Abstract
Reactive oxygen species (ROS) have been reported to play a primary role in triggering the cardioprotective adaptations by some preconditioning procedures, but whether they are required for exercise-induced preconditioning is unclear. Thus in this study we used the free radical scavenger N-(2-mercaptopropionyl)glycine (MPG) to test the hypothesis that ROS is the trigger for exercise-induced preconditioning of the heart against ischemia-reperfusion injury. Male F344 rats were assigned to four groups: sedentary (SED, n = 7), SED/MPG (100 mg/kg ip daily for 2 days, n = 12), exercised on a treadmill for 2 days at 20 m/min, 6° grade, for 60 min (RUN, n = 7), and RUN/MPG with 100 mg/kg MPG injected 15 min before exercise (n = 10). Preliminary experiments verified that MPG administration maintained myocardial redox status during the exercise bout. Twenty-four hours postexercise or MPG treatment isolated perfused working hearts were subjected to global ischemia for 22.5 min followed by reperfusion for 30 min. Recovery of myocardial external work (percentage of preischemic systolic pressure times cardiac output) for SED (50.4 ± 4.5) and SED/RUN (54.7 ± 6.6) was similar and improved in both exercise groups (P < 0.05) to 77.9 ± 3.0 in RUN and 76.7 ± 4.5 in RUN/MPG. A 2 × 2 ANOVA also revealed that exercise decreased lactate dehydrogenase release from the heart during reperfusion (marker of cell damage) without MPG effects or interactions. Expression of the cytoprotective protein inducible heat shock protein 70 increased by similar amounts in the left ventricles of RUN and RUN/MPG compared with sedentary groups (P < 0.05). We conclude that ROS are not a necessary trigger for exercise-induced preconditioning in rats.
Keywords: cardioprotection, ischemia-reperfusion, isolated perfused heart, antioxidant, stress protein
exercise is an effective intervention for both prevention of cardiovascular disease and preconditioning the heart to survive an ischemic event. It is now well established that exercise, along with a variety of other nonpharmacological procedures, can result in de novo synthesis of protective proteins in the heart and provide protection for 72+ h (8, 15, 31 for reviews). This sustained state of protections is known as late preconditioning. Understanding the molecular signals that initiate or trigger the preconditioning adaptations from these procedures is important for the further development of cardioprotective procedures and drugs.
Studies employing a variety of antioxidants have concluded that the generation of reactive oxygen species (ROS) in the heart is the trigger that initiates the development of preconditioning for many preconditioning procedures. However, whether ROS are the primary trigger for exercise-induced cardioprotection remains equivocal. Hamilton and colleagues (11) got mixed results when investigating whether a diet high in antioxidants would affect preconditioning in rats associated with five consecutive days of treadmill exercise for 60 min/day. Exercise alone, antioxidant diet alone, and exercise plus antioxidant diet all significantly improved measures of ischemia-reperfusion (I-R) injury after ischemic bouts of 20 and 60 min. Interpretation of the results is confounded by the dramatic effectiveness of the antioxidant diet in protecting the heart against the I-R bouts. Yamashita and colleagues (41) reported that the short-acting antioxidant N-(2-mercaptopropionyl)glycine (MPG) administered to rats during exercise abrogated late preconditioning. MPG is a potent cell-permeable scavenger of peroxynitrite and hydroxyl radicals that has been widely used as a tool to probe for ROS as a trigger because it scavenges free radicals during a preconditioning procedure but not during the subsequent development and mediation of late preconditioning. For example, MPG has been reported to prevent late preconditioning by brief bouts of ischemia (35, 37), heat stress (2, 42), prooxidant exposure (33, 43), nitric oxide donor exposure (33), and opioid receptor agonist treatment (23). However, the exercise preconditioning study by Yamashita et al. is confounded by at least two important issues. These include a brief window of protection that is inconsistent with late preconditioning by exercise or other procedures (8, 15, 17, 31), and a significant improvement in protection after only a single exercise session that was brief and moderate in intensity compared with other studies demonstrating exercise-induced preconditioning. These inconsistences appear to weaken their conclusion that ROS are the primary trigger for exercise-induced late preconditioning in rats.
Akita et al. (1) followed up with another study using MPG administered to mice to investigate ROS as a trigger for exercise-induced cardioprotection. They exercised mice on a motor-driven treadmill for 60 min/day for 1 wk. Exercise resulted in a significant reduction of infarct size following 30 min of left coronary artery occlusion, and this improved cardioprotection was abolished when MPG was administered before each exercise session. Using nitric oxide synthase (NOS) knockouts and inhibitors, the authors ultimately conclude that ROS triggers cardioprotection by activating endothelial NOS (eNOS), which leads to upregulation of inducible NOS (iNOS), which then acts as a mediator of the protection. While the study by Akita and colleagues is important because it is the first to investigate exercise-induced late cardioprotection in mice, almost all of the other studies investigating exercise-induced cardioprotection were carried out using rats. It is very unlikely that iNOS plays a significant role in exercise-induced late preconditioning in this species because the enzyme is not elevated in the rat heart by chronic exercise programs (7, 14) or 24 h following 2 and 5 consecutive days of treadmill exercise for 60 min/day (24, 36). Furthermore, Taylor et al. (36) found that exercise-induced cardioprotection against I-R injury in rats is not abolished by inhibition of NOS. Thus the study by Akita and colleagues does not confirm the conclusion by Yamashita et al. (41) that ROS are the trigger for exercise-induced cardioprotection in rats.
