modified this method by using a micropipette to puncture the membrane over the cisternum magnum (10). The micropipette was constructed from a 6–7 mm, 30-gauge needle that was inserted 4–5 mm into the lumen of a 10-μl micropipette, and the junction was sealed with a drop of epoxy resin. The needle-pipette assembly was then attached to the mouth suction apparatus supplied with the micropipettes. For sampling, a needle was inserted through the dura over the cisternum magnum, and the CSF was aspirated by gentle mouth suction (10). This technique was subsequently modified by Liu et al. (14) by using a 6-cm glass capillary to penetrate the dura and collect CSF. We started our present study with that technique. After several attempts, we realized that the manually conducted processes of collecting CSF samples had major shortcomings. First, the unstable penetration of a capillary tube with a hand and/or slight movement of the hand after penetration could puncture the fourth ventricle across the inferior medullary velum, which contains capillaries, ependyma, and myelinated axons (36). Puncture of this structure thus risks blood and tissue contamination of CSF. In fact, neither success rate nor blood contamination rate was reported in those papers (10, 14). Second, the manual stabilization of a capillary tube did not allow sampling CSF for a relatively long duration, leading to inconsistent amount of CSF collection. For these reasons, we considered improving the method. Eventually, we found that a probe holder attached to a stereotaxic instrument (Fig. 1A) allows the capillary tube to be held stably and precisely to penetrate the cisternum magnum. The capillary tube could then be maintained in the exact same position for as long a duration as needed. With such improvement, our success rate reached to 92%, and blood contamination rate was reduced to less than 5%. Therefore, this improved method is stable and has minimal adverse consequences.
CSF is derived in part from the extracellular fluid in the CNS, and it is segregated from plasma by the blood-CSF barrier. It is estimated that 80% of CSF proteins come from the plasma, but the protein concentration of whole blood is 200- to 400-fold higher than that in CSF (8, 30). Consequently, even a small proportion of blood contamination during CSF collection can dramatically alter the content profile of some constituents, leading to potential misinterpretation of the experimental data. Conventionally, blood contamination in CSF has been examined by visual observation, and some investigators simply used blood-contaminated CSF after centrifugation of the samples (6). However, those measurements are not sufficiently sensitive to assess a low level of blood contamination in CSF. Therefore, we used a highly sensitive molecular method to screen for potential blood contamination of the CSF by measuring apoB, a plasma-derived protein that is absent in the CNS (29). As depicted in Fig. 2, our detection limit reached as low as 0.0781% (vol/vol) blood contamination in CSF by Western blot analysis. When no apoB is detected, there is a high degree of confidence that the CSF sample is uncontaminated by blood. Although not tested in the current study, a few blood-specific, highly abundant proteins, e.g., hemoglobin, catalase, peroxiredoxin, and carbonic anhydrase I, could be similarly used as blood contamination markers, according to previous reports (21, 37).
Using these improved techniques, we asked whether circulating apoE crosses the BBB and enters CSF. ApoE is primarily produced in the liver of both humans and rodents (19). Roheim and colleagues (24) first reported that apoE is present in the CSF (24), and they suggested that it was either synthesized locally in the brain and then diffused into the CSF, or else that it crossed through the BBB from the plasma. We previously observed apoE mRNA in the hypothalamus (27), implying that at least a portion of the apoE protein present in the brain was actually synthesized in the brain (27), but those findings did not address the possibility that circulating apoE crosses the BBB.
Zlokovic and colleagues (20, 38) administered [125I]-labeled human apoE systemically to guinea pig and then measured radioactive counts of brain tissue. They reported that the apoE did not cross the BBB based on low radioactive counts in the brain. However, such a statement seems inconclusive because of the facts that 1) apoE is quickly metabolized in liver (9) and 2) the authors did not measure the [125I]-labeled apoE levels in the blood at the time when the animals were killed for determining [125I]-labeled radioactivity in homogenized brain tissue. Without knowing the concentration of [125I]-labeled apoE in blood, potential problems for their negative result could occur because of an insufficient amount of [125I]-labeled apoE3 was infused and/or the infused [125I]-labeled apoE3 was rapidly metabolized in the liver, leading to low level of [125I]-labeled apoE3 in the blood and undetectable levels in the brain. Therefore, those studies alone could not rule out the possibility that apoE crosses the BBB.