The purpose of the present study was to clarify whether the administration of MPG will block exercise-induced cardioprotection against I-R injury in rats. The animals exercised for two consecutive days for 60 min/day, which is a more established protocol for inducing persistent cardioprotection against I-R injury than a single bout (13, 36). Response to global ischemia was evaluated using an ex vivo heart model, which eliminates possible exogenous influences that may have impacted the results of both Yamashita et al. (41) and Akita et al. (1).
MATERIALS AND METHODS
Animals and training protocols.
Male 4- to 6-mo-old Fischer 344 rats were used in this study. The animals were maintained on a 12 h:12 h light:dark cycle and fed ad libitum with Harlan Teklad 7013, NIH-31 diet. Before experimentation, rats were randomly assigned to four different treatment groups: sedentary (SED) (N = 7); 2 days of treadmill exercise (RUN) (N = 7); sedentary/injected with 100 mg/kg MPG (SED/MPG) (N = 12); and exercise/injected with MPG (RUN/MPG) (N = 10). Animals were initially familiarized with a motorized treadmill (Collins, Braintree, MA) three times during 1 wk by exercising at low intensity (15 m/min, 0% grade) for 10 min. After this familiarization, they ran on the treadmill for 60 min/day for two consecutive days at a speed of 20 m/min up a 6° grade. This is a previously established protocol for inducing late preconditioning in Fischer 344 rats (36). Groups receiving injections of MPG were administered a single intraperitoneal injection of 100 mg/kg body wt 15 min before each 60-min exercise bout as described by Yamashita et al. (41) and Akita et al. (1) or, for SED/MPG, 48 and 24 h before evaluation of cardiac function. MPG was dissolved in phosphate-buffered saline at a concentration of 200 mg/ml and injected in a volume of 0.5 μl/g body wt. All of the exercised and MPG-treated animals were euthanized 24 h after their last exercise bout or injection. This investigation, approved by the University's Institutional Animal Care and Use Committee, conforms to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996).
Isolated heart perfusions and global ischemia.
All animals in the previous paragraph were subjected to the I-R procedure described in this section. Animals were anesthetized with an intraperitoneal injection of 40 mg/kg body wt of pentobarbital sodium. More was administered as necessary until the animal was unresponsive to a toe pinch on the hind paw. This procedure assured us that a level of anesthesia was reached so that there would be no response from the animal during surgery to excise the heart. Hearts were weighed and myocardial function evaluated at 37°C using an isolated, working heart preparation as previously described (36). The perfusion buffer contained (in mM) 10 glucose, 1.75 CaCl2, 118.5 NaCl, 4.7 KCl, 1.2 MgSO4, 24.7 NaHCO3, 0.5 EDTA, and 12 mU/ml insulin and was gassed with 95% O2-5% CO2. Atrial filling pressure was maintained at 12.5 mmHg and afterload set by an 80-cm high aortic column (ID 3.18 mm). During global, no-flow ischemia for 22.5 min hearts were enclosed in a sealed water-jacketed chamber maintained at 37°C. This ischemia procedure has been established in our lab to result in measureable necrosis and ∼50% recovery of function at the end of the reperfusion period in sedentary, male Fischer 344 rats used herein (36). Upon reperfusion hearts were initially perfused for 10 min in a retrograde, or Langendorff, mode at a perfusion pressure of 80 mmHg, and then returned to the working mode for the final 20 min of reperfusion. At the end of the perfusion period, the beating hearts were freeze-clamped and stored at −80°C until further analysis.
Lactate dehydrogenase assay.
Coronary effluents were collected and immediately placed in a refrigerator (4°C). Lactate dehydrogenase (LDH) activity in the effluents was measured at the end of the daily perfusion protocols as described by Starnes (29). Elevated release of cytosolic proteins from the heart is a widely used biomarker of cell damage and myocardial infarct (4, 5, 10, 44).
Protein blotting for inducible heat shock protein 70.
Post-I-R hearts were used for determination of heat shock protein 70 (HSP70) expression because we previously determined that the I-R procedure used herein does not change HSP70 expression compared with preischemic values. A piece of left ventricle (130–160 mg) was homogenized (1:20 wt/vol) in phosphate buffer (50 mM K2HPO4, 0.1 mM EDTA, and 0.1% TRITON X-100, pH 7.4) using a Teflon-glass Potter-Elvehjem homogenizer and centrifuged at 1,500 g for 10 min. An aliquot was further diluted 1:1 with Laemmli sample buffer (16), protein concentration determined according to Lowry (20), and 80 μg of protein subjected to SDS-PAGE electrophoresis. After electrophoresis, samples were transferred to polyvinyl difuoride (PVDF) membranes (Bio-Rad) and immunoblotted with HSP70 monoclonal IgG (no. sc-024, Santa Cruz Biotechnology). Membranes were then blotted with anti-mouse Ig (NA931V) horseradish peroxidase linked whole antibody (Amersham Pharmacia Biotech) and detected with SuperSignal West Pico chemiluminescent substrate on Kodak Biomax ML imaging film. The densities of resulting bands were quantified using NIH-image software on a Macintosh Power PC, and relative protein content was calculated by comparing the density of each band to the corresponding protein band in a standard tissue homogenate loaded on every gel.
Effect of MPG on redox status during exercise.
Although MPG administration as used herein has been widely used to investigate the role of ROS in a variety of preconditioning procedures (1, 2, 35, 37, 41, 42), we carried out preliminary experiments with 17 additional rats to verify that MPG was effective in maintaining myocardial redox status during the exercise bout. The exercised animals were habituated to the treadmill for 1 wk and then subjected to a single, 60-min exercise bout at a speed of 20 m/min up a 6° grade as described above. Immediately after the exercise bout, rats were anesthetized with 40 mg/kg pentobarbital sodium ip, and hearts were excised and frozen in liquid nitrogen. Nonprotein nonglutathione sulfhydryls (NPNGSH) in the left ventricle were measured as an indicator of redox status as we described previously (25, 28). The NPNGSH pool helps buffer protein sulfhydryl groups and is composed of cysteine, derivatives of cysteine, and other low-molecular-weight molecules containing sulfhydryl groups (19). This pool is a more sensitive marker of mild oxidative stress than total soluble sulfhydryls or GSH because glutathione reductase, which reduces GSSG to GSH, cannot reduce the oxidized forms of NPNGSH, thus it is decreased sooner than GSH and remains decreased for a longer time (19). We previously observed that NPNGSH was decreased in rat heart after exhaustive exercise, whereas total protein sulfhydryls and GSH were unchanged from resting values (28).
Statistical analysis.
Descriptive data (means ± SE) were calculated for each dependent variable. To determine acute effects of MPG, a 1 × 3 ANOVA was used. For other measures, hypotheses were tested in the null form using a two-way ANOVA. If the ANOVA determined that the results were significant, a Tukey honest significant difference (HSD) test was performed. In all tests, a probability level of <0.05 was used as the decision rule for significance testing.
RESULTS
Effect of MPG on redox status during exercise.
The effect of the exercise bout on myocardial redox status is reported in Table 1. A change in the redox state occurred during the 60-min exercise bout as indicated by the 48% decrease in NPNGSH for animals euthanized immediately after a single exercise bout. Administration of MPG before exercise prevented the exercise-induced decrease in NPNGSH levels.
Table 1.
Effect of MPG on oxidative stress following an exercise bout
| Group | NPNGSH, nmol/g wet wt |
|---|---|
| SED | 1,238 ± 120 |
| RUN-1 day | 646 ± 62* |
| RUN/MPG-1 day | 1,063 ± 142 |
Values are means ± SE, n = 5–6. These animals were a companion set to those in Table 2. NPNGSH, Nonprotein nonglutathione sulfhydryls. MPG, N-(2-mercaptopropionyl)glycine; SED, sedentary; RUN-1 day, exercised on a treadmill for 1 day at 20m/min, 6° grade, for 60 min; RUN/MPG-1 day, with 100 mg/kg MMPG injected 15 min before exercise.
P < 0.05 vs. SED and RUN/MPG-1 day.
Animal characteristics.
The body weights, heart weights, and ratio of the two for animals used in the I-R experiments are reported in Table 2. None of these parameters were affected by two doses of MPG alone, two exercise bouts alone, or the interaction of MPG and exercise. There were no significant differences among any of the groups with respect to body weight or heart weight (P > 0.05).
Table 2.
Characteristics of animals used in the ischemia-reperfusion experiments
| n | Body Wt, g | Heart Wt, mg | Heart Wt/Body Wt, mg/g | |
|---|---|---|---|---|
| SED | 7 | 336.6 ± 9.3 | 921 ± 25 | 2.73 ± 0.05 |
| RUN | 7 | 328.0 ± 9.6 | 957 ± 9 | 2.91 ± 0.06 |
| SED/MPG | 12 | 346.7 ± 4.8 | 918 ± 12 | 2.65 ± 0.03 |
| RUN/MPG | 10 | 341.2 ± 4.5 | 972 ± 16 | 2.84 ± 0.04 |
Values are means ± SE; n, number of rats. SED/MPG, 100 mg/kg ip MPG daily for 2 days; RUN, exercised on a treadmill for 2 days at 20 m/min, 6° grade, for 60 min; RUN/MPG, with 100 mg/kg MMPG injected 15 min before exercise. No significant differences (P > 0.05).
Myocardial response during I-R.
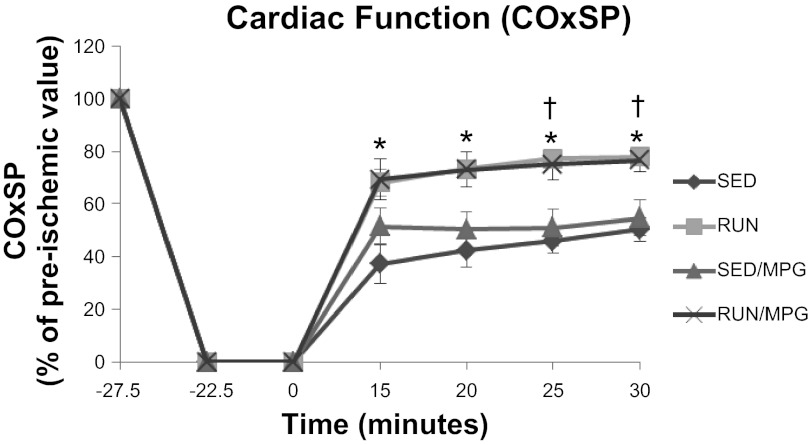
Hemodynamic parameters before and 30 min after ischemia are displayed in Table 3. During the preischemic period, no exercise or MPG main effects or interactions were observed when analyzed by 2 × 2 ANOVA. After 30 min of reperfusion, main effects of exercise were observed without MPG main effects or interactions. Cardiac output and systolic pressure development at this time point were decreased in both sedentary groups compared with preischemia (P < 0.05), whereas these variables were not different from preischemia in the two exercised groups (P > 0.05). Postischemic coronary flow (CF), aortic flow (AF), and cardiac output (CO) for RUN and RUN/MPG were significantly higher than for SED (P < 0.05). However, absolute values for these parameters in RUN/MPG were not different from SED/MPG (P > 0.05) presumably because the preischemic values for SED/MPG tended to be higher than other groups. When preischemic covariance was minimized by normalizing postischemic cardiac function to the preischemic value in each heart (Table 4), the lack of an MPG effect on the exercise-induced cardioprotective response is clear. For almost every parameter, RUN and RUN/MPG recovered better than both SED and SED/MPG; the only exception is systolic pressure (SP) where RUN/MPG was greater than SED only. The time course of recovery of external cardiac work is displayed in Fig. 1.