To address this, we used an animal model with sustained high levels of human apoE3 by adenovirus in the circulating system. This recombinant second-generation adenovirus encoding human apoE3 cDNA has previously been used for somatic, liver-directed gene transfer in mice (31, 32). When injected into mice, the viruses induce a high level of expression of the human apoE3 isoform in the liver within 1 day, and it is still expressed 3 mo after the injection (31). In the present study, we selected day 3 to collect the plasma and CSF samples because previous studies demonstrated that it was the time of peak expression and plasma levels (31).
Consistent with the previous report, there was a high level of human apoE3 in the plasma of AdhapoE3-treated mice relative to that in AdLacZ-treated controls. The liver samples from AdhapoE3-treated mice contained human apoE3 bands, whereas almost none was observed in the liver of AdLacZ-treated controls, confirming the in vivo expression of the human apoE3 protein (Fig. 3). There was a weak “background” in the LacZ control mice when human apoE3 in plasma was determined by ELISA (Fig. 4). This could be caused by 1) a minor cross-binding of the anti-human apoE3 antibody to native mouse apoE protein; and/or 2) an unknown protein induced by the adenovirus, which could nonspecifically interact with human apoE3 antibody at a very low level. Fortunately, this influence was trivial (<2.5%) in terms of the ratio of the background of LacZ control mice to apoE3 levels in the mice treated with human apoE adenovirus. Importantly, this was not an issue because human apoE3 level in the CSF was very low, as assessed by ELISA, and the human apoE3 levels in human apoE3 adenovirus-treated mice were comparable to that in LacZ adenovirus-treated mice (Fig. 4).
Human apoE induced by adenovirus has been broadly used to study endogenous apoE functions in mice (13, 31, 32). Besides having similar molecular size to mouse apoE, the human apoE3 induced by adenovirus in mice has been demonstrated functionally to interact with mouse apoE receptors, e.g., LDL receptor (LDLR) and LDL receptor-related protein 1 (LRP1) (13). Because its molecular weight is 34 kDa, apoE should theoretically be excluded from the CNS by the BBB. To carry the protein into the brain, a transporter would be required. In this case, the apoE receptor(s) are most likely to serve as potential transporters, just like leptin receptor α working as a transporter to carry leptin into the brain. If the human apoE3 could interact with mouse apoE receptors, it seems most likely that the apoE could be transported into mouse brain through the same or similar mechanisms if native mouse apoE could cross the BBB. Because there is no obvious relationship between human apoE3 levels in the plasma and the CSF, the pools of plasma human apoE3 and central apoE3 appear to be distinct, we, therefore, conclude that apoE identified in the brain and/or CSF is produced in the brain and is not derived from peripheral tissues.
Perspectives and Significance
An improved procedure for CSF sampling from the cisternum magnum in mice has been described. Compared with existing techniques, this procedure provides the advantages of consistently yielding large volumes of blood-free CSF and sensitive detection of blood contamination using an assay for apoB. This method could be valuable in studies determining the changes in the levels of specific constituents in the CSF of mice to reveal biological and functional changes in brain activity under various physiological and pathological states. In the present study, through this improved technique, we demonstrated that the human apoE3 induced by adenovirus does not exist in mouse CSF, indicating that peripheral apoE is not able to cross the BBB. Since homozygosity of the ApoE4 allele on chromosome 19 has been associated with an increased risk of sporadic Alzheimer's disease (7), determining whether the plasma-derived apoE4 contributes its level in the CSF due to blood-brain barrier leakage in Alzheimer's disease (2) and understanding the related mechanisms might yield new insights into the prevention and treatment of Alzheimer's disease.
GRANTS
This work was supported by the National Center for Research Resources and the National Institute of Diabetes and Digestive and Kidney Diseases through Grants DK-92779, DK-95440, DK-70992, and DK-17844.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.L., D.Y.H., and S.C.W. conception and design of research; M.L., D.G.K., and L.S. analyzed data; M.L., D.Y.H., and S.C.W. interpreted results of experiments; M.L. prepared figures; M.L. drafted manuscript; M.L. and S.C.W. approved final version of manuscript; D.G.K. and L.S. performed experiments; D.Y.H. and S.C.W. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. Philip Shaul and the Core facility at the University of Texas Southwestern Medical Center, Dallas, Texas for amplifying the adenoviruses used in the present study, and Colleen Goodin for their technical assistance.