Table 3.
Cardiac functional parameters
| Group | CF, ml•min−1•g−1 | AF, ml•min−1•g−1 | CO, ml•min−1•g−1 | SP, mmHg | HR, beats/min |
|---|---|---|---|---|---|
| Preischemia | |||||
| SED | 13.5 ± 0.5 | 41.3 ± 2.6 | 54.8 ± 2.9 | 114.1 ± 2.4 | 277 ± 9 |
| RUN | 14.2 ± 0.6 | 45.6 ± 1.9 | 59.8 ± 2.4 | 111.0 ± 1.4 | 300 ± 5 |
| SED/MPG | 15.4 ± 0.7 | 48.0 ± 1.7 | 63.4 ± 2.0 | 110.8 ± 1.7 | 291 ± 4 |
| RUN/MPG | 13.5 ± 0.5 | 42.7 ± 1.2 | 56.2 ± 1.6 | 108.8 ± 1.5 | 287 ± 5 |
| 30 min Postischemia | |||||
| SED | 11.7 ± 0.7 | 19.2 ± 2.8† | 30.9 ± 2.9† | 101.3 ± 2.7† | 262 ± 13 |
| RUN | 14.2 ± 0.8* | 33.4 ± 2.7*† | 47.7 ± 3.4* | 109.1 ± 1.4 | 298 ± 4 |
| SED/MPG | 14.4 ± 1.0 | 23.7 ± 4.1† | 38.0 ± 4.4† | 99.6 ± 1.5† | 266 ± 13 |
| RUN/MPG | 13.9 ± 0.5* | 31.5 ± 2.5* | 45.4 ± 2.7* | 102.8 ± 1.8 | 285 ± 6 |
Values are means ± SE. CF, coronary flow; AF, aortic flow; CO, cardiac output; SP systolic pressure; HR, heart rate.
P < 0.05 vs. SED at same time point;
P < 0.05 vs. preischemic value.
Table 4.
Percent recovery of function after 30 min reperfusion
| Group | CF | AF | CO | SP | COxSP |
|---|---|---|---|---|---|
| SED | 86.9 ± 3.1 | 46.7 ± 6.0 | 56.7 ± 4.8 | 88.7 ± 1.5 | 50.4 ± 4.5 |
| RUN | 100.3 ± 2.0* | 72.8 ± 3.7* | 79.3 ± 3.1* | 98.4 ± 1.3* | 77.9 ± 3.0* |
| SED/MPG | 92.2 ± 2.7 | 49.9 ± 8.5 | 60.2 ± 6.7 | 90.0 ± 1.6 | 54.7 ± 6.6 |
| RUN/MPG | 103.6 ± 3.3* | 73.5 ± 4.8* | 80.7 ± 4.0* | 94.6 ± 1.8† | 76.7 ± 4.5* |
Values are means ± SE (in %) of 30-min postischemia value compared with preischemic value in same heart. CF, coronary flow; AF, aortic flow; CO, cardiac output; SP systolic pressure.
P < 0.05 vs. SED and SED/MPG;
P < 0.05 vs. SED.
Fig. 1.
Interaction of exercise and N-(2-mercaptopropionyl)glycine (MPG) treatment on cardiac external work (COxSP) in response to ischemia-reperfusion. Values are means ± SE. SED, sedentary; SED/MPG, 100 mg/kg ip MPG daily for 2 days; RUN, exercised on a treadmill for 2 days at 20 m/min, 6° grade, for 60 min; RUN/MPG, with 100 mg/kg MPG injected 15 min before exercise. *P < 0.05, SED vs. RUN and RUN/MPG, †P < 0.05, SED/MPG vs. RUN and RUN/MPG.
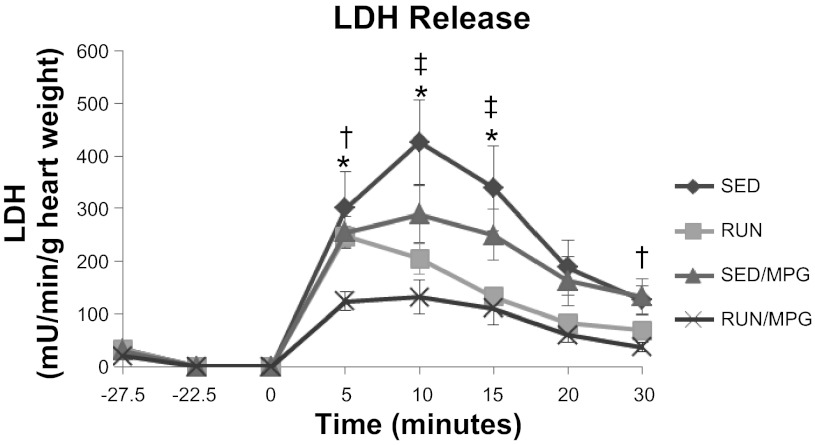
Release of LDH (Fig. 2) was not different in any group before ischemia (P > 0.05) and increased in all groups after ischemia (P < 0.05). A 2 × 2 ANOVA revealed a main effect of exercise for decreasing the amount of LDH released throughout reperfusion without MPG effects or interactions. A post hoc test of the exercise treatment revealed that LDH release was less in RUN/MPG compared with SED or SED/MPG throughout the reperfusion period (Fig. 2).
Fig. 2.
Interaction of exercise and MPG treatment on lactate dehydrogenase (LDH) release into the coronary effluent. Values are means ± SE. All groups are increased from preischemic values 5, 10, and 15 min after ischemia (P < 0.05). *P < 0.05, SED vs. RUN/MPG, †P < 0.05, SED/MPG vs. RUN/MPG; ‡P < 0.05, SED vs. RUN.
Tissue analysis.
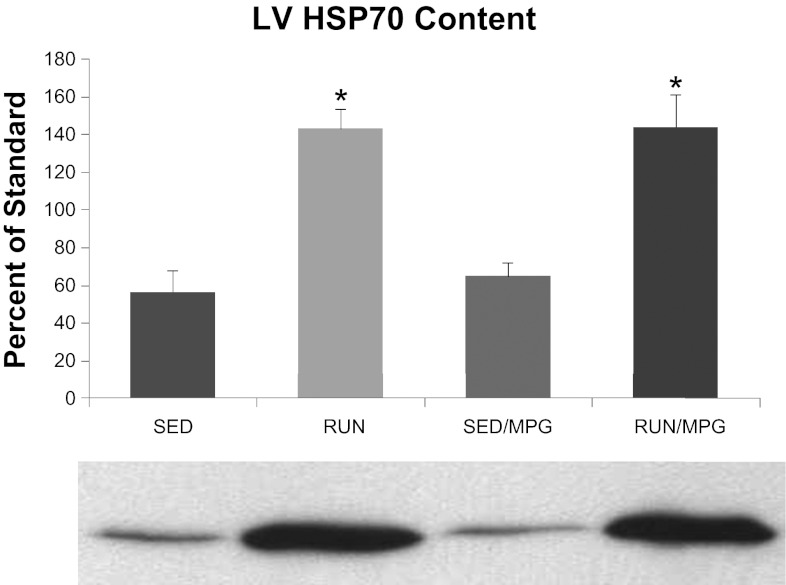
Expression of HSP70 in the left ventricle (Fig. 3) increased by similar amounts in both exercise groups compared with sedentary groups (P < 0.05) and was not affected by MPG treatment.
Fig. 3.
Interaction of exercise and MPG treatment on heat shock protein 70 (HSP70) content of the left ventricle, expressed as a percentage of HSP70 in a standardized tissue homogenate loaded on every gel. Values are means ± SE. *P < 0.05, RUN and RUN/MPG vs. SED and SED/MPG. Representative scan of Western blot below figure.
DISCUSSION
In this study we investigated whether free radicals generated during exercise is a trigger that initiates exercise-induced adaptations mediating cardioprotection against I-R injury. Administration of a short-acting free radical scavenger, MPG, effectively prevented changes in redox status in cardiac tissue during the exercise bout (Table 1). This potent scavenger of peroxynitrite and hydroxyl radicals has been used experimentally in the heart for at least three decades (28 for review) and the dosage selected for the present study (100 mg/kg body wt) is known to block late-phase preconditioning by heat stress (2, 42) and ischemic preconditioning (35, 37). However, unlike those forms of preconditioning, the results presented herein indicate that exercise-induced preconditioning is not attenuated by MPG administration (Figs. 1 and 2).
Our results are in conflict with Yamashita et al. (41), who were the first to report that MPG abrogated exercise-induced cardioprotection. They found that a single, brief exercise bout by male Wistar rats resulted in protection against infarct development after regional ischemia in vivo, and that MPG administered before the exercise prevented this protection. We found that MPG administered in the same manner did not affect exercise-induced protection against I-R injury in isolated perfused hearts of Fischer 344 rats that exercised on a treadmill 60 min/day for two consecutive days at an intensity of ∼70% maximal Vo2. As discussed in the next paragraph, the different exercise protocols used may be the primary reason for the different outcomes.
It now appears that the cardioprotective phenotype resulting from an appropriate and repeated exercise stimulus consists of several adaptations within the heart (9, 22, 24 for reviews). The preconditioning response observed by Yamashita et al. (41) was achieved with a single treadmill exercise bout consisting of a 5-min warm up, 15–20-min moderate intensity exercise, and a 5-min cool down. This is a very low duration and intensity compared with other studies demonstrating exercise-induced preconditioning. Perhaps the stimulus was too low to activate all of the cardioprotective adaptations. There are two reasons to suspect this. First, the nature of the preconditioning response was atypical for late-phase preconditioning. Yamashita et al. observed improved protection against infarct development 48 h postexercise, but there was no difference compared with sedentary control at 24 or 72 h postexercise. This brief window of protection is atypical; preconditioning following short-term exercise has been reported by others to be present within 24 h and to last for several days (17), which is consistent with the general late-phase preconditioning response (8, 15, 31). Second, the protection observed by Yamashita et al. coincided specifically to increased mitochondrial superoxide dismutase (MnSOD), which will certainly help quench the rise in superoxide radicals during reperfusion. However, several more recent studies have reported that exercise-induced protection against both mechanical dysfunction and cell death following I-R can occur without increased MnSOD (3, 12, 17). This indicates that more than one mechanism is involved in the full exercise-induced cardioprotective phenotype. The upregulation of some of these mechanisms may be redox sensitive while others are not redox sensitive (see Ref. 22 for review). If that is the case, it appears that the exercise protocol used by Yamashita and colleagues upregulated only redox-sensitive mechanisms.