REFERENCES
- 1. Beisiegel U. Receptors for triglyceride-rich lipoproteins and their role in lipoprotein metabolism. Curr Opin Lipidol 6: 117–122, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Berzin TM, Zipser BD, Rafii MS, Kuo-Leblanc V, Yancopoulos GD, Glass DJ, Fallon JR, Stopa EG. Agrin and microvascular damage in Alzheimer's disease. Neurobiol Aging 21: 349–355, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Boyles JK, Pitas RE, Wilson E, Mahley RW, Taylor JM. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest 76: 1501–1513, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cabana VG, Feng N, Reardon CA, Lukens J, Webb NR, de Beer FC, Getz GS. Influence of apoA-I and apoE on the formation of serum amyloid A-containing lipoproteins in vivo and in vitro. J Lipid Res 45: 317–325, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Carp RI, Davidson AL, Merz PA. A method for obtaining cerebrospinal fluid from mice. Res Vet Sci 12: 499, 1971 [PubMed] [Google Scholar]
- 6. Cassar SC, Tovcimak AE, Rustay NR, Ellis TA, Hooker BA, Witte DG, Li J, Buck WR, Scharf D, Muller U, Jeromin A, Wang KK, Waring JF. Comparing levels of biochemical markers in CSF from cannulated and non-cannulated rats. J Neurosci Methods 192: 249–253, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261: 921–923, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Cserr H. Potassium exchange between cerebrospinal fluid, plasma, and brain. Am J Physiol 209: 1219–1226, 1965 [DOI] [PubMed] [Google Scholar]
- 9. Fenjves ES, Smith J, Zaradic S, Taichman LB. Systemic delivery of secreted protein by grafts of epidermal keratinocytes: prospects for keratinocyte gene therapy. Hum Gene Ther 5: 1241–1248, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Fleming JO, Ting JY, Stohlman SA, Weiner LP. Improvements in obtaining and characterizing mouse cerebrospinal fluid. Application to mouse hepatitis virus-induced encephalomyelitis. J Neuroimmunol 4: 129–140, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ignatius MJ, Gebicke-Harter PJ, Skene JH, Schilling JW, Weisgraber KH, Mahley RW, Shooter EM. Expression of apolipoprotein E during nerve degeneration and regeneration. Proc Natl Acad Sci USA 83: 1125–1129, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koch S, Donarski N, Goetze K, Kreckel M, Stuerenburg HJ, Buhmann C, Beisiegel U. Characterization of four lipoprotein classes in human cerebrospinal fluid. J Lipid Res 42: 1143–1151, 2001 [PubMed] [Google Scholar]
- 13. Lee SJ, Grosskopf I, Choi SY, Cooper AD. Chylomicron remnant uptake in the livers of mice expressing human apolipoproteins E3, E2 (Arg158→Cys), and E3-Leiden. J Lipid Res 45: 2199–2210, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Liu L, Duff K. A technique for serial collection of cerebrospinal fluid from the cisterna magna in mouse. J Vis Exp 21: 960, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Q, Zhang J, Zerbinatti C, Zhan Y, Kolber BJ, Herz J, Muglia LJ, Bu G. Lipoprotein receptor LRP1 regulates leptin signaling and energy homeostasis in the adult central nervous system. PLoS Biol 9: e1000575, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lo CM, Nordskog BK, Nauli AM, Zheng S, Vonlehmden SB, Yang Q, Lee D, Swift LL, Davidson NO, Tso P. Why does the gut choose apolipoprotein B48 but not B100 for chylomicron formation? Am J Physiol Gastrointest Liver Physiol 294: G344–G352, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240: 622–630, 1988 [DOI] [PubMed] [Google Scholar]
- 19. Mahley RW, Innerarity TL, Rall SC, Jr., Weisgraber KH. Plasma lipoproteins: apolipoprotein structure and function. J Lipid Res 25: 1277–1294, 1984 [PubMed] [Google Scholar]