Several experimental models exist for evaluating myocardial I-R injury. The most widely used models are the in vivo regional ischemia model used by Yamishita et al. (41) and the ex vivo global ischemia model used in the current study. It is very unlikely that differences in experimental models contributed to the conflicting conclusions. A comparison of these models in the context of the present study will be briefly discussed. Yamashita et al. induced regional ischemia in vivo by ligating the left descending coronary artery for 20 min and then measured the size of the infarct that subsequently developed in the area made ischemic by the ligation (area at risk). Regional ischemia, compared with global ischemia, is employed in these models because the heart must be able to pump adequately as soon as the ligature is released or the animal will die. Upon reperfusion the nonischemic tissue functions to take up the slack of the injured ischemic tissue and often there is not a noticeable difference in overall resting mechanical function compared with preischemia. This was the case for the preparation employed by Yamishita and colleagues even when half of the area at risk was infarcted. Thus mechanical function was not compared among groups in their study, and infarct size was the only outcome evaluated. The isolated perfused heart preparation used in the present study eliminates possible extrinsic influences from the blood, nervous system, and hormones that exist in in vivo models. Electron paramagnetic resonance measurements indicate that the oxygenated crystalloid buffers used in these perfused preparations do not cause tissue-wide oxidative stress (40). In the present study the entire heart was subjected to 22.5 min of ischemia, and the outcomes evaluated included subsequent release of LDH and recovery of mechanical function. The amount of LDH release upon reperfusion in isolated hearts is proportional to subsequent infarct size (4, 10, 44), which was the outcome measure used by Yamashita et al. A greater amount of mechanical dysfunction is produced in the globally ischemic heart than in the in vivo regional ischemic model because more tissue is subjected to the ischemic stress. Upon reperfusion following 22.5 min of ischemia, the isolated working heart cannot develop enough systolic pressure to maintain adequate coronary artery perfusion pressure. Therefore, the hearts in the current study were initially reperfused in a retrograde manner at a perfusion pressure of 80 mmHg to keep them viable while recovering enough to maintain adequate perfusion pressure in the working mode. Thus we were able to get an additional outcome measure (mechanical function) that Yamashita and colleagues were not able to obtain.
If ROS were the common trigger to initiate late preconditioning for all nonpharmacological procedures, one might expect the cardioprotective phenotypes to be similar among the various procedures. However, there is emerging evidence that the acquired cardioprotection by exercise is unique and different from other forms of preconditioning (see Ref. 9 for review). For example, upregulation of sarcolemmal ATP-sensitive K+ (KATP) channel density is reported to be central to exercise-induced protection from infarction, whereas the mitochondrial KATP channel is much more important than the sarcolemmal isoform in most other forms of preconditioning (see Ref. 9 for review). However, a recent study provides evidence that the mitochondrial KATP channel may be important to exercise-induced protection from I-R-generated arrhythmias (25). Acute and chronic exercise can also result in a severalfold increase in HSP70 (11, 12, 17, 36, present work), whereas this cytoprotective protein is not altered by some other forms of preconditioning, including ischemic preconditioning. We showed herein that MPG administration does not attenuate exercise-induced increased HSP70 expression (Fig. 3). Interestingly, Arnaud et al. (2) reported that MPG also did not attenuate heat shock-induced increased HSP70 expression but did abrogate the cardioprotection against I-R injury. They conclude that free radical production following hyperthermia plays a role in the heat stress-induced cardioprotection, independently of HSP levels. Furthermore, the findings that MPG does not attenuate exercise-induced or heat shock-induced increases in HSP70 suggests that the synthesis of this protein is not regulated by redox-sensitive mechanisms. As mentioned earlier, exercise does not elevate iNOS in rats, whereas it may be a central mediator of cardioprotection elicited by several other forms of preconditioning (see Ref. 15 for review).
Another important difference between exercise and other forms of preconditioning is that exercise preconditioning is sustained after chronic treatment. For some other precondioning procedures, e.g., prior ischemia and adenosine receptor agonists, the cardioprotective effect is eventually lost after repetitive administration (6, 9, 39). This does not happen for exercise-induced cardioprotection; in fact the protection becomes more robust over time (see Ref. 9 for review) even though antioxidant capacity increases and ROS generation is reported to decrease over time (24, 30).
So what is the trigger for exercise-induced cardioprotective adaptations? Several candidate triggers for exercise-induced preconditioning are discussed in a recent review by Frasier et al. (9). In addition to transient ROS production, these also include activation of adenosine and/or opioid receptors, AMP kinase, and surges in inflammatory cytokines. Also as reviewed by Shiojima and Walsh (27), upregulation of the PI3K/Akt pathway can provide cardioprotection against I-R injury and doxorubicin-induced dysfunction. In a recent paper we reported that MPG administration did not block exercise-induced activation of Akt (21). This activation may have been due to increased insulin-like growth factor rather than elevated ROS (27). Overall, there is still much work to be done before we have a good understanding of the triggers involved in exercise-induced preconditioning.
Limitations.
In this study, an animal model was used to represent the metabolic activity of humans, which limits the applicability of the findings. Inferences beyond the specific data in the current study should be made with caution. For example, preconditioning for arrhythmias associated with I-R was not considered; therefore, inferences regarding triggers and mechanisms associated with anti-arrhythmia preconditioning should not be made. The overall preconditioning effect in vivo is influenced by intrinsic changes within the heart as well as humoral, neural, and hormonal factors. These extrinsic factors are eliminated when using the isolated heart preparation; this is both a strength and a limitation of the preparation. The heart is ischemic during the short period (<2 min) while being transferred from the rat to the perfusion apparatus. Potentially, this could cause a preconditioning effect against the subsequent I-R stress; however, this possibility was minimized by keeping the heart in a cold cardioplegia solution and by providing the same treatment to all hearts.
Perspectives and Significance
The results of the present experiments provide clear evidence that quenching ROS during exercise with MPG does not prevent exercise-induced preconditioning against myocardial I-R injury or the increase of the cytoprotective protein HSP70. The finding that ROS are not a necessary trigger for exercise-induced late preconditioning is consistent with the fact that important differences exist between exercise-induced cardioprotection and other forms of late preconditioning, including heat stress and ischemic preconditioning (9). The finding is also consistent with the findings of Traverse et al. (38) who reported that free radical production by the heart during treadmill exercise could not be detected using EPR spectroscopy and by Liu et al. (19) who reported that even when rats exercise to exhaustion there are no changes in the heart for biomarkers for lipid peroxidation, protein oxidation, or oxidative DNA damage. Finally, by providing the first evidence that ROS are not the trigger of exercise-induced preconditioning, our findings open the door for future investigations aimed at discovering the unique mechanisms underlying exercise-induced cardioprotection.
GRANTS
This work was supported by National Institutes of Health Grant AG-02220 (to J. W. Sarnes) and an American College of Sports Medicine Foundation Research Grant (to R. P. Taylor).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.P.T. and J.W.S. conception and design of research; R.P.T. performed experiments; R.P.T. and J.W.S. analyzed data; R.P.T. and J.W.S. interpreted results of experiments; R.P.T. prepared figures; R.P.T. drafted manuscript; R.P.T. and J.W.S. approved final version of manuscript; J.W.S. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors thank Matthew J. Nelson for the experiments verifying that MPG was effective in reducing exercise-induced oxidative stress and Jennifer Seidman for the creating the figures.
REFERENCES
- 1.Akita Y, Otani H, Matsuhisa S, Kyoi S, Enoki C, Hattori R, Imamura H, Kamihata H, Kimura Y, Iwasaka T. Exercise-induced activation of cardiac sympathetic nerve triggers cardioprotection via redox-sensitive activation of eNOS and upregulation of iNOS. Am J Physiol Heart Circ Physiol 292: H2051–H2059, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Arnaud C, Joyeux M, Garrel C, Godin-Ribuot D, Demenge P, Ribuot C. Free-radical production triggered by hyperthermia contributes to heat stress-induced cardioprotection in isolated rat hearts. Br J Pharmacol 135: 1776–1782, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown DA, Chicco AJ, Jew KN, Johnson MS, Lynch JM, Watson PA, Moore RL. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J Physiol 569:913–924, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao CM, Chen M, Wong TM. The KCa channel as a trigger for the cardioprotection induced by kappa-opioid receptor stimulation–its relationship with protein kinase C. Br J Pharmacol 145: 984–991, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chia S, Senatore F, Raffel OC, Lee H, Wackers FJT, Jang IK. Utility of cardiac biomarkers in predicting infarct size, left ventricular function, and clinical outcome after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 1: 415–423, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Cohen MV, Yang XM, Downey JM. Conscious rabbits become tolerant to multiple episodes of ischemic preconditioning. Circ Res 74: 998–1004, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Criswell DS, Henry KM, DiMarco NM, Grossie VB., Jr Chronic exercise and the pro-inflammatory response to endotoxin in the serum and heart. Immunol Lett 95: 213–220, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Das M, Das DK. Molecular mechanisms of preconditioning. IUBMB Life 60: 199–203, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Frasier CR, Moore RL, Brown DA. Exercise-indiced cardioprotection: How exercise protects your achy-breaky heart. J Appl Physiol 11: 905–915, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Giricz Z, Lalu MM, Csonka C, Bencsik P, Schulz R, Ferdinandy P. Hyperlipidemia attenuates the infarct size-limiting effect of ischemic preconditioning: role of matrix metalloproteinase-2 inhibition. J Pharmacol Exp Ther 316: 154–161, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Hamilton KL, Staib JL, Phillips T, Hess A, Lennon SL, Powers SK. Exercise, antioxidants, and HSP72: protection against myocardial ischemia/reperfusion. Free Radic Biol Med 34: 800–809, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Harris MB, Starnes JW. Effects of body temperature during exercise training on myocardial adaptations. Am J Physiol Heart Circ Physiol 280: H2271–H2280, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Hoshida S, Yamashita N, Otsu K, Hori M. Repeated physiologic stresses provide persistent cardioprotection against ischemia-reperfusion injury in rats. J Am Coll Cardiol 40: 826–831, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Husain K. Interaction of physical training and chronic nitroglycerin treatment on blood pressure, nitric oxide, and oxidants/antioxidants in the rat heart. Pharmacol Res 48: 253–261, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Jones SP, Bolli R. The ubiquitious role of nitric oxide in cardioprotection. J Mol Cell Cardiol 40: 16–23, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970 [DOI] [PubMed] [Google Scholar]
- 17.Lennon SL, Quindry J, Hamilton KL, French J, Staib J, Mehta JL, Powers SK. Loss of exercise-induced cardioprotection after cessation of exercise. J Appl Physiol 96: 1299–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Lesnefsky EJ, Dauber IM, Horwitz LD. Myocardial sulfhydryl pool alterations occur during reperfusion after brief and prolonged myocardial ischemia in vivo. Circ Res 68: 605–613, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Yeo HC, Overvik-Douki E, Hagen T, Doniger SJ, Chu DW, Brooks GA, Ames BN. Chronically and acutely exercised rats: biomarkers of oxidative stress and endogenous antioxidants. J Appl Physiol 89: 21–28, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951 [PubMed] [Google Scholar]