- 20. Martel CL, Mackic JB, Matsubara E, Governale S, Miguel C, Miao W, McComb JG, Frangione B, Ghiso J, Zlokovic BV. Isoform-specific effects of apolipoproteins E2, E3, and E4 on cerebral capillary sequestration and blood-brain barrier transport of circulating Alzheimer's amyloid beta. J Neurochem 69: 1995–2004, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Parkkila AK, Parkkila S, Serlo W, Reunanen M, Vierjoki T, Rajaniemi H. A competitive dual-label time-resolved immunofluorometric assay for simultaneous detection of carbonic anhydrase I and II in cerebrospinal fluid. Clin Chim Acta 230: 81–89, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Pitas RE, Boyles JK, Lee SH, Hui D, Weisgraber KH. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J Biol Chem 262: 14352–14360, 1987 [PubMed] [Google Scholar]
- 23. Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 71: 343–353, 1992 [DOI] [PubMed] [Google Scholar]
- 24. Roheim PS, Carey M, Forte T, Vega GL. Apolipoproteins in human cerebrospinal fluid. Proc Natl Acad Sci USA 76: 4646–4649, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rudick RA, Zirretta DK, Herndon RM. Clearance of albumin from mouse subarachnoid space: a measure of CSF bulk flow. J Neurosci Methods 6: 253–259, 1982 [DOI] [PubMed] [Google Scholar]
- 26. Shen DD, Artru AA, Adkison KK. Principles and applicability of CSF sampling for the assessment of CNS drug delivery and pharmacodynamics. Adv Drug Deliv Rev 56: 1825–1857, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Shen L, Tso P, Woods SC, Clegg DJ, Barber KL, Carey K, Liu M. Brain apolipoprotein E: an important regulator of food intake in rats. Diabetes 57: 2092–2098, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shen L, Wang DQ, Tso P, Jandacek RJ, Woods SC, Liu M. Apolipoprotein E reduces food intake via PI3K/Akt signaling pathway in the hypothalamus. Physiol Behav 105: 124–128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takechi R, Galloway S, Pallebage-Gamarallage MM, Wellington CL, Johnsen RD, Dhaliwal SS, Mamo JC. Differential effects of dietary fatty acids on the cerebral distribution of plasma-derived apo B lipoproteins with amyloid-beta. Br J Nutr 103: 652–662, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Thompson EJ. Cerebrospinal fluid. J Neurol Neurosurg Psychiatry 59: 349–357, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsukamoto K, Smith P, Glick JM, Rader DJ. Liver-directed gene transfer and prolonged expression of three major human ApoE isoforms in ApoE-deficient mice. J Clin Invest 100: 107–114, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsukamoto K, Tangirala R, Chun SH, Pure E, Rader DJ. Rapid regression of atherosclerosis induced by liver-directed gene transfer of ApoE in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 19: 2162–2170, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Utermann G. Isolation and partial characterization of an arginine-rich apolipoprotein from human plasma very-low-density lipoproteins: apolipoprotein E. Hoppe Seylers Z Physiol Chem 356: 1113–1121, 1975 [DOI] [PubMed] [Google Scholar]
- 34. Wahrle SE, Jiang H, Parsadanian M, Legleiter J, Han X, Fryer JD, Kowalewski T, Holtzman DM. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J Biol Chem 279: 40987–40993, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Weisgraber KH. Apolipoprotein E: structure-function relationships. Adv Protein Chem 45: 249–302, 1994 [DOI] [PubMed] [Google Scholar]
- 36. William PL, Warwick R. Functional Neuroanatomy of Man, Churchill Livingstone, London, 1975 [Google Scholar]
- 37. You JS, Gelfanova V, Knierman MD, Witzmann FA, Wang M, Hale JE. The impact of blood contamination on the proteome of cerebrospinal fluid. Proteomics 5: 290–296, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Zlokovic BV, Martel CL, Mackic JB, Matsubara E, Wisniewski T, McComb JG, Frangione B, Ghiso J. Brain uptake of circulating apolipoproteins J and E complexed to Alzheimer's amyloid beta. Biochem Biophys Res Commun 205: 1431–1437, 1994 [DOI] [PubMed] [Google Scholar]