- 21.Nelson MJ, Harris MB, Boluyt MO, Hwang HS, Starnes JW. Effect of N-2-mercaptopropionyl glycine (MPG) on exercise-induced cardiac adaptations. Am J Physiol Regul Integr Comp Physiol 300: R993–R1000, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Peart JN, Headrick JP. Clinical cardioprotection and the value of conditioning responses. Am J Physiol Heart Circ Physiol 296: H1705–H1720, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Patel HH, Hsu A, Gross GJ. Delayed cardioprotection is mediated via a non-peptide delta opioid agonist, SNC-121, independent of opioid receptor stimulation. Basic Res Cardiol 99: 38–45, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Powers SK, Quindry JC, Kavazis AN. Exercise-induced cardioprotection against myocardial ischemia-reperfusion injury. Free Radic Biol Med 44: 193–201, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Quindry JC, Schreiber L, Hosick P, Wrieden J, Irwin JM, Hoyt E. Mitochondrial KATP channel inhibition blunts arrhythmia protection in ischemic exercised hearts. Am J Physiol Heart Circ Physiol 299: H175–H183, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seward SW, Seiler KS, Starnes JW. Intrinsic myocardial function and oxidative stress after exhaustive exercise. J Appl Physiol 79: 251–255, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Shiojima I, Walsh K. Regulation of cardiac growth and angiogenesis by the Akt/PKB signaling pathway. Genes Dev 20: 3347–3365, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Simpson PJ, Mickelson JK, Lucchesi BR. Free radical scavengers in myocardial ischemia. Fed Proc 46: 2413–2421, 1987 [PubMed] [Google Scholar]
- 29.Starnes JW. Effect of storage conditions on lactate dehydrogenase released from perfused hearts. Int J Cardiol 127: 114–116, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Starnes JW, Barnes BD, Olsen ME. Exercise training decreases rat heart mitochondria free radical generation but does not prevent Ca2+-induced dysfunction. J Appl Physiol 102: 1793–1798, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Stein AB, Tang XL, Guo Y, Xuan YT, Dawn B, Bolli R. Delayed adaptation of the heart to stress: late preconditioning. Stroke 35: 2676–2679, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun JZ, Tang XL, Park SW, Qiu Y, Turrens JF, Bolli R. Evidence for an essential role of reactive oxygen species in the genesis of late preconditioning against myocardial stunning in conscious pigs. J Clin Invest 97: 562–576, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takano H, Tang XL, Qiu Y, Guo Y, French BA, Bolli R. Nitric oxide donors induce late preconditioning against myocardial stunning and infarction in conscious rabbits via an antioxidant-sensitive mechanism. Circ Res 83: 73–84, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka M, Fujiwara H, Yamasaki K, Sasayama S. Superoxide dismutase and N-2-mercaptopropionyl glycine attenuate infarct size limitation effect of ischaemic preconditioning in the rabbit. Cardiovasc Res 28: 980–986, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Tang XL, Takano H, Rizvi A, Turrens JF, Qiu Y, Wu WJ, Zhang Q, Bolli R. Oxidant species trigger late preconditioning against myocardial stunning in conscious rabbits. Am J Physiol Heart Circ Physiol 282: H281–H291, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Taylor RP, Olsen ME, Starnes JW. Late preconditioning following acute exercise is not mediated by nitric oxide synthase in the rat heart. Am J Physiol Heart Circ Physiol 292: H601–H607, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Toufektsian MC, Morel S, Tanguy S, Jeunet A, de Leiris J, Boucher F. Involvement of reactive oxygen species in cardiac preconditioning in rats. Antioxid Redox Signal 5: 115–122, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Traverse JH, Nesmelov YE, Crampton M, Lindstrom P, Thomas DD, Bache RJ. Measurement of myocardial free radical production during exercise using EPR spectroscopy. Am J Physiol Heart Circ Physiol 290: H2453–H2458, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Tsuchida A, Thompson R, Olsson RA, Downey JM. The anti-infarct effect of an adenosine A1-selective agonist is diminished after prolonged infusion as is the cardioprotective effect of ischaemic preconditioning in rabbit heart. J Mol Cell Cardiol 26: 303–311, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Wang P, Zweier JL. Measurement of nitric oxide and peroxynitrite generation in the postischemic heart: Evidence for peroxynitrite-mediated reperfusion injury. J Biol Chem 271: 29223–29230, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Yamashita N, Hoshida S, Otsu K, Asahi M, Kuzuya T, Hori M. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J Exp Med 189: 1699–1706, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamashita N, Hoshida S, Taniguchi N, Kuzuya T, Hori M. Whole-body hyperthermia provides biphasic cardioprotection against ischemia/reperfusion injury in the rat. Circulation 98: 1414–1421, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Yue Y, Krenz M, Cohen MV, Downey JM, Critz SD. Menadione mimics the infarct-limiting effect of preconditioning in isolated rat hearts. Am J Physiol Heart Circ Physiol 281: H590–H595, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Zhong B, Wang DH. TRPV1 gene knockout impairs preconditioning protection against myocardial injury in isolated perfused hearts in mice. Am J Physiol Heart Circ Physiol 293: H1791–H1798, 2007 [DOI] [PubMed] [Google Scholar]